Abstract
BACKGROUND
Standard first-line chemotherapy for endometrial cancer is paclitaxel plus carboplatin. The benefit of adding pembrolizumab to chemotherapy remains unclear.
METHODS
In this double-blind, placebo-controlled, randomized, phase 3 trial, we assigned 816 patients with measurable disease (stage III or IVA) or stage IVB or recurrent endometrial cancer in a 1:1 ratio to receive pembrolizumab or placebo along with combination therapy with paclitaxel plus carboplatin. The administration of pembrolizumab or placebo was planned in 6 cycles every 3 weeks, followed by up to 14 maintenance cycles every 6 weeks. The patients were stratified into two cohorts according to whether they had mismatch repair–deficient (dMMR) or mismatch repair–proficient (pMMR) disease. Previous adjuvant chemotherapy was permitted if the treatment-free interval was at least 12 months. The primary outcome was progression-free survival in the two cohorts. Interim analyses were scheduled to be triggered after the occurrence of at least 84 events of death or progression in the dMMR cohort and at least 196 events in the pMMR cohort.
RESULTS
In the 12-month analysis, Kaplan–Meier estimates of progression-free survival in the dMMR cohort were 74% in the pembrolizumab group and 38% in the placebo group (hazard ratio for progression or death, 0.30; 95% confidence interval [CI], 0.19 to 0.48; P<0.001), a 70% difference in relative risk. In the pMMR cohort, median progression-free survival was 13.1 months with pembrolizumab and 8.7 months with placebo (hazard ratio, 0.54; 95% CI, 0.41 to 0.71; P<0.001). Adverse events were as expected for pembrolizumab and combination chemotherapy.
CONCLUSIONS
In patients with advanced or recurrent endometrial cancer, the addition of pembrolizumab to standard chemotherapy resulted in significantly longer progression-free survival than with chemotherapy alone. (Funded by the National Cancer Institute and others; NRG-GY018 ClinicalTrials.gov number, NCT03914612.)
Endometrial cancer is one of only a few malignant conditions for which both incidence and mortality are currently rising. By 2040, it is projected to be the third most prevalent cancer and the fourth leading cause of cancer death among women.1 Survival for women with uterine cancers has not improved during the past 4 decades, unlike the history of many other solid tumors.2,3
Investigators in the Gynecologic Oncology Group Study 209 (GOG0209)4 established the noninferiority of paclitaxel plus carboplatin as compared with paclitaxel–doxorubicin–cisplatin as standard first-line chemotherapy for the treatment of patients with advanced or recurrent endometrial cancer. The selection of therapies beyond first-line drugs is more complex because of the diverse histologic, molecular, and clinical features of endometrial cancers,5 including the use of molecular classification to determine whether the cancer is mismatch repair–deficient (dMMR) or mismatch repair–proficient (pMMR). Researchers in the Cancer Genome Atlas Network found that 28% of endometrial cancers had high microsatellite instability on the basis of genomic assessment of seven repeat loci.5 Monotherapy with an immune checkpoint inhibitor (e.g., pembrolizumab6–8 and dostarlimab-gxly9) has established efficacy as the second line of therapy and beyond in patients with dMMR endometrial cancer with high microsatellite instability. Pembrolizumab in combination with lenvatinib has shown efficacy in pMMR, microsatellite-stable endometrial cancer.10–12
The combination of pembrolizumab and cytotoxic chemotherapy has resulted in clinically significant improvements in progression-free and overall survival in patients with multiple types of solid tumors.13,14 It has been hypothesized that these improvements resulted from increased antigenic diversity in the tumor from failure to repair point mutations and the potential immunogenic effects of cytotoxic chemotherapy, which include inhibition of myeloid-derived suppressor cells, increased antigen cross-presentation after immunogenic-cell death, enhanced dendritic-cell activity through STAT6 pathway inhibition, and an increased ratio of cytotoxic lymphocytes to regulatory T cells.15–18
We performed a phase 3, international, double-blind, randomized, placebo-controlled trial (NRG-GY018) to evaluate standard chemotherapy with paclitaxel plus carboplatin plus either pembrolizumab or placebo in patients with advanced or recurrent endometrial cancer. The two treatment strategies were analyzed in two independent cohorts — patients with dMMR disease and those with pMMR disease — as determined on the basis of central immunohistochemical analysis.
METHODS
TRIAL DESIGN AND OVERSIGHT
The trial was conducted at 395 sites in four countries (the United States, Canada, Japan, and South Korea) in a two-step enrollment process. Step 1 included submission of the results of the institutional or local immunohistochemical analysis of MMR status for examination in a central laboratory (NeoGenomics). If this examination was positive for MMR status, step 2 included registration and randomization. Inconclusive results regarding MMR status necessitated repeat testing. A protocol amendment that was implemented on April 4, 2022, permitted step 2 registration and randomization on the basis of the institutional or local results regarding MMR status. However, central laboratory confirmation continued throughout the trial. For the primary analysis, patients were assigned to either the dMMR or pMMR cohort on the basis of a central review of MMR status.
The trial was funded by the National Cancer Institute (NCI), which provided pembrolizumab and placebo at no charge. Funding was provided by Merck through a cooperative research and developmental agreement with the NCI. Merck also provided supplemental funding to NRG Oncology (part of the National Clinical Trials Network), which conducted the trial. All the patients provided written informed consent.
Institutional review boards or independent ethics committees approved the trial protocol at each site. The trial was monitored by an independent data and safety monitoring committee. All the authors contributed to the preparation of the manuscript and assume responsibility for the accuracy and completeness of the reported data and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org.
PATIENTS
Adult women (≥18 years of age) with confirmed advanced-stage, metastatic, or recurrent endometrial cancer of any histologic subtype except for carcinosarcoma were eligible for enrollment. All the patients had newly diagnosed stage III or IVA disease according to the Response Evaluation Criteria for Solid Tumors (RECIST), version 1.1,19 for measurable disease or stage IVB or recurrent endometrial cancer with or without measurable disease. Patients who had received previous adjuvant chemotherapy were eligible if their chemotherapy-free interval was at least 12 months. The receipt of previous radiation or hormonal therapy was permitted. Among the inclusion criteria were institutional results on immunohistochemical analysis of MMR status (except for sites in Japan), an available biopsy specimen for central immunohistochemical assessment, and an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0, 1, or 2 (on a scale of 0 to 5, with higher scores indicating greater disability). A comprehensive list of inclusion and exclusion criteria is provided in the trial protocol.
RANDOMIZATION AND TREATMENT
Patients in each cohort were randomly assigned in a 1:1 ratio to receive paclitaxel plus carboplatin along with either pembrolizumab or placebo for 6 cycles, which was followed by pembrolizumab or placebo maintenance every 6 weeks for up to 14 cycles; a maximum of 20 cycles of pembrolizumab or placebo could be administered. Patients received 200 mg of pembrolizumab or placebo administered intravenously in a 30-minute infusion every 3 weeks in combination with chemotherapy, followed by 400 mg of pembrolizumab or placebo maintenance, administered intravenously in a 30-minute infusion every 6 weeks. On day 1 in the chemotherapy portion of the therapy, patients received paclitaxel administered intravenously in a 3-hour infusion at a dose of 175 mg per square meter of body-surface area plus carboplatin at an area under the curve of 5 mg per milliliter per minute administered intravenously over 30 to 60 minutes. Patients with measurable disease who had RECIST-defined stable disease or a partial response at the completion of cycle 6 were permitted to receive paclitaxel plus carboplatin (with pembrolizumab or placebo) for up to 10 cycles at the discretion of the treating investigator. Randomization to trial group was stratified according to MMR status, ECOG performance status (0 or 1 vs. 2), and receipt of previous adjuvant chemotherapy (yes or no).
OUTCOMES
The primary outcome was progression-free survival, which was assessed by investigators according to RECIST. Key secondary outcomes included safety, overall survival, and health-related quality of life as assessed with the use of validated and sensitive instruments, including the Trial Outcome Index of the Functional Assessment of Cancer Therapy–Endometrial [FACT-En-TOI],20 the short form of the FACT–GOG Neurotoxicity subscale, the short form of the Patient-Reported Outcomes Measurement Information System (PROMIS)–Fatigue, the short form of the PROMIS–Physical Function, and a single-item survey measuring the level of distress caused by the side effects of cancer therapy. Quality-of-life assessments were performed only in the pMMR cohort. A comprehensive list of secondary outcomes is provided in the protocol.
All outcomes were examined separately in the dMMR and pMMR cohorts. Details regarding tumor assessments are provided in Table S1 in the Supplementary Appendix, available at NEJM.org. Adverse events, as assessed by NCI Common Terminology Criteria for Adverse Events, version 5.0, were recorded and graded from randomization until 30 days after the last administration of the trial drugs.
STATISTICAL ANALYSIS
We determined that the enrollment of 810 patients — 220 in the dMMR cohort and 590 in the pMMR cohort — would provide the trial with 85% power to detect a relative reduction of 40% in the risk of progression or death as compared with chemotherapy alone in the dMMR cohort after the occurrence of 168 primary-outcome events and 90% power to detect a relative reduction of 30% in the pMMR cohort after the occurrence of 394 events. The complete statistical analysis plan is provided in the protocol.
The trial was based on the intention-to-treat principle, except for prerandomization eligibility exclusions and postrandomization adjustments for incomplete central immunohistochemical results regarding MMR status owing to insufficient tumor quantity. The time that patients were considered to be at risk started on the date of randomization and continued until disease progression, death, or the date of the last follow-up.
The null hypothesis was tested separately in each MMR-status cohort at an initial significance level of 0.0125 with a stratified log-rank statistic. The group that had the anticipated number of primary-outcome events was tested first. One interim analysis for futility in each cohort was planned after approximately half the anticipated primary-outcome events had occurred. If the stratified log-rank statistic was greater than zero, indicating an equal or greater risk of failure in the pembrolizumab group, the trial would be considered for early closure due to futility.21 One interim efficacy analysis was planned after each cohort had been closed to enrollment and at least half the anticipated primary-outcome events had occurred. An O’Brien–Fleming stopping boundary22 was used with a Lan–DeMets alpha spending function.23 At the data-cutoff date (December 16, 2022), the analysis in the pMMR cohort had not been adjusted for multiplicity. If the null hypothesis for one cohort was rejected, then all the alpha (0.0125) would be forwarded to the other cohort for analysis. The alpha that was spent on the other group by means of the O’Brien–Fleming function was adjusted according to the rules outlined by Maurer and Bretz.24
At the time of the final analysis of progression-free survival or an interim analysis crossing the efficacy boundary, an interim overall survival analysis for futility was planned. The null hypothesis was that the risk of death would be lower in the pembrolizumab group than in the placebo group. Using a stratified Cox proportional-hazards model, we tested the null hypothesis at a 10% level of significance. A rejection of the null hypothesis would indicate a concern for lower overall survival in the pembrolizumab group.
RESULTS
PATIENTS
From July 2019 through December 2022, a total of 816 patients were enrolled, with 225 in the dMMR cohort and 591 in the pMMR cohort; of these patients, 588 were available for evaluation for efficacy analysis. The trial was closed to enrollment in the dMMR cohort in August 2022. Patients who had local pMMR results that were later determined to be dMMR by central review continued as part of the dMMR cohort. The trial was temporarily paused to enrollment from April 2020 to November 2020 because of the coronavirus disease 2019 pandemic to permit implementation of strategies to mitigate patient risk, given the placebo-controlled nature of the trial. The randomization plan is shown in Figure 1.
Figure 1. Enrollment and Outcomes.
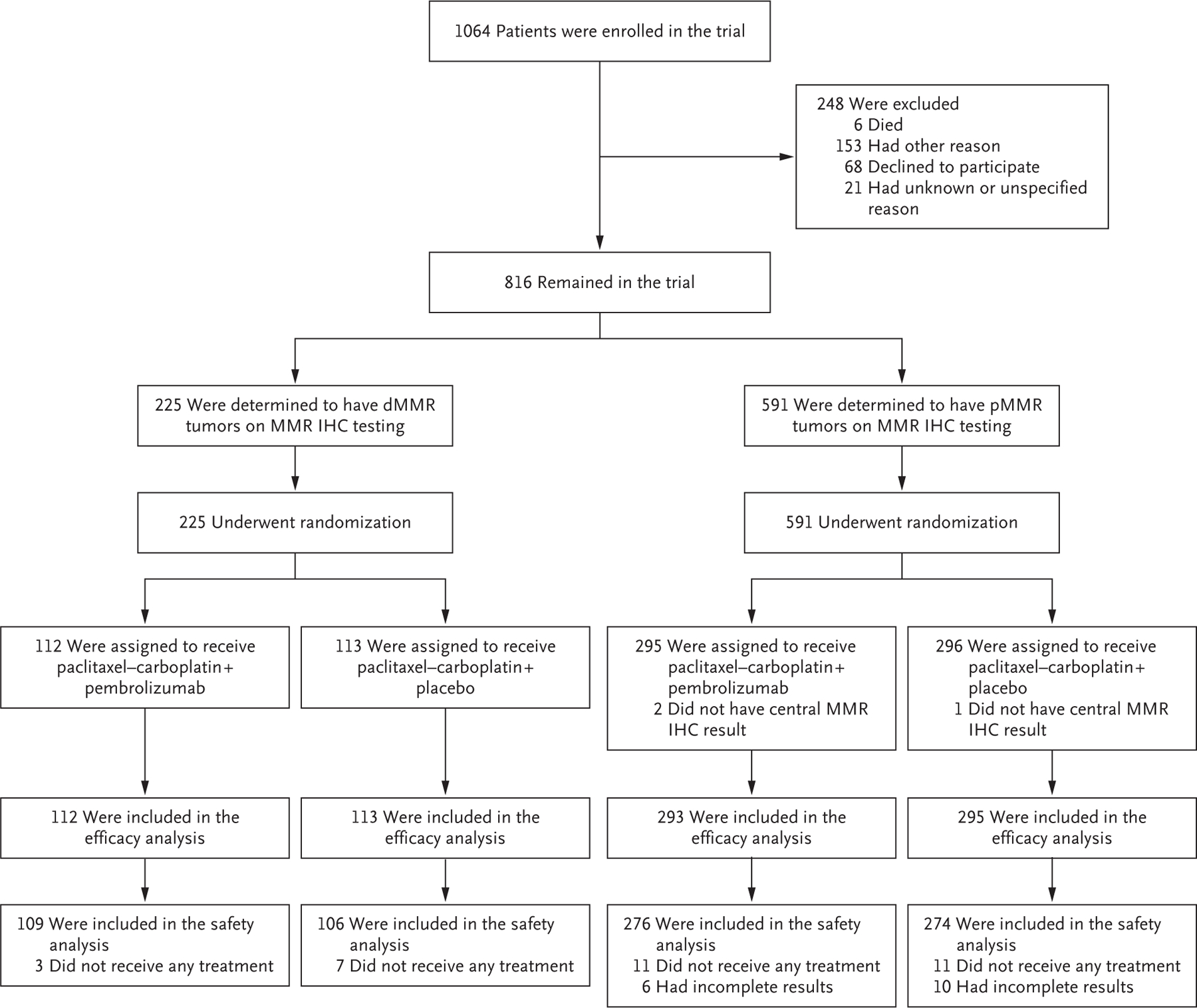
The patients were stratified into two cohorts according to whether they had mismatch repair–deficient (dMMR) or mismatch repair–proficient (pMMR) disease on immunohistochemical (IHC) assessment.
Most demographic characteristics along with clinical and pathological factors were well balanced between patients in the pembrolizumab and placebo groups in both the dMMR and pMMR cohorts (Table 1). Of the 223 patients with centrally determined dMMR status (with 2 confirmations pending at the time of analysis), the results had been locally identified as pMMR in 17 patients (7.6%). Of the 573 patients with centrally determined pMMR status (with 15 confirmations pending), the results had been locally identified as dMMR in 12 patients (2.1%). Approximately 6% of the patients in the dMMR cohort had received adjuvant chemotherapy before enrollment, as compared with 25.3% of the patients in the pMMR cohort. Radiotherapy had been administered to approximately 43% of the patients in the dMMR cohort and to 39.6% of those in the pMMR cohort. Serous histologic analysis was largely restricted to the pMMR cohort.5 The clinical trial population reflected a racially diverse and representative cohort. Patients identified their race as Black in 8.9% of the dMMR cohort and in 16.3% of the pMMR cohort, which reflects the higher incidence of histologic and molecular subtypes associated with poor prognosis among Black women than among women of other races.1,25,26
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | dMMR Cohort | pMMR Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab (N = 112) |
Placebo (N = 113) |
All Patients (N = 225) |
Pembrolizumab (N = 293) |
Placebo (N = 295) |
All Patients (N = 588) |
|||
| Demographic | ||||||||
| Median age (range) — yr | 67 (38–81) | 66 (37–85) | 66 (37–85) | 66 (31–93) | 65 (29–90) | 65.5 (29–93) | ||
| Race or ethnic group — no. (%)† | ||||||||
| White | 92 (82.1) | 86 (76.1) | 178 (79.1) | 212 (72.4) | 212 (71.9) | 424 (72.1) | ||
| Black | 11 (9.8) | 9 (8.0) | 20 (8.9) | 45 (15.4) | 51 (17.3) | 96 (16.3) | ||
| Asian | 3 (2.7) | 4 (3.5) | 7 (3.1) | 17 (5.8) | 14 (4.7) | 31 (5.3) | ||
| American Indian or Alaska Native | 0 | 2 (1.8) | 2 (0.9) | 2 (0.7) | 2 (0.7) | 4 (0.7) | ||
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 1 (0.3) | 3 (1.0) | 4 (0.7) | ||
| Unknown | 3 (2.7) | 9 (8.0) | 12 (5.3) | 8 (2.7) | 7 (2.4) | 15 (2.6) | ||
| Not reported | 3 (2.7) | 3 (2.7) | 6 (2.7) | 7 (2.4) | 5 (1.7) | 12 (2.0) | ||
| Multiracial | 0 | 0 | 0 | 1 (0.3) | 1 (0.3) | 2 (0.3) | ||
| Hispanic ethnic group — no. (%)† | ||||||||
| No | 106 (94.6) | 99 (87.6) | 205 (91.1) | 263 (89.8) | 273 (92.5) | 536 (91.2) | ||
| Yes | 5 (4.5) | 6 (5.3) | 11 (4.9) | 21 (7.2) | 16 (5.4) | 37 (6.3) | ||
| Unknown | 1 (0.9) | 4 (3.5) | 5 (2.2) | 4 (1.4) | 3 (1.0) | 7 (1.2) | ||
| Not reported | 0 | 4 (3.5) | 4 (1.8) | 5 (1.7) | 3 (1.0) | 8 (1.4) | ||
| Medical history | ||||||||
| ECOG performance-status score — no. (%)‡ | ||||||||
| 0 | 72 (64.3) | 73 (64.6) | 145 (64.4) | 196 (66.9) | 198 (67.1) | 394 (67.0) | ||
| 1 | 39 (34.8) | 35 (31.0) | 74 (32.9) | 88 (30.0) | 88 (29.8) | 176 (29.9) | ||
| 2 | 1 (0.9) | 5 (4.4) | 6 (2.7) | 9 (3.1) | 9 (3.1) | 18 (3.1) | ||
| Histologic analysis - no. (%) | ||||||||
| Adenocarcinoma, NOS§ | 12 (10.7) | 14 (12.4) | 26 (11.6) | 24 (8.2) | 33 (11.2) | 57 (9.7) | ||
| Clear cell | 1 (0.9) | 0 | 1 (0.4) | 17 (5.8) | 20 (6.8) | 37 (6.3) | ||
| Dedifferentiated or undifferentiated | 4 (3.6) | 4 (3.5) | 8 (3.6) | 7 (2.4) | 6 (2.0) | 13 (2.2) | ||
| Endometrioid | ||||||||
| G1 | 21 (18.8) | 35 (31.0) | 56 (24.9) | 54 (18.4) | 46 (15.6) | 100 (17.0) | ||
| G2 | 52 (46.4) | 41 (36.3) | 93 (41.3) | 51 (17.4) | 58 (19.7) | 109 (18.5) | ||
| G3 | 15 (13.4) | 16 (14.2) | 31 (13.8) | 53 (18.1) | 42 (14.2) | 95 (16.2) | ||
| Mixed epithelial | 3 (2.7) | 2 (1.8) | 5 (2.2) | 6 (2.0) | 11 (3.7) | 17 (2.9) | ||
| Serous | 4 (3.6) | 1 (0.9) | 5 (2.2) | 78 (26.6) | 72 (24.4) | 150 (25.5) | ||
| Pending | 0 | 0 | 0 | 3 (1.0) | 7 (2.4) | 10 (1.7) | ||
| Previous therapy | ||||||||
| Chemotherapy - no. (%) | ||||||||
| Yes | 5 (4.5) | 8 (7.1) | 13 (5.8) | 72 (24.6) | 77 (26.1) | 149 (25.3) | ||
| No | 107 (95.5) | 105 (92.9) | 212 (94.2) | 221 (75.4) | 218 (73.9) | 439 (74.7) | ||
| Radiotherapy - no. (%) | ||||||||
| Yes | 41 (36.6) | 55 (48.7) | 96 (42.7) | 114 (38.9) | 119 (40.3) | 233 (39.6) | ||
| No | 71 (63.4) | 58 (51.3) | 129 (57.3) | 179 (61.1) | 176 (59.7) | 355 (60.4) | ||
| Surgery - no. (%) | ||||||||
| Yes | 98 (87.5) | 105 (92.9) | 203 (90.2) | 261 (89.1) | 245 (83.1) | 506 (86.1) | ||
| No | 14 (12.5) | 8 (7.1) | 22 (9.8) | 29 (9.9) | 46 (15.6) | 75 (12.8) | ||
| Missing data | 0 | 0 | 0 | 3 (1.0) | 4 (1.4) | 7 (1.2) | ||
Patients who were assigned to receive pembrolizumab or placebo also received combination therapy with paclitaxel plus carboplatin. Percentages may not total 100 because of rounding. The abbreviation dMMR denotes mismatch repair–deficient, and pMMR mismatch repair–proficient.
Race and ethnic group were reported by the patients.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a scale of 0 to 5, with higher scores indicating greater disability.
The “not otherwise specified” (NOS) category included endometrioid adenocarcinoma, grade not specified.
EFFICACY
The data set was closed on December 2, 2022, for analysis of futility in the pMMR cohort; on December 6, 2022, for analysis of efficacy in the pMMR cohort; and on December 16, 2022, for analyses of both futility and efficacy in the dMMR cohort. These separate data sets were analyzed and presented to the data and safety monitoring committee on January 26, 2023.
The median follow-up was 12 months in the dMMR cohort and 7.9 months in the pMMR cohort. Follow-up times were similar according to randomized group in each cohort. At the time of the primary analysis, either pembrolizumab or placebo was still being administered to 39.6% of the patients in the dMMR cohort and to 41.8% of those in the pMMR cohort; either pembrolizumab or placebo was discontinued in 57% of the patients in the trial, largely because of disease progression.
In the 12-month analysis in the dMMR cohort, the risk of disease progression or death was 70% lower with pembrolizumab than with placebo; Kaplan–Meier estimates of freedom from disease progression or death were 74% and 38%, respectively (hazard ratio, 0.30; 95% CI, 0.19 to 0.48; P<0.001) (Fig. 2A). Similarly, at a median follow-up of 7.9 months in the pMMR cohort, median progression-free survival was 13.1 months with pembrolizumab and 8.7 months with placebo (hazard ratio, 0.54; 95% CI, 0.41 to 0.71; P<0.001) (Fig. 2B). Subgroup analyses of progression-free survival in both the dMMR and pMMR cohorts appeared to favor the pembrolizumab group, including during the maintenance phase, although efficacy results appeared to be heterogeneous according to geographic location and the numbers of patients in some subgroups were small (Fig. S2A and S2B).
Figure 2. Progression-free Survival in the Two Cohorts.
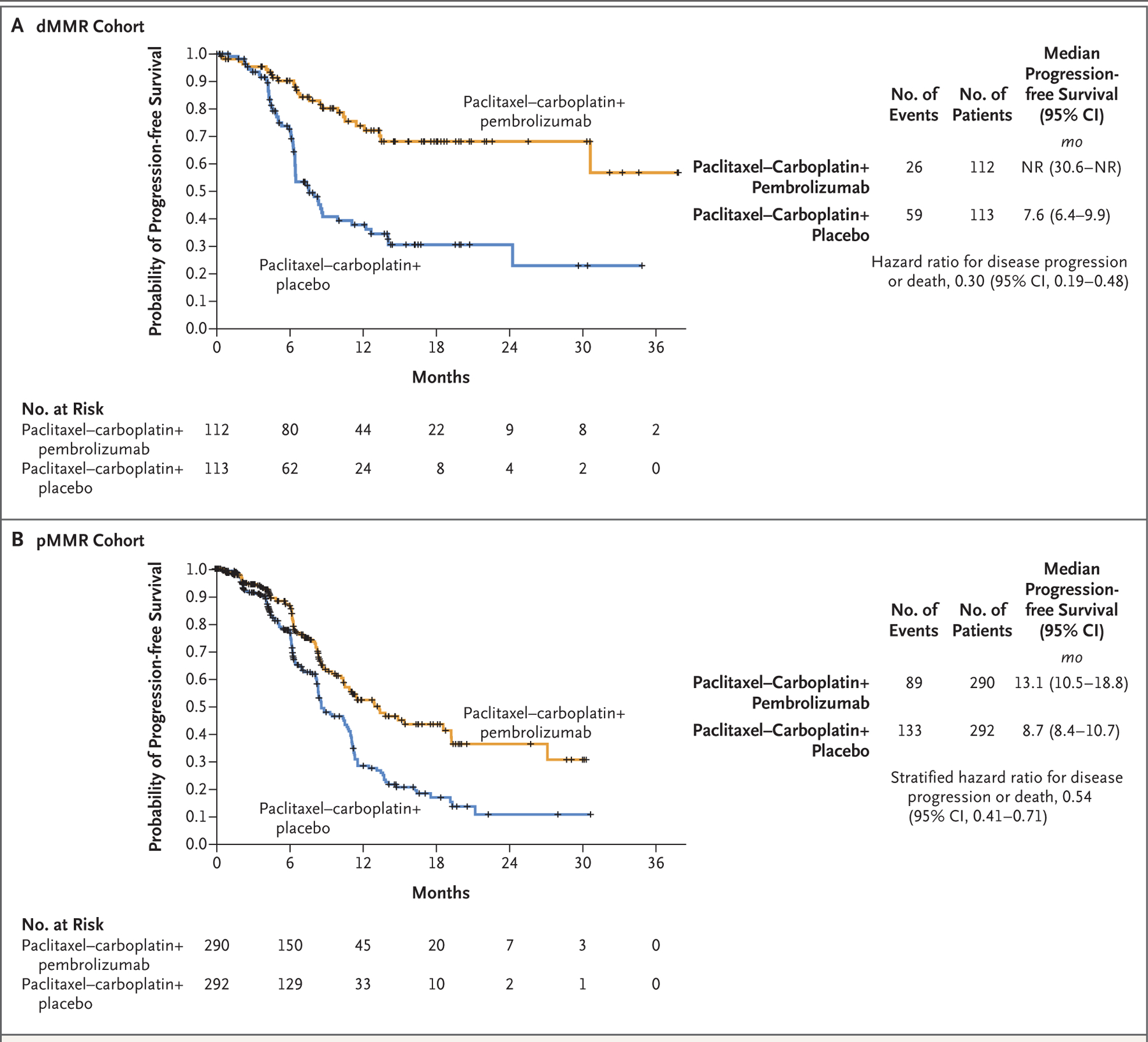
Shown are Kaplan–Meier estimates of progression-free survival in the population of patients with advanced or recurrent endometrial cancer with mismatch repair–deficient (dMMR) disease (Panel A) or mismatch repair–proficient (pMMR) disease (Panel B). Tick marks in both panels indicate censoring of data. Patients in both the pembrolizumab and the placebo groups received combination chemotherapy with paclitaxel and carboplatin. NR denotes not reached.
QUALITY OF LIFE
Quality of life was assessed in 588 patients in the pMMR cohort at baseline and in similar percentages of patients in the pembrolizumab group and the placebo group (86% and 87%, respectively) at 6 weeks after randomization. In the two cohorts, assessments of quality of life at subsequent preplanned intervals (weeks 18, 30, and 54) were in progress at the time of this report.
ADVERSE EVENTS
The most common adverse events (occurring in ≥15% of patients in either trial group) are listed in Table 2; adverse events of interest are listed in Table 3. Similar frequencies of grade 3 or 4 adverse events (regardless of attribution) were identified in the dMMR and pMMR cohorts.
Table 2.
Adverse Events of Any Cause.*
|
Adverse Event |
dMMR Cohort (N = 215) |
pMMR Cohort (N = 550) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pembrolizumab (N = 109) |
Placebo (N = 106) |
Pembrolizumab (N = 276) |
Placebo (N = 274) |
|||||||||||
| Any Grade | Grade ≥3† | Any Grade | Grade ≥3† | Any Grade | Grade ≥3† | Any Grade | Grade ≥3† | |||||||
| number of patients (percentage) | ||||||||||||||
| Any event | 107 (98.2) | 69 (63.3) | 105 (99.1) | 50 (47.2) | 258 (93.5) | 152 (55.1) | 256 (93.4) | 124 (45.3) | ||||||
| Event leading to death | 1 (0.9) | 1 (0.9) | 2 (1.9) | 2 (1.9) | 6 (2.2) | 6 (2.2) | 2 (0.7) | 2 (0.7) | ||||||
| Adverse event | ||||||||||||||
| Fatigue | 78 (71.6) | 1 (0.9) | 59 (55.7) | 3 (2.8) | 175 (63.4) | 5 (1.8) | 165 (60.2) | 7 (2.6) | ||||||
| Peripheral sensory neuropathy | 71 (65.1) | 4 (3.7) | 66 (62.3) | 0 | 153 (55.4) | 3 (1.1) | 157 (57.3) | 5 (1.8) | ||||||
| Anemia | 63 (57.8) | 21 (19.3) | 58 (54.7) | 11 (10.4) | 152 (55.1) | 38 (13.8) | 144 (52.6) | 25 (9.1) | ||||||
| Nausea | 55 (50.5) | 3 (2.8) | 44 (41.5) | 1 (0.9) | 121 (43.8) | 3 (1.1) | 114 (41.6) | 3 (1.1) | ||||||
| Constipation | 47 (43.1) | 1 (0.9) | 40 (37.7) | 0 | 120 (43.5) | 1 (0.4) | 106 (38.7) | 1 (0.4) | ||||||
| Diarrhea | 46 (42.2) | 5 (4.6) | 36 (34.0) | 1 (0.9) | 99 (35.9) | 4 (1.4) | 88 (32.1) | 3 (1.1) | ||||||
| Thrombocytopenia | 38 (34.9) | 5 (4.6) | 31 (29.2) | 2 (1.9) | 83 (30.1) | 12 (4.3) | 59 (21.5) | 7 (2.6) | ||||||
| Arthralgia | 32 (29.4) | 0 | 31 (29.2) | 1 (0.9) | 62 (22.5) | 3 (1.1) | 75 (27.4) | 2 (0.7) | ||||||
| Dyspnea | 30 (27.5) | 3 (2.8) | 21 (19.8) | 1 (0.9) | 58 (21.0) | 5 (1.8) | 52 (19.0) | 0 | ||||||
| Myalgia | 29 (26.6) | 0 | 19 (17.9) | 1 (0.9) | 45 (16.3) | 2 (0.7) | 46 (16.8) | 4 (1.5) | ||||||
| Neutropenia | 28 (25.7) | 13 (11.9) | 34 (32.1) | 18 (17.0) | 87 (31.5) | 51 (18.5) | 73 (26.6) | 33 (12.0) | ||||||
| Vomiting | 22 (20.2) | 2 (1.8) | 9 (8.5) | 2 (1.9) | 53 (19.2) | 2 (0.7) | 38 (13.9) | 2 (0.7) | ||||||
| Weight loss | 16 (14.7) | 1 (0.9) | 6 (5.7) | 1 (0.9) | 19 (6.9) | 1 (0.4) | 21 (7.7) | 2 (0.7) | ||||||
| Rash | 7 (6.4) | 1 (0.9) | 13 (12.3) | 1 (0.9) | 57 (20.7) | 6 (2.2) | 27 (9.9) | 2 (0.7) | ||||||
Listed are adverse events with a rounded incidence of at least 15% in all the patients in either trial group, according to preferred term.
In the dMMR cohort, 3 patients (1.4%) — 1 in the pembrolizumab group and 2 in the placebo group — died from grade 5 adverse events: cardiac arrest, sepsis, and lower gastrointestinal hemorrhage in 1 patient each. In the pMMR cohort, 8 patients (1.5%) — 6 in the pembrolizumab group and 2 in the placebo group — died from grade 5 adverse events: sepsis in 4 patients, cardiac arrest in 2 patients, and small intestinal obstruction and sudden death not otherwise specified in 1 patient each.
Table 3.
Adverse Events of Interest.*
|
Adverse Event |
dMMR Cohort (N = 215) |
pMMR Cohort (N = 550) |
||||||
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab (N = 109) |
Placebo (N = 106) |
Pembrolizumab (N = 276) |
Placebo (N = 274) |
|||||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| number of patients (percentage | ||||||||
| Any event | 42 (38.5) | 9 (8.3) | 28 (26.4) | 6 (5.7) | 92 (33.3) | 10 (3.6) | 54 (19.7) | 7 (2.6) |
| Infusion reaction | 16 (14.7) | 4 (3.7) | 16 (15.1) | 3 (2.8) | 41 (14.9) | 4 (1.4) | 35 (12.8) | 5 (1.8) |
| Hypothyroidism | 14 (12.8) | 0 | 10 (9.4) | 0 | 37 (13.4) | 0 | 7 (2.6) | 0 |
| Hyperthyroidism | 10 (9.2) | 0 | 1 (0.9) | 0 | 16 (5.8) | 0 | 10 (3.6) | 0 |
| Colitis | 7 (6.4) | 0 | 0 | 0 | 4 (1.4) | 0 | 4 (1.5) | 1 (0.4) |
| Pneumonitis | 3 (2.8) | 2 (1.8) | 2 (1.9) | 1 (0.9) | 2 (0.7) | 0 | 1 (0.4) | 0 |
| Glucose intolerance | 2 (1.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acute kidney injury | 2 (1.8) | 2 (1.8) | 2 (1.9) | 2 (1.9) | 5 (1.8) | 5 (1.8) | 1 (0.4) | 1 (0.4) |
| Hepatic failure | 1 (0.9) | 1 (0.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Myositis | 1 (0.9) | 0 | 1 (0.9) | 0 | 1 (0.4) | 0 | 0 | 0 |
| Hypophysitis | 0 | 0 | 0 | 0 | 2 (0.7) | 2 (0.7) | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 1 (0.4) | 0 | 0 | 0 |
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 4 (1.4) | 0 | 1 (0.4) | 0 |
The events of interest are those with a possible immune-related cause and are considered regardless of attribution by the investigator. Some patients may have had more than one adverse event of interest. The events are listed in descending order of frequency in the pembrolizumab group in the dMMR cohort.
In the dMMR cohort, adverse events of any cause (regardless of attribution by the treating physician) occurred in 98.2% of the patients in the pembrolizumab group and in 99.1% of those in the placebo group (Table 2). In the pMMR cohort, these frequencies were 93.5% and 93.4%, respectively. In the dMMR cohort, grade 3 or higher adverse events were reported in 63.3% of patients in the pembrolizumab group and in 47.2% of those in the placebo group. In the pMMR cohort, these frequencies were 55.1% and 45.3%, respectively.
Adverse events leading to death (grade 5) were reported in 3 of 215 patients (1.4%) in the dMMR cohort and included one each of cardiac arrest, sepsis, and lower gastrointestinal hemorrhage. One of these patients was in the pembrolizumab group and two were in the placebo group, and an association with the trial group was considered to be unlikely by the treating physician. In the pMMR cohort, 8 patients (1.5%) had grade 5 adverse events (6 in the pembrolizumab group and 2 in the placebo group), which included sepsis in 4 patients, cardiac arrest in 2 patients, and small intestinal obstruction or sudden death not otherwise specified in 1 patient each. Grade 5 cardiac arrest was deemed to be possibly related to pembrolizumab in 1 patient in the pMMR cohort.
DISCUSSION
In this phase 3 trial, we found that the addition of pembrolizumab to standard chemotherapy, followed by pembrolizumab maintenance, resulted in a 70% lower risk of disease progression or death in patients in the dMMR cohort and a 46% lower risk in the pMMR cohort than in the placebo group. These data suggest that the incorporation of immunotherapy into the first-line treatment of patients with advanced or recurrent endometrial cancer translates into improved oncologic outcomes, regardless of MMR status or histologic findings. Previous trials of monotherapy drugs against programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in patients with recurrent or metastatic pMMR endometrial cancer resulted in only modest improvement.27–30
In our trial, the efficacy curves in the two MMR cohorts separated early in the course of treatment, with a preserved separation throughout the evaluation period. This benefit was observed in most subgroups, including among patients who had received previous adjuvant chemotherapy or radiation and among those with less common histologic subtypes. The question of whether pembrolizumab plus chemotherapy has greater efficacy than pembrolizumab monotherapy in patients with newly diagnosed, advanced-stage or recurrent dMMR endometrial cancer warrants additional study.
The addition of pembrolizumab did not appear to increase the frequency of adverse events that are commonly associated with combination chemotherapy. Furthermore, the incidence of immune-mediated adverse events was not greater than that observed in previous studies of pembrolizumab monotherapy in patients with endometrial cancer.8
A limitation of this trial is the relatively short duration of follow-up. Although protocol-specified criteria were met for the primary efficacy analysis of progression-free survival in both the dMMR and pMMR cohorts, safety and efficacy monitoring are ongoing.
Our results show that pembrolizumab in combination with chemotherapy and continued as maintenance therapy led to significantly longer progression-free survival than placebo in patients with dMMR and pMMR endometrial cancers.
Supplementary Material
Acknowledgments
Supported by grants (U10CA180868 and U10CA180822) from the National Cancer Institute (NCI). Funding was provided by Merck through a cooperative research and developmental agreement with the NCI. Merck also provided supplemental funding to NRG Oncology for this trial. Drs. Aghajanian and O’Cearbhaill are supported in part by a grant (P30CA008748) from the NCI.
Footnotes
Contributor Information
Ramez N. Eskander, Division of Gynecologic Oncology, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Diego, Rebecca and John Moores Cancer Center, La Jolla, California
Michael W. Sill, Clinical Trial Development Division, Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, New York
Lindsey Beffa, Case Comprehensive Cancer Center, Cleveland Clinic Foundation, Cleveland
Richard G. Moore, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York
Joanie M. Hope, Pacific Cancer Research Consortium, NCORP, Alaska Women’s Cancer Care, and Providence Alaska Cancer Center, Anchorage
Fernanda B. Musa, Pacific Cancer Research Consortium, NCORP, Swedish Medical Center-First Hill, Seattle
Robert Mannel, University of Oklahoma Health Sciences Center, Oklahoma City
Mark S. Shahin, Jefferson Abington Hospital, Asplundh Cancer Pavilion of Sidney Kimmel Cancer Center, Jefferson Health, Willow Grove, PA
Guilherme H. Cantuaria, Georgia NCORP, Atlanta
Eugenia Girda, Rutgers Cancer Institute of New Jersey, New Brunswick
Cara Mathews, Women and Infants Hospital, Legoretta Cancer Center, Alpert Medical School, Brown University, Providence, RI
Juraj Kavecansky, Kaiser Permanente National Cancer Institute Community Oncology Research Program (NCORP), Antioch Medical Center, Antioch, California
Charles A. Leath, University of Alabama at Birmingham-Deep South Research Consortium, O’Neal Comprehensive Cancer Center, University of Alabama Hospital, Birmingham
Lilian T. Gien, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Canada
Emily M. Hinchcliff, Feinberg School of Medicine and the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago
Shashikant B. Lele, Clinical Trial Development Division, Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, New York
Lisa M. Landrum, Indiana University Health Simon Cancer Center, Indianapolis
Floor Backes, Ohio State University Wexner Medical Center and James Cancer Hospital, Columbus
Roisin E. O’Cearbhaill, Gynecologic Medical Oncology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, and the Department of Medicine, Weill Cornell Medical College, New York, New York
Tareq Al Baghdadi, Michigan Cancer Research Consortium, NCORP, Trinity Health IHA Medical Group, Ypsilanti
Emily K. Hill, University of Iowa Hospitals and Clinics, Iowa City
Premal H. Thaker, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis
Veena S. John, Northwell Health Cancer Institute, New Hyde Park, New York
Stephen Welch, London Regional Cancer Program, London, ON, Canada
Amanda N. Fader, Division of Gynecologic Oncology, Department of Gynecology and Obstetrics, Johns Hopkins School of Medicine, Baltimore
Matthew A. Powell, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Washington University School of Medicine, St. Louis
Carol Aghajanian, Gynecologic Medical Oncology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, and the Department of Medicine, Weill Cornell Medical College, New York, New York
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- 2.McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother’s cancer. Cancer 2016;122:2787–98. [DOI] [PubMed] [Google Scholar]
- 3.Wan YL, Beverley-Stevenson R, Carlisle D, et al. Working together to shape the endometrial cancer research agenda: the top ten unanswered research questions. Gynecol Oncol 2016;143:287–93. [DOI] [PubMed] [Google Scholar]
- 4.Miller DS, Filiaci VL, Mannel RS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol 2020;38:3841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine DA, The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol 2022;40:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol 2020;6:1766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020;38:2981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 2022;386:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 14.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 15.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 2017;6(7):e1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roselli M, Cereda V, di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013;2(10):e27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesterhuis WJ, Punt CJA, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 20.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wieand S, Schroeder G, O’Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med 1994;13:1453–8. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–56. [PubMed] [Google Scholar]
- 23.Gordon Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63. [Google Scholar]
- 24.Maurer W, Bretz F. Multiple testing in group sequential trials using graphical approaches. Stat Biopharm Res 2013;5:311–20. [Google Scholar]
- 25.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of non-endometrioid cancers. J Clin Oncol 2019;37:1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol 2022;8:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott PA, Bang Y-J, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 Study. J Clin Oncol 2017;35:2535–41. [DOI] [PubMed] [Google Scholar]
- 28.Antill YC, Kok PS, Robledo K, et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol 2019;37(15):Suppl:5501. abstract. [Google Scholar]
- 29.Oaknin A, Gilbert L, Tinker AV, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GAR-NET-a phase I, single-arm study. J Immunother Cancer 2022;10(1):e003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinopoulos PA, Luo W, Liu JF, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol 2019;37:2786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


