Introduction
Influenza, also referred to as the “flu,” is a respiratory infectious disease caused by RNA viruses within the Orthomyxoviridae family. Influenza viruses are classified into four types: A, B, C, and D, according to their nucleoprotein and matrix protein M1 antigenicity. Only influenza A and B viruses can cause clinically significant disease and seasonal outbreaks in humans, and influenza A viruses have become one of the most threatening viruses to global health and have caused numerous global pandemics. Influenza A viruses are divided into different subtypes according to their HA and NA protein antigenicity. Historically, some notable influenza A virus that have caused pandemics include H1N1, H2N2, and H3N2 subtypes.[1] Given the infectious nature of influenza viruses, global and seasonal pandemics are a threat to human health. A range of 291,245–645,832 deaths from seasonal influenza-related illnesses are reported globally each year (4.0–8.8 cases per 100,000 people). Patients aged 75 years or older are at particularly high risk (51.3–99.4 deaths per 100,000 people annually). In children under age 5, a range of 9,243–105,690 deaths occur each year.[2] During the 2018–2019 influenza season in the United States, there were approximately 3.5 million cases, 1.6 million clinical visits, 500,000 hospitalizations, and 30,000 deaths.[3]
Influenza pandemics in Taiwan can be traced back to the global pandemic of 1918, with subsequent pandemics in 1957, 1968, and 2009. In the current century, annual seasonal pandemics were predominantly H1N1 and H3N2, with influenza B emerging as the predominant strain in 2000, 2006, 2011, and 2017. In recent years, cases of H5N1 and H7N9 avian influenza viruses have also emerged.[4] In the 2019–2020 influenza season, the cumulative mortality rate was 0.5 per 10,000 people in the general population, and 1.8 per 10,000 in patients age 75 years or older.[5] In 2020, influenza-like symptoms accounted for a staggering 120,000 or more weekly outpatient emergency department visits and at its peak, more than 20% of emergency department visits.[6] Thus, it is important to accurately and efficiently diagnose influenza, as missed or delayed diagnoses may result in inappropriate or delayed treatment and lead to further influenza spread in hospitals and communities.
There are various methods for laboratory diagnosis of influenza including rapid nucleic acid amplification tests (rapid NAATs), rapid antigen tests, and reverse transcription polymerase chain reaction (RT-PCR). Each method has different characteristics, and the diagnostic process needs to be further standardized. Internationally, there are clinical guidelines for influenza management[7] with corresponding recommended diagnostic methods. However, in Taiwan, there is no consensus on the diagnostic approach of influenza for patients in emergency settings. Therefore, it is important to develop diagnostic guidelines for emergency medicine physicians to facilitate early diagnosis and treatment of influenza patients and thereby minimize the hazards posed by influenza to society.
The purpose of these guidelines is to provide guidance regarding diagnostic testing for influenza in emergency clinical settings. This does address influenza management, treatment, or community outbreak prevention and control.
Overview of the Primary Laboratory Diagnostic Methods for Influenza
Rapid NAAT: This is a relatively novel method for identifying influenza A and B RNA via examination of respiratory specimens. Rapid NAAT is fast and easy to operate at bedside; multiplex nucleic acid amplification platforms are also available for the screening of other prevalent clinically relevant respiratory viral and bacterial pathogens. While rapid NAATs have high sensitivity and specificity, the cost is higher compared to other methods.[7-12]
Rapid influenza diagnostic tests (RIDTs, or rapid influenza antigen tests, RIATs): These tests identify the nucleoprotein antigens of influenza A and B viruses. Similar to rapid NAAT, RIDT is also quick and simple to operate at the bedside. While more cost-effective than rapid NAAT diagnostics, RIDTs are less sensitive.[7-12]
RT-PCR: This method has high sensitivity and specificity. Different primer kits are available for detecting various viruses and can be used to serotype influenza viruses. However, RT-PCR tests require significantly more time and are therefore less convenient.[7-12]
Serological assay: This method allows for the dynamic detection of serum immunoglobulin M (IgM) and IgG antibody titers in both acute and recovered cases of influenza. A patient is considered recovered when the serum IgG antibody titer is 4-fold or higher than that of the acute phase. However, serum IgM and IgG titers have limited utility for early diagnosis.[7-12]
Viral culture: This involves culturing inoculated cell lines from respiratory specimens (e.g., chicken embryo or monkey embryonic kidney cells) and subsequent isolation and identification of viral strains. This may provide accurate information on the types and subtypes of prevalent viral strains. However, these cultures are not suitable for early clinical diagnosis.[7-12]
Recommendations
1. During influenza prevalent seasons, screening is not required for all emergency room visits with influenza-like symptoms. However, testing should be performed on pregnant women, those with high-risk comorbidities involving the heart, lung, kidney, liver, metabolic disease, or immunodeficiency, and those requiring hospitalization to prevent nosocomial or institutional spread of infection.
2. During periods of low prevalence, testing of all emergency patients with influenza-like symptoms is recommended, especially for those who are at high risk of hospitalization, co-morbidities, and the need to prevent nosocomial or institutional spread of infection.
It is recommended that all patients with influenza-like symptoms be tested during low influenza prevalence periods, particularly those at high hospitalization risk or with high-risk co-morbidities, as well as those who are at risk of nosocomial or institutional transmission.
3. Patients aged 65 years or older often show atypical symptoms including shortness of breath and altered mental status, and do not necessarily have classic influenza-like symptoms. Thus, it is recommended to test clinically suspected patients in this group irrespective of influenza prevalence.
4. Vaccination history of the patients should not be considered a basis to exclude diagnosis or testing.
5. The sensitivity of rapid influenza antigen tests ranges from 40% to 80%. Thus, to improve diagnostic accuracy, rapid NAATs should be the influenza test of choice in emergency clinical settings.
6. If a laboratory is unable to process a NAAT report at the time of the emergency visit, then point-of-care rapid NAAT equipment should be considered to improve the timeliness of results. This is applicable to adults and minor patients, including children under 5 years of age.
7. Blood tests for influenza antibodies should not be used as an emergency influenza test during the window of availability.
8. Upper respiratory specimen is acceptable to be used as a standard approach for diagnostic testing considering the practicality. However, a lower respiratory specimen has higher sensitivity. Patients who refuse to have a nasopharyngeal swab test, or who have a high clinical probability but a negative result from upper respiratory specimen, can have sputum or saliva samples collected.
Rationale and Description
Influenza can manifest with fever, cough, myalgia, malaise, laryngitis, and rhinorrhea. Some patients, especially children, also experience gastrointestinal symptoms such as vomiting and diarrhea.[13] These symptoms are not specific, although fever and cough correlate relatively strongly with influenza.[14-17] High fever (≥ 40°C) is associated with a higher likelihood of influenza.[18] In a study of 3,744 patients with influenza-like symptoms in which 66% were ultimately diagnosed with influenza, fever, and cough had a positive predictive value of 79%.[17] A Taiwanese prospective study evaluated 158 adults (aged 28 to 50 years, of whom 71 had a confirmed diagnosis of influenza) during two influenza seasons and found that fever and cough had a positive predictive value of 62% for influenza, with a likelihood ratio of 2.0219. A systematic review suggested that the positive probability ratio for any individual symptom did not exceed 2 without age stratification and that the positive probability ratio for fever and cough could reach 5.0 in patients aged 60 or older.[19]
Overall, influenza-like symptoms can be nonspecific, and similar symptoms may also be present in bacterial pneumonia and other viral respiratory infections. The severity of symptoms varies depending on the patient's immune response and other chronic conditions. Furthermore, the prevalence of influenza affects the positive predictive value of influenza-like symptoms in diagnosing influenza. Thus, when someone decides to test patients, the implications of the results for treatment and management, the prevalence of the disease, and the patient's underlying medical conditions all should be considered. A prospective study of 292 patients with emergency influenza-like symptoms during an influenza prevalent season in the United States were tested with rapid NAAT, and 29% were diagnosed with influenza.[20] Of the 292 patients enrolled, 143 were classified as requiring urgent treatment (43% with influenza), and 61% had a change in clinical management due to influenza NAAT results (e.g., changes in antiviral therapy, antibiotic therapy, or hospitalization), resulting in a savings of $200.40 per emergency visit.
According to the Taiwan Centers for Disease Control,[21] during influenza prevalent seasons, the detection rate of influenza viruses in respiratory specimens can reach as high as 40%-50%. Thus, if a patient has the typical clinical symptoms of influenza, and testing would therefore not affect clinical decision-making, then no testing is needed, and the patient can be treated according to the clinical diagnosis. However, influenza testing should be prioritized in the following situations: (1) the patient has influenza-like symptoms and would meet criteria for antiviral influenza therapy (e.g., within 48 hours of fever); (2) the patient has atypical symptoms or a complex clinical presentation that requires a definitive diagnosis to direct treatment, especially antibiotic therapy is being considered; (3) the patient has chronic diseases (such as heart, lung, kidney, liver, and metabolic diseases), high-risk co-morbidities such as immunosuppression, pregnant or within 2 weeks postpartum, belongs to a high risk age group (elderly, infants, and young children), or has developed clinical manifestations of influenza-related complications such as pneumonia; (4) the patient requires hospitalization; (5) the patient has household contact with high-risk groups such as the elderly, infants, and young children who warrant protection or prophylactic antiviral therapy; (6) the patient has history that suggests possible infection with highly pathogenic avian influenza or other novel viruses that pose high epidemiological risk.[22-28] These populations would benefit from antiviral therapy but could be harmed by the possibly false negative testing results as the negative predictive value is low during prevalent seasons. During non-influenza prevalent seasons, multi-pathogen PCR testing may be considered in emergency patients with influenza-like symptoms. Recommendations for clinical testing and interpretation of results are provided in Table 1 and Table 2. [7]
Table 1. Recommendations for clinical use of rapid nucleic acid amplification tests for influenza.
| Clinical assay recommendation | Influenza prevalent season | Non-influenza prevalent season |
|---|---|---|
| High (should be tested) |
1. Patients presenting with influenza-like symptoms and with high-risk factors (such as senility, immunosuppression, etc.) 2. Patients with respiratory distress and high clinical probability of complications (pneumonia, shock, myocarditis, encephalitis, etc.) |
|
| Medium (consider testing) |
1. Patients without risk factors but with influenza-like symptoms and (1) require a decision on antiviral or antibiotic therapy; (2) require hospitalization 2. Elderly patients without influenza-like symptoms and with shortness of breath, confusion, heart failure, or acute deterioration of chronic obstructive pulmonary disease |
No high-risk factors, with influenza-like symptoms |
| Low (In principle, no testing is required) | Patients without high-risk factors but with influenza-like symptoms are eligible for direct clinical diagnosis without testing | Patients with respiratory tract infections without influenza-like symptoms without high-risk factors |
Table 2. Interpretation of test resultsa .
aDerived from Uyeki et al.[7]
NAAT: nucleic acid amplification test; RIDT: rapid influenza diagnostic test; RT-PCR: reverse transcription polymerase chain reaction.
| Influenza prevalent season | Non-influenza prevalent season | |||
|---|---|---|---|---|
| RIDT | Negative results:
Negative predictive values are low
● Possible false negative, primarily if the test is performed four days after the onset of disease ● A negative result cannot be used as a basis for not initiating treatment if antiviral therapy is clinically indicated ● Confirmation with a more sensitive test is required |
Positive results:
Positive predictive values are high
● Possible influenza infection |
Negative results:
Negative predictive values are high
● Possible true negative ● In cases where the epidemiologic history suggests an influenza pandemic outbreak, consider confirming with a more sensitive test |
Positive results:
Positive predictive values are low
● Possible false positive ● Confirmation with a more sensitive test is warranted |
| Rapid NAAT, RT-PCR | Negative results:
Negative predictive
values are low
● Possible true negative result if the patient does not present with lower respiratory tract infection ● Consider the possibility of a false negative if the result is from an upper respiratory specimen from an inpatient ● Consider obtaining a lower respiratory specimen if the upper respiratory specimen is negative in an inpatient requiring mechanical ventilation |
Positive results:
Positive predictive values
are high
● Possible influenza infection |
Negative results:
Negative predictive
values are high
● High probability of a true negative result |
Positive result:
Positive predictive values
are low
● Possible false positive |
Vaccine effectiveness (VE) varies across subtypes of influenza viruses. A study using data from the US Flu VE Network, which included 20,022 adult emergency visits over five consecutive influenza prevalent seasons, suggested that VE was 49% for the H1N1 subtype and only 14% for the H3N2 subtype in patients age 65 years and older.[29] Another study in Europe showed similar results but also suggested that the vaccine was effective against influenza B. When H3N2 is the predominant strain, vaccines are less effective.[30] Influenza vaccines administered in a given year do not necessarily target the prevalent strain in that particular year. In a study that analyzed data from the 2011-2012 influenza season in Taiwan, the trivalent vaccine administered did not provide protection as it did not cover the prevalent influenza B virus (B/Yamagata-lineage).[31] A retrospective study utilizing national health insurance data from 2002 to 2009 showed that influenza vaccination rates ranged from 40% to 60% among patients aged 65 years and older and suggested that influenza vaccination reduces the risk of hospitalization from influenza or pneumonia, but does not decrease outpatient visits.[32] Thus, influenza vaccination status should not be used as a criterion for excluding a patient from testing.
Due to their simplicity, rapid NAAT and RIDT are exempt from the FDA's CLIA (Clinical Laboratory Improvement Amendments) requirements and can be performed in emergency and point-of-care settings without laboratory equipment or specialized personnel, allowing for rapid results in 10-30 minutes.[33,34] The sensitivity of different RIDT detection systems ranges from 40% to 80%, and specificities are above 90%.[7,35,36] In general, RIDT detection systems utilize subjective and low- sensitivity readings with visual interpretation, which can be improved by using digital readers; however, the sensitivity is still lower than that of NAATs. An extensive meta-analysis in 2017 demonstrated a maximum sensitivity of 83% (95% confidence interval [CI]: 73.4%, 90.1%) for RIATs using digital readers (BD Veritor System), which remains well below the sensitivity of 97.1% (95% CI: 92.9%, 98.9%) for rapid point-of-care NAATs (Liat, Roche)[36] The Liat system was more sensitive than the Alere system for detecting influenza A and B.[35] Compared to RIDT, rapid NAAT is more sensitive for detecting influenza A and B in the general population. One meta-analysis demonstrated that the sensitivity of RIDT for influenza A and influenza B was 54.4% (95% CI: 48.9%, 59.8%) and 53.2% (95% CI: 41.7%, 64.4%) respectively, compared with 91.6% (95% CI: 84.9%, 95.9%) and 95.4% (95% CI: 95.4%) for rapid NAAT. The difference in specificity between the two methods was not significant.[36] It should be noted that in this meta-analysis, for NAAT, comparing with sponsored studies, pooled results from non-sponsored studies showed lower sensitivity (80.0 [95% CrI: 53.0-94.7]) than that from sponsored studies (97.2 [95% CrI: 92.7-99.0]) in diagnosing influenza B (difference: 16.9 [95% CrI: 1.7-44.1]). Sensitivities of different NAAT methods can vary. In 2017, the US FDA upgraded the RIDT detection system from a Class I risk medical device to a Class II risk medical device. This requires the device to have a sensitivity of at least 90% (with a lower 95% CI
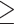 80%) for influenza A, and a sensitivity of at least 80% (with a lower 95% CI
80%) for influenza A, and a sensitivity of at least 80% (with a lower 95% CI
 70%) for influenza B.[37] Annual testing of devices is required, and non-compliant devices are not permitted for use.[37] FDA-approved testing products and the characteristics of several products are listed in Table 3 and Table 4
[38-41] and are available on the official FDA website.[42] The traditional RT-PCR method is often regarded as the "gold standard" for diagnosing influenza because of its high sensitivity and specificity. In addition, it is valuable due to its ability to identify virus serotype. However, it is less useful as an early screening method as it requires a longer period of time (1-8 hours) to yield results, laboratory equipment, and specialized personnel. Compared to conventional RT-PCR, rapid NAAT offers faster detection and can also diagnose other viral and bacterial pathogens, thus potentially decreasing misdiagnosis and further spread of infection due to delayed diagnosis.
70%) for influenza B.[37] Annual testing of devices is required, and non-compliant devices are not permitted for use.[37] FDA-approved testing products and the characteristics of several products are listed in Table 3 and Table 4
[38-41] and are available on the official FDA website.[42] The traditional RT-PCR method is often regarded as the "gold standard" for diagnosing influenza because of its high sensitivity and specificity. In addition, it is valuable due to its ability to identify virus serotype. However, it is less useful as an early screening method as it requires a longer period of time (1-8 hours) to yield results, laboratory equipment, and specialized personnel. Compared to conventional RT-PCR, rapid NAAT offers faster detection and can also diagnose other viral and bacterial pathogens, thus potentially decreasing misdiagnosis and further spread of infection due to delayed diagnosis.
Table 3. Sensitivities of some clinical laboratory improvement amendments waived rapid NAAT tests for influenzaa .
aDerived from Babady et al.[38], Chen et al.[39], and Popowitch et al.[40]. NAAT: rapid nucleic acid amplification test.
| Rapid NAAT test | Sensitivity for influenza A | Sensitivity for influenza B |
|---|---|---|
| Alere™ i (ID NOW™) | 63.8%–99.3% | 81.5%–100% |
| Cobas Liat | 99.2%–100% | 97.9%–100% |
| Xpert Xpress | 98.6%–100% | 96.3%–97.9% |
| bioFire FilmArray | 86.2%–100% | 77.3%–100% |
Table 4. Clinical laboratory improvement amendments waived RIDT and NAAT testsa .
aDerived from Babady et al.[38] and Al salmi et al.[41] BAL: bronchoalveolar lavage; D: direct specimen; ETA: endotracheal aspirate; NA: nasal aspirates; NAAT: nucleic acid amplification test; NPA: nasopharyngeal aspirates; NPS: nasopharyngeal swabs; NPW: nasopharyngeal wash; NS: nasal swabs; NW: nasal washes; RIDT: rapid influenza diagnostic test; RSV: respiratory syncytial virus; VTM: viral transport media.
| Tests | Specimen type | Testing time (minutes) |
|---|---|---|
| RIDT | ||
| Alere Influenza A/B | NS | 10 |
| Binax Now Influenza A/B card 2 | NS | 15 |
| BD Veritor Flu A+B | NS, NPS, | 10 |
| Sofia Influenza A+B | ||
| Sofia Influenza A+B 2 | NS, NPS, NPA, NPW (VTM/D) | 15 |
| QuickVue Influenza A+B | NS, NPS, NA, NW | 10 |
| Rapid NAAT | ||
| Alere i Influenza A/B | NPS, NPS (VTM) | < 15 |
| Alere i Influenza A/B v2 | NS (D/VTM), NPS (VTM) | < 15 |
| Xpert Xpress Flu | NPS, NS | < 30 |
| Xpert Xpress Flu + RSV | NPS | < 30 |
| Cobas Liat Influenza A/B | NPS | < 20 |
| Cobas Liat Influenza A/B, RSV | NPS | < 20 |
| FilmArray Respiratory panel EZ | NPS | < 45 |
| FilmArray Respiratory panel 2 | NPS | < 45 |
| FilmArray Pneumonia panel | Sputum, ETA, BAL | 60 |
| Accula Flu A/Flu B | NS | < 30 |
Several studies have compared the efficacy of different assays in emergency clinics or similar settings. Compared with conventional PCR, RIDT or rapid NAAT impacts clinical decision-making and may increase the use of antiviral drugs.[22,26,43] An open randomized controlled trial in a UK emergency clinic compared 362 patients who underwent point-of-care testing (POCT via rapid NAAT in this particular study) and 358 patients who underwent RT-PCR, and showed that those who underwent POCT were more likely to receive the correct antiviral therapy and that more patents in the POCT group received only single-dose or brief antibiotic therapy. However, there was no difference in the number of patients receiving antibiotic therapy and the duration of antibiotic use between the two groups.[43] In a retrospective study comparing rapid NAAT and RIDT in 620 patients in the United States, a positive rapid NAAT significantly increased antiviral use (82.4% vs. 69.9%, p < 0.05) and a negative NAAT significantly decreased antiviral use (2.3% vs. 13.1%, p < 0.005). However, the difference in antibiotic use was not statistically significant.[44] An additional study comparing rapid NAAT and RIDT also demonstrated that utilizing rapid NAAT may prevent unnecessary additional testing and hospitalization and may increase rates of antiviral therapy use and patient isolation to reduce transmission.[27] Although rapid NAAT has positively affected clinical decision-making and patient management, the current studies do not suggest that the use of rapid NAAT improves patient prognosis, such as mortality.
The economic benefit of utilizing rapid NAAT is yet to be determined. Despite the higher cost of testing, there is potential for savings in overall healthcare expenditures due to accurate diagnosis and improved treatment outcomes, reduced antibiotic use, and the prevention of nosocomial infections. One Danish group investigated POCT for influenza and multiple respiratory viruses in emergency clinics in 180 minor and 334 adult patients and demonstrated that early testing shortened antibiotic use and increased treatment of antiviral respiratory infections. Based on these findings, they recommended point-of-care rapid NAAT testing in emergency clinics for children under five years of age.[45] Currently, cost-effectiveness analyses are being conducted in Europe and the United States, although these may not be applicable in Taiwan; willingness to pay thresholds also vary according to socioeconomic conditions.[46] Overall, there is a lack of data regarding the cost-effectiveness of various diagnostic tests in Taiwan, and therefore the optimal testing method should be determined by combining local epidemiological and socioeconomic conditions. In the future, further research should be undertaken to address these questions.
Influenza virus serum IgM antibodies are detectable in the blood within one week of infection.[47,48] However, the rate of IgM positivity in the early phase of the disease is relatively low and is therefore an unreliable biomarker for early diagnosis.[49] Changes in serum IgG antibody titers are significant in the recovery phase when compared with the acute phase, but can only be used for retrospective diagnosis because of the need for double blood sampling.[12]
The viral load and viral shedding capacity of influenza patients vary depending on the onset, underlying conditions, and virus type. It is generally recognized that there is a correlation between influenza viral load, shedding, and symptoms.[50-52] One study conducted in Hong Kong suggested that the shedding of influenza A is the highest within 1-2 days of symptom onset and gradually subsides after 6-7 days, rendering the virus difficult to be detected, whereas influenza B has the ability to disseminate 2 days prior to symptom onset and can persist until 6-7 days after symptom onset. Most influenza transmission occurs within the first 2-3 days of symptoms.[52] In immunosuppressed patients, viral shedding may occur for extended periods.[53] Therefore, testing within 2-3 days of symptom onset is more likely to yield a positive result.
Viral loads vary depending on specimen collection methods. In the 2009 H1N1 pandemic, the specimens in the order of decreasing viral load were: nasopharyngeal lavage, nasal swabs, and pharyngeal swabs.[54]5 Other studies have shown similar diagnostic accuracy for nasopharyngeal lavage, nasopharyngeal swabs, and both nasal and pharyngeal swabs.[55,56] The accuracy of saliva and nasopharygeal samples for detecting influenza may be comparable.[57] The latest COVID-19 pooled analysis found that saliva or mouthwash and nasopharyngeal samples had comparable accuracy for COVID-19 detection.[58,59] Multiple sampling may increase the accuracy of virus detection.[60,61] Lower respiratory specimens, including sputum, tracheal intubation aspirates, and alveolar lavage fluid, are more sensitive than upper respiratory specimens for diagnosis. Lower respiratory specimens should be obtained in hospitalized critically ill patients or those who have an artificial airway.[7,62] To evaluate concomitant or coinfection with other bacterial or viral pathogens, equipment such as multiple rapid NAAT can be used. However, attention should be paid to which particular pathogens can be detected with rapid NAAT.[63]
References
- Iuliano A Danielle, Roguski Katherine M, Chang Howard H, Muscatello David J, Palekar Rakhee, Tempia Stefano, Cohen Cheryl, Gran Jon Michael, Schanzer Dena, Cowling Benjamin J, Wu Peng, Kyncl Jan, Ang Li Wei, Park Minah, Redlberger-Fritz Monika, Yu Hongjie, Espenhain Laura, Krishnan Anand, Emukule Gideon, van Asten Liselotte, Pereira da Silva Susana, Aungkulanon Suchunya, Buchholz Udo, Widdowson Marc-Alain, Bresee Joseph S, Azziz-Baumgartner Eduardo, Cheng Po-Yung, Dawood Fatimah, Foppa Ivo, Olsen Sonja, Haber Michael, Jeffers Caprichia, MacIntyre C Raina, Newall Anthony T, Wood James G, Kundi Michael, Popow-Kraupp Therese, Ahmed Makhdum, Rahman Mahmudur, Marinho Fatima, Sotomayor Proschle C Viviana, Vergara Mallegas Natalia, Luzhao Feng, Sa Li, Barbosa-Ramírez Juliana, Sanchez Diana Malo, Gomez Leandra Abarca, Vargas Xiomara Badilla, Acosta Herrera aBetsy, Llanés María Josefa, Fischer Thea Kølsen, Krause Tyra Grove, Mølbak Kåre, Nielsen Jens, Trebbien Ramona, Bruno Alfredo, Ojeda Jenny, Ramos Hector, an der Heiden Matthias, del Carmen Castillo Signor Leticia, Serrano Carlos Enrique, Bhardwaj Rohit, Chadha Mandeep, Narayan Venkatesh, Kosen Soewarta, Bromberg Michal, Glatman-Freedman Aharona, Kaufman Zalman, Arima Yuzo, Oishi Kazunori, Chaves Sandra, Nyawanda Bryan, Al-Jarallah Reem Abdullah, Kuri-Morales Pablo A, Matus Cuitláhuac Ruiz, Corona Maria Eugenia Jimenez, Burmaa Alexander, Darmaa Oyungerel, Obtel Majdouline, Cherkaoui Imad, van den Wijngaard Cees C, van der Hoek Wim, Baker Michael, Bandaranayake Don, Bissielo Ange, Huang Sue, Lopez Liza, Newbern Claire, Flem Elmira, Grøneng Gry M, Hauge Siri, de Cosío Federico G, de Moltó Yadira, Castillo Lourdes Moreno, Cabello Maria Agueda, von Horoch Marta, Medina Osis Jose, Machado Ausenda, Nunes Baltazar, Rodrigues Ana Paula, Rodrigues Emanuel, Calomfirescu Cristian, Lupulescu Emilia, Popescu Rodica, Popovici Odette, Bogdanovic Dragan, Kostic Marina, Lazarevic Konstansa, Milosevic Zoran, Tiodorovic Branislav, Chen Mark, Cutter Jeffery, Lee Vernon, Lin Raymond, Ma Stefan, Cohen Adam L, Treurnicht Florette, Kim Woo Joo, Delgado-Sanz Concha, de mateo Ontañón Salvador, Larrauri Amparo, León Inmaculada León, Vallejo Fernando, Born Rita, Junker Christoph, Koch Daniel, Chuang Jen-Hsiang, Huang Wan-Ting, Kuo Hung-Wei, Tsai Yi-Chen, Bundhamcharoen Kanitta, Chittaganpitch Malinee, Green Helen K, Pebody Richard, Goñi Natalia, Chiparelli Hector, Brammer Lynnette, Mustaquim Desiree. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet. 2018 Mar;391(10127) doi: 10.1016/s0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Yu-Nong, Kuo Rei-Lin, Chen Guang-Wu, Shih Shin-Ru. Centennial review of influenza in Taiwan. Biomedical Journal. 2018 Aug;41(4) doi: 10.1016/j.bj.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki Timothy M, Bernstein Henry H, Bradley John S, Englund Janet A, File Thomas M, Fry Alicia M, Gravenstein Stefan, Hayden Frederick G, Harper Scott A, Hirshon Jon Mark, Ison Michael G, Johnston B Lynn, Knight Shandra L, McGeer Allison, Riley Laura E, Wolfe Cameron R, Alexander Paul E, Pavia Andrew T. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clinical Infectious Diseases. 2018 Dec 19;68(6) doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand Caroline, Leeflang Mariska M.G., Minion Jessica, Brewer Timothy, Pai Madhukar. Accuracy of Rapid Influenza Diagnostic Tests. Annals of Internal Medicine. 2012 Apr 03;156(7) doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- Chu Haitao, Lofgren Eric T., Halloran M. Elizabeth, Kuan Pei F., Hudgens Michael, Cole Stephen R. Performance of rapid influenza H1N1 diagnostic tests: a meta-analysis. Influenza and Other Respiratory Viruses. 2011 Aug 31;6(2) doi: 10.1111/j.1750-2659.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green Daniel A., StGeorge Kirsten. Kraft Colleen Suzanne., editor. Rapid Antigen Tests for Influenza: Rationale and Significance of the FDA Reclassification. Journal of Clinical Microbiology. 2018 Oct;56(10) doi: 10.1128/jcm.00711-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry Marie Louise. Diagnostic tests for influenza infection. Current Opinion in Pediatrics. 2011 Feb;23(1) doi: 10.1097/mop.0b013e328341ebd9. [DOI] [PubMed] [Google Scholar]
- Petric Martin, Comanor Lorraine, Petti Cathy A. Role of the Laboratory in Diagnosis of Influenza during Seasonal Epidemics and Potential Pandemics. The Journal of Infectious Diseases. 2006 Nov;194(s2) doi: 10.1086/507554. [DOI] [PubMed] [Google Scholar]
- Monto Arnold S., Gravenstein Stefan, Elliott Michael, Colopy Michael, Schweinle Jo. Clinical Signs and Symptoms Predicting Influenza Infection. Archives of Internal Medicine. 2000 Nov 27;160(21) doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- Tang Julian Wei-Tze. Differing Symptom Patterns in Early Pandemic vs Seasonal Influenza Infections. Archives of Internal Medicine. 2010 May 24;170(10) doi: 10.1001/archinternmed.2010.108. [DOI] [PubMed] [Google Scholar]
- Call Stephanie A. Does This Patient Have Influenza? JAMA. 2005 Feb 23;293(8) doi: 10.1001/jama.293.8.987. [DOI] [Google Scholar]
- Zambon Maria, Hays John, Webster Alison, Newman Robert, Keene Oliver. Diagnosis of Influenza in the Community. Archives of Internal Medicine. 2001 Sep 24;161(17) doi: 10.1001/archinte.161.17.2116. [DOI] [PubMed] [Google Scholar]
- Heinonen S., Peltola V., Silvennoinen H., Vahlberg T., Heikkinen T. Signs and symptoms predicting influenza in children: a matched case–control analysis of prospectively collected clinical data. European Journal of Clinical Microbiology & Infectious Diseases. 2011 Nov 13;31(7) doi: 10.1007/s10096-011-1479-4. [DOI] [PubMed] [Google Scholar]
- Yang Jeng-How, Huang Po-Yen, Shie Shian-Sen, Yang Shuan, Tsao Kuo-Chien, Wu Tsu-Lan, Leu Hsieh-Shong, Huang Ching-Tai. Predictive Symptoms and Signs of Laboratory-confirmed Influenza. Medicine. 2015 Nov;94(44) doi: 10.1097/md.0000000000001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen Glen T., Moore Johanna, Herding Emily, Gooch Tami, Hirigoyen Diane, Hanson Kevan, Deike Marcia. Clinical decision making in the emergency department setting using rapid PCR: Results of the CLADE study group. Journal of Clinical Virology. 2018 May;102 doi: 10.1016/j.jcv.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impact of Rapid Diagnosis on Management of Adults Hospitalized With Influenza. Archives of Internal Medicine. 2007 Feb 26;167(4) doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- Varkey Jay B, Varkey Basil. Viral infections in patients with chronic obstructive pulmonary disease. Current Opinion in Pulmonary Medicine. 2008 Mar;14(2) doi: 10.1097/mcp.0b013e3282f4a99f. [DOI] [PubMed] [Google Scholar]
- Muthuri Stella G, Venkatesan Sudhir, Myles Puja R, Leonardi-Bee Jo, Al Khuwaitir Tarig S A, Al Mamun Adbullah, Anovadiya Ashish P, Azziz-Baumgartner Eduardo, Báez Clarisa, Bassetti Matteo, Beovic Bojana, Bertisch Barbara, Bonmarin Isabelle, Booy Robert, Borja-Aburto Victor H, Burgmann Heinz, Cao Bin, Carratala Jordi, Denholm Justin T, Dominguez Samuel R, Duarte Pericles A D, Dubnov-Raz Gal, Echavarria Marcela, Fanella Sergio, Gao Zhancheng, Gérardin Patrick, Giannella Maddalena, Gubbels Sophie, Herberg Jethro, Iglesias Anjarath L Higuera, Hoger Peter H, Hu Xiaoyun, Islam Quazi T, Jiménez Mirela F, Kandeel Amr, Keijzers Gerben, Khalili Hossein, Knight Marian, Kudo Koichiro, Kusznierz Gabriela, Kuzman Ilija, Kwan Arthur M C, Amine Idriss Lahlou, Langenegger Eduard, Lankarani Kamran B, Leo Yee-Sin, Linko Rita, Liu Pei, Madanat Faris, Mayo-Montero Elga, McGeer Allison, Memish Ziad, Metan Gokhan, Mickiene Auksė, Mikić Dragan, Mohn Kristin G I, Moradi Ahmadreza, Nymadawa Pagbajabyn, Oliva Maria E, Ozkan Mehpare, Parekh Dhruv, Paul Mical, Polack Fernando P, Rath Barbara A, Rodríguez Alejandro H, Sarrouf Elena B, Seale Anna C, Sertogullarindan Bunyamin, Siqueira Marilda M, Skręt-Magierło Joanna, Stephan Frank, Talarek Ewa, Tang Julian W, To Kelvin K W, Torres Antoni, Törün Selda H, Tran Dat, Uyeki Timothy M, Van Zwol Annelies, Vaudry Wendy, Vidmar Tjasa, Yokota Renata T C, Zarogoulidis Paul, Nguyen-Van-Tam Jonathan S. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. The Lancet Respiratory Medicine. 2014 May;2(5) doi: 10.1016/s2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli M. J., Athota R., Reed S., Czajkowski L., Bristol T., Proudfoot K., Hagey R., Voell J., Fiorentino C., Ademposi A., Shoham S., Taubenberger J. K. The Natural History of Influenza Infection in the Severely Immunocompromised vs Nonimmunocompromised Hosts. Clinical Infectious Diseases. 2013 Nov 01;58(2) doi: 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke A. J., Shapiro D. J., Pavia A. T., Byington C. L., Ampofo K., Stockmann C., Hersh A. L. A National Study of the Impact of Rapid Influenza Testing on Clinical Care in the Emergency Department. Journal of the Pediatric Infectious Diseases Society. 2013 Nov 13;3(2) doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson Laurent, Mahadeb Bhavna, De Foor Marc, Vandenberg Olivier, Hallin Marie. Contribution of a rapid influenza diagnostic test to manage hospitalized patients with suspected influenza. Diagnostic Microbiology and Infectious Disease. 2017 Mar;87(3) doi: 10.1016/j.diagmicrobio.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Hayden Frederick G., Atmar Robert L., Schilling Margo, Johnson Casey, Poretz Donald, Paar David, Huson Les, Ward Penelope, Mills Roger G. Use of the Selective Oral Neuraminidase Inhibitor Oseltamivir to Prevent Influenza. New England Journal of Medicine. 1999 Oct 28;341(18) doi: 10.1056/nejm199910283411802. [DOI] [PubMed] [Google Scholar]
- Russell Kate, Chung Jessie R., Monto Arnold S., Martin Emily T., Belongia Edward A., McLean Huong Q., Gaglani Manjusha, Murthy Kempapura, Zimmerman Richard K., Nowalk Mary Patricia, Jackson Michael L., Jackson Lisa A., Flannery Brendan. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018 Feb;36(10) doi: 10.1016/j.vaccine.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla Jesús, Martínez-Baz Iván, Navascués Ana, Casado Itziar, Aguinaga Aitziber, Díaz-González Jorge, Delfrade Josu, Guevara Marcela, Ezpeleta Carmen. Comparison of influenza vaccine effectiveness in preventing outpatient and inpatient influenza cases in older adults, northern Spain, 2010/11 to 2015/16. Eurosurveillance. 2018 Jan 11;23(2) doi: 10.2807/1560-7917.es.2018.23.2.16-00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Yi-Chun, Chuang Jen-Hsiang, Kuo Hung-Wei, Huang Wan-Ting, Hsu Yu-Fen, Liu Ming-Tsan, Chen Chang-Hsun, Huang Hui-Hsun, Chang Chi-Hsi, Chou Jih-Haw, Chang Feng-Yee, Lin Tzou-Yien, Chiu Wen-Ta. McVernon Jodie., editor. Surveillance and Vaccine Effectiveness of an Influenza Epidemic Predominated by Vaccine-Mismatched Influenza B/Yamagata-Lineage Viruses in Taiwan, 2011−12 Season. PLoS ONE. 2013 Mar 05;8(3) doi: 10.1371/journal.pone.0058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Po-Ju, Chen Chang-Hsun, Chih Yi-Chien. Effectiveness of the influenza vaccination program for the elderly in Taiwan. Vaccine. 2013 Jan;31(4) doi: 10.1016/j.vaccine.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Nolte Frederick S., Gauld Lori, Barrett Susan B. McAdam A. J., editor. Direct Comparison of Alere i and cobas Liat Influenza A and B Tests for Rapid Detection of Influenza Virus Infection. Journal of Clinical Microbiology. 2016 Nov;54(11) doi: 10.1128/jcm.01586-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx Joanna, Wali Rehab, Schiller Ian, Caya Chelsea, Gore Genevieve C., Chartrand Caroline, Dendukuri Nandini, Papenburg Jesse. Diagnostic Accuracy of Novel and Traditional Rapid Tests for Influenza Infection Compared With Reverse Transcriptase Polymerase Chain Reaction. Annals of Internal Medicine. 2017 Sep 05;167(6) doi: 10.7326/m17-0848. [DOI] [PubMed] [Google Scholar]
- Babady N. Esther, Dunn James J., Madej Roberta. CLIA-waived molecular influenza testing in the emergency department and outpatient settings. Journal of Clinical Virology. 2019 Jul;116 doi: 10.1016/j.jcv.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Huanzhu, Weng Huilan, Lin Meirui, He Ping, Li Yazhen, Xie Qingdong, Ke Changwen, Jiao Xiaoyang. The Clinical Significance of FilmArray Respiratory Panel in Diagnosing Community-Acquired Pneumonia. BioMed Research International. 2017;2017 doi: 10.1155/2017/7320859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popowitch Elena B., O'Neill Stacey S., Miller Melissa B. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP Fast Multiplex Assays for Detection of Respiratory Viruses. Journal of Clinical Microbiology. 2013 May;51(5) doi: 10.1128/jcm.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al salmi T., Algothmi A., Alshehri A., Aljohani S. Performance of FilmArray RP2 Multiplex Panel for identification of MERS CoV Journal of Infection and Public Health. 2019 Jan;12(1) doi: 10.1016/j.jiph.2018.10.019. [DOI] [Google Scholar]
- Brendish Nathan J, Malachira Ahalya K, Armstrong Lawrence, Houghton Rebecca, Aitken Sandra, Nyimbili Esther, Ewings Sean, Lillie Patrick J, Clark Tristan W. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. The Lancet Respiratory Medicine. 2017 May;5(5) doi: 10.1016/s2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke Robert C., McElvania Erin, Thomson Richard B., Kaul Karen L., Das Sanchita. Miller Melissa B., editor. Clinical Impact of Rapid Point-of-Care PCR Influenza Testing in an Urgent Care Setting: a Single-Center Study. Journal of Clinical Microbiology. 2019 Mar;57(3) doi: 10.1128/jcm.01281-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Uffe Vest, Holm Mona Katrine Alberthe, Bang Didi, Petersen Randi Føns, Mortensen Shila, Trebbien Ramona, Lisby Jan Gorm. Point-of-care tests for influenza A and B viruses and RSV in emergency departments – indications, impact on patient management and possible gains by syndromic respiratory testing, Capital Region, Denmark, 2018. Eurosurveillance. 2020 Nov 05;25(44) doi: 10.2807/1560-7917.es.2020.25.44.1900430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann Peter J., Cohen Joshua T., Weinstein Milton C. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. New England Journal of Medicine. 2014 Aug 28;371(9) doi: 10.1056/nejmp1405158. [DOI] [PubMed] [Google Scholar]
- Buchner Y I, Heath R B, Collins J V, Pattison J R. Serum IgM antibody and influenza A infection. Journal of Clinical Pathology. 1977 Aug 01;30(8) doi: 10.1136/jcp.30.8.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikerfors T, Lindegren G, Grandien M, van der Logt J. Diagnosis of influenza A virus infections by detection of specific immunoglobulins M, A, and G in serum. Journal of Clinical Microbiology. 1989 Mar;27(3) doi: 10.1128/jcm.27.3.453-458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Yao, Zhipeng Zhao, Wenqi Song, Runqing Li, Dong Zhu, Kun Qin, Xiuying Zhao. Jin Dong-Yan., editor. Unreliable usage of a single influenza virus IgM antibody assay in influenza-like illness: A retrospective study of the 2016–2018 flu epidemic. PLOS ONE. 2019 Apr 22;14(4) doi: 10.1371/journal.pone.0215514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Lincoln L. H., Cowling Benjamin J., Fang Vicky J., Chan Kwok‐Hung, Lau Eric H. Y., Lipsitch Marc, Cheng Calvin K. Y., Houck Peter M., Uyeki Timothy M., Peiris J. S. Malik, Leung Gabriel M. Viral Shedding and Clinical Illness in Naturally Acquired Influenza Virus Infections. The Journal of Infectious Diseases. 2010 May 15;201(10) doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrat Fabrice, Vergu Elisabeta, Ferguson Neil M., Lemaitre Magali, Cauchemez Simon, Leach Steve, Valleron Alain-Jacques. Time Lines of Infection and Disease in Human Influenza: A Review of Volunteer Challenge Studies. American Journal of Epidemiology. 2008 Jan 29;167(7) doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- Ip Dennis K. M., Lau Lincoln L. H., Chan Kwok-Hung, Fang Vicky J., Leung Gabriel M., Peiris Malik J. S., Cowling Benjamin J. The Dynamic Relationship Between Clinical Symptomatology and Viral Shedding in Naturally Acquired Seasonal and Pandemic Influenza Virus Infections. Clinical Infectious Diseases. 2015 Oct 30; doi: 10.1093/cid/civ909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock David M., Gubareva Larisa V., Zuccotti Gianna. Prolonged Shedding of Multidrug-Resistant Influenza A Virus in an Immunocompromised Patient. New England Journal of Medicine. 2003 Feb 27;348(9) doi: 10.1056/nejm200302273480923. [DOI] [PubMed] [Google Scholar]
- Ngaosuwankul Nathamon, Noisumdaeng Pirom, Komolsiri Pisut, Pooruk Phisanu, Chokephaibulkit Kulkanya, Chotpitayasunondh Tawee, Sangsajja Chariya, Chuchottaworn Charoen, Farrar Jeremy, Puthavathana Pilaipan. Influenza A viral loads in respiratory samples collected from patients infected with pandemic H1N1, seasonal H1N1 and H3N2 viruses. Virology Journal. 2010 Apr 20;7(1) doi: 10.1186/1743-422x-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeByle Carolynn, Bulkow Lisa, Miernyk Karen, Chikoyak Lori, Hummel Kimberlee Boyd, Hennessy Thomas, Singleton Rosalyn. Comparison of nasopharyngeal flocked swabs and nasopharyngeal wash collection methods for respiratory virus detection in hospitalized children using real-time polymerase chain reaction. Journal of Virological Methods. 2012 Oct;185(1) doi: 10.1016/j.jviromet.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert Stephen B., Whiley David M., O'Neill Nicholas T., Andrews Emily C., Canavan Fiona M., Bletchly Cheryl, Siebert David J., Sloots Theo P., Nissen Michael D. Comparing Nose-Throat Swabs and Nasopharyngeal Aspirates Collected From Children With Symptoms for Respiratory Virus Identification Using Real-Time Polymerase Chain Reaction. Pediatrics. 2008 Sep 01;122(3) doi: 10.1542/peds.2008-0691. [DOI] [PubMed] [Google Scholar]
- To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P., Ng A.C.K., Leung K.-H., Poon R.W.S., Chan K.-H., Cheng V.C.C., Hung I.F.N., Yuen K.-Y. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clinical Microbiology and Infection. 2019 Mar;25(3) doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Goldfarb David M., Tilley Peter, Al-Rawahi Ghada N., Srigley Jocelyn A., Ford Geoffrey, Pedersen Heather, Pabbi Abhilasha, Hannam-Clark Stephanie, Charles Marthe, Dittrick Michelle, Gadkar Vijay J., Pernica Jeffrey M., Hoang Linda M. N. Tang Yi-Wei., editor. Self-Collected Saline Gargle Samples as an Alternative to Health Care Worker-Collected Nasopharyngeal Swabs for COVID-19 Diagnosis in Outpatients. Journal of Clinical Microbiology. 2021 Mar 19;59(4) doi: 10.1128/jcm.02427-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Laporte Guillaume, Lawandi Alexander, Schiller Ian, Yao Mandy, Dendukuri Nandini, McDonald Emily G., Lee Todd C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2. JAMA Internal Medicine. 2021 Mar 01;181(3) doi: 10.1001/jamainternmed.2020.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de la Tabla Victoria, Masiá Mar, Antequera Pedro, Martin Coral, Gazquez Gregoria, Buñuel Fernando, Gutiérrez Félix. Comparison of Combined Nose-Throat Swabs with Nasopharyngeal Aspirates for Detection of Pandemic Influenza A/H1N1 2009 Virus by Real-Time Reverse Transcriptase PCR. Journal of Clinical Microbiology. 2010 Oct;48(10) doi: 10.1128/jcm.01105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood Fatimah S., Jara Jorge, Estripeaut Dora, Vergara Ofelina, Luciani Kathia, Corro Mary, de León Tirza, Saldaña Ricardo, Castillo Baires Juan Miguel, Rauda Flores Rafael, Cazares Rafael A., Brizuela de Fuentes Yarisa Sujey, Franco Danilo, Gaitan Melissa, Schneider Eileen, Berman LaShondra, Azziz-Baumgartner Eduardo, Widdowson Marc-Alain. What Is the Added Benefit of Oropharyngeal Swabs Compared to Nasal Swabs Alone for Respiratory Virus Detection in Hospitalized Children Aged <10 Years?: Table 1. Journal of Infectious Diseases. 2015 May 05;212(10) doi: 10.1093/infdis/jiv265. [DOI] [PubMed] [Google Scholar]
- Roa Paula López, Rodríguez-Sánchez Belen, Catalán Pilar, Giannella Maddalena, Alcalá Luis, Padilla Belén, Viedma Darío García, Muñoz Patricia, Bouza Emilio. Diagnosis of Influenza in Intensive Care Units: Lower Respiratory Tract Samples Are Better than Nose–Throat Swabs. American Journal of Respiratory and Critical Care Medicine. 2012 Nov 01;186(9) doi: 10.1164/ajrccm.186.9.929. [DOI] [PubMed] [Google Scholar]
- Goldstein E.J., Gunson R.N. In-house validation of the cobas Liat influenza A/B and RSV assay for use with gargles, sputa and endotracheal secretions. Journal of Hospital Infection. 2019 Mar;101(3) doi: 10.1016/j.jhin.2018.10.025. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Past pandemics Centers for Disease Control and Prevention (CDC) Feb 1, https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html. 2021. https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html
- Centers for Disease Control and Prevention (CDC) Estimated flu-related illnesses, medical visits, hospitalizations, and deaths in the United States—2018–2019 flu season Centers for Disease Control and Prevention (CDC) Feb 1, https://www.cdc.gov/flu/about/burden/2018-2019.html. 2021. https://www.cdc.gov/flu/about/burden/2018-2019.html
- Taiwan Centers for Disease Control Influenza express [流感速訊] Taiwan Centers for Disease Control. Feb 2, https://www.cdc.gov.tw/Category/MPage/7cc1CTepnQ7B7yBAdq6z7A. 2021. https://www.cdc.gov.tw/Category/MPage/7cc1CTepnQ7B7yBAdq6z7A
- Taiwan Centers for Disease Control Taiwan national infectious disease statistics system Taiwan Centers for Disease Control. Feb 2, https://nidss.cdc.gov.tw/en. 2021. https://nidss.cdc.gov.tw/en
- Centers for Disease Control and Prevention Flu symptoms & complications Centers for Disease Control and Prevention. Feb 2, https://www.cdc.gov/flu/symptoms/symptoms.htm. 2021. https://www.cdc.gov/flu/symptoms/symptoms.htm
- Taiwan Centers of Disease Control Influenza express Taiwan Centers of Disease Control. Feb 2, https://www.cdc.gov.tw/En/Category/MPage/Utv3lzlSnTK-t6inZrBZsw. 2021. https://www.cdc.gov.tw/En/Category/MPage/Utv3lzlSnTK-t6inZrBZsw
- Centers for Disease Control and Prevention (CDC) Rapid Influenza Diagnostic Tests (RIDTs) Centers for Disease Control and Prevention (CDC) Feb 3, https://www.cdc.gov/flu/professionals/diagnosis/table-ridt.html. 2021. https://www.cdc.gov/flu/professionals/diagnosis/table-ridt.html
- Centers for Disease Control and Prevention (CDC) Table 3. Nucleic Acid Detection Based Tests Centers for Disease Control and Prevention (CDC) Feb 3, https://www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html. 2021. https://www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html
- Food and Drug Administration Microbiology Devices; Reclassification of Influenza Virus Antigen Detection Test Systems Intended for Use Directly With Clinical Specimens. Fed Regist 2017;82:3609-3619. [PubMed]
- U.S. Food and Drug Administration Devices@FDA U.S. Food and Drug Administration. Feb 3, https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm. 2021. https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm


