Abstract
Understanding how environmental drivers influence shark and ray spatial and temporal patterns can provide crucial knowledge for their evidence-based protection and long-term monitoring. However, information on which drivers of variation are most important for elasmobranch communities on soft sediments is limited. Using baited remote underwater stereo-video systems (stereo-BRUVs), we investigated how seasonal and environmental variables affected the elasmobranchs of the iSimangaliso Wetland Park marine protected area (MPA) in South Africa (SA). In total, 11 species were identified from 48 sites between 12 m and 33 m water depth in a sandy habitat. While species richness was similar across seasons, the total abundance of elasmobranchs recorded was higher in winter than summer. The species assemblage composition varied significantly between seasons, with the Human’s whaler shark Carcharhinus humani prevalent in summer and the Critically Endangered whitespotted wedgefish Rhynchobatus djiddensis more abundant during winter. Most species were sighted throughout the entire depth range, but rays were more common in shallower waters (< 25 m depth), while C. humani and R. djiddensis were more common in the deeper depth zone of this study. This research provides baseline information about this previously unexplored sandy habitat for elasmobranchs in a site of regional and global significance. Records of species of conservation concern in the sampling area highlight the importance of protecting sand environments within an MPA.
Keywords: Elasmobranchs, Species diversity, Relative abundance, Sandy habitat, Environmental drivers, Seasonality, Stereo-BRUVs
Introduction
The impacts of changing seasons in the marine environment can be perceived at several scales, from local ecosystems (Scrosati & Ellrich, 2020) to entire oceans (Lee & Jin, 2021; Liao et al., 2022). In turn, environmental variations can impact different trophic levels, from microorganisms (Kudryavtseva et al., 2019) to megafauna (Rayment, Dawson & Slooten, 2010), including elasmobranchs (Blaison et al., 2015; Ketchum et al., 2014).
A change in temperature in the ocean is one of the most obvious and predictable seasonal variations (Jo et al., 2022). Since most sharks and rays are ectotherms (rely on the environment to determine their body temperature), environmental temperature variations can be a key driver for their physiology (Bernal et al., 2018; Silva-Garay & Lowe, 2021) and spatial/temporal distribution (Carlisle & Starr, 2009; Elston et al., 2022; Schlaff, Heupel & Simpfendorfer, 2014). For example, De Vos et al. (2015) found temperate shark diversity to be higher in summer than winter, and Brooks et al. (2013) encountered the same seasonal pattern for the abundance of Caribbean reef sharks (Carcharhinus perezi). Elasmobranchs also respond to biological cues to exploit seasonally abundant food sources (Hutchings et al., 2010; Dudley & Cliff, 2010), and mate/pup in specific locations and times of the year (Jirik & Lowe, 2012; Dicken, 2006; Olbers & Cliff, 2017). Accordingly, seasonality reveals itself as a proxy for a host of environmental and biological cues, which makes it an influencing factor of changes or patterns in elasmobranch communities.
However, not all sharks and rays are expected to move in response to seasons. Environmental variables such as depth, water quality and habitat availability also drive the community structure and species diversity of elasmobranch assemblages (Heupel et al., 2014). Nevertheless, rays and benthic sharks are most likely to be present year-round (Tilley & Strindberg, 2013), while the populations of more mobile elasmobranchs can fluctuate throughout time (Taylor, Sumpton & Ham, 2011; Ruiz-Abierno et al., 2021). Alternatively, some species exhibit site fidelity by remaining in the same region all year, but their distribution within the area can change (Hammerschlag et al., 2012; Vaudo & Heithaus, 2012); for instance, between shallower and deeper zones (Ketchum et al., 2014; Le Port, Lavery & Montgomery, 2012; Vianna et al., 2014). Studies have shown that the species richness and diversity of shark assemblages change with depth (Lucifora et al., 2012), and that larger apex shark species, such as tiger sharks (Galeocerdo cuvier), are more common in deeper waters (Lester et al., 2022). Additionally, this species and sandbar sharks (Carcharhinus plumbeus) demonstrate ontogenetic changes in depth distributions (Afonso & Hazin, 2015; McAuley et al., 2007). Understanding how environmental drivers affect sharks and rays is important in the context of their conservation (Peterson & Grubbs, 2020; Schlaff, Heupel & Simpfendorfer, 2014), especially to inform species-specific regulations.
Several studies suggest that well-enforced MPAs effectively protect elasmobranch populations (Ferretti et al., 2008; Heupel et al., 2014; MacNeil et al., 2020; Micheli et al., 2012), and even present 14 times more shark biomass than fished areas (Edgar et al., 2014). The management of elasmobranchs is increasingly being incorporated into the planning of MPAs (Bergmann et al., 2022; MacKeracher, Diedrich & Simpfendorfer, 2019), and the positive effect of no-take zones on endangered shark and ray species diversity and abundance is clear (Albano et al., 2021; Da Silva et al., 2013). The long-term monitoring of biodiversity and key species groups is important to demonstrate the effectiveness of MPAs and to guide adaptive management.
In the iSimangaliso Wetland Park (iSimangaliso) and World Heritage Site MPA, on the east coast of SA, sharks and rays remain a significantly under-studied group, with few elasmobranch studies undertaken in this region (Cliff et al., 2007; Daly et al., 2021; Dicken, 2014; Gifford et al., 2007; Olbers & Cliff, 2017). While coastal coral reef ecosystems are the focus (Floros, Samways & Armstrong, 2004; Floros, Schleyer & Maggs, 2013; Schleyer et al., 2018), there is a lack of similar attention on soft sediment areas, which can be valuable to elasmobranchs, particularly to bottom-dwellers (Pennino et al., 2013; Pierce, Scott-Holland & Bennett, 2011; Vaudo & Heithaus, 2009; Vaudo & Heithaus, 2012). For instance, sandy habitats provide sharks with foraging and reproduction areas, and offer juvenile rays important nursery areas (Martins et al., 2020; Parton et al., 2023). Thus, understanding the importance of different habitats for shark and ray species can inform conservation management strategies (Knip, Heupel & Simpfendorfer, 2012; White et al., 2015).
This study consisted of seasonal surveys (winter of 2021 and summer of 2022) of elasmobranch communities in a shallow sandy area within the iSimangaliso MPA. The main objectives were to assess shark and ray assemblages, and to evaluate the influence of season, depth, temperature, and current strength on species composition. To achieve this, species richness and relative abundance of elasmobranchs were assessed using baited remote underwater stereo-video systems (stereo-BRUVs).
Materials & Methods
Study area
This research was conducted in the iSimangaliso Wetland Park, an MPA on the north-eastern coast of SA, in KwaZulu-Natal (KZN), in the Western Indian Ocean (WIO). iSimangaliso is a large and transboundary MPA encompassing different protection levels, each permitting varying degrees of human activity. For logistical purposes, a small subsection of the MPA was sampled in this study. The study area is approximately 15 km in length and 2 km wide, and is a shallow zone with a northern limit at Jesser Point, a boat launch site in Sodwana Bay. It is located in the iSimangaliso Offshore Controlled Pelagic Linefish Zone North (IOCPLZN) management zone, in which diving and pelagic game fishing are allowed, but anchoring, bottom-fishing, night fishing and fishing for elasmobranchs are forbidden.
Unlike the surrounding zones where the substrate type is mainly shallow coral reefs, this area’s seafloor is primarily sand and has a submarine canyon in the middle of its length known as the Diepgat canyon. Although the study area has never been researched, photic soft sediments of KZN are known to be dominated by macrobenthic invertebrates (such as crabs and echinoderms) and also Sciaenidae and Haemulidae fish species (MacKay & Untiedt, 2014; Mann & Fennessy, 2014). In turn, Diepgat and other canyons of this region have revealed a great species richness of fish, including rays and coelacanths (Geldenhuys, 2015; Roberts et al., 2006).
This research was fully approved by the Department of Environment, Forestry and Fisheries of the Republic of South Africa (permit for the purposes of a scientific investigation or practical experiment in terms of section 83 of the Marine Living Resources Act, 1998 (act no. 18 of 1998), Res2021-25 and Res2022-60), and by the iSimangaliso Wetland Park Authority (Research Agreement with Sharklife Conservation Group and with Wildlands Conservation Trust (WILDTRUST) for the Oceans Alive project). This research was used for a Master’s thesis (Ferreira, 2022).
Baited remote underwater stereo-video system (stereo-BRUVs) surveys
Surveys using stereo-BRUVs were performed during winter (July–September 2021) and summer (January–March 2022). To guarantee similar sampling efforts throughout the photic zone, the sampling sites were selected following depth contour lines while driving the boat from shallower to deeper areas. These sites were later divided into three categories: “shallow”, between 12 and 20 m; “mid-depth” between 20.1 and 25 m; and “deep”, between 25.1 and 33 m.1 This sampling approach was the same for both seasons, as comparable locations were sampled in winter and summer. To ensure that the comparisons across seasons were consistent, only samples of equivalent depths were included in the final analysis. Consequently, 48 stereo-BRUVs from each season were used for analysis (Fig. 1). Within these 48 sites for summer and winter, 19 sites were in the north zone, 17 were located near the submarine canyon (distance less than 1.8 km), and 12 were in the south zone of the study area.
Figure 1. Winter and summer baited remote underwater stereo-video systems (stereo-BRUVs) sampling sites.
The overlap between winter (white) and summer (black) sites is due to their similar geographic coordinates. Map data from OpenStreetMap (2022). Bathymetry data from GEBCO (2021). Borders from Municipal Demarcation Board (2018).
The deployments performed during the same day (up to six deployments) were located at least 350 m apart in an inshore to offshore direction to establish a sufficient minimum distance between sampling sites to prevent individual elasmobranchs from being recounted, and thereby avoiding pseudo-replication (Haggitt, Freeman & Lily, 2014; Langlois et al., 2020). In the longshore direction, each deployment was located 1 km apart from the next in order to fully sample the study area. Near the canyon, the longshore distance was reduced to 500 m to increase sampling efforts in that region.
The stereo-BRUVs were equipped with pairs of GoPro Hero 6 or 7 cameras (50 or 60 frames per second, resolution of 1080p, and linear setting). Calibration using SeaGIS CAL software (SeaGIS, 2022a) was performed before sampling to ensure the cameras’ overlapping field of view enabled stereo-measurement (Harvey & Shortis, 1995). The cameras were installed in a steel trapezoidal structure, with one 1 kg weight attached to each leg for stability on the seafloor. In front of the cameras, a 1 m length rod with a perforated PVC bait box at the end contained 1 kg of chopped sardines Sardinops sagax. The stereo-BRUVs were manually deployed to the seafloor through a rope with a surface buoy.
At the time of each deployment, geographic coordinates were registered by the vessel’s GPS (Hook-7 Lowrance GPS system, Lowrance Ltd, Tulsa, OK, USA), and depth (m) and sea surface temperature (SST (°C)) were taken by the inbuilt transducer. Thirty-eight winter stereo-BRUVs and 17 summer stereo-BRUVs were fitted with a temperature logger (iButton, Maxim Integrated DS1921H-F5#, Digital Temperature Sensor; Whitewater, WI, USA) to record sea bottom temperature (SBT (°C)), while 41 winter stereo-BRUVs and 38 summer stereo-BRUVs had current strength (km/h) registered by a current meter (Garmin Colorado 300 GPS, Olathe, KS, USA). The standard soak time was one hour minimum (Langlois et al., 2020), which allowed for multiple deployments across the study area on a day.
Video analysis
All recorded videos were analysed until the 60 minutes of footage were completed. During that time, most elasmobranchs were identified to species level (using Ebert, Dando & Fowler, 2021; Last et al., 2016) but some could only be identified to family level. The latter records were omitted from the data analysis. It was not possible to assess to species level some rays of the Himantura uarnak-leoparda species complex with honeycomb/spotted patterning. As such, these were identified as Himantura spp. and were included in the data analysis. To avoid double counting the same individual, MaxN was registered for each species, for each stereo-BRUVs sample. The MaxN measure is the maximum number of individuals of a species seen during the 60-minute sample in the same video frame (Cappo et al., 2003). The sum of all MaxN for each species divided by the total number of samples determined relative abundance per species. Species diversity was considered species richness (number of species). Underwater visibility (cm) was considered the furthest visible distance possible that still allowed for an animal to be identified, and it was measured through SeaGIS EventMeasure 5.71 software (SeaGIS, 2022b).
Statistical analysis
Kruskal-Wallis tests and Dunn’s multiple comparisons tests (uncorrected Dunn’s test for comparisons between winter and summer SBT and within summer SBT, given that the corrected p-values did not allow to assess the significant differences) were used to analyse environmental variables and assess significant differences according to season, location and depth (p < 0.05 for significant differences). Unpaired Mann–Whitney U tests were adopted to check for significant seasonal differences in underwater visibility, and also in the number of elasmobranchs sighted per deployment. These analyses were performed in GraphPad Prism 9 (GraphPad, 2022).
To determine the effect of season and environmental variables in elasmobranch composition, multivariate statistics were performed. Biological data were log(x+1) transformed to ensure the trend was still detectable in spite of dominant species. Missing values in SBT and current strength data were replaced with the correspondent seasonal mean value. A canonical correspondence analysis (CCA) was used to visualise the relationship between the elasmobranch assemblages and SBT, depth and current strength (SST was excluded since SBT reflected the exact temperature of the species sightings). All explanatory variables were included, but summarizing effects allowed to identify which ones contributed significantly to species composition (p < 0.05). The CCA was preceded by a detrended correspondence analysis (DCA) to assess the main gradients along the species matrix (not including environmental variables). The results of the DCA suggested preference for a CCA (a unimodal ordination method) instead of a redundancy analysis (RDA) (a linear ordination method) (Zelený, 2022). These two analyses were run in Canoco 5.1 (Ter Braak & Smilauer, 2012).
A Bray-Curtis resemblance matrix (with a dummy variable = 1) was created among samples to obtain similarity in elasmobranch composition and abundance. Sequential permutational multivariate analyses of variance (PERMANOVAs) were run on this matrix to assess the effect of depth (shallow/mid-depth/deep), location (North/canyon/South), season (winter/summer), and the interactions of these variables in elasmobranch composition. By employing a sequential PERMANOVA, the effect of season could be tested after accounting for variation in the assemblage caused by depth and location. A PERMDISP was used to check if the levels of the factors differed in terms of the variability within each level, and pairwise tests were run for the significant factors in PERMANOVA. The results were assessed under the pseudo-F statistic with 999 random permutations (p < 0.05). These analyses were conducted in PERMANOVA+, an extension software in PRIMER-e 6 (Anderson, Gorley & Clarke, 2008).
One-way similarity percentages analyses (SIMPERs) were run, also in PRIMER-e 6, on the transformed biological data to identify the contribution of each species to the Bray-Curtis dissimilarity between depth zones and the two seasons (Clarke, 1993).
Results
Sample sites and environmental variables
Depth ranged from 12.2 to 33.0 m in winter (mean [x] ± standard deviation [SD] = 22.30 ± 6.50 m) and from 12.7 to 32.0 m in summer (x ± SD = 21.97 ± 6.54 m). A total of 21 winter and summer deployments were in the “shallow” depth category, 11 winter and summer deployments were in the “mid-depth” depth category, and 16 winter and summer deployments were deployed in the “deep” depth category.
The average SST was lower during winter (22.21 ± 0.54 °C) than summer (27.45 ± 0.70 °C), and the same pattern was verified for SBT (21.34 ± 0.52 °C for winter; 25.49 ± 1.35 °C for summer). Although there were no significant differences across locations and depth categories for winter SST (Kruskal-Wallis statistic (K-W) = 9.88; p = 0.273), winter SBT (SBT: K-W = 8.37, p = 0.301) or summer SST (K-W = 15.18, p = 0.056), summer SBT was significantly different (K-W = 10.04, p = 0.011) between the “shallow” and “deep” zones near the canyon region (p = 0.0288) (Fig. 2). Winter SST and SBT were significantly different (K-W = 42.89, p = 0.0003), but not within the same location and depth category, while summer SST and SBT were significantly different (K-W = 37.19, p = 0.0002) in the “shallow” zone in the North (p = 0.0006). Both SST (K-W = 76.66, p < 0.001) and SBT (K-W = 36.14, p = 0.0003) were significant different between winter and summer, for example in the “shallow” zone near the canyon (SST: p = 0.0004; SBT: p = 0.001). In addition, SBT also varied considerably across seasons in the “shallow” zone in the South (p = 0.009), as well as in the “deep” zone in the North (p = 0.022) and South (p = 0.025).
Figure 2. Seasonal sea bottom temperature (SBT) and sea surface temperature (SST).

Winter and summer temperatures in the north, near the canyon, and south zones of the study area, within the “shallow”, “mid-depth” and “deep” depth categories.
Current strength (1.08 ± 0.79 km/h for winter; 1.23 ± 0.73 km/h for summer) was significantly affected by location (K-W = 16.26, p = 0.006), with the North and South zones differing during winter (p = 0.015), and the North during winter differing from the South during summer (p = 0.022) (Fig. 3). Underwater visibility (768.1 ± 528.9 cm for winter; 684.0 ± 325.7 cm for summer) was not significantly different between seasons (Mann–Whitney U [M-W U] =1026, p = 0.675).
Figure 3. Seasonal current strength.
Winter and summer current strength in the north, near the canyon, and south zones of the study area.
Species richness and relative abundance
Approximately 77.08% of winter deployments (n = 37) and 66.67% of summer deployments (n = 32) recorded at least one sighting of an elasmobranch. The number of observed individual elasmobranchs per deployment (1.40 ± 1.12 individuals/deployment in winter; 1.23 ± 1.17 individuals/deployment in summer) was not significantly different between seasons (M-W U = 1042, p = 0.408).
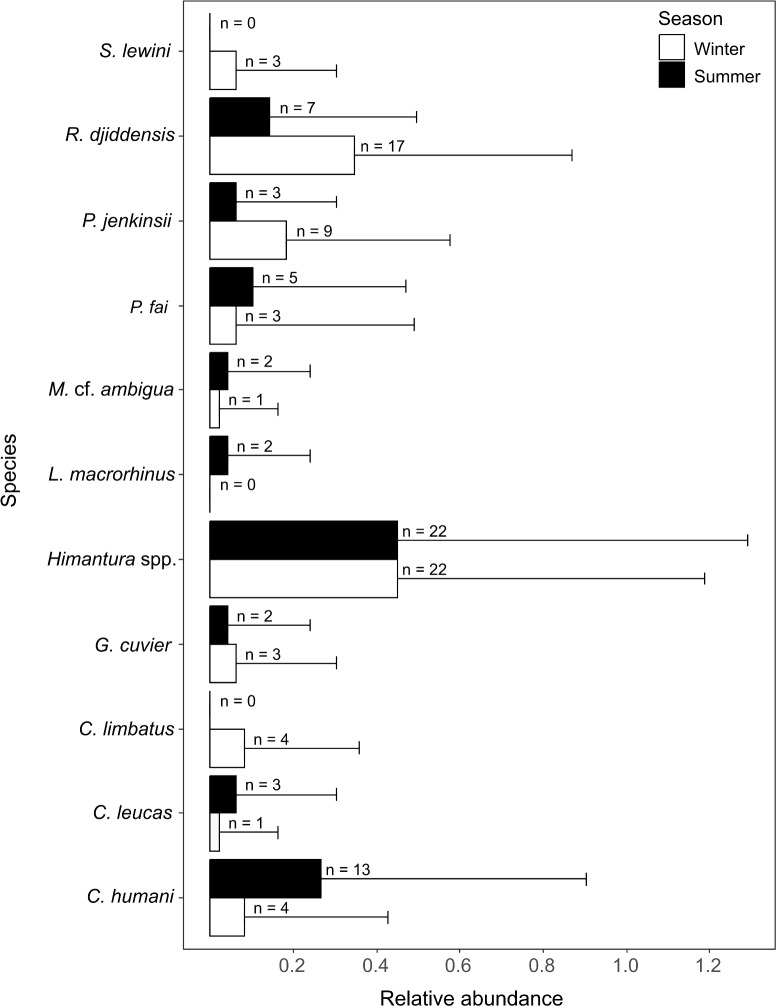
A total of 10 species were identified in winter (five shark species, n = 15; five ray species, n = 52) and nine were identified in summer (four shark species, n = 20; five ray species, n = 39). All rays and three species of sharks were present in both seasons. The common blacktip shark Carcharhinus limbatus and the scalloped hammerhead shark Sphyrna lewini were only recorded in winter, and the sliteye shark Loxodon macrorhinus was only seen in summer (Fig. 4).
Figure 4. Relative abundance (±SD) of each elasmobranch species found during winter and summer.
The n values in front of the bars represent the sum of MaxN of each species during each season.
Elasmobranch community analyses
A DCA on the biological data revealed that species constituted a heterogenous dataset, since there was a strong ecological gradient (gradient length of axis 1 = 4.28, given that a dataset is considered heterogenous if gradient length of axis 1 > 4). The CCA analysis (eigenvalue of axis 1 = 0.261; eigenvalue of axis 2 = 0.162; explained fitted variation of axis 1 = 55.63%; explained fitted variation of axis 2 = 90.06%) revealed that the continuous environmental variables accounted for 7.35% of the variation in species composition (Fig. 5). Depth (explained 3.50% of the variation, p = 0.012) and SBT (explained 2.80% of the variation, p = 0.036) were variables that contributed significantly to elasmobranch variation, while current strength (explained 1.00% of the variation, p = 0.608) was not considered a significant explanatory variable to species composition. The depth vector was directed towards quadrant 3 (negative segments of axes 1 and 2), while the SBT vector varied towards quadrant 2 (negative segment of axis 1 and positive segment of axis 2), and the current strength vector varied towards quadrant 4 (positive segment of axis 1 and negative segment of axis 2).
Figure 5. Canonical correspondence analysis (CCA) biplot representing the effect of environmental variables—current strength (current), depth and sea bottom temperature (SBT)—on species composition.
Environmental variables are represented by black arrows and species are represented by dark (shark) and light (ray) grey triangles.
Most species (including all rays except the whitespotted wedgefish Rhynchobatus djiddensis) were located between quadrants 1 and 4, which corresponded to shallower depths. On the contrary, the Human’s whaler shark Carcharhinus humani and L. macrorhinus showed up on quadrant 2, linked to deeper waters. The bull shark Carcharhinus leucas and R. djiddensis were also detached in quadrant 3, in deeper zones. In turn, the pink whipray Pateobatis fai and L. macrorhinus, and to a lesser extent C. humani and the sharpnose whipray Maculabatis cf. ambigua, seemed to be associated with higher SBT (summer), while C. limbatus and S. lewini were associated with lower SBT (winter).
The three-factor sequential PERMANOVA with categorical variables showed that depth and season (in this sequence) had a significant effect on elasmobranch composition (Table 1). The PERMDISP tests comparing variability within factors revealed non-significant results, which indicated that the variability in elasmobranch composition was similar among the factor levels.
Table 1. Permutacional analysis of variance (PERMANOVA) results testing the effect of season, depth, location, and the interactions of the three factors in elasmobranch composition.
| Model input factors | df | Pseudo-F | P |
|---|---|---|---|
| Depth | 2 | 3.165 | 0.005 |
| Location | 2 | 1.584 | 0.139 |
| Season | 1 | 3.093 | 0.016 |
| Depth × Location | 4 | 1.040 | 0.404 |
| Depth × Season | 2 | 1.261 | 0.293 |
| Location × Season | 2 | 1.694 | 0.100 |
| Depth × Location × Season | 4 | 1.254 | 0.262 |
Notes.
- df
- degrees of freedom
Underlined p-values indicate significance at p < 0.05.
Pairwise tests revealed that the effect of depth was driven by the difference between “shallow” and “deep” zones (p = 0.002), while there were no significant differences between the “mid-depth” zone and either the “shallow” (p = 0.279) or “deep” zone (p = 0.058). The SIMPER analysis revealed that the difference between the “shallow” and “deep” zones was driven by higher occurrence of R. djiddensis (dissimilarity x, x/SD: 24.47, 0.77) and C. humani (dissimilarity: 12.26, 0.54) in the “deep” zone, and higher occurrence of Himantura spp. (dissimilarity: 22.89, 0.81) and the Jenkin’s whipray Pateobatis jenkinsii (dissimilarity: 10.76, 0.51) in the “shallow” zone (Fig. 6).
Figure 6. Distribution along depth of the sighted shark and ray species.
The white triangles (winter) and black dots (summer) provide the depths of the samples where each species was detected.
The SIMPER analysis indicated that the seasonal variation in the elasmobranch assemblage was driven by higher rates of detection for R. djiddensis (dissimilarity: 20.04, 0.68) and P. jenkinsii (dissimilarity: 9.88, 0.48) in winter than summer (Fig. 4). On the other hand, C. humani was a characteristic species of the elasmobranch assemblage in summer (dissimilarity: 10.23, 0.50), but not winter (Fig. 4). Himantura. spp. was the most abundant elasmobranch recorded during the study with similar abundances in winter (x ± SD = 0.46 ± 0.74) and summer (0.46 ± 0.85).
Discussion
General diversity and species findings
This study documented 11 species of elasmobranchs with a similar diversity of sharks (six species) and rays (five species). Rays were more common than sharks, highlighting the importance of unconsolidated habitats for these organisms. It has been demonstrated that sandy areas can be crucial to batoid species, especially juveniles, as they seem to concentrate more in soft sediment than in coral reefs (Martin et al., 2012; Martins et al., 2020), while mature individuals are more easily found in both types of habitat (Aguiar, Valentin & Rosa, 2009).
This study found that R. djiddensis was a notably abundant species in the study area during winter. This wedgefish is listed as globally “Critically Endangered” and has suffered an estimated population decline of over 80% over the past 45 years (Kyne, Gledhill & Jabado, 2019). In SA, its population has been described as “Endangered”, with the main threats in KZN being fisheries—e.g., shore angling—and the bather protection nets (locally known as shark nets) located south of the study area (Daly et al., 2020; Pradervand et al., 2007). Since the mature individuals of this species can move long distances, they are also vulnerable to threats across the border in Mozambique (Jordaan et al., 2021; SAAMBR, 2022). Additionally, an illegal fin trade of R. djiddensis and other endangered sharks in southern Africa has been recently unveiled (Asbury et al., 2021). Accordingly, the current results strengthen the fact that the iSimangaliso MPA is important to the conservation of this species (Jordaan et al., 2021). Finding endangered elasmobranchs in this region supports the prohibition of elasmobranch fishing in the study area. This level of protection is particularly relevant considering that there is no legislation that forbids shore angling in the “Controlled” zones of iSimangaliso (iSimangaliso Wetland Park Authority, 2022).
Himantura rays with a honeycomb/spotted pattern were the predominant elasmobranchs in the area. In this study, elasmobranch identification was based on characteristics visible in the video footage, and rays of the H. uarnak-leoparda species complex could not be specifically categorized under one species. In fact, the genetic relationships within this species complex—to which the reticulate whipray Himantura uarnak and the leopard whipray Himantura leoparda belong (both extant in SA)—are still to be clarified (Arlyza et al., 2013; Borsa et al., 2021). The study area provides the opportunity for taxonomic studies to elucidate on whipray identification.
Carcharhinus humani was the most abundant of the six shark species. The Human’s whaler shark, previously thought to be the widespread C. sealei, was first described in 2014 and so far, it is the only species from the C. sealei subgroup to inhabit the WIO (White & Weigmann, 2014). This shark is a poorly known species, which reflects in its “Data Deficient” conservation status (Pollom et al., 2019), but this study confirmed that, as with C. sealei, C. humani occurs in photic and soft sediment habitats (Dulvy et al., 2021; Pollom et al., 2019). The larger species of sharks were also not abundant in the study area, which makes sense since these species are highly mobile, with average travelling distances between 86 and 267 km, as evidenced by the Oceanographic Research Institute (ORI) tagging and recapture programme in SA (iSimangaliso Wetland Park Authority, 2022).
Seasonality and environmental predictors of elasmobranch diversity and abundance
Sightings of sharks and rays were higher in winter than in summer. Species richness and diversity was similar across seasons, as the majority of species were common to both. While the univariate metrics were relatively stable, when species identity was considered, a significant seasonal pattern was evident with the “Critically Endangered” R. djiddensis being more common in winter and the relatively unknown C. humani being more common in summer. The remaining species showed little seasonal variation or had insufficient data to confidently identify patterns.
Rhynchobatus djiddensis is known to be most abundant in central and southern KZN during summer and to concentrate northwards, possibly into Mozambique, during winter (Daly et al., 2020; Jordaan et al., 2021). The current findings suggest that at least some wedgefishes remain in SA waters during winter, where they are under the protection of the iSimangaliso MPA, rather than being exposed to threats in Mozambique. The study area therefore forms a template that can inform management strategies and research priorities.
The seasonality effect is not expected to strongly predict elasmobranch communities in a subtropical zone like iSimangaliso, due to considerably homogeneous environmental conditions through time. In this study, it seemed most elasmobranchs, in particular rays, were residents in the area year-round, with two ray species and one small-bodied shark species showing seasonal variation. Large-bodied mobile sharks were rarely detected with no obvious environmental correlations. Therefore, sampling throughout both seasons is necessary to assess the full spectrum of the shark and ray assemblages. The association of some species with higher or lower SBT can be easily explained by the seasonality of their observations, given that temperature is a proxy for season. It is difficult to accurately determine if temperature or some other variables were driving the species seasonal patterns.
Most species were sighted in the entire depth range of this study (from 12 to 33 m depth), but whiprays seemed to be more common between shallow and intermediate depths (<25 m depth). Conversely, C. humani was more common in the deeper zone in this study. This species is known to reach 43 m deep (Pollom et al., 2019; White & Weigmann, 2014) and past research in iSimangaliso found this shark to be common deeper than 30 m, with the deepest record at 90 m depth (Bernard, personal observation, 28 March 2023). This study only sampled the shallow portion of the depth range for this species due to logistical constraints associated with the methodology. Carcharhinus humani showed a distinct seasonal pattern with individuals more common in shallower zones during summer. This pattern may reflect a seasonal depth change that warrants further investigation, potentially incorporating acoustic telemetry tracking.
In spite of continuous environmental variables only contributing to 7.35% of variation in elasmobranch composition in this study, environmental drivers may present influence on a species-specific scale. There may be unexplored factors which are stronger drivers for shark and ray assemblages and assessing other abiotic variables (e.g., current direction) could provide further insights.
Location was not a significant driver of elasmobranch species composition. Nevertheless, submarine canyons can have a recognized impact on abiotic conditions (e.g., upwelling of cooler deep waters) (Allen et al., 2001), and it is known that big pelagic sharks can be associated with these geomorphic features (Bradford et al., 2020; Klimley et al., 2002; Yano et al., 2007). Further research in the study area is necessary to assess the possible effect of the Diepgat canyon on the local elasmobranch communities.
Applicability of the methodology
The number of identified elasmobranch species in this research was lower when compared to other stereo-BRUVs assessments in South Africa, which may be due to other studies having larger sampling sizes over a longer period, the inclusion of assessments of different habitats besides sand, the inclusion of greater depths, and a higher diversity of endemic species in the study areas (Cortelezzi et al., 2022; De Vos et al., 2015; Osgood, McCord & Baum, 2019). Thus, it would be of interest that future research near the sampling area compares iSimangaliso’s sand and reef habitats.
In this study, underwater visibility was not significantly different between seasons, which suggests water clarity is suitable enough year-round for monitoring using this methodology. Footages of stereo-BRUVs allowed for the visualisation of biological variables, such as size and sex, which can be assessed in further studies. Although the deployments allowed for the observation and counting of elasmobranch individuals, stationary equipment may underestimate true relative abundance and diversity when using the conservative measure MaxN (Kilfoil et al., 2017; Sherman et al., 2018). It should therefore not be unquestionably assumed that the species only sighted in a certain season or depth zone in this study are completely restricted to those features. Thus, longer-term monitoring studies using stereo-BRUVs will help create a stronger database to assess patterns in elasmobranch communities; for instance, recognizing interannual variability in the presence of large and highly mobile species.
Conclusions
This study contributed to the understanding of the elasmobranch communities of a sandy habitat, as well as its relationship with season and the surrounding environmental conditions. In this ray-dominated study area, season and its proxy temperature, together with water depth were significant in shaping elasmobranch assemblage composition. However, almost all species were present in both seasons. This finding supports the concept of MPAs which provide year-round protection as opposed to time-area closures which, despite being beneficial during feeding/mating/pupping events, cannot ensure adequate continuous protection to elasmobranch species. Most species sighted during the surveys had at least a “Vulnerable” conservation status, and the “Critically Endangered” R. djiddensis was notably abundant. Thus, it is essential to ensure the enforcement of the MPA rules in this “Controlled” area, in order to achieve an effective preservation of all the sharks and rays that either reside in or pass through this region. The stereo-BRUVs methodology proved to be an efficient technique to survey elasmobranchs in a quick, non-invasive and repeatable manner, which is particularly important in seasonal studies and in the management of MPAs where resources are often constrained. As the first study in this area, further research is necessary to incorporate adjacent deeper habitats and assess long-term seasonal trends of the local elasmobranch communities.
Supplemental Information
Deployment details (including measurements of environmental variables), and the species MaxN associated with each sampling site, during winter and summer seasons.
Acknowledgments
The authors would like to thank Ezemvelo KZN Wildlife, which allowed the fulfilment of this study in the iSimangaliso Wetland Park. This work was also possible due to equipment from WILDOCEANS, a programme of the WILDTRUST through its Blue Action Fund (BAF) OCEANS ALIVE project, together with logistical support from the South African Institute for Aquatic Biodiversity (SAIAB), and the time donated by the Save our Seas Foundation. The authors would also like to thank Dr. Richard Furnas for granting access to Canoco 5.1.
Funding Statement
This work was supported by the Blue Action Fund through the Wildlands Conservation Trust (WILDTRUST). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Depth contour lines in Fig. 1 are visual representations and continuously change with tides and seasons, with the only certain depth being the one taken at the moment of sampling.
Additional Information and Declarations
Competing Interests
Lauren de Vos is employed by the Save Our Seas Foundation, and Grant Smith is the managing director at Sharklife Conservation Group.
Author Contributions
Jessica A. Ferreira performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Julie A. Alberts performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Grant Smith conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Anthony T.F. Bernard analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Mário J. Pereira analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Lauren De Vos analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Department of Environment, Forestry and Fisheries (DEFF) of the Republic of South Africa (Res2021-25 and Res2022-60) fully approved this research, which is within the scope of the Oceans Alive project, implemented by the Wildlands Conservation Trust (WILDTRUST).
Permit for the purposes of a scientific investigation or practical experiment in terms of section 83 of the Marine Living Resources Act, 1998 (Act no. 18 of 1998).
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments were fully approved by the iSimangaliso Wetland Park Authority.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Afonso & Hazin (2015).Afonso AS, Hazin FHV. Vertical movement patterns and ontogenetic niche expansion in the Tiger Shark, Galeocerdo cuvier. PLOS ONE. 2015;10(1):e0116720. doi: 10.1371/JOURNAL.PONE.0116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar, Valentin & Rosa (2009).Aguiar AA, Valentin JL, Rosa RS. Habitat use by Dasyatis americana in a south-western Atlantic oceanic island. Journal of the Marine Biological Association of the United Kingdom. 2009;89(6):1147–1152. doi: 10.1017/S0025315409000058. [DOI] [Google Scholar]
- Albano et al. (2021).Albano PS, Fallows C, Fallows M, Schuitema O, Bernard ATF, Sedgwick O, Hammerschlag N. Successful parks for sharks: no-take marine reserve provides conservation benefits to endemic and threatened sharks off South Africa. Biological Conservation. 2021;261:109302. doi: 10.1016/J.BIOCON.2021.109302. [DOI] [Google Scholar]
- Allen et al. (2001).Allen SE, Vindeirinho C, Thomson RE, Foreman MGG, Mackas DL. Physical and biological processes over a submarine canyon during an upwelling event. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58(4):671–684. doi: 10.1139/CJFAS-58-4-671. [DOI] [Google Scholar]
- Anderson, Gorley & Clarke (2008).Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E. https://www.researchgate.net/publication/285237419_PERMANOVA_for_primer_Guide_to_software_and_statistical_methods 2008
- Arlyza et al. (2013).Arlyza IS, Shen KN, Solihin DD, Soedharma D, Berrebi P, Borsa P. Species boundaries in the Himantura uarnak species complex (Myliobatiformes: Dasyatidae) Molecular Phylogenetics and Evolution. 2013;66(1):429–435. doi: 10.1016/J.YMPEV.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Asbury et al. (2021).Asbury TA, Bennett R, da Price AS, Silva C, Bürgener M, Klein JD, Maduna SN, Sidat N, Fernando S, Bester-van der Merwe AE. Application of DNA mini-barcoding reveals illegal trade in endangered shark products in southern Africa. African Journal of Marine Science. 2021;43(4):511–520. doi: 10.2989/1814232X.2021.1996459. [DOI] [Google Scholar]
- Bergmann et al. (2022).Bergmann MPMZ, Guttridge TL, Smukall MJ, Adams VM, Bond ME, Burke PJ, Fuentes MMPB, Heinrich DDU, Huveneers C, Gruber SH, Papastamatiou YP. Using movement models and systematic conservation planning to inform marine protected area design for a multi-species predator community. Biological Conservation. 2022;266:109469. doi: 10.1016/J.BIOCON.2022.109469. [DOI] [Google Scholar]
- Bernal et al. (2018).Bernal D, Reid JP, Roessig JM, Matsumoto S, Sepulveda CA, Cech JJ, Graham JB. Temperature effects on the blood oxygen affinity in sharks. Fish Physiology and Biochemistry. 2018;44(3):949–967. doi: 10.1007/s10695-018-0484-2. [DOI] [PubMed] [Google Scholar]
- Blaison et al. (2015).Blaison A, Jaquemet S, Guyomard D, Vangrevelynghe G, Gazzo T, Cliff G, Cotel P, Soria M. Seasonal variability of bull and tiger shark presence on the west coast of Reunion Island, western Indian Ocean. African Journal of Marine Science. 2015;37(2):199–208. doi: 10.2989/1814232X.2015.1050453. [DOI] [Google Scholar]
- Borsa et al. (2021).Borsa P, Williams CT, McIvor AJ, Hoareau TB, Berumen ML. Neotype designation and re-description of Forsskål’s reticulate whipray Himantura uarnak. Marine Biodiversity. 2021;51(2):1–10. doi: 10.1007/s12526-021-01180-1. [DOI] [Google Scholar]
- Bradford et al. (2020).Bradford R, Patterson TA, Rogers PJ, McAuley R, Mountford S, Huveneers C, Robbins R, Fox A, Bruce BD. Evidence of diverse movement strategies and habitat use by white sharks, Carcharodon carcharias, off southern Australia. Marine Biology. 2020;167(7):1–12. doi: 10.1007/s00227-020-03712-y. [DOI] [Google Scholar]
- Brooks et al. (2013).Brooks EJ, Sims DW, Danylchuk AJ, Sloman KA. Seasonal abundance, philopatry and demographic structure of Caribbean reef shark (Carcharhinus perezi) assemblages in the north-east Exuma Sound, The Bahamas. Marine Biology. 2013;160(10):2535–2546. doi: 10.1007/S00227-013-2246-0. [DOI] [Google Scholar]
- Cappo et al. (2003).Cappo M, Harvey ES, Malcolm H, Speare P. Potential of video techniques to monitor diversity, abundance and size of fish in studies of Marine Protected Areas. In: Beumer JP, Grant A, Smith DC, editors. Aquatic Protected Areas - what works best and how do we know. vol. 1. University of Queensland; 2003. pp. 455–464. . Australian Society for Fish Biology. North Beach, WA, Australia. http://www.pelagicos.net/MARS6910_spring2019/readings/Cappo_et_al..pdf. [Google Scholar]
- Carlisle & Starr (2009).Carlisle AB, Starr RM. Habitat use, residency, and seasonal distribution of female leopard sharks Triakis semifasciata in Elkhorn Slough, California. Marine Ecology Progress Series. 2009;380:213–228. doi: 10.3354/MEPS07907. [DOI] [Google Scholar]
- Clarke (1993).Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecology. 1993;18(1):117–142. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- Cliff et al. (2007).Cliff G, Anderson-Reade MD, Aitken AP, Charter GE, Peddemors VM. Aerial census of whale sharks (Rhincodon typus) on the northern KwaZulu-Natal coast, South Africa. Fisheries Research. 2007;84(1):41–46. doi: 10.1016/J.FISHRES.2006.11.012. [DOI] [Google Scholar]
- Cortelezzi et al. (2022).Cortelezzi P, Paulet TG, Olbers JM, Harris JM, Bernard ATF. Conservation benefits of a marine protected area on South African chondrichthyans. Journal of Environmental Management. 2022;319:115691. doi: 10.1016/J.JENVMAN.2022.115691. [DOI] [PubMed] [Google Scholar]
- Da Silva et al. (2013).Da Silva C, Kerwath S, Attwood C, Thorstad E, Cowley P, Økland F, Wilke C, Næsje T. Quantifying the degree of protection afforded by a no-take marine reserve on an exploited shark. African Journal of Marine Science. 2013;35(1):57–66. doi: 10.2989/1814232X.2013.769911. [DOI] [Google Scholar]
- Daly et al. (2021).Daly R, Le Noury P, Hempson TN, Ziembicki MGM, Brokensha Olbers JM, Mann BQ. Bull shark Carcharhinus leucas recruitment into the St Lucia Estuary, South Africa, after prolonged mouth closure, and the first observation of a neonate bull shark preyed on by a Nile crocodile Crocodylus niloticus. African Journal of Marine Science. 2021;43(3):417–421. doi: 10.2989/1814232X.2021.1964599. [DOI] [Google Scholar]
- Daly et al. (2020).Daly R, Parker D, Cliff G, Jordaan GL, Nomfundo N, Bennett RH, Mann BQ. Long-term catch trends and risk assessment of the Critically Endangered white-spotted wedgefish (Rhynchobatus djiddensis) from South Africa. Aquatic Conservation: Marine and Freshwater Ecosystems. 2020;31(4):777–788. doi: 10.1002/AQC.3483. [DOI] [Google Scholar]
- De Vos et al. (2015).De Vos L, Watson R, Götz A, Attwood C. Baited remote underwater video system (BRUVs) survey of chondrichthyan diversity in False Bay, South Africa. African Journal of Marine Science. 2015;37(2):209–218. doi: 10.2989/1814232X.2015.1036119. [DOI] [Google Scholar]
- Dicken (2006).Dicken ML. Doctoral dissertation. 2006. Population dynamics of the raggedtooth shark (Carcharias taurus) along the east coast of South Africa. [Google Scholar]
- Dicken (2014).Dicken M. Socio-economic aspects of the Sodwana Bay SCUBA diving industry, with a specific focus on sharks. African Journal of Marine Science. 2014;36(1):39–47. doi: 10.2989/1814232X.2014.893257. [DOI] [Google Scholar]
- Dudley & Cliff (2010).Dudley SFJ, Cliff G. Influence of the annual sardine run on catches of large sharks in the protective gillnets off KwaZulu-Natal, South Africa, and the occurrence of sardine in shark diet. African Journal of Marine Science. 2010;32(2):383–397. doi: 10.2989/1814232X.2010.502641. [DOI] [Google Scholar]
- Dulvy et al. (2021).Dulvy NK, Ali B, Bineesh KK, Derrick D, Seyha L, Tanay D, VanderWright WJ, Vo VQ, Yuneni RR, Maung A, Utzurrum JAT. Carcharhinus sealei . 2021. [27 October 2022]. The IUCN Red List of Threatened Species 2021: e.T41738A68613628. https://dx.doi.org/10.2305/IUCN.UK.2021-2.RLTS.T41738A68613628.en.
- Ebert, Dando & Fowler (2021).Ebert DA, Dando M, Fowler S. Sharks of the world: a complete guide. (Volume 22 of Wild Nature Press series) Princeton University Press; Princeton, NJ, EUA: 2021. [Google Scholar]
- Edgar et al. (2014).Edgar GJ, Stuart-Smith RD, Willis TJ, Kininmonth S, Baker SC, Banks S, Barrett NS, Becerro MA, Bernard ATF, Berkhout J, Buxton CD, Campbell SJ, Cooper AT, Davey M, Edgar SC, Försterra G, Galván DE, Irigoyen AJ, Kushner DJ, Thomson RJ. Global conservation outcomes depend on marine protected areas with five key features. Nature. 2014;506(7487):216–220. doi: 10.1038/nature13022. [DOI] [PubMed] [Google Scholar]
- Elston et al. (2022).Elston C, Cowley PD, von Brandis RG, Lea J. Stingray habitat use is dynamically influenced by temperature and tides. Frontiers in Marine Science. 2022;8:754404. doi: 10.3389/FMARS.2021.754404. [DOI] [Google Scholar]
- Ferreira (2022).Ferreira JA. Master’s Thesis. 2022. Seasonal assessment of elasmobranch communities in iSimangaliso, a South African marine protected area. [Google Scholar]
- Ferretti et al. (2008).Ferretti F, Myers RA, Serena F, Lotze HK. Loss of large predatory sharks from the Mediterranean Sea. Conservation Biology. 2008;22(4):952–964. doi: 10.1111/j.1523-1739.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- Floros, Samways & Armstrong (2004).Floros CD, Samways MJ, Armstrong B. Taxonomic patterns of bleaching within a South African coral assemblage. Biodiversity and Conservation. 2004;13(6):1175–1194. doi: 10.1023/B:BIOC.0000018151.67412.C7. [DOI] [Google Scholar]
- Floros, Schleyer & Maggs (2013).Floros C, Schleyer MH, Maggs JQ. Fish as indicators of diving and fishing pressure on high-latitude coral reefs. Ocean & Coastal Management. 2013;84:130–139. doi: 10.1016/j.ocecoaman.2013.08.005. [DOI] [Google Scholar]
- Geldenhuys (2015).Geldenhuys DA. Master’s Thesis. 2015. Quantitative fish survey of the submarine canyons of the iSimangaliso Wetland Park. [Google Scholar]
- GEBCO (2021).General Bathymetry Chart of the Oceans [GEBCO] compilation group GEBCO 2021 Grid. 2021. https://doi.org/10.5285/c6612cbe-50b3-0cff-e053-6c86abc09f8f https://doi.org/10.5285/c6612cbe-50b3-0cff-e053-6c86abc09f8f
- Gifford et al. (2007).Gifford A, Compagno LJV, Levine M, Antoniou A. Satellite tracking of whale sharks using tethered tags. Fisheries Research. 2007;84(1):17–24. doi: 10.1016/J.FISHRES.2006.11.011. [DOI] [Google Scholar]
- GraphPad (2022).GraphPad Welcome to the prism 9 statistics guide. 2022. https://www.graphpad.com/guides/prism/latest/statistics/index.htm. [27 October 2022]. https://www.graphpad.com/guides/prism/latest/statistics/index.htm
- Haggitt, Freeman & Lily (2014).Haggitt T, Freeman D, Lily C. Baited Remote Underwater Video Guidelines. eCoast. 2014. https://www.doc.govt.nz/documents/science-and-technical/inventory-monitoring/im-toolbox-marine-baited-remote-underwater-video-guidelines.pdf https://www.doc.govt.nz/documents/science-and-technical/inventory-monitoring/im-toolbox-marine-baited-remote-underwater-video-guidelines.pdf
- Hammerschlag et al. (2012).Hammerschlag N, Luo J, Irschick DJ, Ault JS. A comparison of spatial and movement patterns between sympatric predators: bull sharks (Carcharhinus leucas) and Atlantic Tarpon (Megalops atlanticus) PLOS ONE. 2012;7(9):e45958. doi: 10.1371/JOURNAL.PONE.0045958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey & Shortis (1995).Harvey ES, Shortis MR. A system for stereo-video measurement of sub-tidal organisms. Marine Technology Society Journal. 1995;29(4):10–22. [Google Scholar]
- Heupel et al. (2014).Heupel MR, Knip DM, Simpfendorfer CA, Dulvy NK. Sizing up the ecological role of sharks as predators. Marine Ecology Progress Series. 2014;495:291–298. doi: 10.3354/meps10597. [DOI] [Google Scholar]
- Hutchings et al. (2010).Hutchings L, Morris T, van der Lingen CD, Lamberth SJ, Connell AD, Taljaard S, van Niekerk L. Ecosystem considerations of the KwaZulu-Natal sardine run. African Journal of Marine Science. 2010;32(2):413–421. doi: 10.2989/1814232X.2010.502644. [DOI] [Google Scholar]
- iSimangaliso Wetland Park Authority (2022).iSimangaliso Wetland Park Authority iSimangaliso Wetland Park integrated management plan (2022–2031). Draft. 2022. https://sahris.sahra.org.za/sites/default/files/additionaldocs/iSimangaliso%20Integrated%20Management%20Plan.pdf https://sahris.sahra.org.za/sites/default/files/additionaldocs/iSimangaliso%20Integrated%20Management%20Plan.pdf
- Jirik & Lowe (2012).Jirik KE, Lowe CG. An elasmobranch maternity ward: female round stingrays Urobatis halleri use warm, restored estuarine habitat during gestation. Journal of Fish Biology. 2012;80(5):1227–1245. doi: 10.1111/J.1095-8649.2011.03208.X. [DOI] [PubMed] [Google Scholar]
- Jo et al. (2022).Jo AR, Lee J-Y, Timmermann A, Jin F-F, Yamaguchi R, Gallego A. Future amplification of sea surface temperature seasonality due to enhanced ocean stratification. Geophysical Research Letters. 2022;49(9):e2022GL098607. doi: 10.1029/2022GL098607. [DOI] [Google Scholar]
- Jordaan et al. (2021).Jordaan GL, Mann BQ, Daly R, Dunlop SW, Cowley PD. Movement patterns and growth rate of the whitespotted wedgefish Rhynchobatus djiddensis in southern Africa based on tag-recapture data. African Journal of Marine Science. 2021;43(2):201–213. doi: 10.2989/1814232X.2021.1906318. [DOI] [Google Scholar]
- Ketchum et al. (2014).Ketchum JT, Hearn A, Klimley AP, Espinoza E, Peñaherrera C, Largier JL. Seasonal changes in movements and habitat preferences of the scalloped hammerhead shark (Sphyrna lewini) while refuging near an oceanic island. Marine Biology. 2014;161(4):755–767. doi: 10.1007/S00227-013-2375-5. [DOI] [Google Scholar]
- Kilfoil et al. (2017).Kilfoil JP, Wirsing AJ, Campbell MD, Kiszka JJ, Gastrich KR, Heithaus MR, Zhang Y, Bond ME. Baited Remote Underwater Video surveys undercount sharks at high densities: insights from full-spherical camera technologies. Marine Ecology Progress Series. 2017;585:113–121. doi: 10.3354/MEPS12395. [DOI] [Google Scholar]
- Klimley et al. (2002).Klimley AP, Beavers SC, Curtis TH, Jorgensen SJ. Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environmental Biology of Fishes. 2002;63(2):117–135. doi: 10.1023/A:1014200301213. [DOI] [Google Scholar]
- Knip, Heupel & Simpfendorfer (2012).Knip DM, Heupel MR, Simpfendorfer CA. Evaluating marine protected areas for the conservation of tropical coastal sharks. Biological Conservation. 2012;148(1):200–209. doi: 10.1016/j.biocon.2012.01.008. [DOI] [Google Scholar]
- Kudryavtseva et al. (2019).Kudryavtseva E, Aleksandrov S, Bukanova T, Dmitrieva O, Rusanov I. Relationship between seasonal variations of primary production, abiotic factors and phytoplankton composition in the coastal zone of the south-eastern part of the baltic sea. Regional Studies in Marine Science. 2019;32:100862. doi: 10.1016/J.RSMA.2019.100862. [DOI] [Google Scholar]
- Kyne, Gledhill & Jabado (2019).Kyne PM, Gledhill K, Jabado RW. Rhynchobatus djiddensis. The IUCN Red List of Threatened Species 2019, e.T39394A121035795. 2019. https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T39394A121035795.en. [27 October 2022]. https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T39394A121035795.en
- Langlois et al. (2020).Langlois T, Goetze J, Bond T, Monk J, Abesamis RA, Asher J, Barrett N, Bernard ATF, Bouchet PJ, Birt MJ, Cappo M, Currey-Randall LM, Driessen D, Fairclough DV, Fullwood LAF, Gibbons BA, Harasti D, Heupel MR, Hicks J, Holmes TH, Huveneers C, Ierodiaconou D, Jordan A, Knott NA, Lindfield S, Malcolm HA, McLean D, Meekan M, Miller D, Mitchell PJ, Newman SJ, Radford B, Rolim FA, Saunders BJ, Stowar M, Smith ANH, Travers MJ, Wakefield CB, Whitmarsh SK, Williams J, Harvey ES, Codling E. A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods in Ecology and Evolution. 2020;11(11):1401–1409. doi: 10.1111/2041-210X.13470. [DOI] [Google Scholar]
- Last et al. (2016).Last PR, White WT, deCarvalho MR, Séret B, Stehamnn MFW, Naylor GJP, editors. Rays of the World. CSIRO Publishing; Melbourne, Australia: 2016. [Google Scholar]
- Le Port, Lavery & Montgomery (2012).Le Port A, Lavery S, Montgomery JC. Conservation of coastal stingrays: seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area. ICES Journal of Marine Science. 2012;69(8):1427–1435. doi: 10.1093/ICESJMS/FSS120. [DOI] [Google Scholar]
- Lee & Jin (2021).Lee H-J, Jin E-K. Seasonality and dynamics of atmospheric teleconnection from the tropical indian ocean and the western pacific to West Antarctica. Atmosphere. 2021;12(7):849. doi: 10.3390/atmos12070849. [DOI] [Google Scholar]
- Lester et al. (2022).Lester E, Langlois T, Lindgren I, Birt M, Bond T, McLean D, Vaughan B, Holmes TH, Meekan M. Drivers of variation in occurrence, abundance, and behaviour of sharks on coral reefs. Scientific Reports. 2022;12:728. doi: 10.1038/s41598-021-04024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2022).Liao F, Gao G, Zhan P, Wang Y. Seasonality and trend of the global upper-ocean vertical velocity over 1998–2017. Progress in Oceanography. 2022;204:102804. doi: 10.1016/J.POCEAN.2022.102804. [DOI] [Google Scholar]
- Lucifora et al. (2012).Lucifora LO, García VB, Menni RC, Worm B. Spatial patterns in the diversity of sharks, rays, and chimaeras (Chondrichthyes) in the Southwest Atlantic. Biodiversity Conservation. 2012;21:407–419. doi: 10.1007/s10531-011-0189-7. [DOI] [Google Scholar]
- MacKay & Untiedt (2014).MacKay F, Untiedt C. Subtidal Soft Sediments. In: Goble BJ, Van Der Elst RP, Oellermann LK, editors. Ugu Lwethu –Our Coast. A profile of coastal KwaZulu-Natal, Coastal and Marine Ecosystems. KwaZulu-Natal Department of Agriculture and Environmental Affairs and the Oceanographic Research Institute; Cedara, KwaZulu-Natal, South Africa: 2014. pp. 50–51. [Google Scholar]
- MacKeracher, Diedrich & Simpfendorfer (2019).MacKeracher T, Diedrich A, Simpfendorfer CA. Sharks, rays and marine protected areas: a critical evaluation of current perspectives. Fish and Fisheries. 2019;20(2):255–267. doi: 10.1111/FAF.12337. [DOI] [Google Scholar]
- MacNeil et al. (2020).MacNeil MA, Chapman DD, Heupel M, Simpfendorfer CA, Heithaus M, Meekan M, Harvey E, Goetze J, Kiszka J, Bond ME, Currey-Randall LM, Speed CW, Sherman CS, Rees MJ, Udyawer V, Flowers KI, Clementi G, Valentin-Albanese J, Gorham T, Adam MS, Ali K, Pina-Amargós F, Angulo-Valdés JA, Asher J, Barcia LG, Beaufort O, Benjamin C, Bernard ATF, Berumen ML, Bierwagen S, Bonnema E, Bown RMK, Bradley D, Brooks E, Brown JJ, Buddo D, Burke P, Cáceres C, Cardeñosa D, Carrier JC, Caselle JE, Charloo V, Claverie T, Clua E, Cochran JEM, Cook N, Cramp J, D’Alberto B, de Graaf M, Dornhege M, Estep A, Fanovich L, Farabaugh NF, Fernando D, Flam AL, Floros C, Fourqurean V, Garla R, Gastrich K, George L, Graham R, Guttridge T, Hardenstine RS, Heck S, Henderson AC, Hertler H, Hueter R, Johnson M, Jupiter S, Kasana D, Kessel ST, Kiilu B, Kirata T, Kuguru B, Kyne F, Langlois T, Lédée EJI, Lindfield S, Luna-Acosta A, Maggs J, Manjaji-Matsumoto BM, Marshall A, Matich P, McCombs E, McLean D, Meggs L, Moore S, Mukherji S, Murray R, Kaimuddin M, Newman SJ, Nogués J, Obota C, O’Shea O, Osuka K, Papastamatiou YP, Perera N, Peterson B, Ponzo A, Prasetyo A, Quamar LMS, Quinlan J, Ruiz-Abierno A, Sala E, Samoilys M, Schärer-Umpierre M, Schlaff A, Simpson N, Smith ANH, Sparks L, Tanna A, Torres R, Travers MJ, van Zinnicq Bergmann M, Vigliola L, Ward J, Watts AM, Wen C, Whitman E, Wirsing AJ, Wothke A, Zarza-Gonzâlez E, Cinner JE. Global status and conservation potential of reef sharks. Nature. 2020;583(7814):801–806. doi: 10.1038/s41586-020-2519-y. [DOI] [PubMed] [Google Scholar]
- Mann & Fennessy (2014).Mann B, Fennessy S. Fishes. In: Goble B, van der Elst R, Oellermann L, editors. Ugu Lwethu –Our Coast. A profile of coastal KwaZulu-Natal, Coastal and Marine Species. KwaZulu-Natal Department of Agriculture and Environmental Affairs and the Oceanographic Research Institute; 2014. pp. 64–67. [Google Scholar]
- Martin et al. (2012).Martin CS, Vaz S, Ellis JR, Lauria V, Coppin F, Carpentier A. Modelled distributions of ten demersal elasmobranchs of the eastern English Channel in relation to the environment. Journal of Experimental Marine Biology and Ecology. 2012;418–419:91–103. doi: 10.1016/J.JEMBE.2012.03.010. [DOI] [Google Scholar]
- Martins et al. (2020).Martins APB, Heupel MR, Bierwagen SL, Chin A, Simpfendorfer C. Diurnal activity patterns and habitat use of juvenile Pastinachus ater in a coral reef flat environment. PLOS ONE. 2020;15(2):e0228280. doi: 10.1371/JOURNAL.PONE.0228280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley et al. (2007).McAuley RB, Simpfendorfer CA, Hyndes GA, Lenanton RCJ. Distribution and reproductive biology of the sandbar shark, Carcharhinus plumbeus (Nardo), in Western Australian waters. Marine and Freshwater Research. 2007;58(1):116–126. doi: 10.1071/MF05234. [DOI] [Google Scholar]
- Micheli et al. (2012).Micheli F, Saenz-Arroyo A, Greenley A, Vazquez L, Montes JAE, Rossetto M, De Leo GA. Evidence that marine reserves enhance resilience to climatic impacts. PLOS ONE. 2012;7(7):e40832. doi: 10.1371/journal.pone.0040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Municipal Demarcation Board (2018).Municipal Demarcation Board MDB District Municipalities 2018. 2018. https://www.arcgis.com/sharing/rest/content/items/23e1b3458c704f65bc764168ae8557b8/info/metadata/metadata.xml?format=defaultoutput=html. [27 October 2022]. https://www.arcgis.com/sharing/rest/content/items/23e1b3458c704f65bc764168ae8557b8/info/metadata/metadata.xml?format=defaultoutput=html
- Olbers & Cliff (2017).Olbers JM, Cliff G. https://www.researchgate.net/publication/317400565_A_brief_survey_of_ragged-tooth_shark_Carcharias_taurus_abundance_and_behaviour_during_their_annual_gestation_period_on_Quarter-mile_reef_Sodwana_Bay_iSimangaliso_Wetland_Park_South_Africa_Season_Repor A brief survey of ragged-tooth shark (Carcharias taurus) abundance and behaviour during their annual gestation period on Quarter-mile reef, Sodwana Bay, iSimangaliso Wetland Park, South Africa. Season Report for 2016/2017, Ezemvelo KZN Wildlife Internal Report. 2017
- OpenStreetMap (2022).OpenStreetMap Data available under the Open Database License. 2022. https://www.openstreetmap.org/copyright/en. [27 October 2022]. https://www.openstreetmap.org/copyright/en
- Osgood, McCord & Baum (2019).Osgood GJ, McCord ME, Baum JK. Using baited remote underwater videos (BRUVs) to characterize chondrichthyan communities in a global biodiversity hotspot. PLOS ONE. 2019;14(12):e0225859. doi: 10.1371/JOURNAL.PONE.0225859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton et al. (2023).Parton KJ, Doherty PD, Parrish M, Shearer P, Myrick K, Shipley ON, Gallagher AJ. Opportunistic camera surveys provide insight into discrete foraging behaviours in nurse sharks (Ginglymostoma cirratum) Environmental Biology of Fishes. 2023;106:19–30. doi: 10.1007/s10641-022-01366-x. [DOI] [Google Scholar]
- Pennino et al. (2013).Pennino MG, Muñoz F, Conesa D, López-Qúlez A, Bellido JM. Modeling sensitive elasmobranch habitats. Journal of Sea Research. 2013;83:209–218. doi: 10.1016/J.SEARES.2013.03.005. [DOI] [Google Scholar]
- Peterson & Grubbs (2020).Peterson CT, Grubbs RD. Distribution and abundance of elasmobranchs and large teleost fishes in a subtropical seagrass ecosystem: community structure along environmental and spatial gradients. Environmental Biology of Fishes. 2020;103(4):319–338. doi: 10.1007/S10641-020-00959-8. [DOI] [Google Scholar]
- Pierce, Scott-Holland & Bennett (2011).Pierce SJ, Scott-Holland TB, Bennett MB. Community composition of elasmobranch fishes utilizing intertidal sand flats in Moreton Bay, Queensland, Australia. Pacific Science. 2011;65(2):235–247. doi: 10.2984/65.2.235. [DOI] [Google Scholar]
- Pollom et al. (2019).Pollom R, Gledhill K, Simpfendorfer C, Jabado RW, Moore A, Elhassan I. Carcharhinus humani. The IUCN Red List of Threatened Species 2019, e.T110834677A139929300. 2019. https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T110834677A139929300.en. [27 October 2022]. https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T110834677A139929300.en
- Pradervand et al. (2007).Pradervand P, Mann BQ, Bellis MF, Mann B, Bellis M. Long-term trends in the competitive shore fishery along the KwaZulu-Natal coast, South Africa. African Zoology. 2007;42(2):216–236. doi: 10.1080/15627020.2007.11407399. [DOI] [Google Scholar]
- Rayment, Dawson & Slooten (2010).Rayment W, Dawson S, Slooten E. Seasonal changes in distribution of Hector’s dolphin at Banks Peninsula, New Zealand: implications for protected area design. Aquatic Conservation: Marine and Freshwater Ecosystems. 2010;20(1):106–116. doi: 10.1002/AQC.1049. [DOI] [Google Scholar]
- Roberts et al. (2006).Roberts MJ, Ribbink AJ, Morris T, Van DenBerg MA, Engelbrecht DC, Harding RT. Oceanographic environment of the Sodwana Bay coelacanths (Latimeria chalumnae), South Africa. https://core.ac.uk/download/pdf/90214487 South African Journal of Science. 2006;102:435–443. [Google Scholar]
- Ruiz-Abierno et al. (2021).Ruiz-Abierno A, Márquez-Farías JF, Rojas-Corzo A, Miller V, Angulo-Valdés JA, Hueter RE. Seasonal abundance and size structure of sharks taken in the pelagic longline fishery off Northwestern Cuba. Marine and Coastal Fisheries. 2021;13(3):275–291. doi: 10.1002/MCF2.10152. [DOI] [Google Scholar]
- Schlaff, Heupel & Simpfendorfer (2014).Schlaff AM, Heupel MR, Simpfendorfer CA. Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Reviews in Fish Biology and Fisheries. 2014;24(4):1089–1103. doi: 10.1007/S11160-014-9364-8. [DOI] [Google Scholar]
- Schleyer et al. (2018).Schleyer MH, Floros C, Laing SCS, Macdonald AHH, Montoya-Maya PH, Morris T, Porter SN, Seré MG. What can South African reefs tell us about the future of high-latitude coral systems? Marine Pollution Bulletin. 2018;136:491–507. doi: 10.1016/J.MARPOLBUL.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Scrosati & Ellrich (2020).Scrosati RA, Ellrich JA. Latitudinal and seasonal changes in intertidal sea surface temperature along the atlantic coast of Nova Scotia, Canada. Frontiers in Marine Science. 2020;7:592. doi: 10.3389/fmars.2020.00592. [DOI] [Google Scholar]
- SeaGIS (2022a).SeaGIS 2022a. [27 October 2022]. CAL - Stereo Camera Calibration. https://www.seagis.com.au/bundle.html
- SeaGIS (2022b).SeaGIS 2022b. [27 October 2022]. EventMeasure - Event logging & 3D measurement. https://www.seagis.com.au/event.html
- Sherman et al. (2018).Sherman CS, Chin A, Heupel MR, Simpfendorfer CA. Are we underestimating elasmobranch abundances on baited remote underwater video systems (BRUVS) using traditional metrics? Journal of Experimental Marine Biology and Ecology. 2018;503:80–85. doi: 10.1016/J.JEMBE.2018.03.002. [DOI] [Google Scholar]
- Silva-Garay & Lowe (2021).Silva-Garay L, Lowe CG. Effects of temperature and body-mass on the standard metabolic rates of the round stingray, Urobatis halleri (Cooper, 1863) Journal of Experimental Marine Biology and Ecology. 2021;540:151564. doi: 10.1016/J.JEMBE.2021.151564. [DOI] [Google Scholar]
- SAAMBR (2022).South African Association for Marine Biological Research (SAAMBR) ORI Cooperative fish tagging project: tagging News 2021. 2022. https://issuu.com/saambr/docs/tagging-news-2021-final https://issuu.com/saambr/docs/tagging-news-2021-final
- Taylor, Sumpton & Ham (2011).Taylor S, Sumpton W, Ham T. Fine-scale spatial and seasonal partitioning among large sharks and other elasmobranchs in south-eastern Queensland, Australia. Marine and Freshwater Research. 2011;62:638–647. doi: 10.1071/MF10154. [DOI] [Google Scholar]
- Ter Braak & Smilauer (2012).Ter Braak CJF, Smilauer P. 2012. Canoco reference manual and user’s guide: software for ordination, version 5.1. Microcomputer Power. https://research.wur.nl/en/publications/canoco-reference-manual-and-users-guide-software-for-ordination-v
- Tilley & Strindberg (2013).Tilley A, Strindberg S. Population density estimation of southern stingrays Dasyatis americana on a Caribbean atoll using distance sampling. Aquatic Conservation: Marine and Freshwater Ecosystems. 2013;23(2):202–209. doi: 10.1002/AQC.2317. [DOI] [Google Scholar]
- Vaudo & Heithaus (2009).Vaudo JJ, Heithaus MR. Spatiotemporal variability in a sandflat elasmobranch fauna in Shark Bay, Australia. Marine Biology. 2009;156(12):2579–2590. doi: 10.1007/s00227-009-1282-2. [DOI] [Google Scholar]
- Vaudo & Heithaus (2012).Vaudo JJ, Heithaus MR. Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system. Marine and Freshwater Research. 2012;63(11):1077–1084. doi: 10.1071/mf11226. [DOI] [Google Scholar]
- Vianna et al. (2014).Vianna GMS, Meekan MG, Bornovski TH, Meeuwig JJ. Acoustic telemetry validates a citizen science approach for monitoring sharks on coral reefs. PLOS ONE. 2014;9(4):e95565. doi: 10.1371/JOURNAL.PONE.0095565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White et al. (2015).White TD, Carlisle AB, Kroodsma DA, Block BA, Casagrandi R, De Leo GA, Gatto M, Micheli F, McCauley DJ. Assessing the effectiveness of a large marine protected area for reef shark conservation. Biological Conservation. 2015;207:64–71. doi: 10.1016/j.biocon.2017.01.009. [DOI] [Google Scholar]
- White & Weigmann (2014).White WT, Weigmann S. Carcharhinus humani sp. nov., a new whaler shark (Carcharhiniformes: Carcharhinidae) from the western Indian Ocean. Zootaxa. 2014;3821(1):71–87. doi: 10.11646/ZOOTAXA.3821.1.5. [DOI] [PubMed] [Google Scholar]
- Yano et al. (2007).Yano K, Miya M, Aizawa M, Noichi T. Some aspects of the biology of the goblin shark, Mitsukurina owstoni, collected from the Tokyo Submarine Canyon and adjacent waters, Japan. Ichthyological Research. 2007;54(4):388–398. doi: 10.1007/S10228-007-0414-2. [DOI] [Google Scholar]
- Zelený (2022).Zelený D. 2022. [27 October 2022]. Ordination analysis. Analysis of community ecology data in R. https://www.davidzeleny.net/anadat-r/doku.php/en:ordination
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- SeaGIS 2022a. [27 October 2022]. CAL - Stereo Camera Calibration. https://www.seagis.com.au/bundle.html
- SeaGIS 2022b. [27 October 2022]. EventMeasure - Event logging & 3D measurement. https://www.seagis.com.au/event.html
- Ter Braak CJF, Smilauer P. 2012. Canoco reference manual and user’s guide: software for ordination, version 5.1. Microcomputer Power. https://research.wur.nl/en/publications/canoco-reference-manual-and-users-guide-software-for-ordination-v
- Zelený D. 2022. [27 October 2022]. Ordination analysis. Analysis of community ecology data in R. https://www.davidzeleny.net/anadat-r/doku.php/en:ordination
Supplementary Materials
Deployment details (including measurements of environmental variables), and the species MaxN associated with each sampling site, during winter and summer seasons.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.