Abstract
Objective
To identify the predictive factors for renal response in patients with lupus nephritis (LN).
Methods
Patients and data were extracted from a prospective systemic lupus erythematosus cohort in Korea, in which clinical data were collected at 0, 3, 6, and 12 months after induction therapy. Treatment response of LN were evaluated as a complete response (CR), partial response (PR), or non-response (NR) at 3, 6, and 12 months, respectively. Predictive factors for CR at 6 months were evaluated using multivariable Poisson regression analysis.
Results
A total of 75 patients with LN who underwent biopsy was enrolled. The mean age at diagnosis of LN was 28.9±9.7 years, and 68 (90.7%) were female. The frequencies of classes III, IV, III+V, IV+V, and V were 20.0%, 44.0%, 16.0%, 12.0%, and 8.0%, respectively. Compared to relapsed LN, new-onset LN showed a lower percentage of glomerulosclerosis (45.5% vs. 76.2%, p=0.013). The overall proportions of CR, PR, and NR at 6 and 12 months were 52.0%, 26.7%, 21.3% and 50.7%, 24.0%, 25.3%, respectively. In multivariate analysis, age at enrollment (odds ratio [OR]=1.02, p=0.022), relapsed LN (OR=0.71, p=0.037), anti-Ro antibody (OR=0.67, p=0.014), and class III LN (OR=1.48, p=0.001) were associated with CR at 6 months.
Conclusion
In our prospective cohort, class III LN was a good predictive factor for CR at 6 months in patients with LN, whereas younger age, relapsed LN, and anti-Ro antibody were poor predictive factors.
Keywords: Systemic lupus erythematosus, Lupus nephritis, Outcome assessment, Risk factor
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with heterogeneous features. Lupus nephritis (LN) is one of the common manifestations of SLE and is observed in 38.3%~68.9% of SLE cases [1-3]. The target of LN treatment is complete remission (CR), which is accomplished through a combination therapy of immunosuppressive agents and glucocorticoids. However, up to 40% of LN patients who undergo induction therapy do not respond to treatment [4-6].
Several studies were conducted to identify predictive factors for remission after induction therapy in an effort to reduce the proportion of non-responders. Luis et al. found that <2 g/day of proteinuria at 3 months after induction treatment in proliferative LN patients was a reliable predictor for CR [7]. Park et al. [8] showed that glomerulosclerosis in the chronicity index was negatively associated with achieving CR in LN patients who had received the induction treatment. Another retrospective study demonstrated that the anti-La antibody was a good predictive factor for a CR after 12 months, but that higher levels of serum blood urea nitrogen (BUN) and β2-microglobulin levels were poor predictive factors for a CR at 12 months [4]. A separate retrospective study identified that the chronicity index was a poor predictive factor for a 12-month CR in proliferative LN, and a high CH50 level represented a good predictive factor in patients with membranous LN [9]. Moreover, a ≥59% reduction in the urine protein-to-creatinine ratio (UPCR) and an absolute albumin serum level of ≥3.29 mg/dL at 3 months after induction therapy were also reported as predictive factors for a CR after 6 months [10].
While there is a consensus that some factors such as proteinuria are associated with a treatment response after LN patients are given induction treatment, it is difficult to determine whether other factors such as glomerulosclerosis, BUN, CH50, and anti-La antibody derived from each retrospective cohort can be predictive factors for treatment response in patients with LN. This is because of the limitations of some retrospective studies such as, selection bias, where non-responders are more likely to be excluded than responders, different criteria for treatment response, and differing periods of observation [4,8,9].
Therefore, we aim to identify the predictive factors for treatment response after induction treatment, including already known factors but with a prospective cohort where the observation period is constant, patient compliance is high, and selection and information bias can be reduced.
MATERIALS AND METHODS
Study population
Patients with LN were recruited from a prospective Korean Unlimited multi-Dimensional Omics research in SLE (KUDOS) cohort conducted at a single center. Although the KUDOS cohort continuously enrolls patient subjects, all LN patients included in this study were enrolled between March 2018 and March 2020. All patients were ≥18 years of age and fulfilled the 1997 revised American College of Rheumatology (ACR) [11] or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) clinical classification criteria [12]. Patients with kidney transplantations and pregnant women, were excluded from the study. Renal biopsy was performed in patients with new-onset and relapsed LN who had persistent proteinuria of ≥500 mg/day (UPCR or 24-hour urine collection) and/or active sediment (>5 RBC or WBC/HPF and/or ≥1 cellular cast). The study was approved by the Institutional Review Board (IRB) of the Hanyang University Medical Center (IRB No. HYUH2017-08-035) and was performed in accordance with the Declaration of Helsinki. All subjects provided written informed consent.
Data collection
The baseline clinical data collected were serum creatinine levels, estimated glomerular filtration rate (eGFR), BUN, spot UPCR (or 24-hour urine collection), complements (C3, C4, and CH50), SLE-related autoantibodies (anti-double-stranded DNA antibody, anti-Smith antibody, anti-ribonucleoprotein antibody, anti-Ro/La antibody, anti-phospholipid antibodies such as lupus anticoagulant, anti-cardiolipin antibody, and β2-glycoprotein 1 (β2-GP1) IgM/IgG antibody and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [13]. Classifications of LN and the activity index and chronicity index were based on the International Society of Nephrology/Renal Pathology Society (ISN/RPS) guidelines. Our hospital’s pathology department has adopted the 2018 revised ISN/RPS classification since December 2018 [14]. Hence, 26 patients who had previously reported with 2003 ISN/RPS classifications were recategorized based on the revised 2018 ISN/RPS classifications. Blood and urine samples and SLEDAI were collected after the kidney biopsy at 0, 3, 6, and 12 months.
Induction treatment and clinical response
All patients underwent induction treatment based on the current LN treatment guidelines from the ACR and EULAR recommendations, either after the initial LN diagnosis or after an acute renal flare [15,16]. Mycophenolate mofetil (MMF; up to 2 g/day) or a combination of low-dose MMF (500~1,000 mg) and tacrolimus (TAC; 1~3 mg) was administered as an oral immunosuppressant to patients with proliferative LN, depending on their tolerance. In patients with membranous LN, MMF or TAC were administered as an immunosuppressant for patients that had nephrotic-range proteinuria. Prednisolone (0.5~1 mg/kg/day) was administered during the induction treatment, with or without intravenous methylprednisolone pulse therapy. Additionally, MMF or MMF plus TAC, received as part of induction treatment, was used in equal or lower doses of induction treatment agents as a maintenance treatment.
The renal response status was evaluated at 3, 6, and 12 months after the induction treatment and was categorized as either a CR, partial response (PR), or non-response (NR) based on the ACR guidelines [17]. When there was a nephrotic-range of proteinuria (≥3,000 mg/day), the PR definition of the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline was adapted to determine the renal response criteria [18]. A CR is defined as “a UPCR (or 24-hour urine collection) of 500 mg/day or less if the serum creatinine (and/or eGFR) is normal; or if abnormal, is 125% less than baseline.” A PR was defined as “at least a 50% UPCR (or 24-hour urine collection) reduction compared with the baseline but above a UPCR of 500 mg/day. In the presence of nephrotic-range proteinuria (≥3,000 mg/day) at baseline, then a 50% reduction in UPCR and less than a UPCR 3,000 mg/day were required; the same as the creatinine level (and/or eGFR) of a CR definition”. A NR is defined as “neither a CR nor PR.”
Statistical analysis
For the continuous variables, the differences between groups were assessed by a Mann–Whitney U-test, while Pearson chi-square test and Fisher’s exact test were used to assess the categorical data. A modified Poisson regression model, using variables significant in the univariate analysis was used to evaluate associated factors for CR at 6 months after induction treatment. Additionally, clinical and histopathological characteristics of LN patients were compared between new-onset and relapsed LN. In all analyses, p-values were two-tailed, and all p-values less than 0.05 were considered statistically significant. Factors with a p-value of less than 0.10 in the univariate analysis were included in the multivariate analysis. All patients completed follow-up, and there were no missing data in this study. Data were analyzed using Statistical Analysis System (SAS) 9.4 statistical software (SAS Institute, Cary, NC, USA).
RESULTS
Baseline clinical characteristics of patients
Of the 78 patients enrolled between March 2018 and March 2020, three were excluded (two with LN class I and one with missing data at 12 months), and a total of 75 patients participated in this study. All patients completed a one-year follow-up period. Baseline demographic, laboratory, and histopathological characteristics are summarized in Tables 1 and 2. In total, 68 patients were female (90.7%), and the mean age at diagnosis of LN was 28.9 years (standard deviation [SD]=±9.7). All 75 patients underwent a kidney biopsy and 33 (44.0%) of these were diagnosed with new-onset LN. In baseline renal histology (ISN/RPS), the frequencies of classes III, IV, III+V, IV+V, and V were 15 (20.0%), 33 (44.0%), 12 (16.0%), 9 (12.0%), and 6 (8.0%), respectively. The baseline proteinuria level in classes III, IV, III+V, IV+V, V was 1,304.7 mg/g (±614.2), 1,525.9 mg/g (±932.5), 2,901.1 mg/g (±2,074.1), 5,600.7 mg/g (±4,234.0), and 2,327.9 mg/g (±1,411.1), respectively. The means of the activity index and chronicity index (CI) were 7.0 (SD=±3.9) of 24 and 1.9 (SD=±1.7) of 12, respectively. Most patients had low C3 (94.7%) and low C4 (69.3%) and were positive for anti-ds DNA antibodies (70.7%). The mean baseline SLEDAI score was 15.6 points (SD=±4.6), and in terms of renal symptoms, proteinuria, hematuria, pyuria, and urinary casts were noted in 100%, 90.7%, 64.0%, and 14.7% of the patients, respectively. Other SLE-associated symptoms included arthritis, skin rashes, alopecia, fever, and leukopenia in 14.7%, 10.7%, 10.7%, 8.0%, and 12.0% of the patients, respectively.
Table 1.
Demographic, laboratory baseline characteristics of all patients and induction treatment
| n=75 | |
|---|---|
| Female sex | 68 (90.7) |
| Age at enrollment (yr) | 34.9±9.8 |
| Age at diagnosis of SLE (yr) | 23.8±7.8 |
| Age at diagnosis of LN (yr) | 28.9±9.7 |
| LN duration (yr) | 6.0±6.5 |
| SLE duration (yr) | 11.9±5.9 |
| Renal flare | |
| 1st | 33 (44.0) |
| 2nd | 31 (41.3) |
| 3rd | 10 (13.3) |
| 4th | 1 (1.3) |
| Laboratory findings | |
| WBC count (cells ×103/mm3) | 5.7±2.4 |
| Neutrophil count (cells ×103/mm3) | 4.2±2.2 |
| Lymphocytes (cells ×103/mm3) | 1.1±0.7 |
| Creatinine (mg/dL) | 0.7±0.3 |
| eGFR (mL/min/1.73 m2) | 110.1±25.8 |
| Active urinary sediment* | 70 (93.3) |
| C3 (g/L) | 52.3±18.6 |
| Decreased C3 | 71 (94.7) |
| C4 (g/L) | 9.3±6.8 |
| Decreased C4 | 52 (69.3) |
| Proteinuria (mg/day) | 2,639.9±2,408.5 |
| Sub-nephrotic range | 54 (72.0) |
| Nephrotic range | 21 (28.0) |
| Positivity for auto-antibody | |
| Anti-dsDNA | 53 (70.7) |
| Anti-Sm | 30 (40.0) |
| Anti-Ro | 47 (62.7) |
| Anti-La | 11 (14.7) |
| Antiphospholipid antibody | |
| Lupus anticoagulant antibody | 9 (12.0) |
| Anti-Cardiolipin IgM & IgG antibody | 5 (6.7) |
| β2-GP1 IgM & IgG antibody | 3 (4.0) |
| APL antibody† | 12 (16.0) |
| SLEDAI | 15.6±4.6 |
| Hypertension | 8 (10.7) |
| Induction treatment | |
| MMF | 55 (73.3) |
| MMF+TAC | 12 (16.0) |
| TAC | 2 (2.7) |
| Others‡ | 6 (8.0) |
Values are presented as number (%) or mean±standard deviation unless otherwise indicated. SLE: systemic lupus erythematosus, LN: lupus nephritis, WBC: white blood cells, eGFR: estimated glomerular filtration rate, C3: complement 3, C4: complement 4, Anti-dsDNA: anti-double-stranded deoxyribonucleic acid, Anti-Sm: anti-Smith, IgM: immunoglobulin M, IgG: immunoglobulin G, β2-GP1: β2-glycoprotein 1, APL antibody: antiphospholipid antibody, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index, MMF: mycophenolate mofetil, TAC: tacrolimus, RBC: red blood cell, HPF: high power field. *>5 RBC or WBC/HPF and/or ≥1 cellular cast. †Have at least one antiphospholipid antibody. ‡Cyclosporine, Rituximab, and Steroid only, number (%): 1 (1.3), 1 (1.3), and 4 (6.7), respectively.
Table 2.
Histopathological baseline characteristics of all patients
| n=75 | |
|---|---|
| Biopsy class | |
| III | 15 (20.0) |
| IV | 33 (44.0) |
| III+V | 12 (16.0) |
| IV+V | 9 (12.0) |
| V | 6 (8.0) |
| Renal histology (ISN/RPS) | |
| Activity index (/24) | 7.0±3.9 |
| Endocapillary hypercellularity | 69 (92.0) |
| Neutrophils/karyorrhexis | 67 (89.3) |
| Fibrinoid necrosis | 28 (37.3) |
| Hyaline deposits | 43 (57.3) |
| Cellular/fibrocellular crescents | 31 (41.3) |
| Interstitial inflammation | 42 (56.0) |
| Chronicity index (/12) | 1.9±1.7 |
| Glomerulosclerosis | 47 (62.7) |
| Fibrous crescents | 2 (2.7) |
| Tubular atrophy | 34 (45.3) |
| Interstitial fibrosis | 32 (42.7) |
Values are presented as number (%) or mean±standard deviation unless otherwise indicated. ISN/RPS: International Society of Nephrology/Renal Pathology Society.
Profile of treatment for LN
At enrollment, 55 patients (73.3%) were treated with MMF, twelve (16.0%) with MMF plus TAC, and two (2.7%) with TAC as induction agents (Table 1). The remaining six patients were treated with rituximab (n=1), cyclosporin (n=1), and glucocorticoid only (n=4). Two of the patients were administered rituximab or cyclosporin for the induction treatment as they were previously diagnosed with LN and suffered adverse effects after the administration of several immunosuppressants. In the four cases that were treated solely with glucocorticoid, two patients were class V while the others were unable to use the typical induction agents such as MMF or cyclophosphamide (CYC) due to a high risk of infection or a high possibility of undergoing surgery at the time of LN diagnosis. For the class V patients (n=6), two used TAC, two used MMF, and the other two used only glucocorticoid.
For the maintenance treatment, 90.7% (n=68) of the patients maintained equivalent or reduced doses of induction treatment agents with a low dose of glucocorticoid. For the adjunctive therapy, 87% of patients received hydroxychloroquine, which was recommended unless there were previous side effects or contraindications.
Before being enrolled in the KUDOS cohort, CYC and MMF were previously administered as part of the induction treatment to 18 (42.9%) and 10 (23.8%) of the 42 relapsed LN patients, respectively, followed by azathioprine which was given to 6 (16%) patients. In the two patients, however, clinical information could not be confirmed because they underwent renal biopsies and treatments at different hospitals.
Renal response rates at 3, 6, and 12 months after induction therapy
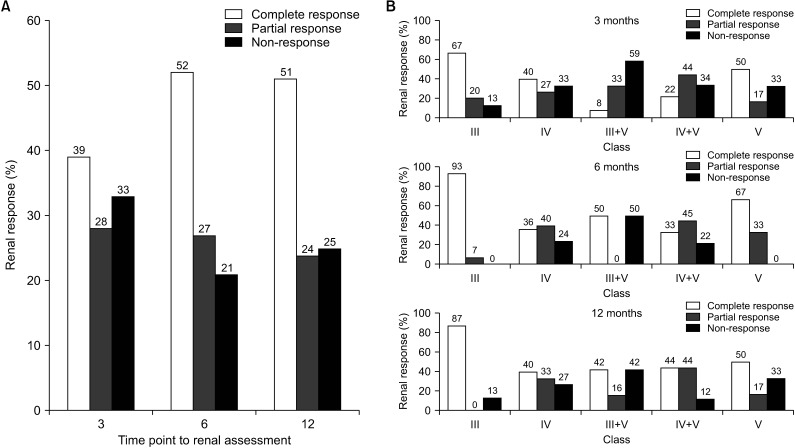
Renal response rates were assessed at 3, 6, and 12 months after the induction therapy and the overall proportion of either CR or PR was 67%, 79%, and 75%, respectively (Figure 1A). The proportion of CR at 3 months was 39%. At 6 and 12 months, the rates increased further to 52% and 51%, respectively. The proportion of NR patients was 33% at 3 months. However, at 6 and 12 months, the rates decreased (21% and 25%, respectively). The renal response also showed class-specific differences; in particular, the CR rates in class III patients were 93% and 87% at 6 and 12 months, respectively (Figure 1B), while the CR, PR, and NR rates of class V patients were 67%, 33%, and 0% at 6 months and 50%, 17%, and 33% at 12 months, respectively.
Fig. 1.
Comparison of renal response rates at 3, 6, and 12 months after induction treatment (A). Comparison of renal response rates by lupus nephritis class at 3, 6, and 12 months (B).
Predictive factors for renal response after induction therapy
For predicting renal response, the two groups of CR and NR were compared at 6 months. The univariate modified Poisson regression model showed that age at enrollment (odds ratio [OR]=1.02, 95% confidence interval [95% CI]=1.01~1.04, p=0.003), age at SLE diagnosis (OR=1.03, 95% CI=1.01~1.05, p=0.006), age at LN diagnosis (OR=1.02, 95% CI=1.01~1.04, p=0.001), relapsed LN (versus new-onset LN, OR=0.71, 95% CI=0.51~1.00, p=0.051), anti-Ro (OR=0.76, 95% CI=0.55~1.04, p=0.084), and class III LN (versus classes IV, III+V, IV+V, and V; OR=1.64, 95% CI=1.28~2.10, p<0.001) were all predictors for CR at 6 months (Table 3). According to the ISN/RPS classification of LN, six activity indices and four chronicity indices were not statistically significant as predictors of CR (Supplementary Table 1). Although statistically significant results are presented in Supplementary Table 1, they cannot be interpreted as predictive factors, as shown in the footnote. A multivariable Poisson regression model showed that class III LN (OR=1.48, 95% CI=1.17~1.87, p=0.001) was a predictive factor for CR at 6 months compared with the other classes except for classes I and II. A younger age at enrollment (OR=1.02, 95% CI=1.00~1.04, p=0.022), relapsed LN (OR=0.71, 95% CI=0.51~0.98, p=0.037) compared with new-onset LN, and anti-Ro antibody positivity (OR=0.67, 95% CI=0.49~0.92, p=0.014) were all shown to be predictive factors for NR at 6 months after induction treatment. The Poisson regression model for CR at 6 months was also conducted on 51 patients with proliferative LN and mixed LN, excluding pure membranous LN patients, and showed the same results in univariate and multivariate analysis with or without class V.
Table 3.
Univariate and multivariate analyses of predictive factors for complete response at 6 months
| Variable | Univariate regression | Multivariate regression | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Female sex | 1.07 | 0.59~1.94 | 0.820 | 1.09 | 0.62~1.92 | 0.759 | |
| Age at enrollment | 1.02 | 1.01~1.04 | 0.003 | 1.02‡ | 1.00~1.04‡ | 0.022‡ | |
| Age at diagnosis of SLE | 1.03 | 1.01~1.05 | 0.006 | ||||
| Age at diagnosis of LN | 1.02 | 1.01~1.04 | 0.001 | ||||
| LN duration | 1.00 | 0.98~1.02 | 0.997 | ||||
| SLE duration | 1.02 | 1.00~1.04 | 0.073 | 1.00 | 0.98~1.02 | 0.912 | |
| Relapsed LN | 0.71 | 0.51~1.00 | 0.051 | 0.71‡ | 0.51~0.98‡ | 0.037‡ | |
| Laboratory findings | |||||||
| Creatinine | 0.61 | 0.26~1.47 | 0.270 | ||||
| eGFR | 1.00 | 1.00~1.01 | 0.545 | ||||
| C3 | 1.00 | 1.00~1.01 | 0.975 | ||||
| C4 | 1.00 | 0.97~1.02 | 0.778 | ||||
| Proteinuria | 1.00 | 1.00~1.00 | 0.241 | ||||
| Positivity of auto-antibody | |||||||
| Anti-dsDNA | 1.14 | 0.76~1.70 | 0.524 | ||||
| Anti-Sm | 0.84 | 0.58~1.22 | 0.356 | ||||
| Anti-RNP | 1.09 | 0.78~1.53 | 0.612 | ||||
| Anti-Ro | 0.76 | 0.55~1.04 | 0.084 | 0.67‡ | 0.49~0.92‡ | 0.014‡ | |
| Anti-La | 1.12 | 0.75~1.66 | 0.583 | ||||
| APL antibody* | 0.67 | 0.33~1.37 | 0.273 | ||||
| Renal histology (ISN/RPS) | |||||||
| Activity index | 0.97 | 0.92~1.02 | 0.203 | ||||
| Chronicity index | 0.98 | 0.89~1.09 | 0.765 | ||||
| Class III† | 1.64 | 1.28~2.10 | <0.001 | 1.48‡ | 1.17~1.87‡ | 0.001‡ | |
| SLEDAI | 1.00 | 0.97~1.04 | 0.947 | ||||
| Hypertension | 0.93 | 0.52~1.69 | 0.820 | ||||
OR: odds ratio, 95% CI: 95% confidence interval, SLE: systemic lupus erythematosus, LN: lupus nephritis, eGFR: estimated glomerular filtration rate, C3: complement 3, C4: complement 4, Anti-dsDNA: anti-double-stranded deoxyribonucleic acid, Anti-Sm: anti-Smith, Anti-RNP: anti-ribonucleoprotein, APL antibody: antiphospholipid antibody, ISN/RPS: International Society of Nephrology/Renal Pathology Society, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index. *Have at least one antiphospholipid antibody. †Class III vs. class III+V, IV, IV+V, and V. ‡Values are considered significant.
DISCUSSION
This study evaluated clinical and histopathologic predictors for renal responses in 75 patients with proliferative (classes III and IV), mixed (classes III/V and IV/V), or pure membranous (class V) LN. Class III LN was identified as a good predictor for CR at 6 months when compared with the other proliferative, mixed, and pure membranous LN classes. In contrast, a younger age, relapsed LN, and the presence of anti-Ro antibody were all poor predictors.
For this study, a six-month time point was chosen to assess renal response after receiving induction treatment in order to minimize bias regarding patient compliance and confounding factors. Drug adherence to the appropriate dose inevitably decreased at 12 months rather than 6 months due to the discomfort associated with taking a multitude of tablets or because symptoms have resolved to a certain extent in some LN patients. Additionally, the long-term use of high-dose steroids and immunosuppressants could increase the risk of infections and drug complications, which can lead to the drug regimen being temporary suspended. Therefore, evaluating renal response at 6 months rather than 12 months can reduce these confounding factors.
In our cohort, CR, PR, NR rates at 6 months and 12 months were 52.0%, 26.7%, 21.3% and 50.7%, 24.0%, 25.3%, respectively. In 2019, Choi et al. [19] reported renal response rates after induction treatment in Korean LN patients treated with MMF, in which the CR, PR, and the NR rates at 6 and 12 months after induction therapy were 43.8%, 25.0%, 31.2% and 56.2%, 18.8%, 25.0%, respectively. Although the induction treatment agents in our cohort were not the same, the renal response rates of the patients in our cohort were similar to those reported by Choi et al. [19]. Moreover, the CR rate was also similar to those previously reported in studies in the United States, Japan and China, ranging from 43% to 58% [4,20,21]. Regarding LN histologic class, the rate of CR in class III showed the highest response rate of 93% at 6 months and 87% at 12 months, while the CR rate for class V was 67% at 6 months and 50% at 12 months, which were similar to the results of previous studies [21].
This study showed an inverse association of age with renal response after induction treatment. A younger age at SLE or LN diagnosis as well as at enrollment in the study were all associated with NR after induction treatment. These results contrast with the findings reported by Park et al. [8] and Ichinose et al. [4] in which the age at onset of LN did not show an association with renal response at 12 months. However, there were some differences in study design between the two studies and our study. For example, CR as a study outcome was compared with PR or NR in the two studies, whereas it was only compared with NR in ours. Additionally, the renal response was assessed 12 months after induction treatment in the two studies, and after 6 months in ours. Also, patients with juvenile onset SLE were included in our study, but not in the other two studies. These differences between the two studies and ours have to be considered when the results are interpreted.
Compared with new-onset LN, relapsed LN was identified as a poor predictive factor for short-term renal response in this study. As the number of relapses increased, the rate of CR decreased to 62.5%, 50%, and 0.0%. However, the sample size was too small for the differences to be significant. Differences were also observed in the histopathology between patients with new-onset LN and relapsed LN. A higher chronicity index in patients with relapsed LN was marginally significant (p=0.059), as shown in Supplementary Table 2. In particular, there was a significant difference in glomerulosclerosis (p=0.013). Marinaki et al. [22] illustrated that the CI was also found to increase in conjunction with the number of kidney biopsies, demonstrating that despite the use of appropriate immunosuppressants, time-accumulated, chronic, and irreversible damage can still occur. Thus, chronic and irreversible histologic changes in relapsed LN patients appear to be associated with a poor renal response after induction treatment.
The positivity of the anti-Ro antibody was a poor predictive factor within our cohort. Anti-Ro antibody, alongside anti-La antibody, are the main serological markers of Sjogren’s disease, which is known to be associated with SLE. Several associations between SLE and anti-Ro antibodies have been reported, but the association with LN is controversial. Korbet et al. [23] found predictable factors for the long-term prognosis of LN in a total of 86 LN patients (54 White, 21 Black, and 11 from other races). In this study the presence of the anti-Ro antibody (relative risk=3.0, 95% CI=1.4~6.4, p<0.01) was associated with a greater potential for progressing to ESRD in multivariate analysis. However, Moon et al. [24] revealed that being seronegative for anti-Ro antibody (hazard ratio (HR)=3.51, 95% CI=1.40~8.81, p=0.007) was a predictor of relapse in 108 LN patients [24]. In another study by Sule et al. [25], the presence of antibodies against anti-Ro was a protective factor against renal disease (HR=0.2, 95% CI=0.05~0.5) in LN patients aged ≤19 years. Further studies on the role of anti-Ro antibody on LN are necessary in order to make robust conclusions.
There may be concerns that a lower level of proteinuria in class III compared with other classes could prompt favorable responses in LN. In the mixed or membranous pattern, the baseline proteinuria level was higher than the pure proliferative pattern, while no differences were noted between classes III and IV. Therefore, baseline proteinuria levels do not seem to influence the high CR rate in class III LN.
This study has several strengths and limitations. One strength of this study is a prospective cohort study, which can minimize bias throughout the study period. Additionally, the treatment for LN was performed in a single center, which provide relative consistency throughout the study period and reduce the risk of confounding factors that can occur if multiple clinical settings are used. Conversely, the total number of patients was small, particularly for the multivariate analysis. There were 39 CR patients but only 19 NR patients, which might result in low statistical power. Nevertheless, we did find statistically significant predictive factors for the renal response. Another limitation is that class V showed different characteristics in clinical course and treatment from proliferative nephritis, meaning that the tool used for evaluating the renal response may not be suitable. Investigating these two groups separately would be a way to reduce bias in future studies.
CONCLUSION
In this prospective study, class III LN was identified as a good predictor for CR at 6 months after induction treatment compared with proliferative, mixed, and pure membranous LN classes. In contrast, a younger age, relapsed LN and anti-Ro antibody were poor predictors. In LN patients with these kinds of poor predictors, more promising and precise therapeutic strategies that produce better renal response will need to be found.
SUPPLEMENTARY DATA
Supplementary data can be found with this article online at https://doi.org/10.4078/jrd.22.0006.
ACKNOWLEDGMENTS
None.
Funding Statement
FUNDING Dr. Sang-Cheol Bae (corresponding author) is supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2021R1A6A1A03038899).
Footnotes
CONFLICT OF INTEREST
Young Bin Joo has been an Editorial Board member of the Journal of Rheumatic Diseases since 2022. But she did not involve in the peer review process. Except for that, no potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hanly JG, O'Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016;55:252–62. doi: 10.1093/rheumatology/kev311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian HM, Roseman JM, McGwin G, Jr, Alarcón GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 3.Kalloo S, Aggarwal N, Mohan P, Radhakrishnan J. Lupus nephritis: treatment of resistant disease. Clin J Am Soc Nephrol. 2013;8:154–61. doi: 10.2215/CJN.05870612. [DOI] [PubMed] [Google Scholar]
- 4.Ichinose K, Kitamura M, Sato S, Eguchi M, Okamoto M, Endo Y, et al. Complete renal response at 12 months after induction therapy is associated with renal relapse-free rate in lupus nephritis: a single-center, retrospective cohort study. Lupus. 2019;28:501–9. doi: 10.1177/0961203319829827. [DOI] [PubMed] [Google Scholar]
- 5.Vajgel G, Oliveira CBL, Costa DMN, Cavalcante MAGM, Valente LM, Sesso R, et al. Initial renal histology and early response predict outcomes of Brazilian lupus nephritis patients. Lupus. 2020;29:83–91. doi: 10.1177/0961203319890681. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JE, Fu Q, Ji B, Rao S, Roth D, Magder LS, et al. Renal remission status and longterm renal survival in patients with lupus nephritis: a retrospective cohort analysis. J Rheumatol. 2018;45:671–7. doi: 10.3899/jrheum.161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luís MSF, Bultink IEM, da Silva JAP, Voskuyl AE, Inês LS. Early predictors of renal outcome in patients with proliferative lupus nephritis: a 36-month cohort study. Rheumatology (Oxford) 2021;60:5134–41. doi: 10.1093/rheumatology/keab126. [DOI] [PubMed] [Google Scholar]
- 8.Park DJ, Choi SE, Xu H, Kang JH, Lee KE, Lee JS, et al. Chronicity index, especially glomerular sclerosis, is the most powerful predictor of renal response following immunosuppressive treatment in patients with lupus nephritis. Int J Rheum Dis. 2018;21:458–67. doi: 10.1111/1756-185X.13254. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto M, Kitamura M, Sato S, Fujikawa K, Horai Y, Matsuoka N, et al. Life prognosis and renal relapse after induction therapy in Japanese patients with proliferative and pure membranous lupus nephritis. Rheumatology (Oxford) 2021;60:2333–41. doi: 10.1093/rheumatology/keaa599. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Wang H, Le J, Lan L, Xu Y, Yang Y, et al. Early-stage predictors for treatment responses in patients with active lupus nephritis. Lupus. 2019;28:283–9. doi: 10.1177/0961203319826703. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 14.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–96. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–23. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 16.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria, author. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421–32. doi: 10.1002/art.21625. [DOI] [PubMed] [Google Scholar]
- 18.Cattran DC, Feehally J, Cook HT, Liu ZH, Fervenza FC, Mezzano SA, et al. Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 19.Choi SE, Park DJ, Kang JH, Lee KE, Xu H, Lee JS, et al. Comparison of renal responses to cyclophosphamide and mycophenolate mofetil used as induction therapies in Korean patients with lupus nephritis. J Rheum Dis. 2019;26:57–65. doi: 10.4078/jrd.2019.26.1.57. [DOI] [Google Scholar]
- 20.Korbet SM, Lewis EJ. Complete remission in severe lupus nephritis: assessing the rate of loss in proteinuria. Nephrol Dial Transplant. 2012;27:2813–9. doi: 10.1093/ndt/gfr741. [DOI] [PubMed] [Google Scholar]
- 21.Mok CC, To CH, Yu KL, Ho LY. Combined low-dose mycophenolate mofetil and tacrolimus for lupus nephritis with suboptimal response to standard therapy: a 12-month prospective study. Lupus. 2013;22:1135–41. doi: 10.1177/0961203313502864. [DOI] [PubMed] [Google Scholar]
- 22.Marinaki S, Kapsia E, Liapis G, Gakiopoulou H, Skalioti C, Kolovou K, et al. Clinical impact of repeat renal biopsies in patients with lupus nephritis: renal biopsy is essential especially later in the course of the disease. Eur J Rheumatol. 2020;7:2–8. doi: 10.5152/eurjrheum.2019.18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, Rohde RD. Factors predictive of outcome in severe lupus nephritis. Lupus Nephritis Collaborative Study Group. Am J Kidney Dis. 2000;35:904–14. doi: 10.1016/S0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 24.Moon SJ, Park HS, Kwok SK, Ju JH, Choi BS, Park KS, et al. Predictors of renal relapse in Korean patients with lupus nephritis who achieved remission six months following induction therapy. Lupus. 2013;22:527–37. doi: 10.1177/0961203313476357. [DOI] [PubMed] [Google Scholar]
- 25.Sule SD, Moodalbail DG, Burnham J, Fivush B, Furth SL. Predictors of kidney disease in a cohort of pediatric patients with lupus. Lupus. 2015;24:862–8. doi: 10.1177/0961203315570162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.