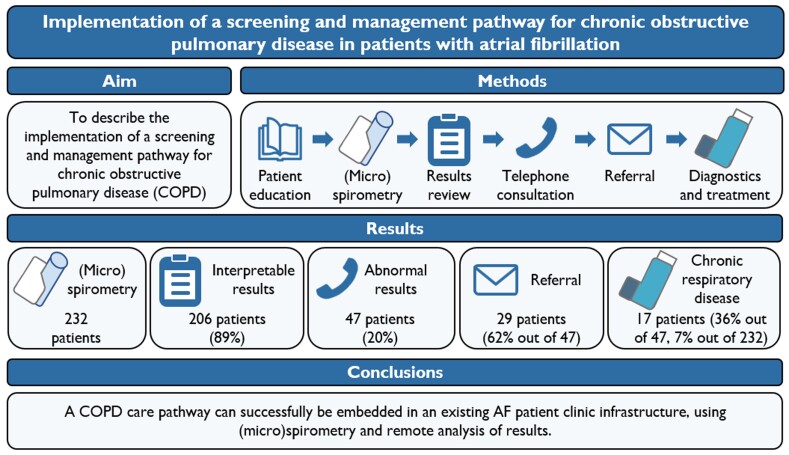
Abstract
Aims
Chronic obstructive pulmonary disease (COPD) negatively impacts the efficacy of heart rhythm control treatments in patients with atrial fibrillation (AF). Although COPD is recognized as a risk factor for AF, practical guidance about how and when to screen for COPD is not available. Herein, we describe the implementation of an integrated screening and management pathway for COPD into the existing pre-ablation work-up in an AF outpatient clinic infrastructure.
Methods and results
Consecutive unselected patients accepted for AF catheter ablation in the Maastricht University Medical Center+ were prospectively screened for airflow limitation using handheld (micro)spirometry at the pre-ablation outpatient clinic supervised by an AF nurse. Patients with results suggestive of airflow limitation were offered referral to the pulmonologist. Handheld (micro)spirometry was performed in 232 AF patients, which provided interpretable results in 206 (88.8%) patients. Airflow limitation was observed in 47 patients (20.3%). Out of these 47 patients, 29 (62%) opted for referral to the pulmonologist. The primary reason for non-referral was low perceived symptom burden. Using this screening strategy 17 (out of 232; 7.3%) ultimately received a diagnosis of chronic respiratory disease, either COPD or asthma.
Conclusion
A COPD care pathway can successfully be embedded in an existing AF outpatient clinic infrastructure, using (micro)spirometry and remote analysis of results. Although one out of five patients had results suggestive of an underlying chronic respiratory disease, only 62% of these patients opted for a referral. Pre-selection of patients as well as patient education might increase the diagnostic yield and requires further research.
Keywords: Atrial fibrillation, Chronic obstructive pulmonary disease, Screening, Microspirometry, Care pathway
Graphical Abstract
Graphical abstract.
What’s new?
One out of five AF patients at the pre-ablation outpatient clinic had airflow limitation on (micro)spirometry.
Screening with (micro)spirometry in this patient population is feasible, with 88.8% of patients being able to achieve interpretable results.
A screening and management pathway for chronic obstructive pulmonary disease can successfully be embedded in an existing AF outpatient clinic infrastructure.
Introduction
Detection and management of comorbidities associated with atrial fibrillation (AF) is recommended in the European Society of Cardiology (ESC) AF guidelines as one of the pillars of the comprehensive Atrial fibrillation Better Care (ABC) management pathway.1 Besides many other comorbidities, chronic obstructive pulmonary disease (COPD), present in approximately 13% of AF patients,2–4 has recently been introduced as an emerging AF risk factor. COPD is associated with higher symptom burden, worse quality of life, worse cardiovascular outcomes, and increased hospital admissions and all-cause mortality in AF patients.5–7 Moreover, it is also associated with AF progression and reduced efficacy of rhythm control treatments such as cardioversion and catheter ablation.8–12
Despite the fact that COPD is highly prevalent worldwide and is recognized as a relevant AF risk factor in international AF management guidelines, practical guidance as well as recommendations about how and when to test and screen for COPD are not available.1,3,13,14 Such screening strategies could identify patients at risk of a poor outcome and adequate diagnosis and treatment of COPD will likely improve symptom burden through improvement of overlapping symptoms such as dyspnoea.15 Nevertheless, whether treatment of COPD improves the outcome of AF therapy has yet to be determined. Developing and employing screening and diagnostic pathways is necessary to appraise the impact of treatment strategies targeting comorbidities in the aging multi-morbid AF population. A recent survey performed within the European Heart Rhythm Association (EHRA)-PATHS project, which is aimed at developing such new care pathways, demonstrated challenges such as the lack of an integrated care model, organizational or institutional issues and issues with patient adherence, and the consortium consequently calls for a systematic, integrated management of AF-related comorbidities, including COPD.14,16
The aim of this study was to describe the implementation of a screening and management pathway for COPD into the existing pre-ablation work-up in an AF outpatient clinic infrastructure and the integration of the results of the pulmonary assessment within a multidisciplinary team.
Methods
Study design and population
From August 2021 until June 2022, all consecutive patients with electrocardiogram (ECG)-documented symptomatic AF accepted for AF ablation according to local protocol17 in the Maastricht University Medical Center+ (MUMC+, Maastricht, the Netherlands) were prospectively screened for COPD during a visit to the AF ablation clinic. Such a visit consists of all necessary investigations before ablation and a consultation with a specialized AF nurse and is described in detail elsewhere.18 The screening strategy consisted of (micro)spirometry supervised by trained AF nurses and is described in more detail below. If patients had airflow limitation on (micro)spirometry, they were offered a referral to a pulmonologist, and extensive pulmonary function testing was performed to confirm the diagnosis of COPD. Patients with previously diagnosed COPD were not excluded from the pathway but were only referred if the patients had not been systematically assessed by formal lung function tests according to the criteria we used in the study, if their last follow-up by a pulmonologist was more than 2 years ago and/or if they reported uncontrolled dyspnoea as a marker of potentially uncontrolled COPD.
This implementation study was part of the prospective ‘IntenSive mOlecular and eLectropathological chAracterization of patienTs undergoIng atrial fibrillatiOn ablatioN’ (ISOLATION) (ClinicalTrials.gov identifier: NCT04342312) and ‘clinical electrophysiology registry of MUMC+ and Radboudumc’ studies.17 The studies were approved by the ethical review board MUMC+/Maastricht University [UM, NL number: 70787.068.19/METC number: 19-052] and complied with the Declaration of Helsinki. All participants provided written informed consent.
Chronic obstructive pulmonary disease screening and management pathway
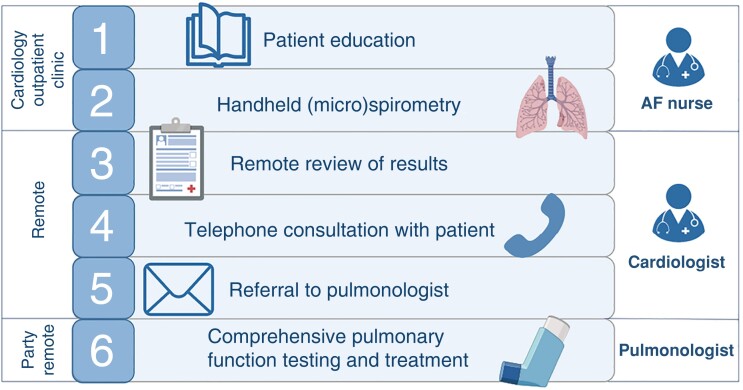
The COPD screening and management pathway comprises several steps (Figure 1). During a patient’s visit to the AF outpatient clinic for a pre-ablation work-up, a specialized AF nurse informed the patient about the conceivably negative impact of COPD on symptom burden, quality of life, and the efficacy of rhythm control interventions such as ablation (Step 1). Subsequently, the AF nurse performed a handheld pre-bronchodilator (micro)spirometry in each patient according to a standardized protocol as described below (Step 2). These results were then uploaded into the hospital’s electronic patient record and reviewed by a cardiologist (Step 3). In case of airflow limitation, patients were contacted to discuss the results and referral to a pulmonologist (Step 4). To avoid referral bias based on a pre-existing diagnosis, the cardiologist contacted patients solely based on the (micro)spirometry results without considering pre-existing pulmonary diagnoses in the patient’s medical history. However, patients with a previous COPD diagnosis were only referred if one of the predefined criteria for referral in these patients was met. If the patient opted for a referral, the cardiologist sent a request to the pulmonology department (Step 5). The patient was then scheduled for a full pulmonary function test and for consultation with a pulmonologist to discuss the results and further analysis. Pulmonary function testing included spirometry before and after bronchodilation, testing of diffusion capacity, body plethysmography, and if indicated methacholine provocation testing.19 If asthma was suspected as the underlying cause of airflow limitation, this was further characterized using laboratory testing (eosinophils, Immunoglobulin E), airway inflammation (fractional exhaled nitric oxide), and allergy testing (skin prick or Phadiatop®). Pulmonary function testing was performed by pulmonary function analysists who were blinded to the results of the handheld (micro)spirometry device. The pulmonologist initiated inhaler treatment based on the GOLD strategy20 and the preferences of the patient, with the patient actively involved in the decision-making process (Step 6).
Figure 1.
Chronic obstructive pulmonary disease screening and management pathway.
Handheld devices for chronic obstructive pulmonary disease screening
From August 2021 until December 2021 the micro-spirometry Vitalograph COPD-6 device (Vitalograph, Ireland) was used for COPD screening. From January 2022 onwards, this was replaced by the handheld spirometry AioCare device (Healthup, Poland) in order to enable a more digital and remote assessment. Handheld (micro)spirometry was supervised by a specialized AF nurse who had been trained according to the device-specific instructions beforehand. This training was given once and comprised of a short introduction to the device and a tutorial on how to perform spirometry adequately. Patients were asked to perform three measurements. If the device indicated the measurements to be of insufficient quality, the patient was asked to perform additional measurements up to a maximum of eight attempts, according to American Thoracic Society guidelines.21 The best out of three measurements was used for clinical decision-making. The micro-spirometry Vitalograph COPD-6 device measures the forced expiratory volume (FEV) during the first second (FEV1) and during the first six seconds (FEV6). Subsequently, a ratio between FEV1/FEV6 is calculated. Based on previous studies, a cut-off value of ≤0.73 was used.22–25 The AioCare device is a handheld spirometry device and thus measures FEV1 and forced vital capacity (FVC) and calculates the FEV1/FVC ratio.26 According to spirometry guidelines, a cut-off value of <0.70 was used. The diagnostic accuracy of both devices is good when compared to routine spirometry26,27 and both devices have previously been tested for their validity and efficacy to screen for COPD.22,28
Questionnaires and classifications
The EHRA classification was used to assess and quantify AF-related symptoms.29 Several dyspnoea and COPD-related questionnaires were incorporated into the pre-ablation AF clinic to test a posteriori different screening scenarios (either only handheld (micro)spirometry or handheld (micro)spirometry with questionnaires). The modified Medical Research Council (mMRC) dyspnoea scale was used to measure the impact of dyspnoea on daily activities on a scale from 0 to 4, with a mMRC score of 0 meaning ‘breathlessness only on strenuous exercise’ and a mMRC score of 4 meaning ‘too breathless to leave the house, or breathless when dressing or undressing’.30 A Respiratory Health Screening Questionnaire (RHSQ) from the Dutch Lung Foundation, also named ‘COPD risk test’, was used to assess the risk of COPD.31 This validated questionnaire contains 10 simple questions about age (0, 4, or 8 points), smoking history (0, 2, 3, or 7 points), body mass index (0, 1, or 5 points), and five different respiratory complaints (0 or 3–4 points each). The risk of COPD is then calculated by adding the points of the individual items (score 0–36), with <16.5 points being low risk, 16.5–19.5 points being medium risk, and >19.5 points being high risk. The COPD assessment test (CAT) was used to quantify potential COPD-related symptoms.32 It consists of eight items presented as a six-point scale, with a minimum score of 0 (‘this symptom is not present at all’) and a maximum score of 5 (‘this symptom is very disabling’) per item. Total scores may range from 0 to 40 points. Scores of <10 points indicate mild clinical impact, whereas scores of ≥10 points indicate moderate to more severe clinical impact.20
Clinical diagnosis of chronic obstructive pulmonary disease
We defined airflow limitation as the pathological reduction in airflow from the lungs that leads to a reduced FEV1/FEV6 or FEV1/FVC ratio, depending on the device.19 Since screening (micro)spirometry only included pre-bronchodilator spirometry without any clinical evaluation, only a presumptive diagnosis of ‘obstructive pulmonary disease’ or ‘airflow limitation’ could be given after abnormal (micro)spirometry results.
Patients received a final diagnosis of COPD after full pulmonary function testing and clinical evaluation by a pulmonologist. A diagnostic strategy for pulmonary function interpretation was followed according to international guidelines.19 Following GOLD recommendations, COPD was defined as an FEV1/FVC ratio below 0.7 after bronchodilation in a patient with persistent respiratory symptoms, a history of smoking (or other relevant exposure), and the absence of other known causes of obstructive pulmonary disease (such as asthma or bronchiectasis).20 The severity of the airflow obstruction was defined as the percentage predicted and divided into mild (>80%), moderate (50–80%), severe (30–50%), and very severe (<30%) airflow obstruction.20 Though initially set up to screen for COPD, other lung diseases such as asthma were also considered as a part of pulmonology work-up. Asthma was diagnosed following international guidelines.33,34
Statistical analysis
The primary outcome was the percentage of patients with airflow limitation that could be identified using the abovementioned single (micro)spirometry screening strategy. Feasibility of the screening strategy was defined as the percentage of patients who were able to perform at least one correct measurement. Diagnostic yield of the care pathway was defined as the percentage of patients with airflow limitation on handheld (micro)spirometry correctly identified with a definite clinical diagnosis of obstructive pulmonary disease. A sensitivity analysis was performed a posteriori to evaluate whether different screening questionnaires could enhance the detection of underlying obstructive pulmonary disease.
All continuous variables were tested for normality with the Shapiro–Wilk test. Continuous normally distributed variables were presented as mean (standard deviation, SD), whereas continuous non-normally distributed variables were presented as median (interquartile range, IQR). Categorical variables were presented as counts (no.) with percentages (%). Continuous variables were analysed using an independent samples t-test or a Mann–Whitney U test, as appropriate. Categorical variables were analysed with a chi-square test or a Fisher’s exact test, as appropriate. A P-value of <0.05 was considered statistically significant. All analyses were conducted using IBM SPSS version 28.
Results
Study population
From August 2021 until June 2022, a total of 232 patients were screened using handheld (micro)spirometry devices. In total, 92 (39.7%) patients were screened using the Vitalograph COPD-6 device, whereas the remaining 140 (60.3%) patients were screened using the AioCare device. The baseline characteristics of these patients are presented in Table 1. There were no significant differences in patient characteristics between the patients who used the COPD-6 device and those who used the AioCare device.
Table 1.
Baseline characteristics of included patients
| Total (N = 232) | Vitalograph COPD-6 (N = 92) | AioCare (N = 140) | P-value | |
|---|---|---|---|---|
| Age (years) | 66 [59–71] | 66 [59–71] | 65 [59–71] | 0.907 |
| Male | 158 (68.1) | 60 (65.2) | 98 (70.0) | 0.445 |
| BMI (kg/m2) | 27.8 [25.4–30.5] | 27.7 [25.4–30.3] | 27.8 [25.3–31.1] | 0.614 |
| Hypertension | 106 (45.7) | 42 (45.7) | 64 (45.7) | 0.993 |
| Diabetes mellitus | 21 (9.1) | 8 (8.7) | 13 (9.3) | 0.878 |
| Hypercholesterolemia | 55 (23.7) | 17 (18.5) | 38 (27.1) | 0.129 |
| Coronary artery disease | 30 (12.9) | 13 (14.1) | 17 (12.1) | 0.659 |
| Heart failure | 36 (15.7) n = 229 | 12 (13.0) | 24 (17.1) n = 137 | 0.362 |
| Thromboembolic events | 26 (11.2) | 10 (10.9) | 16 (11.4) | 0.895 |
| COPD (previously diagnosed) | 14 (6.0) | 7 (7.6) | 7 (5.0) | 0.414 |
| Smoking—currently or previously | 88 (39.1) n = 225 | 31 (33.7) n = 88 | 57 (40.7) n = 137 | 0.339 |
| CHA2DS2-VASc score | 2 [1–3] | 2 [1–3] | 2 [1–3] | 0.974 |
| AF characteristics | ||||
| AF type | Paroxysmal—146 (63.5) | Paroxysmal—60 (65.9) | Paroxysmal—86 (61.9) | 0.531 |
| Persistent—84 (36.5) n = 230 | Persistent—31 (34.1) n = 91 | Persistent—53 (37.9) n = 139 | ||
| EHRA classification | I–12 (5.2) | I–6 (6.5) | I–6 (4.3) | 0.750 |
| II–167 (72.0) | II–65 (70.7) | II–102 (72.9) | ||
| III–53 (22.8) | III–21 (22.8) | III–32 (22.9) | ||
| Self-reported dyspnoea | 78 (33.6) | 31 (33.7) | 47 (33.6) | 0.938 |
| Cardiovascular medication | ||||
| Beta-blockers | 104 (44.8) | 45 (48.9) | 59 (42.1) | 0.310 |
| Digitalis | 18 (7.8) | 9 (9.8) | 9 (6.4) | 0.350 |
| Antiarrhythmic drugsa | 117 (50.4) | 45 (48.9) | 72 (51.4) | 0.708 |
| NCCB | 13 (5.6) | 5 (5.4) | 8 (5.7) | 0.928 |
| RAS inhibitors | 92 (39.7) | 39 (42.4) | 53 (37.9) | 0.490 |
| MRA | 1 (0.4) | 0 | 1 (0.7) | 1.000 |
| Diuretics | 39 (16.8) | 15 (16.3) | 24 (17.1) | 0.867 |
| DCCB | 20 (8.6) | 8 (8.7) | 12 (8.6) | 0.974 |
| Statins | 85 (36.6) | 34 (37.0) | 51 (36.4) | 0.935 |
| Vasodilators | 12 (5.2) | 3 (3.3) | 9 (6.4) | 0.287 |
| VKA | 3 (1.3) | 0 | 3 (2.1) | 0.279 |
| NOAC | 221 (96.5) n = 228 | 88 (96.7) n = 91 | 133 (96.4) n = 137 | 1.000 |
Number provided in italics indicates the total number of patients available for that variable. Data is presented as no. (%) or median [IQR].
Abbreviations: AF, atrial fibrillation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DCCB, dihydropyridine calcium channel blocker; EHRA, European Heart Rhythm Association; MRA, mineralocorticoid receptor antagonist; NOAC, non-vitamin K antagonist oral anticoagulant; NCCB, non dihydropyridine calcium channel blocker; RAS, renin–angiotensin system; VKA, vitamin K antagonist.
Either flecainide, amiodarone, or sotalol.
Handheld (micro)spirometry in the AF outpatient clinic
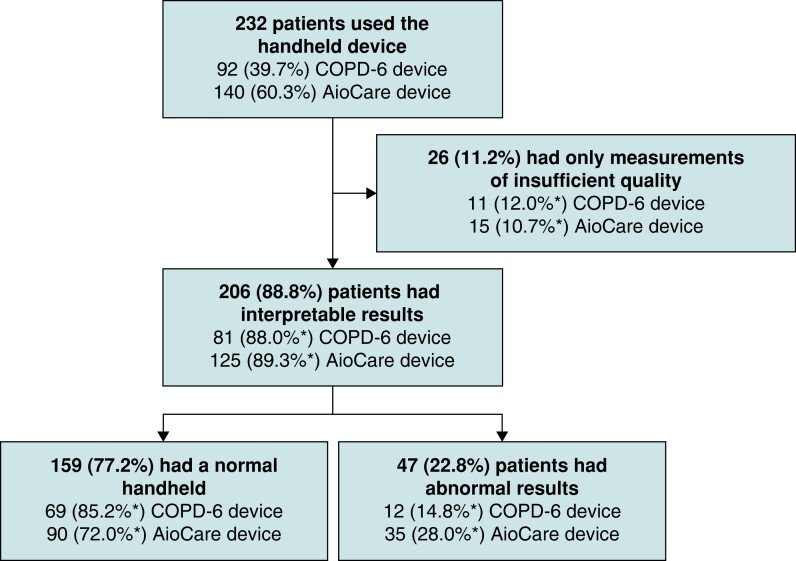
An overview of the results of the handheld (micro)spirometry is presented in Figure 2.
Figure 2.
Flowchart of handheld (micro)spirometry screening for chronic obstructive pulmonary disease. *percentages per device.
The majority of patients (n = 206, 88.8%) were able to perform the handheld (micro)spirometry measurements correctly at least once and thus had interpretable results. Twenty-six patients did not have any correctly performed measurements and were therefore excluded from further analysis, resulting in feasibility of this diagnostic care pathway of 88.8%. Characteristics of these patients are presented in Supplementary material online, Table S1.
In 47 patients (22.8% out of 206), the best-out-of-three value on handheld (micro)spirometry was below the cut-off value and thus suggestive of airflow limitation. Thirty-five of these patients were identified using the AioCare device. Characteristics of patients with vs. patients without airflow limitation on (micro)spirometry are presented in Table 2. Except for the fact that patients with airflow limitation were more likely to have persistent AF (51.1% vs. 32.3%, P = 0.019), there were no significant differences in characteristics.
Table 2.
Characteristics of patients with vs. without airflow limitation on (micro)spirometry and characteristics of patients with a final diagnosis of obstructive pulmonary disease
| Normal screening (micro)spirometry (N = 159) | Airflow limitation (N = 47) | P-value | Obstructive pulmonary disease (N = 17) | |
|---|---|---|---|---|
| Age (years) | 64 [58–71] | 68 [63–72] | 0.056 | 66 [62–72] |
| Male | 104 (65.4) | 36 (76.6) | 0.149 | 12 (70.6) |
| BMI (kg/m2) | 28.0 [25.5–30.8] | 27.4 [25.3–30.5] | 0.537 | 29.4 [25.1–31.0] |
| Hypertension | 71 (44.7) | 20 (42.6) | 0.799 | 9 (52.9) |
| Diabetes mellitus | 15 (9.4) | 4 (8.5) | 1.000 | 1 (5.9) |
| Hypercholesterolemia | 42 (26.4) | 9 (19.1) | 0.311 | 5 (29.4) |
| Coronary artery disease | 21 (13.2) | 9 (19.1) | 0.310 | 3 (17.6) |
| Heart failure | 24 (15.4) n = 156 | 9 (19.1) | 0.540 | 3 (17.6) |
| Thromboembolic events | 23 (14.5) | 3 (6.4) | 0.210 | 1 (5.9) |
| Smoking—currently or previously | 60 (39.0) n = 154 | 21 (45.7) n = 46 | 0.417 | 9 (52.9) |
| CHA2DS2-VASc score | 2 [1–3] | 2 [1–3] | 0.579 | 2 [1–3] |
| AF characteristics | ||||
| AF type | Paroxysmal—107 (67.7) | Paroxysmal—23 (48.9) | 0.019 | Paroxysmal—7 (41.2) |
| Persistent—51 (32.3) n = 158 | Persistent—24 (51.1) | Persistent—10 (58.8) | ||
| EHRA classification | I–7 (4.4) | I–3 (6.4) | 0.380 | I–1 (5.9) |
| II–118 (74.2) | II–30 (63.8) | II–12 (70.6) | ||
| III–34 (21.4) | III–14 (29.8) | III–4 (23.5) | ||
| Self-reported dyspnoea | 57 (35.8) | 17 (36.2) | 0.991 | 7 (41.2) |
| Questionnaires | ||||
| mMRC score ≥1 | 47 (45.2) n = 104 | 20 (52.6) n = 38 | 0.432 | 12 (75.0) n = 16 |
| CAT score ≥10 | 56 (50.9) n = 110 | 22 (55.0) n = 40 | 0.657 | 12 (80.0) n = 15 |
| COPD risk score | Moderate risk—31 (27.4) | Moderate risk—14 (35.9) | 0.002 | Moderate risk—5 (29.4) |
| High risk—16 (14.2) n = 113 | High risk—14 (35.9) n = 39 | High risk—9 (52.9) | ||
Bold values indicate P-values of <0.05. Number provided in italics indicates the total number of patients available for that variable. Data is presented as no. (%) or median [IQR].
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; EHRA, European Heart Rhythm Association; mMRC, (modified) Medical Research Council Dyspnoea questionnaire.
Patients with airflow limitation on screening (micro)spirometry showed a significant respiratory-related disease burden. On average, they had dyspnoea when hurrying or walking up a slight hill at one’s own pace [median mMRC = 1, (0–1)] and their respiratory symptoms had a significant impact on quality of life [mean CAT = 11.6 (7.2) points], although this respiratory-related symptom burden was equally elevated in patients without airflow limitation (Table 2), confirming that dyspnoea is a common symptom in most AF patients.15 At the time of questionnaire completion, 63 patients were in AF, 120 were not in AF and in 21 the AF status was unknown. Although the proportion of patients who had an mMRC score ≥1 did not differ between the group of patients in AF compared to the group of patients not in AF (41.9% vs. 40.9%, P = 0.107), patients in AF more often scored ≥10 points on the CAT score (69.4% vs. 40.4%, P = 0.004). Further analysis of the independent categories of the mMRC score did not show a correlation between the severity of dyspnoea and the risk of airflow limitation.
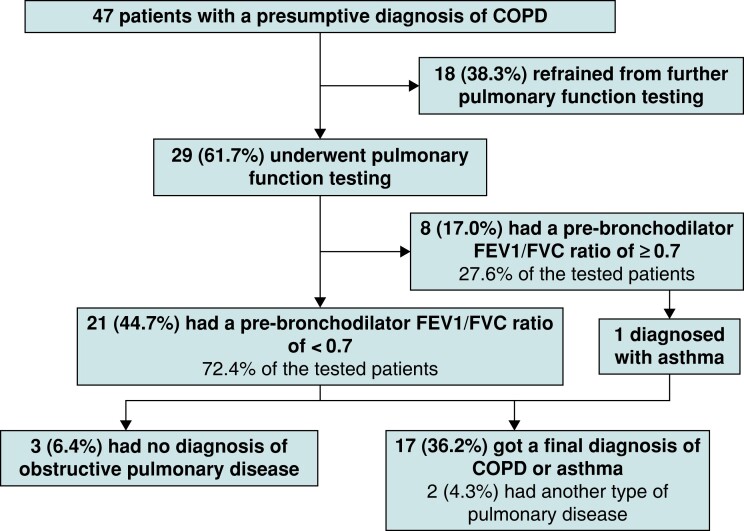
Of the 47 patients with airflow limitation, only 29 (61.7%) chose to be referred to the pulmonologist for further testing and a final clinical diagnosis. The other 18 patients (38.3%) did not wish to undergo further investigations, mainly because of a low perceived disease burden (11, 61.1%). Other reasons were a preoccupation with AF treatment (3, 16.7%), not wanting to undergo a further consultation for other unknown reasons (3, 16.7%), or already having a pulmonary diagnosis and not wanting to undergo re-analysis (1, 5.6%) (Figure 3). Of the 18 patients who did not wish to undergo further testing and who had scores available, nine had a mMRC score of 0, and 12 had a CAT score of <10 points, indeed suggesting a low disease burden. If selection for referral to a pulmonologist had been done based on any dyspnoea present (mMRC ≥ 1) referral would have been advised in 20 patients, whereas in 18 patients referral would not be advised and in nine patients the mMRC score was unknown.
Figure 3.
Flowchart of the clinical evaluation part of the chronic obstructive pulmonary disease screening and management pathway.
Final results and clinical consequences of the pulmonary assessment
In total, 29 of all 232 included patients were referred for pulmonary assessment. Of these referred patients, 8 had a pre-bronchodilator FEV1/FVC ratio of ≥0.7 on routine spirometry, of whom 1 was diagnosed with asthma and the others had no obstructive pulmonary disease. The remaining 21 patients indeed also had a pre-bronchodilator FEV1/FVC ratio of <0.7 on routine spirometry. Of these patients, three had a post-bronchodilator FEV1/FVC ratio of ≥0.7 and no signs of obstructive pulmonary disease, nine patients were diagnosed with COPD, seven with asthma, and two with another type of pulmonary disease. Thus, pulmonary analysis of patients with abnormal (micro)spirometry revealed an underlying obstructive pulmonary disease in 17 patients (36.2%) (9 COPD, 8 asthma), of whom 11 were newly diagnosed (Figure 3). In conclusion, the diagnostic yield of this care pathway for diagnosing obstructive pulmonary disease was 36% (17 out of 47), mainly caused by non-referral. When including only referred patients, this rose to 59% (17 out of 29). In all but two patients, treatment with inhalation medication was recommended by the treating pulmonologist. Details on treatment are provided in Supplementary material online, Table S2. Of the previously diagnosed patients, one was found to be unstable, whereas, in the others, treatment adaptation was not necessary. In 7 of the 11 newly diagnosed patients (63.6%), treatment with inhalation medication was initiated.
There were no differences in clinical characteristics nor symptoms (CAT, mMRC) between patients with and patients without obstructive pulmonary disease (see Supplementary material online, Table S3). Had only the 20 patients with signs of airflow limitation and a mMRC score ≥1 been referred, 12 patients (70.6%) would have been correctly identified with obstructive pulmonary disease and 4 patients (23.5%) would have been false negative (in one patient score was unknown). Since the majority (14 out of 17, 82%) had a moderately or highly increased COPD risk score, and combining the two scores would have resulted in only two false negative patients, applying such a score might have helped in preselecting patients for referral.
Time investment
The time investment to train the specialized AF nurses was small, and compromised of two training sessions of half an hour each, one for the COPD-6 device and one for the AioCare device. Since the AioCare device is a digital device, the exact time of the measurements was stored in the device. Patients performed a median of four (IQR 3–5) measurements, which took patients a median of 1 min 58 s (IQR 1 min 27 s–2 min 57 s). Although the exact time from the COPD-6 device is not available, it is expected to be similar. A review of the results could be done very quickly and took less than a minute per patient. With 10 patients visiting the pre-ablation clinic on a weekly basis, this corresponded to a maximum of 10 min per week.
A telephone consultation with a patient to discuss abnormal results took the cardiologist 5–10 min, depending on the amount of questions. Since 47 patients corresponded to on average one patient per week, this was an additional 5–10 min per week.
Discussion
Herein we describe the implementation of a multidisciplinary integrated screening and management care pathway for COPD, which was embedded in the existing AF clinic infrastructure. Primarily, the results of our study show that implementation of such a pathway into the infrastructure of the cardiology department is feasible, with AF nurses being able to supervise the handheld (micro) spirometry and achieving valid results according to device standards in 89% of patients, comparable to conventional spirometry.21,35,36 Moreover, airflow limitation was identified in one out of five patients.
The ongoing EHRA-PATHS project aims to develop generic, multidisciplinary, and evidence-based care pathways for multi-morbid AF patients, targeting AF-related comorbidities through systematic, integrated management.14,16 The herein presented COPD screening and management pathway may contribute to more structurally implemented and uniform care of COPD; an under-recognized comorbidity potentially impacting symptom and AF burden in patients scheduled for AF ablation. It includes an interdisciplinary care component for the comprehensive treatment of AF patients and can be implemented in existing infrastructures, overcoming organizational issues. Moreover, our care pathway has been designed in such a way that remote diagnosing and management of patients is attainable.
The diagnostic yield was lower than anticipated potentially due to the fact that 1 out of 3 patients with detected airflow limitation refrained from further pulmonological investigations. This could be a potential risk to the cost-effectiveness of such a screening strategy. Since non-referral was mainly caused by a lack of (perceived) symptoms, the performance of this pathway could be enhanced by performing handheld (micro)spirometry only in those with respiratory symptoms. Indeed, almost all patients with chronic respiratory diseases exhibited symptoms. We would therefore suggest a stepwise approach, first assessing respiratory symptoms in each AF patient and subsequently performing screening handheld (micro)spirometry at the AF clinic. Previous meta-analysis suggests that such a combination reduces the need for diagnostic assessment.37 However, one should keep in mind that patients in AF more often have higher CAT scores and that AF-related symptoms might therefore impact the CAT score, unrelated to respiratory disease. In addition, it is known that AF patients can present with respiratory-related symptoms such as dyspnoea and chest discomfort and our results give credence to the hypothesis that screening with symptoms alone will likely be insufficient to diagnose respiratory diseases in an AF population.15 The presence of (respiratory) symptoms should therefore be seen as complimentary to (micro)spirometry, but likely cannot in itself replace it.
An alternative explanation could be the unawareness of patients of the importance of screening for respiratory diseases. Although the initial education provided by the AF nurse, presumably in combination with the immediate availability of the screening test, persuaded all invited patients to perform handheld (micro)spirometry, some patients did not recognize the potential benefits of a more time-consuming referral. Therefore, more extensive patient education, and therewith increased patient empowerment and involvement, might also help to increase screening efficacy. A third hurdle might have been that patients had to revisit the hospital for final pulmonary diagnostics. Remote diagnostic systems, such as the AioCare device, might assist in home diagnosis and mitigate the hindrance of repeated hospital visits.
Work-up of the remaining patients revealed an underlying chronic respiratory disease in 36%. This diagnosis was of clinical importance since respiratory-related disease burden was moderate to high and treatment was indicated in most of these patients. Two findings should be noted here. First, the yield of COPD in our study population was lower than expected. In previous population studies, the prevalence of COPD in AF patients is estimated to be around 13%, with a wide range of 1%–35%,4 whereas we found COPD in 4% of our study population. Several factors may explain this difference. First, our study should not be seen as a true prevalence study since we adopted a hierarchical approach (first screening, then diagnosis) to diagnose COPD. Thus, some patients with COPD might have been missed due to false negative results on the (micro)spirometry measurements. Moreover, our study population consisted of patients referred for catheter ablation who overall had fewer comorbidities compared to patients from general AF registries.38–40 Finally, although previous literature on the accuracy of both (micro)spirometry devices, in general, showed good and comparable results for both devices,22,28 the fact that three out of four patients with airflow limitation were identified with the AioCare device suggests that there might be a difference in accuracy between the devices, which should be evaluated in follow-up studies.
Second, one unexpected yet important finding was the relatively frequent occurrence of (undiagnosed) asthma found through our screening strategy. Although this finding could be merely a coincidence since asthma is highly prevalent worldwide,41 studies have shown an association between asthma, especially poorly controlled, and the incidence of AF, suggesting a causative role of (under-recognized) asthma.42,43 Since we did not systematically screen for asthma and only included patients with abnormal (micro)spirometry results, whereas asthma patients can have normal spirometry, the actual prevalence of asthma in our population might even be higher than currently found. Moreover, patients with asthma—particularly elderly patients—can present with similar symptoms as AF patients, such as paroxysmal dyspnoea or chest discomfort, and uncontrolled asthma might even lead to palpitations due to increased inhaler use.34 It, therefore, merits further investigation to evaluate if screening for (uncontrolled) asthma might be beneficial for patients with AF and if treatment of asthma can also improve the AF symptom burden.
Future perspectives
In the next step of the pathway, the specialized AF nurses could be trained to integrate review of results and referral into the AF outpatient clinic in order to further optimize the pathway, which would spare time for the cardiologist and therewith costs. In addition, most patients with a previously diagnosed lung disease were found to be stable and no treatment adaptation was necessary. Therefore, these patients could in the future likely be excluded from the pathway.
Our screening and management pathway has the potential for remote and digital care by the pulmonologist when using the AioCare device. Comparable to and learning from previously integrated care pathways for the management of AF, we propose a remote approach using the cloud-based AioCare spirometry solution.44,45 Remote diagnostics with bronchodilation response performed by pulmonary function technicians and analysed afterward by the pulmonologist via the cloud might change screening for COPD into remote diagnosis of COPD. This would have the additional benefit of synchronizing COPD screening with AF work-up, so optimal treatment can be discussed during the multidisciplinary meetings without any delay in AF management. In addition, these novel remote pathways may contribute to patient convenience and subsequently to patient acceptance and involvement in their own AF management. Whether this proposed digital remote pathway is feasible and whether structured testing for obstructive pulmonary disease and earlier initiation of treatment is also cost-effective and improves AF outcomes needs to be investigated in further studies.
Strengths and limitations
This is the first study in AF evaluating an implementation strategy to screen for COPD. In addition, owing to the integration of a pulmonologist in the care pathway, a final diagnosis of COPD could be provided with certainty, whereas asthma was uncovered as an important coincidental finding. This study also has several limitations. First, selection bias may occur due to the fact that a significant proportion of patients refused to undergo full lung function testing. Second, this pathway was implemented in a pre-ablation AF outpatient clinic. Naturally, implementation into general AF clinics with the potential for diagnosis and treatment initiation at an earlier stage, as well as the inclusion of non-ablation AF patients who may have more comorbidities overall may be more beneficial, but whether this pathway can be implemented in a general AF outpatient clinic in the same way, warrants further study. Third, no patient surveys on e.g. the user-friendliness of the handheld devices were applied. However, previously collected patient experiences were found to be positive.46 Fourth, setting up such a screening strategy necessitates financial and infrastructural resources. This might be challenging given the fact that it is not yet proven that this screening and management pathway is cost-effective or results in better outcomes of rhythm control strategies. Indeed, in this study, the pathway existed parallel to the ablation trajectory, without influencing the AF treatment. Follow-up data on outcomes was not part of this study but needs to be investigated in further studies. Moreover, this study presents only one screening approach using (micro)spirometry. Whether other screening strategies would yield similar results was currently not investigated. Finally, the study incorporated two screening devices with potentially different sensitivity and specificity, which could impact the screening results.
Conclusions
Implementation of handheld (micro)spirometry into the pre-existing pre-ablation AF outpatient clinic is feasible with patients being able to perform handheld (micro)spirometry measurements. Airflow limitation was detected in one out of five patients. In order to ensure patient acceptance of the complete screening and management pathway, patient education and involvement are of utmost importance. Whether pre-selection of patients based on COPD and dyspnoea questionnaires further improves patient acceptance and the yield of the screening pathway and whether this screening and management pathway results in better rhythm outcomes warrants further study.
Supplementary Material
Contributor Information
Rachel M J van der Velden, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
Maartje J M Hereijgers, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
Nazia Arman, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
Naomi van Middendorp, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands.
Frits M E Franssen, Department of Research and Development, Ciro, 6085 NM Horn, the Netherlands; NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, 6200 MD Maastricht, the Netherlands; Department of Respiratory Medicine, Maastricht University Medical Centre, 6229 HX Maastricht, the Netherlands.
Monika Gawalko, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands; 1st Department of Cardiology, Medical University of Warsaw, 02-091 Warsaw, Poland.
Dominique V M Verhaert, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands; Department of Cardiology, Radboud University Medical Centre, 6525 GA Nijmegen, the Netherlands.
Zarina Habibi, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands; Department of Cardiology, Radboud University Medical Centre, 6525 GA Nijmegen, the Netherlands.
Kevin Vernooy, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands; Department of Cardiology, Radboud University Medical Centre, 6525 GA Nijmegen, the Netherlands.
Lukasz Koltowski, 1st Department of Cardiology, Medical University of Warsaw, 02-091 Warsaw, Poland.
Jeroen M Hendriks, Caring Futures Institute, College of Nursing and Health Sciences, Flinders University, 5001 Adelaide, Australia; Centre for Heart Rhythm Disorders, University of Adelaide and Royal Adelaide Hospital, 5000 Adelaide, Australia.
Hein Heidbuchel, Department of Cardiology, Antwerp University Hospital, 2650 Antwerp, Belgium; Research Group Cardiovascular Diseases, University of Antwerp, 2650 Antwerp, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, 3590 Hasselt, Belgium.
Lien Desteghe, Department of Cardiology, Antwerp University Hospital, 2650 Antwerp, Belgium; Research Group Cardiovascular Diseases, University of Antwerp, 2650 Antwerp, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, 3590 Hasselt, Belgium; Heart Center Hasselt, Jessa Hospital, 3500 Hasselt, Belgium.
Sami O Simons, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, 6200 MD Maastricht, the Netherlands; Department of Respiratory Medicine, Maastricht University Medical Centre, 6229 HX Maastricht, the Netherlands.
Dominik Linz, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, P. Debyelaan 25, 6229 HX Maastricht, the Netherlands; Department of Cardiology, Radboud University Medical Centre, 6525 GA Nijmegen, the Netherlands; Centre for Heart Rhythm Disorders, University of Adelaide and Royal Adelaide Hospital, 5000 Adelaide, Australia; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The ISOLATION study and the analysis of the collected material are supported by grants from the Dutch Heart Foundation (CVON2014-09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hypercoagulability, Electrical remodelling, and Vascular Destabilization in the Progression of AF) and the European Commission (ITN Network Personalize AF: Personalized Therapies for Atrial Fibrillation: a translational network, grant no. 860974; CATCH ME: Characterizing Atrial fibrillation by Translating its Causes into Health Modifiers in the Elderly, grant no. 633196; MAESTRIA: Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation, grant no. 965286). In addition, this project is supported by funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945260.
Data availability
Data are available on reasonable request. Data will be made available after an application at carim-office@maastrichtuniversity.nl.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Simons SO, Elliott A, Sastry M, Hendriks JM, Arzt M, Rienstra Met al. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur Heart J 2021;42:532–40. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jret al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 4. Romiti GF, Corica B, Pipitone E, Vitolo M, Raparelli V, Basili Set al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. Eur Heart J 2021;42:3541–54. [DOI] [PubMed] [Google Scholar]
- 5. Durheim MT, Holmes DN, Blanco RG, Allen LA, Chan PS, Freeman JVet al. Characteristics and outcomes of adults with chronic obstructive pulmonary disease and atrial fibrillation. Heart 2018;104:1850–8. [DOI] [PubMed] [Google Scholar]
- 6. Proietti M, Laroche C, Drozd M, Vijgen J, Cozma DC, Drozdz Jet al. Impact of chronic obstructive pulmonary disease on prognosis in atrial fibrillation: a report from the EURObservational research programme pilot survey on atrial fibrillation (EORP-AF) general registry. Am Heart J 2016;181:83–91. [DOI] [PubMed] [Google Scholar]
- 7. Warming PE, Garcia R, Hansen CJ, Simons SO, Torp-Pedersen C, Linz Det al. The association of temporal sequence in atrial fibrillation and chronic obstructive pulmonary disease diagnosis and mortality risk. Eur Heart J Qual Care Clin Outcomes 2023;9:e2. [DOI] [PubMed] [Google Scholar]
- 8. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJet al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 9. Ecker V, Knoery C, Rushworth G, Rudd I, Ortner A, Begley Det al. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin Cardiol 2018;41:862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pisters R, Nieuwlaat R, Prins MH, Le Heuzey JY, Maggioni AP, Camm AJet al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the euro heart survey. Europace 2012;14:666–74. [DOI] [PubMed] [Google Scholar]
- 11. Gu J, Liu X, Tan H, Zhou L, Jiang W, Wang Yet al. Impact of chronic obstructive pulmonary disease on procedural outcomes and quality of life in patients with atrial fibrillation undergoing catheter ablation. J Cardiovasc Electrophysiol 2013;24:148–54. [DOI] [PubMed] [Google Scholar]
- 12. Seara JG, Roubin SR, Gude Sampedro F, Barreiro VB, Sande JM, Mañero MRet al. Risk of atrial fibrillation, stroke, and death after radiofrequency catheter ablation of typical atrial flutter. Clin Res Cardiol 2014;103:543–52. [DOI] [PubMed] [Google Scholar]
- 13. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CCet al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Canad J Cardiol 2020;36:1847–948. [DOI] [PubMed] [Google Scholar]
- 14. Lee G, Baker E, Desteghe L, Heidbuchel H, Collins R, Merino JL. The challenge of managing multimorbid atrial fibrillation: a pan-European European Heart Rhythm Association (EHRA) member survey of current management practices and clinical priorities. Europace 2022;24:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Velden RMJ, Hermans ANL, Pluymaekers N, Gawalko M, Elliott A, Hendriks JMet al. Dyspnea in patients with atrial fibrillation: mechanisms, assessment and an interdisciplinary and integrated care approach. Int J Cardiol Heart Vasc 2022;42:101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heidbuchel H, Van Gelder IC, Desteghe L. ESC And EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the horizon 2020 EHRA-PATHS project. Eur Heart J 2022;43:1450–2. [DOI] [PubMed] [Google Scholar]
- 17. Verhaert DVM, Linz D, Chaldoupi SM, Westra SW, den Uijl DW, Philippens Set al. Rationale and design of the ISOLATION study: a multicenter prospective cohort study identifying predictors for successful atrial fibrillation ablation in an integrated clinical care and research pathway. Front Cardiovasc Med 2022;9:879139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verhaert DVM, Linz D, Wassink GF, Weijs B, Philippens S, Luermans JGLMet al. A new efficient and integrated pathway for patient evaluation prior to atrial fibrillation ablation. Eur J Cardiovasc Nurs 2022:zvac095. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 19. Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic Iet al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022;60:2101499. [DOI] [PubMed] [Google Scholar]
- 20. Global Initiative for Chronic Obstructive Lung Disease . 2023 GOLD Report. Deer Park, IL, USA; 2022.
- 21. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GLet al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickens AP, Fitzmaurice DA, Adab P, Sitch A, Riley RD, Enocson Aet al. Accuracy of vitalograph lung monitor as a screening test for COPD in primary care. NPJ Prim Care Respir Med 2020;30:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Represas-Represas C, Fernández-Villar A, Ruano-Raviña A, Priegue-Carrera A, Botana-Rial M. Screening for chronic obstructive pulmonary disease: validity and reliability of a portable device in non-specialized healthcare settings. PLoS One 2016;11:e0145571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kjeldgaard P, Lykkegaard J, Spillemose H, Ulrik CS. Multicenter study of the COPD-6 screening device: feasible for early detection of chronic obstructive pulmonary disease in primary care? Int J Chron Obstruct Pulmon Dis 2017;12:2323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorn J, Tilling B, Lisspers K, Jörgensen L, Stenling A, Stratelis G. Improved prediction of COPD in at-risk patients using lung function pre-screening in primary care: a real-life study and cost-effectiveness analysis. Prim Care Respir J 2012;21:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boros PW, Maciejewski A, Nowicki MM, Wesołowski S. Comparability of portable and desktop spirometry: a randomized, parallel assignment, open-label clinical trial. Adv Respir Med 2022;90:60–7. [DOI] [PubMed] [Google Scholar]
- 27. Zhou J, Li X, Wang X, Yu N, Wang W. Accuracy of portable spirometers in the diagnosis of chronic obstructive pulmonary disease: a meta-analysis. NPJ Prim Care Respir Med 2022;32:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mycroft K, Korczynski P, Jankowski P, Kutka M, Zelazna O, Zagaja Met al. Active screening for COPD among hospitalized smokers—a feasibility study. Ther Adv Chronic Dis 2020;11:2040622320971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H-Cet al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German atrial fibrillation competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. [DOI] [PubMed] [Google Scholar]
- 30. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–6. [DOI] [PubMed] [Google Scholar]
- 31. Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov Det al. Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006;73:285–95. [DOI] [PubMed] [Google Scholar]
- 32. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J 2009;34:648–54. [DOI] [PubMed] [Google Scholar]
- 33. Louis R, Satia I, Ojanguren I, Schleich F, Bonini M, Tonia Tet al. European respiratory society guidelines for the diagnosis of asthma in adults. Eur Respir J 2022;60:2101585. [DOI] [PubMed] [Google Scholar]
- 34. Global Initiative For Asthma . 2022 GINA main report. 2022.
- 35. Enright P, Vollmer WM, Lamprecht B, Jensen R, Jithoo A, Tan Wet al. Quality of spirometry tests performed by 9893 adults in 14 countries: the BOLD study. Respir Med 2011;105:1507–15. [DOI] [PubMed] [Google Scholar]
- 36. Torre-Bouscoulet L, Velázquez-Uncal M, García-Torrentera R, Gochicoa-Rangel L, Fernández-Plata R, Enright Pet al. Spirometry quality in adults with very severe lung function impairment. Respir Care 2015;60:740. [DOI] [PubMed] [Google Scholar]
- 37. Haroon S, Jordan R, Takwoingi Y, Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ Open 2015;5:e008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guerra F, Brambatti M, Nieuwlaat R, Marcucci M, Dudink E, Crijns HJGMet al. Symptomatic atrial fibrillation and risk of cardiovascular events: data from the euro heart survey. Europace 2017;19:1922–9. [DOI] [PubMed] [Google Scholar]
- 39. Lip GYH, Laroche C, Boriani G, Cimaglia P, Dan G-A, Santini Met al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the euro observational research programme pilot survey on atrial fibrillation. Europace 2015;17:24–31. [DOI] [PubMed] [Google Scholar]
- 40. Schnabel RB, Pecen L, Rzayeva N, Lucerna M, Purmah Y, Ojeda FMet al. Symptom burden of atrial fibrillation and its relation to interventions and outcome in Europe. J Am Heart Assoc 2018;7:e007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med 2020; 8:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tattersall MC, Dasiewicz AS, McClelland RL, Gepner AD, Kalscheur MM, Field MEet al. Persistent asthma is associated with increased risk for incident atrial fibrillation in the MESA. Circ Arrhythm Electrophysiol 2020;13:e007685. [DOI] [PubMed] [Google Scholar]
- 43. Cepelis A, Brumpton BM, Malmo V, Laugsand LE, Loennechen JP, Ellekjær Het al. Associations of asthma and asthma control with atrial fibrillation risk: results from the Nord-Trøndelag health study (HUNT). JAMA Cardiology 2018;3:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verhaert DVM, Betz K, Gawałko M, Hermans ANL, Pluymaekers NAHA, van der Velden RMJet al. A VIRTUAL sleep apnoea management pathway for the work-up of atrial fibrillation patients in a digital remote infrastructure: VIRTUAL-SAFARI. Europace 2022;24:565–75. [DOI] [PubMed] [Google Scholar]
- 45. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes Set al. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace 2021;23:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kupczyk M, Hofman A, Kołtowski Ł, Kuna P, Łukaszyk M, Buczyłko Ket al. Home self-monitoring in patients with asthma using a mobile spirometry system. J Asthma 2021;58:505–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Data will be made available after an application at carim-office@maastrichtuniversity.nl.