Abstract
Background:
Charcoal-based preparations have recently gained popularity, particularly in oral hygiene products such as tooth whitening dentifrices, owing to their abrasive and adsorptive properties. The present in vitro study evaluates the efficacy of a charcoal-based tooth whitening dentifrice compared with a non-charcoal-based whitening dentifrice in removing coffee stains on tooth surfaces.
Methods:
Thirty-three human extracted tooth specimens were randomly assigned to 1 of 3 study groups: Group 1 (charcoal-based whitening dentifrice [CBWD]), Group 2 (non-charcoal-based whitening dentifrice [NCBWD]), and Group 3 (distilled water [DW]). All tooth specimens were immersed in a prepared coffee extract for 4 weeks to facilitate staining and then mounted on blocks where they were brushed with an electric toothbrush daily for 8 seconds with 1 of the 2 allocated dentifrices or with DW for 4 weeks following staining. Spectrophotometric analysis was conducted using the CIELAB system to measure the L*, a*, and b* values at 3 time points: before staining, after staining, and following the brushing protocol. These values were used to calculate the colour change (ΔE) between time points.
Results:
Following the coffee staining, the tooth samples’ whiteness (ΔL) decreased with the overall colour change (ΔE). Next, there was a significant improvement in the degree of tooth whiteness (ΔL) values following the brushing protocol in all 3 groups (p = 0.003), with the greatest improvement occurring in the CBWD group. However, the overall colour change (ΔE) was not significantly different between the groups.
Conclusion:
CBWD, NCBWD, and DW were effective in removing coffee stains from the tooth surface. However, the amount of colour change (ΔE) produced by CBWD was not significantly different from NCBWD or DW.
Keywords: charcoal dentifrice, coffee, teeth stain, toothbrushing, whitening
Abstract
Introduction :
Les préparations à base de charbon ont récemment gagné en popularité, en particulier dans les produits d’hygiène buccale comme les dentifrices blanchissants, en raison de leurs propriétés d’abrasion et d’adsorption. La présente étude in vitro évalue l’efficacité d’un dentifrice blanchissant à base de charbon par rapport à un dentifrice blanchissant sans charbon pour éliminer les taches de café sur la surface des dents.
Méthodes :
Trente-trois spécimens de dents humaines extraites ont été répartis aléatoirement dans 3 groupes d’étude : groupe 1 (dentifrice blanchissant à base de charbon [DBBC]), groupe 2 (dentifrice blanchissant sans charbon [DSC]) et groupe 3 (eau distillée [ED]). Tous les spécimens de dents ont été immergés dans une préparation de café pendant 4 semaines pour permettre la coloration, puis montés sur des blocs où ils ont été brossés quotidiennement à la brosse à dents électrique pendant 8 secondes avec l’un des deux dentifrices testés ou avec de l’eau distillée pour une période de 4 semaines après la coloration. Une analyse spectrophotométrique a été effectuée à l’aide du système CIELAB pour mesurer les valeurs L*, a* et b* à 3 moments précis : avant la coloration, après la coloration et après le protocole de brossage. Ces valeurs ont été utilisées pour calculer le changement de couleur (ΔE) entre les moments précis.
Résultats :
Après la coloration du café, la blancheur des échantillons de dents (ΔL) a diminué en raison du changement global de couleur (ΔE). Ensuite, il y a eu une amélioration significative du degré de blancheur des dents (ΔL) suivant le protocole de brossage dans les 3 groupes (p = 0,003), la plus grande amélioration ayant eu lieu dans le groupe DBBC. Toutefois, le changement global de couleur (ΔE) n’était pas significativement différent d’un groupe à l’autre.
Conclusion :
Les DBBC, DSC et l’ED se sont montrés efficaces pour éliminer les taches de café sur la surface des dents. Toutefois, le changement de couleur (ΔE) produit par le DBBC n’était pas significativement différent de celui produit par le DSC ou l’ED.
PRACTICAL IMPLICATIONS OF THIS RESEARCH.
The coffee stain removal efficacy of a charcoal-based whitening dentifrice was not significantly different from a non-charcoal-based whitening dentifrice or distilled water.
Dental hygienists should exercise caution in recommending charcoal-based whitening dentifrices owing to their abrasive potential and often lack of fluoride, which is essential for tooth protection.
INTRODUCTION
Tooth discolouration, in the form of extrinsic stain mainly caused by the dietary intake of tea, coffee or red wine, is a frequent complaint encountered by oral health professionals in their practices.1 Extrinsic tooth staining is associated with the deposition of chemical compounds on the acquired pellicle on tooth surfaces.2 Studies have shown that consumers and patients alike are usually displeased with their current tooth colour, with personal dissatisfaction ranging from 17.9% to 52.6%.3
Tooth colour mainly depends on the enamel’s colour, thickness, and translucency.4 Enamel generally appears white with varying levels of translucency, owing to the mineral composition of hydroxyapatite.5 Continuous enamel wear due to erosion and abrasion reduces its thickness, thus exposing the darker, yellowish underlying dentin.5, 6 Various chromophores incorporated into the dental hard tissues alter the tooth colour.7 These compounds absorb visible light and reflect complementary colours indistinguishable by the human eye, typically yellow or brown in the case of teeth.5
Coffee consumption is one of the main causes of staining and significant alterations in the tooth structure.8, 9 The outermost layer of enamel is highly vulnerable to staining with eventual demineralization leaving the enamel prisms and dentinal tubules open.9 Besides changing the opalescent colour of teeth, the low pH of coffee reduces the concentration of calcium and phosphorous through enamel sequestration.9 Moreover, consuming acidic drinks further increases susceptibility to coffee staining.10
The management of tooth discolouration varies from professional scaling, polishing with an abrasive paste, bleaching or fabrication of veneers or crowns.1 These measures are performed by an oral health professional and are labour-intensive and costly.1 A wide range of products addressing the issue of tooth discolouration are currently commercially available to satisfy the expectations of patients and consumers.11 These products include dentifrices for use with manual or electric toothbrushes.5
Contemporary dentifrices contain highly complex formulations, incorporating varying forms of fluorides (such as sodium and stannous), calcium phosphates (such as hydroxyapatite), zinc salts, surfactants, and abrasives for effective plaque control.5 Most whitening dentifrices contain solid cleansing abrasive materials, solubilizing humectants, hydrogen peroxide, thickening agents, foam generating surfactants, and fluorides.11 They may also contain sweeteners, flavouring, buffering and opacifying agents, and preservatives.11 Among these ingredients, the abrasives help to remove extrinsic stains.12, 13 These particles are interposed between the toothbrush bristle and the tooth surface, and are physically harder, which facilitates stain removal.11 Abrasives commonly incorporated in dentifrices include hydrated silica, sodium bicarbonate, calcium carbonate, calcium pyrophosphate, dicalcium phosphate dihydrate, alumina, and perlite.14
Whitening dentifrices have demonstrated significant reductions of extrinsic stains on natural teeth.1 These include charcoal-based dentifrices (CBWD) that have emerged as popular oral hygiene products for toothbrushing, extrinsic stain removal, and tooth whitening.15 Charcoal is available in various forms such as activated, organic, black, premium, raw, white, coconut, bamboo, medical-grade, harmful ionic charged, active pine tree, virgin carbon hardwood-derived or pure hardwood.15 Charcoal-based oral care products include toothpowders, dentifrices, chewing sticks, tooth tabs, capsules, dental creams, and tooth polish wherein charcoal is the main ingredient in combination with other compounds.15, 16 They have been widely,advertised in countries such as the United Kingdom, India, Nigeria, Italy, Cameroon, Malaysia, and Bangladesh.16
The whitening mechanism of CBWD is mainly related to their adsorption capacity and porosity.17 The nanocrystalline form of carbon in activated charcoal produces a large surface area and porosity which adsorbs pigments, chromophores or staining responsible for the colour alteration of natural teeth.4 The adsorption property combined with the abrasive action of these particles removes the extrinsic stains of coffee and tea from the tooth surface.4 However, there is a scarcity of scientific evidence supporting the adsorbent action of charcoal, and its stain reduction mechanism is mainly attributed to its abrasive action.4, 16
Research on numerous commercially available CBWD has produced conflicting results regarding their whitening properties.4, 17, 18 For example, Dionysopoulos and colleagues4 conducted a study to evaluate the influence of a novel CBWD (1% charcoal) used alone or in combination with a whitening mouthwash (1% active charcoal and 0.5% hydrogen peroxide) on tooth colour and enamel surface after toothbrushing for 90 days. It was found that the whitening dentifrice significantly increased the tooth colour change and produced a smoother surface after toothbrushing, but the surface was more heterogeneous with large craters. The whitening mouthwash did not cause any surface morphological changes. It was suggested that CBWD enhanced the whiteness of the teeth but should be used with caution owing to their ability to cause enamel surface changes. The charcoal-containing mouthwash did not produce any additional whitening effect.4 Ghajari and colleagues17 agreed with these findings. They observed that charcoal dentifrices had a whitening and abrasive effect on permanent teeth.17 Conversely, another in vitro study compared human enamel’s colour, surface roughness, and microhardness after applying different CBWD for 12 weeks. The researchers observed similar effects on enamel colour after using CBWD and regular fluoridated dentifrice. Moreover, the surface roughness was increased while microhardness was not affected with CBWD.18
The properties of charcoal that promote extrinsic stain removal led to the hypothesis that CBWD have superior extrinsic tooth stain removal properties compared to NCBWD. This study was conceived to enable oral health practitioners, specifically dental hygienists, to decide whether whitening dentifrices with additives such as charcoal particles would be superior or at par with the other commercially available NCBWD. With this background, this in vitro study aimed to evaluate the efficacy of one commercially available CBWD on coffee stains on tooth surfaces compared with a NCBWD.
METHODS
The present in vitro study was conducted according to the CRIS guidelines following approval from the Institutional Ethics Committee (IEC NO. – 08/2021).19
Sample size calculation
The sample size for the study was calculated using the following formula: N = (r+1) (Zœ/2 + Z1-ß)2 (σ)2 / rd2, at 0.05 level of significance.20 It resulted in a sample size of 11 per group. As this study consisted of 3 groups, a sample size of 33 specimens was deemed appropriate to carry out the study.
Inclusion and exclusion criteria
Anonymous, previously extracted permanent maxillary human incisors were used for the study. Teeth with caries, restorations, developmental defects, enamel discolorations, cracks, fractures or calcifications were excluded.
Randomization and sample preparation
The 33 tooth specimens were equally divided and mounted onto acrylic blocks. Each was allocated an individual code using the block randomization method. The first 5 blocks each contained 6 coded teeth and the sixth block contained the remaining 3 coded sample teeth. Accordingly, on each of the first 5 blocks, there were 2 samples coded to receive the CBWD; 2 to receive the NCBWD; and 2 to receive the DW. The sixth block, which contained only 3 teeth, had been randomly coded to receive 1 of the 3 treatments. As the CBWD had a distinctive black colour, allocation concealment was not achieved.
Thirty-three teeth were randomly allocated into 1 of the 3 groups: Group 1 (Colgate Charcoal Clean dentifrice [charcoal whitening dentifrice/CBWD]); Group 2 (Colgate Visible White dentifrice [non-charcoal whitening dentifrice/NCBWD]); Group 3 (distilled water/DW). The composition of the dentifrices employed is listed in Table 1. An ultrasonic scaler removed the debris, calculus, and soft tissue remnants from the tooth surfaces. The samples were disinfected with a 5.25% sodium hypochlorite solution for 30 minutes.21 They were polished with a prophylaxis paste to eliminate any previously developed extrinsic stains on the tooth surfaces.22 The samples were then stored in an allocated compartment within the group to avoid any specimen shuffling and ensure that the same sample area was recorded during spectrophotometric analysis. All specimens were stored in artificial saliva.
Table 1.
Composition of the charcoal and non-charcoal-based dentifrices
|
Dentifrice |
Composition |
|
Colgate Charcoal Clean dentifrice |
sorbitol, water, silica, sodium lauryl sulphate, flavour, cocoamidopropyl betaine, polyethylene glycol 600, sodium carboxymethyl cellulose, sodium saccharin, sodium fluoride, charcoal, benzyl alcohol, eugenol |
|
Colgate Visible White |
silica, sorbitol, glycerin, polyethylene, glycol, sodium tripolyphosphate, tetrapotassium pyrophosphate, sodium lauryl sulphate, flavour, cocamidopropyl betaine, sodium carboxymethyl cellulose, sodium fluoride, xantham, sodium hydroxide, sorbosil bgf51 blue, titanium dioxide in aqueous base |
Coffee extract preparation and immersion
The coffee extract solution was prepared by dissolving 5.5 grams of coffee powder (Bru Instant Coffee) in 80 mL of boiling water at 100˚C.23 The water temperature was standardized with the help of a portable digital probe thermometer. The coffee extract was cooled to 85˚C before sample immersion to mimic the typical temperature of a coffee serving.24
As the average daily consumption of coffee lasts 15 minutes, approximately, the samples were immersed in the coffee extract for 15 minutes per day and then stored in artificial saliva until the next day.25 This procedure was repeated daily for 4 weeks. The coffee extract was replaced with a new solution every day before immersion.
Brushing protocol
A brushing time of 120 seconds twice a day was applied; the most extended contact period for a single tooth was 8 seconds per day.26 Dentifrice slurries were prepared by mixing artificial saliva with the allocated dentifrice in the ratio of 3:1.27 The slurry was then applied to the surface of the samples and brushed for 8 seconds per day, 4 seconds each on the buccal and palatal surfaces, and then stored in artificial saliva until the next day. A pressure-sensitive electric toothbrush (Oral B® PRO 2 2000 cross action electric rechargeable toothbrush) was used to standardize the brushing force. It had a round brush head with bristles angled at 16 degrees, which operated in oscillatory, rotatory, and pulsatory movements. The pressure control technology alerted the researcher if increased force was applied. The toothbrush head was held parallel to the sample surface, and 3 separate heads were used for each group. Brushing strokes were applied as suggested by the manufacturer and were standardized for all specimens. The samples were stored in artificial saliva until the next day. The procedure was repeated daily for 4 weeks, and the same researcher performed all the procedures.
Spectrophotometric analysis
Under illumination, the colour measurements were conducted with a digital spectrophotometer (X-rite i1 pro device) over a white background (Figure 1). The CIELAB-CIE1976 (L*a*b*) system (Commission Internationale de l’Eclairage‒CIE) was utilized for these measurements. The spectrophotometer was calibrated before each session using a white reference supplied by the manufacturer. Before any colour measurement, the samples were dried with blotting paper. A white polystyrene sheet, 3.93 inches thick, was used to hold the teeth in place while being subjected to the spectrophotometric analysis. Each tooth sample had a specific segment on the polystyrene sheet to ensure that the same sample area was recorded at all 3 time points. Markings were made on this sheet corresponding to each tooth sample and the spectrophotometer, such that the rays emitted from the sensor would enter the same segment on the buccal surface of each tooth sample at all time points (Figure 2). This procedure was repeated, thrice for each reading, and the average was used for data analysis.
Figure 1.
Digital spectrophotometer
Figure 2.
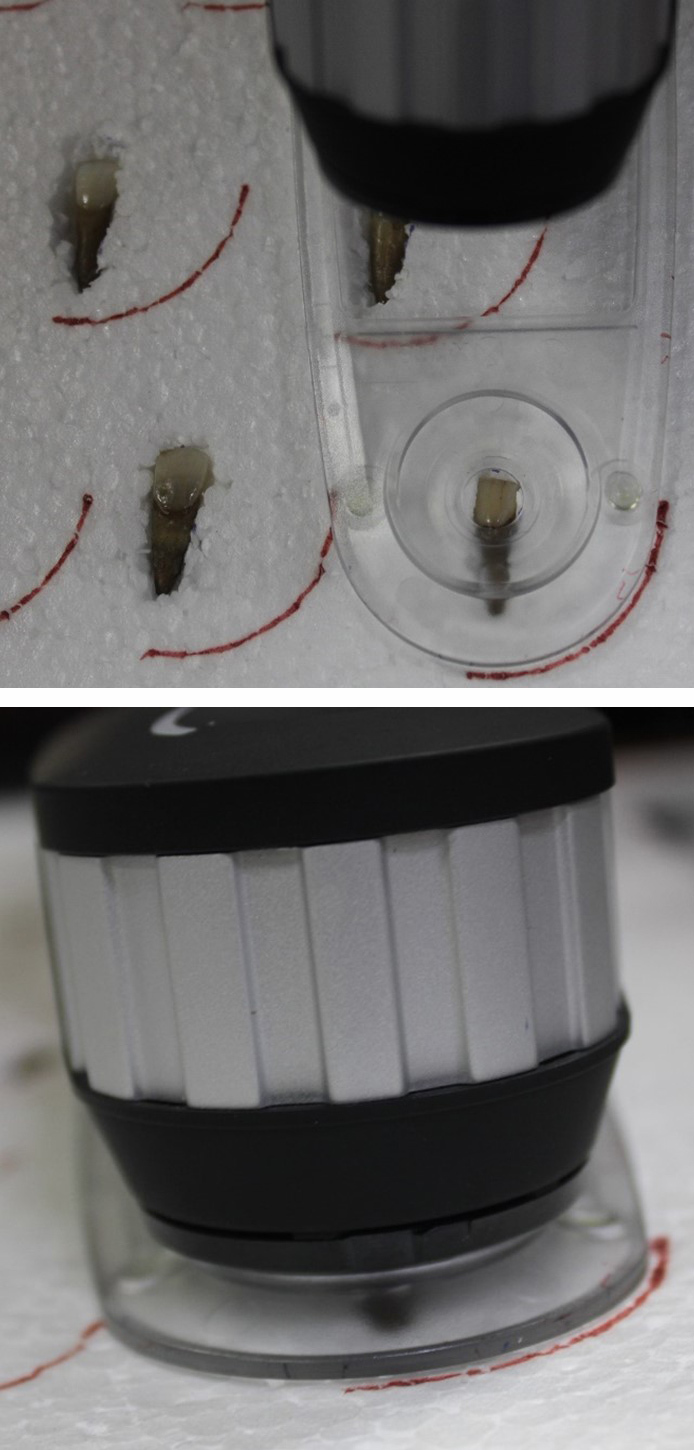
Digital spectrophotometer positioning and analysis
Data collection
The L*, a*, and b* values of specimens were measured prior to coffee staining (T0), after 4 weeks of coffee staining (T1), and after 4 weeks of brushing (T2). The L* value illustrates the degree of whiteness and varies from black to white (0 to 100). The a* value depicts the degree of redness (+a) or greenness (‒a). The b* value represents the degree of yellowness (+b) or blueness (‒b).21 ΔL, Δa, and Δb values were calculated from baseline to coffee staining (pre-test) and from coffee staining to the brushing protocol (post-test). The ΔLpre and ΔLpost were calculated as (L1 – L0) and (L2 – L1), respectively. Likewise, the values for pre and post, Δa and Δb, were calculated. The colour changes from baseline to coffee staining (ΔEpre) and from coffee staining to the brushing protocol (ΔEpost) were calculated by the following formulae:
ΔEpre = {(ΔLpre*)2 + (Δapre*)2 + (Δbpre*)2}1/2
ΔEpost = {(ΔLpost*)2 + (Δapost*)2 + (Δbpost*)2}1/2
Statistical analysis
Data were analysed using the statistical package SPSS 22.0 (SPSS Inc., Chicago, IL), and the significance level was set at p < 0.05. Descriptive statistics were performed to assess the mean and standard deviation of the respective groups. The normality of the data was assessed using the Shapiro–Wilk test. The inferential statistics to determine the differences between the pre- and post-test values of the parameters were made with the Wilcoxon signed-rank test. The intergroup comparisons were made using the Kruskal–Wallis test followed by the Dunn’s test post hoc analysis to determine any differences between the pair of groups.
RESULTS
The mean ΔLpre-test (whiteness) values (before the brushing protocol) were negative in all groups, while the ΔLpost-test (whiteness) values (after the brushing protocol) were positive (Tables 2 and 3). Intragroup comparisons showed a statistically significant difference in the mean ΔLpre and ΔLpost values in all groups (p < 0.05) (Table 2; Figure 3). The mean pre-test Δa (redness) values were positive after coffee staining (Tables 2 and 3). After the brushing protocol, the mean Δapost (redness) values were negative in Group 1 and Group 3 while positive in Group 2. Intragroup comparisons showed a significant difference between the mean Δapre and Δapost values for Group 1 and Group 3 only (p < 0.05) (Table 2; Figure 4). The mean Δbpre (yellowness) values were negative following the coffee staining in all groups. After the brushing protocol, the mean Δbpost (yellowness) values were negative in Group 1 and Group 3, while those in Group 2 showed a positive value. Intragroup comparisons showed a statistically significant difference between the mean Δbpre and Δbpost values only for Group 1 and Group 2 (p < 0.05) (Table 2; Figure 5). Accordingly, there was a significant difference between the mean ΔEpre and ΔEpost values across all 3 groups (p < 0.05) (Table 2; Figure 6) as ΔE is calculated from ΔL, Δa, and Δb values.
Table 2.
Intragroup comparison of ΔL, Δa, Δb, and ΔE (mean ± SD) between pre-test (from baseline to coffee staining) and post-test (from coffee staining to the brushing protocol) using Wilcoxon signed rank test
|
ΔL |
Δa |
Δb |
ΔE |
|||||||||
|
ΔLpre |
ΔLpost |
p value |
Δapre |
Δapost |
p value |
Δbpre |
Δbpost |
p value |
ΔEpre |
ΔEpost |
p value |
|
|
Group 1 |
–15.44 ± 5.11 |
0.58 ± 2.20 |
0.003a |
1.12 ± 0.73 |
–0.22 ± 0.78 |
0.009a |
–3.00 ± 2.26 |
–0.7 ± 1.59 |
0.02a |
15.93 ± 5.17 |
2.38 ± 1.35 |
0.003a |
|
Group 2 |
–15.01 ± 5.16 |
0.38 ± 2.68 |
0.003a |
0.71 ± 1.17 |
0.54 ± 0.97 |
0.65 |
–2.27 ± 3.47 |
1.32 ± 3.29 |
0.02a |
15.53 ± 5.29 |
3.63 ± 2.64 |
0.003a |
|
Group 3 |
–13.61 ± 5.04 |
0.23 ± 1.79 |
0.003a |
0.7 ± 0.55 |
–0.21 ± 0.93 |
0.018a |
–0.98 ± 2.24 |
–1.34 ± 2.35 |
0.92 |
13.89 ± 4.88 |
2.81 ± 1.75 |
0.003a |
aDenotes statistical significance
Table 3.
Intergroup comparison of ΔL, Δa, Δb and ΔE (mean ± SD) between pre-test (from baseline to coffee staining) and post-test (from coffee staining to the brushing protocol) using Kruskal–Wallis test.
|
Group 1 |
Group 2 |
Group 3 |
p value |
||
|
ΔL |
ΔLpre |
–15.44 ± 5.11 |
–15.01 ± 5.16 |
–13.61 ± 5.04 |
0.88 |
|
ΔLpost |
0.58 ± 2.02 |
0.38 ± 2.68 |
0.23 ± 1.79 |
0.86 |
|
|
Δa |
Δapre |
1.12 ± 0.73 |
0.71 ± 1.17 |
0.7 ± 0.55 |
0.27 |
|
Δapost |
–0.22 ± 0.78 |
0.54 ± 0.97 |
–0.21 ± 0.93 |
0.15 |
|
|
Δb |
Δbpre |
–3.00 ± 2.26 |
–2.27 ± 3.47 |
–0.98 ± 2.24 |
0.08 |
|
Δbpost |
–0.7 ± 1.59 |
1.32 ± 3.29 |
–1.34 ± 2.35 |
0.06 |
|
|
ΔE |
ΔEpre |
15.93 ± 5.17 |
15.53 ± 5.29 |
13.89 ± 4.88 |
0.74 |
|
ΔEpost |
2.38 ± 1.35 |
3.63 ± 2.64 |
2.81 ± 1.75 |
0.43 |
|
However, intergroup comparisons showed no significant differences between the groups (p > 0.05) (Table 3). Among the 3 groups, the highest increase in whiteness (ΔLpost) was observed in Group 1, followed by Group 2 and Group 3, although these differences were not statistically significant (p > 0.05). Group 1 and Group 3 showed a negative Δapost value, while Group 2 showed a positive Δapost value. Group 2 showed the highest mean value of ΔEpost (Colour change), although it was was not significantly different between the groups (Table 3).
DISCUSSION
The present study showed that brushing with a CBWD removed coffee stains similarly to brushing with either a NCBWD or DW. Similar results were observed in a recent in vitro study where the colour change produced by a charcoal and non-charcoal-based whitening dentifrice was not significantly different.17 It has been suggested that the whitening properties are mainly due to the size and shape of abrasive particles such as silica, which help remove surface stains. In the current study, hydrated silica was a common ingredient in whitening dentifrices. In addition, charcoal was present in the CBWD, which may help to remove the coffee stains.
Usually, a dentifrice with a high relative dentin abrasiveness (RDA) causes more abrasion.18 In the current study, the abrasive potential of dentifrices was not evaluated. However, a previous study has shown that charcoal dentifrices are more abrasive (RDA >76) than the NCBWD (RDA <70).17 The amount of wear produced by them increases with the size of charcoal particles.28 Moreover, in another study by Koc Vural and colleagues,18 brushing with either a charcoal-based or a fluoridated dentifrice for 12 weeks had similar effects on the enamel colour. The charcoal-based dentifrices increased the surface roughness with no change in the microhardness. They did not whiten the teeth and caused enamel abrasion.18 As Ghajari and colleagues reported,17 both charcoal-based and non-charcoal-based whitening dentifrices change the tooth’s primary and secondary surface profile due to their abrasive properties.
Figure 3.
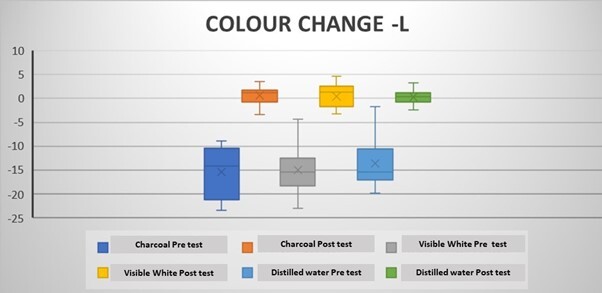
Change in L values in the groups following the brushing protocol
Figure 4.
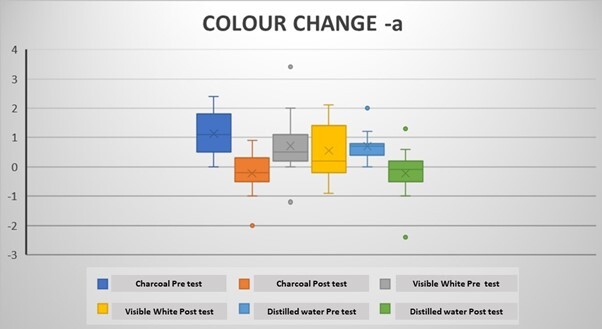
Change in a value in the groups following the brushing protocol
Figure 5.
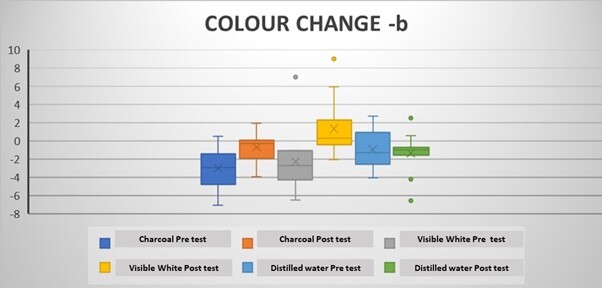
Change in b value in the groups following the brushing protocol
Figure 6.
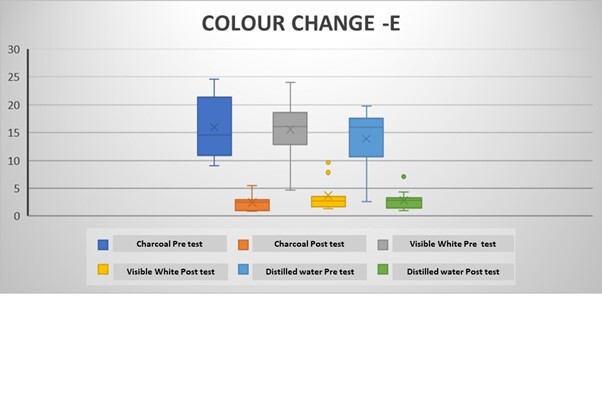
Change in tooth colour (E) value in the groups following the brushing protocol
A recent study by Rostamzadeh et al.29 also compared the effects of whitening dentifrices containing activated charcoal, abrasive particles or hydrogen peroxide on the colour of aged microhybrid composite. In this study, even though the maximum colour change was observed with a charcoal dentifrice, it was not significantly different from other whitening agents, including hydrogen peroxide.29 It was proposed that hydrogen peroxide in dentifrices decreased the yellowness and increased the brightness of the teeth by its chemical action.29 The authors suggested that the silica particles enhanced the abrasive action of the nanocrystalline-activated charcoal. Additionally, they concluded that the high surface area of the charcoal particles supported the adsorption of the chromophores in the oral cavity producing a superior cleaning action on the dentition.29 The authors also suggested that the,sodium lauryl sulphate and cocoamidopropyl betaine increased the availability of the hydrophobic agents and promoted the distribution of the dentifrice particles in the oral cavity, which improved its action. Furthermore, the hydrated silica, titanium dioxide, and sodium hydroxide in non-charcoal-based dentifrice led to a higher pH and lower abrasiveness, producing the lowest colour change. In contrast, the dentifrice with hydrogen peroxide produced an intermediate colour change due to its synergistic action with silica.29 In the current study, the silica particles, sodium lauryl sulfate, and cocoamidopropyl betaine in the applied dentifrices could be responsible for the colour change observed following the brushing protocol.
Wetter and colleagues30 have suggested that the L* value is a primary parameter for assessing the degree of whiteness. A higher L* value following the application of CBWD represents increased tooth whitening.30 Similarly, Palandi and colleagues31 suggested that a negative post-Δa and Δb after using charcoal dentifrices implies reduced redness and yellowness of the teeth. In the present study, a positive post-ΔL and reduced post-Δa and Δb values were observed, indicative of increased whitening and reduced redness and yellowness in all the samples. Although a significant colour change (ΔEpost) was observed in all the samples, the difference was insignificant between the groups. The CBWD had a lower mean ΔEpost than the NCBWD. As the overall colour change (ΔE) is dependent on L*, a*, and b*, higher a* and b* values in the non-charcoal group could be responsible for this effect.
It has been suggested that the human eye cannot detect ∆E values less than 1.5.25 A person trained in colour recognition may detect a ∆E value of 1.5 to 2.5. Furthermore, as the ∆E values were not significantly different between the groups, changes produced by different dentifrices would appear similar and equally satisfy the patients.17
Numerous studies have produced contradictory results about the tooth whitening effects of charcoal dentifrices.4, 17, 18, 31, 32 For instance, some in vitro studies have reported increased whitening of teeth stained with black tea or coffee after using charcoal dentifrices,4, 17, 32 while others have not reported these outcomes.31 The variations in whitening effect between studies, besides using different staining protocols, may be related to a different type of charcoal dentifrice used in the experiments.
In the present study, the coffee extract contained tannins at about 9.65%, responsible for staining.33 Usually, physiological mechanisms of ingestion of fluids prevent their retention in the oral cavity.34 However, in vitro models involve static immersion of specimens in the concoction of these beverages for prolonged periods (hours to days) without disturbing the medium.34 Beverages such as coffee are colloidal suspensions that precipitate sediments with a prolonged immersion period when left stagnant.34 Subsequent staining outcomes may not resemble clinical realities.34 In the present study, immersion of the specimens in the coffee extract stained them. It produced negative ΔL values, positive Δa, and negative Δb pre-test values, indicating that all the teeth specimens displayed reduced whiteness, yellowness, and increased redness, respectively. Similar results were reported in earlier in vitro studies following daily coffee or tea intake.34, 35 Ren and colleagues34 suggested that immersion in coffee for prolonged periods may lead to a high ΔE value (>15) and deposition of dark sediments leading to increased negative ΔL values, which was also observed in the present study. Overall, the coffee staining significantly changed the colour and decreased the whiteness of all specimens, which was not significantly different between the groups showing that all specimens were stained uniformly and were comparable.
Apart from the dentifrices, an electric toothbrush was used in the present study as it has been shown to have a better stain removing and sustained tooth whitening ability than manual toothbrushes.36-38 Moreover, the pressure sensor standardized the brushing forces applied to the tooth surfaces.
Although the results from the present study suggest that CBWD produce a colour change, it was not significantly different from the colour change that occurred in the NCBWD or the DW control.
Limitations
Limitations of this study include the lack of evaluation of the potential abrasive effects of CBWD as well as the alterations in surface morphology, such as increased surface roughness, that some researchers have suggested.17, 31 Another major limitation of this study is the lack of blinding and the fact that all measurements were made by a single investigator which may have affected the interpretation of the results. Although the CIELAB system of colour measurement has been traditionally used in the majority of colour studies, the CIEDE 2000 system is a more current and more accurate measure of tooth colour and could have produced different results.39 Finally, rather than using DW as a negative control, it would have been better to use a non-whitening dentifrice which may have better explained the role of charcoal in whitening dentifrices. These limitations should be considered before recommending a charcoal-based dentifrice for tooth whitening.
CONCLUSION
The charcoal-based whitening dentifrice used in the present study was effective in reducing extrinsic stains caused by coffee consumption. However, the colour change was not significantly different from that produced by the non-charcoal-based whitening dentifrice. Future, more rigorous in vivo clinical trials are required to clarify the safety and efficacy of charcoal-based dentifrices before they are recommended as tooth whitening dentifrices.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest with respect to this article.
Footnotes
CDHA Research Agenda category: risk assessment management
References
- Walsh TF , Rawlinson A , Wildgoose D , Marlow I , Haywood J , Ward JM Clinical evaluation of the stain removing ability of a whitening dentifrice and stain controlling system J Dent 2005 ; 33 ( 5 ): 413 – 418 [DOI] [PubMed] [Google Scholar]
- Joiner A , Luo W Tooth colour and whiteness: a review J Dent 2017 ; 67 ( Suppl 1 ): S3 – S10 [DOI] [PubMed] [Google Scholar]
- Xiao J , Zhou XD , Zhu WC , Zhang B , Li JY , Xu X The prevalence of tooth discolouration and the self-satisfaction with tooth colour in a Chinese urban population J Oral Rehabil 2007 ; 34 ( 5 ): 351 – 360 [DOI] [PubMed] [Google Scholar]
- Dionysopoulos D , Papageorgiou S , Malletzidou L , Gerasimidou O , Tolidis K Effect of novel charcoal-containing whitening toothpaste and mouthwash on color change and surface morphology of enamel J Conserv Dent 2020 ; 23 ( 6 ): 624 – 631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple M , Meyer F , Enax J A critical review of modern concepts for teeth whitening Dent J (Basel) 2019 ; 7 ( 3 ): 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algarni AA , Ungar PS , Lippert F , Martínez-Mier EA , Eckert GJ , González-Cabezas C , et al. Trend-analysis of dental hard-tissue conditions as function of tooth age J Dent 2018 ; 74 : 107 – 112 [DOI] [PubMed] [Google Scholar]
- Liu H, Tu J Reduction of extrinsic tooth stain by a toothpaste containing 10% high cleaning silica, 0.5% sodium phytate and 0.5% sodium pyrophosphate: an 8-week randomised clinical trial BMC Oral Health 2021;21(1):113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A , Addy M Tooth discolouration and staining: a review of the literature Br Dent J 2001 ; 190 ( 6 ): 309 – 316 [DOI] [PubMed] [Google Scholar]
- Manno SHC , Manno FAM , Ahmed I , Ahmed R , Shu L , Li L , et al. Spectroscopic examination of enamel staining by coffee indicates dentin erosion by sequestration of elements Talanta 2018 ; 189 : 550 – 559 [DOI] [PubMed] [Google Scholar]
- Carlos NR , Pinto A , do Amaral F , França F , Turssi CP , Basting RT Influence of staining solutions on color change and enamel surface properties during at-home and in-office dental bleaching: an in situ study Oper Dent 2019 ; 44 ( 6 ): 595 – 608 [DOI] [PubMed] [Google Scholar]
- Joiner A Whitening toothpastes: a review of the literature J Dent 2010 ; 38 ( Suppl 2 ): S17 – S24 [DOI] [PubMed] [Google Scholar]
- Stookey GK , Burkhard TA , Schemehorn BR In vitro removal of stain with dentifrices J Dent Res 1982 ; 61 ( 11 ): 1236 – 1239 [DOI] [PubMed] [Google Scholar]
- Joiner A , Pickles MJ , Tanner C , Weader E , Doyle P An in situ model to study the toothpaste abrasion of enamel J Clin Periodontol 2004 ; 31 ( 6 ): 434 – 438 [DOI] [PubMed] [Google Scholar]
- Hefferren JJ Historical view of dentifrice functionality methods J Clin Dent 1998 ; 9 ( 3 ): 53 – 56 [PubMed] [Google Scholar]
- Brooks JK , Bashirelahi N , Reynolds MA Charcoal and charcoal-based dentifrices: a literature review J Am Dent Assoc 2017 ; 148 : 661 – 670 [DOI] [PubMed] [Google Scholar]
- Greenwall LH , Greenwall-Cohen J , Wilson NHF Charcoal-containing dentifrices Br Dent J 2019 ; 226 ( 9 ): 697 – 700 [DOI] [PubMed] [Google Scholar]
- Ghajari MF , Shamsaei M , Basandeh K , Galouyak MS Abrasiveness and whitening effect of charcoal-containing whitening toothpastes in permanent teeth Dent Res J (Isfahan) 2021 ; 18 : 51 [PMC free article] [PubMed] [Google Scholar]
- Koc Vural U , Bagdatli Z , Yilmaz AE , Yalçın Çakır F , Altundaşar E , Gurgan S Effects of charcoal-based whitening toothpastes on human enamel in terms of color, surface roughness, and microhardness: an in vitro study Clin Oral Investig 2021 ; 25 ( 10 ): 5977 – 5985 [DOI] [PubMed] [Google Scholar]
- Krithikadatta J , Gopikrishna V , Datta M CRIS Guidelines (checklist for reporting in-vitro studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research J Conserv Dent 2014 ; 17 ( 4 ): 301 – 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C , Lucas R , Smith AJ , Cooper PR An in vitro screening assay for dental stain cleaning BMC Oral Health 2017 ; 17 ( 1 ): 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SV , Tiwari R , Bhullar RK , Bansal H , Bhandari R , Kakkar T , et al. Sterilization of extracted human teeth: a comparative analysis J Oral Biol Craniofac Res 2012 ; 2 ( 3 ): 170 – 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadas M , Seven N The effect of different drinks on tooth color after home bleaching Eur J Dent 2014 ; 8 ( 2 ): 249 – 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraj PS , Raguganesh V Ultraviolet–visible spectrophotometric analysis of tooth whitening efficiency of ultraphosphate J Contemp Med Dent 2020 ; 8 ( 1 ): 48 – 53 [Google Scholar]
- Brown F , Diller KR Calculating the optimum temperature for serving hot beverages Burns 2008 ; 34 ( 5 ): 648 – 654 [DOI] [PubMed] [Google Scholar]
- Bazzi JZ , Bindo MJ , Rached RN , Mazur RF , Vieira S , de Souza EM The effect of at-home bleaching and toothbrushing on removal of coffee and cigarette smoke stains and color stability of enamel J Am Dent Assoc 2012 ; 143 ( 5 ): 1 – 7 [DOI] [PubMed] [Google Scholar]
- Bizhang M , Schmidt I , Chun YP , Arnold WH , Zimmer S Toothbrush abrasivity in a long-term simulation on human dentin depends on brushing mode and bristle arrangement PLoS One 2017 ; 12 ( 2 ): 0172060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykut-Yetkiner A , Attin T , Wiegand A Prevention of dentine erosion by brushing with anti-erosive toothpastes J Dent 2014 ; 42 ( 7 ): 856 – 861 [DOI] [PubMed] [Google Scholar]
- Pertiwi U , Eriwati Y , Irwan B Surface changes of enamel after brushing with charcoal toothpaste J Phys Conf Ser 2017 ; 884 : 012002 [Google Scholar]
- Rostamzadeh P , Omrani LR , Abbasi M , Yekaninejad MS , Ahmadi E Effect of whitening toothpastes containing activated charcoal, abrasive particles, or hydrogen peroxide on the color of aged microhybrid composite Dent Res J (Isfahan) 2021 ; 18 : 106 [PMC free article] [PubMed] [Google Scholar]
- Wetter NU , Branco EP , Deana AM , Pelino JE Color differences of canines and incisors in a comparative long-term clinical trial of three bleaching systems Lasers Med Sci 2009 ; 24 ( 6 ): 941 – 947 [DOI] [PubMed] [Google Scholar]
- Palandi SDS , Kury M , Picolo MZD , Coelho CSS , Cavalli V Effects of activated charcoal powder combined with toothpastes on enamel color change and surface properties J Esthet Restor Dent 2020 ; 32 : 783 – 790 [DOI] [PubMed] [Google Scholar]
- Vaz VTP, Jubilato DP, Oliveira MRM, Bortolatto JF, Floros MC, Dantas AA, et al. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: which one is the most effective? J Appl Oral Sci 2019; 27:20180051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushir Y , Luha A , Abhang S , Vadalia K Estimation of secondary metabolites in different tea and coffee brands from Indian market Int J Pharm Life Sci 2011 ; 2 : 599 – 600 [Google Scholar]
- Ren YF , Feng L , Serban D , Malmstrom HS Effects of common beverage colorants on color stability of dental composite resins: the utility of a thermocycling stain challenge model in vitro J Dent 2012 ; 40 ( Suppl 1 ): S48 – S56 [DOI] [PubMed] [Google Scholar]
- Arruda BM , Bassi JC , Vitti RP , Scatolin RS Color stability of bulk fill composite resins submitted to coffee staining Braz Dent Sci 2021 ; 24 : 1 – 7 [Google Scholar]
- Moran JM , Addy M , Newcombe RG A comparative study of stain removal with two electric toothbrushes and a manual brush J Clin Dent 1995 ; 6 : 188 – 193 [PubMed] [Google Scholar]
- Schemehorn BR , Keil JC The effect of an oscillating/rotating electric toothbrush and a sonic toothbrush on removal of stain from enamel surfaces J Clin Dent 1995 ; 6 : 194 – 197 [PubMed] [Google Scholar]
- Grossman E , Cronin M , Dembling W , Proskin H A comparative clinical study of extrinsic tooth stain removal with two electric toothbrushes [Braun D7 and D9] and a manual brush Am J Dent 1996 ; 9 : S25 – S29 [PubMed] [Google Scholar]
- Paravina RD , Ghinea R , Herrera LJ , Bona AD , Igiel C , Linninger M , et al. Color difference thresholds in dentistry J Esthet Restor Dent 2015 ; 27 ( Suppl 1 ): S1-S9 [DOI] [PubMed] [Google Scholar]



