Abstract
Purpose
Routinely collected healthcare data on the comparative effectiveness of the long-acting muscarinic antagonist/long-acting β2-agonist combination umeclidinium/vilanterol (UMEC/VI) versus tiotropium bromide/olodaterol (TIO/OLO) for chronic obstructive pulmonary disease (COPD) is limited. This study compared rescue medication prescriptions in patients with COPD in England receiving UMEC/VI versus TIO/OLO.
Patients and Methods
This retrospective cohort study used primary care data from the Clinical Practice Research Datalink Aurum database linked with secondary care administrative data from Hospital Episode Statistics. Patients with a COPD diagnosis at age ≥35 years were included (indexed) following initiation of single-inhaler UMEC/VI or TIO/OLO between July 1, 2015, and September 30, 2019. Outcomes included the number of rescue medication prescriptions at 12-months (primary), and at 6-, 18- and 24-months (secondary), adherence at 6-, 12-, 18- and 24-months post-index, defined as proportion of days covered ≥80% (secondary), and time-to-initiation of triple therapy (exploratory). Inverse probability of treatment weighting (IPTW) was used to balance potential confounding baseline characteristics. Superiority of UMEC/VI versus TIO/OLO for the primary outcome of rescue medication prescriptions was assessed using an intention-to-treat analysis with a p-value < 0.05.
Results
In total, 8603 patients were eligible (UMEC/VI: n = 6536; TIO/OLO: n = 2067). Following IPTW, covariates were well balanced across groups. Patients initiating UMEC/VI had statistically significantly fewer (mean [standard deviation]; p-value) rescue medication prescriptions versus TIO/OLO in both the unweighted (4.84 [4.78] vs 5.68 [5.00]; p < 0.001) and weighted comparison (4.91 [4.81] vs 5.48 [5.02]; p = 0.0032) at 12 months; consistent results were seen at all timepoints. Adherence was numerically higher for TIO/OLO versus UMEC/VI at all timepoints. Time-to-triple therapy was similar between treatment groups.
Conclusion
UMEC/VI was superior to TIO/OLO in reducing rescue medication prescriptions at 12 months after treatment initiation in a primary care cohort in England, potentially suggesting improvements in symptom control with UMEC/VI compared with TIO/OLO.
Keywords: COPD treatment, LABA/LAMA, primary care setting, rescue medication, treatment escalation
Plain Language Summary
People with chronic obstructive pulmonary disease (COPD) have symptoms of breathlessness, cough and phlegm. When symptoms get worse in the short term, a “rescue” medication inhaler may be needed. In the long term, more rescue medication prescriptions indicate poorer disease control. As such, treatments that lower the need for rescue medication may lead to better control of COPD and improved outcomes for patients.
In the long term, COPD symptoms are treated using therapies called bronchodilators, which may lower the need for rescue medicine. As there are several different bronchodilators available, comparing them may help doctors decide which one to prescribe to patients. Two options are umeclidinium/vilanterol (UMEC/VI) and tiotropium bromide/olodaterol (TIO/OLO). The primary goal of this study was to compare how many rescue medication prescriptions people with COPD in primary care in England had after starting treatment with these medicines.
Clinical Practice Research Datalink Aurum database healthcare records were used and linked to hospital records via Hospital Episode Statistics. Only patients with COPD at least 35 years of age starting treatment with UMEC/VI or TIO/OLO were included, of whom 6536 received UMEC/VI and 2067 received TIO/OLO. This study found people treated with UMEC/VI had significantly fewer prescriptions for rescue medication than people treated with TIO/OLO at 12 months after starting treatment. Consistent results were seen at 6-, 18- and 24-months. UMEC/VI was assessed to be a superior treatment to TIO/OLO at lowering rescue medication prescriptions, suggesting patients may have better improvements for COPD when starting treatment with UMEC/VI over TIO/OLO.
Introduction
Chronic obstructive pulmonary disease (COPD) has an estimated global prevalence of 10.3% affecting 391.1 million people aged 30–79 years, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) definition of COPD as a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) fixed ratio < 0.7.1 COPD is the third leading cause of mortality globally, responsible for 3.23 million deaths, and continues to be a leading cause of hospital visits.2–4 Common COPD symptoms including dyspnea, cough and sputum production may worsen during exacerbations.5 Consequently, COPD exacerbations increase the risk of hospitalizations and mortality,6–9 and may warrant a change in regular medication.5,10
For patients with persistent exacerbations or symptoms while receiving maintenance therapy with long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) monotherapy, the GOLD 2023 strategy report recommends stepping-up treatment to dual therapy (LAMA/LABA).5 Similarly, in the UK, the National Institute for Health and Care Excellence (NICE) guidelines recommend offering LAMA/LABA dual therapy for patients with dyspnea or exacerbations despite use of a short-acting bronchodilator.11 For patients who continue to experience exacerbations despite dual therapy or who have blood eosinophil counts ≥300 cells/µL, the GOLD strategy report recommends escalation to inhaled corticosteroid (ICS)/LAMA/LABA triple therapy.5 Accordingly, the NICE guidelines recommend escalation to triple therapy in patients who experience at least one severe or two moderate exacerbations in a year.11
For short-term treatment of exacerbations, both GOLD and NICE recommend rescue medication prescriptions with short-acting β2-agonists (SABA), short-acting muscarinic antagonists (SAMA) or SAMA/SABA combinations as the initial bronchodilator in all patients.5,11 In clinical trials, increasing rescue medication prescriptions has been associated with more frequent exacerbations and breathlessness, and worse lung function and health status.12–14 Consequently, rescue medication can be used as a surrogate marker for the effectiveness of maintenance therapy in COPD.14
Network meta-analyses have shown numerically reduced rescue medication prescriptions for single-inhaler LAMA/LABA combinations versus placebo,15 and significantly reduced rescue use with the LAMA/LABA combination umeclidinium/vilanterol (UMEC/VI) compared with aclidinium/formoterol (ACL/FOR).16 Additionally, a previous randomized clinical study and US-based retrospective study demonstrated a significant decrease in rescue medication prescriptions with the single-inhaler LAMA/LABA UMEC/VI compared with single-inhaler tiotropium bromide/olodaterol (TIO/OLO) in patients with COPD.17,18 However, to date, no comparative real-world effectiveness studies have been conducted in the UK comparing these treatments.
The objective of this study was to compare the real-world effectiveness of UMEC/VI versus TIO/OLO on rescue medication prescriptions, adherence and time-to-triple therapy among patients with COPD newly initiating UMEC/VI or TIO/OLO in a primary care setting in England.
Materials and Methods
Study Design
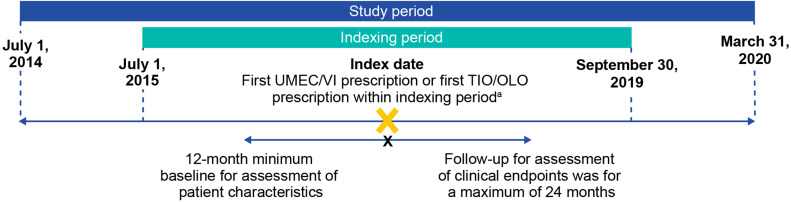
This was a new-user, retrospective, cohort study (GSK study 217815) using primary healthcare records that are collected directly from general practitioners (GPs) during consultations and stored as electronical medical record data within the Clinical Practice Research Datalink (CPRD) Aurum, as well as linked on a patient level to secondary care Hospital Episode Statistics (HES) data in England (July 1, 2014, to March 31, 2020; Figure 1). The index date was defined as the first single-inhaler UMEC/VI or TIO/OLO prescription during the indexing period (July 1, 2015, to September 30, 2019; study dates were chosen to exclude the COVID-19 pandemic as COPD patient management would likely differ during the pandemic and the change in healthcare service was not under study). The minimum baseline for assessment of patient characteristics was 12 months pre-index, and the follow-up period for assessment of clinical endpoints spanned from the index date to a maximum of 24 months post-index until the end date of the study (March 31, 2020), end of data availability (the earliest date the patient left the physician practice or last data collection date of the practice), or patient death.
Figure 1.
Study design. aThe first UMEC/VI or TIO/OLO prescription within the indexing period was defined as the index date. Only patients with no LAMA/LABA prescriptions prior to the index date were included in the analysis.
Abbreviations: LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OLO, olodaterol; TIO, tiotropium bromide; UMEC; umeclidinium; VI, vilanterol.
Study Population
Included patients had one or more primary care diagnostic codes for COPD at ≥35 years of age, ≥1 prescription of single-inhaler UMEC/VI or TIO/OLO within the indexing period, a trough FEV1/ FVC ratio < 0.7 at any time prior to the index date (inclusive), ≥12 months of continuous registration with a GP pre-index and data eligible for linkage to HES. Patients were excluded if they had a diagnosis for medical conditions that can interfere with a clinical COPD diagnosis or change the natural history of the disease, for example, bronchial developmental anomalies, degenerative pulmonary diseases, such as cystic fibrosis, pulmonary fibrosis, or pulmonary resection, at any time prior to and including index date. Patients were also excluded if they had a prescription for both UMEC/VI and TIO/OLO, or concomitant ICS use at index (two ICS prescriptions with ≤30 days gap between end of last supply date to subsequent new supply, overlapping with index therapy by ≥1 day and which started before or up to 30 days post-index date), or ≥1 prescription of any single-inhaler or open combinations of LAMA/LABA, or ICS- or LABA-containing medication prior to index. Patients were classified by indexed therapy (UMEC/VI or TIO/OLO).
Outcomes
Outcomes were assessed for up to a maximum of 24 months during the follow-up period.
The primary outcome was the mean number of rescue medication prescriptions in the 12 months following index, defined as the number of prescriptions of inhaled or nebulized SABA- or SAMA-containing medication excluding those prescribed on the index date. Secondary outcomes were the mean number of rescue medication prescriptions in the 6-, 18-, and 24-months following index date, and medication adherence at 6-, 12-, 18- and 24-months after index date. Adherence was defined as proportion of days covered (PDC) ≥80%, which is based on the threshold typically accepted in the literature.19 PDC represented the proportion of time over the course of the patients’ treatment that they theoretically were in possession of the medication; PDC was calculated by dividing the days covered by a fixed time interval: PDC= (number of covered days in period/number of days in period) x 100. Patients were considered “covered” for any given day in which they had a valid prescription for the relevant dual therapy. Any days with concomitant ICS use (triple therapy) were not included in the numerator for PDC. The exploratory outcome was time-to-triple therapy initiation, administered via multiple or single inhalers. Multiple-inhaler triple therapy initiation was defined as ≥1 day overlap in the supply of all three triple therapy (ICS, LAMA, LABA) components, with patients considered continuous users if they had two prescriptions with ≤30 days gap between the end of last supply date to subsequent new supply.
Statistical Analysis
The superiority of UMEC/VI versus TIO/OLO in reducing the mean number of rescue medication prescriptions at 12 months post-index was defined with a margin of 0. If superiority was not met, non-inferiority in the primary outcome was assessed and defined with a margin of 0.3, based on clinical relevance and the findings of a post hoc analysis of UMEC/VI versus TIO/OLO.20 Therefore, the UMEC/VI cohort could have at most 0.30 more prescriptions of rescue medication on average compared with the TIO/OLO cohort in order to meet the criteria of non-inferiority. For superiority, a sample size of 908–1612 and 227–403 patients receiving UMEC/VI and TIO/OLO (4:1 ratio), respectively, was determined to provide 80% power (two-sided alpha: 0.05) to detect a mean difference in rescue medication prescription; for inferiority, sample sizes were 424–648 and 106–162 for 80% power (one-sided alpha: 0.05) to determine a mean difference in rescue medication prescription.
To minimize potential confounding due to differences in baseline demographics and clinical characteristics when determining treatment in the primary outcome, a propensity score (PS)-based methodology was implemented. Covariates were selected based on background knowledge of the association of pre-treatment variables on the outcomes of interest. Where the causal influence of any pre-treatment variable was not fully known and to avoid over-parameterizing the model, the strength of any association between such variables and the given endpoint to be conducted were explored using univariable logistic regressions to obtain the estimated coefficient, the estimated standard error and the likelihood ratio test for the significance of the coefficient. Only variables whose regression showed a p-value < 0.1 were selected for inclusion in the PS model. Covariates included in the PS model were age, sex, body mass index (BMI), socioeconomic status, practice region, smoking status, and baseline variables including %predicted FEV1, modified Medical Research Council Grade, current asthma diagnosis, comorbidities, exacerbations of COPD, respiratory treatment, number of respiratory classes, COPD-related healthcare resource utilization and COPD-related costs. Following PS modeling, inverse probability of treatment weighting (IPTW) was used, whereby weights were derived from the PS to create a pseudo-population in which the distribution of covariates in the population is independent of treatment assignment. This allowed estimation of the average treatment effect (ATE) in the entire population by accounting for the effects of the covariates on the results. A standardized mean difference (SMD) >10 in a covariate was deemed to represent insufficient balance.
For the secondary outcome of proportion of adherent patients (PDC ≥ 80%) at 6-, 12-, 18- and 24-months following the index date, results were compared between unweighted and weighted treatment cohorts, with and without stockpiling (early ordering of prescriptions for later use), using IPTW. Following this, a post hoc analysis to describe rescue medication prescription stratified by adherence at 12-months post-index and to describe demographics and baseline characteristics stratified by adherence was performed to facilitate the interpretation of the results.
For the primary outcome, as not to increase the probability of a type 1 error, an intention-to-treat (ITT) analysis was conducted where patients were classified into treatment groups at index and remained in their indexed treatment group for the entire follow-up period (maximum 24 months) and were not censored for any reason other than a switch to the comparator treatment. An on-treatment sensitivity analysis was also conducted where patients were classified into treatment groups at index and censored at the time of the first prescription for any non-indexed, long-acting, open or single device maintenance medication, or discontinuation of their index therapy. Only an on-treatment analysis was done to assess adherence. Time-to-triple therapy was determined using a Kaplan–Meier survival analysis. When reporting outcomes, results based on small numbers of patients (n < 5) were suppressed to protect patient confidentiality, as well as related values to protect primary suppression.
Results
Study Population and Baseline Characteristics
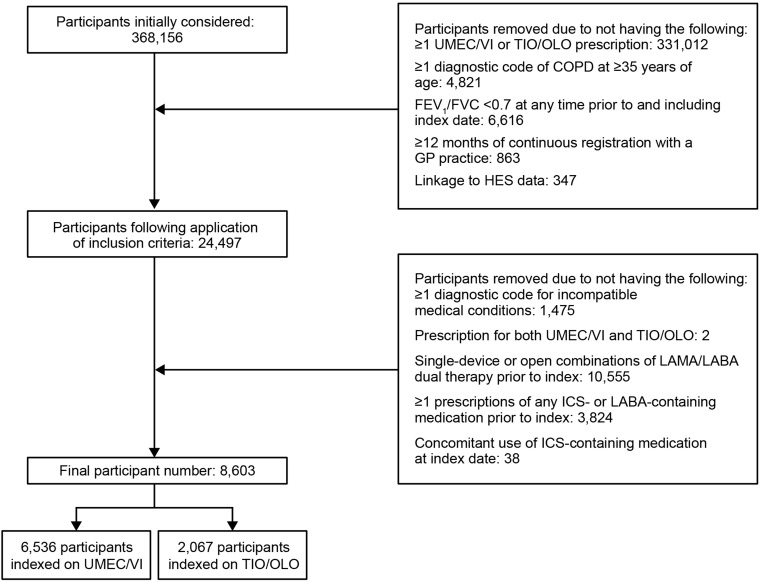
Of the 368,156 patients identified, 8603 patients met the study eligibility criteria and were included in the study (UMEC/VI: n = 6536; TIO/OLO: n = 2067) (Figure 2).
Figure 2.
Sample attrition.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced volume capacity; GP, general practitioner; HES, Health Episode Statistics; ICS, inhaled corticosteroid; IND, indacaterol; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OLO, olodaterol; TIO, tiotropium bromide; UMEC, umeclidinium; VI, vilanterol.
Patient demographics and baseline characteristics were generally similar between the two treatment groups including the severity of COPD and prevalence of comorbidities (Table 1). Mean (standard deviation [SD]) age was 69.5 (10.6) and 70.0 (10.3) years and 43.7% and 47.1% of patients were female in the UMEC/VI and TIO/OLO groups, respectively. In the UMEC/VI and TIO/OLO groups, 59.9% and 60.7% of patients had GOLD grade 2 (FEV1% predicted ≥50– < 80%); 22.8% and 21.5% GOLD grade 3 (FEV1% predicted ≥30– < 50%); 42.1% and 40.9% GOLD group A; and 37.0% and 36.7% GOLD group B. The proportion of patients receiving UMEC/VI with GOLD D and 4–5 group MRC dyspnea scale was 10.2% and 12.6%, respectively. For patients receiving TIO/OLO, corresponding proportions were 12.4% and 14.7%, respectively. Some variations were noted in prescribing patterns between regions, for example, 1.9% of patients indexed on UMEC/VI were from the North East compared with 10.1% of patients indexed on TIO/OLO. Additionally, a lower proportion of patients indexed on UMEC/VI versus TIO/OLO were prescribed a SABA (79.9% vs 85.4%) or a LAMA (56.1% vs 60.0%).
Table 1.
Patient Demographics and Baseline Characteristics
| Characteristic | UMEC/VI (n=6536) | TIO/OLO (n=2067) |
|---|---|---|
| Age, years, mean (SD)a | 69.5 (10.6) | 70.0 (10.3) |
| Female, n (%) | 2857 (43.7) | 973 (47.1) |
| BMI, mean (SD)a,b | 27.2 (6.2) | 27.3 (6.4) |
| Patient region, n (%) | ||
| East Midlands | 72 (1.1) | 20 (1.0) |
| East of England | 172 (2.7) | 52 (2.5) |
| London | 493 (7.6) | 103 (5.0) |
| North East | 124 (1.9) | 207 (10.1) |
| North West | 1480 (22.8) | 542 (26.4) |
| South Central | 718 (11.1) | 460 (22.4) |
| South East Coast | 423 (6.5) | 126 (6.1) |
| South West | 1323 (20.4) | 333 (16.2) |
| West Midlands | 1075 (16.6) | 103 (5.0) |
| Yorkshire and the Humber | 601 (9.3) | 109 (5.3) |
| FEV1% predicted in baseline period, n (%)c | ||
| GOLD grade 1: FEV1% predicted ≥80% | 747 (14.7) | 240 (14.5) |
| GOLD grade 2: FEV1% predicted ≥50– < 80% | 3039 (59.9) | 1002 (60.7) |
| GOLD grade 3: FEV1% predicted ≥30– < 50% | 1156 (22.8) | 355 (21.5) |
| GOLD grade 4: FEV1% predicted < 30% | 135 (2.7) | 54 (3.3) |
| GOLD 2019 group, n (%)d | ||
| A | 2555 (42.1) | 786 (40.9) |
| B | 2249 (37.0) | 705 (36.7) |
| C | 652 (10.7) | 194 (10.1) |
| D | 618 (10.2) | 238 (12.4) |
| MRC Dyspnea Scale score, n (%)e | ||
| 1f | 611 (9.3) | 215 (10.4) |
| 2 | 2596 (39.7) | 765 (37.0) |
| 3 | 2043 (31.3) | 640 (31.0) |
| 4 | 734 (11.2) | 266 (12.9) |
| 5f | 90 (1.4) | 37 (1.8) |
| Unknown | 462 (7.1) | 144 (7.0) |
| Smoking status, n (%)a | ||
| Current smoker | 3690 (56.5) | 1160 (56.1) |
| Former smoker | 2721 (41.6) | 854 (41.3) |
| Non-smoker | 117 (1.8) | NRg |
| Unknown | 8 (0.1) | NRg |
| Comorbidities, n (%)h | ||
| Rheumatoid/osteoarthritis | 2211 (33.8) | 734 (35.5) |
| Depression | 2100 (32.1) | 682 (33.0) |
| Diabetes | 1383 (21.2) | 431 (20.9) |
| Anxiety | 1326 (20.3) | 396 (19.2) |
| Gastroesophageal reflux disease | 1222 (18.7) | 393 (19.0) |
| Stroke | 739 (11.3) | 234 (11.3) |
| Acute myocardial infarction | 716 (11.0) | 244 (11.8) |
| Congestive heart failure | 629 (9.6) | 193 (9.3) |
| Dementia/cognitive impairment | 487 (7.5) | 192 (9.3) |
| Bronchiectasis | 176 (2.7) | 65 (3.1) |
| Lung cancer | 62 (0.9) | 19 (0.9) |
| Current asthma diagnosis, n (%)i | 518 (8.1) | 194 (9.6) |
| Historical asthma diagnosis, n (%)j | 1106 (17.2) | 356 (17.5) |
| Moderate-to-severe exacerbations, n (%)k | 2014 (30.8) | 668 (32.3) |
| Respiratory therapies received in baseline period, n (%) | ||
| No use | 702 (10.7) | 141 (6.8) |
| Any use | 5834 (89.3) | 1926 (93.2) |
| LAMAl | 3669 (56.1) | 1240 (60.0) |
| SABA (not overlapping SAMA)l | 5220 (79.9) | 1765 (85.4) |
| SAMA (not overlapping SABA)l | 123 (1.9) | 29 (1.4) |
| SAMA/SABA (FDC and open)l | 158 (2.4) | NRg |
| Xanthinel | 14 (0.2) | NRg |
Notes: aAt index; bUMEC/VI: n=6438; TIO/OLO: n=2036; cUMEC/VI: n=5077; TIO/OLO: n=1651; dA composite measure of COPD severity using AECOPD events in the prior 12 months and MRC grade in the prior 24 months; eMost recent score in the 24 months prior to index; f1Not troubled by breathlessness except on strenuous exercise; f5Too breathless to leave the house; gResults based on small numbers of patients (n < 5) were suppressed, as well as the next-smallest value to protect primary suppression; hAt any time in the patients history; iAt any time in the 24 months pre-index (inclusive); j≥24 months pre-index; kBased on a validated algorithm, defined as the presence of one of the following events: i. Antibiotic and OCS prescriptions for 5–14 days with the same start date; ii. ≥2 symptoms of breathlessness, cough, sputum volume or purulence and an antibiotic or OCS prescription on the same day; iii. Lower respiratory tract infection code; iv. Exacerbation specific medical code; lPercentages calculated for the full cohort.
Abbreviations: AECOPD, acute exacerbations of COPD; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LAMA, long-acting muscarinic antagonist; MRC, Medical Research Council; NR, not reported; OCS, oral corticosteroid; OLO, olodaterol; SABA, short-acting β2-agonist; SD, standard deviation; SAMA, short-acting muscarinic antagonist; TIO, tiotropium bromide; UMEC; umeclidinium; VI, vilanterol.
Rescue Medication Prescriptions Over 12 Months
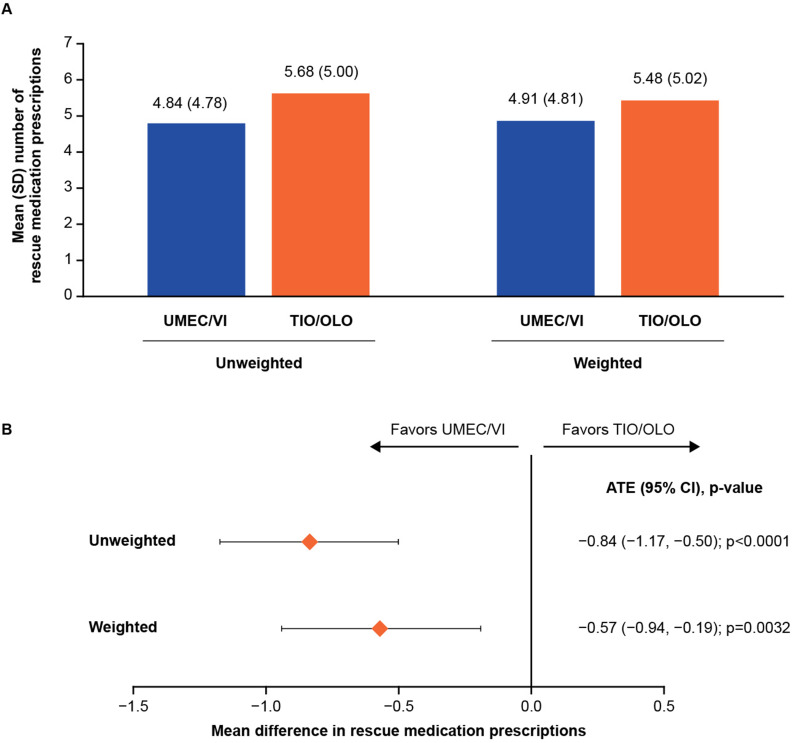
Following PS and IPTW application, model balance was satisfactory at the 12-month timepoint, with none of the covariates showing any imbalance (ie, SMD < 10%) (Supplementary Table 1). Patients indexed on UMEC/VI versus TIO/OLO had a statistically significantly lower mean number of rescue medication prescriptions in the 12 months following treatment initiation, for both unweighted (4.84 vs 5.68) and weighted (4.91 vs 5.48) estimates (Figure 3A). In the ITT analysis, the difference observed for mean number of rescue medication prescriptions in both the unweighted and weighted ATE values were statistically significant and less than 0 (–0.84 [p < 0.0001] and –0.57 [p = 0.0032], respectively) (Figure 3B), favoring UMEC/VI versus TIO/OLO; therefore, superiority was demonstrated. In the on-treatment sensitivity analysis, the weighted mean difference (–0.26) indicated fewer mean rescue medication prescriptions among those indexed on UMEC/VI versus TIO/OLO in the 12 months following treatment initiation; the difference between the two groups was not statistically significant (p = 0.1393) (Supplementary Table 2).
Figure 3.
(A) Mean number of rescue medication prescriptions between patients newly initiating UMEC/VI versus TIO/OLO in the 12 months following treatment initiation and (B) treatment differences for rescue medication prescriptions outcome in the ITT analysis.
Abbreviations: ATE, average treatment effect; CI, confidence interval; ITT, intention-to-treat; OLO, olodaterol; SD, standard deviation; TIO, tiotropium bromide; UMEC; umeclidinium; VI, vilanterol.
Rescue Medication Prescriptions Over 6-, 18-, and 24-Months
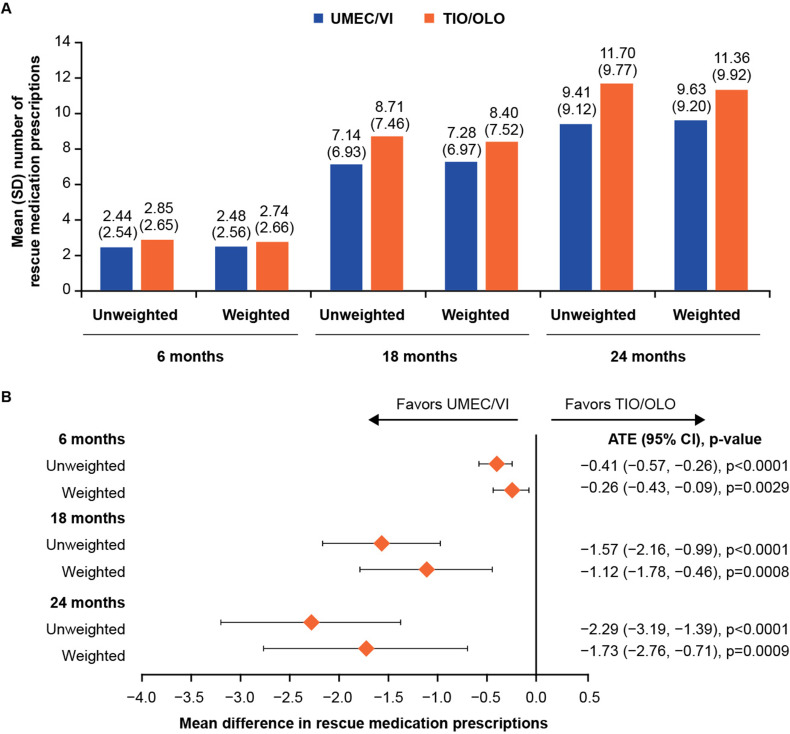
Model balance was satisfactory at the 6-, 18- and 24-month timepoints, with only the 24-month timepoint demonstrating insufficient balance in one covariate, practice region, after model weighting (Supplementary Table 1). The mean number of rescue medication prescriptions was lower in the UMEC/VI than in the TIO/OLO group, in unweighted and weighted analyses and across all timepoints (Figure 4A). In the ITT analysis, all unweighted and weighted ATE values were statistically significantly different between treatments and less than 0 (range: –0.41, –2.29, and –0.26, –1.73, respectively), favoring UMEC/VI over TIO/OLO (Figure 4B). On-treatment sensitivity analyses were consistent with the ITT analysis, although the weighted ATE value was not significantly different between treatments at the 6-month timepoint (Supplementary Table 2).
Figure 4.
(A) Mean number of rescue medication prescriptions between patients newly initiating UMEC/VI versus TIO/OLO in the 6-, 18- and 24- months following treatment initiation and (B) Treatment differences for rescue medication prescriptions outcome in the ITT analysis.
Abbreviations: ATE, average treatment effect; CI, confidence interval; ITT, intention-to-treat; OLO, olodaterol; TIO, tiotropium bromide; UMEC; umeclidinium; VI, vilanterol.
Adherence
Model balance was insufficient in one covariate at the 6- and 24-month timepoints but sufficient at the 12- and 18-month timepoints (Supplementary Table 1). Patient demographics and baseline characteristics were generally similar between the two treatment groups when stratified by level of adherence (PDC < 80%, PDC ≥ 80%) (Supplementary Table 3). Across all timepoints, the proportion of patients adherent to UMEC/VI was consistently lower than that for TIO/OLO in both unweighted and weighted analyses without (Table 2) and with stockpiling (Supplementary Table 4). When stratified by adherence, the mean number of rescue medication prescriptions was higher for adherent patients in both UMEC/VI and TIO/OLO treatment groups at 12 months, regardless of weighting (Table 3).
Table 2.
Adherent Patients (PDC ≥80%) at 6-, 12-, 18- and 24-Months Post-Index Without Stockpiling
| Patients with PDC ≥80% (%) | Unweighted | Weighted | ||
|---|---|---|---|---|
| UMEC/VI | TIO/OLO | UMEC/VI | TIO/OLO | |
| 6 months | 37.8 | 41.3 | 38.0 | 41.6 |
| 12 months | 31.2 | 35.9 | 31.5 | 36.7 |
| 18 months | 27.1 | 32.8 | 27.5 | 32.5 |
| 24 months | 25.0 | 31.1 | 25.5 | 30.9 |
Abbreviations: OLO, olodaterol; PDC, proportion of days covered; TIO, tiotropium bromide; UMEC, umeclidinium; VI, vilanterol.
Table 3.
Mean Number of Rescue Medication Prescriptions for Patients Newly Initiating UMEC/VI versus TIO/OLO in the 12 Months Following Treatment Initiation, Stratified by Adherence (PDC < 80%, PDC ≥ 80%), Without Stockpiling
| 12 Months Post-Index | Mean Number of Rescue Medication Prescriptions in Adherent Patients (PDC ≥80%) | Mean Number of Rescue Medication Prescriptions in Non-Adherent Patients (PDC < 80%) | ||
|---|---|---|---|---|
| ITT | On-Treatment | ITT | On-Treatment | |
| Unweighted, mean (SD) | ||||
| UMEC/VI | 6.7 (5.39) | 6.1 (5.39) | 4.0 (4.24) | 2.6 (3.27) |
| TIO/OLO | 7.4 (5.33) | 6.6 (5.42) | 4.7 (4.53) | 2.9 (3.63) |
| Weighted, mean (SD) | ||||
| UMEC/VI | 6.7 (5.38) | 6.2 (5.40) | 4.1 (4.28) | 2.6 (3.28) |
| TIO/OLO | 7.1 (5.37) | 6.3 (5.45) | 4.5 (4.55) | 2.7 (3.46) |
Abbreviations: ITT, intention-to-treat; OLO, olodaterol; PDC, proportion of days covered; TIO, tiotropium bromide; UMEC, umeclidinium; VI, vilanterol.
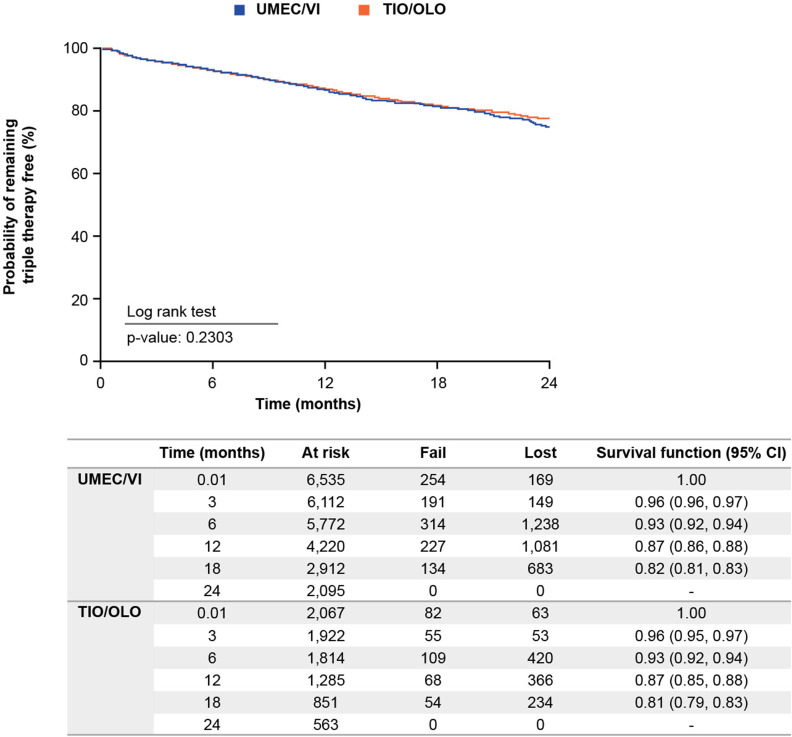
Time-to-Triple Therapy
Initiation of triple therapy occurred at a steady and relatively unchanging rate from index until 24 months in both treatment groups; median was not reached for either group (Figure 5). There was no statistical difference between groups (log rank test, p = 0.2303).
Figure 5.
Kaplan–Meier survival analysis for time to initiation of triple.
Abbreviations: CI, confidence interval; OLO, olodaterol; TIO, tiotropium bromide; UMEC; umeclidinium; VI, vilanterol.
Discussion
This is the first study to demonstrate the superiority of UMEC/VI over TIO/OLO in reducing rescue medication prescriptions over 12 months from treatment initiation in a primary care cohort in England. Additionally, statistical reductions in rescue medication prescriptions were seen from 6 months post-UMEC/VI initiation compared with TIO/OLO and this reduction persisted until the final study timepoint, at 24 months. Given the association between rescue medication prescriptions and clinical outcomes,12–14 these results suggest that patients initiating treatment with UMEC/VI may achieve better symptom control over patients initiating treatment with TIO/OLO.
The number of rescue medication prescriptions at 12 months with UMEC/VI was reduced by 0.84 (17.4%) in the unweighted comparison and 0.57 (11.6%) in the weighted comparison versus TIO/OLO. This reduction in rescue medication prescriptions is greater than in a previous US-based, real-world retrospective study comparing UMEC/VI with TIO/OLO, which found a 0.16 reduction in favor of UMEC/VI at 12 months.18 Our study’s long-term findings of control over 24 months are also further to a previous short-term randomized crossover study, which found that patients receiving UMEC/VI used significantly less rescue medication compared with those receiving TIO/OLO over 8 weeks (a difference of –0.25 puffs per day). In a recent network meta-analysis including 49 randomized controlled trials, UMEC/VI significantly reduced rescue medication prescriptions by –0.25 puffs compared with TIO/OLO at 12 weeks; and versus ACL/FOR 400/12 µg (with a difference of –0.46) at 24 weeks.16 Therefore, collectively across three different study designs, including randomized crossover, network meta-analysis and real-world evidence, evidence suggests that patients receiving UMEC/VI require lower rescue medication prescriptions, compared with TIO/OLO.
Improvements in rescue medication prescriptions across all timepoints may indicate that UMEC/VI is more effective than TIO/OLO at long-term symptom control, in particular reducing dyspnea. As rescue medication prescription is associated with clinical outcomes including exacerbation frequency, breathlessness, worse lung function and health status,12–14 improved symptom control with UMEC/VI may potentially lead to greater improvements in the burden of disease over TIO/OLO. Indeed, a previous study found that greater symptom improvements with UMEC/VI over TIO/OLO were accompanied by superior improvement in trough FEV1,20 and patients demonstrated twice the odds of achieving a clinically important improvement with UMEC/VI versus TIO/OLO.17 Whilst in this study, patients who were prescribed TIO/OLO appeared to be slightly more severe at baseline than those prescribed UMEC/VI, as indicated by the proportion of patients with GOLD D and 4–5 group MRC dyspnea scale, a PS and IPTW was done to account for the differences observed between groups and to allow a proper comparison.
Beyond comparing dual bronchodilators, in a previous multicenter, prospective study, patients with improvements in health-related quality of life had significantly fewer symptoms including dyspnea, coughing and sputum compared with patients with a deterioration in health-related quality of life.21 Fewer symptoms may also improve the ability of patients to participate in normal activities throughout the day and physical activity.22 Morning symptoms in particular are reported by patients to be a key barrier to performing daily activities.23,24 Further investigation is required to determine the benefits of UMEC/VI versus TIO/OLO beyond decreased rescue therapy prescriptions.
Our results suggest that patients taking once-daily UMEC/VI were less adherent than those taking once-daily TIO/OLO after 12 months of treatment. Additionally, post hoc sensitivity analyses showed mean rescue medication prescriptions were higher among patients who were adherent (PDC ≥ 80%) in both treatment groups. These findings suggest that patients who experience more symptoms, such as breathlessness, and therefore require increased number of prescriptions for rescue medication, may be more adherent and similarly, those who are feeling better may be less adherent to the medication. This finding however differs to a previous US claims study that found a significantly higher proportion of patients achieved PDC ≥80% among patients initiating UMEC/VI (28.6%) compared with TIO/OLO (22.7%; p < 0.001).18 Potential reasons for this discrepancy could be the location, as the population in the US may be different in terms of adherence to those in the UK, but also differences in the databases, or in the definitions employed, which may affect the results. Furthermore, it should be noted that while more patients in the TIO/OLO group received a greater number of prescriptions for rescue medication, this does not necessarily correlate with the use of these medications.
Time-to-triple therapy was similar between treatments, with approximately 18% of patients switching over 18 months. This would be expected given both comparator treatments are LAMA/LABA combinations, and step-up to triple therapy is recommended by both GOLD and NICE in patients who continue to experience exacerbations while receiving any treatment from the LAMA/LABA combination class of drugs.5,11
This study has several strengths and limitations, which should be considered when interpreting the results. One strength was the use of an extensive and robust process to identify suitable covariates for inclusion into the PS models. This ensured that covariate balance in all models was sufficient for effective comparison of treatment groups and confounding limited, as supported by the finding that across all objectives, PS models achieved high or satisfactory balance across all covariates once weighting had been applied. Despite this, the impact of unseen confounding cannot be ruled out, although most relevant variables are captured within the CPRD, limiting the potential for this impact. A second strength is that electronic medical databases such as the CPRD-Aurum represent an observation of effects in the real world rather than under highly controlled conditions. Study limitations include the use of rescue medication prescription as a proxy for short-term worsening of symptoms, as the frequency of rescue medication prescriptions may not directly correlate with worsened symptoms. Prescriptions may not be dispensed and/or not consumed as prescribed, particularly if patients stockpile rescue medicine in advance of when it is required; however, we do not anticipate that this would be differential between UMEC/VI and TIO/OLO. This is also a limitation in terms of the classification of patients into an index treatment group, which may result in an underestimation of treatment effects if patients switched therapies during follow-up. A second limitation is that patients were excluded if they had received ICS- or LABA-containing medications in the 12 months pre-index. However, as a recent study found that only approximately 10% of patients received one or more ICS- or LABA-containing medications in the 12 months prior to initiating TIO/OLO or UMEC/VI, the impact of this eligibility criteria on result generalizability is likely minimal.25 Additionally, the generalizability of the study results to the wider UK population could be impacted by CPRD-Aurum data only covering 15% of UK practices and HES linkage reducing the patient sample to only those registered at a GP practice in England. However, as the sample population is considered highly representative of the whole UK population,26 the results can be considered generalizable. Finally, these findings may not be broadly generalizable to countries which have a significantly different healthcare system to England, due to global variety in the structure of healthcare systems. However, our findings for rescue medication prescriptions are consistent with a US-based retrospective study,18 which suggests these data may be generalizable across different settings.
Conclusion
Superiority of UMEC/VI was demonstrated over TIO/OLO with respect to mean rescue medication prescriptions over 12 months among patients with COPD newly initiating LAMA/LABA therapy in primary care in England. The mean number of rescue medication prescriptions was also lower in the UMEC/VI than TIO/OLO group at 6-, 18-, and 24-months following treatment initiation. However, adherence was lower for UMEC/VI versus TIO/OLO. Further investigation is required as to whether reduced rescue medication prescriptions may be associated with other clinical improvements for UMEC/VI versus TIO/OLO.
Acknowledgments
Editorial support (in the form of writing assistance including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Alexandra Berry, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK. An abstract based on this study was previously presented as a poster presentation at the ERS 2022 Congress, 4–6 September 2022, Barcelona, Spain.
Funding Statement
This study was funded by GSK (GSK study 217815) and approved by Clinical Practice Research Datalink (CPRD) Research Data Governance process. GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report and GSK funded the article processing charges and open access fee.
Abbreviations
AECOPD, acute exacerbations of COPD; ACL, aclidinium; ATE, average treatment effect; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FOR, formoterol; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HES, Hospital Episode Statistics; ICS, inhaled corticosteroid; IPTW, inverse probability of treatment weighting; ITT, intention-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MRC, Medical Research Council; NICE, National Institute for Health and Care Excellence; NR, not reported; OLO, olodaterol; PDC, proportion of days covered; PS, propensity score; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation; SMD, standardized mean difference; TIO, tiotropium bromide; UMEC, umeclidinium; VI, vilanterol.
Data Sharing Statement
This study is based in part on data from the CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Data from HES Copyright© (2022), re-used with the permission of the Health and Social Care Information Centre. All rights reserved. Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to each database can be requested via the respective websites (EMIS Web® electronic patient record system and CPRD-Aurum).
Ethics Approval and Informed Consent
Approval of this study was provided by the GSK Protocol Review Committee and by the Clinical Practice Research Datalink (CPRD) Research Data Governance, which reviewed the protocol and approved access to CPRD data (protocol no. 21_000618). This study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. Personal identifiers, and personal identifiable information was removed by the database provider prior to receipt by the study team. Study results are in tabular form and aggregate analyses that omits subject identification, therefore informed consent, ethics committee or institutional review board approval were not required. This study was designed, implemented, and reported in accordance with the 2008 Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology, the Strengthening the Reporting of Observational Studies in Epidemiology guidelines, and the ethical principles laid down in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
GR, AC, CC, KJR, and ASI are employees of GSK and hold stock and shares at GSK. ASI also holds an unpaid faculty position at McMaster University. FH is an employee of the Translational Lung Research Center Heidelberg, part of the Germany lung research Foundation (DZL). JKQ holds a position at Imperial College London. CMC, TT, RWo, and RWi are employees of Adelphi Real World. VB is currently an employee of Bayer AG UK, and holds stock and shares in Bayer AG UK. VB and JY were employees of Adelphi Real World at the time of the study. Adelphi Real World is a business that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations which received funding from GSK to conduct the study. Adelphi Real World employees work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. The authors report no other conflicts of interest in this work.
References
- 1.Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/s2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The top 10 causes of death; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed September 9, 2022.
- 3.Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679. doi: 10.1136/bmj-2021-069679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–2012 and nationwide emergency department sample 2006–2011. Chest. 2015;147(4):989–998. doi: 10.1378/chest.14-2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease; 2022. Available from: https://goldcopd.org/archived-reports/. Accessed September 9, 2022.
- 6.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çolak Y, Afzal S, Marott JL, et al. Prognosis of COPD depends on severity of exacerbation history: a population-based analysis. Respir Med. 2019;155:141–147. doi: 10.1016/j.rmed.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105. doi: 10.1136/thorax.58.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson G, Mushnikov V, Bäckström T, et al. Exacerbations and healthcare resource utilization among COPD patients in a Swedish registry-based nation-wide study. BMC Pulm Med. 2018;18(1):17. doi: 10.1186/s12890-018-0573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2018. Available from: https://www.nice.org.uk/guidance/ng115. Accessed June 16, 2022. [PubMed]
- 12.Jenkins CR, Postma DS, Anzueto AR, et al. Reliever salbutamol use as a measure of exacerbation risk in chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:97. doi: 10.1186/s12890-015-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Anderson JA, Calverley PM, et al. Health status in the TORCH study of COPD: treatment efficacy and other determinants of change. Respir Res. 2011;12(1):71. doi: 10.1186/1465-9921-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punekar YS, Sharma S, Pahwa A, Takyar J, Naya I, Jones PW. Rescue medication use as a patient-reported outcome in COPD: a systematic review and regression analysis. Respir Res. 2017;18(1):86. doi: 10.1186/s12931-017-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sion KYJ, Huisman EL, Punekar YS, Naya I, Ismaila AS. A network meta-analysis of Long-Acting Muscarinic Antagonist (LAMA) and Long-Acting β2-Agonist (LABA) combinations in COPD. Pulm Ther. 2017;3(2):297–316. doi: 10.1007/s41030-017-0048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismaila AS, Haeussler K, Czira A, et al. Comparative efficacy of umeclidinium/vilanterol versus other bronchodilators for the treatment of chronic obstructive pulmonary disease: a network meta-analysis. Adv Ther. 2022. doi: 10.1007/s12325-022-02234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34(11):2518–2533. doi: 10.1007/s12325-017-0626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moretz C, Bengtson LG, Sharpsten L, et al. Evaluation of rescue medication use and medication adherence receiving umeclidinium/vilanterol versus tiotropium bromide/olodaterol. Int J Chron Obstruct Pulmon Dis. 2019;14:2047–2060. doi: 10.2147/copd.S213520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moretz C, Cole AL, Mu G, et al. Evaluation of medication adherence and rescue medication use in non-exacerbating patients with COPD receiving umeclidinium/vilanterol or budesonide/formoterol as initial maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2020;15:2207–2215. doi: 10.2147/copd.s259850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcázar Navarrete B, Boucot I, Naya I, et al. Umeclidinium/vilanterol versus tiotropium/olodaterol in maintenance-naïve patients with moderate symptomatic chronic obstructive pulmonary disease: a post hoc analysis. Pulm Ther. 2018;4(2):171–183. doi: 10.1007/s41030-018-0057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteagudo M, Rodríguez-Blanco T, Llagostera M, et al. Factors associated with changes in quality of life of COPD patients: a prospective study in primary care. Respir Med. 2013;107(10):1589–1597. doi: 10.1016/j.rmed.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 22.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi: 10.1186/s12931-017-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi: 10.1183/09031936.00051110 [DOI] [PubMed] [Google Scholar]
- 24.O’Hagan P, Chavannes NH. The impact of morning symptoms on daily activities in chronic obstructive pulmonary disease. Curr Med Res Opin. 2014;30(2):301–314. doi: 10.1185/03007995.2013.857648 [DOI] [PubMed] [Google Scholar]
- 25.Czira A, Banks V, Requena G, et al. Characteristics and treatment pathways of patients with COPD receiving an inhaled corticosteroid/long-acting ß2-agonist (ICS/LABA) in the UK. Eur Respir J. 2021;58(suppl 65):PA2411. doi: 10.1183/13993003.congress-2021.PA2411 [DOI] [Google Scholar]
- 26.Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740–1740g. doi: 10.1093/ije/dyz034 [DOI] [PMC free article] [PubMed] [Google Scholar]