Abstract
With the progressive aging of the world’s population, prolongation of a healthy lifespan in old age has become a medical research priority. The presence of depressive symptoms in later life is associated with poor health prognosis and increased mortality1,2. Here we explore distinct trajectories of depressive symptoms in later life and their association with several health-related outcomes in 19,110 older individuals followed for a median of 4.7 years. Using a latent class, mixed-modeling approach we identified four distinct trajectories of depressive symptoms with scoring patterns of consistently low, moderate, emerging and persistently high. Compared to those with minimal depressive symptoms, membership of any other class was associated with specific patterns of baseline sociodemographic and medical factors. Membership of any group with depressive symptoms was associated with a higher likelihood of health events, including physical disability, cancer and major bleeding episodes. Membership of the persistently depressed class was associated with increased mortality, while a diagnosis of dementia was generally limited to the class with initially low and progressively rising symptoms. The course of depressive symptoms in older individuals can vary widely and depend on several factors. The presence of depressive symptoms, including those that do not meet criteria for major depression, can flag a poor prognosis and risk for specific health conditions. Systematic assessment of depressive symptoms may facilitate early identification of at-risk populations.
Forecasting the course and potential consequences of an illness in subgroups of people who share risk factors and prognosis is an important step towards precision medicine and early intervention. The presence of depressive symptoms in later life, including those that do not meet criteria for major depression, is associated with poorer outcomes for a myriad of medical conditions3,4. Despite the marked effect of depression on physical health, depressive symptoms are often undiagnosed and untreated in the presence of comorbidities common in old age5. Evidence suggests that tackling depression in this context is effective and might improve clinical outcomes6.
However, most studies to date have relied on a single assessment of depression and/or focus on individual disease outcomes. Few longitudinal studies have investigated how distinct trajectories of depressive symptoms in older adults might be associated with differential clinical outcomes.
The very large ASPirin in Reducing Events in the Elderly (ASPREE) trial enabled modeling of distinct trajectories of depressive symptoms in later life and their association with a range of health outcomes documented in the study. We also explored sociodemographic and medical factors associated with each trajectory. Understanding factors that may be associated with resilience or vulnerability might inform early recognition and intervention opportunities, with the potential to alter outcomes and increase quality of life in an aging society.
Results
Individual participant data were used to model trajectories of depressive symptoms (assessed by the short version of the Center for Epidemiological Studies Depression (CES-D-10) scale), using latent class mixed models (LCMMs) for curvilinear longitudinal outcomes. Descriptive statistics of participants according to latent class are displayed in Table 1. Of the 19,110 participants included in this study (four did not have a CES-D measure at baseline and were excluded), 18,238 had at least one follow-up CES-D score, 10,779 (56.4%) were female and the mean age was 75 years.
Table 1 |.
Baseline characteristics of participants by latent class membership according to trajectory of depressive symptoms
| Nondepressed (n = 8,630) | Subthreshold depression (n = 7,449) | Persistent depression (n = 1,775) | Emerging depression (n = 1,256) | Total (n = 19,110) | |
|---|---|---|---|---|---|
|
| |||||
| Age (mean (s.d.)) | 75.0 (4.5) | 75.2 (4.6) | 74.9 (4.5) | 75.5 (4.7) | 75.1 (4.5) |
| Gender | |||||
| Male | 3,817 (44.2%) | 3,234 (43.4%) | 731 (41.2%) | 549 (43.7%) | 8,332 (43.6%) |
| Female | 4,813 (55.8%) | 4,215 (56.6%) | 1,044 (58.8%) | 707 (56.3%) | 10,779 (56.4%) |
| Living status | |||||
| At home alone or in a residential home | 2,709 (31.4%) | 2,555 (34.3%) | 708 (39.9%) | 361 (28.7%) | 6,333 (33.1%) |
| At home with someone | 5,921 (68.6%) | 4,894 (65.7%) | 1,067 (60.1%) | 895 (71.3%) | 12.777 (66.9%) |
| Race/ethnicity | |||||
| White/Caucasian | 8,012 (93.9%) | 6,900 (93.5%) | 1,603 (91.2%) | 1,179 (95.2%) | 17,694 (93.6%) |
| Other | 525 (6.1%) | 480 (6.5%) | 154 (8.8%) | 60 (4.8%) | 1,219 (6.4%) |
| Education | |||||
| ≤12 years | 4,761 (55.2%) | 4,318 (58.0%) | 1,106 (62.3%) | 766 (61.0%) | 10,951 (57.3%) |
| >12 years | 3,869 (44.8%) | 3.131 (42.0%) | 668 (37.7%) | 490 (39.0%) | 8,158 (42.7%) |
| Smoking status | |||||
| Current | 281 (3.3%) | 288 (3.9%) | 115 (6.5%) | 50 (4.0%) | 734 (3.8%) |
| Former | 3,311 (38.4%) | 3,198 (42.9%) | 769 (43.3%) | 519 (41.3%) | 7,797 (40.8%) |
| Never | 5,038 (58.4%) | 3,963 (53.2%) | 891 (50.2%) | 687 (54.7%) | 10,579 (55.4%) |
| Alcohol use | |||||
| Current | 6,566 (76.1%) | 5,830 (78.3%) | 1,295 (73.0%) | 947 (75.4%) | 14,638 (76.6%) |
| Former | 456 (5.3%) | 443 (5.9%) | 156 (8.8%) | 81 (6.4%) | 1,136 (5.9%) |
| Never | 1,608 (18.6%) | 1,176 (15.8%) | 324 (18.3%) | 228 (18.2%) | 3,336 (17.5%) |
| Body mass index (kg m−2) | |||||
| ≤25 | 2,291 (26.7%) | 1,944 (26.2%) | 409 (23.2%) | 332 (26.5%) | 4,976 (26.2%) |
| 25–30 | 3,944 (45.9%) | 3,203 (43.2%) | 737 (41.8%) | 555 (44.3%) | 8,439 (44.4%) |
| 30–35 | 1,746 (20.3%) | 1,625 (21.9%) | 424 (24.0%) | 267 (21.3%) | 4,062 (21.4%) |
| >35 | 603 (7%) | 647 (8.7%) | 195 (11.0%) | 99 (7.9%) | 1,544 (8.1%) |
| Quality of life score (measured on SF-12 scale) | |||||
| Physical component (mean (s.d.)) | 50.2 (7.7) | 47.1 (9.1) | 44.6 (10.2) | 48.2 (8.6) | 48.2 (8.8) |
| Mental component (mean (s.d.)) | 58.2 (5.2) | 54.5 (7.0) | 47.8 (9.1) | 56.7 (6.0) | 55.7 (7.1) |
| Number of medical comorbidities | |||||
| 0 | 488 (5.7%) | 337 (4.5%) | 53 (3.0%) | 53 (4.2%) | 931 (4.9%) |
| 1 | 1,919 (22.2%) | 1,379 (18.5%) | 287 (16.2%) | 243 (19.3%) | 3,828 (20.0%) |
| 2 | 2,988 (34.6%) | 2,349 (31.5%) | 527 (29.7%) | 394 (31.4%) | 6,258 (32.7%) |
| 3 | 2,037 (23.6%) | 1,934 (26.0%) | 486 (27.4%) | 329 (26.2%) | 4,786 (25.0%) |
| ≥4 | 1,198 (13.9%) | 1,450 (19.5%) | 422 (23.8%) | 237 (18.9%) | 3,307 (17.3%) |
| Polypharmacy (≥5 medications) | 1,322 (15.3%) | 1,685 (22.6%) | 549 (30.9%) | 285 (22.7%) | 3,841 (20.1%) |
| Antidepressant use | 593 (6.9%) | 932 (12.5%) | 457 (25.7%) | 162 (12.9%) | 2.144 (11.2%) |
| Aspirin arm | 4.345 (50.3%) | 3.767 (50.6%) | 871 (49.1%) | 603 (48.0%) | 9.586 (50.2%) |
Trajectory class identification.
As shown in Fig. 1, we found four distinct trajectories of depressive symptoms, reflecting patterns of consistently low, consistently moderate, consistently high and initially low but emerging symptoms of depression. Thus, we labeled the four trajectories as: “nondepressed” (n = 8,631, 45%; mean (s.d.) CES-D at baseline: 1.3 (1.6)) (reference group); “subthreshold depression” (n = 7,451, 39%; 4.5 (2.6)); “persistent depression” (n = 1,776, 9.3%; 8.5 (4.4)); and “emerging depression” (n = 1,256, 6.6%; 1.06 (1.3)). Figure 1 also compares the trajectories extracted from latent class analyses with summary descriptive of classes across waves.
Fig. 1 |. Trajectory of depressive symptoms.
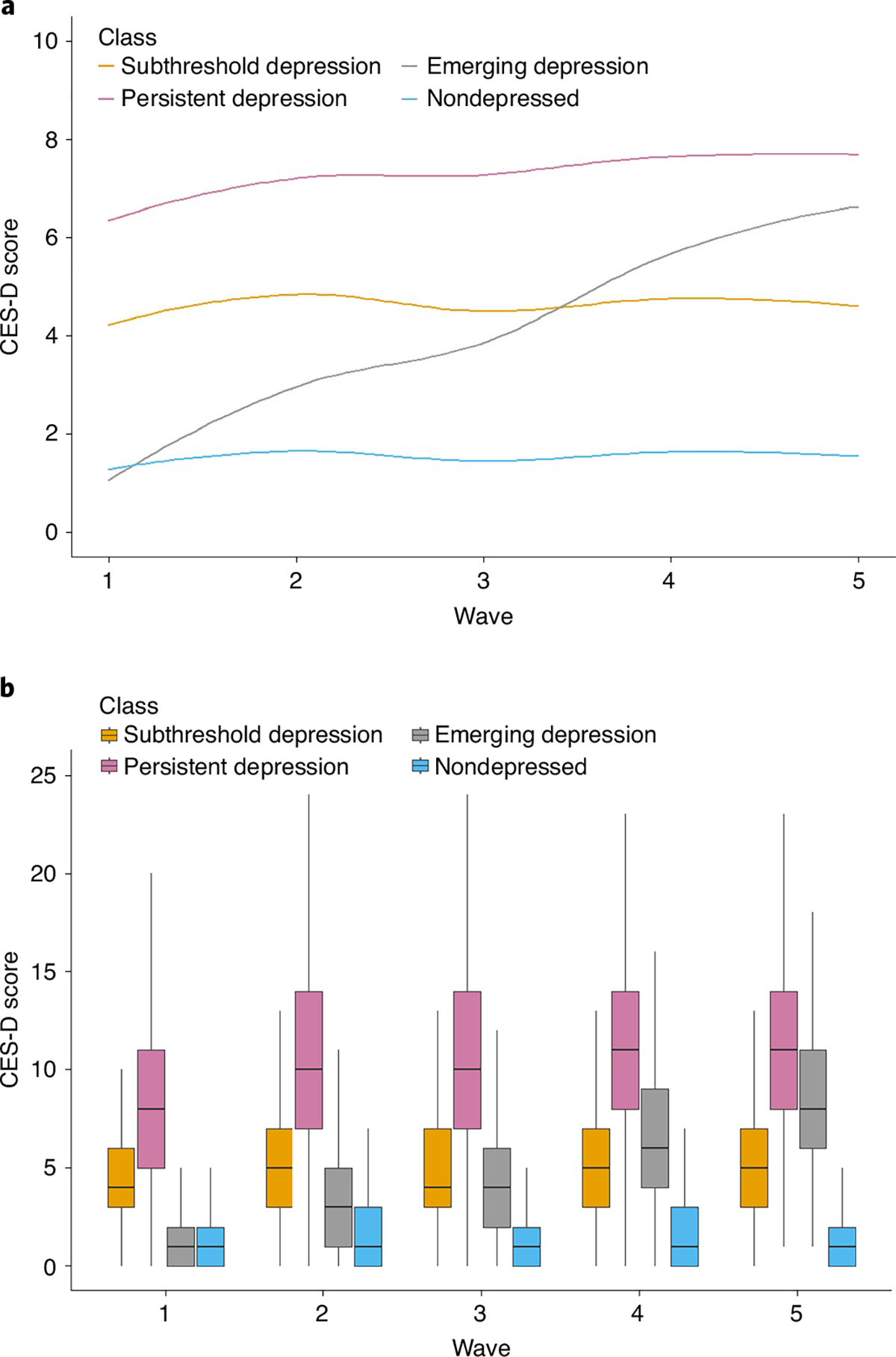
a, Model-based trajectory patterns according to latent class model. b, Summary descriptive of classes across the waves. Data are presented as Loess curves (a) and box plot graphs (b) showing the relationship between group membership and CES-D scores across follow-up (n = 19,110). b, Boxes show interquartile range, with solid horizontal lines representinging the median. Upper whiskers extend from the hinge to the highest value no further than 1.5× interquartile from the hinge; lower whiskers extend from the hinge to the lowest value no further than 1.5× interquartile from the hinge.
Profile of trajectory class members.
Participants’ characteristics according to their trajectory class are shown in Table 1. Compared with the nondepressed class, membership in any of the other classes was predicted by lower educational levels and lower quality of life scores (mental and physical components) at baseline. Female gender was a predictor for both subthreshold and persistently high classes. Persistently high symptoms were also associated with smoking, alcohol use and living alone or in a residential home. Conversely, the emergent group was associated with living with someone at baseline (Table 1 and Supplementary Table 2).
Figure 2 shows the association of latent class membership with the presence of medical comorbidities at baseline. Compared with the nondepressed class, significant baseline predictors of membership in any other class included polypharmacy and a history of depression. A history of cancer was associated with membership of the persistent class.
Fig. 2 |. Association between latent class membership and medical comorbidities at baseline.
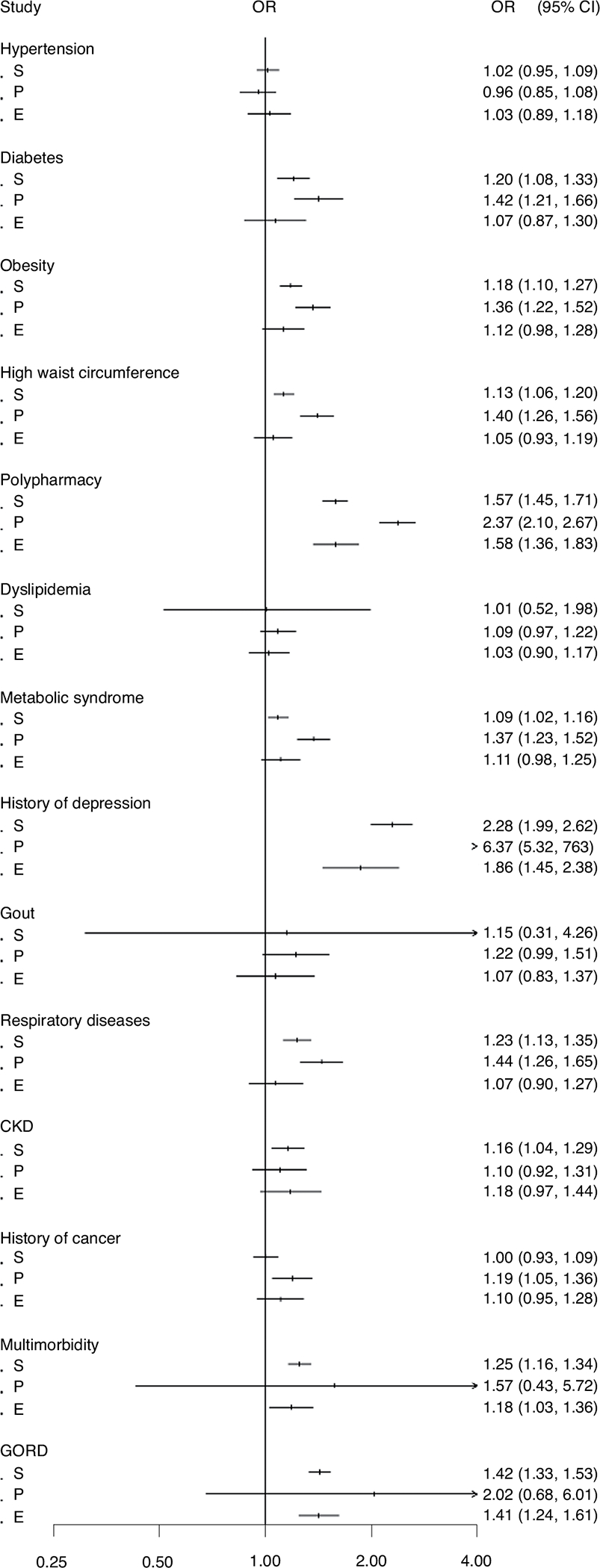
Data are presented as a forest plot reporting OR as dots and 95% CI as error bars from the logistic regression model, with comorbidity at baseline as the dependent variable and group membership as the independent variable (n = 19,110). The model was adjusted for age, gender, living status, race, education, smoking, alcohol, body mass index, PCS, MCS, number of comorbidities and polypharmacy. S, subthreshold depression class; P, persistent depression class; E, emerging depression class.
Membership of trajectories with chronic depressive symptoms (that is, subthreshold and persistently high class) was predicted by the presence of distinct medical comorbidities at baseline, most associated with metabolic abnormalities. These include diabetes, obesity, metabolic syndrome, high waist circumference, respiratory conditions and multimorbidity (Fig. 2). The presence of gastroesophageal reflux disease (GORD) was associated with both subthreshold and emerging trajectories, while chronic kidney disease (CKD) was a membership predictor only for the subthreshold class. The presence of hypertension, dyslipidemia and gout was not associated with membership in any other group when compared with the nondepressed population.
Trajectory of depressive symptoms and associated outcomes.
Results of regression models for the association between distinct trajectories of depressive symptoms and prespecified outcomes are displayed in Table 2. Compared with the nondepressed class, all other trajectories were significantly associated with increased odds of developing clinical depression, being diagnosed with cancer, developing physical disability (higher in the persistent class: OR: 4.96 (3.68, 6.69)) and/or having a major bleeding event (higher for the emergent trajectory: OR: 1.42 (1.02–1.97)) (Table 2). There was no statistically significant association between cardiovascular disease (CVD) events and any trajectory of depressive symptoms when compared with the nondepressed class.
Table 2 |.
OR for prespecified outcomes according to trajectory of depressive symptoms
| Outcome | Events (n (%)) | Model 1: OR (95% CI) | Model 2: OR (95% CI) | Model 3: OR (95% CI) |
|---|---|---|---|---|
|
| ||||
| Any outcome | 1,847 (9.7) | |||
| Nondepressed | 700 (8.1) | REF | REF | REF |
| Subthreshold depression | 747 (10) | 1.25 (1.11, 1.40) | 1.21 (1.07, 1.36) | 1.20 (1.06, 1.34) |
| Persistent depression | 258 (14.5) | 1.98 (1.70, 2,30) | 2.05 (1.76, 2.38) | 1.93 (1.65, 2.25) |
| Emerging depression | 142 (11.3) | 1.52 (1.26, 1.84) | 1.43 (1.18, 1.74) | 1.41 (1.16, 1.71) |
| Disability | 412 (2.2) | |||
| Nondepressed | 104 (1.2) | REF | REF | REF |
| Subthreshold depression | 179 (2.4) | 2.09 (1.61, 2.71) | 2.03 (1.56, 2.63) | 2.00 (1.54, 2.60) |
| Persistent depression | 93 (5.2) | 5.04 (3.75, 6.77) | 5.18 (3.86, 6.95) | 4.96 (3.68, 6.69) |
| Emerging depression | 36 (2.9) | 2.85 (1.92, 4.18) | 2.70 (1.83, 3.99) | 2.61 (1.77, 3.86) |
| Death | 1,052 (5.5) | |||
| Nondepressed | 429 (5.0) | REF | REF | REF |
| Subthreshold depression | 417 (5.6) | 1.10 (0.95, 1.28) | 1.07 (0.92, 1.24) | 1.04 (0.89, 1.21) |
| Persistent depression | 126 (7.1) | 1.51 (1.22, 1.87) | 1.57 (1.27, 1.95) | 1.43 (1.15, 1.78) |
| Emerging depression | 80 (6.4) | 1.34 (1.04, 1.73) | 1.25 (0.97, 1.62) | 1.23 (0.95, 1.59) |
| CVD | 782 (4.1) | |||
| Nondepressed | 327 (3.8) | REF | REF | REF |
| Subthreshold depression | 319 (4.3) | 1.16 (0.98, 1.37) | 1.14 (0.97, 1.35) | 1.12 (0.95, 1.32) |
| Persistent depression | 75 (4.2) | 1.26 (0.99, 1.64) | 1.31 (1.01, 1.70) | 1.23 (0.96, 1.59) |
| Emerging depression | 61 (4.9) | 1.36 (1.03, 1.82) | 1.30 (0.98, 1.73) | 1.28 (0.98, 1.71) |
| Dementia | 575 (3.0) | |||
| Nondepressed | 231 (2.7) | REF | REF | REF |
| Subthreshold depression | 233 (3.1) | 1.14 (0.95, 1.39) | 1.11 (0.91, 1.35) | 1.11 (0.92, 1.35) |
| Persistent depression | 62 (3.5) | 1.30 (0.96, 1.76) | 1.33 (0.98, 1.80) | 1.32 (0.97, 1.79) |
| Emerging depression | 49 (3.9) | 1.50 (1.08, 2.08) | 1.41 (1.02, 1.96) | 1.42 (1.02, 1.97) |
| Cancer | 1,932 (10.1) | |||
| Nondepressed | 811 (9.4) | REF | REF | REF |
| Subthreshold depression | 777 (10.4) | 1.13(1.02, 1.26) | 1.13 (1.02, 1.26) | 1.12 (1.01, 1.24) |
| Persistent depression | 192 (10.8) | 1.22 (1.04, 1.45) | 1.25 (1.06, 1.48) | 1.22 (1.03, 1.44) |
| Emerging depression | 152 (12.1) | 1.33 (1.11, 1,60) | 1.32 (1.10, 1.58) | 1.30 (1.09, 1.57) |
| Depression a | 6,007 (31.4) | |||
| Nondepressed | 447 (5.2) | REF | REF | REF |
| Subthreshold depression | 3,258 (43.7) | 14.11 (12.61, 15.79) | 14.24 (12.73, 15.93) | 14.18 (12.68, 15.86) |
| Persistent depression | 1,604 (90.4) | 91.2 (80.74, 1013.15) | 93.08 (82.2, 105.29) | 92.07 (81.53, 104.43) |
| Emerging depression | 698 (55.6) | 18.41 (16.24, 20.87) | 18.49 (16.31, 20.96) | 18.35 (16.18, 20.81) |
| Major hemorrhage | 623 (3.3) | |||
| Nondepressed | 229 (2.7) | REF | REF | REF |
| Subthreshold depression | 269 (3.6) | 1.31 (1.09, 1.58) | 1.28 (1.06, 1.54) | 1.26 (1.04, 1.52) |
| Persistent depression | 65 (3.7) | 1.47 (1.11, 1.95) | 1.51 (1.14, 2.01) | 1.45 (1.09, 1.94) |
| Emerging depression | 60 (4.8) | 1.94 (1.45, 2.59) | 1.85 (1.38, 2.47) | 1.84 (1.37, 2.46) |
Results from logistic regressions using GEEs with robust variance estimator and within-group exchangeable correlation matrix to account for clustered nature of data. Model 1 was unadjusted, model 2 was adjusted for gender and age and model 3 was adjusted for age, gender, race, smoking, alcohol consumption, education and living arrangement. REF, reference group (non depressed).
Clinically relevant depression was characterized as reaching a score of eight or more points at any time on the CES-D-10 scale.
Membership in the persistently depressed class was significantly associated with increased mortality (OR: 1.43 (1.15–1.78)), while a diagnosis of dementia was generally limited to the class with emerging symptoms (OR: 1.42 (1.02–1.97)) (although statistical significance was lost after adjusting for multiple comparisons). Also, a trend was seen for the association between dementia and membership in the persistent depression class (OR: 1.32 (0.97–1.79)).
In subgroup analyses divided by gender (Supplementary Table 1), we found that women in the persistently depressed class had higher odds of mortality (OR: 1.58 (1.51–2.17)) and dementia (OR: 1.53 (1.03–2.26)), while in males a similar pattern was seen for the emerging depression class, with odds for mortality (OR: 1.35 (0.99–1.87) and dementia (OR: 1.48 (0.94–2.35)) showing a trend for statistical significance in this class, but not in others. Males were also more likely to have a major bleeding episode if they were either in the persistently depressed class (OR: 1.65 (1.11–2.46)) or the emerging depression class (OR: 2.30 (1.57–3.37)), with higher odds seen for the latter. Cancer was statistically significant in males in the emerging depression class (OR: 1.53 (1.21–1.94)) but not in other classes and not in females, although a trend was seen for women in the persistently depressed class (OR: 1.21 (0.95–1.54)). As a sensitivity analysis, model 3 was employed without adjusting for sex and age since these factors were used in developing latent classes. The findings remained unchanged although there was a trend for larger effects.
Effects of aspirin.
Subgroup analyses divided by treatment arm (aspirin versus placebo) showed that aspirin users had increased odds of death in both persistently depressed (OR: 1.85 (1.34–2.56)) and emerging depression (OR: 1.51 (1.05–2.16)) classes, with no statistically significant association seen for placebo users. Aspirin users who were members of any of the three classes with depressive symptoms were also more likely to have a diagnosis of cancer, while in placebo users this association was seen only in the emerging depression trajectory. Conversely, membership in the emerging depression class was associated with increased bleeding only in placebo users (OR: 2.17 (1.35–3.46)), with no significant association for other classes or for the aspirin subgroup (Supplementary Table 2).
Discussion
This study used a large sample to investigate the longitudinal association of distinct depressive trajectories in later life and many serious health-related outcomes. We were able to identify four distinct trajectories of late-life depressive symptoms. Compared with the nondepressed class, membership in any class with depressive symptoms (including subthreshold) was associated with serious health-related outcomes, including a greater risk of persistent physical disability, cancer and major bleeding episodes. Persistently high scores were associated with increased mortality and increased odds of developing a persistent physical disability. A pattern of initially low but consistently rising (emerging) depressive symptoms was associated with a diagnosis of dementia. These results extend the notion that the presence of depressive symptoms, including those that do not meet criteria for major depression, may be signals of overall health and prognosis in later life. Consequently, attention to and follow-up of depressive symptomatology in clinical settings might increase recognition of individuals at risk for serious health-related events and facilitate prompt intervention.
Our results agree with most studies showing that depression in later life tends to stability and chronicity. Over 90% of our sample was included in classes with stable trajectories, be it nondepressed, subthreshold or persistent depression. Unlike other studies, mostly conducted in younger populations7–10, we did not find a group with decreasing symptoms, suggesting that risk factors for depression might already be established in this older adult sample. Symptoms of depression (as measured by CES-D scores) tend to follow a U-shaped pattern across adulthood and consistently rise after the seventh decade, when sex differences in prevalence also tend to converge11. Notably, physical limitation, disease burden and/or impending death seem to explain only part of the increase in depressive symptoms in this age group11.
Our results concur with extensive data linking late-life depression and poor health-related outcomes12–15. Here we found that membership in any class with chronic depressive symptoms (both subthreshold and persistent) was predicted by higher levels of medical comorbidities at baseline, most characterized by metabolic dysfunction and chronic, low-grade inflammation16. Mounting evidence suggests that depression might independently potentiate the chronic effects of these conditions via inflammation and oxidative stress17, potentially accelerating age-related biological processes that can lead to cellular senescence and reduced capacity of organ regeneration and/or promote activation of oncogenic factors linked to increased cellular replication and cancer18. Late-life depression is associated with increased biological aging and increased markers of cellular senescence19, as well as higher levels of inflammation20 and oxidative stress21, known contributors to frailty, vascular damage and their inherent consequences, including physical disability and death16.
We found that mortality was significantly associated with membership of only the persistently depressed class. This might reflect a short follow-up period, since it contrasts with a 12-year longitudinal study which found that increasing (but not persistent) depressive symptoms were associated with increased mortality, although that was conducted in a younger and smaller population9. Besides having higher rates of medical comorbidities and obesity, persistently depressed people in our study were also more likely to be smokers, drink alcohol and live alone, factors previously associated with both mortality and late-life depression22. Notably, this class has a greater history of depression at baseline. Emerging data show that previous depression is associated with higher levels of inflammatory and metabolic disturbances later in life (especially in women)23, and increased risk of subsequent somatic diseases and premature death24. This highlights the importance of early detection and effective management of depression earlier in life.
On the other hand, dementia was associated with membership of only the emerging depression class and lost statistical significance after adjusting for multiple comparisons. This is in line with previous studies showing that a pattern of low, but progressively increasing, depressive symptoms that emerge later in life is more strongly related to dementia and cognitive decline than persistently high depressive symptoms8,10,25. One recent neuroimaging study found that those who develop increasing symptoms later in life have more neurological abnormalities and a higher load of vascular risk factors than those with persistently high scores25. Nevertheless, persistent depression is also associated with increased risk of dementia when compared with groups having subthreshold and/or minimal symptoms10,26. This might reflect different biological processes, and there is a debate as to whether depression is a prodromal stage of dementia or a modifiable factor, with most evidence suggesting the former27. While vascular changes might drive specific subtypes of both depression and dementia, other factors such as neuroinflammation, impaired neurogenesis, changes in gut and brain membrane permeability and associated dysbiosis can all contribute to brain dysfunction and progressive mood and cognitive decline28.
Our findings of increased major bleeding in all groups (but greater in the emergent symptoms class) might also hint that covert gastrointestinal lesions and resulting dysbiosis may play a role in this association29. Depression has been genetically and epidemiologically linked to increased risk of inflammatory and hemorrhagic gastrointestinal diseases30. Depression is also associated with increased markers of intestinal permeability which, in turn, correlate with severity and clinical response31,32. This study documents risk for major bleeding episodes only, but it is possible that smaller, clinically undetectable, lesions are more frequent. While these might be of little clinical significance, their potential for altering gut permeability can have important consequences in the gut microenvironment, possibly accelerating biological processes related to dysbiosis, inflammation and microvascular disease33,34.
We recently published data showing that aspirin may have a deleterious effect on the mood of depressed older adults, and we speculated that changes in intestinal permeability could be one possible biological driver of this association29. Here we found that aspirin users with depressive symptoms have an increased association with cancer and death when compared with aspirin users with no depression. The ASPREE main trial previously reported an increase in mortality, primarily driven by cancer-related death, in those taking aspirin35. Interestingly, colon cancer had the strongest correlation with death in that study. If our contention is correct, aspirin’s potential to alter intestinal permeability in those with existing vulnerabilities (that is, depressed individuals) might facilitate metastasis and further explain this association.
In exploratory analyses, we found some small differences in outcomes when we divided the sample by sex. Women in the persistently depressed class were at increased risk of death, dementia and cancer, while the strongest associations for these outcomes (and bleeding events) were found in men with low but emerging depressive symptoms. Sex differences are important in biological research but, due to the exploratory nature of these findings and the loss of statistical power in subgroup analyses, we recommend caution when interpreting these results.
The strengths of this study include its very large sample size, which gave us power to test our hypotheses in well-powered models using a sophisticated statistical method that considers interindividual variability and the nonlinear pattern of trajectories. The relatively long follow-up period and the rigorous methods used to document described outcomes are other strengths. Nevertheless, it is known that midlife lifestyle and health can have a significant impact on late-life outcomes, and these could not be accounted for in this study. However, the stringent exclusion criteria of the trial allowed us to presume that this is a somewhat homogeneous sample regarding previous health status. While this is certainly a strength, it limits generalizability to other (less healthy) populations.
Other limitations include the use of a scale to define depression rather than a structured clinical interview. Self-report scales, however, reduce inter-rater bias, and the fact that we used depressive scores as continuous (other than categorical) variables increases the validity of this method for our purposes. Due to the bidirectional relationship between depression and the investigated outcomes, we cannot exclude that the same underlying processes driving depression are also involved in the initial (preclinical) stages related to the investigated outcomes. This precluded any causal conclusions and limited interpretation of the findings as associations (that is, noncausal). Furthermore, because we explore several prespecified outcomes, inflation of type 1 error due to multiple comparisons cannot be excluded (although we did correct for false discovery rate)36. Despite proposing mechanistic explanations for our findings, we do not yet have data on biomarkers of inflammation, gut permeability or neuroimaging to confirm our hypotheses. Future studies might integrate these data to provide a clearer picture of the biological mechanisms underpinning these associations.
In conclusion, we found that depressive symptoms in later life tend to present distinct trajectories. The presence of depressive symptoms, including those that do not meet criteria for depressive episodes, can flag up several critical health-related outcomes. These results should drive clinical and public health efforts for systematic assessment and follow-up of depressive symptoms in later life to allow identification and early intervention for at-risk populations.
Methods
Study population.
Study participants were community-dwelling older adults enrolled in the ASPREE trial. ASPREE was a large multicentre, population-based, double-blind, placebo-controlled randomized trial investigating the effects of low-dose aspirin on several endpoints in older adults living in Australia and the United States. The ASPREE trial recruited 19,114 participants between 2010 and 2014 from primary care services in Australia and through clinic-based mailing lists, electronic records and advertisements in the United States. Methods and baseline characteristics of ASPREE participants have been described in detail elsewhere37,38.
Eligibility criteria for ASPREE included community-dwelling men and women aged 70 years and older (65 years of age and older for US minorities), who gave written informed consent. Participants were excluded if they had a current indication for, or contraindication to, the use of aspirin (trial drug) or had any component of the composite primary outcomes. The following were exclusion criteria: a previous cardiovascular event or established CVD or atrial fibrillation; diagnosed dementia or a score of <78 on the Modified Mini-Mental State examination; the presence of significant physical disability (defined by severe difficulty or inability to perform any one of the basic activities of daily living); a condition with a high current or recurrent risk of bleeding; anemia; a condition likely to cause death within 5 years; current use of other antiplatelet or antithrombotic medication; current use of aspirin for secondary prevention; or severe uncontrolled hypertension (that is, systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥105 mmHg). Notably, the stringent exclusion criteria for the ASPREE study resulted in an overall sample generally healthier than their population counterparts. Correspondingly, baseline quality of life scores were slightly higher than those reported in population-based studies of older individuals39.
The trial was conducted according to the Australian National Statement on Ethical Conduct in Human Research, the Australian Code for the Responsible Conduct of Research, the 2008 Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice E6, and was approved by institutional review boards at all sites. The protocol was published40, developed in accordance with Standard Protocol Items Recommendations for Intervention Trials 2013 guidelines, reported using the Consolidated Standards of Reporting Trials guidelines and according to the ICH E9 Statistical Principles for Clinical Trials and was registered on ClinicalTrials.gov (identifier: NCT01038583). Institutional review boards at each participating institution approved the trial, and these are listed on the clinicaltrials.gov site for ASPREE.
Measures.
Baseline instruments.
Sociodemographic questionnaires were administered at baseline. Information obtained included age, gender, education, race, smoking status, alcohol use, living status, number and type of current medications used and self-reported presence and/or history of medical conditions.
The presence of medical comorbidities at baseline was ascertained from self-report, medication use and direct physical and laboratory measures (with thresholds defined in medical guidelines). A list of all medical comorbidities investigated and their definitions was published41 and included hypertension, diabetes, obesity, dyslipidemia, metabolic syndrome, GORD, respiratory disorders, CKD and gout. Polypharmacy was defined as the simultaneous use of five or more medications41.
The Quality-of-Life Short Form 12 (SF-12) questionnaire was used to rate quality of life at baseline42. SF-12 is composed of a physical component summary (PCS) and a mental component summary score (MCS) designed to provide an indication of the physical and mental health of respondents, respectively.
Assessment of depressive symptoms.
Depressive symptoms were assessed annually using the short version of the CES-D-10 scale. CES-D-10 is a self-rated questionnaire that scores the severity of depressive symptoms “during the past week”. This instrument has performance comparable to the full version of CES-D (kappa = 0.97) in classifying participants with depressive symptoms43. Our assessment of the construct validity of CES-D-10 showed that a single score was a reliable and valid measure of depression in this population44. When compared with a formal psychiatric diagnosis of depression in old age, the scale demonstrated a sensitivity of 97% and specificity of 84% (ref. 45). In line with previous research, a cut-off of ≥8 was operationalized as a positive screen for depression in ASPREE22.
Outcomes of interest.
Outcomes of interest included every prespecified primary and secondary endpoint of the ASPREE trial, namely persistent physical disability, dementia, CVD events, cancer, major bleeding and death. All clinical and safety endpoints were adjudicated by blinded endpoint adjudication committees who were provided with deidentified clinical information. Criteria for each of these events are described in detail in the respective ASPREE papers35,46,47, but can be summarized as:
Primary outcome: primary composite derived from first occurrence of the endpoint of death, dementia or persistent physical disability.
Persistent physical disability: inability to perform or severe difficulty in performing at least one of the six basic activities of daily living that had persisted for at least 6 months.
Death: confirmation of death via two independent sources.
Dementia: cognitive decline in two domains associated with functional decline, adjudicated according to Diagnostic and Statistical Manual of Mental Disorders (4th edition) criteria.
CVD: composite of fatal coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal stroke or hospitalization for heart failure.
Cancer: histopathological confirmation.
Major depression: clinically relevant depression was characterized as reaching a score of ≥8 points at any time in the CES-D scale.
Major bleeding: composite of hemorrhagic stroke, symptomatic intracranial bleeding or clinically significant extracranial bleeding (defined as bleeding that led to transfusion, hospitalization, surgery or death).
Statistical analysis.
Individual participant data were utilized to model trajectories of depressive symptoms in up to five follow-up waves using LCMMs for curvilinear longitudinal outcomes according to specific trajectories of CES-D scores across annual visits (number of CES-D assessments in each wave of study: wave 1, n = 19,110; wave 2, n = 18,097; wave 3, n = 7,129; wave 4, n = 15,167; wave 5, n = 10,406). Missing data in one or more follow-up waves were imputed using single imputation with fully conditional specification implemented by predictive mean matching, using age and sex as auxiliary variables48.
We implemented nonlinear LCMMs to handle a nonlinear pattern of CES-D trajectories49. To find the best model fit, we evaluated a wide range of linear and nonlinear LCMMs following recommended procedures, as follows:49
LCMMs with different numbers of latent classes, ranging from one to five classes, were investigated.
We examined the role of gender and age interactions with follow-up time points to improve class membership prediction.
LCMMs with linear and two spline link functions (that is, either three or five knots placed in percentiles of the outcome variable) were considered.
We compared models using log-likelihood, entropy, the Akaike information criterion (AIC) and the Bayesian information criterion (BIC)49. Entropy was used to measure the accuracy of classification, ranging from 0 to 1, with higher values indicating better classification. AIC and BIC provide information on how well each model fits the data, with lower values indicating better model fit50. Log-likelihood represents the combination of model parameter values that maximize the probability of drawing the sample obtained, with higher log-likelihood indicating better consistency between model and obtained data51.
We ran each model seven times through a grid of different sets of initial values, multiplied by a random number to ensure that likelihood solutions were not locally optimized. Maximum-likelihood estimators were obtained using a modified Marquardt algorithm with strict convergence criteria based on the parameters and likelihood stability, and on the negativity of the second derivatives49.
Our vigorous model search strategy indicated that a model with four latent classes, with a five-knot spline link function and without gender and time interaction, was the best fit for these data and was therefore used in subsequent analyses (Supplementary Table 3). Participants were then classified according to their maximum-likelihood class membership, using gender and age as auxiliary variables (posterior classification probabilities are available in Supplementary Table 4). We further conducted visual data inspection of trajectories divided by gender and found no significant differences, and thus we used the whole sample for our primary outcomes.
Baseline characteristics were summarized in each latent class (based on trajectory) by mean and s.d. for continuous variables or frequency (%) for categorical variables. The criterion validity of the model was confirmed by assessing sociodemographic associations with group classification membership through multinominal logistic regression modeling (Supplementary Table 5). We further investigated the association between class membership and the presence of medical comorbidities at baseline, and present these in a forest plot (Fig. 2).
To examine the association between depressive symptom trajectories and any of the outcomes of interest, we fitted multivariable logistic regressions using GEEs with robust variance estimator and a within-group exchangeable correlation matrix to account for the clustered nature of data. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. We selected a GEE model with unstructured covariance matrix and robust standard error to account for covariance misspecification52. For each outcome, three models were presented: model 1 was unadjusted; model 2 was adjusted for gender and age while model 3 was adjusted for age, gender, ethnicity/race, smoking, alcohol consumption, education and living arrangements, factors previously associated with depression at baseline in this population22. To account for the multiple comparison problem, we adjusted P values in model 3 (main model for interpretation) using the Benjamini-Hochberg method36.
Due to this study being part of a randomized clinical trial, and after our findings showing that aspirin could adversely impact depressive symptoms29, we ran subgroup analyses divided by treatment arm (aspirin versus placebo), antidepressant use and the combination of antidepressant and aspirin. In exploratory analyses based on previous literature, we further investigated outcomes divided by gender. Because age and gender were accounted for in the development of latent classes, we further fitted model 3 for each outcome without adjusting for age and gender as a sensitivity analysis to evaluate potential overadjustment bias for age and gender. All GEE models were fitted using STATA software, v.15.0. (StataCorp), multiple imputation with “mice” package48 and LCMMs with the “lcmm” package in R49.
Supplementary Material
Acknowledgements
The ASPREE trial was supported by grants from the National Institute on Aging and the National Cancer Institute at the US National Institutes of Health (NIH, nos. U01AG029824 and U19AG062682); the National Health and Medical Research Council of Australia (NHMRC) (nos. 334047, 1081901 and 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia). M.B. is supported by a NHMRC Senior Principal Research Fellowship (no. 1156072), C.M.R. by a NHMRC Principal Research Fellowship (no. 1136372) and L.J.W. by a NHMRC Emerging Leadership Fellowship (no. 1174060). M.L. is funded by the Alfred Deakin Postdoctoral Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank the ASPREE participants who volunteered for this study, the general practitioners and staff of the medical clinics who support the study participants and the trial staff and management team of the ASPREE study in Australia and the United States (www.aspree.org).
Footnotes
Competing interests
The authors declare the following potential competing interests. M.B. has received grant/research support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, NHMRC, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation; has been a speaker for AstraZeneca, Lundbeck, Merck and Pfizer; and served as a consultant to Allergan, AstraZeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. M.R.N. is member of the Novartis lipids advisory board and received travel and advisory board support from Bayer AG, who provided product for the ASPREE study. A.T. has received honoraria for Safety Monitoring Committee or Advisory Board participation, or lectures from Amgen, Boehringer-Ingelheim, The Medicines Group, Novartis, Pfizer and Merck; and research support from Bayer for materials in ASPREE—all unrelated to this work. These funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Code availability
Codes are stored in the ASPREE web-based data portal safe haven, based at Monash University. They are available upon request following the procedures described above and on www.ASPREE.org.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s43587-022-00203-1.
Peer review information Nature Aging thanks Gindo Tampubolon, Mark Ward and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Reprints and permissions information is available at www.nature.com/reprints.
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The individual participant data that underlie the results reported in this article will be made available after deidentification. Requests for data access will be via the ASPREE Principal Investigators, with details for applications provided through the website, www.ASPREE.org, and in accordance with the NIH policy on data sharing: details available at https://grants.nih.gov/grants/policy/data_sharing/. Data availability will commence on publication of this article. The supporting Protocol and Statistical Analysis Plan is already available as an independently published article53. These data will be available upon request to investigators whose proposed use of the data, registered as a project through the ASPREE Access Management Site: https://ams.aspree.org/public/, has been approved by a review committee. These data will be available through a web-based data portal safe haven, based at Monash University, Australia.
References
- 1.Moussavi S et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Wei J et al. The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: systematic review and meta-analysis. Br. J. Psychiatry 215, 449–455 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS Mechanisms and treatment of late-life depression. Transl. Psychiatry 9, 188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold SM, et al. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers 6, 69 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Menear M et al. The influence of comorbid chronic physical conditions on depression recognition in primary care: a systematic review. J. Psychosom. Res. 78, 304–313 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Unützer J et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. J. Am. Med. Assoc. 288, 2836–2845 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Kuchibhatla MN, Fillenbaum GG, Hybels CF & Blazer DG Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatr. Scand. 125, 492–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza SS et al. 10-Year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 3, 628–635 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Saeed Mirza S et al. 12 Year trajectories of depressive symptoms in community-dwelling older adults and the subsequent risk of death over 13 years. J. Gerontol. A Biol. Sci. Med. Sci. 73, 820–827 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Formánek T et al. Trajectories of depressive symptoms and associated patterns of cognitive decline. Sci. Rep. 10, 20888 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutin AR et al. The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry 70, 803–811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Silva SA, Scazufca M & Menezes PR Population impact of depression on functional disability in elderly: results from “São Paulo Ageing & Health Study” (SPAH). Eur. Arch. Psychiatry Clin. Neurosci. 263, 153–158 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Ly M et al. Late-life depression and increased risk of dementia: a longitudinal cohort study. Transl. Psychiatry 11, 147 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YH et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol. Psychiatry 25, 1487–1499 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Machado MO et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. 16, 112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrucci L & Fabbri E Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mclaughlin AP et al. The influence of comorbid depression and overweight status on peripheral inflammation and cortisol levels. Psychol. Med. 18, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman D et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diniz BS, Iii CFR, Sibille E, Bot M & Penninx BWJH. Major depression and enhanced molecular senescence abnormalities in young and middle-aged adults. Transl. Psychiatry 9, 198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Cengotitabengoa M et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int. J. Mol. Sci. 17, 2022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diniz BS et al. Oxidative stress markers imbalance in late-life depression. J. Psychiatr. Res. 102, 29–33 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Mohebbi M et al. Prevalence of depressive symptoms and its associated factors among healthy community-dwelling older adults living in Australia and the United States. Int. J. Geriatr. Psychiatry 34, 1208–1216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ploubidis GB, Batty GD, Patalay P, Bann D & Goodman A Association of early-life mental health with biomarkers in midlife and premature mortality: evidence from the 1958 British birth cohort. JAMA Psychiatry 78, 38–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leone M et al. Association of youth depression with subsequent somatic diseases and premature death. JAMA Psychiatry 78, 302–310 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demnitz N et al. Association of trajectories of depressive symptoms with vascular risk, cognitive function and adverse brain outcomes: the Whitehall II MRI sub-study. J. Psychiatr. Res. 131, 85–93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaup AR et al. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 73, 525–531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida OP, Hankey GJ, Yeap BB, Golledge J & Flicker L Depression as a modifiable factor to decrease the risk of dementia. Transl. Psychiatry 7, e1117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley EMM Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 17, 94 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Berk M et al. Effects of aspirin on the long-term management of depression in older people: a double-blind randomised placebo-controlled trial. Mol. Psychiatry 26, 5161–5170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulugeta A, Zhou A, King C & Hyppönen E Association between major depressive disorder and multiple disease outcomes: a phenome-wide Mendelian randomisation study in the UK Biobank. Mol. Psychiatry 25, 1469–1476 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson L et al. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 139, 185–193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liśkiewicz P et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 11076 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Snelson M et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 7, eabe4841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgueño JF & Abreu MT Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 17, 263–278 (2020). [DOI] [PubMed] [Google Scholar]
- 35.McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. New Engl. J. Med. 379, 1519–1528 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 57, 289–300 (1995). [Google Scholar]
- 37.Group AI Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp. Clin. Trials 36, 555–564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeil JJ et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1586–1593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stocks NP et al. Quality of life for 19,114 participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study and their association with sociodemographic and modifiable lifestyle risk factors. Qual. Life Res. 28, 935–946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berk M et al. ASPREE-D: aspirin for the prevention of depression in the elderly. Int. Psychogeriatr. 28, 1741–1748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agustini B et al. Patterns of association between depressive symptoms and chronic medical morbidities in older adults. J. Am. Geriatr. Soc. 68, 1834–1841 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandek B et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J. Clin. Epidemiol. 51, 1171–1178 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Chang K-F & Weng L-J Screening for depressive symptoms among older adults in Taiwan: cutoff of a short form of the Center for Epidemiologic Studies Depression Scale. Health (Irvine) 5, 588–594 (2013). [Google Scholar]
- 44.Mohebbi M et al. Psychometric properties of a short form of the Center for Epidemiologic Studies Depression (CES-D-10) scale for screening depressive symptoms in healthy community dwelling older adults. Gen. Hosp. Psychiatry 51, 118–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irwin M, Artin KH & Oxman MN Screening for depression in the older adult. Arch. Intern Med. 159, 1701–1704 (1999). [DOI] [PubMed] [Google Scholar]
- 46.McNeil JJ et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. New Engl. J. Med. 379, 1509–1518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNeil JJ et al. Effect of aspirin on disability-free survival in the healthy elderly. New Engl. J. Med. 379, 1499–1508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Buuren S & Groothuis-Oudshoorn K mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 49.Proust-Lima C, Philipps V & Liquet B Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J. Stat. Softw. 78, 1–56 (2017). [Google Scholar]
- 50.Berlin KS, Williams NA & Parra GR An introduction to latent variable mixture modeling (Part 1): overview and cross-sectional latent class and latent profile analyses. J. Pediatr. Psychol. 39, 174–187 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Myung IJ Tutorial on maximum likelihood estimation. J. Math. Psychol. 47, 90–100 (2003). [Google Scholar]
- 52.Hubbard AE et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 21, 467–474 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Wolfe R et al. The aspirin in reducing events in the elderly trial: statistical analysis plan. Int. J. Stroke 13, 335–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant data that underlie the results reported in this article will be made available after deidentification. Requests for data access will be via the ASPREE Principal Investigators, with details for applications provided through the website, www.ASPREE.org, and in accordance with the NIH policy on data sharing: details available at https://grants.nih.gov/grants/policy/data_sharing/. Data availability will commence on publication of this article. The supporting Protocol and Statistical Analysis Plan is already available as an independently published article53. These data will be available upon request to investigators whose proposed use of the data, registered as a project through the ASPREE Access Management Site: https://ams.aspree.org/public/, has been approved by a review committee. These data will be available through a web-based data portal safe haven, based at Monash University, Australia.


