Abstract
Background and Objectives
Spinal cord infarction (SCInf) is a rare condition where consensus regarding diagnostic criteria is lacking, and misdiagnosis or delayed diagnosis can be detrimental. The aim of this study was to describe baseline findings and predictors of long-term functional outcome in a population-based cohort of patients with SCInf.
Methods
All adult patients (aged 18 years or older) treated at the spinal cord injury unit of the study center, between 2006 and 2019, and discharged with a G95 diagnosis (other and unspecified disease of the spinal cord) were screened for inclusion. The diagnostic criteria proposed by Zalewski et al. were retrospectively applied to evaluate the certainty of the SCInf diagnosis.
Results
A total of 270 patients were screened and 57 were included in the study, of whom 30 had a spontaneous SCInf and 27 had a periprocedural SCInf. The median American Spinal Cord Injury Association Impairment Scale (AIS) on admission was C, which at a median follow-up of 2.1 years had improved to D (p = 0.002). Compared with periprocedural cases, those with spontaneous SCInf showed significantly better admission AIS (median AIS D vs B, p < 0.001), fewer multilevel SCInf (27% vs 59%, p = 0.029), shorter hospital stay (median 22 vs 44 days, p < 0.001), and better AIS (median AIS D vs C, p < 0.001) and ambulatory status on long-term follow-up (66% vs 1%, p < 0.001). Regression analyses revealed that spontaneous SCInfs (odds ratio [OR] 5.91 [1.92–18.1], p = 0.002) and more favorable admission AIS (OR 33.6 [7.72–146], p < 0.001) were significant predictors of more favorable AIS at follow-up, with admission AIS demonstrating independent predictive ability (OR 35.9 [8.05–160], p < 0.001).
Discussion
SCInf is a rare neurologic emergency lacking specific management guidelines. While the presumptive diagnosis is based on the typical presentation and clinical findings, T2-weighted and diffusion-weighted MRI were the most useful diagnostic tools in establishing a definitive diagnosis. Our data show that spontaneous SCInf mostly affected a single spinal cord segment, whereas periprocedural cases were more extensive, had poorer AIS on admission, poorer ambulatory function, and longer hospital stays. Regardless of the etiology, significant neurologic improvements were seen at long-term follow-up, highlighting the importance of active rehabilitation.
Spinal cord infarction (SCInf) is a rare occurrence representing 1.2% of all ischemic strokes and approximately 6% of all acute myelopathies.1-4 However, incorrectly diagnosed SCInfs have been found to make up 14%–16% of transverse myelitis cohorts, suggesting that the incidence of SCInf is greatly underestimated.5,6 SCInf occurs either spontaneously or in a periprocedural or traumatic setting.7,8 While most cases are secondary to aortic disease and repair,7,8 the etiology is unknown in up to one-third of cases.7,8
Patients with SCInf may present with a wide array of clinical symptoms, reflecting the distribution of the spinal cord injury.9 Symptoms may range from back pain, described in up to 70% of patients,10 to different degrees of sensory or motor deficits including tetraplegia or paraplegia.11,12 Disruption of bladder, bowel, and autonomic system functions are often reported.4,7,13 The diversity of possible symptoms makes the diagnosis of SCInf challenging and difficult to differentiate from other neurologic conditions such as multiple sclerosis, inflammatory myelopathies, and infectious or malignant processes.4,7 MRI plays a crucial role in the diagnostic workup and may assist in the differentiation of SCInf from other myelopathies.14 However, an acute onset and subsequent rapid neurologic deterioration is characteristic of the condition15 and suggestive of a poor prognosis.11 Of importance, there is no definitive diagnostic criteria or consensus on the optimal management of SCInf, putting patients at risk of a delayed diagnosis and disadvantageous treatments.16
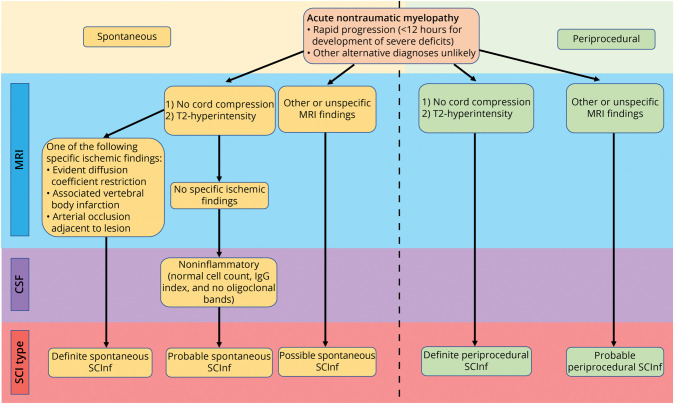
The establishment of agreed-upon diagnostic criteria is a prerequisite for the subsequent development of treatment guidelines. Zalewski et al.15 proposed a list of criteria for the diagnosis of SCInf based on the clinical presentation, MRI, and CSF findings. In this classification, SCInf is divided into spontaneous or periprocedural depending on the etiology. Based on the specificity of the diagnostic findings, spontaneous SCInf is further classified as definite, probable, or possible and periprocedural SCInf as either definite or probable (Figure 1).
Figure 1. Flowchart Illustrating the Categorization SCInf Based on the Certainty of Diagnosis, as Defined by Zalewski et al.
IgG = immunoglobulin G; SCI = spinal cord injury; SCInf = spinal cord infarction.
For periprocedural SCInf, preventive measures have been suggested, including CSF drainage to lower the intrathecal pressure to improve the spinal cord perfusion pressure.17 Recent reviews have highlighted the importance of maintaining adequate spinal cord perfusion to protect the spinal cord during and after aortic procedures.18 However, CSF drainage is associated with severe complications,14,17,19 and its effect on spinal cord perfusion and oxygenation is disputed.20 Treatment with thrombolysis has also been described in the acute phase of spontaneous SCInf,21 where thrombolytic therapy in the first hours after onset of symptoms resulted in partial recovery in a patient with anterior spinal cord syndrome22 and full recovery in a patient with a posterior spinal artery syndrome.23 Because only sporadic cases have been described in the literature, additional evidence is required before the introduction of this therapy as a standard of care. The use of corticosteroids to reduce oxidative stress in SCInfs has been suggested but support is limited to case reports.8,24,25
The current practice in the management of SCInf relies heavily on the treatment guidelines for ischemic cerebral stroke and myocardial infarction. Consequently, focus is placed on the reduction of cardiovascular risk factors23 and antiplatelet therapy in eligible patients.26
Prompted by the lack of definitive guidelines for the diagnosis and management of SCInf, this study aims to review our institutional experience of SCInf in a population-based cohort, focusing on risk factors and outcome predictors. The findings are evaluated in relation to the diagnostic criteria proposed by Zalewski et al.
Methods
Patient Selection and Study Setting
This retrospective study of a population-based cohort of patients consecutively diagnosed with SCInf at the Karolinska University Hospital (Solna, Stockholm, Sweden) is in accordance with the RECORD reporting guidelines (eTable 1, links.lww.com/WNL/C817). The study hospital is a publicly funded and owned tertiary care center serving a region of roughly 2.3 million inhabitants and the only neurologic spinal cord injury unit (SCIU) in the region. Patients were identified using the health record software TakeCare (CompuGroup Medical Sweden AB, Farsta, Sweden) and regional electronic archives. All data were subsequently extracted from the patients’ electronic charts.
All adult patients (aged 18 years or older) treated at the SCIU of the study center between 2006 and 2019 and discharged with a G95 diagnosis (other and unspecified diseases of the spinal cord), according to the International Classification of Diseases, were eligible for inclusion. Patients were excluded when diagnoses other than SCInf were established as the cause of the presenting symptoms.
Classification of SCInf
The initial diagnosis of SCInf was made through a thorough decision-making process by specialists in neurology with an extensive experience of stroke and spinal cord injury rehabilitation. For this study, the Zalewski classification scheme15 was then used to retrospectively evaluate the type and certainty of the diagnosis of SCInf.
SCIU Patient Management and Follow-up Routine
Patients were initially admitted to either the study center or any of the major hospitals in the region. After initial evaluation, patients with suspected SCInf were transferred to the SCIU at the study center. Typical diagnoses that are admitted to our SCIU, and subsequently provided access to a lifelong spinal cord rehabilitation program, include traumatic spinal cord injury, degenerative spinal disease, inflammation, infection, benign tumors, or vascular conditions. Spinal cord injury or progressive myelitis due to underlying malignancy or multiple sclerosis may receive in-patient hospital care at the SCIU but are not provided lifelong follow-up because they are managed by other specialists.
In the included cohort of patients with SCInf, MRI was performed in 93% including diffusion-weighted imaging (DWI) in 63%. Spinal tap with CSF analysis was performed in 80% of patients with spontaneous SCInf, while no spinal taps were performed for those with periprocedural SCInf. Additional routine evaluations included laboratory analyses, echocardiography, and tests for vascular risk factors (especially hyperlipidemia and diabetes mellitus). The initial treatment consisted of aspirin (75 mg/d) and dalteparin (7,500 IU/d) along with an appropriate pharmacologic management of vascular risk factors. In-hospital care and rehabilitation were tailored to meet the needs of each patient. The goals at the SCIU include establishing strategies for the following: (1) respiration, including personalized ventilator regimens; (2) ambulation, mobility, and transfers including walking aids and wheelchairs; (3) voiding and defecation, including intermittent self-catheterization, suprapubic catheters, and laxatives; (4) spasticity, including physiotherapy, pharmacologic treatment, orthoses, and pain management; (5) autonomic dysfunction; and (6) the prevention of pressure ulcers. In addition, the necessary aids and equipment and the need for personal assistance are evaluated and planned for. Patients remained at the SCIU until deemed ready for management at a secondary rehabilitation center.
On discharge from the SCIU, the patients are admitted to secondary rehabilitation institutions within the greater Stockholm region for continuous inpatient rehabilitation lasting from a few weeks to several months. After the inpatient rehabilitation period, the patients are scheduled for outpatient rehabilitation at a dedicated spinal cord injury outpatient clinic. This provides lifelong support for patients with spinal cord injuries by a multidisciplinary team of health care professionals.
Patients are assessed on arrival to and discharge from the SCIU and then regularly at the dedicated outpatient clinic. Clinical and imaging evaluations are performed including the AIS evaluation, quality-of-life assessments, and MRI when indicated. The evaluations are performed at yearly intervals until the neurologic function has stabilized, at which point the intervals are extended. In this study, the latest neurologic status examinations were obtained at a median of 2.1 years after discharge, while the survival status of patients was examined at a median of 8 years after discharge (i.e., during data collection).
Neurologic Function Assessment
The severity of the spinal cord injury was reported using the American Spinal Cord Injury Association Impairment Scale (AIS), a scale that ranges from A to E, where A represents complete injury to the spinal cord and E represents normal neurologic function.27
The Functional Independence Measure (FIM) is a tool that measures the disability of patients irrespective of underlying comorbidities. This instrument is commonly used to assess patients in hospital settings or rehabilitation centers in Europe and the United States. It mainly addresses cognitive function and covers dependence and self-care in relation to everyday activities such as dressing, toileting, mobility, and eating. The allocated score ranges from 18 to 126 and contains 2 parts: cognitive (5–35) and a motor component (13–91).28-30
Statistical Analysis
The normality of the data was evaluated using the Shapiro-Wilk test. Because the distribution of all continuous data deviated significantly from a normal distribution (p value <0.05), median values and interquartile ranges (IQRs) were used. Categorical data are presented using numbers and proportions. Comparisons between periprocedural and spontaneous SCInf were performed using the Mann-Whitney U test (continuous nonparametric data), χ2 test (categorical data with sample size >5), or Fisher’ exact test (categorical data with sample size ≤5). The Wilcoxon signed rank test was used to determine the significance levels associated with changes in the AIS and FIM motor score between admission and long-term follow-up. Finally, a univariable and forced-entry multivariable proportional odds logistic regression model was used to determine predictors of long-term AIS, using listwise deletion to handle missing data. In the multivariable model, we included variables that showed a trend toward significance (p < 0.1) in the univariable analysis. All analyses were conducted using R (version 4.1.2). Statistical significance was set to p < 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was conducted according to the guidelines of the Declaration of Helsinki. The study was also approved by the Swedish Ethical Review Authority (Dnr: 2020-02086). In accordance with Swedish law, the ethical review board waived the need for informed consent due to the retrospective nature of the study and the anonymized dataset used.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Baseline Data
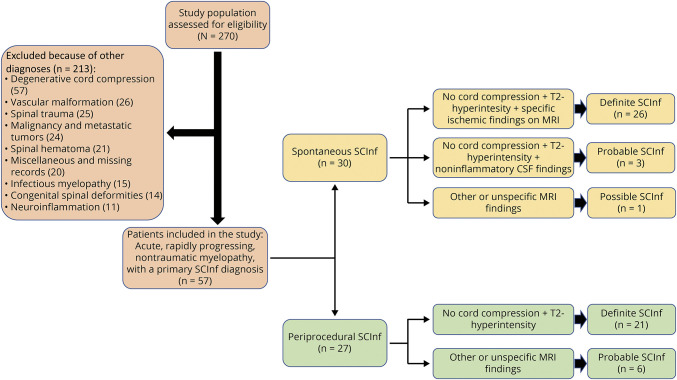
A total of 270 patients were screened for inclusion. After removal of patients lacking an SCInf diagnosis, 57 patients remained and were included in the study: 30 (53%) had spontaneous and 27 (47%) had periprocedural infarctions.
MRI was performed in 93% including DWI in 63%. CSF analyses were performed in 24 (80%) of the patients with spontaneous SCInf, while none were performed in the periprocedural cases. White cell counts were available for all 24 patients, while CSF protein results were available for 23. Pleocytosis was reported in only 1 case (4%), and CSF proteins were elevated in 14 patients (61%) and normal in 9 (39%).
The application of the diagnostic criteria by Zalewski et al. to this cohort of patients identified 82% (47/57) of cases as definite SCInf (Figure 2). However, 4 spontaneous and 6 periprocedural cases could not be classified as definite (eTable 2, links.lww.com/WNL/C818).
Figure 2. Flowchart Describing the Patient Inclusion Process and the Classification of SCInf According to Zalewski et al.
SCInf = spinal cord infarction.
The 4 cases of nondefinite spontaneous SCInf were primarily admitted to hospitals lacking DWI protocols then. No other MRI-based diagnostic criteria (vertebral body infarction or adjacent arterial dissection/occlusion) were met. Instead, based on the clinical findings and CSF analyses, 3 of the cases were classified as probable and 1 as possible. In the 6 cases of nondefinite periprocedural SCInf, MRI examinations or findings were lacking: in 3 cases, no MRI was performed because of the presence of aortic stents; in 1 case, no MRI was performed because the patient was claustrophobic; in 2 cases, the MRIs were not of sufficient diagnostic quality due to artefacts caused by aortic stents. All these cases were classified as probable. However, statistical analysis did not reveal any significant differences between definite and probable or possible SCInf for sex, age, etiology, or AIS score on admission (eTable 3, links.lww.com/WNL/C819).
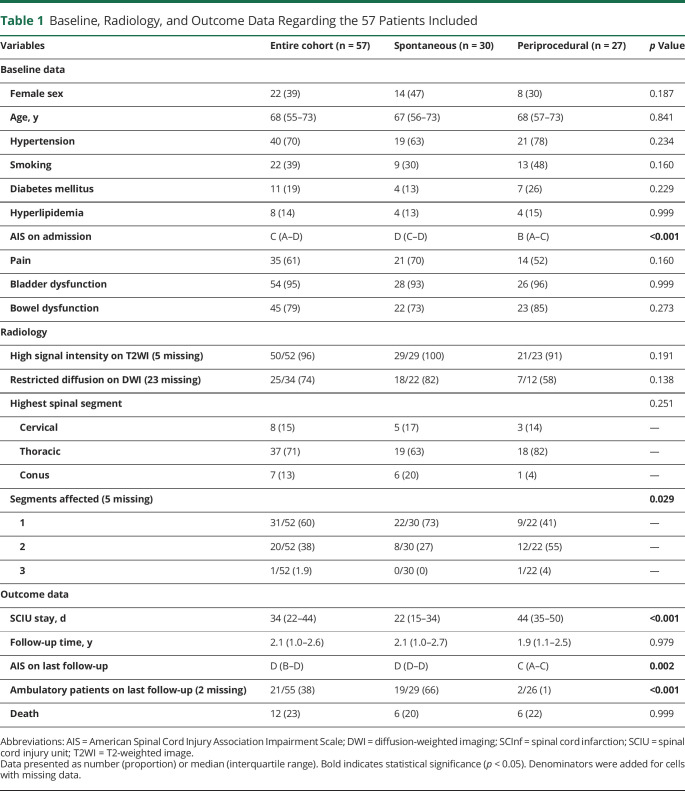
The median age for the entire cohort was 68 years (IQR 55–73), and 39% were women. Among the periprocedural infarctions, 24 were due to procedures involving aortic manipulation and the remaining 3 cases were related to surgery for hip fracture, scoliosis correction, and renal-pancreas transplantation, respectively. Hypertension, smoking, diabetes, and hyperlipidemia were reported in 70%, 39%, 19%, and 14% of patients in the entire cohort, with balanced proportions between spontaneous and periprocedural subgroups. The following coexisting cardiovascular conditions were identified in the entire cohort: coronary artery disease (n = 6; 11%), atrial fibrillation (n = 7, 12%), and a history of cerebral insult including cerebral infarction and transitory ischemic attack (n = 8, 14%) (Table 1).
Table 1.
Baseline, Radiology, and Outcome Data Regarding the 57 Patients Included
In 48 (91%) patients, the MRI findings supported or confirmed the diagnosis of SCInf by showing an intramedullary lesion with hyperintensity on T2 imaging and/or apparent restriction in the DWI (Table 2).
Table 2.
Proportional Odds Logistic Regression Predicting More Favorable Follow-up AIS
Most of the cases were thoracic (71%), followed by cervical (15%) and the conus regions (13%). One spinal segment was affected in 31 (60%) patients, 2 segments in 20 (38%) patients, and 3 segments in 1 (1.9%) patient. On admission, the median AIS was C. Bladder dysfunction was present in 54 (95%), bowel dysfunction in 45 (79%), and pain in 35 (61%) patients.
Compared with spontaneous cases, periprocedural SCInf affected a larger number of spinal segments (median 1 vs 2, p = 0.029) and were associated with a poorer AIS on admission (median AIS D vs B; p < 0.001). The median stay at the SCIU was 34 days (IQR 22–44), significantly longer for patients with periprocedural SCInf (22 vs 44; p < 0.001) (Table 1).
Outcome: AIS
The median follow-up time was 2.1 years (IQR 1.0–2.6), at which point the median functional status had improved from AIS C to D (Table 1). Paired testing showed a significant improvement in AIS between admission and long-term follow-up, with 15 patients improved, 37 unchanged, and 1 worsened (Figure 3, p = 0.002). At follow-up, patients with spontaneous SCInf had more favorable AIS scores when compared with those with periprocedural SCInf (median AIS D vs C, p = 0.002). Regarding walking ability, 21 patients (38%) were ambulatory on follow-up, 12 of whom required no walking aids. Among the remaining 9 patients, 2 were dependent on crutches, 7 on walking frames, and 1 on a standing support frame. Patients with spontaneous SCInf were more likely to regain ambulatory functions compared with those with periprocedural SCInf (66% vs 1%, p < 0.001).
Figure 3. Stacked Bar Chart Showing the AIS on Admission, Discharge, and Follow-up.
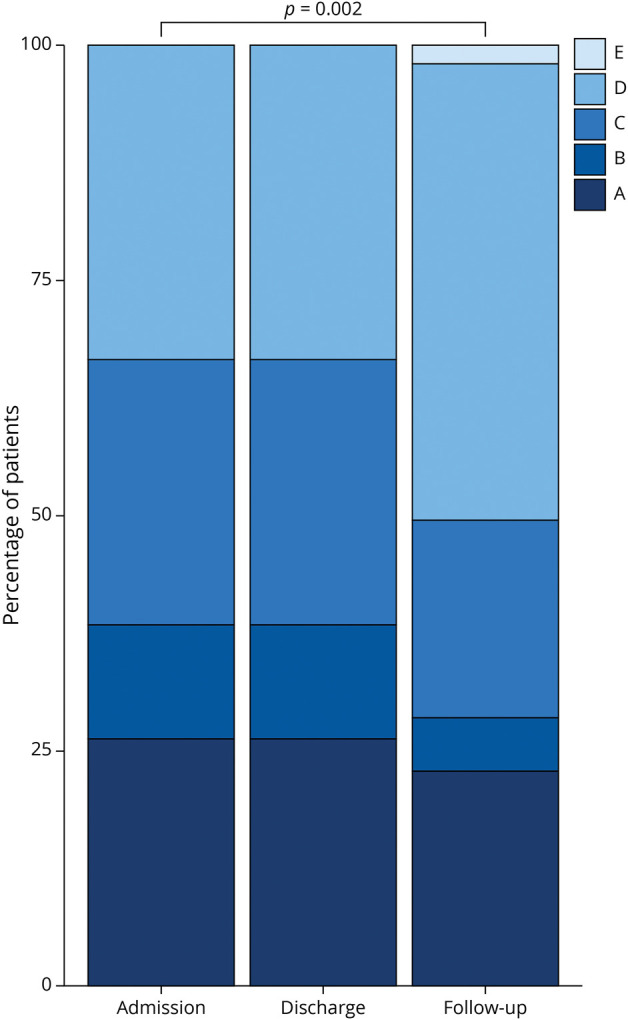
AIS = American Spinal Cord Injury Association Impairment Scale.
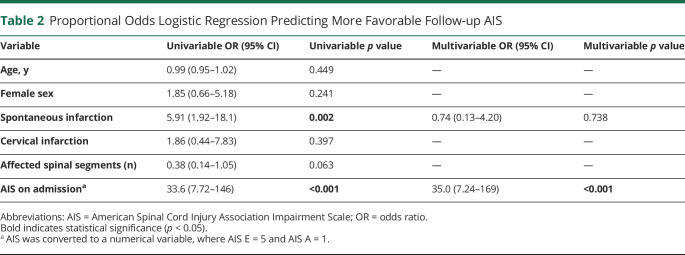
In the proportional odds logistic regression predicting long-term outcome, patients with more favorable AIS on admission (odds ratio [OR] 33.6, p < 0.001) and with spontaneous infarctions (OR 5.91, p = 0.002) were more likely to present with more favorable AIS on long-term follow-up. Of these, more favorable AIS on admission showed independent predictive ability in the multivariable analysis (OR 35.0, p < 0.001) (Table 2).
Outcome: FIM Motor Score
There were 25 patients with FIM motor scores on both discharge and at follow-up, recorded at a median of 1.2 years later. The median FIM motor score at SCIU discharge was 46 (IQR 36–59), and the median score at follow-up was 76 (IQR 64–82). Paired testing showed a significant improvement in FIM motor score between discharge and follow-up (p < 0.001, Figure 4).
Figure 4. Boxplot Showing FIM Motor Score on Discharge From the Spinal Cord Injury Unit and at the Median Follow-up of 1.2 Years.
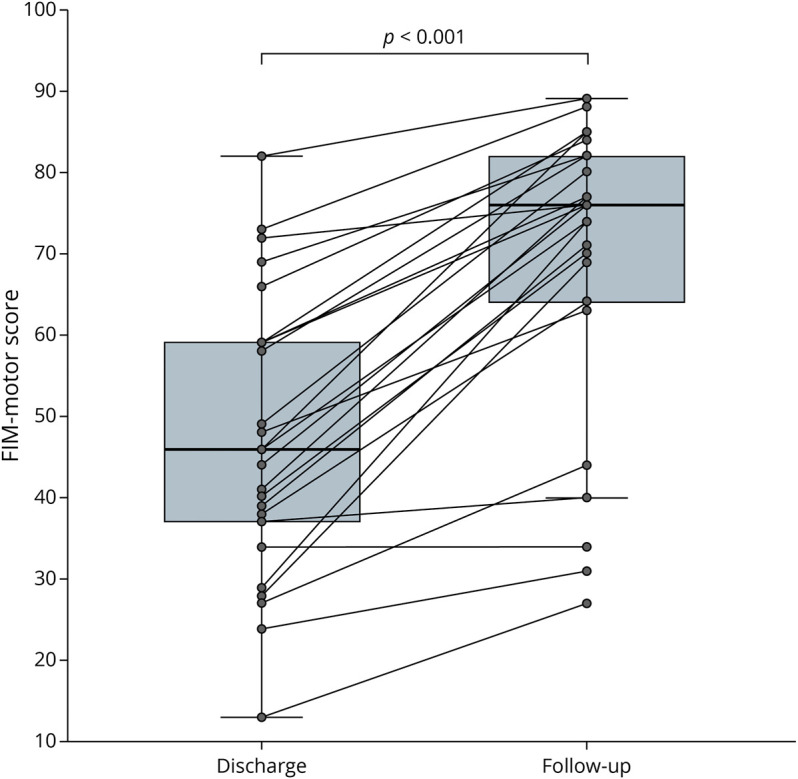
FIM = Functional Independence Measure.
Outcome: Mortality
At a median follow-up of 6.5 months after the event, 12 deaths had been recorded, with 6 occurring in each of the spontaneous (20%) and periprocedural (22%) groups. All but 2 of the deaths occurred within 31 months. The remaining 2 occurred at 70 and 87 months, respectively. Causes of death were present for all but 2 patients. Sepsis and respiratory failure were identified as the cause of death in 3 patients each, while cardiac arrest and thromboembolic events caused death in 2 patients each (eFigure 1, links.lww.com/WNL/C816).
Discussion
In this study, we reviewed our institutional experience with SCInf and evaluated predictors of outcome. In line with the diagnostic criteria proposed by Zalewski et al., the cohort of SCInf was divided into spontaneous and periprocedural. Spontaneous SCInf tended to affect a single spinal cord segment and was associated with better AIS on admission, while periprocedural cases affected 2 or more segments and were associated with a poorer AIS on admission. On follow-up, significant improvements were seen in the AIS and FIM motor scores. Patients with spontaneous SCInf presented with more favorable AIS on follow-up, compared with patients with periprocedural SCInf.
The European Quality of Life 5 Dimensions 3 Level Version measuring Health-Related Quality of Life,31 available for 11 patients, revealed that most of the responders had difficulties walking, were bedridden, and were unable to perform activities of daily living. Seven patients also reported moderate or severe anxiety/depression. This reflects the impact of SCInf on the quality of life of patients despite measurable neurologic improvements.
Previous studies have shown that 17%–45% of imaging workups are normal in patients where there is a clinical suspicion of SCInf.32 Spinal cord lesions may not be discernible on T2-weighted imaging within the first 12–24 hours after onset of symptoms.16,33 In this study, the exact time interval from symptom onset to MRI examination could not be defined. However, the first available MRI showed imaging findings supporting or confirming the SCInf diagnosis in most of the patients (91%). As previously described,3,4,15 a “pencil-like” appearance on sagittal T2-weighted sequences with bilateral hyperintense lesions in the anterior horns creating the “owl’s eyes” pattern on axial images and hyperintensities in the anterior spinal artery territory were characteristic. Our data confirm the utility of MRI in the diagnosis of SCInf.
DWI has been recognized as a useful tool in the diagnostic workup of cerebral ischemia. Similarly, in the context of SCInf, DWI has been identified as a useful and feasible technique for the early detection of SCInf.34 However, parameters such as blood and CSF pulsations, the small dimensions of the infarction, and the heterogeneity of the field in the spinal cord region constitute some of the obstacles to the generalizability of this method.4 In our study, the DWI findings were conclusive of SCInf in 63% of the patients where this modality had been used. Both T2-weighted imaging and DWI helped exclude other differential diagnoses and establish the diagnosis of SCInf early in the course of the disease.
Due to the similarity to cerebral stroke, spontaneous SCInf has been postulated to result from analogous disease mechanisms, mainly vasculopathies. However, unlike cerebral stroke, the implication of vascular disease processes in the pathophysiology of spontaneous SCInf has rarely been studied.35 Yet the prevalence of vascular risk factors among patients with spontaneous SCInf is well described.15 In this study, 67% of patients with spontaneous SCInf presented with at least 1 vascular risk factor. Hypertension was present in 63% and diabetes mellitus in 13%. Previous studies analyzing the prevalence of vascular risk factors at similar ages in the Swedish population found hypertension in approximately 55% and diabetes mellitus in 7.5%.36,37 Hence, the overrepresentation of these risk factors in our cohort hints at their importance in the disease processes leading to SCInf.
In agreement with previous literature, our study shows that the improvement of neurologic function after SCInf occurs progressively over an extended period.38,39 At final follow-up, 15 (26%) patients had improved in their AIS, and 37 (65%) remained unchanged. Neurologic recovery was only partial, indicating that permanent sequalae after SCInf are very likely. At follow-up, only 1 patient had recovered to an AIS score of E, while all other patients had some degree of disability. Analysis of the predictors of outcomes using proportional odds logistic regression revealed a more favorable AIS on admission and spontaneous SCInf (vs periprocedural) as significant predictors of a more favorable AIS at follow-up.
In another study,39 the authors reported that evidence was lacking to differentiate the prognosis depending on the etiology of the ischemia. Although a trend toward more favorable outcomes for patients with spontaneous SCInf was found in a study conducted by Salvador de la Barrera et al.,2 the association did not reach statistical significance. However, older studies previously suggested that periprocedural SCInfs were associated with worse outcomes when compared with spontaneous or idiopathic SCInfs.40,41 Similarly, this study revealed that periprocedural SCInf, when compared with spontaneous SCInfs, are associated with significantly worse AIS on both admission (median D vs B, p < 0.001) and follow-up (median D vs C, p = 0.002). The fact that multilevel ischemia was more often seen in periprocedural SCInf (median 1 vs 2, p = 0.029) and that AIS was generally poorer in these patients (p < 0.001) is suggestive of a more extensive ischemia in these cases. However, we could not correlate multiple segment SCInf to poorer AIS on follow-up. Nonetheless, multivariable analysis revealed AIS on admission as the only significant predictor of unfavorable outcomes. Thus, we interpret the association between periprocedural SCInf and poor outcome to reflect the poor admission AIS in this patient group. Nedeltchev et al.32 also associated poor AIS at presentation with worse follow-up AIS. In another study, age was identified as a significant prognostic marker.2 However, neither our study nor the one performed by Nedeltchev et al.32 could demonstrate any significant relation between age and AIS at follow-up.
At long-term follow-up, 38% of patients were able to walk with or without walking aids. Similar studies have reported that the ability to walk with or without walking aids is regained in 38%–70% of patients.7,32 These differences may be explained by the relative contribution of periprocedural cases in the studies. In fact, our study demonstrates a clear predilection of cases with spontaneous SCInf among patients who regained ambulatory function at last follow-up (66% vs 1%, p < 0.001). In support of this, the greatest proportion of patients regaining their ambulatory function (70%) was seen in the study conducted by Nedeltchev et al.,32 where patients with periprocedural SCInf constituted only 16% of the total. Inversely, the lowest proportion of patients regaining ambulation (38%) was found in both our cohort and in the one in the study conducted by Cheshire et al.,7 where the corresponding share of periprocedural SCInf among all cases reached 47% and 43%, respectively.
In this study, the survival status of all patients was retrieved during data collection, that is, on average 6.5 years after the diagnosis. During that period, 12 (21%) patients had died. Despite having poorer outcomes, the overall survival in patients with periprocedural SCInf did not differ from that of those with spontaneous SCInf (22% vs 20%, p = 0.77, eFigure 1, links.lww.com/WNL/C816). Considering similar lengths of follow-up, the mortality rate in this study was comparable with that in other studies in the literature.2,38,39 Nedeltchev et al.32 showed a lower mortality rate of 9% at an average of 4 years of follow-up. In addition to the shorter follow-up time, patients in their cohort had better AIS scores at baseline which, at least partly, may explain the lower mortality. In our cohort, 3 of the patients had died within the first year, and almost all the deaths occurred within 2.5 years of the diagnosis. Only 2 patients died later, 1 of whom was older than 85 years. Taken together, the available data suggest that there is a peak in mortality early in the course of the disease. This warrants further investigations to identify manageable risks during the first 2–3 years after diagnosis.
The overall agreement between the certainty of the diagnosis as established at the study center and as suggested by the diagnostic criteria proposed Zalewski et al. lend support to their general adoption. The application of the diagnostic criteria to this cohort of patients identified 82% (47/57) of cases as definite SCInf (Figure 2). However, 4 spontaneous and 6 periprocedural cases could not be classified as definite (eTable 2, links.lww.com/WNL/C818). In the 10 nondefinite cases of SCInf, MRI examination or findings were lacking, and the diagnosis was instead based on clinical findings and CSF analyses. Statistical analysis did not reveal any significant differences between definite and probable or possible SCInf for sex, age, etiology, or AIS score on admission (eTable 3, links.lww.com/WNL/C819). This underscores the importance of clinical examination and alternative diagnostic tools, such as CSF analysis, to support the diagnosis of SCInf in cases where MRI is either unavailable or yields inconclusive results. Furthermore, this calls for the need to develop specific diagnostic tools, such as tests or markers, to confirm the diagnosis of SCInf on high suspicion.
The main limitation of this study is the small sample size and the retrospective study design. The sample size is comparable with that of other studies in the field, highlighting the rarity of SCInf. Strengths of this study include the population-based design and the standardized patient management including in-hospital care, rehabilitation, and long-term follow-up. The comparison with the Zalewski criteria was made retrospectively to a cohort of patients already diagnosed with SCInf. Hence, the relative agreement between our data and the diagnostic criteria must be evaluated as such. Moreover, this work reveals major differences regarding the etiology of SCInf that were unaddressed by previous literature. We found that periprocedural SCInfs were associated with larger infarcts and worse neurologic status on admission, when compared with spontaneous ones. Possible explanations include more proximal or more prolonged vessel obstructions in periprocedural SCInf. Another explanation could be that in endovascular aortic procedures, stenting may result in obstruction of several aortic branches to the spinal cord, while spontaneous infarctions result from more localized insults. Aside from indicating differences in pathophysiologic mechanisms, our findings also reveal discrepancies in outcomes between the 2 groups, suggesting that a tailored approach may be warranted in the management of patients based on the nature of their SCInf.
In this retrospective population-based cohort study, spontaneous and periprocedural SCInfs were evaluated, and recently proposed diagnostic criteria were applied. Overall, the findings match those reported in the literature and support the use of the diagnostic criteria.
SCInf is a rare neurologic emergency lacking specific treatment, and the management aims at preventing secondary complications. While the presumptive diagnosis is based on the typical presentation and clinical findings, T2-weighted and diffusion-weighted MRI are the most useful diagnostic tools in establishing a definite diagnosis. Spontaneously occurring SCInf mostly affected a single spinal cord segment, whereas periprocedural cases were more extensive, had poorer AIS on admission, and longer hospital stays. Spontaneous infarction and better AIS on admission were identified as predictors of more favorable outcomes. Regardless of the etiology, both AIS and FIM motor scores significantly improved at long-term follow-up. The high incidence of vascular risk factors compared with that in the general population indicates that stroke mechanisms play an important role in the pathophysiology of SCInf. Long-term improvements highlight the importance of active rehabilitation.
Glossary
- AIS
American Spinal Cord Injury Association Impairment Scale
- DWI
diffusion-weighted imaging
- FIM
Functional Independence Measure
- IQR
interquartile range
- OR
odds ratio
- SCInf
spinal cord infarction
- SCIU
spinal cord injury unit
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
A. Elmi-Terander was supported by Region Stockholm (clinical research appointment). None of the other authors received funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Masson C, Pruvo JP, Meder JF, et al. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry. 2004;75(10):1431-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvador de la Barrera S, Barca-Buyo A, Montoto-Marques A, Ferreiro-Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord. 2001;39(10):520-525. [DOI] [PubMed] [Google Scholar]
- 3.Hsu JL, Cheng MY, Liao MF, et al. The etiologies and prognosis associated with spinal cord infarction. Ann Clin Transl Neurol. 2019;6(8):1456-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav N, Pendharkar H, Kulkarni GB. Spinal cord infarction: clinical and radiological features. J Stroke Cerebrovasc Dis. 2018;27(10):2810-2821. [DOI] [PubMed] [Google Scholar]
- 5.Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology. 2018;90(2):e96-e102. [DOI] [PubMed] [Google Scholar]
- 6.Barreras P, Fitzgerald KC, Mealy MA, et al. Clinical biomarkers differentiate myelitis from vascular and other causes of myelopathy. Neurology. 2018;90(1):e12-e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996;47(2):321-330. [DOI] [PubMed] [Google Scholar]
- 8.Lin WP, Kuan TS, Lin CI, Hsu LC, Lin YC. Spinal cord infarction during physical exertion due to polycythemia vera and aortoiliac occlusive disease A case report. Medicine (Baltimore). 2018;97(35):e12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76(3-4):95-98. [DOI] [PubMed] [Google Scholar]
- 10.Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63(8):1113-1120. [DOI] [PubMed] [Google Scholar]
- 11.Vargas MI, Gariani J, Sztajzel R, et al. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR Am J Neuroradiol. 2015;36(5):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vongveeranonchai N, Zawahreh M, Strbian D, Sundararajan S. Evaluation of a patient with spinal cord infarction after a hypotensive episode. Stroke. 2014;45(10):e203-e205. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AT, Weiss SJ, McGarvey ML, et al. Interventions for reversing delayed-onset postoperative paraplegia after thoracic aortic reconstruction. Ann Thorac Surg. 2002;74(2):413-419; discussion 420-421. [DOI] [PubMed] [Google Scholar]
- 14.Ros Castello V, Sanchez Sanchez A, Natera Villalba E, et al. Spinal cord infarction: aetiology, imaging findings, and prognostic factors in a series of 41 patients. Neurologia (Engl Ed). 2021. doi: 10.1016/j.nrleng.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 15.Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76(1):56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein NE. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: a review. Surg Neurol Int. 2018;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lella SK, Waller HD, Pendleton A, Latz CA, Boitano LT, Dua A. A systematic review of spinal cord ischemia prevention and management after open and endovascular aortic repair. J Vasc Surg. 2022;75(3):1091-1106. [DOI] [PubMed] [Google Scholar]
- 19.Kramer CL. Vascular disorders of the spinal cord. Continuum (Minneap Minn). 2018;24(2, spinal cord disorders):407-426. [DOI] [PubMed] [Google Scholar]
- 20.Visagan R, Hogg FRA, Gallagher MJ, et al. Monitoring spinal cord tissue oxygen in patients with acute, severe traumatic spinal cord injuries. Crit Care Med. 2022;50(5):e477-e86. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic J, Rey Bataillard V, Mercier N, Bonvin C, Michel P. Acute ischemic myelopathy treated with intravenous thrombolysis: four new cases and literature review. Int J Stroke. 2019;14(9):893-897. [DOI] [PubMed] [Google Scholar]
- 22.Muller KI, Steffensen LH, Johnsen SH. Thrombolysis in anterior spinal artery syndrome. BMJ Case Rep. 2012;2012:bcr2012006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes J, Shapiro M, Raz E, Czeisler B, Nossek E. Intra-arterial thrombolytic therapy for acute anterior spinal artery stroke. J Clin Neurosci. 2021;84:102-105. [DOI] [PubMed] [Google Scholar]
- 24.Robertson CS, Foltz R, Grossman RG, Goodman JC. Protection against experimental ischemic spinal cord injury. J Neurosurg. 1986;64(4):633-642. [DOI] [PubMed] [Google Scholar]
- 25.Lee DW, Choi YH. Spinal cord infarction mimicking ischemic heart disease. Clin Exp Emerg Med. 2017;4(2):109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch K, Oster J, Apetauerova D, Hreib K. Spinal cord stroke: acute imaging and intervention. Case Rep Neurol Med. 2012;2012:706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priebe MM, Waring WP. The interobserver reliability of the revised American Spinal Injury Association standards for neurological classification of spinal injury patients. Am J Phys Med Rehabil. 1991;70(5):268-270. [DOI] [PubMed] [Google Scholar]
- 28.Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132. [PubMed] [Google Scholar]
- 29.Heinemann AW, Linacre JM, Wright BD, Hamilton BB, Granger C. Relationships between impairment and physical-disability as measured by the functional independence measure. Arch Phys Med Rehab. 1993;74(6):566-573. [DOI] [PubMed] [Google Scholar]
- 30.Harvey LA, Glinsky JV, Chu J. Do any physiotherapy interventions increase spinal cord independence measure or functional independence measure scores in people with spinal cord injuries? A systematic review. Spinal Cord. 2021;59(7):705-715. [DOI] [PubMed] [Google Scholar]
- 31.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343. [DOI] [PubMed] [Google Scholar]
- 32.Nedeltchev K, Loher TJ, Stepper F, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35(2):560-565. [DOI] [PubMed] [Google Scholar]
- 33.Pikija S, Kunz AB, Nardone R, et al. Spontaneous spinal cord infarction in Austria: a two-center comparative study. Ther Adv Neurol Disord. 2022;15:17562864221076321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48(11):795-801. [DOI] [PubMed] [Google Scholar]
- 35.Tubbs RS, Blouir MC, Romeo AK, Mortazavi MM, Cohen-Gadol AA. Spinal cord ischemia and atherosclerosis: a review of the literature. Br J Neurosurg. 2011;25(6):666-670. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson AC, Wandell PE, de Faire U, Hellenius ML. Prevalence of hypertension in immigrants and Swedish-born individuals, a cross-sectional study of 60-year-old men and women in Sweden. J Hypertens. 2008;26(12):2295-2302. [DOI] [PubMed] [Google Scholar]
- 37.Wandell PE, Wajngot A, de Faire U, Hellenius ML. Increased prevalence of diabetes among immigrants from non-European countries in 60-year-old men and women in Sweden. Diabetes Metab. 2007;33(1):30-36. [DOI] [PubMed] [Google Scholar]
- 38.Hanson SR, Romi F, Rekand T, Naess H. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131(4):253-257. [DOI] [PubMed] [Google Scholar]
- 39.Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78(2):114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foo D, Rossier AB. Anterior spinal artery syndrome and its natural history. Paraplegia. 1983;21(1):1-10. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro L, Leite I, Pinto JA, Stocker A. Spontaneous thoracolumbar spinal cord infarction: report of six cases. Acta Neurol Scand. 1992;86(6):563-566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.