Abstract
Aims
Cardiac device–related infective endocarditis (CDRIE) is a severe complication of cardiac device (CD) implantation and is usually treated by antibiotic therapy and percutaneous device extraction. Few studies report the management and prognosis of CDRIE in real life. In particular, the rate of device extraction in clinical practice and the management of patients with left heart infective endocarditis (LHIE) and an apparently non-infected CD (LHIE+CDRIE−) are not well described.
Methods and results
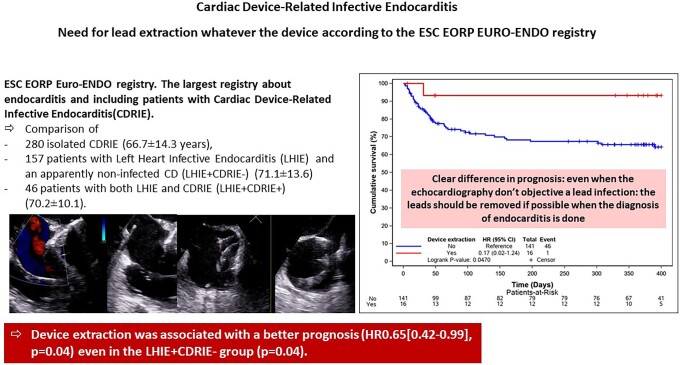
We sought to study in EURO-ENDO, the characteristics, prognosis, and management of 483 patients with a CD included in the European Society of Cardiology EurObservational Research Programme EURO-ENDO registry. Three populations were compared: 280 isolated CDRIE (66.7 ± 14.3 years), 157 patients with LHIE and an apparently non-infected CD (LHIE+CDRIE−) (71.1 ± 13.6), and 46 patients with both LHIE and CDRIE (LHIE+CDRIE+) (70.2 ± 10.1). Echocardiography was not always transoesophageal echography (TOE); it was transthoracic echography (TTE) for isolated CDRIE in 88.4% (TOE = 67.6%), for LHIE+CDRIE− TTE = 93.0% (TOE = 58.6%), and for CDRIE+LHIE+ TTE = 87.0% (TOE = 63.0%). Nuclear imaging was performed in 135 patients (positive for 75.6%). In-hospital mortality was lower in isolated CDRIE 13.2% vs. 22.3% and 30.4% for LHIE+CDRIE− and LHIE+CDRIE+ (P = 0004). Device extraction was performed in 62.1% patients with isolated CDRIE, 10.2% of LHIE+CDRIE− patients, and 45.7% of CDRIE+LHIE+ patients. Device extraction was associated with a better prognosis [hazard ratio 0.59 (0.40–0.87), P = 0.0068] even in the LHIE+CDRIE− group (P = 0.047).
Conclusion
Prognosis of endocarditis in patients with a CD remains poor, particularly in the presence of an associated LHIE. Although recommended by guidelines, device extraction is not always performed. Device removal was associated with better prognosis, even in the LHIE+CDRIE− group.
Keywords: Cardiac device, Pacemaker, Implantable defibrillator, Infective endocarditis, staphylococci, Prognosis
Infective endocarditis associated with the presence of a cardiac device remains a serious disease and should be treated with device extraction, even in patients with left heart infective endocarditis and an apparently non-infected device.
Graphical Abstract
Graphical abstract.
Introduction
Cardiovascular implantable electronic devices are widely used [pacemakers (PM) and defibrillator]. Devices are implanted in many patients and can require replacement throughout the patient’s life. As these patients age, they accumulate comorbidities. Therefore, the epidemiology and the prognosis of cardiac device–related infective endocarditis (CDRIE) has changed and remains poorly defined.1 Imaging techniques have progressed; previously limited to two-dimensional (2D) echocardiography has moved to three-dimensional (3D) echocardiography, cardiac tomography, and positron emission tomography/computed tomography (PET/CT).2–4 Guidelines have move forward and integrate the evolution.5 But evidence of their implementation in clinical practice is lacking. Also, there are still clinical challenges. For instance, characterization of patients who have a diagnosis of left heart infective endocarditis (IE) and an apparently non-infected intracardiac device is missing.6
Main results of the European Society of Cardiology (ESC) EurObservational Research Programme (EORP) European Endocarditis (EURO-ENDO) registry have been published.7 This large registry has been conducted in order to develop a contemporary international investigation of the care and outcomes of IE in Europe and abroad.7,8 We thought that patients with intracardiac devices require specific attention.7,8 Thus, we sought:
To report current profile of all patients who have cardiovascular implantable electronic devices and IE implanted, in Europe and abroad.
To assess the use of new imaging techniques.
To assess the prognosis in isolated CDRIE+, left heart infective endocarditis (LHIE)+ CDRIE− and LHIE+CDRIE+.
How the guidelines are implemented.
Methods
Study patients
A prospective cohort of 3116 adult patients (2470 from Europe and 646 from non-ESC countries) admitted to 156 hospitals in 40 countries between January 2016 and March 2018 with a diagnosis of IE based on ESC 2015 diagnostic criteria.7–9 Clinical, biological, microbiological, and imaging [echocardiography, CT scan, 18F-fluorodeoxyglucose PET/CT (18F-FDG PET/CT)] data were collected. Infective endocarditis was native valve endocarditis (NVE) in 1764 (56.6%) patients, prosthetic valve endocarditis (PVIE) in 939 (30.1%), and device-related (CDRIE) in 326 (10.5%). We focused on a total number of 483 patients with cardiovascular implantable electronic devices. The study complies with the Declaration of Helsinki, with locally appointed ethics committee having approved the research protocol and that informed consent has been obtained from the subjects (or their legally authorized representative).
Diagnostic criteria and definitions
Inclusion criteria were a diagnosis of definite IE (or considered probable IE and treated as IE) based on the ESC 2015 IE diagnostic criteria.9 In the present study, we considered all the patients with IE and a CD. Three populations were compared, including 280 isolated CDRIE (CDRIE+LHIE−)(66.7 ± 14.3 years), 157 patients with LHIE and an apparently non-infected CD (LHIE+CDRIE−) (71.1 ± 13.6) and 46 patients with both LHIE and CDRIE (CDRIE+LHIE+) (70.2 ± 10.1).
The diagnosis is based on guidelines using blood cultures, echocardiography [transthoracic echography (TTE) and transoesophageal echography (TOE)] and if needed by cardiac tomography and nuclear medicine imaging techniques. After informed consent, data were collected at inclusion and during hospitalization, including demographics, patient history, Charlson index (this score is considered to describe the population but not for the statistical analysis on its own), considering age and several comorbidities, clinical, biological, microbiological, and echocardiographic findings. The use of other imaging techniques (CT scan, 18F-FDG PET/CT, and leucocyte scintigraphy), medical therapy, complications (embolic event, infectious, and haemodynamic complications), theoretical indications for surgery, and in-hospital mortality were collected.
Management of CDRIE
The frequency and mode of device removal was reported. The decision to remove the CD was based on the judgement of the endocarditis team,8,9 and the reason for no extraction of the CD was assessed.10,11
Statistical analysis
All data were collected by the collecting officers or investigators at the participating centres and included in an electronic case report form for online data entry. All patients enrolled with possible or definite IE were included in the analyses. Univariable analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± standard deviation (SD) or as median and interquartile range (IQR). Among-group comparisons were made using a non-parametric test (Kruskal–Wallis test). Categorical variables were reported as counts and percentages. Among-group 2 × 2 comparisons were made using a χ2 test or Fisher’s exact test if any expected cell count was <5. In other cases, the Monte-Carlo estimate of the exact P-value was used. Plots of the Kaplan–Meier curves for time to all causes of deaths were performed and log-rank test was calculated. The Kaplan–Meier curve of time to death was also adjusted for the covariates from the Cox proportional hazard model. Pairwise correlations between all candidate variables (variables with P < 0.10 in univariate) within the model and variables considered of relevant clinical interest were tested before proceeding to the multi-variable model. In case of correlation, some criteria were not taken into account. A backward multi-variable Cox regression analysis was performed to identify the independent predictors of 400-day all-cause mortality. Significance levels of 0.05 were required to allow a variable to stay (SLSTAY = 0.05) within the model. Some measures of model fit were considered: concordance and the goodness of fit test proposed by May and Hosmer. In addition, the proportional hazard ratios assumptions were verified graphically and with the Schoenfeld residuals test. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographics and clinical characteristics
During the study period, 111 out of 120 centres recruited CDRIE in EURO-ENDO.7,8 A total number of 483 patients with cardiovascular implantable electronic devices were included: 280 isolated CDRIE, 157 LHIE+CDRIE−, and 46 CDRIE+LHIE+. The median number of endocarditis treated per centre was 30 (20–60) (Tables 1 and 2 and Supplementary material online, Table S1).
Table 1.
Demography and patient medical history in patients with device
| CDRIE+LHIE− (1) (n = 280) |
LHIE+CDRIE− (2) (n = 157) |
CDRIE+LHIE+ (3) (n = 46) |
P-value (1) vs. (2) |
P-value (2) vs. (3) |
|
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | |||||
| N | 280 | 157 | 46 | ||
| Mean ± SD | 66.69 ± 14.31 | 71.08 ± 13.59 | 70.22 ± 10.11 | 0.0002 | 0.1 |
| Females | 75/280 (26.8%) | 39/157 (24.8%) | 19/46 (41.3%) | 0.6 | 0.02 |
| Body mass index (kg/m²) | |||||
| N | 257 | 142 | 40 | ||
| Mean ± SD | 27.48 ± 6.21 | 27.53 ± 5.58 | 28.32 ± 5.68 | 0.6 | 0.4 |
| History of previous endocarditis | |||||
| Previous endocarditis | 16/280 (5.7%) | 23/157 (14.6%) | 4/46 (8.7%) | 0.001 | 0.2 |
| Type of microorganism | |||||
| Staphylococcus aureus | 6/12 (50.0%) | 6/15 (40.0%) | 2/3 (66.7%) | 0.6 | 0.5 |
| Methi-S Staphylococcus aureus | 6/12 (50.0%) | 5/15 (33.3%) | 1/3 (33.3%) | 0.4 | >0.9 |
| Chronic renal failure | 71/280 (25.4%) | 52/157 (33.1%) | 14/46 (30.4%) | 0.08 | 0.7 |
| Diabetes mellitus | 110/280 (39.3%) | 51/157 (32.5%) | 21/46 (45.7%) | 0.1 | 0.1 |
| Heart failure | 114/245 (46.5%) | 80/141 (56.7%) | 14/43 (32.6%) | 0.05 | 0.005 |
| Charlson index | |||||
| N | 232 | 136 | 40 | ||
| Mean ± SD | 4.42 ± 3.16 | 5.41 ± 2.83 | 4.93 ± 2.04 | <0.0001 | 0.3 |
| Antithrombotic treatment on admission | 206/274 (75.2%) | 135/155 (87.1%) | 40/45 (88.9%) | 0.003 | 0.7 |
| Device therapy | |||||
| Pacemaker | 150/280 (53.6%) | 118/157 (75.2%) | 28/46 (60.9%) | <0.0001 | 0.1 |
| ICD (defibrillator) | 84/280 (30.0%) | 23/157 (14.6%) | 8/46 (17.4%) | ||
| CRT-D (with ICD) | 42/280 (15.0%) | 11/157 (7.0%) | 6/46 (13.0%) | ||
| CRT-P (pacing only) | 4/280 (1.4%) | 5/157 (3.2%) | 4/46 (8.7%) | ||
CDRIE+ corresponds to patient with at least the infective endocarditis is on ICD/PM only or on ICD/PM+ (pulmonary or tricuspid). CDRIE− corresponds to patient with device and without CDRIE or pulmonary or tricuspid location of IE. Kruskal–Wallis test is used for quantitative data. χ2 or Fisher’s exact test [a] is used for binary variables.
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LHIE+, left heart infective endocarditis; LHIE−, no left heart infective endocarditis.
Table 2.
Echocardiography findings in patients with device
| CDRIE+LHIE− (1) (n = 280) |
LHIE+CDRIE− (2) (n = 157) |
CDRIE+LHIE+ (3) (n = 46) |
P-value (1) vs. (2) |
P-value (2) vs. (3) |
|
|---|---|---|---|---|---|
| Transthoracic echocardiography | 243/275 (88.4%) | 146/157 (93.0%) | 40/46 (87.0%) | 0.1 | 0.2 |
| Transoesophageal echocardiography | 186/275 (67.6%) | 92/157 (58.6%) | 29/46 (63.0%) | 0.05 | 0.5 |
| Location of findings | |||||
| Aortic | 19/275 (6.9%) | 78/157 (49.7%) | 16/46 (34.8%) | <0.0001 | 0.07 |
| Mitral | 16/275 (5.8%) | 75/157 (47.8%) | 15/46 (32.6%) | <0.0001 | 0.06 |
| Tricuspid | 42/275 (15.3%) | 12/157 (7.6%) | 7/46 (15.2%) | 0.02 | 0.1 |
| Pulmonary | 3/275 (1.1%) | 0/157 | 1/46 (2.2%) | 0.5 | 0.2 |
| ICD/PM/other | 205/275 (74.5%) | 5/157 (3.2%) | 25/46 (54.3%) | <0.0001 | <0.0001 |
| Any pericardial effusion | 15/240 (6.3%) | 5/141 (3.5%) | 2/43 (4.7%) | 0.5 | 0.1 |
| Any right ventricular dysfunction | 24/240 (10.0%) | 15/141 (10.6%) | 5/43 (11.6%) | 0.4 | 0.6 |
| Elevating filling pressure | 63/240 (26.3%) | 49/141 (34.8%) | 10/43 (23.3%) | 0.07 | 0.1 |
| Right ventricular systolic pressure (mmHg) | |||||
| N | 159 | 104 | 24 | ||
| Median (IQR) | 38.0 (29.0–45.0) | 40.0 (32.5–48.0) | 41.5 (33.5–52.5) | 0.1 | 0.5 |
| Left ventricular ejection fraction (%) | |||||
| N | 240 | 132 | 37 | ||
| Median (IQR) | 48.5 (33.5–60.0) | 50.0 (40.0–58.5) | 50.0 (40.0–60.0) | 0.1 | 0.9 |
Kruskal–Wallis test is used for quantitative data. χ2 or Fisher’s exact test [a] is used for binary variables. Results are those of the first echocardiography performed after the date of first hospital admission/medical consultation.
ICD, implantable cardioverter-defibrillator.
The three groups of patients are displayed in Tables1 and 2 and Supplementary material online, Table S1. The CDRIE was associated with a tricuspid valve involvement (10.0%).
Imaging
Echocardiography was not always TOE. It was TTE for isolated CDRIE in 88.4% (TOE = 67.6%), for LHIE+CDRIE− TTE = 93.0% (TOE = 58.6%) and for CDRIE+LHIE+, TTE = 87.0% (TOE = 63.0%). By far the majorin cases with IE and a device (CDRIE+), the leading abnormality was a vegetation on the lead. Table 2 is displaying the main echocardiographic features.
Nuclear imaging (PET scan) was used in 135 (28.0%) patients; it was positive in 75.6%.
Management and prognosis
The staphylococcus were dominant and the antibiotic regimen mostly consisted of vancomycin (see Supplementary material online, Tables S2 and S3). A device extraction was required for 62.1% CDRIE+ patients, 10.2% of the LHIE+CDRIE− and 45.7% of CDRIE+LHIE+ according to local endocarditis teams. When device extraction was indicated by the local endocarditis team, CD was removed percutaneously for 57.5% of the CDRIE+LHIE− but 37.5% for the LHIE+CDRIE− and 38.1% of the CDRIE+LHIE+ (Tables 3 and 4 and Supplementary material online, Table S3).
Table 3.
In-hospital follow-up under treatment in patients with device
| CDRIE+LHIE− (1) (n = 280) |
LHIE+CDRIE− (2) (n = 157) |
CDRIE+LHIE+ (3) (n = 46) |
P-value (1) vs. (2) |
P-value (2) vs. (3) |
|
|---|---|---|---|---|---|
| Complications under therapy | |||||
| Embolic events | 35/280 (12.5%) | 25/157 (15.9%) | 12/46 (26.1%) | 0.3 | 0.1 |
| CHF | 39/280 (13.9%) | 28/157 (17.8%) | 5/46 (10.9%) | 0.2 | 0.2 |
| Cardiogenic shock | 17/245 (6.9%) | 14/141 (9.9%) | 1/43 (2.3%) | 0.2 | 0.1 |
| Septic shock | 24/280 (8.6%) | 17/157 (10.8%) | 9/46 (19.6%) | 0.4 | 0.1 |
| Acute renal failure | 54/280 (19.3%) | 42/157 (26.8%) | 15/46 (32.6%) | 0.07 | 0.4 |
| Persistent fever (>7 days) | 30/245 (12.2%) | 12/141 (8.5%) | 6/43 (14.0%) | 0.2 | 0.3 |
| Positive blood cultures after 48h | 45/278 (16.2%) | 23/152 (15.1%) | 16/46 (34.8%) | 0.7 | 0.003 |
| Increasing vegetation size | 12/280 (4.3%) | 9/157 (5.7%) | 5/46 (10.9%) | 0.4 | 0.3 |
| New abscess | 4/280 (1.4%) | 8/157 (5.1%) | 1/46 (2.2%) | 0.03 | 0.6 |
| AV block | 2/245 (0.8%) | 1/141 (0.7%) | 5/43 (11.6%) | >0.9 | 0.002 |
| Thrombopenia (<100 000) | 14/245 (5.7%) | 13/141 (9.2%) | 6/43 (14.0%) | 0.1 | 0.3 |
| Device extraction | 174/280 (62.1%) | 16/157 (10.2%) | 21/46 (45.7%) | <0.0001 | <0.0001 |
| Percutaneous lead extraction | 103/280 (36.8%) | 6/157 (3.8%) | 8/46 (17.4%) | <0.0001 | 0.004 |
| Surgical lead extraction | 74/280 (26.4%) | 10/157 (6.4%) | 13/46 (28.3%) | <0.0001 | <0.0001 |
| Valvular surgery | 12/280 (4.3%) | 55/157 (35.0%) | 12/46 (26.1%) | <0.0001 | 0.2 |
| Positive valve or lead culture | |||||
| No | 133/248 (53.6%) | 67/148 (45.3%) | 22/44 (50.0%) | <0.0001 | 0.6 |
| Positive PCR of valve or lead culture | |||||
| No | 191/280 (68.2%) | 85/157 (54.1%) | 32/46 (69.6%) | 0.004 | 0.1 |
| Suspected source of Infection | |||||
| Health care–associated IE | 66/256 (25.8%) | 25/145 (17.2%) | 11/45 (24.4%) | 0.04 | 0.2 |
Kruskal–Wallis test is used for quantitative data. χ2 or Fisher’s exact test [a] is used for binary variables.
CHF, chronic heart failure; IE, infective endocarditis; PCR, polymerase chain reaction.
Table 4.
(A) Univariate cox regression analysis for all causes of death (until 400 days) with device forced in the model and (B) final cox regression analysis for all causes of death (until 400 days) with status of device forced in the model
| A | Effect | Hazard ratio | 95% CI | P Wald |
|---|---|---|---|---|
| Device status | CDRIE+LHIE+ | 1.81 | [1.04–3.16] | 0.02 |
| LHIE+CDRIE− | 1.59 | [1.08–2.35] | . | |
| Source of infection | Non-nosocomial | 0.85 | [0.41–1.78] | 0.03 |
| Nosocomial | 1.68 | [1.09–2.59] | . | |
| Age (per 10 years) | 1.18 | [1.01–1.37] | 0.03 | |
| Female gender | 0.91 | [0.60–1.37] | 0.6 | |
| Charlson index | 1.11 | [1.06–1.17] | <0.0001 | |
| Creatinine >2 mg/dL | 2.59 | [1.76–3.83] | <0.0001 | |
| Staphylococcus aureus | 1.74 | [1.19–2.55] | 0.004 | |
| Congestive heart failure | 2.07 | [1.36–3.17] | 0.0007 | |
| Vegetation length >10 mm | 1.42 | [0.96–2.11] | 0.07 | |
| Cerebral complication | 3.48 | [1.90–6.36] | <0.0001 | |
| Abscess | 1.07 | [0.54–2.10] | 0.8 | |
| Positive PET (whatever location) | 0.85 | [0.37–1.92] | 0.6 | |
| Heart failure | 1.84 | [1.24–2.72] | 0.002 | |
| Congenital disease | 0.71 | [0.33–1.53] | 0.3 | |
| Ischaemic heart disease | 0.90 | [0.62–1.32] | 0.5 | |
| Chronic renal failure | 2.18 | [1.52–3.14] | <0.0001 | |
| Cancer | 1.29 | [0.81–2.05] | 0.2 | |
| Intravenous drug dependency | 0.89 | [0.12–6.42] | 0.9 | |
| Alcohol abuse | 0.57 | [0.21–1.55] | 0.2 | |
| Diabetes mellitus | 1.39 | [0.97–2.00] | 0.07 | |
| Device extraction | 0.65 | [0.42–0.99] | 0.04 |
| B | Hazard ratio | 95% CI | P Wald |
|---|---|---|---|
| CDRIE+LHIE+ | 1.82 | [1.02–3.22] | 0.04 |
| LHIE+CDRIE− | 1.35 | [0.89–2.04] | 0.1 |
| Creatinine >2 mg/dL | 1.96 | [1.27–3.03] | 0.002 |
| Congestive heart failure | 1.64 | [1.04–2.57] | 0.03 |
| Cerebral complication | 3.41 | [1.85–6.27] | <0.0001 |
| Chronic renal failure | 1.56 | [1.02–2.39] | 0.04 |
Status of device and effects with a P-value of <0.10 in the univariate analysis are taken into account except Charlson index. No correlation was found among these variables. Cox analysis is performed with a backward procedure with SLSTAY = 0.05. Only 400-day survival data were taken into account: deaths occurring after 400 days were censored at 401 days. For type of IE, the reference is CDRIE+LHIE−. Goodness of fit test: P = 0.04. Concordance = 0.69—Global Schoenfeld residual test P = 0.93.
In-hospital mortality was lower in isolated CDRIE 13.2% vs. 22.3% and 30.4% for LHIE+CDRIE− and LHIE+CDRIE+, respectively (P = 0.004).
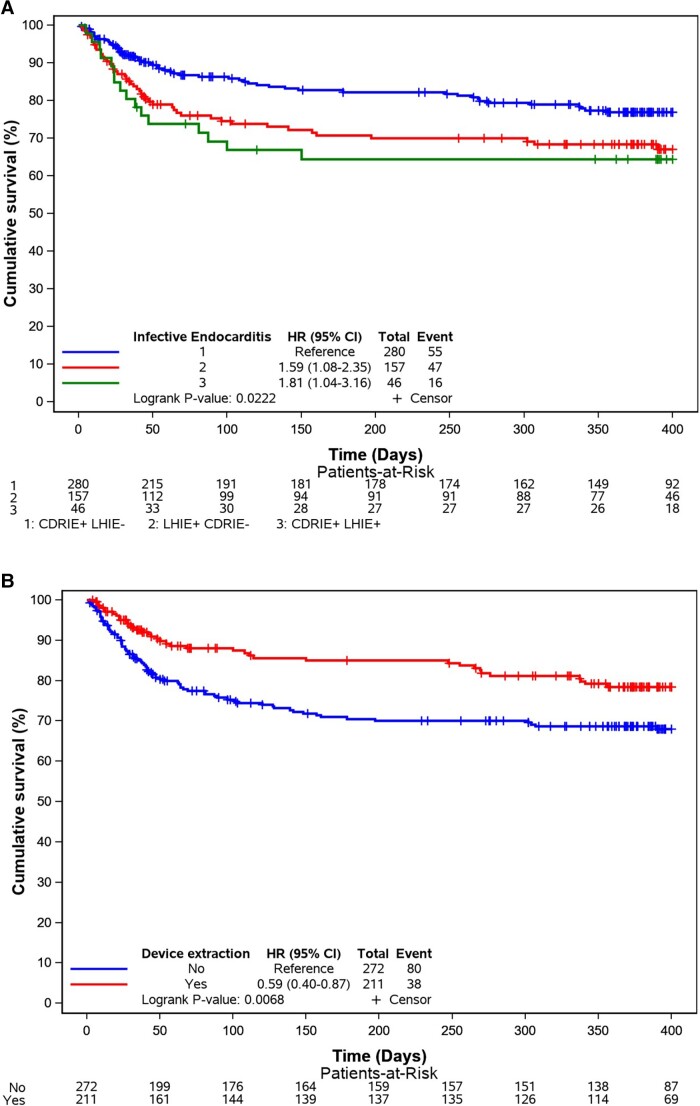
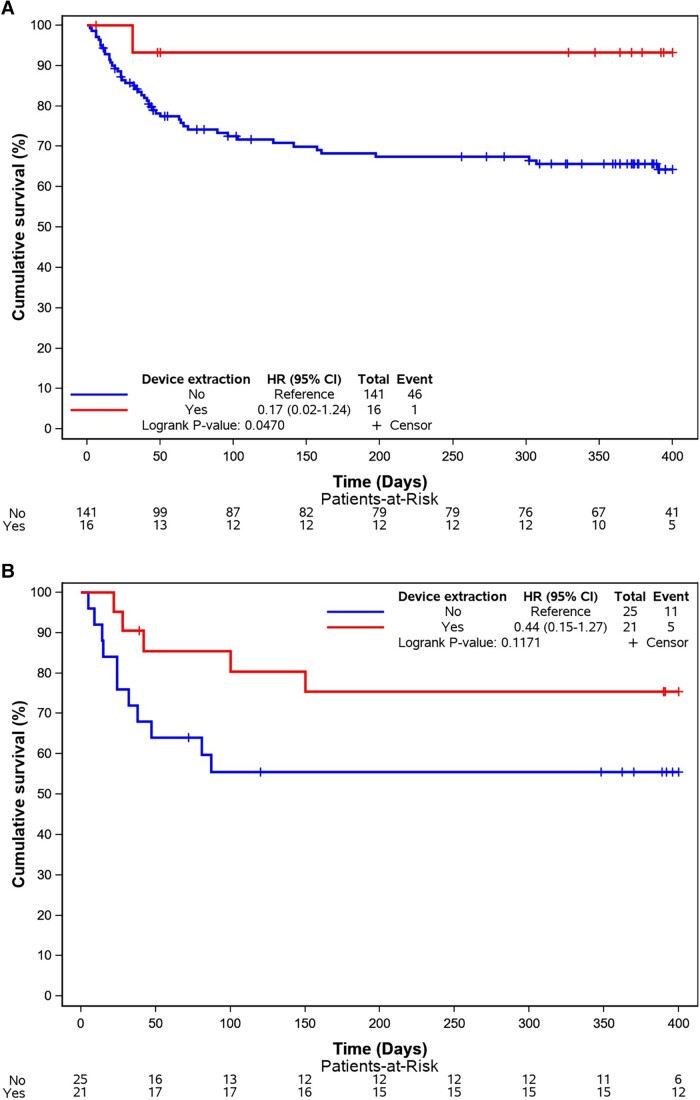
At 400-day follow-up, death occurred for 19.6% (in-hospital death 13.2%) in the CDRIE+LHIE−. 29.9% (in hospital 22.3%) for the CDRIE−LHIE+ and 34.8% (in hospital = 30.4%) for the CDRIE+LHIE+. Univariate and multi-variable Cox regression analysis for 400-day mortality is displayed in Table 4. The Kaplan–Meier curves are displayed in Figures 1 and 2.
Figure 1.
(A) Kaplan–Meier curves for all causes of death according to the three groups. (B) Kaplan–Meier curves for all causes of death according to device extraction—overall. CI, confidence interval; CDRIE, cardiac device–related infective endocarditis; HR, hazard ratio; LHIE, left heart infective endocarditis.
Figure 2.
(A) Kaplan–Meier curves for all causes of death according to device extraction—LHIE+CDRIE. (B) Kaplan–Meier curves for all causes of death according to device extraction—LHIE+CDRIE+. CI, confidence interval; CDRIE, cardiac device–related infective endocarditis; HR, hazard ratio; LHIE, left heart infective endocarditis.
Device extraction was associated with a better prognosis [HR 0.59 (0.40–0.87), P = 0.0068] even in the LHIE+CDRIE− group (P = 0.047).
Discussion
The major findings of this large observational study are the following:
Prognosis of endocarditis in patients with a CD remains poor, particularly when associated with a left-sided IE.
Although recommended by guidelines, device extraction is not always performed in the real life.
Lack of device removal is associated with worse prognosis, even in the LHIE+CDRIE− group
Prognosis of cardiac device–related infective endocarditis
Endocarditis associated with CDs has a high mortality. The risk of death at 1 year has been reported up to 30%. Patients with CDRIE+ are older and have more comorbidities. Factors associated with increased 1-year mortality include left-sided endocarditis and CDRIE removal/reimplantation.12,13 Overall mortality has been reported in EURO-ENDO higher in ≥80 years old but was not different from that of <80 years old among those who had surgery (1 year: 27.3% vs. 25.5%).14 The importance of identifying the microorganism responsible has been underlined previously.15
Importance of device extraction
Our cohort is the largest prospective multi-centre cohort reflecting a worldwide reality of this severe infectious disease. EURO-ENDO underlines, like others,16 the difficulties in following guidelines in daily practice, where both technical and human considerations interfere with their strict appliance. Although S. aureus has gradually replaced streptococci as the primary pathogen for IE overall in different cohorts.17–19 Independent of the pathogen, device extraction is recommended and extremely important for the prognosis. We observed that there is room for improvement and physician should be more aggressive in extracting the devices as much as they can even in LHIE+CDRIE−. Unfortunately, the precise reason(s) for not pursuing extraction have not been reported in EURO-ENDO. The main observation is that no extraction is a risk factor for death at 400 days. This is fundamental and is underscored in the main results of the EURO-ENDO registry. Also the socio-economic condition by countries might impact.7,20 Technical possibility according the hospital have to be taken into account, but still, lead extraction should be push for as much as possible during the endocarditis team discussions. It is really important to extract more the devices in order to improve the prognosis. Data presented were reported by the investigating centres, and patients managed palliatively were also included which impacts the results.20
Importance of imaging
Diagnosis was made based on positive imaging results for at least 85% of the patients. Despite TOE being more sensitive than TTE, it was not performed in every patient. Other imaging tools (cardiac computed tomography, 18 FDG PET-CT, or leucocyte scintigraphy) are valuable in the case of difficult diagnosis.9 The blood cultures were positive of 78.3% of the patients included and a definite positive diagnosis of IE was possible for 82.2% of the patients, with 17.8% not reaching definite diagnostic criteria. New 3D capabilities of echocardiography should be incorporated and applied in every single patient with suspected endocarditis.21 In case of high clinical suspicion without echocardiographic evidence, we should encourage the use of the new imaging modalities.5,7 Applying stringent imaging assessment of patients with endocarditis and device allowed us to define a subgroup of patients who had a valvular infection but no device-related infection. These patients are not exactly the same to the CDRIE, and the device could probably be maintained. It is thus important that more than observed in EURO-ENDO, endocarditis teams should consider, as recommended, a multi-modality approach for improving the diagnosis and best managing the treatment of CDRIE or endocarditis in patients with CDs.5,9
Difficult diagnosis and management but important prognostic implications
EURO-ENDO underlines, like others,16 the difficulties in following guidelines in daily practice, where both technical and human considerations interact with their strict application. The clinical presentation of endocarditis and especially CDRIE is heterogeneous, and the time taken to reach the diagnosis is long and is seen in all centres in this large ESC registry involving centres from all over the world. The prognosis is poor with in-hospital death of 17.8% of included patients (19.8% of exclusively cardiovascular death). This is in line with most cohorts.1,22,23 Most CDRIE are related to interventions (medical or paramedical). Few studies have analysed risk factors for 1-year (400-day) mortality, in patients with CDRIE. Baman et al. found that systemic embolization (HR 7.11), moderate or severe tricuspid regurgitation (HR 4.24), abnormal right ventricular function (HR 3.59), and abnormal renal function (HR 2.98) were the four independent factors associated with 6-month mortality. For Kim et al., only methicillin-resistant S. aureus infection and concomitant valve endocarditis independently predicted mortality. In the International Collaboration on Endocarditis (ICE) registry, the only factor independently associated with 1-year survival was device removal, and this was also the only factor predictive of survival in smaller sample size studies.
In EURO-ENDO, device removal was a fundamental parameter to improve the prognosis. When removal was indicated but could not be performed due to patients’ condition or technical issues, most patients were prescribed chronic suppressive antimicrobial therapy and had a worse prognosis, seen in other studies.1 We demonstrate that the lead extraction should be done but not only when a pre-operative CDRIE is made. In the case of LHIE+CDRIE−, it seems relevant to treat the LHIE but also to extract the intracardiac device.
Limitations
EURO-ENDO is a prospective and large registry involving centres worldwide.8 Some data were not collected as they were focused on the application of the guidelines and especially the integration of imaging techniques in the diagnosis and management of the endocarditis,7–9 for instance, the time duration between the device implantation and the occurrence of CDRIE and the pocket description. In EURO-ENDO, we were not able to distinguish early and late IE after the device implantation. Also, as previously mentioned, the precise reasons for not performing the extraction are not reported extensively in the registry as well as the details about the techniques available for performing the extraction.
Conclusion
Prognosis of endocarditis in patients with a CD remains poor. Device extraction is key to improving outcomes, even when CDRIE is not diagnosed in a LHIE+ patient.
Supplementary Material
Acknowledgements
The authors acknowledge EORP Oversight Committee, Registry Executive, and Steering Committees. The data collection was conducted by the EORP department of the ESC: Emanuela Fiorucci, as Project Officer, and Viviane Missiamenou, Florian Larras, and Rachid Mir Hassaine, as Data Managers. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Dr Aldo P. Maggioni (EORP Scientific Coordinator).
Special thanks to the EACVI (European Association of CardioVascular Imaging) and to the ESC Working Group on Valvular Heart Disease for their support.
Contributor Information
Erwan Donal, Cardiologie, CHU de RENNES, LTSI UMR1099, INSERM, Université de Rennes-1, hopital pontchaillou, 35000 Rennes, France.
Christophe Tribouilloy, Department of Cardiology, Amiens University Hospital Amiens, Amiens 80000, France.
Anita Sadeghpour, Echocardiography Research Centre, Rajaie Cardiovascular Medical and Research Centre, Iran University of Medical Sciences, Tehran, Iran.
Cécile Laroche, European Society of Cardiology, EORP, Sophia-Antipolis, France.
Ana Clara Tude Rodrigues, servico de Echocardiografia—InRad-HC—Faculdade de Medicina, Universidade de Sao Paulo, SP, Brazil.
Maria do Carmo Pereira Nunes, Serviço de Cardiologia e Cirurgia Cardiovascular e Centro de Telessaúde, Hospital das Clínicas da Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil.
Duk-Hyun Kang, Department of Cardiology, Asan Medical Center, University of Ulsan College of Medicine, 388-1, Poongnap-dong, Songpa-gu, Seoul 138-736, Korea.
Marta Hernadez-Meneses, Infectious Diseases Service, Hospital Clinic-IDIBAPS, University of Barcelona, Barcelona, Spain.
Zhanna Kobalava, Department of Cardiology, RUDN Univerisity, Moscow, Russia.
Michele De Bonis, Cardiac Surgery, Innovation and Research, ‘Vita-Salute’ San Raffaele University Hospital, Milan 20132, Italy.
Rafal Dworakowski, Department of Cardiology, Kings College Hospital and King's College London, Denmark Hill, London SE5 9RS, UK.
Branislava Ivanovic, Clinical Center of Serbia, Clinic of Cardiology, Belgrade, Serbia.
Maria Holicka, Department of Cardiology, University Hospital Brno, Jihlavska 20, Brno 62500, Czech Republic.
Takeshi Kitai, Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, Kobe, Japan.
Ines Cruz, Departamento de Cardiologia, Hospital Garcia de Orta, Almada, Portugal.
Olivier Huttin, F-CRIN INI-CRCT Cardiovascular and Renal Clinical Trialists Network, INSERM 1116, CHRU de Nancy, Nancy, France.
Paolo Colonna, Department of Cardiology, Polyclinic of Bari—Hospital, Bari 70124, Italy.
Patrizio Lancellotti, Department of Cardiology, Heart Valve Clinic, GIGA Cardiovascular Sciences, CHU Sart Tilman, University of Liege Hospital, Liege, Belgium; Department of Cardiology, Gruppo Villa Maria Care and Research, Anthea Hospital, Bari, Italy.
Gilbert Habib, APHM, Cardiology Department, La Timone Hospital, Marseille, France; IRD, APHM, MEPHI, IHU-Méditerranée Infection, Aix Marseille University, Marseille, France.
Lead author biography
 Prof. Erwan Donal, at the University Hospital of RENNES, is a senior cardiologist for heart valve diseases and cardiomyopathies, head of the echocardiography laboratory of the cardiology department (23000 exams /year), and head of the imaging Core Lab at the CIC-IT INSERM 1414, accredited by ISO 9001 and certified by large industry partners; senior researcher at the LTSI (laboratoire du traitement du signal et de l’image) INSERM 1099 University Rennes-1 (www.ltsi.univ-rennes1.fr); current secretary of the EACVI Board; and current president of the French group for valvular heart disease.
Prof. Erwan Donal, at the University Hospital of RENNES, is a senior cardiologist for heart valve diseases and cardiomyopathies, head of the echocardiography laboratory of the cardiology department (23000 exams /year), and head of the imaging Core Lab at the CIC-IT INSERM 1414, accredited by ISO 9001 and certified by large industry partners; senior researcher at the LTSI (laboratoire du traitement du signal et de l’image) INSERM 1099 University Rennes-1 (www.ltsi.univ-rennes1.fr); current secretary of the EACVI Board; and current president of the French group for valvular heart disease.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
Abbott Vascular Int (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), The Bristol Myers Squibb and Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards (2016–2019), Gedeon Richter Plc. (2014–2016), Menarini Int. Op. (2009–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi (2009–2011), SERVIER (2009–2021), and Vifor (2019–2022)
References
- 1. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha Y-M, Clancy J, Deharo J-C, Ellenbogen KA, Exner D, Hussein AA, Kennergren C, Krahn A, Lee R, Love CJ, Madden RA, Mazzetti HA, Moore JC, Parsonnet J, Patton KK, Rozner MA, Selzman KA, Shoda M, Srivathsan K, Strathmore NF, Swerdlow CD, Tompkins C, Wazni O. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–ee51. [DOI] [PubMed] [Google Scholar]
- 2. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NAM, Gewitz M, Newburger JW, Schron EB, Taubert KA. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American heart association. Circulation 2010;121:458–477. [DOI] [PubMed] [Google Scholar]
- 3. Deshmukh A, Patel N, Noseworthy PA, Patel AA, Patel N, Arora S, Kapa S, Noheria A, Mulpuru S, Badheka A, Fischer A, Coffey JO, Cha YM, Friedman P, Asirvatham S, Viles-Gonzalez JF. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation 2015;132:2363–2371. [DOI] [PubMed] [Google Scholar]
- 4. Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythmia Electrophysiol 2016;9:e003929. [DOI] [PubMed] [Google Scholar]
- 5. Habib G, Lancellotti P, Iung B. 2015 ESC guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart 2016;102:992–994. [DOI] [PubMed] [Google Scholar]
- 6. Rigau PV, Moral S, Bosch D, Morales M, Frigola JM, Albert X, Robles R, Ballesteros E, Roqué M, Aboal J, Brugada R. Clinical prognosis of right-sided infective endocarditis not associated with cardiac devices or intravenous drug use: a cohort study and meta-analysis. Sci Rep 2020;10:7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, Popescu BA, Prendergast B, Tornos Pilar, Sadeghpour A, Oliver L, Vaskelyte J-J, Sow R, Axler O, Maggioni AP, Lancellotti P, Gale CP, Beleslin B, Budaj A, Chioncel O, Dagres N, Danchin N, Emberson J, Erlinge D, Glikson M, Gray A, Kayikcioglu M, Maggioni A P, Nagy VK, Nedoshivin A, Petronio A-S, Roos-Hesselink J, Wallentin L, Zeymer U, Habib G, Lancellotti P; EURO-ENDO Investigators . Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J 2019;40:3222–3232. [DOI] [PubMed] [Google Scholar]
- 8. Habib G, Lancellotti P, Erba PA, Sadeghpour A, Meshaal M, Sambola A, Furnaz S, Citro R, Ternacle J, Donal E, Cosyns B, Popescu B, Iung B, Prendergast B, Laroche C, Tornos P, Pazdernik M, Maggioni A, Gale CP; EURO-ENDO Investigators . The ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur Heart J Qual Care Clin Outcomes 2019;5:202–207. [DOI] [PubMed] [Google Scholar]
- 9. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, Khoury GE, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Mas PT, Vilacosta I, Zamorano JL. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 10. Dumont E, Camus C, Victor F, de Place C, Pavin D, Alonso C, Mabo P, Daubert JC. Suspected pacemaker or defibrillator transvenous lead infection. Prospective assessment of a TEE-guided therapeutic strategy. Eur Heart J 2003;24:1779–1787. [DOI] [PubMed] [Google Scholar]
- 11. Camus C, Donal E, Bodi S, Tattevin P. Pacemaker and implantable cardioverter defibrillator infections. Med Mal Infect 2010;40:429–439. [DOI] [PubMed] [Google Scholar]
- 12. Gaitan R M, Boix-Palop L, Munoz Garcia P, Mestres CA, Marin Arriaza M, Pedraz Prieto A, Boix-Palop L, Muñoz García P, Mestres CA, Marín Arriaza M, Pedraz Prieto Á, de Alarcón Gonzalez A, Gutiérrez Carretero E, Hernández Meneses M, Goenaga Sánchez MÁ, Cobo Belaustegui M, Oteo Revuelta JA, Gainzarain Arana JC, García Vázquez E, Martínez-Sellés M. Infective endocarditis in patients with cardiac implantable electronic devices: a nationwide study. Europace 2020;22:1062–1070. [DOI] [PubMed] [Google Scholar]
- 13. Urien JM, Camus C, Leclercq C, Dejoies L, Mabo P, Martins R, Boukthir S, Bénézit F, Behar N, Revest M, Bodi S, Bila J, Donal E, Tattevin P. The emergence of Staphylococcus aureus as the primary cause of cardiac device–related infective endocarditis. Infection 2021;49:999–1006. [DOI] [PubMed] [Google Scholar]
- 14. Pazdernik M, Iung B, Mutlu B, Alla F, Riezebos R, KongLa W, Nunes MCP, Pierard L, Srdanovic I, Yamada H, De Martino A, Miglioranza MH, Magne J, Piper C, roche C, Maggioni AP, Lancellotti P, Habib G, Selton-Suty C; EURO-ENDO Investigators . Surgery and outcome of infective endocarditis in octogenarians: prospective data from the ESC EORP EURO-ENDO registry. Infection 2022;50:1191–1202. [DOI] [PubMed] [Google Scholar]
- 15. Kong WKF, Salsano A, Giacobbe DR, Popescu BA, Laroche C, Duval X, Schueler Robert, Moreo A, Colonna P, Piper C, Calvo-Iglesias F, Badano LP, Srdanovic I, Boutoille D, Huttin O, Stöhr E, Timóteo AT, Vaskelyte JJ, Sadeghpour A, Tornos P, Abid L, Poh KK, Habib G, Lancellotti P, Habib G. Outcomes of culture-negative vs. Culture-positive infective endocarditis: the ESC-EORP EURO-ENDO registry. Eur Heart J 2022;43:2770–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Ciancio G, Erpelding ML, Filippetti L, Goehringer F, Blangy H, Huttin O, Agrinier N, Juillière Y, Sadoul N, Selton-Suty C. Adherence to diagnostic and therapeutic practice guidelines for suspected cardiac implantable electronic device infections. Arch Cardiovasc Dis 2021;114:634–646. [DOI] [PubMed] [Google Scholar]
- 17. Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230–1239. [DOI] [PubMed] [Google Scholar]
- 18. Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JTM, Elliott TSJ, Levine DP, Bayer AS; ICE Investigators . Staphylococcus aureus endocarditis: a consequence of medical progress. Jama 2005;293:3012–3021. [DOI] [PubMed] [Google Scholar]
- 19. Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators . Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sengupta SP, Prendergast B, Laroche C, Furnaz S, Ronderos R, Almaghraby A, Asch FM, Blechova K, Zaky H, Strahilevitz J, Dworakowski R, Miyasaka Y, Sebag I, Izumi C, Axler O, Jamiel A, Philip M, Campos Vieira ML, Lancellotti P, Habib G. Socioeconomic variations determine the clinical presentation, aetiology, and outcome of infective endocarditis: a prospective cohort study from the ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur Heart J Qual Care Clin Outcomes 2022;9:85–96. [DOI] [PubMed] [Google Scholar]
- 21. Sifaoui I, Oliver L, Tacher V, Fiore A, Lepeule R, Moussafeur A, Huguet R, Teiger E, Audureau E, Derbel H, Luciani A, Kobeiter H, Lim P, Ternacle J, Deux J-F. Diagnostic performance of transesophageal echocardiography and cardiac computed tomography in infective endocarditis. J Am Soc Echocardiogr 2020;33:1442–1453. [DOI] [PubMed] [Google Scholar]
- 22. Rohacek M, Baddour LM. Cardiovascular implantable electronic device infections: associated risk factors and prevention. Swiss Med Wkly 2015;145:w14157. [DOI] [PubMed] [Google Scholar]
- 23. Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, Spry M. J., Steeds R. P, Tayebjee M. H., Watkin R; British Society for Antimicrobial Chemotherapy, British Heart Rhythm Society; British Cardiovascular Society, British Heart Valve Society; British Society for Echocardiography . Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint working party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemotherapy 2015;70:325–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author