Abstract
Signaling pathways involving second messenger c-di-GMP regulate various aspects of bacterial physiology and behavior. We describe the use of a red light-activated diguanylate cyclase (c-di-GMP synthase) and a blue light-activated c-di-GMP phosphodiesterase (hydrolase) for manipulating intracellular c-di-GMP levels in bacterial cells. We illustrate the application of these enzymes in regulating several c-di-GMP-dependent phenotypes, i.e. motility and biofilm phenotypes in E. coli and chemotactic behavior in the alphaproteobacterium Azospirillum brasilense. We expect these light-activated enzymes to be also useful in regulating c-di-GMP-dependent processes occurring at the fast timescale, for spatial control of bacterial populations, as well as for analyzing c-di-GMP-dependent phenomena at the single-cell level.
Keywords: cyclic dimeric GMP, optogenetics, photoactivation, motility, biofilm, chemotaxis, diguanylate cyclase, phosphodiesterase
1. Introduction
Cyclic dimeric GMP (c-di-GMP) regulates various aspects of bacterial physiology and behavior including motility, surface attachment, formation and dispersion of biofilms, cell cycle, differentiation, production of secondary metabolites, and virulence [1]. The ability to manipulate intracellular c-di-GMP levels in real time may provide important insights into these processes. Inducible expression of the genes encoding enzymes involved in c-di-GMP synthesis and hydrolysis, i.e. diguanylate cyclases (DGCs) or c-di-GMP phosphodiesterases (PDEs), may not be adequate for this purpose because of the delays associated with gene expression. Further, removing gene expression inducer to reverse the process is difficult without disturbing the system. In contrast, the DGCs and PDEs whose activities are turned on by light of specific wavelengths can be controlled at high temporal resolution. These enzymes are also well suited for interrogating biological processes at high spatial resolution because light can be focused on a subpopulation of bacteria or even on individual cells. We describe the use of the recently engineered light-responsive DGC and a PDE whose activities are turned on by red and blue light, respectively [2-4]. Either one or both enzymes can be expressed in the desired bacterial cells or other types of cells for unidirectional or bidirectional modulation of c-di-GMP levels.
At the beginning of the chapter we describe the use of the photoactivated enzymes for modulating c-di-GMP concentrations in Escherichia coli that result in changes in motility and biofilm phenotypes. These static assays, which are easy to perform, intend to train an inexperienced user in handling light-sensitive bacteria and observing straightforward light-dependent phenotypic changes. The more challenging applications of these enzymes involve manipulating c-di-GMP-dependent processes that occur at fast (seconds-to-minutes) timescales, which are illustrated by the effect of changing c-di-GMP levels on oxygen-dependent chemotaxis (aerotaxis) in the alphaproteobacterium Azospirillum brasilense [5]. The photo-activated enzymes involved in c-di-GMP turnover can also be adapted for regulating gene expression [3]; however, such modality is not discussed here.
The two light-activated enzymes described in this chapter are BphS and EB1. BphS [3] is a red/near-infrared light controlled DGC, which synthesizes c-di-GMP from the intracellular pool of GTP. Light enhances DGC activity of BphS by ~11-fold (at room temperature in vitro), compared to the protein activity in the dark. As a photoreceptor of the bacteriophytochrome class, BphS uses biliverdin IXα as a light absorbing chromophore [6]. Biliverdin IXα is a linear tetrapyrrole derivative of heme. Most bacteria, including E. coli and A. brasilense, synthesize heme; however, they lack a specific heme oxygenase for making biliverdin IXα. Therefore, biliverdin IXα has to be added to the media, or a heme oxygenase gene involved in its production needs to be introduced along with the bphS gene. In the experiments described below, we use the heme oxygenase gene bphO from Rhodobacter sphaeroides, the organism from which the photoreceptor module of BphS was derived [2]. BphS has spectral absorption maxima in the red-to-near-infrared and violet-to-blue spectral regions. The optimal wavelength for its activation is 712 nm [3]; however, commonly available sources of red light (630-660 nm) are perfectly suitable. Upon irradiation, a fraction of BphS undergoes a conformational change resulting in a lit form with an absorption maximum of 756 nm and increased DGC activity [3]. This form gradually decays to the less active, dark state.
The blue light-activated PDE, EB1 [4], hydrolyzes c-di-GMP to a linear dimeric GMP (pGpG), which is subsequently degraded to GMP by cellular nucleases [7, 8]. Upon irradiation, the PDE activity of the MBP-EB1 fusion protein, where MBP is maltose binding protein [9], is increased by >34-fold (in vitro at room temperature). Currently this is the highest dynamic range for a photoactivated c-di-GMP PDE. As a photoreceptor of the BLUF domain family, EB1 captures light via a flavin chromophore (FAD or FMN) [10, 11]. Since all cells synthesize flavins, neither chromophore addition nor introduction of flavin synthesis genes is necessary. The absorption maxima of flavins lie in the UV-B-to-blue spectrum. Prolonged exposure of cells to such light is deleterious [12]. To avoid cell damage, induction of the PDE activity is accomplished by irradiation with short pulses of blue light. Importantly, at the wavelength of approximately 465 nm, the spectral overlap between BphS and EB1 is minimal, which allows for simultaneous use of these two enzymes for the dichromatic, bidirectional modulation of intracellular c-di-GMP levels [4].
2. Materials
2.1. Strains and plasmids
-
1
E. coli MG1655[DE3] [3], a motile derivative of strain MG1655 that expresses a T7 Polymerase gene from an IPTG-inducible promoter (see Note 1).
-
2
E. coli MG1655 ΔyhjH (Kmr) [13], a MG1655 mutant with high intracellular c-di-GMP levels due to the lack of the major c-di-GMP PDE, YhjH/PdeH [14].
-
3
E. coli BL21 [DE3] (NEB), strain producing curli fimbriae in a c-di-GMP-dependent manner; contains a T7 Polymerase gene expressed from an IPTG-inducible promoter.
-
4
A. brasilense Sp7 (ATCC 29145).
-
5
A. brasilense tlp1 (strain SG323), a mutant derived from strain Sp7, impaired in the chemotaxis receptor Tlp1 [15].
-
6
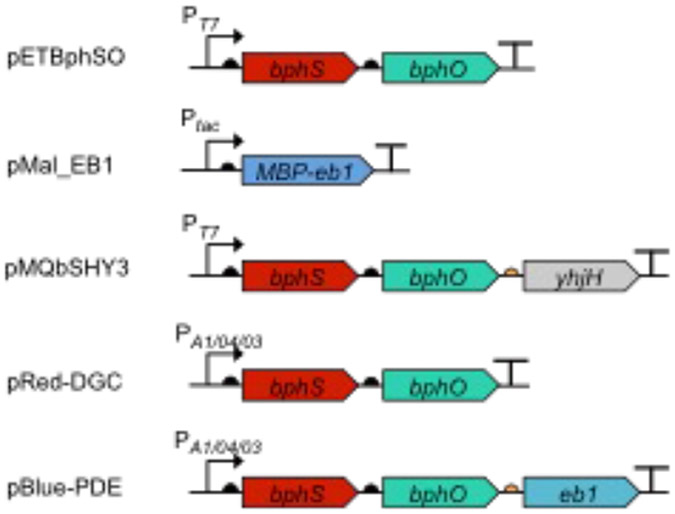
Plasmids expressing light-activated DGC and PDE (Fig. 1) (see Note 2).
-
6.1
pETBphSO [3]: pET23a(+) containing the bphS-bphO operon downstream of the T7 promoter; used for elevating c-di-GMP levels with red light.
-
6.2
pET23a(+) (EMD Millipore): vector control to be used with pETBphSO.
-
6.3
pMal_EB1 [4]: pMal-c5x expressing an MBP-EB1 fusion; used for lowering c-di-GMP levels with blue light.
-
6.4
pMAL-c5x (NEB): vector control to be used with pMal_EB1.
-
6.5
pMQbSHY3 [3]: pMQ56 [16] encoding BphS, BphO and YhjH (RBS3) under the control of T7 promoter.
-
6.5
pRed-DGC [5]: pIND4 containing the bphS-bphO operon downstream of the IPTG-inducible promoter.
-
6.6
pBlue-PDE [5]: pIND4 containing the bphS-bphO-eb1 operon downstream of the IPTG-inducible promoter.
-
6.7
pIND4 [17]: broad-host range vector to be used with pRed-DGC and/or pBlue-PDE.
Fig. 1.
Organization of the genes encoding the light-activated enzymes, DGC (BphS) and PDE (EB1 and MBP-EB1), in the plasmids described in this chapter.
2.2. Equipment and supplies for experiments in E. coli
Incubation room at 30 °C and illuminated with safe green light (see Note 3).
Shaking incubator set up to 200 rpm and 30 °C unless indicated otherwise.
Centrifuge(s) capable of handling 50-mL and 2-mL centrifugation tubes.
Two metal wire shelving units (racks).
- Light panels (see Note 4)
- All-red (660 nm) LED Grow light panels 225 (30.5 × 30.5 cm; LED Wholesalers).
- All-blue (465 nm) LED Grow light panel 225 (30.5 × 30.5 cm; LED Wholesalers).
Light-impenetrable shield (e.g. cardboard, plastic, metal sheet).
Timer: CT-1 Digital Short Cycle Timer (Innovative Grower) (see Note 5).
Aluminum foil.
Sterile toothpicks.
50-mL and 2-mL centrifugation tubes.
2.3. Equipment and supplies for experiments in A. brasilense
Phase contrast microscope (e.g. Nikon E200) equipped with a digital camera (e.g. Nikon Coolpix).
Hollow rectangular glass capillary tubes, 0.1 x 1 x 50 mm. These tubes are commercially available from Vitro Dynamics, Inc., Rockaway, N.J.
Gas equilibration chamber, 3.8 x 8 x 0.4 cm, with a cover slip top and glass slide bottom. The gas equilibration chamber was 3-D printed at the University of Tennessee following the specifications in reference [18].
Compressed N2 gas.
Remotely controlled Magic Light LED light bulb.
Optical filters: green (505-575 nm); red (610-730 nm) and blue light (450 nm) (Andover, Inc.). These filters are placed over the microscope white light source.
2.4. Media and solutions for motility and biofilm experiments in E. coli
Prepare all solutions using deionized water. Autoclave media and solutions at 121 °C for 20 min. Add antibiotics and chemical inducers to the autoclaved media when it is cooled to approximately 55 °C. Store all media and solutions at 4 °C, unless indicated otherwise.
LB medium: Add 5 g Yeast extract, 10 g Tryptone, 5 g NaCl into 1 L of deionized water. Autoclave.
LB agar: Add 15 g/L agar to LB medium. Autoclave. Add 100 μg/mL ampicillin.
SOC medium: Add 15 g of SOC powder into 1 L of deionized water. Autoclave.
Congo red dye solution: Dissolve 200 mg Congo red powder (Sigma-Aldrich) in 10 mL deionized water. Do not autoclave. Store at room temperature.
Congo red agar: To LB agar add 20 μg/mL Congo red dye solution, 100 μg/mL ampicillin and 10 μM IPTG.
Ampicillin 1000x stock solution: Dissolve 1 g of ampicillin powder in 10 mL sterile deionized water making 100 mg/mL stock solution. Store in aliquots at −20 °C.
IPTG stock 1 M solution: Dissolve 2.38 g IPTG in 10 mL deionized water. Store in aliquots at −20 °C.
Semisolid agar for E. coli motility assays: Add 5 g NaCl, 10 g Tryptone and 2.5 g agar in 1000 mL of deionized water. Autoclave. Add 100 μg/mL ampicillin. For motility assays involving BphS, also add 10 μM IPTG (final concentration) (see Notes 6, 7).
100 mM CaCl2: Dissolve 1.11 g CaCl2 in 100 mL deionized water
100 mM CaCl2 +15 % glycerol solution: Dissolve 1.11 g CaCl2 in 85 mL deionized water; add 15 mL glycerol. Autoclave.
2.5. Media and solutions for c-di-GMP extraction
C-di-GMP extraction buffer: 40% methanol (v/v), 40% acetonitrile (v/v) in 0.1 N formic acid. Do not sterilize (see Note 8).
Neutralization solution: 15% NH4HCO3 (w/v). Do not sterilize. Store at room temperature for no longer than 1 week.
2.6. Media and solutions for aerotaxis experiments in A. brasilense
Minimal medium containing malate minus salts (MMAB-salts). Dissolve 3 g K2HPO4, 1 g NaH2PO4, 0.15 g KCl, 0.05 g Na2MoO4, 5 g malate, 1 g NH4Cl in 995 mL deionized water. Adjust pH to 6.85-7.00 with NaOH. Autoclave.
MMAB medium: Prepare the following 3 salt solutions, autoclave them separately, and sterilely add the specified volumes to 1000 mL of the MMAB-salts medium prepared in section 2.6, step 1 to make MMAB medium. (i) 60 g MgSO4 in 1000 mL water; add 5 mL; (ii) 0.631 g FeSO4 x 7 H2O and 0.592 g EDTA in 50 mL water; add 250 uL; (iii) 20 g CaCl2 in 1000 mL water; add 500 μL.
MMAB medium with 200 μg/mL ampicillin and 30 μg/mL kanamycin
MMAB agar: Add 15 g agar to 1000 mL MMAB medium. Autoclave.
Tryptone-yeast medium: Dissolve 10 g Tryptone and 5 g Yeast extract in 1000 mL deionized water. Adjust pH to 7.00 with NaOH. Autoclave.
Tryptone-yeast agar. Add 15 g agar to 1000 mL Tryptone-yeast medium. Autoclave.
Chemotaxis buffer: Dissolve 1.7 g K2HPO4, 1.36 g KH2PO4 in 900 mL deionized water. Add EDTA to 0.1 mM (final concentration). Adjust pH to 6.85-7.00 with NaOH. Add water to 1000 mL. Autoclave.
Chemotaxis buffer with 1 mM malate: Add 0.134 g malate to 1000 mL of chemotaxis buffer prepared as described in section 2.6 step 7. Autoclave.
3. Methods
3.1. Preparation of chemically competent E. coli cells and transformation
Grow an overnight culture of E. coli in LB medium at 37 °C with shaking. This method is applicable to all E. coli strains and plasmids described in this chapter (see Note 9).
Transfer 1 mL of the overnight culture into 100 mL fresh LB medium (use a 750-mL flask). Grow bacteria at 37 °C with shaking until cell optical density reaches OD600, 0.6.
Pre-cool the centrifuge capable of handling 50-mL conical tubes to 4 °C.
Transfer bacterial culture on ice and incubate for 15 min. In all subsequent steps keep cells on ice and perform all manipulations in a cold room, if available.
Transfer cells into the pre-cooled 50-mL conical tubes.
Collect cells by centrifugation at 4500 g for 10 min at 4 °C. Discard the supernatant as completely as possible.
Add 10 mL 100 mM CaCl2 solution in each 50-mL tube, resuspend cell pellets.
Combine cells into a single tube and incubate it on ice for 15 min.
Spin cells down by centrifugation at 4500 g for 10 min. Discard the supernatant.
Resuspend the pellet in 3 mL of cold 100 mM CaCl2 + 15% glycerol solution.
Aliquot 100 μL into 1.5 mL tubes and proceed to step 14 for immediate transformation. Alternatively, flash freeze tubes by dropping them into −80 °C ethanol or liquid nitrogen.
Store aliquots of chemically competent cells at −80 °C.
For transformation, defrost competent cells on ice for approximately 5 min.
Add 10-20 ng of plasmid DNA to competent cells, mix gently and incubate on ice for 5 min.
Heat shock cells by placing the tube into a 42 °C water bath for 30 s, then place the tube back on ice for 60 s.
Add 900 μL SOC medium and incubate for 1 h at 37 °C.
Plate 20 μL on LB plates containing ampicillin. Incubate plate at 37 °C until colonies appear.
3.2. Incubation room setup for light experiments using E. coli
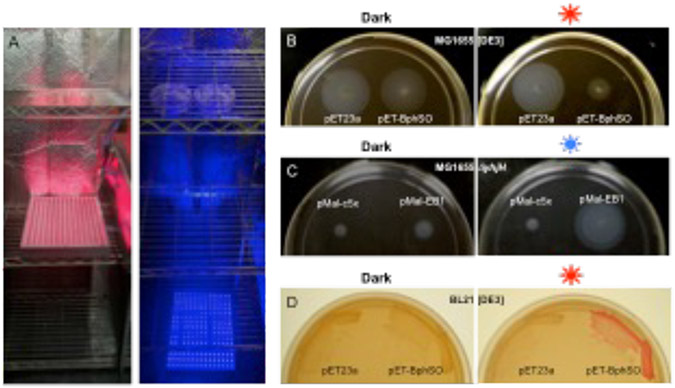
In a 30 °C incubation room, set up 2 metal wire shelving units (racks), preferably at a distance from each other. Place All-red Grow panel(s) on a shelf of one unit, and All-blue Grow panel(s) on a floor or a lower shelf of another unit (Fig. 2a).
Place a light-impenetrable shield between the shelving units to prevent cross-irradiation between blue and red light panels.
Program timers for the following irradiation regiments: 30 s light/120 s dark cycles for red light; and 10 s light/ 50 s dark for blue light.
Set up green light via flexible green LED strip for illuminating the incubation room (see Note 10).
Fig. 2.
Light regulation of c-di-GMP-dependent motility and biofilm phenotypes in E. coli. a, Setup of the red and blue light panels. b, Inhibition of swimming in the semi-solid agar in strain MG1655[DE3] expressing the red light-activated DGC, BphS (plasmid pETBphSO). c, Restoration of swimming in the semi-solid agar in strain MG1655 ΔyhjH expressing the blue light-activated PDE, MBP-EB1 (plasmid pMal-EB1). d, Induction of curli fimbria formation in BL21[DE3] expressing the red light-activated DGC, BphS, (plasmid pETBphSO).
3.3. Light-regulated motility assays in E. coli
A red light-induced increase in c-di-GMP levels in a motile strain MG1655[DE3] inhibits its ability to swim in the semi-solid (soft) agar [3]. Similarly, a blue light-induced decrease in intracellular c-di-GMP levels restores motility in the MG1655 yhjH mutant that has approximately 10-fold elevated intracellular c-di-GMP levels, compared to MG1655, and therefore is impaired in swimming in the semi-solid agar [4]. The experiments described below illustrate the effect of BphS and EB1 on modulating motility patterns in these strains.
Inoculate colonies of the freshly grown MG1655[DE3] transformants containing pETBphSO and pET23a (negative control) into the culture tubes containing 3 mL LB + ampicillin.
Inoculate colonies of the freshly grown MG1655 yhjH transformants containing pMal_EB1 and pMal-c5x (negative control) into the culture tubes containing 3 mL LB + ampicillin.
Grow cultures overnight (~16 h) at 30 °C in the dark (cover with aluminum foil) on a shaker (200 rpm) or culture wheel.
Drop 2 μL from each overnight bacterial culture onto 2 motility agar plates for subsequent incubation under red, blue and no light [dark] conditions. Keep approximately 2-cm distance between the drops to avoid overlap between the neighboring swim zones (see Note 11).
For exposure to red light, place a plate (upside up) on a shelf of a red light wire shelving unit approximately 30 cm above the shelf with the All-red Grow panel.
For exposure to blue light, place a plate on a shelf of the blue light wire shelving unit, approximately 100 cm away from the All-blue Grow panel.
Wrap the control “dark” plate in aluminum foil to eliminate access to light and place it next to the plate exposed to light.
Turn the light panels on (via pre-programmed timers) and incubate plates at 30 °C for 8-10 h (see Note 12).
Observe diameters of swim zones (Fig. 2b, 2c). For quantitative analysis, measure swim zone diameters from at least 3 transformants and average the results. Repeat the experiment for obtaining statistically significant results.
3.4. Light-regulated curli fimbriae formation in E. coli BL21[DE3]
In E. coli strains producing curli fimbriae, such as BL21[DE3], red light-induced increase in c-di-GMP levels results in red-pigmented colonies grown on the agar medium in the presence of the Congo red dye [3]. All steps below are carried out in the incubation room at 30 °C.
Using sterile toothpicks, streak colonies of the freshly grown BL21[DE3] transformants containing pETBphSO and pET23a onto 2 Congo red agar plates (for incubation under red light and dark conditions) (see Note 13).
For exposure to red light, place a plate (inverted, i.e. upside down) on a shelf of a red light wire shelving unit approximately 20 cm above the shelf with the All-red Grow panel.
Wrap the second plate (“dark” control) in aluminum foil to eliminate access to light and place it next to the plate exposed to light.
Turn the light panels on (via pre-programmed timers) and incubate plates at 30 °C for 3-4 days until red colony pigmentation develops (Fig. 2d).
3.5. Manipulating intracellular c-di-GMP levels in liquid-grown E. coli cultures using a red light-activated DGC
In this section, we describe a system for red light-dependent control of intracellular c-di-GMP levels in liquid-grown E. coli. To overpower the background activity of BphS in the dark, the construct used in these experiments (pMQbSHY3) contains the bphS-bphO operon along with a gene for a constitutive c-di-GMP PDE, YhjH/PdeH (the bphS-bphO-yhjH operon) [3]. The role of YhjH/PdeH is to keep the intracellular c-di-GMP levels at near-zero levels in the absence of red light (see Note 14). All steps below are carried out in the incubation room at 30 °C.
Inoculate a colony of MG1655[DE3] (pMQbSHY3) expressing the bphS-bphO-yhjH operon in 3 mL liquid LB medium containing ampicillin.
Wrap the tube in aluminum foil and grow on a shaker (200 rpm) set at 30 °C overnight.
Place an All-red Grow light panel close to the shaker (~10 cm away) so that tubes can be exposed to red light (see Note 15). Do not turn on the light yet.
In a room illuminated with safe green light, measure OD600 of the overnight culture, and inoculate 6 tubes with 3 mL fresh LB medium containing ampicillin to OD600, 0.2.
Wrap all tubes in foil
Place wrapped tubes in a wire rack on a shaker (200 rpm) and grow for an additional 1 h at 30 °C.
Following the 1-h incubation, take 2 tubes off the shaker, place then on ice and proceed to nucleotide extraction. These 2 cultures will correspond to the initial (0 h irradiation) time point.
At the same time point, unwrap 2 tubes and turn the light panel on via a timer (pre-set for the following regimen: 30 s light, 120 s dark). Grow remaining cultures upon shaking.
At each desired time point during irradiation (e.g. 3 h and 6 h), collect 2 cultures -- one wrapped (dark), another one exposed to light and proceed with nucleotide extraction (see Note 16).
Repeat the experiments several times for statistical analysis.
3.6. Nucleotide extraction from bacterial biomass and c-di-GMP measurements
Weigh empty 2-mL microtubes (one tube per bacterial sample) and record their weights.
Centrifuge 2 mL of cells prepared in section 3.5 for 1 min at 13,000 g at 4 °C. Discard supernatant and weigh the tubes with wet biomass (see Note 17).
Preserve the remaining culture (approximately 1 mL) on ice for determining viable cell count, which will be needed for normalizing c-di-GMP concentrations.
Add 100 μL of extraction buffer per 50 mg of wet pellet from step 1. Vortex vigorously to fully resuspend cells.
Incubate slurries for 30 min at −20 °C.
Spin down the suspension at −20 °C, 13,000 g, for 3 min.
Place supernatant into a new tube and neutralize it with 4 μL 15% NH4HCO3 per 100 μL of sample.
Extract remaining nucleotides from the insoluble material by repeating steps 4-7.
Combine two neutralized supernatants and measure the sample volume.
Send samples to a LC-MS/MS facility for c-di-GMP measurements (see Note 18).
Calculate approximate intracellular c-di-GMP levels using c-di-GMP values determined by mass-spectrometry, numbers of viable cells and average cell volume (see Note 19).
3.7. Spatial gradient assay for aerotaxis in A. brasilense
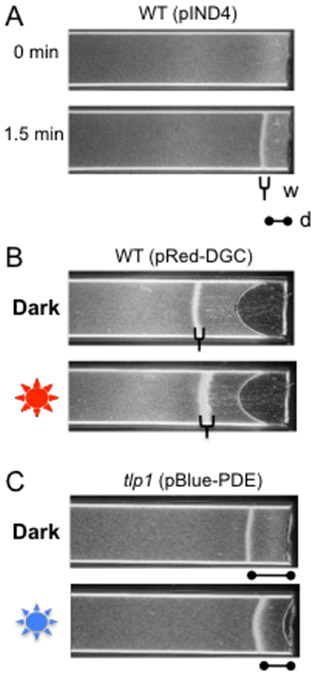
In a capillary containing liquid medium, A. brasilense forms a stable aerotactic band at a specific position corresponding to the preferred oxygen concentration [19] (Fig. 3a). The aerotactic band forms because A. brasilense senses both high and lower oxygen concentrations as repellents while the preferred, low oxygen concentration (~0.4% dissolved oxygen) as an attractant [20]. Intracellular c-di-GMP levels can modulate aerotaxis by affecting activity of at least one chemotaxis receptor, Tlp1 [21]. Expression of BphS, the red light activated DGC from a broad-host-range plasmid, pRed-DGC, or a blue light activated PDE from plasmid pBlue-PDE can be used to characterize the role of intracellular c-di-GMP levels in aerotactic behavior in A. brasilense [5]. These assays can be adapted to characterize other chemotaxis responses or other reversible behaviors occurring in chemical gradients.
Fig. 3.
C-di-GMP effects on aerotactic band formation in A. brasilense. a, Aerotactic band formation after introduction of an oxygen gradient in the wild-type strain Sp7 containing an empty vector pIND4. Two parameters, distance from the band to the meniscus (d) and band width (w) are used to characterize oxygen sensitivity. b, The red light-activated DGC, BphS, expressed from pRed-DGC increases band thickness. c, The blue light-activated PDE, EB1, expressed from pBlue-PDE changes band position in regard to the meniscus in the tlp1 mutant.
Grow A. brasilense strains Sp7 and tlp1 containing pRed-DGC, pBle-PDE and pIND4 plasmids in MMAB medium with 200 μg/mL ampicillin and 30 μg/mL kanamycin in the dark, at 28 °C, with shaking at 180 rpm to OD600, 0.6 (see Notes 10, 20, 21).
Collect 1 mL of cells at OD600, 0.6 by centrifugation at 13,000 g for 3 min. at room temperature, decant supernatant and suspend cells in 1mL of chemotaxis buffer. After suspending, centrifuge again to wash and pellet cells. Repeat wash 3 times.
Suspend the washed cell pellet in 100 μL chemotaxis buffer with 1 mM malate by vortexing.
Take a small aliquot and observe cells under the light microscope, with 200x (or higher) magnification to ensure that they are motile. Over 80 % motile cells are desirable.
Let washed cells equilibrate in dark for 10 min, at room temperature, to avoid induction of enzymes by exposure to white light.
Use forceps to place capillary tube into the suspension of washed motile cells. Allow capillary action to draw up the cell suspension into the capillary tube.
Wipe the outside of the capillary tube to remove excess liquid and place the filled capillary tube, horizontally, on a microscope slide, in the gas equilibration chamber [22].
Turn on N2 gas and allow cells to equilibrate under an atmosphere of gaseous N2 for 3 min (Fig. 3a).
Keep cells in the dark on the microscope stage. Then place green filter over microscope white light source.
Turn on the digital camera and start recording cells’ behavior within the capillary tubes if downstream subsequent motion or image analysis is performed.
3.7.1. Modulating c-di-GMP levels before air gradient establishment
We describe two experimental conditions. In 3.7.1, the effects of light-induced changes in intracellular c-di-GMP levels are assayed prior to exposure to the gradient and thus before aerotactic band formation. In 3.7.2, the effects of light-induced changes in intracellular c-di-GMP levels on an aerotactic band are assayed after the band has formed. These two assays discriminate between the response of cells newly exposed to an air gradient (3.7.1) and the response of cells adapted to specific aeration conditions (3.7.2).
Prepare capillary tubes as described in section 3.7 and allow cells to equilibrate under an atmosphere of gaseous N2 (Fig. 3a).
Expose cells to red or blue light, using appropriate optic filters, during last 10 seconds of N2 equilibration. Avoid exposing the cells suspension to white light.
After an exposure of 10 s to red or blue light, return to green light using the appropriate optic lens filter. Avoid exposing the cells suspension to white light
After 3 min, turn gas off and allow air to flow into the chamber and record the time to formation of the aerotaxis band and its position relative to the meniscus. For a given cell density, carbon source and physiological stage, the aerotactic band forms at a similar position and within the same time frame (see Note 22 and Fig. 3b).
3.7.2. Modulating c-di-GMP levels after establishment of the air gradient
Prepare capillary tubes as described in section 3.7 and allow cells to equilibrate under an atmosphere of gaseous N2 for 3 min (Fig. 3a).
After 3 min, turn off gas and allow air to flow into the chamber.
Start digital recording of cells’ behavior within the capillary tube.
Allow the aerotaxis band to form and record time to formation and position of the aerotactic band relative to meniscus.
After the band has formed and is stable, expose cells to red of blue light once or twice for 10 seconds and continue recording under green light (Fig. 3c). Avoid exposing the cells suspension to white light, which could induce enzyme activity.
4. Notes
Intracellular c-di-GMP levels in E. coli strains MG1655 and MG1655 ΔyhjH differ by ~10-fold [23], which make them convenient for illustrating the effect of light-activated DGCs and PDEs. In the described assays, we use MG1655[DE3], as opposed to MG1655, to drive expression of the bphS-bphO operon from the T7 promoter. However, alternative promoters for bphS expression can and have been successfully used in E. coli, in other bacterial species [24] and in mammalian cells [25].
Various other light-activated enzymes involved in c-di-GMP synthesis and hydrolysis have been described [26-29]; however, in our opinion, they are not as convenient for optogenetic application as BphS and EB1. For further discussion of this issue, see [4].
Place the shelving units, shaker and timer in the incubation room. In the absence of such room, the experiments in E. coli can be performed in a regular plate incubator. The disadvantage of an incubator is a risk of overheating. If performing an experiment in the incubator, place a light panel inside, turn it on in the desired irradiation regimen before the experiment, and test whether or not pulsed irradiation overheats the incubator beyond acceptable temperature fluctuation. Placing a small fan inside the incubator may improve air mixing and solve the overheating issue.
We use light panels because they are affordable and convenient for uniform irradiation of large number of Petri dishes. However, various alternative sources of red (635-660 nm) or blue (465 nm) light are appropriate.
More affordable timer models can also be used. To avoid the inhibitory effect of light on bacterial growth, chose a timer allowing short (less than 1 min) irradiation periods.
For assays involving red light-induced motility inhibition, add 10 μM IPTG to the semi-solid agar medium to induce bphS-bphO operon expression. If experiments are performed in different E. coli strains, optimizing IPTG concentration may be necessary. Adding IPTG for blue light-dependent induction of swimming involving pMal_EB1 is unnecessary because leaky MBP-EB1 expression from pMal_EB1 is sufficient.
For testing multiple strains, use large, 15-cm diameter, Petri dishes. Store semi-solid agar plates at room temperature upright for no longer than 2 days prior to use.
It is advisable to prepare extraction buffer fresh for every extraction to avoid methanol evaporation. Alternatively, store this buffer in a gas-tight container.
This method can be replaced with any other method of preparing chemically competent or electroporation ready cells.
Avoid exposing bacteria in liquid cultures or on plates to white light. Perform handling of bacterial cultures in safe green light.
Do not invert semi-solid (motility) agar plates. Keep them upside up (lid on the top) at all times.
For experiments performed at different temperatures, e.g. room temperature or 37 °C, incubation periods will differ, e.g. approximately 12-14 h and 4-6 h, respectively.
Note that curli fimbriae in strain BL21[DE3] as well as in most other E. coli strains are not expressed at temperatures higher than 30 °C. It is possible to perform these experiments at room temperature but not at 37 °C.
Several bphS-bphO-yhjH operons have been engineered where expression of yhjH/pdeH varies due to different ribosome-binding site strength [3]. It is expected that a proper construct can be found to keep intracellular c-di-GMP levels at the near-zero levels in a desired E. coli strain.
Secure the light panel at a safe distance from a shaker. Note that a red light panel can be replaced with a red light bulb; however, it would be important to ensure to even light fluency of the irradiated cultures.
In our hands intracellular c-di-GMP concentrations rose from ~4 nM in the dark at time point 0 h to ~84 nM after 3 h of irradiation (>20-fold increase) and ~210 nM after 6 h of irradiation (>50-fold increase) [3].
This method is based on [30]. It is applicable to both E. coli or A. brasilense. Alternative methods for calculating c-di-GMP concentrations that rely on c-di-GMP detection via specific chemicals or riboswitches have been described [31, 32]. Furthermore, c-di-GMP-specific ELISA kits are commercially available.
We and other laboratories have been successfully using the Michigan State University Mass Spectrometry and Metabolomics Core Facility. If using a different facility you may be asked to supply c-di-GMP standards for quantification purposes. C-di-GMP is available from various vendors.
Use the remaining E. coli culture to determine viable cell count, e.g. by plating serial dilutions onto LB agar containing ampicillin. Use the number of cells submitted for LC-MS/MS to calculate approximate intracellular c-di-GMP concentration. Example: Assume that c-di-GMP was extracted from 5 × 1010 cells (this corresponds to ~50 mg of wet biomass). Assume that c-di-GMP concentration in the 200 μL (2 × 10−4 L) buffer submitted for LC-MS/MS is 50 nM (50 × 10−9 M). Based on these data, the total molar c-di-GMP amount extracted from bacterial sample is (50 × 10−9 M) × (2 × 10−4 L) = 1 × 10−11 mole. Since 1 mole corresponds to 6.02 × 1023 molecules (Avogadro number), 1 × 10−11 mole corresponds to (6.02 × 1023 molecules/M) × (1 × 10−11 moles) = 6.02 × 1012 c-di-GMP molecules. By dividing the number of molecules by the number of cells from which c-di-GMP was extracted we obtain an approximate number of c-di-GMP molecules per cell: (6.02 × 1012 molecules) / (5 × 1010 cells) = 120 molecules/cell. Based on the assumption that 1 molecule in an average E. coli cell corresponds to ~1 nM concentration [33], the approximate intracellular concentration of c-di-GMP is 120 × 1 nM = 120 nM. An alternative method of normalizing c-di-GMP concentration may use total soluble protein, which can be measured based on the remaining 1 mL of E. coli culture from step 3.6. Protein concentration can be determined using various commercial kits.
While pBlue-PDE contains both BphS and EB1, expression levels of BphS in A. brasilense are apparently low in the absence of induction, therefore pBlue-PDE can be used as a source of the blue light-dependent PDE [5]. It is important to note that for bidirectional control of c-di-GMP levels, expression levels for BphS and EB1 need to be carefully optimized for any given strain. Further, MBP-EB1 is preferred over EB1 because of its superior dynamic range (fold-activation) by blue light.
Strains carrying the pRed-DGC grow slower than strains carrying pIND4 or pBlue-PDE. The cause for this effect on growth is unknown. Strains carrying pRed-DGC should thus be inoculated at least one day prior to strains carrying empty vectors or pBlue-PDE to ensure equivalent growth at the planned time for the experiment.
Aerotaxis bands typically form in less than 2 min after exposure to the air gradient in the wild type strain and the bands are pretty stable and cells remain motile for at least 25 min under the conditions described here.
ACKNOWLEDGEMENTS
This work was supported in part by National Science Foundation grants MCB1052575 (to MG) and MCB13330344 (to GA). LON was supported by the National Institutes of Health grant R25GM086761.
References
- 1.Römling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial secondary messenger. Microbiol Mol Biol Rev 77:1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarutina M, Ryjenkov DA, Gomelsky M (2006) An unorthodox bacteriophytochrome from Rhodobacter sphaeroides involved in turnover of the second messenger c-di-GMP. J Biol Chem 281:34751–34758 [DOI] [PubMed] [Google Scholar]
- 3.Ryu MH, Gomelsky M (2014) Synthetic second messenger module controlled by near-infrared window light. ACS Synth Biol 3:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu MH, Fomicheva A, Moskvin OM, Gomelsky M (2017) Optogenetic module for dichromatic control of c-di-GMP signaling. J Bacteriol doi: 10.1128/JB.00014-17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neal L, Ryu MH, Gomelsky M, Alexandre G (2017) Optogenetic manipulation of c-di-GMP levels reveals the role of c-di-GMP in regulating aerotaxis receptor activity in Azospirillum brasilense. J Bacteriol doi: 10.1128/JB.00020-17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwell NC, Su YS, Lagarias JC (2006) Phytochome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr MW, Donaldson GP, Severin GB, Wang J, Sintim HO, Waters CM, Lee VT (2015) Oligoribonuclease is the primary degradative enzyme for pGpG in Pseudomonas aeruginosa that is required for cyclic-di-GMP turnover. Proc Natl Acad Sci USA 112:E5048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen D, Mechold U, Nevenzal H, Yarmiyhu Y, Randall TE, Bay DC, Rich JD, Parsek MR, Kaever V, Harrison JJ, Banin E (2015) Oligoribonuclease is a central feature of cyclic diguanylate signaling in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 112:11359–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–33 [DOI] [PubMed] [Google Scholar]
- 10.Gomelsky M, Klug G (2002) BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci 27:497–500 [DOI] [PubMed] [Google Scholar]
- 11.Conrad KS, Manahan CC, Crane BR (2014) Photochemistry of flavoprotein light sensors. Nat Chem Biol 10:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai T (2017) The antimicrobial effect of blue light: what are behind? Virulence 4:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Gomelsky M (2010) A post-translational, c-di-GMP-dependent mechanism regulating bacterial flagellar motility. Mol Microbiol 76:1295–1305 [DOI] [PubMed] [Google Scholar]
- 14.Hengge R, Galperin MY, Ghigo JM, Gomelsky M, Green J, Hughes KT, Jenal U, Landini P (2015) Systematic nomenclature for GGDEF and EAL domain-containing c-di-GMP turnover proteins of Escherichia coli. J Bacteriol 198:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greer-Phillips S, Stephens B, Alexandre G (2004) An energy taxis transducer promotes root colonization by Azospirillum brasilense. J Bacteriol 186:6595–6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanks RMQ, Kadouri DE, MacEachran DP, O’Toole GA (2009) New yeast recombineering tools for bacteria. Plasmid 62, 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ind AC, Porter SL, Brown MT, Byles ED, de Beyer JA, Godfrey SA, Armitage JP (2009) Inducible-expression plasmid for Rhodobacter sphaeorides and Paracoccus denitrificans. Appl Environ Microbiol 75:6613–6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laszlo DJ, Taylor BL (1981) Aerotaxis in Salmonella typhimurium. Role of electron transport. J Bacteriol 145:990–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharf BE, Hynes MF, Alexandre GM (2016) Chemotaxis signaling systems in model beneficial plant–bacteria associations. Plant Mol Biol 90:549–559 [DOI] [PubMed] [Google Scholar]
- 20.Zhulin IB, Bespalov VA, Johnson MS, Taylor BL (1996) Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol 178:5199–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell MH1, Bible AN, Fang X, Gooding JR, Campagna SR, Gomelsky M, Alexandre G (2013) Integration of the second messenger c-di-GMP into the chemotactic signaling pathway. mBio 4:e00001–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor BL, Watts KJ, Johnson MS (2007) Oxygen and redox sensing by two-component systems that regulate behavioral responses: Behavioral assays and structural studies of Aer using in vivo disulfide cross-linking. Methods Enzymol 422:190–232 [DOI] [PubMed] [Google Scholar]
- 23.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U (2010) Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–16. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Wu Y, Mukherjee M, Cao B (2017) A near-infrared light responsive c-di-GMP module-based AND logic gate in Shewanella oneidensis. Chem Commun (Camb) 53:1646–1648 [DOI] [PubMed] [Google Scholar]
- 25.Folcher M, Oesterle S, Zwicky K, Thekkottil T, Heymoz J, Hohmann M, Christen M, Daoud El-Baba M, Buchmann P, Fussenegger M (2014) Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nat Commun 5:5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barends TRM, Hartmann E, Griese J, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I (2009) Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459:1015–1018 [DOI] [PubMed] [Google Scholar]
- 27.Savakis P, De Causmaecker S, Angerer V, Ruppert U, Anders K, Essen LO, Wilde A (2012) Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol Microbiol 85:239–51 [DOI] [PubMed] [Google Scholar]
- 28.Enomoto G, Nomura R, Shimada T, Ni-Ni-Win, Narikawa R, Ikeuchi M (2014) Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus. J Biol Chem 289:24801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enomoto G, Ni-Ni-Win, Narikawa R, Ikeuchi M (2015) Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc Natl Acad Sci USA 112:8082–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM (2012) Quantification of high-specificity cyclic diguanylate signaling. Proc Natl Acad Sci USA 109:12746–12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuji G, Sintim HO (2016) Cyclic dinucleotide detection with riboswitch-G-quadruplex hybrid. Mol Biosyst 12:773–777 [DOI] [PubMed] [Google Scholar]
- 32.Kellenberger CA, Sales-Lee J, Pan Y, Gassaway MM, Herr AE, Hammond MC (2015) A minimalist biosensor: Quantitation of cyclic di-GMP using the conformational change of a riboswitch aptamer. RNA Biol 12:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran U, Phillips R, Milo R (2010) SnapShot: key numbers in biology. Cell 141:1262–1262.e1 [DOI] [PubMed] [Google Scholar]