Abstract
Fatty liver is the earliest response to excessive ethanol consumption, which increases the susceptibility of the liver to develop advanced stage of liver disease. Our previous studies have revealed that chronic alcohol administration alters metabolic hormone levels and their functions. Of current interest to our laboratory is glucagon-like peptide 1 (GLP-1), a widely studied hormone known to reduce insulin resistance and hepatic fat accumulation in patients with metabolic-associated fatty liver disease. In this study, we examined the beneficial effects of exendin-4 (a GLP-1 receptor agonist) in an experimental rat model of ALD. Male Wistar rats were pair-fed the Lieber-DeCarli control or ethanol diet. After 4 weeks of this feeding regimen, a subset of rats in each group were intraperitoneally injected every other day with either saline or exendin-4 at a dose of 3nmol/kg/day (total 13 doses) while still being fed their respective diet. At the end of the treatment, rats were fasted for 6h and glucose tolerance test was conducted. The following day, the rats were euthanized, and the blood and tissue samples collected for subsequent analysis. We found that exendin-4 treatment had no significant effect on body weight gain among the experimental groups. Exendin-4-treated ethanol rats exhibited improved alcohol-induced alterations in liver/body weight and adipose/body weight ratio, serum ALT, NEFA, insulin, adiponectin and hepatic triglyceride levels. Reduction in indices of hepatic steatosis in exendin-4 treated ethanol-fed rats was attributed to improved insulin signaling and fat metabolism. These results strongly suggest that exendin-4 mitigates alcohol-associated hepatic steatosis by regulating fat metabolism.
Keywords: Chronic alcohol, Fatty liver disease, Organ crosstalk, GLP-1, Exendin-4, Insulin sensitivity
Graphical Abstract

1. Introduction
Excessive alcohol consumption is a global healthcare problem. Being a major metabolic site for alcohol metabolism, the liver sustains the greatest degree of tissue injury by chronic heavy drinking [1]. The prevalence alcohol-associated liver disease (ALD) is gradually increasing in the developing countries with a rise in daily intake of alcohol owing partly to improved economic status [2]. Recent statistical studies report that ALD is one of the leading preventable causes of illness and death from liver disease worldwide [1]. However, currently there is no US Food and Drug Administration approved therapeutics available to treat ALD and the only available options are abstinence or liver transplantation [3].
ALD comprises a wide spectrum of pathology, the most characteristic of which are steatosis, hepatitis, and fibrosis/cirrhosis [4]. Fatty liver (steatosis), characterized by the presence of excess (>5%) lipid accumulation in hepatocytes, is the earliest and most common response of the liver to excessive ethanol consumption. Once the liver becomes steatotic, its susceptibility to develop progressive injury including alcoholic steatohepatitis (ASH), hepatic fibrosis, cirrhosis and even hepatocellular carcinoma is enhanced [4, 5]. Pathophysiological mechanisms involved in development of ALD are complex and multifactorial, including gut, pancreas and adipose tissue dysfunctions that promotes liver injury and disease progression [6-8]. Accumulating evidence demonstrates that gut, adipose, pancreas, and liver interplay is regulated by peptide hormones [7-9]. Thus, understanding the biology and the role of these peptides is important for developing effective therapeutics for the treatment/prevention of metabolic diseases including ALD.
Of interest to our laboratory are gut peptide hormones, such as ghrelin and glucagon-like peptide 1 (GLP-1), and their roles in ALD pathogenesis. Ghrelin (majorly secreted from stomach) and GLP-1 (majorly secreted from intestine) are neuropeptide hormones that play key roles in appetite regulation and energy metabolism [10, 11]. Recent experimental and clinical studies have demonstrated that these two gut hormones additionally modulate various aspects of addiction processes. Regarding alcohol, ghrelin promotes its craving and intake, while pharmacological suppression of the ghrelin receptor attenuates the alcohol craving and alcohol intake in experimental and clinical settings [3]. GLP-1, an appetite suppressor and enhancer of glucose-stimulated insulin release and insulin sensitivity [10], has recently gained attention for reducing alcohol craving and alcohol intake [12-15]. Our previous studies revealed that an alcohol-induced increase in serum ghrelin levels contributes to the development of hepatic steatosis via impairing insulin secretion that by reducing circulating insulin levels ultimately promotes adipose lipolysis and mobilization of fatty acids to the liver. Further studies from our laboratory using ghrelin receptor antagonist [D-Lys-3] GHRP-6 treatment, which reduced the alcohol-induced steatosis, confirmed the role of ghrelin in steatosis development [7, 8]. Similarly, many clinical and experimental studies have shown the efficacy of GLP-1 receptor (GLP-1R) agonists or GLP-1 analogs in the treatment of obesity, metabolism-associated fatty liver disease (MAFLD) and nonalcoholic steatohepatitis (NASH) [16-21]. The mechanism of action includes improving insulin sensitivity, increasing hepatic lipolysis and reducing hepatic lipogenesis, inflammation and fibrosis [10, 16, 22]. However, no studies to date have examined the efficacy of GLP-1 agonist as a treatment modality for ALD. This study is the first to investigate whether a GLP-1 analog/agonist for the GLP-1 receptor has the potential to therapeutically reduce ALD pathogenesis. In this study, we utilized extendin-4 (a GLP-1 analog) to examine its effect on alcohol-induced fatty liver disease and its mechanism of action.
2. Materials and methods
2.1. Antibodies and reagents
Antibodies and reagents were purchased from the following companies: Ethanol was purchased from Pharmaco-AAPER (Brookfield, CT) and exendin-4 (Cat. No. E7144) was from Sigma-Aldrich Corp (St. Louis, MO). Antibodies to IRS-1 (Cat. No. 2382), pIRS-1(Cat. No. 2580), AKT (Cat. No. 4691S), pAKT, (Cat. No. 4060S) HSL (Cat. No. 4107S), pHSL (Cat. No. 4139S), and ATGL (Cat. No. 2138S) were purchased from Cell Signaling (Danvers, MA, USA). CGI58 (Cat. No. NB110-41576) was purchased from Novus Biologicals (Centennial, CO, USA). HRP Conjugated secondary antibodies were purchased from Promega (Madison, WI, USA). All other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA) unless stated otherwise. RIN-m5F cells were generously provided by Robert G. Bennett’s lab, University of Nebraska Medical Center and the Department of Veterans Affairs, Omaha, NE. Insulinoma derived INS-1E β-cells were provided by Dr. Pierre Maechler (University Medical Center, Geneva, Switzerland). VA-13 cells, recombinant Hep G2 cells stably express ADH1 and metabolize alcohol through this enzyme, were obtained from Dr. Dahn Clemens of University of Nebraska Medical Center, Omaha. De-identified human blood and tissue samples from control and alcohol-associated liver disease patients (selection criteria for the samples are male and female patients with alcohol-associated HCC without obesity, diabetes or viral infection) are obtained from Nebraska Biobank and Liver Tissue Cell Distribution System (LTCDS); National Institutes of Health (NIH) service.
2.2. Cell culture
RIN-m5F insulinoma cells were cultured in RPMI-1640 media (Cat. No. SH30027.02, Cytiva HyClone™, Marlborough, MA, USA) supplemented with 10% FBS 1mM sodium pyruvate, 10mM HEPES, 4500 mg/L glucose, 1500 mg/L sodium bicarbonate and 100U/mL penicillin, and 100 μg/mL streptomycin as described previously [23]. Insulinoma derived INS-1E β-cells were cultured as described previously [24] using RPMI-1640 media (Cat. No. 11995065; Gibco, USA) supplemented with 10% fetal calf serum (FCS), 1mM sodium pyruvate, 10mM HEPES, 50μM 2-mercaptoethanol, 100U/mL penicillin, and 100 μg/mL streptomycin. VA-13 cells (recombinant Hep G2 cells stably express ADH1 and metabolize alcohol through this enzyme) were maintained in Dulbecco’s modified Eagle’s medium (DMEM high glucose; Cat No. 11995065; Gibco, MA, USA) with 10% FBS, penicillin/streptomycin [25].
2.3. Exendin-4 labeling and cellular uptake
Exendin-4 peptide (hereafter referred as exendin) were fluorescently tagged with rhodamine using Pierce NHS-Rhodamine Labeling kit (Cat. No. 53031; Thermo Fisher Scientific, USA) as per manufacturer’s instructions. Briefly, 1 mg of exendin mixed with a vial of NHS-Rhodamine reagent and pipetted up-and-down 10 times until the complete dissolution of the dye. Reaction mixture was further incubated (protected from light) at room temperature for 60 minutes followed by purification of the rhodamine-labelled exendin using the spin column provided with the kit and NHS-rhodamine to peptide ratio was calculated by diluting small amount of labeled purified peptide in PBS and measuring absorbance at A280 and A552 (i.e., Amax of NHS-rhodamine). Exendin concentration was also calculated using molar extinction coefficient as detailed in kit protocol. The aliquots of the rhodamine-labelled exendin were stored at −20°C.
Primary hepatocytes isolated from chow-fed rats were cultured with Williams’ media containing 5% FBS on collagen-coated coverslips as described previously [26]. For INS-1E cells and VA-13 cells, nearly 0.05 million cells were seeded in 6-well plate with sterile 22 x 22 mm glass coverslip and cultured until cells reach confluence. Then, cells were washed with their respective serum free media (without FBS or FCS) and incubated for 1h. The cells were then either left untreated or treated with 100 nM of rhodamine-tagged exendin in serum free media for 5 min. After aspirating the media, the cells were washed 3 times with cold sterile 1XPBS and fixed immediately with 4% PFA for 20 min. Further, cells were washed 2 times with PBS and water and mounted with DAPI (ProLong Diamond mountant with DAPI, Cat. No. P36962, Invitrogen). Confocal images were collected using Confocal Laser Scanning Microscopy (Zeiss 710 CLSM, Carl Zeiss Inc. White Plains, NY, USA).
2.4. Assessment of Exendin-4 effect on hepatic fat accumulation in in vitro
Rat primary hepatocytes and VA-13 cells were treated with appropriate serum-free media containing 1.5% fat free BSA or oleic acid-BSA conjugate (500 μM oleic acid-1.5% BSA) in the presence or absence of 50 mM ethanol and 20 nM exendin as described [8]. After 24h treatment for primary hepatocytes and 48 h treatment for VA-13 cells, cellular triglycerides were extracted and estimated as detailed later.
2.5. Animals
Male Wistar rats (175–200 g) were purchased from Charles River Laboratories (Portage, MI, United States). Rats were weight-matched and pair-fed Lieber-DeCarli ethanol liquid diet (contains 18% of total energy as protein, 35% as fat, 11% as carbohydrates, and 36% as ethanol) or control diet (same calorie as control except ethanol was replaced isocalorically with maltodextrin) as described previously [27]. After 4 weeks of this feeding regimen, while still being fed their respective diet, half of the rats in both groups received an intraperitoneal injection of exendin at a dose of 3 nmol/kg/day every other day for 25 days (13 doses) [28, 29]. The other half received the saline injection. Since GLP-1 analogs have been shown to reduce ethanol intake [12, 13, 30], the saline treated control (here onwards referred as control), exendin-treated control, saline-treated ethanol (here onwards referred as ethanol) were pair-fed to the exendin-treated ethanol rat during this treatment period to maintain nutritional equality among all rats in each experimental group. Body weight was monitored once in a week throughout the experimental period.
After 36 h of last injection, rats were fasted for 6 h to perform oral glucose tolerance test (OGTT) as described [31]. The rats were fed their respective diets after the OGTT and the following morning to ensure that all rats are in the “fed” state. The rats were euthanized under isoflurane anesthesia after 2 hours of feeding. Blood was collected from the vena cava. The liver, epididymal adipose, pancreas and intestine were excised and either immediately processed for histopathological studies or stored at −80°C till processed for subsequent biochemical analyses. All animals received humane care in accordance with the guidelines established by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee at the VA NWIHCS Research Service.
2.6. Histopathological examination
Paraffin-embedded liver was deparaffinized and stained with hematoxylin followed by counter-staining with eosin.
2.7. Serum enzymes and hormones
Serum level of alanine aminotransferase (ALT) was measured in clinical laboratory at VA NWIHCS. Serum non-esterified fatty acids (NEFA) were measured in an enzymatic colorimetric assay using a HR Series NEFA-HR2 kit (Wako Chemicals, Richmond, VA, USA). Serum cholesterol was quantified using a colorimetric diagnostic kit (Cat. No. TR2242, Thermo Fisher Scientific, Middletown, VA, USA). Specific ELISA kits were used for measuring serum levels of insulin (Cat. No. 10-1251-01; Mercodia, Uppsala, Sweden), GLP-1 (Cat. No. EIAR-GLP1; RayBiotech, Peachtree Corners, GA, USA) and adiponectin (Cat. No. RRP300; R&D Systems, Inc., Minneapolis, MN, USA).
2.8. Hepatic triglycerides
Hepatic and cellular lipids were extracted according to the Folch procedure (Folch et al., 1957) [32]. Aliquots of the lipid extract were saponified to quantify the triglyceride mass using the triglyceride diagnostics kit (Cat. No.TR22421, Thermo Fisher Scientific, Middletown, VA, USA). Triglyceride levels were normalized to grams of liver tissue or ug DNA.
2.9. Total RNA extraction and Real-Time PCR
RNA was isolated from liver, epididymal adipose tissue and pancreatic β cells (INS-1E and RIN-m5F) using the PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA, USA). Further, cDNA was synthesized by reverse-transcribing 1μg of total RNA by using the High-Capacity cDNA Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). For Quantitative PCR (qPCR), synthesized cDNA was amplified by real-time PCR (7500 Fast Real-Time PCR system, Applied Biosystems, Waltham, MA, USA) using TaqMan™ Universal Master Mix II (Thermo Fisher Scientific, Waltham, MA, USA) or iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and specific primers from Applied Biosystems or Integrated DNA Technologies (Coralville, IA, USA). The ΔΔCt method was used to determine the fold change using actin for normalization. The sequence of specific primers obtained from Integrated DNA Technologies are listed in Table 1.
Table 1:
Primer sets used in RT-PCR analysis
| Protein | Gene Symbol |
Forward (5’ to 3’) | Reverse (5’ to 3’) | Accession Number |
|---|---|---|---|---|
| GLP1R | Glp1r | AATGGCGCTGGTCATCCTGCTT | GGATGACAAACAGCAGGGGAG | NM_012728.2 |
| GLP1R | GLP1R | AAGTCCTAGAGTGGGGGTGG | TTGGGACACCTGGGGATACT | NM_002062.5 |
| CPT1a | Cpt1a | CCGAGAAGGGAGGACAGAGA | GTACAGGTGCTGGTGCTTCT | NM_031559.2 |
| PPARG | Pparg | GAGATCCTCCTGTTGACCCAG | CCACAGAGCTGATTCCGAAGT | NM_013124.3 |
| PPARA | Ppara | GTCCTCTGGTTGTCCCCTTG | GTCAGTTCACAGGGAAGGCA | NM_013196.2 |
| FAS | Fasn | TCCCAGGTCTTGCCGTGC | GCGGATGCCTAGGATGTGTGC | NM_017332.2 |
| SREBP1 | Srebf1 | CGAGGTGTGCGAAATGGACG | GCACGGACGGGTACATCTTTA | NM_001276707.1 |
| SREBP2 | Srebf2 | CGAACTGGGCGATGGATGAG | GGTAGACAATGGGACCTGGC | NM_001033694.2 |
| FATP2 | Slc27a2 | AGTACATCGGTGAACTGCTTCGGT | TGCCTTCAGTGGAAGCGTAGAACT | NM_031736.2 |
| CD36 | Cd36 | AACCCAGAGGAAGTGGCAAAG | GACAGTGAAGGCTCAAAGATGG | NM_031561.2 |
| ACC | Acac | TGAGGAGGACCGCATTTATC | GAAGCTTCCTTCGTGACCAG | AB004329.1 |
| GYS | Gys | AAACTCCCCATCGCCACAAT | ATTCGTGCACCGCAGAAAAC | AF346902.1 |
| FATP5 | Slc27a5 | GTAATGTCCCAGGGCAACCA | GGCTCTGCCGTCTCTATGTC | NM_024143.2 |
For Semi-quantitative Reverse Transcriptase-PCR (sqRT-PCR) analysis, cDNA was amplified in gradient thermal cycler (Eppendorf, Hamburg, Germany) by using a reaction mixture (20 ng cDNA, 9.2μl of all-in-one PCR Master Mix (GoTaq® G2 Master Mixes, Promega, Madison, WI, USA) and 0.6 μM of forward and reverse primers). After PCR reaction was completed, 10 μl of the PCR product was electrophoresed in a 0.8 % agarose gel. The ethidium bromide-stained gel was photographed with Bio-Rad ChemiDoc™ MP Imaging System (Hercules, CA, USA).
2.10. Western blotting analysis
Tissue homogenates were prepared in ice-cold Tris-EDTA buffer containing protease (Cat. No. P2714, Sigma, St. Louis, MO, USA) and phosphatase inhibitors inhibitor cocktail (sodium fluoride, sodium orthovanadate and sodium pyrophosphate). Homogenates were subjected to SDS-PAGE, followed by blotting of proteins to nitrocellulose membranes. The protein of interest was detected with specific primary antibodies and HRP conjugated secondary antibodies. β-actin was used as an internal control. The protein bands were visualized using Bio-Rad ChemiDoc MP Imaging System. The intensities of immunoreactive protein bands were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
2.11. Statistical Analysis
Results are expressed as mean values ± SD. Data was analyzed using one-way ANOVA, followed by Tukey test. Comparison between 2 groups was analyzed using the Student’s t-test. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Validation of liver GLP-1 receptor expression and exendin-4 uptake
Before conducting the in vivo studies for investigating the protective liver effects of exendin, we first examined for the presence of a functional GLP-1 receptor (GLP-1R) in hepatocytes. We used pancreatic β-cells (INS-1E and RIN-m5F rat insulinoma cells) as positive controls. GLP-1R receptor gene amplified as a 369 bp product in 0.8% agarose gel electrophoresis in rat liver samples similar to that seen in the positive controls (Fig. 1A). The human GLP-1R receptor gene amplified as a 143 bp product (Fig. 1B). These results revealed that both rat and human liver indeed expresses GLP-1R. Further confirmation that GLP-1R is present on hepatocytes that facilitates the uptake of GLP-1 was demonstrated by the Confocal images showing similar intracellular uptake of the fluorescently tagged (rhodamine tagged) exendin into the primary rat hepatocytes (Fig. 1D) and VA-13 (Fig. 1F) as the pancreatic INS-1E β-cells (Fig. 1H). In addition to exendin uptake by hepatocytes, we further observed a protective role of exendin in significantly reducing oleic acid (P ≤ 0.02) and/or ethanol-induced triglyceride accumulation (P ≤ 0.02) in both rat primary hepatocytes (Fig. 1I) and VA-13 cells (Fig. 1J).
Figure 1. Validation of GLP-1 receptor expression in liver and exendin-4 uptake in hepatocytes.
A) Agarose gel electrophoresis showing the GLP-1 receptor gene expression in rat liver. Rat pancreatic β-cell line (RIN-m5F and INS-1E) were used as positive control cells. B) Agarose gel electrophoresis showing the GLP-1 receptor gene expression in human liver. C-H) Confocal microscopic pictures (400X) of rat primary hepatocytes, VA-13 (recombinant Hep G2) cells and INS-1E pancreatic β-cells treated with or without rhodamine labeled exendin-4. C, E, G – untreated cells; D, F, H – cells treated with rhodamine labeled exendin-4. I & J) Triglyceride (TG) levels in primary hepatocytes and VA-13 cells treated with 500 μM oleic acid in the presence or absence of ethanol (50 mM) and exendin (20 nM).
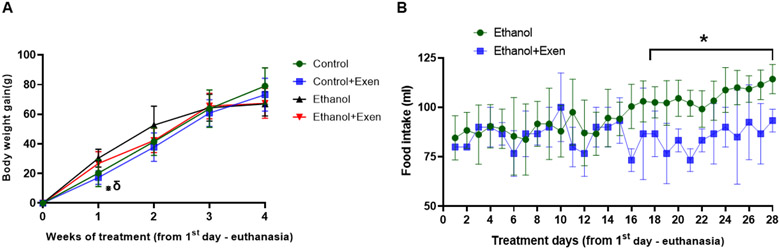
3.2. Exendin-4 treatment reduces ethanol intake
In the current study, weight matched control rats were pair-fed a nutritionally balanced isocaloric diet with ethanol-fed rats for 4 weeks. As reported previously [8, 33], we observed a similar body weight gain between control and ethanol fed rats measured at weekly intervals during this feeding period. Exendin-treated control rats showed significant lower body weight compared to ethanol and exendin-treated ethanol rats at the end of the first week of treatment. However, no significant changes in body weight gain in between the groups were observed at subsequent time points (Fig. 2A). To calculate the actual effect of exendin on intake of the ethanol diet, we compared the data from previous study of average food intake in ethanol rats fed the diet ad libitum during 4-8 week of feeding period vs average food intake in exendin-treated ethanol rats also fed the diet ad libitum during the same treatment period. As shown in Figure 2B, the exendin treatment significantly (p ≤ 0.05) reduced ethanol intake during the last 14 days of the experimental period.
Figure 2. Body weight gain and diet intake during Exendin-4 treatment.
Body weight gain and diet intake during last three weeks of exendin-4 treatment. A) Body weight gain (grams) in all experimental groups. Values are means ± SD (n=4-6). * δ represents exendin-treated control group (Control+Exen) is significantly different (p ≤ 0.05) from ethanol-fed and exendin-treated ethanol group (Ethanol+Exen) respectively. B) Comparison of ethanol-diet intake between plain ethanol-fed rats and exendin-treated ethanol group (Ethanol+Exen) in last three weeks of experimental period (26 days). Values are means ± SD (n=4-6). * represents significant differences (p ≤ 0.05) between ethanol-fed group and exendin-treated ethanol group.
3.3. Exendin-4 improves glucose tolerance in ethanol-fed rats
We have consistently observed impaired glucose tolerance in chronic ethanol-fed rats in our previous studies [7, 8]. In this study, OGTT corroborated our previous results revealing impaired glucose tolerance as demonstrated by significantly higher area under curve (AUC) in chronic ethanol-fed rats compared with control animals (P = 0.004) [7, 8]. However, exendin treatment improved the glucose tolerance in ethanol-fed rats (P = 0.004) as evident by the decreased AUC to that seen in control rats (Fig.3).
Figure 3. Exendin-4 improved glucose tolerance in ethanol-fed rats.
Oral glucose tolerance tests (OGTT) were performed in fasted rats, as described under experimental procedures. A) OGTT, (B) Area under the curve (AUC) from OGTT. Values are means ± SD (n=4-6). * represents exendin-treated control (Control+Exen) and exendin-treated ethanol (Ethanol+Exen) groups were significantly different (p ≤ 0.05) from control and ethanol groups. δ represents ethanol group is significantly different (p ≤ 0.05) from control and exendin-treated ethanol (Ethanol+Exen) group. Φ represents exendin-treated ethanol (Ethanol+Exen) group is significantly different (p ≤ 0.05) from ethanol group. Ψ represents ethanol group is significantly different (p ≤ 0.05) from all other three groups.
3.4. Serum GLP-1 levels and intestinal GLP-1 mRNA expression
GLP-1, a 30-amino acid peptide hormone is secreted from intestinal epithelial endocrine L-cells located mainly in the ileum. GLP-1 synthesized as proglucagon and cleaved by prohormone convertase 3 into GLP-1 [10]. We first measured expression of mRNA encoding for proglucagon and proprotein convertase in ilea of experimental rats, followed by measuring circulating GLP-1 levels and hepatic GLP-1R expression. We observed an increase in the expression of mRNA encoding for ileal proglucagon and proprotein convertase required for GLP-1 synthesis and maturation with a concurrent rise in that of hepatic GLP-1R and serum GLP-1 in ethanol-fed rats compared to control rats (P ≤ 0.02) (Fig. 4A-4D). Similar changes in serum GLP-1 and hepatic GLP-1R as seen in our rat model of ALD were also seen in patients with alcohol-associated hepatocellular carcinoma (HCC) compared to controls (P ≤ 0.01) (Fig. 4E and F). In exendin-treated ethanol rats, the expression of mRNA encoding for ileal proglucagon was normalized to control levels while that of ileal proprotein convertase (P = 0.03), hepatic GLP-1R (P = 0.01) and serum GLP-1 levels (P = 0.004) were significantly reduced compared to ethanol-fed rats.
Figure 4. GLP-1 expression and serum GLP-1 levels.
Expression of mRNA encoding for rat A) ileal proglucagon, (B) ileal proprotein convertase and C) hepatic GLP-1R expression by qRT–PCR analysis. D) Rat GLP-1 levels in serum. Values are means ± SD (n=4-6). Values not sharing a common letter are statistically different, p ≤ 0.05. E) Expression of mRNA encoding for human GLP-1R by qRT–PCR analysis. (F) human serum GLP-1 levels. Values are means ± SD (n=6-8). * Represents significantly different (p ≤ 0.05).
3.5. Effect of exendin-4 treatment on alcohol-induced altered liver and adipose wight and serum parameters
Studies from our lab and other reported that chronic alcohol feeding increases liver to body weight ratio and decreases adipose to liver weight [8, 27, 34]. In this study, as expected, we observed a significant increase in liver weight and decrease in adipose weight in ethanol-fed rats compared to control rats (P ≤ 0.01). However, exendin-treated ethanol rats exhibited insignificant improvement in the liver and adipose weight (Table 2). Similarly, serum ALT, a marker for liver injury, was significantly higher in ethanol-fed rats (P = 0.01) compared to controls, while exendin treatment significantly decreased alcohol-induced increased serum ALT levels (P = 0.03) (Table 2). The ethanol-induced rise in serum NEFA and cholesterol levels were significantly decreased in exendin-treated ethanol rats to levels seen in control rats (P ≤ 0.03). Interestingly, as shown in Table 2, serum NEFA levels were even reduced in exendin-treated controls compared to control rats.
Table 2:
General characteristics and biochemical parameters
| Control | Ethanol | Control+Exen | Ethanol+Exen | |
|---|---|---|---|---|
| Liver weight (g) | 12.55 ± 0.44a | 14.47 ± 0.92b | 12.97 ± 1.08a | 13.93 ± 1.10b |
| Relative liver weight (g/100 g body weight) | 2.93 ± 0.19a | 3.76 ± 0.23b | 2.903 ± 0.25a | 3.35 ± 0.24b |
| Adipose weight (g) | 9.44 ± 1.16a | 5.007 ± 1.25b | 9.30 ± 0.67a | 6.55 ± 1.17b |
| Relative adipose weight (g/100 g body weight) | 2.10 ± 0.16a | 1.30 ± 0.21b | 2.01 ± 0.11a | 1.67 ± 0.34 a,b |
| Serum ALT (U/L) | 47.0 ± 4.37a | 85.4 ± 18.70b | 35.0 ± 8.66c | 59.67 ± 4.58d |
| Serum NEFA (nM/ml) | 230 ± 65.5a | 338 ± 38.03b | 199 ± 67.88a | 201 ± 42.21a |
| Serum cholesterol (mg/dl) | 238 ± 21.3a | 326 ± 50.5b | 233 ± 17.4a | 237 ± 46.1a |
| Serum glucose (mg/dl) | 162 ± 17.3a | 191 ± 25.5a,b | 152 ± 8.26a,c | 141 ± 11.7a,c |
| Serum insulin (ng/ml) | 1.15 ± 0.06a | 0.63 ± 0.14b | 0.90 ± 0.34a,b | 0.80 ± 0.31a,b |
| Serum adiponectin (μg/ml) | 4.61 ± 0.44a | 3.54 ± 0.13b | 6.16 ± 0.11c | 6.25 ± 0.33c |
| Serum ghrelin (pg/ml) | 52.25 ± 29.36a | 132.4 ± 13.5b | 67.38 ± 2.17a | 90.44 ± 7.81c |
Values are means ± SD (n=4-6). Values not sharing a common letter are statistically different, p ≤ 0.05.
Further, we measured serum glucose and levels of metabolic hormones insulin, ghrelin and adiponectin (Table 2). We observed that ethanol-fed rats had the highest glucose levels compared to the other three groups. Regarding hormone levels, as expected the ethanol-fed rats showed decreased insulin and adiponectin levels and increased ghrelin levels compared to control rats (P ≤ 0.03). Further, whereas exendin treatment significantly improved serum insulin (P = 0.002) and decreased ghrelin (P = 0.01) levels in exendin-treated ethanol rats, adiponectin levels were significantly increased in both exendin-treated control and exendin-treated ethanol rats (P = 0.002) compared even to control rats (Table 2).
3.6. Exendin-4 treatment reduces chronic alcohol-induced hepatic lipid accumulation in rats
As shown in Figure 5A, hematoxylin and eosin-stained liver sections of ethanol-fed rats revealed increased fat accumulation which was significantly decreased in exendin-treated ethanol rats (P ≤ 0.03). Further, similar liver histology was observed in control and exendin-treated control rats. In line with histological analysis, quantitative analysis revealed increased hepatic TG content with chronic alcohol feeding which was significantly reduced in exendin-treated ethanol rats (Fig. 5B).
Figure 5. Exendin-4 treatment reduces the chronic alcohol-induced hepatic steatosis.
Hematoxylin and eosin staining of paraffin sections from experimental animals. Images are representative of each pair-fed group of n = 4-6. Magnification, 200X. B) Quantitative analysis of hepatic TG content. Values are means ± SD (n=4-6). Values not sharing a common letter are statistically different, p ≤ 0.05.
3.7. Effects of exendin-4 treatment on hepatic insulin signaling and glucose metabolism
GLP-1 analogs are known to improve insulin signaling and glucose metabolism [10, 16, 35, 36]. In this study, we examined insulin signaling by measuring activation of insulin receptor and its key downstream protein AKT [37] by western blot analysis. We also measured the expression of mRNA encoding for proteins which are involved in glucose homeostasis. As shown in Figure 6A & 6B, we observed increased phosphorylation (indicating activation) of insulin receptor and its downstream protein AKT (P ≤ 0.03). Further, we measured the expression of mRNA encoding for enzymes that are involved in glycogen synthesis and gluconeogenesis. Chronic alcohol administration significantly decreased (P ≤ 0.001) the expression of mRNA encoding for glycogen synthase (GYS) (Fig. 6C), while increasing the levels of phosphoenolpyruvate carboxykinase 2 (PCK2) and glucose-6-phosphatase (G6PC) involved in gluconeogenesis (Fig. 6D). These expression levels were normalized in exendin-treated ethanol-fed rats (Fig. 6C & 6D).
Figure 6. Exendin-4 treatment improves hepatic insulin sensitivity and carbohydrate metabolism ethanol-fed rats.
A) Representative Western Blots from livers of control (Con), ethanol (EtOH), exendin-treated control (Con+Ex) and exendin-treated ethanol (EtOH+Ex) rats. B) Densitometric ratios of insulin receptor substrate-1 (IRS-1), pIRS-1 (active IRS-1), AKT and pAKT (active AKT) to β-actin. Expression of mRNA encoding for hepatic C) glycogen synthase (GYS), phosphoenolpyruvate carboxy kinase 2 (PCK2) and glucose-6-phosphatase (G6PC) by qRT-PCR analysis Values expressed as a relative to the control values. Values are means ± SD, n=4-6, Values not sharing a common letter are statistically different, p ≤ 0.05.
3.8. Exendin-4 reduces hepatic fatty acid uptake and synthesis
To determine the possible mechanism by which exendin-4 reduces the alcohol induced fat accumulation in the liver, real-time quantitative analysis of hepatic expression of mRNA encoding for proteins related to fatty acid uptake and synthesis was performed. As shown Figure 7A, chronic alcohol-feeding increased (P ≤ 0.005) the relative expression of mRNA encoding for sterol regulatory element-binding protein 1 and 2 (SREBP1 & 2), transcription factors that promotes the expression of enzyme involved in fatty acid synthesis. Significant reduction in the relative expression of mRNA encoding for SREBP1 & 2 to the below the normal levels was observed in exendin-treated ethanol rats (P ≤ 0.005). In addition, we also measured the expression of mRNA encoding for plasma membrane associated long-chain fatty acid transporters, cluster of differentiation 36 (CD36) and fatty acid transport protein 2 (FATP2). Further we measured the expression levels of mRNA encoding for acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS), enzymes involved in de novo fatty acid synthesis, and diacylglycerol O-transferase 1 (DGAT1) that catalyzes fatty acid esterification. As observed previously (9), chronic alcohol feeding significantly increased the expression of mRNA encoding for CD36, FATP2, FAS, ACC and DGAT1 (P ≤ 0.004) (Fig. 7B & 7C). However, their expression was reduced significantly to near-normal to normal levels in exendin-treated ethanol rats as seen in controls.
Figure 7. Effect of exendin-4 treatment on hepatic fatty acid metabolism.
Expression of mRNA encoding for hepatic A) sterol regulatory element-binding protein 1 & 2 (SREBP1 & 2), (B) cluster of differentiation 36 (CD36) and fatty acid transport protein 2 (FATP2), (acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and diacylglyceride acyltransferase 1 (DGAT1) by qRT–PCR analysis. D) Representative Western Blots from livers of control (Con), ethanol (EtOH), exendin-treated control (Con+Ex) and exendin-treated ethanol (EtOH+Ex) rats. E) Densitometric ratios of ATGL, CGI58, HSL and pHSL. F) Expression of mRNA encoding for hepatic PPARα and CPT1a by qRT–PCR analysis. Values are expressed as a relative to the control values. Values are means ± SD, n = 4-6. Values not sharing a common letter are statistically different, p ≤ 0.05.
3.9. Exendin-4 treatment improved the hepatic fatty acid oxidation
To identify the levels of fatty acid oxidation breakdown, we measured the total content of hormone sensitive lipase (HSL) and adipose triacylglycerol lipase (ATGL), the two main lipases responsible for a breakdown of hepatic triglycerides. As expected, we did not observe any significant difference in the total protein content of HSL and ATGL among all the groups. However, we saw decreased levels of active HSL (phosphorylated HSL; pHSL) and CGI58 (an activator of ATGL) in ethanol-fed rats (P ≤ 0.04). Activation of both HSL and ATGL (as evident by increased pHSL and CGI58) was observed in both exendin-treated control and exendin-treated ethanol rats (Fig. 7D & 7E). Further, we observed reduced fatty acid oxidation in ethanol-fed rats as indicated by decreased expression of mRNA encoding for peroxisome proliferator-activated receptor alpha (PPARα, a transcriptional regulator of β-oxidation) (P = 0.04), and carnitine palmitoyl transferase I (CPT1a, a rate-limiting enzyme of the mitochondrial fatty acid β-oxidation) (P = 0.001). Their expression levels were normalized to the control levels in exendin-treated ethanol rats (Fig. 7F).
3.10. Exendin-4 treatment improved insulin sensitivity and lipid metabolism in adipose tissue
Insulin has profound effects on lipid metabolism in adipose tissue. Insulin promotes adipocyte differentiation and inhibits adipose lipolysis, thus allowing for increased lipid storage in adipocytes, which are meant for lipid storage [38]. Studies from our group and others have demonstrated that chronic alcohol administration reduces circulating insulin levels and insulin sensitivity that by promoting adipocyte lipolysis increases circulating NEFA contributing to fat accumulation in the liver [7, 34, 39, 40]. In this study, we measured insulin signaling in adipose tissue by Western Blotting. As shown in Figure 8A & 8B, we found decreased insulin receptor and its downstream signaling protein AKT activation (phosphorylation) in ethanol-fed rats (P ≤ 0.04). As reported previously [41], increased insulin signaling was observed in both exendin-treated control and exendin-treated ethanol rats compared to control rats (P ≤ 0.02) (Fig. 8A & 8B).
Figure 8. Exendin-4 treatment on adipose insulin sensitivity, adipose differentiation and adipose lipolysis.
A) Representative Western Blots from adipose tissue of control (Con), ethanol (EtOH), exendin-treated control (Con+Ex) and exendin-treated ethanol (EtOH+Ex) rats. B) Densitometric ratios of insulin receptor substrate-1 (IRS-1), pIRS-1 (active IRS-1), AKT and pAKT (active AKT) to β-actin in adipose tissue. C) Expression of mRNA encoding for adipose PPARγ and FATP5 by qRT–PCR analysis. D) Representative western blots for measuring HSL and ATGL activity. E) Densitometric ratios of ATGL, CGI58, HSL and pHSL. Values expressed as a relative to the control values. Values are means ± SD, n=4-6, Values not sharing a common letter are statistically different, p ≤ 0.05.
Since insulin regulates adipose differentiation and lipolysis, we further measured expression of mRNA encoding for PPARγ, a key player in adipocyte differentiation and fat storage, which is regulated by insulin [42]. As shown previously, in this study we also observed decreased expression of mRNA encoding for PPARγ in the ethanol-diet fed rats (P = 0.003) (Fig. 8C). However, exendin-treated ethanol-fed rats showed increased expression of mRNA encoding for PPARγ in adipose tissue (Fig. 8C). Further, we measured the adipose lipolysis by measuring protein content of enzymes related to adipose lipolysis (HSL and ATGL). In line with increased serum NEFA in ethanol-fed rats, we observed increased adipose lipolysis as evident by increased contents of pHSL and CGI58 in ethanol-fed rats compared to control rats (P ≤ 0.04) (Fig. 8D & 8E). Further, we observed increased expression of mRNA encoding for fatty acid transporter protein 5 (FATP5) in adipose tissue of ethanol-fed rats (P = 0.003) compared to control rats (Fig. 8C). Since exendin treatment improved insulin signaling, as predicted, we observed decreased activation of HSL and ATGL and decreased expression of mRNA encoding for FATP5 in both exendin-treated control and exendin-treated ethanol rats (Fig. 8C & 8D).
4. Discussion
GLP-1, a gut neuropeptide hormone involved in the regulation of food intake and glucose homeostasis by improving insulin release and insulin sensitivity [10, 16, 36, 43]. Recent experimental and clinical studies have shown that GLP-1 analogs reduce alcohol craving and alcohol intake and targeting GLP-1Rs by using GLP-1 analogs could be the novel and promising pharmacotherapeutic target for AUD [12, 13]. Further, when it comes to metabolic diseases such as diabetes and associated fatty liver disease, various GLP-1 receptor agonists are used as treatment for metabolic disease (type II diabetes) and are in clinical trials for MAFLD and NASH. The mechanism of action includes improved insulin secretion and its sensitivity, reduced inflammation [16, 20, 22, 36, 43, 44]. Decreased serum insulin levels, increased insulin resistance and hepatic inflammation have been reported in chronic ethanol-fed rats and alcohol-associated ALD patient [4, 8, 39]. These pathological changes occur despite elevated hepatic GLP-1R expression and increased serum GLP-1 levels observed here. Incidentally, an increased GLP-1 serum levels have also been reported before in alcohol-associated HCC patients [45]. This suggests that possibly the GLP-1-mediated signaling may be impaired by ethanol administration or that the increases in GLP-1 levels is not sufficient to compensate for alcohol-induced altered fat metabolism in liver, adipose tissue and other organs. We believe that improving GLP-1 function by treatment with GLP-1 analogs could be an effective tool to treat ALD akin to insulin injections overcoming insulin resistance in obese patients [46, 47]. Based on the literature, we conducted the present study on the premise that treatment with a GLP-1 analog, exendin-4 (a long acting GLP-1 analogue that is an agonist for the GLP-1R) could improve insulin secretion and insulin sensitivity that can lead to improved adipose and liver metabolism and prevents the development of alcohol-induced liver injury.
In this study, since there are mixed reports available about the GLP-1R expression in the liver [10], we first validated that GLP-1R is expressed in the liver using GLP-1R specific primers and positive control (rat pancreatic β-cells RIN-m5F and INS-1E) by conducting sqPCR analysis. In addition to confirming GLP-1R expression, we further showed exendin uptake by rat and human hepatocytes and its role in reducing oleic acid and/or alcohol-induced triglyceride accumulation. We then conducted the in vivo studies using exendin treatment in a rat model of alcohol-associated liver injury using Lieber-DeCarli control or ethanol diet. After 4 weeks of this feeding regimen, we injected exendin or saline every other day for 26 days (13 doses) to half the animals while still being on their respective diet. Since studies have been shown that exendin-4/GLP-1 analogs reduce food and alcohol intake [10, 12, 14, 30], throughout our treatment, all rats were pair-fed to the exendin-treated ethanol rat. In addition, during the treatment period we assessed differences in food intake between current exendin-treated ethanol rats fed ad libitum and our previous experimental rats with similar body weights fed ethanol diet ad libitum for up to 8 weeks. We observed that ethanol rats exhibited reduced calorie intake (ethanol-diet intake) during the last 14 days before sacrifice. This indicated that either the exendin dose we used may not be effective immediately in reducing their intake when on Lieber-DeCarli liquid diet or that such effect on reducing food or ethanol craving does take time. In addition to food intake, when we assessed body weight gain during the treatment, we found that exendin-treated control rats weighed less compared with ethanol and exendin-treated ethanol rats during the first week of the treatment. However, other than that, all experimental groups showed similar body weight gain during the rest of the treatment period. This could be due to the pair-feeding which ensures same caloric intake among all the experimental groups.
Previous studies from our lab and others demonstrated that chronic alcohol administration associates with impaired glucose tolerance because of impaired insulin secretion and insulin signaling [8, 39]. Since GLP-1 analogs stimulate insulin release and improves insulin sensitivity, we predicted that exendin treatment could improve ethanol-induced impaired glucose tolerance. In this current study, and as previously reported by us [7, 8], we observed impaired glucose tolerance with a concomitant increased AUC in ethanol-fed rats compared to controls. Whereas exendin treatment of ethanol rats normalized the glucose tolerance and AUC to control levels, the exendin-treated control rats exhibited lower AUC compared to their untreated controls. Interestingly, the blood glucose levels in both exendin groups peaked at 60 min (OGGT test), possibly due to the known effect of GLP-1 analogs to reduce gastric emptying [48].
As shown in Table 2, all experimental groups showed similar body weight gain during the experimental period as expected. However, ethanol-fed rats exhibited a significant increase in liver/body weight ratio and a decreased adipose/body weight due to increased fat accumulation in the liver and fat loss in the adipose tissue as explained previously. While exendin treatment slightly improved ethanol-induced changes in adipose and liver weight and serum ALT levels (Table 2), a significant improvement in lipid metabolism in liver and adipose tissue was noted as discussed below. This reduction in serum hepatic injury makers with exendin treatment was consistent with previous studies on NAFLD [18, 49]. Regarding serum hormones, chronic alcohol-feeding reduced the serum insulin and adiponectin levels and increased serum ghrelin levels as we reported before [7, 8]. Exendin is known to directly induce glucose-stimulated insulin secretion from pancreas, increase adiponectin secretion from adipose tissue and decrease fasting induced-ghrelin secretion from stomach [36, 43, 50, 51]. In this study, exendin treatment moderately increased serum insulin levels, significantly reduced alcohol-induced serum ghrelin levels in ethanol-fed rats. Further, exendin treatment significantly increased serum adiponectin levels in both control and ethanol-fed rats compared to control and ethanol rats. Though the GLP-1 analogs are known to induce insulin secretion, exendin-treated control rats showed moderately lower serum insulin levels than control animals. This is likely due to improved insulin sensitivity by exendin treatment as evident by moderately lowered AUC during OGTT in exendin-treated control compared to control rats.
Liver is a major insulin responsive organ and plays a major role in glucose storage and glucose synthesis by promoting glycogen synthesis and suppressing gluconeogenesis. Previous studies have shown that chronic alcohol exposure decreases insulin levels and increases insulin resistance [8, 39]. Parallel to these changes, chronic alcohol exposure decreases the hepatic glycogen levels by reducing synthesis and increasing its breakdown [52]. In line with our previous reports, here we observed decreased phosphorylated IRS-1 and AKT levels, indicating decreased insulin mediated signaling in ethanol-fed rats. Consequent to these events, decreased glycogen synthesis and increased gluconeogenesis were observed in ethanol-fed rats. Exendin improved insulin signaling as evident by increased phosphorylation status of IRS-1 and AKT with a consequent increase in glycogen synthesis and decrease in gluconeogenesis in exendin-treated ethanol rats.
GLP-1 analogs have been shown to reduce hepatic steatosis in preclinical and clinical experimental NAFLD studies. The mechanism of action includes improved hepatic insulin sensitivity and modulating genes involved in de novo lipogenesis, triglyceride storage and fatty acid oxidation [18, 36]. Consistent with all our previous studies [8, 27], chronic alcohol-fed rats exhibited significantly higher accumulation of hepatic triglycerides than their pair-fed controls (Fig. 5). This increased accumulation of fats in liver associated with increased expression of mRNA encoding for proteins related to hepatic fatty acid uptake (CD 36 and FATP2 mRNA increase) and transcription factors (SREBP1 and SREBP2) which play a key role in the induction of lipogenesis. We also observed increased hepatic de novo fatty acid synthesis and esterification (as evidenced by increased expression of mRNA encoding for FAS, ACC and DGAT1) that eventually increased the hepatic fat content in the ethanol-fed rats. On the other hand, decreased TG breakdown by HSL and ATGL (as shown by lower levels of pHSL and CGI58, the activator of ATGL) and reduced expression of mRNA encoding for proteins related to fatty acid oxidation (PPARα and CPT1a), all contribute to the accumulation of fat in the liver. As anticipated, exendin treatment reduced ethanol-induced hepatic TG accumulation by normalizing the hepatic fatty acid uptake, synthesis, breakdown and oxidation to the control levels (Fig. 7). These results are in accordance with other studies reporting that GLP-1 analogs reduce hepatic fat accumulation mainly by improving hepatic glucose metabolism, reducing lipogenesis and improving PPARα mediated fatty acid oxidation [18, 29, 36]. Further, studies showed that addition to improving insulin levels and insulin secretion, GLP-1R agonists improves adiponectin secretion [51], which we also observed in this study, can results in increased adiponectin-mediated hepatic fatty acid oxidation and consequent reduction in hepatic steatosis.
Liver and adipose tissue are major regulators of energy homeostasis that act as caloric reservoirs. In particular, liver engages in glucose homeostasis by storing or synthesizing glucose by glycogen synthesis and gluconeogenesis respectively. Conversely, adipose tissue plays a critical role in energy homeostasis by storing the excessive calories/energy in the form of lipids. During energy needs, adipose tissue releases NEFA into the circulation, which can be taken up by other organs including liver and there they can be utilized for oxidative phosphorylation to generate ATP or re-esterified to form TG and stored in lipid droplets. Insulin plays a major role in this energy homeostasis. Insulin promotes glucose utilization, glycogen storage and very-low-density lipoprotein secretion while suppressing gluconeogenesis in the liver. However in adipose tissue insulin promotes glucose utilization and inhibits lipolysis at the adipose tissue, thus allowing for increased storage site for excess lipids [53]. We previously reported that chronic alcohol-induced decreased insulin secretion resulting in increasing circulating level of adipose-derived NEFA, promoted hepatic fatty acid uptake and de novo lipogenesis, thereby contributing to the development of fatty liver [8, 34, 39]. Similar to our previous studies, we observed decreased serum insulin levels (Table 2 and Fig. 8) and subsequent decrease in insulin signaling (decreased pIRS and pAKT levels), followed by increase in adipose lipolysis (increased pHSL and CGI58 levels) and NEFA release (from adipose tissue of ethanol-fed rats. Further, we observed decreased expression of mRNA encoding for PPARγ, which plays a crucial role in IRS-1 and AKT mediated adipose differentiation and fat storage [54, 55]. Not surprisingly, improved serum insulin levels observed in the exendin-treated ethanol rats associated with improved insulin signaling, PPARγ expression and decreased adipose lipolysis and NEFA release. This decrease in circulating NEFA levels contributed to reduced hepatic fat accumulation in exendin-treated ethanol rats. Our results are in accordance with other reports showing that GLP-1 analogs increase adipogenesis, glucose/lipid uptake at the adipocytes and reduces NEFA release from the adipose tissue [56, 57].
To summarize, first, we validated that GLP-1R is expressed and functionally active in the liver. Further, we showed that chronic alcohol administration is associated with decreased serum insulin levels and decreased insulin signaling at both adipose and liver tissue. Decreased insulin signaling results in increased adipose lipolysis and NEFA release into circulation and delivery to the liver. This increased NEFA uptake combined with increased hepatic fatty acid synthesis/esterification and decreased fatty acid oxidation contributes to hepatic steatosis. Exendin-4 treatment by increasing insulin signaling at the adipose and the liver improved downstream events that eventually reduces fat accumulation in the liver. Since there is substantial evidence available that GLP-1 analogs are a viable therapeutic strategy for AUD, our data add credence that GLP-1 analogs are new therapeutics that target both AUD and ALD.
Acknowledgements
We thank Dr. Pierre Maechler (University Medical Center, Geneva, Switzerland) for providing INS-1E pancreatic cells. We thank Robert G. Bennett (University of Nebraska Medical Center and the Department of Veterans Affairs, Omaha, NE) for sharing RIN-m5F cells. We thank Dr. Dahn Clemens (University of Nebraska Medical Center, Omaha, NE) for sharing VA-13 cells. We thank Dr. Paul Thomes (University of Nebraska Medical Center, Omaha) for kindly sharing human hepatic tissue samples of controls and alcohol-associated HCC patients obtained from Liver Tissue and Cell Distribution System (LTCDS); National Institutes of Health (NIH) service.
Funding source
This work was supported by the National Institutes of Health/NIAAA funding R01 AA028504
List of abbreviations/symbols
- ACC
Acetyl-CoA carboxylase
- AKT
Serine/threonine kinase
- ALD
Alcohol-associated fatty liver disease
- ALT
Alanine aminotransferase
- ASH
Alcoholic steatohepatitis
- ATGL
Adipose triglyceride lipase
- AUC
Area under curve
- AUD
Alcohol used disorder
- DGAT1
Diacylglyceride acyltransferase 1
- FAS
Fatty acid synthase
- G6PC
Glucose-6-phosphatase
- GLP-1
Glucagon-like peptide 1
- GYS
Glycogen synthase
- HCC
Hepatocellular carcinoma
- HSL
Hormone-sensitive lipase
- IRS1
Insulin receptor substrate-1
- NEFA
Non-esterified free fatty acids
- OGTT
Oral glucose tolerance test
- PCK2
Phosphoenolpyruvate carboxy kinase 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Declaration of Interest Statement:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
All authors have read and approved the revised article for submission to Biochemical Pharmacology; have made a substantial contribution to the conception, design, gathering, analysis and/or interpretation of data and a contribution to the writing and intellectual content of the article; and acknowledge that they have exercised due care in ensuring the integrity of the work. In addition, none of the original material contained in the manuscript has been submitted for consideration nor will any of it be published elsewhere except in abstract form in connection with scientific meetings. All authors acknowledge that they have exercised due care in ensuring the integrity of the work they presented.
References
- [1].Axley PD, Richardson CT, Singal AK, Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease, Clin Liver Dis 23(1) (2019) 39–50. [DOI] [PubMed] [Google Scholar]
- [2].Mitra S, De A, Chowdhury A, Epidemiology of non-alcoholic and alcoholic fatty liver diseases, Transl Gastroenterol Hepatol 5 (2020) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kharbanda KK, Farokhnia M, Deschaine SL, Bhargava R, Rodriguez-Flores M, Casey CA, Goldstone AP, Jerlhag E, Leggio L, Rasineni K, Role of the ghrelin system in alcohol use disorder and alcohol-associated liver disease: A narrative review, Alcohol Clin Exp Res 46(12) (2022) 2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gao B, Ahmad MF, Nagy LE, Tsukamoto H, Inflammatory pathways in alcoholic steatohepatitis, J Hepatol 70(2) (2019) 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel R, Gossman W, Alcoholic Liver Disease, StatPearls, Treasure Island (FL), 2020. [Google Scholar]
- [6].Hartmann P, Seebauer CT, Schnabl B, Alcoholic liver disease: the gut microbiome and liver cross talk, Alcohol Clin Exp Res 39(5) (2015) 763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rasineni K, Kubik JL, Casey CA, Kharbanda KK, Inhibition of Ghrelin Activity by Receptor Antagonist [d-Lys-3] GHRP-6 Attenuates Alcohol-Induced Hepatic Steatosis by Regulating Hepatic Lipid Metabolism, Biomolecules 9(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rasineni K, Thomes PG, Kubik JL, Harris EN, Kharbanda KK, Casey CA, Chronic alcohol exposure alters circulating insulin and ghrelin levels: role of ghrelin in hepatic steatosis, Am J Physiol Gastrointest Liver Physiol 316(4) (2019) G453–G461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parker R, Kim SJ, Gao B, Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations, Nat Rev Gastroenterol Hepatol 15(1) (2018) 50–59. [DOI] [PubMed] [Google Scholar]
- [10].Muller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschop MH, Glucagon-like peptide 1 (GLP-1), Mol Metab 30 (2019) 72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH, Ghrelin, Mol Metab 4(6) (2015) 437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jerlhag E, Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1, Brain Res 1727 (2020) 146562. [DOI] [PubMed] [Google Scholar]
- [13].Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker HC, Goldman D, Heilig M, Ramchandani VA, Leggio L, The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence, Transl Psychiatry 5 (2015) e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marty VN, Farokhnia M, Munier JJ, Mulpuri Y, Leggio L, Spigelman I, Long-Acting Glucagon-Like Peptide-1 Receptor Agonists Suppress Voluntary Alcohol Intake in Male Wistar Rats, Front Neurosci 14 (2020) 599646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Farokhnia M, Fede SJ, Grodin EN, Browning BD, Crozier ME, Schwandt ML, Hodgkinson CA, Momenan R, Leggio L, Differential association between the GLP1R gene variants and brain functional connectivity according to the severity of alcohol use, Sci Rep 12(1) (2022) 13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arvanitakis K, Koufakis T, Kotsa K, Germanidis G, How Far beyond Diabetes Can the Benefits of Glucagon-like Peptide-1 Receptor Agonists Go? A Review of the Evidence on Their Effects on Hepatocellular Carcinoma, Cancers (Basel) 14(19) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ, The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases, Hepatology 67(1) (2018) 328–357. [DOI] [PubMed] [Google Scholar]
- [18].Ding X, Saxena NK, Lin S, Gupta NA, Anania FA, Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice, Hepatology 43(1) (2006) 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G, Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials, Metabolites 11(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Patel Chavez C, Cusi K, Kadiyala S, The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD, J Clin Endocrinol Metab 107(1) (2022) 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hinnen D, Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes, Diabetes Spectr 30(3) (2017) 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, Nasiri M, Yu J, Gough SC, Newsome PN, Tomlinson JW, Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis, J Hepatol 64(2) (2016) 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bennett RG, Hamel FG, Duckworth WC, An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures, Diabetes 52(9) (2003) 2315–20. [DOI] [PubMed] [Google Scholar]
- [24].Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P, Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells, Endocrinology 145(2) (2004) 667–78. [DOI] [PubMed] [Google Scholar]
- [25].Clemens DL, Tuma DJ, Casey CA, Cyanamide potentiates the ethanol-induced impairment of receptor-mediated endocytosis in a recombinant hepatic cell line expressing alcohol dehydrogenase activity, Int J Hepatol 2012 (2012) 954157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rasineni K, Donohue TM Jr., Thomes PG, Yang L, Tuma DJ, McNiven MA, Casey CA, Ethanol-induced steatosis involves impairment of lipophagy, associated with reduced Dynamin2 activity, Hepatol Commun 1(6) (2017) 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rasineni K, Penrice DD, Natarajan SK, McNiven MA, McVicker BL, Kharbanda KK, Casey CA, Harris EN, Alcoholic vs non-alcoholic fatty liver in rats: distinct differences in endocytosis and vesicle trafficking despite similar pathology, BMC Gastroenterol 16 (2016) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H, Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes, Diabetologia 54(4) (2011) 965–78. [DOI] [PubMed] [Google Scholar]
- [29].Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Lee WY, Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice, PLoS One 7(2) (2012) e31394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM, Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats, Endocrinology 141(6) (2000) 1936–41. [DOI] [PubMed] [Google Scholar]
- [31].Reddy SS, Karuna R, Baskar R, Saralakumari D, Prevention of insulin resistance by ingesting aqueous extract of Ocimum sanctum to fructose-fed rats, Horm Metab Res 40(1) (2008) 44–9. [DOI] [PubMed] [Google Scholar]
- [32].Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues, J Biol Chem 226(1) (1957) 497–509. [PubMed] [Google Scholar]
- [33].Rasineni K, McVicker BL, Tuma DJ, McNiven MA, Casey CA, Rab GTPases associate with isolated lipid droplets (LDs) and show altered content after ethanol administration: potential role in alcohol-impaired LD metabolism, Alcoholism, clinical and experimental research 38(2) (2014) 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z, Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis, Am J Pathol 180(3) (2012) 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo C, Huang T, Chen A, Chen X, Wang L, Shen F, Gu X, Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages, Braz J Med Biol Res 49(12) (2016) e5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu H, Sui C, Xu H, Xia F, Zhai H, Zhang H, Weng P, Han B, Du S, Lu Y, The GLP-1 analogue exenatide improves hepatic and muscle insulin sensitivity in diabetic rats: tracer studies in the basal state and during hyperinsulinemic-euglycemic clamp, J Diabetes Res 2014 (2014) 524517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mackenzie RW, Elliott BT, Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes, Diabetes Metab Syndr Obes 7 (2014) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao J, Wu Y, Rong X, Zheng C, Guo J, Anti-Lipolysis Induced by Insulin in Diverse Pathophysiologic Conditions of Adipose Tissue, Diabetes Metab Syndr Obes 13 (2020) 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE, Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines, Alcohol Clin Exp Res 31(9) (2007) 1581–8. [DOI] [PubMed] [Google Scholar]
- [40].Steiner JL, Lang CH, Alcohol, Adipose Tissue and Lipid Dysregulation, Biomolecules 7(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang Y, Choi PP, Smith WW, Xu W, Ma D, Cordner ZA, Liang NC, Moran TH, Exendin-4 reduces food intake via the PI3K/AKT signaling pathway in the hypothalamus, Sci Rep 7(1) (2017) 6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kintscher U, Law RE, PPARgamma-mediated insulin sensitization: the importance of fat versus muscle, Am J Physiol Endocrinol Metab 288(2) (2005) E287–91. [DOI] [PubMed] [Google Scholar]
- [43].Meloni AR, DeYoung MB, Lowe C, Parkes DG, GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence, Diabetes Obes Metab 15(1) (2013) 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nauck MA, Quast DR, Wefers J, Meier JJ, GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art, Mol Metab 46 (2021) 101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang Z, Kusumanchi P, Ross RA, Heathers L, Chandler K, Oshodi A, Thoudam T, Li F, Wang L, Liangpunsakul S, Serum Metabolomic Profiling Identifies Key Metabolic Signatures Associated With Pathogenesis of Alcoholic Liver Disease in Humans, Hepatol Commun 3(4) (2019) 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Church TJ, Haines ST, Treatment Approach to Patients With Severe Insulin Resistance, Clin Diabetes 34(2) (2016) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Larsen J, Goldner W, Approach to the hospitalized patient with severe insulin resistance, J Clin Endocrinol Metab 96(9) (2011) 2652–62. [DOI] [PubMed] [Google Scholar]
- [48].Maselli DB, Camilleri M, Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity, Adv Exp Med Biol 1307 (2021) 171–192. [DOI] [PubMed] [Google Scholar]
- [49].Ahangarpour A, Oroojan AA, Badavi M, Exendin-4 protects mice from D-galactose-induced hepatic and pancreatic dysfunction, Pathobiol Aging Age Relat Dis 8(1) (2018) 1418593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, Mallo F, Exendin-4 potently decreases ghrelin levels in fasting rats, Diabetes 56(1) (2007) 143–51. [DOI] [PubMed] [Google Scholar]
- [51].Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y, Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression, Biochem Biophys Res Commun 390(3) (2009) 613–8. [DOI] [PubMed] [Google Scholar]
- [52].Gu J, Zhang Y, Xu D, Zhao Z, Zhang Y, Pan Y, Cao P, Wang Z, Chen Y, Ethanol-induced hepatic steatosis is modulated by glycogen level in the liver, J Lipid Res 56(7) (2015) 1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Saltiel AR, Kahn CR, Insulin signalling and the regulation of glucose and lipid metabolism, Nature 414(6865) (2001) 799–806. [DOI] [PubMed] [Google Scholar]
- [54].Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C, Regulation of adipocyte formation by GLP-1/GLP-1R signaling, J Biol Chem 287(9) (2012) 6421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lowell BB, PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function, Cell 99(3) (1999) 239–42. [DOI] [PubMed] [Google Scholar]
- [56].Gao H, Wang X, Zhang Z, Yang Y, Yang J, Li X, Ning G, GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytes, Endocrine 32(1) (2007) 90–5. [DOI] [PubMed] [Google Scholar]
- [57].Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Ferno J, Salvador J, Escalada J, Dieguez C, Lopez M, Fruhbeck G, Nogueiras R, GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK, Diabetes 63(10) (2014) 3346–58. [DOI] [PubMed] [Google Scholar]