Abstract
Objective
To explore whether MRI‐visible enlarged perivascular spaces (EPVS) are associated with migraine and may serve as a predictor of migraine. Then further explore its correlation with migraine chronification.
Methods
A total of 231 participants (healthy control [HC] = 57, episodic migraine [EM] = 59, chronic migraine [CM] = 115) were included in this case–control study. A 3T MRI device and the validated visual rating scale were used to assess the grades of EPVS in centrum semiovale (CSO), midbrain (MB), and basal ganglia (BG). Comparisons between the two groups were made using the chi‐square or Fisher's exact tests to initially determine whether high‐grade EPVS were associated with migraine and migraine chronification. A multivariate logistic regression model was constructed to further investigate the role of high‐grade EPVS in migraine.
Results
The prevalence of high‐grade EPVS in CSO and MB were significantly higher in patients with migraine than in HCs (CSO: 64.94% vs. 42.11%, P = 0.002; MB: 55.75% vs. 29.82%, P = 0.001). Subgroup analysis showed no statistical difference between patients with EM and CM (CSO: 69.94% vs. 62.61%, P = 0.368; MB: 50.85% vs. 58.26%, P = 0.351). Individuals with high‐grade EPVS in CSO (odds ratio [OR]: 2.324; 95% confidence interval [CI]: 1.136–4.754; P = 0.021) and MB (OR: 3.261; 95% CI: 1.534–6.935; P = 0.002) were more likely to suffer from migraine.
Interpretation
This case–control study showed that high‐grade EPVS in CSO and MB in clinical practice with the underlying mechanism of dysfunction of the glymphatic system could be a predictor of migraine, but no significant correlation had been found with migraine chronification.
Introduction
Migraine is a highly disabling disease with years lived with disability (YLDs) in 2019 ranking second out of 369 human diseases and first among women aged 15–49, 1 affecting approximately 14% of the general population. 2 Genetic, environmental, dietary, and endocrine factors are considered to be risk factors for migraine. 3 In clinical practice, avoidance of these risk factors, dietary and lifestyle interventions, and early identification of clinically based indicators associated with migraine can prevent the onset and progression of migraine. However, there is still a lack of clinical predictors of migraine to enable timely intervention.
Recently, the proposal of the glymphatic system has opened up a new insight into the brain. The brain's glymphatic system is currently considered to be a perivascular pathway in the central nervous system that promotes the exchange of cerebrospinal and intercellular fluids to ultimately carry solutes and waste products out of the brain. 4 Studies have suggested that the impairment of the glymphatic system may be an underlying cause of sleep disorders, headaches, and cortical spreading depression (CSD). 5 , 6 , 7 , 8 A diffusion tensor imaging (DTI) analysis of the perivascular space (DTI‐ALPS) in patients with cluster headache found that the DTI‐ALPS index was lower in patients than in healthy controls (HCs), suggesting a dysfunction of the glymphatic system in patients with cluster headache. 9 However, the same method used in the migraine population found no difference between patients with migraine and HCs. 10 Various methods are available for evaluating the glymphatic system's function, such as 2‐photon laser scanning microscopy, DTI‐ALPS in imaging, and MRI‐visible perivascular spaces (PVS). PVS, one of the imaging markers of cerebral small vessel disease, have been demonstrated to be associated with headaches. 11 , 12
As an important component of the glymphatic system, the PVS are interstitial fluid‐filled spaces around perforating arteries that pass from the subarachnoid space through the soft meninges into the brain parenchyma, serving as drainage pathways for the interstitial fluid recycle pathway. 13 Normal PVS are not visible on MRI; MRI‐visible enlarged perivascular spaces (EPVS) provide us with a non‐invasive method of indirectly reflecting the dysfunction of glymphatic drainage, 14 which could be widely used in clinical practice. Several prior studies have focused on the relationship between EPVS and migraine. 15 , 16 , 17 , 18 For instance, one of these studies found that accentuated Virchow‐Robin spaces (VRS) were significantly more common in children with migraine than in those with tension‐type headache (TTH) or headache‐free controls. 16 A case report of a 15‐year‐old boy indicated that hemiplegic migraine might be caused by giant tumefactive PVS. 18 Interestingly, a population‐based study of HUNT‐MRI revealed a decrease in dilated PVS among headache sufferers. 17 Thus, no consistent conclusions have been reached about the association between migraine and EPVS. Whether migraine progression is accompanied by an exacerbation of the EPVS has also not been investigated.
In this study, we aimed to evaluate the distribution and severity of EPVS, which represent the damage to the glymphatic system in patients with migraine and HCs based on the Chinese population. We further explored whether higher grade EPVS would contribute to migraine and as a consequence of migraine progression. We hypothesized that patients with migraine would have more EPVS at different locations compared to HCs and that chronic migraine (CM) was also more severe compared to episodic migraine (EM).
Methods
Study design and patients
This was a case–control study supported by a single‐center hospital. Patients who visited the headache department of Beijing Tiantan Hospital between January 2021 and December 2022, with a final diagnosis of migraine without aura and completed brain MRI scans, were recruited. The diagnosis of migraine was performed by at least two headache specialists with consistent results. This study included 174 patients with migraine (59 EM and 115 CM) and 57 age‐ and sex‐matched HCs (Fig. 1). The inclusion criteria were as follows: (1) age 18–60 years; (2) all diagnoses of migraine without aura were made according to the International Classification of Headache Disorders 3rd edition (ICHD‐3) 19 and the age of first migraine onset should be <50 years; (3) no previous history of prophylactic medication. In contrast, the exclusion criteria were: (1) patients with any neurological diseases, psychiatric disorders, or other headache subtypes; (2) pregnancy or menstrual period in women; (3) contraindications to MRI scanning or inadequate imaging data. HCs who met the criteria of having no previous history of headaches and the exclusion criteria above were recruited for this study through recruitment advertisements and posters.
Figure 1.
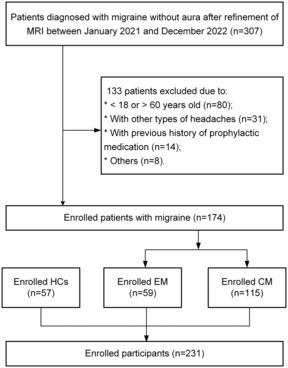
Flowchart of the participant inclusion process. HCs, healthy controls; CM, chronic migraine; EM, episodic migraine.
This case–control study was a branch of the ongoing China HeadAche DIsorders RegiStry Study (CHAIRS, unique identifier: NCT05334927) with the study protocols and procedures approved by the institutional review board of Beijing Tiantan Hospital. All participants voluntarily signed a written informed consent before enrollment following the Helsinki Declaration.
Clinical characteristics of participants
To make a definitive diagnosis, all patients underwent a complete history taking, physical examinations, routine laboratory tests, and MRI scans before the diagnosis. Each of the enrolled participants then completed a detailed headache questionnaire covering the duration of headache history, headache characteristics (location, severity, duration, frequency of attacks, etc.), medication use over the past 3 months, history of smoking and alcohol consumption, and sleep quality based on Pittsburgh Sleep Quality Index (PSQI). The PSQI consists of 7 components with total scores ranging from 0 to 21, with higher scores associated with poorer sleep quality. Although most researchers defined a PSQI score >5 as poor sleep quality, we used stricter criteria to determine a sleep disturbance with a PSQI score ≥8 according to a previous study in the Chinese population. 20
MRI acquisition and assessment
All patients with migraine underwent brain MRI scans before diagnosis and HCs completed the MRI scans after enrolling. All MRI scans were obtained by the 3.0T PET/MR system (SIGNA PET/MR, GE Healthcare), which included MRI sequences of axial three‐dimensional T1‐weighted brain volume (T1w‐BRAVO), two‐dimensional T2‐weighted periodically rotated overlapping parallel lines with enhanced reconstruction (T2w‐PROPELLER), and T2 fluid‐attenuated inversion recovery (T2‐FLAIR) images. The detailed acquisition parameters of the sequences are listed in Table S1.
Furthermore, the assessments of the EPVS were carried out simultaneously by two experienced and specially trained neuroradiologists who were blinded to any clinical information about the participants. Notably, EPVS appear linear when parallel to the MRI image plane and dot‐like when perpendicular to the plane because it follows the track of perforating arterioles. Several studies have shown that EPVS are visible on MRI images with a diameter of <3 mm, which is different from lacunar infarction, which usually ranges from 3 to 15 mm in diameter. 14 , 21 , 22 EPVS were assessed on the sequence of T2w‐PROPELLER and T2‐FLAIR, as demonstrated by small (<3 mm) punctate or linear hyperintensities on T2w‐PROPELLER images with corresponding hypointensities on FLAIR images. Meanwhile, we added the sequence of T1w‐BRAVO images to further differentiate from other imaging changes. EPVS were visually evaluated in three different brain axial planes: basal ganglia (BG), midbrain (MB), and centrum semiovale (CSO) levels. In addition, the number of EPVS was rated based on the hemisphere with the most EPVS. Meanwhile, we needed to review all relevant slices in each location, then selected the slice with the maximum number of EPVS. A widely used visual rating scale was applied for the grade of the EPVS (https://www.ed.ac.uk/sites/default/files/imports/fileManager/epvs‐rating‐scale‐user‐guide.pdf), 21 with the following detailed classification criteria: In CSO and BG, 0 = no EPVS, 1 = ≤10 EPVS, 2 = 11–20 EPVS, 3 = 21–40 EPVS, and 4 = >40 EPVS; in MB, 0 = no EVPS visible, 1 = EVPS visible. We designated CSO‐ and BG‐EPVS > 10 or the presence of MB‐EPVS as high‐grade EPVS based on previous literature (Fig. 2). 22
Figure 2.

Examples of high‐grade EPVS distributed in three regions in patients with migraine based on the axial T2w‐PROPELLER sequence of 3T MRI. (A) High‐grade EPVS in MB; (B) High‐grade EPVS in BG; (C) High‐grade EPVS in CSO. EPVS, enlarged perivascular spaces; MB, midbrain; BG, basal ganglia; CSO, centrum semiovale.
Statistical analysis
Statistics was conducted using the IBM SPSS statistical software (version 26.0). Non‐normally distributed continuous data (age, body mass index [BMI], duration of headache history, migraine days per month, visual analogue scale [VAS]) were compared between groups by Mann–Whitney U tests summarized as medians (interquartile range [IQR]). The categorical variable, including sex, the scale of PVS, the presence of sleep disturbance, and the history of smoking and alcohol consumption, were used the chi‐square or Fisher's exact tests for analysis and presented as numbers with percentages. Baseline variables with univariate analyses with P < 0.05 or clinically considered to be relevant with migraines such as high‐grade CSO EPVS, high‐grade MB EPVS, sleep disturbance, sex, and age were included in the multivariate logistic regression analysis. Given the sample size limitations, we carefully selected the included variables and calculated the variance inflation factor (VIF). The VIF as a diagnostic tool of multicollinearity in our data was all <1.20, meaning there is no multicollinearity between the variables in the model. The Hosmer‐Lemeshow test was conducted to assess the goodness of fit, and its value was 0.8.
Results
Demographics and clinical characteristics of the study participants
As shown in Fig. 1, 231 participants were ultimately enrolled in the study. There were no significant differences between migraine groups and HCs in age, sex, BMI, and the proportion of smokers and drinkers (Table 1). The prevalence of comorbid sleep disturbance was higher in the patients with migraine (100, 57.47%) than the HCs (7, 12.28%) (P < 0.001), and the same for CM (73, 63.48%) than EM (27, 45.76%) (P = 0.025). Following the previous study, patients with CM generally had a longer history of headache than EM (Table 2).
Table 1.
Participant demographics and clinical characteristics.
| Healthy control (n = 57) | Migraine (n = 174) | P value | |
|---|---|---|---|
| Age, years | 41 (29.50–47) | 40 (32–47) | 0.952 a |
| Female, n (%) | 38 (66.67) | 125 (71.84) | 0.457 b |
| Body mass index | 22.58 (20.49–24.62) | 22.86 (20.50–25.87) | 0.252 a |
| Current smoker, n (%) | 5 (8.77) | 21 (12.07) | 0.494 b |
| Current drinker, n (%) | 12 (21.05) | 30 (17.24) | 0.517 b |
| Sleep disturbance, PSQI ≥8, n (%) | 7 (12.28) | 100 (57.47) | <0.001 b |
| MRI‐visible EPVS | |||
| High‐grade EPVS in CSO, n (%) | 24 (42.11) | 113 (64.94) | 0.002 b |
| High‐grade EPVS in BG, n (%) | 2 (3.51) | 9 (5.17) | >0.999 c |
| High‐grade EPVS in MB, n (%) | 17(29.82) | 97 (55.75) | 0.001 b |
| Visual analogue scale | – | 7 (6–8) | – |
| History of migraine, years | – | 16 (8–24) | – |
| Migraine frequency, days per month | – | 17 (8–30) | – |
Note: Values are reported as median (interquartile range) for the continuous variables and as n (% of total) for the categorical variables.
PSQI, Pittsburgh Sleep Quality Index; EPVS, enlarged perivascular spaces; CSO, centrum semiovale; BG, basal ganglia; MB, midbrain.
Mann–Whitney U test.
Chi‐square test.
Fisher's exact test.
Table 2.
Participant demographics and clinical characteristics of patients with episodic migraine and chronic migraine.
| Episodic migraine (n = 59) | Chronic migraine (n = 115) | P value | |
|---|---|---|---|
| Age, years | 39 (32–43) | 42 (33–48) | 0.127 a |
| Female, n (%) | 43 (72.88) | 82 (71.30) | 0.827 b |
| Body mass index | 22.68 (20.31–25.39) | 23.03 (20.70–26.03) | 0.576 a |
| Current smoker, n (%) | 9 (15.25) | 12 (10.43) | 0.356 b |
| Current drinker, n (%) | 10 (16.95) | 20 (17.39) | 0.942 b |
| Sleep disturbance, PSQI ≥ 8, n (%) | 27 (45.76) | 73 (63.48) | 0.25 b |
| MRI‐visible EPVS | |||
| High‐grade EPVS in CSO, n (%) | 41 (69.49) | 72 (62.61) | 0.368 b |
| High‐grade EPVS in BG, n (%) | 2 (3.39) | 7 (6.07) | 0.720 c |
| High‐grade EPVS in MB, n (%) | 30 (50.85) | 67 (58.26) | 0.351 b |
| Visual analogue scale | 7 (5–8) | 7 (6–8) | 0.156 a |
| History of migraine, years | 13 (5–20) | 19 (9–26) | 0.007 a |
| Migraine frequency, days per month | 5 (4–8) | 25 (18–30) | <0.001 a |
| Medication overuse, n (%) | – | 59 (51.30) | – |
Values are reported as median (IQR) for the continuous variables and as n (% of total) for the categorical variables. PSQI, Pittsburgh Sleep Quality Index; EPVS, enlarged perivascular spaces; CSO, centrum semiovale; BG, basal ganglia; MB, midbrain.
Mann–Whitney U test.
Chi‐square test.
Fisher's exact test.
MRI features between groups
The examples of high‐grade EPVS in three regions among the participants are shown in Fig. 2, the distribution and prevalence of EPVS by grade are summarized in Table 3. Our findings suggested that there was a higher prevalence of high‐grade EPVS distributed in CSO and MB in patients with migraine compared with HCs (CSO: 64.94% vs. 42.11%, P = 0.002; MB: 55.75% vs. 29.82%, P = 0.001), while there was no significant difference in BG high‐grade EPVS (5.17% vs. 3.51%, P > 0.999). When comparing the condition of high‐grade EPVS according to the type of migraine, there were no significant differences between patients with EM and CM (CSO: 69.49% vs. 62.61%, P = 0.368; MB: 50.85% vs. 58.26%, P = 0.351; BG: 3.39% vs. 6.07%, P = 0.720).
Table 3.
Distribution and grades of EPVS.
| Healthy control (n = 57) | Episodic migraine (n = 59) | Chronic migraine (n = 115) | |
|---|---|---|---|
| CSO‐EPVS level, n (%) | |||
| 0 | 1 (1.75) | 0 | 1 (0.87) |
| 1 | 32 (56.14) | 18 (30.51) | 42 (36.52) |
| 2 | 21 (36.84) | 35 (59.32) | 57 (49.57) |
| 3 | 3 (5.26) | 6 (10.17) | 15 (13.04) |
| 4 | 0 | 0 | 0 |
| BG‐EPVS level, n (%) | |||
| 0 | 21 (36.84) | 9 (15.25) | 20 (17.39) |
| 1 | 34 (59.65) | 48 (81.36) | 88 (76.52) |
| 2 | 1 (1.75) | 1 (1.69) | 7 (6.09) |
| 3 | 1 (1.75) | 1 (1,69) | 0 |
| 4 | 0 | 0 | 0 |
| MB‐EPVS level, n (%) | |||
| 0 | 40 (70.18) | 29 (49.15) | 48 (41.74) |
| 1 | 17 (29.82) | 30 (50.85) | 67 (58.26) |
EPVS, enlarged perivascular spaces; CSO, centrum semiovale; BG, basal ganglia; MB, midbrain.
Associations of migraine and high‐grade EPVS
Using univariate analysis, we observed that sleep disturbance and high‐grade EPVS in two regions (CSO, MB) were significantly associated with migraine compared with HCs. Next, two logistic regression analyses for exploring the probable migraine risk factors were performed and the results are listed in Table 4. Adjusted by age, sex, and sleep disturbance, high‐grade EPVS in CSO [odds ratio (OR): 2.324; 95% confidence interval (95% CI): 1.136–4.754; P = 0.021] and MB (OR: 3.261; 95% CI: 1.534–6.935; P = 0.002) were independently associated with migraine. Sleep disturbance (OR: 9.756; 95% CI: 4.046–23.524; P < 0.001) was also demonstrated to be associated with migraine and no significant association was found for other factors.
Table 4.
Association between high‐grade EPVS and migraine by logistic regression analysis.
| Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|
| Female | 1.276 (0.671–2.424) | 0.458 | 1.456 (0.681–3.112) | 0.333 |
| Age | 0.996 (0.966–1.027) | 0.805 | 0.966 (0.931–1.002) | 0.065 |
| Sleep disturbance | 9.653 (4.142–22.496) | 0.001 | 9.756 (4.046–23.524) | <0.001 |
| High‐grade EPVS in CSO | 2.547 (1.382–4.693) | 0.003 | 2.324 (1.136–4.754) | 0.021 |
| High‐grade EPVS in MB | 2.964 (1.561–5.630) | 0.001 | 3.261 (1.534–6.935) | 0.002 |
EPVS, enlarged perivascular spaces; CSO, centrum semiovale; MB, midbrain; OR, odds ratio; CI, confidence interval.
Discussion
In this study, we aimed to explore the role of EPVS in migraine. Our key findings can be summarized as follows: (1) Patients with migraine had a significantly higher prevalence of high‐grade EPVS in CSO and MB than HCs, suggesting that high‐grade EPVS in CSO and MB may be implicated in the development of migraine; (2) Multivariate logistic regression analysis revealed that high‐grade EPVS in CSO and MB associated with a higher likelihood of having migraine; and (3) Interestingly, we found no significant difference in the incidence of high‐grade EPVS between the EM and CM groups, indicating that there is no clear association between high‐grade EPVS and migraine progression.
The association between EPVS and migraine
Thus far, only a few studies have focused on the relationship between EPVS and adult migraine patients and the existing conclusions remain controversial. Our study, which utilized a 3T MRI device to investigate the Chinese adult migraine population, is consistent with two earlier studies that used a 1.5T MRI device, indicating the involvement of EPVS in migraine. For instance, the first study focused on a migraine population aged 13–56 years and observed a widening of the VRS in 28 (40.00%) patients with migraine compared with only 5 (7.10%) in the controls. 15 Moreover, the second study observed accentuated VRS in 61.00% of children with migraine aged 3–14 years, but only in 22.00% of children with TTH and 27.00% of children without headaches. 16 Despite differences in MRI field strength, the age range of the population, and the definition and interpretation of EPVS, the overall trend of the results is identical. Additionally, a case report described a patient with hemiplegic migraine whose MRI showed the presence of giant PVS, which was considered a possible trigger for initiating migraine. 18 Only one study presented contrary findings. Nevertheless, the researcher considered these to be spurious. 17 The basic mechanisms underlying association between EPVS and migraine are not been well elucidated. Therefore, additional research is warranted to underlie the possible mechanisms behind it.
A growing number of studies have provided us with the theoretical basis that MRI‐visible EPVS tend to reflect the impairment of the glymphatic system and may serve as a reliable imaging marker for some neurological disorders, 13 , 22 although the exact underlying pathophysiological mechanism is unknown. The brain's glymphatic system is responsible for the removal of metabolic waste and the drainage of fluid that contributes to the maintenance of the brain's internal environmental homeostasis. 4 When the glymphatic system is damaged, it disrupts the brain's microenvironment, leading to an imbalance in the homeostasis of its internal environment, which inversely causes an increase in pro‐inflammatory substances in the brain, 23 , 24 resulting in headaches. Calcitonin gene‐related peptide (CGRP) is a neuropeptide that is widely distributed in the central and peripheral nervous system and can be present in three different locations upon release: the venous blood plasma, cerebrospinal fluid (CSF), and possibly the glymphatic system. 7 , 25 As an essential element of headaches, especially migraine, CGRP is a vasodilator and regulator of neuronal excitability. 26 CGRP primarily mediates neurogenic inflammation and regulates injurious input, with the possible underlying mechanism being upregulation of the production of receptor proteins and pro‐nociceptive molecules. 7 , 25 Notably, CGRP released from nerve fibers cannot readily penetrate the blood–brain barrier but instead diffuses into the PVS and thus into the CSF of the subarachnoid space. 27 , 28 Furthermore, CGRP enters the PVS of the venous vessels through the CSF‐interstitial fluid (ISF) flow and is then removed from the brain. 27 This regulatory mechanism is thought to be mediated by the glymphatic system, which can maintain CGRP concentration in the brain. 27 , 28 Evidence has shown that plasma CGRP levels were significantly increased during migraine attacks 26 and that CGRP concentrations in CSF were five times higher than those in plasma. 29 These findings indicate that the glymphatic system dysfunction may contribute to the accumulation of CGRP in the PVS and thus to the development of migraine. MRI‐visible EPVS have also become a critical research issue as a marker of neuroinflammatory activity in the brain. In addition, recurrent inflammation around blood vessels may lead to EPVS, which has been proven in patients with multiple sclerosis and systemic lupus erythematosus. 30 , 31 Consequently, the association between EPVS and migraine can also be explained by their similar pathophysiological mechanism of neuroinflammation.
On the other hand, our population showed that 57.47% of patients with migraine had sleep disturbance, which was higher than 12.28% in HCs. For patients with migraine, the prevalence of sleep disturbance was larger in those with CM than in EM. A prior study pointed out that 48.00–74.00% of patients with migraine reported poor sleep quality as a common trigger for migraine, which was also associated with a higher frequency of headaches. 32 Most patients with headaches would gradually experience sleep problems. 33 Moreover, sleep disturbance can also lead to a decrease in substance exchange through the glymphatic system. 7 It is thought that awakening elicits the discharge of norepinephrine, resulting in the contraction of the interstitial space, which then inhibits substance exchange. 23 Thus, the accumulation of metabolic waste and pro‐inflammatory substances can cause headaches and further aggravate sleep disturbance. 8 , 34 There is a complex relationship between migraine, sleep disturbance, and glymphatic system dysfunction. We tend to hypothesize glymphatic system dysfunction as a common pathophysiological mechanism of sleep disorders and migraine. Depression, a common risk factor for headaches and sleep disorders, is thought to impair glymphatic function. 35 However, whether depression further triggers headaches and sleep disorders by reducing glymphatic function has not been studied.
Furthermore, substantial evidence has indicated that CSD underlies the migraine aura and that certain migraine preventive therapies can lower susceptibility to CSD. 36 However, the role of CSD in migraine is still significantly uncertain. Recently, a study on an animal model of migraine found that CSD could induce a rapid and temporary closure of the PVS around penetrating cortical arteries and veins. 8 The authors proposed that CSD impaired the function of the glymphatic system, which provided a new perspective on the association between the glymphatic system and migraine. Combined with the trigeminovascular theory of migraine and the discovery of increased intracranial vascular pulsation leading to more EPVS, 37 we further hypothesized that the presence of migraine was associated with EPVS.
Only high‐grade EPVS in CSO and MB showed an association with migraine but not in BG. Certain structures in the MB are thought to be one of the structures that may drive or generate migraine. 38 , 39 Several studies have demonstrated the involvement of the periaqueductal gray matter of the MB and the dorsal raphe in migraine pathophysiology. 38 , 39 The greater the number of EPVS around the penetrating arteries that innervate the midbrain may also affect the function of these migraine‐related structures. The mechanism associating CSO‐EPVS with migraine should be investigated further. Earlier studies have demonstrated that BG‐EPVS correlated with routine vascular risk factors, and severe EPVS in BG are usually observed in cerebrovascular disease. 40 Over 90% of participants in one study reported dilated PVS of the BG with a grade of 2 or less, 41 which is in line with our data. We presume that there is no association between BG‐EPVS and migraine. However, this is only a conjecture, and the lack of research on the relationship between different parts of the penetrating arteries and migraine makes it difficult to elucidate the mechanisms involved.
Predictor variables for migraine
Results of univariate regression analysis and multivariate logistic regression analysis indicated that high‐grade EPVS in CSO and MB were an independent risk factor for predicting the presence of migraine in adults. These results further strengthened the evidence that there was a potential association between the glymphatic system dysfunction and migraine. In contrast to the results of a recent study using DTI‐ALPS analysis, the ALPS index was not a significant variable in predicting migraineurs or migraineurs with aura. Similarly using multivariate regression analysis suggested migraine with a normal glymphatic system function. 10 However, variations in analytical methods and limitations in sample size may lead to differences in results. Compared to the DTI‐ALPS technique, MRI‐visible PVS are more intuitive and feasible for clinical use. In the future, the two approaches can be combined. Notably, migraine is more prevalent in women and patients with sleep disturbance, and there is an age range for the peak incidence of migraine. 2 , 32 Moreover, EPVS are affected by aging and sleep disturbance. 13 , 41 Therefore, we included the effects of age, sex, and sleep disturbance to exclude confounding factors in our multivariate regression model. Our findings highlighted the clinical relevance of high‐grade EPVS in migraine and showed that sleep disturbance was an independent risk factor for migraine.
The association between EPVS and migraine chronification
Our results disproved the initial hypothesis that EPVS might be associated with the migraine chronification. According to previous studies, we believed that the relationship between the severity of EPVS and migraine chronification was bidirectional. Higher grades of EPVS may imply more impairment of the glymphatic system, which may lead to more severe headaches and more frequent attacks, leading to migraine progression. An earlier study suggested that chronic pain states might also lead to several microstructural and functional changes in the brain. It showed that patients with cancer pain had significantly impaired ISF drainage compared to healthy people. 42 In addition, worse subjective sleep quality is more common in CM people, and studies have established the effectiveness of sleep interventions in reversing CM into EM. 43 Both state of pain and lack of sleep time can cause increased adrenergic signaling, inhibiting the function of the glymphatic system. 23 , 44 It has also been suggested that recurrent episodes of perivascular inflammation can result in a higher rate of glymphatic drainage. 10 How chronic pain affects the glymphatic system is still an open question. The results might not be a sensitive reflection of the variation in EPVS in a real situation because we used a graded scale of assessment rather than a direct comparison of the number of EPVS. Therefore, automated quantitative methods could be used to improve the research.
Strength and limitations
Our study investigated the relationship between EPVS and migraine and migraine chronification in a population of Chinese adults with migraine, which provides new insights into the diagnosis, treatment, and prevention of migraine in clinical practice. Meanwhile, the interpretation of our findings needs to consider the following limitations. First, the relatively small sample size may affect the stability of the results. Future studies with large sample sizes are necessary to validate our conclusions further. Second, validated visual rating scales in this study, which assess EPVS severity by grade, do not sensitively indicate the changes in EPVS; therefore, the conclusion that EPVS severity is not related to migraine chronification may be inaccurate. With the advancement of imaging analysis technology, automated quantitative methods can be used in the future to continue the investigation of the migraine population. Third, our participants were derived from the Chinese adult population; therefore, the conclusions drawn may not necessarily apply to other ethnic populations. Lastly, the limitation of the cross‐sectional study determined that we could not conclude if there were any causal relationship between EPVS and migraine. Future longitudinal studies with large sample sizes are needed to verify the association.
Conclusion
In conclusion, our data demonstrated that high‐grade EPVS distributed in CSO and MB were significantly more common in the migraine groups. The dysfunction of the glymphatic system might be the underlying mechanism of the association between EPVS and migraine. High‐grade EPVS in CSO and MB and sleep disturbance could serve as promising predictors of migraine occurrence in clinical settings. However, we did not find a correlation between EPVS and migraine chronification.
Conflict of Interest
There is no potential competing interest to be declared by the authors.
Supporting information
Table S1. The detailed MRI scan parameters in the present study. T1w‐BRAVO, T1‐weighted brain volume; FLAIR, fluid‐attenuated inversion recovery; T2w‐PROPELLER, T2‐weighted periodically rotated overlapping parallel lines with enhanced reconstruction.
Acknowledgments
Our main acknowledgment is to the Department of Nuclear Medicine at Beijing Tiantan Hospital for providing us with instrumentation and equipment support and to the headache and imaging specialists at Tiantan Hospital for their great contribution to our enrolled patients and imaging assessment. We acknowledge all subjects for their participation.
Funding Information
This study was supported by the National Natural Science Foundation of China (grant numbers 32170752, 91849104, and 31770800) and the National Natural Science Foundation of Beijing (Z200024).
Ziyu Yuan contributed to the manuscript as the first author.
Funding Statement
This work was funded by National Natural Science Foundation of Beijing grant Z200024; National Natural Science Foundation of China grants 31770800, 32170752, and 91849104.
Contributor Information
Yonggang Wang, Email: w100yg@gmail.com.
Xueying Yu, Email: yuxueying6@aliyun.com.
References
- 1. GBD 2019 Diseases and Injuries Collaborators . Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: a Systematic Analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396(10258):1204‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174‐182. [DOI] [PubMed] [Google Scholar]
- 4. Hablitz LM, Nedergaard M. The glymphatic system. Curr Biol. 2021;31(20):R1371‐R1375. [DOI] [PubMed] [Google Scholar]
- 5. Piantino J, Lim MM, Newgard CD, Iliff J. Linking traumatic brain injury, sleep disruption and post‐traumatic headache: a potential role for glymphatic pathway dysfunction. Curr Pain Headache Rep. 2019;23(9):62. [DOI] [PubMed] [Google Scholar]
- 6. Toriello M, González‐Quintanilla V, Pérez‐Pereda S, Fontanillas N, Pascual J. The potential role of the glymphatic system in headache disorders. Pain Med. 2021;22(12):3098‐3100. [DOI] [PubMed] [Google Scholar]
- 7. Yi T, Gao P, Zhu T, Yin H, Jin S. Glymphatic system dysfunction: a novel mediator of sleep disorders and headaches. Front Neurol. 2022;13:885020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schain AJ, Melo‐Carrillo A, Strassman AM, Burstein R. Cortical spreading depression closes Paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci. 2017;37(11):2904‐2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim J, Lee DA, Lee HJ, et al. Glymphatic system dysfunction in patients with cluster headache. Brain Behav. 2022;12(6):e2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee DA, Lee HJ, Park KM. Normal glymphatic system function in patients with migraine: a pilot study. Headache. 2022;62(6):718‐725. [DOI] [PubMed] [Google Scholar]
- 11. Huisman T. Unraveling the mystery of the perivascular spaces and glymphatic system of the neonatal central nervous system. Radiology. 2023;307:223009. [DOI] [PubMed] [Google Scholar]
- 12. Zhang DD, Cao Y, Mu JY, et al. Inflammatory biomarkers and cerebral small vessel disease: a community‐based cohort study. Stroke Vasc Neurol. 2022;7(4):302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137‐153. [DOI] [PubMed] [Google Scholar]
- 14. Moses J, Sinclair B, Law M, O'Brien TJ, Vivash L. Automated methods for detecting and quantitation of enlarged perivascular spaces on MRI. J Magn Reson Imaging. 2023;57(1):11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machado MA Jr, Matos AS, Goyanna F, et al. Dilatation of Virchow‐Robin spaces in patients with migraine. Arq Neuropsiquiatr. 2001;59(2‐A):206‐209. [PubMed] [Google Scholar]
- 16. Schick S, Gahleitner A, Wöber‐Bingöl C, et al. Virchow‐Robin spaces in childhood migraine. Neuroradiology. 1999;41(4):283‐287. [DOI] [PubMed] [Google Scholar]
- 17. Husøy AK, Indergaard MK, Honningsvåg LM, et al. Perivascular spaces and headache: a population‐based imaging study (HUNT‐MRI). Cephalalgia. 2016;36(3):232‐239. [DOI] [PubMed] [Google Scholar]
- 18. Samanta D. Sporadic hemiplegic migraine and giant tumefactive perivascular space: is there an association? Acta Neurol Belg. 2016;116(4):619‐620. [DOI] [PubMed] [Google Scholar]
- 19. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. [DOI] [PubMed] [Google Scholar]
- 20. Reilly‐Spong M, Park T, Gross CR. Poor sleep in organ transplant recipients: self‐reports and actigraphy. Clin Transplant. 2013;27(6):901‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riba‐Llena I, Nafría C, Mundet X, et al. Assessment of enlarged perivascular spaces and their relation to target organ damage and mild cognitive impairment in patients with hypertension. Eur J Neurol. 2016;23(6):1044‐1050. [DOI] [PubMed] [Google Scholar]
- 23. Goldman N, Hablitz LM, Mori Y, Nedergaard M. The glymphatic system and pain. Med Acupunct. 2020;32(6):373‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosic B, Dukefoss DB, Åbjørsbråten KS, et al. Aquaporin‐4‐independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia. 2019;67(6):1113‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Messlinger K. The big CGRP flood ‐ sources, sinks and signalling sites in the trigeminovascular system. J Headache Pain. 2018;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183‐187. [DOI] [PubMed] [Google Scholar]
- 27. Charles A, Pozo‐Rosich P. Targeting calcitonin gene‐related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765‐1774. [DOI] [PubMed] [Google Scholar]
- 28. Tarasoff‐Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain‐implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dux M, Will C, Eberhardt M, Fischer MJM, Messlinger K. Stimulation of rat cranial dura mater with potassium chloride causes CGRP release into the cerebrospinal fluid and increases medullary blood flow. Neuropeptides. 2017;64:61‐68. [DOI] [PubMed] [Google Scholar]
- 30. Miyata M, Kakeda S, Iwata S, et al. Enlarged perivascular spaces are associated with the disease activity in systemic lupus erythematosus. Sci Rep. 2017;7(1):12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conforti R, Cirillo M, Saturnino PP, et al. Dilated Virchow‐Robin spaces and multiple sclerosis: 3 T magnetic resonance study. Radiol Med. 2014;119(6):408‐414. [DOI] [PubMed] [Google Scholar]
- 32. Fernández‐de‐Las‐Peñas C, Fernández‐Muñoz JJ, Palacios‐Ceña M, et al. Sleep disturbances in tension‐type headache and migraine. Ther Adv Neurol Disord. 2018;11:1756285617745444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lovati C, Peruzzo S, Pecis M, Santus P, Pantoni L. Sleep, headache and sleep breathing disturbances: a polisomnographic study. Neurol Sci. 2020;41(Suppl 2):473‐474. [DOI] [PubMed] [Google Scholar]
- 34. Komaroff AL. Does sleep flush wastes from the brain? JAMA. 2021;325(21):2153‐2155. [DOI] [PubMed] [Google Scholar]
- 35. Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer's disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl). 2017;234(3):365‐379. [DOI] [PubMed] [Google Scholar]
- 36. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9(11):637‐644. [DOI] [PubMed] [Google Scholar]
- 37. Shi Y, Thrippleton MJ, Blair GW, et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab. 2020;40(1):85‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waung MW, Margolis EB, Charbit AR, Fields HL. A midbrain circuit that mediates headache aversiveness in rats. Cell Rep. 2019;28(11):2739‐47.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deen M, Hansen HD, Hougaard A, et al. Low 5‐HT(1B) receptor binding in the migraine brain: a PET study. Cephalalgia. 2018;38(3):519‐527. [DOI] [PubMed] [Google Scholar]
- 40. Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim HG, Shin NY, Nam Y, et al. MRI‐visible dilated perivascular space in the brain by age: the human connectome project. Radiology. 2023;306(3):e213254. [DOI] [PubMed] [Google Scholar]
- 42. Wang A, Chen L, Tian C, et al. Evaluation of the glymphatic system with diffusion tensor imaging‐along the perivascular space in cancer pain. Front Neurosci. 2022;16:823701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calhoun AH, Ford S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47(8):1178‐1183. [DOI] [PubMed] [Google Scholar]
- 44. Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator. J Neurosci Res. 2017;95(6):1336‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The detailed MRI scan parameters in the present study. T1w‐BRAVO, T1‐weighted brain volume; FLAIR, fluid‐attenuated inversion recovery; T2w‐PROPELLER, T2‐weighted periodically rotated overlapping parallel lines with enhanced reconstruction.


