BACKGROUND:
Compared with patients with hypertension only, those with hypertension and diabetes (HTN/DM) have worse prognosis. We aimed to characterize morphological differences between hypertension and HTN/DM using cardiovascular magnetic resonance; and compare differentially expressed proteins associated with myocardial fibrosis using high throughput multiplex assays.
METHODS:
Asymptomatic patients underwent cardiovascular magnetic resonance: 438 patients with hypertension (60±8 years; 59% males) and 167 age- and sex-matched patients with HTN/DM (60±10 years; 64% males). Replacement myocardial fibrosis was defined as nonischemic late gadolinium enhancement on cardiovascular magnetic resonance. Extracellular volume fraction was used as a marker of diffuse myocardial fibrosis. A total of 184 serum proteins (Olink Target Cardiovascular Disease II and III panels) were measured to identify unique signatures associated with myocardial fibrosis in all patients.
RESULTS:
Despite similar left ventricular mass (P=0.344) and systolic blood pressure (P=0.086), patients with HTN/DM had increased concentricity and worse multidirectional strain (P<0.001 for comparison of all strain measures) compared to hypertension only. Replacement myocardial fibrosis was present in 28% of patients with HTN/DM compared to 16% of those with hypertension (P<0.001). NT-proBNP (N-terminal pro-B-type natriuretic peptide) was the only protein differentially upregulated in hypertension patients with replacement myocardial fibrosis and independently associated with extracellular volume. In patients with HTN/DM, GDF-15 (growth differentiation factor 15) was independently associated with replacement myocardial fibrosis and extracellular volume. Ingenuity Pathway Analysis demonstrated a strong association between increased inflammatory response/immune cell trafficking and myocardial fibrosis in patients with HTN/DM.
CONCLUSIONS:
Adverse cardiac remodeling was observed in patients with HTN/DM. The novel proteomic signatures and associated biological activities of increased immune and inflammatory response may partly explain these observations.
Keywords: magnetic resonance imaging, myocardial fibrosis, heart disease, hypertension
CLINICAL PERSPECTIVE
Myocardial fibrosis is a hallmark of heart failure and predicts worse cardiovascular outcomes in patients with hypertension. There is increasing interest to target the myocardial interstitium to improve diagnosis, risk stratification and to discover novel therapies. Our study demonstrates that myocardial fibrosis is a heterogeneous pathology resulting from cardiac disease-specific biology. In patients with hypertension, concomitant diabetes accelerates adverse cardiac remodeling. This observation endorses the importance to consider early risk stratification by imaging or biomarker profiling in these patients. Whether patients with hypertension and diabetes would derive incremental anti-fibrotic benefits from therapies targeting inflammation/immune activate require further investigations.
See Editorial by Paulus
Myocardial fibrosis is a pathological hallmark of heart failure that can be assessed noninvasively with cardiovascular magnetic resonance (CMR).1,2 In hypertensive heart disease, myocardial fibrosis from advanced left ventricular hypertrophy (LVH) eventually leads to cardiac decompensation and complications such as heart failure.3–5 Patients with hypertension and diabetes (HTN/DM) have worse cardiac function and higher mortality than those with hypertension only (hypertension).6,7
These observations suggest in patients with HTN/DM with heart failure, adverse cardiac remodeling associated with diabetes goes beyond the hemodynamic and neuro-hormonal consequences of hypertension. In this context, systematic profiling of circulating proteins may provide an unbiased insight into pathophysiological associations; and guide improvements in diagnosis, risk stratification, and development of novel therapies. Emerging technologies have made it technically feasible to simultaneously measure ~100 different proteins on an assay chip using small serum samples.8
We aimed to characterize morphological and functional differences between asymptomatic patients with hypertension and HTN/DM using CMR; and to compare differentially expressed proteins associated with myocardial fibrosis between HTN/DM and hypertension using the multiplex Proximity Extension Assay technology.
METHODS
The data supporting the findings are available from the corresponding author upon reasonable request.
Study Population
The study population consisted of asymptomatic patients with hypertension from the REMODEL (Response of the Myocardium to Hypertrophic Conditions in the Adult Population; https://www.clinicaltrials.gov; Unique identifier: NCT02670031) study. REMODEL is a prospective, observational study of subjects 21 years and above with essential hypertension. Exclusion criteria included secondary causes of hypertension (such as pheochromocytoma, bilateral renal artery stenosis, and polycystic kidney disease), cardiovascular diseases (such as ischemic heart disease and heart failure), previous cerebrovascular events, atrial fibrillation, and contraindications to gadolinium contrast and CMR.9 Patients with incidental myocardial infarction and cardiomyopathies on CMR were excluded from current analysis.
Average systolic blood pressure (SBP)/diastolic blood pressure over a 24-hour period was measured with the OnTrak 90227 device (SpaceLabs Healthcare, Snoqualmie, WA). A properly sized cuff was selected and placed with the monitor for the patient on the day of CMR after scan was performed. Resting blood pressure was obtained after the monitor was placed to confirm correct function of the monitor. Out-of-clinic blood pressure measurements were obtained every 20 minutes from 6 am to 10 pm and 30 minutes from 10 pm to 6 am.
All participants provided written informed consent. The study was conducted according to the Declaration of Helsinki and approved by the SingHealth Centralized Institutional Review Board (2015/2603).
CMR Imaging and Analysis
All participants underwent CMR following a standardized imaging protocol (Siemens Aera 1.5T, Siemens Healthineers, Erlangen, Germany). Balanced steady-state free precession cine images were acquired in the long-axis 2-, 3- and 4-chamber views (acquired voxel size 1.6×1.3×8.0 mm; 30 phases per cardiac cycle). Short-axis cines extending from the mitral valve annulus to the apex were also acquired (acquired voxel size 1.6×1.3×8.0 mm; 30 phases per cardiac cycle).
Myocardial fibrosis was assessed using late gadolinium–enhanced (LGE) imaging (for nonischemic focal replacement fibrosis) and myocardial T1 mapping (for diffuse myocardial fibrosis). LGE imaging was performed 8 minutes after administration of 0.1 mmol/kg of gadobutrol (Gadovist; Bayer Pharma AG, Germany). An inversion-recovery fast gradient echo sequence (FOV: 340×276 mm; 8 mm slice thickness with 2 mm slice gap; acquired matrix size: 256 x 156 pixels; acquired voxel size: 1.3×1.8×8.0 mm3; TE/TR: 3.24/8.35 ms; acceleration factor of 2) was used, and the inversion time was optimized to achieve appropriate nulling of the myocardium. The Modified Look-Locker inversion-recovery sequence was used to perform myocardial T1 mapping. Native and postcontrast myocardial T1 (15 minutes after contrast administration) were acquired using a heartbeat acquisition scheme of 5(3)3 and 4(1)3(1)2, respectively. Extracellular volume (ECV) fraction was quantified using the native and 15-minute postcontrast T1 map, analyzed using the T1 mapping module (CVI42, Circle Cardiovascular Imaging, Calgary, Canada). Interstitial volume was defined as extracellular volume fraction×myocardial volume, where myocardial volume (mL) was defined as myocardial mass (g)/1.05 g/mL.
Deidentified imaging data were analyzed according to standardized protocols at the National Heart Research Institute of Singapore CMR Core Laboratory using CVI42 (Circle Cardiovascular Imaging, Calgary, Canada) by trained fellows who were blinded to the clinical and proteomic data.10,11 Nonischemic LGE was assessed qualitatively according to the recommendations by the Society of Cardiovascular Magnetic Resonance.12 The presence of LGE detected on the magnitude reconstructed images were confirmed on phase-sensitive inversion-recovery reconstructed images. End-diastolic myocardial wall thickness was measured semiautomatically in the short-axis views (50 chords per myocardial slice), according to the standard 16-segment model. Short-axis slices that did not have a complete myocardium ring were excluded from the wall thickness analysis. Concentricity was defined as LV mass/end-diastolic volume ratio. Recently, we have derived the novel remodeling index ( where EDV is the LV end-diastolic volume and t is the maximal wall thickness across the 16 myocardial segments) as a surrogate marker of global myocardial wall stress.9 A low RI (implying worse global wall stress) predicted worse outcomes in patients with hypertensive LVH.13
Serum N-Terminal Pro-B-Type Natriuretic Peptide and Growth Differentiation Factor 15
Blood samples were collected on the day of CMR and stored at −80 °C. Serum NT-proBNP (N-terminal pro-B-type natriuretic peptide; proBNP II STAT, Roche Diagnostics, Pensberg, Germany) was assayed using electrochemiluminescence immunoassay on the Cobas E602 analyzer (Roche Diagnostics Asia-Pacific, Singapore). The manufacturer reported lower limit of detection was 10 pg/mL. Concentrations lower than the detection levels in the participants were assigned a value equivalent to half the limit of detection. Serum GDF-15 (growth differentiation factor 15) concentration was measured using Human GDF-15 Quantikine ELISA Kit (DGD150; R&D Systems, Minneapolis) in duplicates according to the manufacturer’s protocol. The manufacturer reported minimum detectable dose of human GDF-15 was between 0.0 and 4.4 pg/mL (mean, 2.0 pg/mL).
Multiplex Proteomic Analysis
Measurement of 184 serum proteins putatively related to cardiovascular diseases was performed using 2 commercially available multiplex immunoassays (Olink Target Cardiovascular Disease [CVD] II and III, Olink Proteomics, Uppsala, Sweden; Table S1) across 3 batches in all patients. Among the 184 biomarkers, 3 biomarkers (ITGB1BP2 [Melusin] and PARP-1 [Poly (ADP-ribose) polymerase 1] from CVDII; CHIT1 [Chitotriosidase-1] from CVD III) had expression levels lower than the Limit of Detection in more than 30% of the samples and these were removed from subsequent analyses. Sixteen samples that passed QC and had high detectability (a maximum of 10% data below limit of detection per sample) were randomly selected using OlinkAnalyze R package v3.4.1, olink_bridgeselector function. To correct for batch effects, we utilized the removeBatchEffect function from the limma R package v.3.52.4, performing the batch correction across 3 runs.
The Normalized Protein Expression (NPX; an arbitrary unit in log2 scale) was used as the relative quantification unit of protein concentration. Multivariable logistic regressions with adjustment for clinically important variables (age, sex, SBP, body mass index, hypertension treatment, and duration) were performed to study the associations of the 181 proteins with replacement myocardial fibrosis. A protein with a fold change value of greater/less than 0.5 NPX and a false discovery rate of ≤5% was considered significant. Multivariable linear regression analyses were used to examine the association between the proteins and ECV, adjusting for the same potential confounders listed above.
Ingenuity Pathway Analyses of Biological Functions and Diseases
To examine biological/pathological activities of the proteins associated with myocardial fibrosis, the differential protein signatures were determined by subtracting the median NPX values of HTN/DM and hypertension patients with replacement myocardial fibrosis from the NPX values of hypertension patients without replacement myocardial fibrosis. All protein IDs were converted to gene IDs using UniProtKB except NT-proBNP, which represented the N-terminal cleavage product of BNP and not a gene. The differential protein signatures (NPXtarge –NPXcontrol) were subsequently imported into the ingenuity pathway analysis Core Analysis module (QIAGEN Inc, https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis) and annotated using the benchmarked biological functions and diseases dataset. The likelihood and direction of enrichment was represented by Z score. Z scores of <−2 or >2 were considered significantly inhibited or activated, respectively. The algorithms used in IPA had been described previously.14
Statistical Analysis
Categorical variables were presented using frequencies and percentages; and continuous variables were presented using means with standard deviations or medians with interquartile ranges depending on normality. The Shapiro-Wilk test was used to assess the distribution of continuous variables. Differences in characteristics between the groups were analyzed by the Student t test and 1way ANOVA (with post hoc Bonferroni adjustment for pairwise comparison) or the nonparametric Mann-Whitney U test and Kruskal-Wallis test for continuous data, depending on the normality of the data. Categorical data were compared using the χ2 test. Multivariable linear and logistic regression analyses were used to examine the association between hypertension, HTN/DM, and markers of cardiac remodeling, adjusting for the same potential confounders listed above.
Statistical analyses were performed using GraphPad Prism 8.1.2 (GraphPad Software Inc, San Diego, CA) and R (version 4.2.0), assuming a 2-sided test with a 5% level of significance.
RESULTS
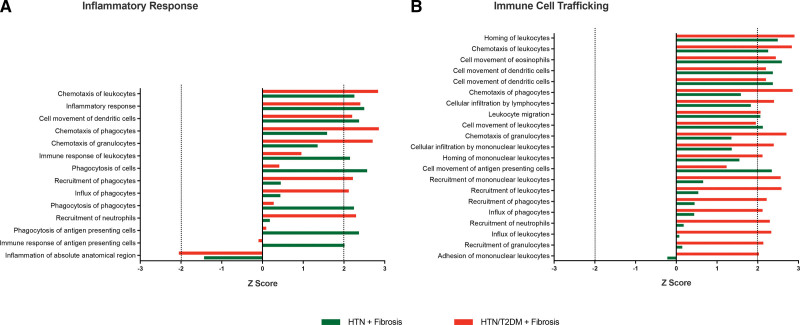
This study included 605 patients: 438 with hypertension (60±8 years; 59% males) and 167 age- and sex-matched patients with HTN/DM (60±10 years; 64% males; Hemoglobin A1c [HbA1c]: 7.0 [6.6 to 7.7]%). There was no significant difference in 24-hour SBP (130±14 versus 132±16 mm Hg, respectively; P=0.086). Compared with those with hypertension only, patients with HTN/DM had other features of metabolic syndrome: they weighed more and had a higher prevalence of dyslipidemia (Table 1).
Table 1.
Characteristics of Patients With Hypertension Stratified by Diabetes Status
CMR Findings in Patients With Hypertension and Diabetes
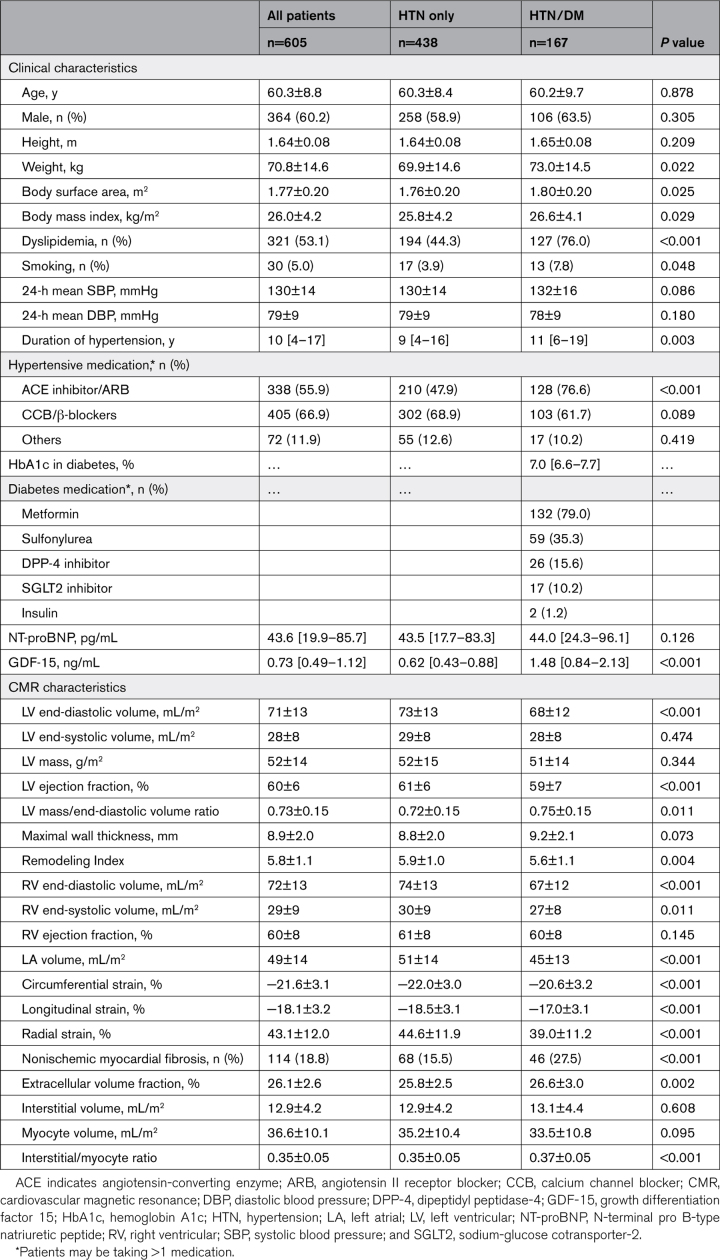
Despite similar LV mass (52±15 versus 51±14 g/m2; P=0.344), patients with HTN/DM had increased concentricity (mass/end-diastolic volume ratio: 0.75±0.15 versus 0.72±0.15, respectively; P=0.011) and worse RI (a surrogate marker of global wall stress; 5.6±1.1 versus 5.9±1.0, respectively; P=0.004) compared with the hypertension group. A higher concentricity and worse RI was significantly associated with LV mass in patients with HTN/DM compared to those with hypertension, after adjusting for age, sex, SBP, body mass index, hypertensive therapy, and treatment duration (Figure 1). Patients with HTN/DM had smaller LV/right ventricular volumes, accompanied by smaller left atrial volumes compared to those with hypertension. In addition, they had worse multidirectional strain and slightly lower LVEF compared with those with hypertension (P<0.001 for all measures; Table 1). Findings remained statistically significant after adjusting for age, sex, SBP, body mass index, hypertensive therapy, and duration.
Figure 1.
Association between concentricity, systolic blood pressure, and left ventricular (LV) mass. For the same systolic blood pressure, patients with hypertension (HTN) and diabetes (HTN/DM, red) have higher concentricity compared to those with hypertension alone (HTN, blue; A). Similarly, for the same left ventricular mass, patients with hypertension and diabetes have higher concentricity and worse Remodeling Index (a surrogate marker of global wall stress) compared to those with hypertension alone (B and C). Analyses were adjusted for age, sex, systolic blood pressure, and body mass index, hypertensive therapy, and treatment duration. EDV indicates LV end-diastolic volume.
Replacement myocardial fibrosis (nonischemic LGE on CMR) was present in 28% of patients with HTN/DM compared to 16% of patients with hypertension (P<0.001; Table 1). Across tertiles of LV mass, higher proportions of patients with HTN/DM had replacement myocardial fibrosis compared to hypertension: tertile 1: 13 versus 8%, P=0.282; tertile 2: 21 versus 7%, P=0.004; tertile 3: 48 versus 30%, P=0.014. Limiting the analyses to those with normal LV mass based on age and sex-specific thresholds established in Asians (HTN/DM; n=126; hypertension, n=316), 25% of patients with HTN/DM (n=32) had replacement myocardial fibrosis compared with 8.5% of patients with hypertension (n=27; P<0.001). Similar findings were observed with CMR markers of diffuse fibrosis and LV function (Table 1). Patients with HTN/DM had higher ECV compared to those with hypertension (26.6±3.0 versus 25.8±2.5, respectively; P=0.002).
Moderate correlations were observed between SBP and LV mass (r=0.47; P<0.0001), global longitudinal strain (r=0.27; P<0.0001), and fibrosis volume index (r=0.46; P<0.0001). However, a weak correlation was found between SBP and left ventricular ejection fraction (r=−0.12; P=0.0039). Additional analyses demonstrated an independent association between DM and increased concentricity, worse RI, myocardial fibrosis, and worse multidirectional strain after adjusting for age, sex, SBP, body mass index, hypertensive therapy, treatment duration, and LV mass (Table S2).
Association Between Myocardial Fibrosis and Proteomic Signatures in Patients With Hypertension and Diabetes
In patients with HTN/DM, 21 central proteins were independently associated with ECV, and 7 unique proteins were upregulated in replacement myocardial fibrosis (Figure S1; Table S3). Of these proteins, GDF-15 was the only common protein upregulated in replacement myocardial fibrosis and independently associated with ECV. DM therapies had no effect on proteomic findings.
In patients with hypertension alone, 39 proteins were independently associated with ECV and only one protein was upregulated (NT-proBNP) in replacement fibrosis (Figure S1; Table S3). NT-proBNP is one of these 39 proteins independently associated with ECV.
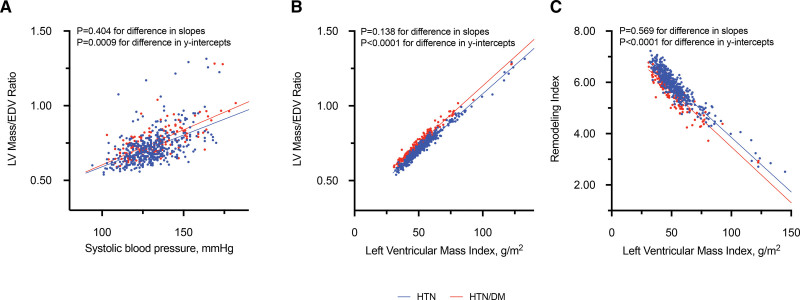
We validated Olink findings of NT-proBNP and GDF-15 with commercially available assays. Strong correlations were observed with NT-proBNP (r=0.82; P<0.0001) and GDF-15 concentrations (r=0.91; P<0.0001). The associations between NT-proBNP, GDF-15, and myocardial fibrosis in patients with hypertension with and without diabetes were similar using Olink and commercially available assays (Figure 2).
Figure 2.
Validating Olink proteomics with commercially available assays. Olink NT-proBNP (N-terminal-pro B-type natriuretic peptide) demonstrated a strong correlation with commercially available assay (A) and elevated in patients with hypertension (HTN) with myocardial fibrosis assessed with Olink and commercially available assay (B and C). In patients with hypertension and diabetes (HTN/DM), Olink GDF-15 (growth differentiation factor 15) had an excellent correlation with commercially available assay (D) and elevated in those with myocardial fibrosis using Olink and commercially available assay (E and F). Normalized Protein Expression (NPX) values for Olink NT-proBNP (A) and GDF-15 (D) were linearized (2NPX). Results in B, C, E, and F were presented in median and interquartile range. HTN/DM-Fib indicates HTN/DM and fibrosis; and HTN-Fib, hypertension and fibrosis.
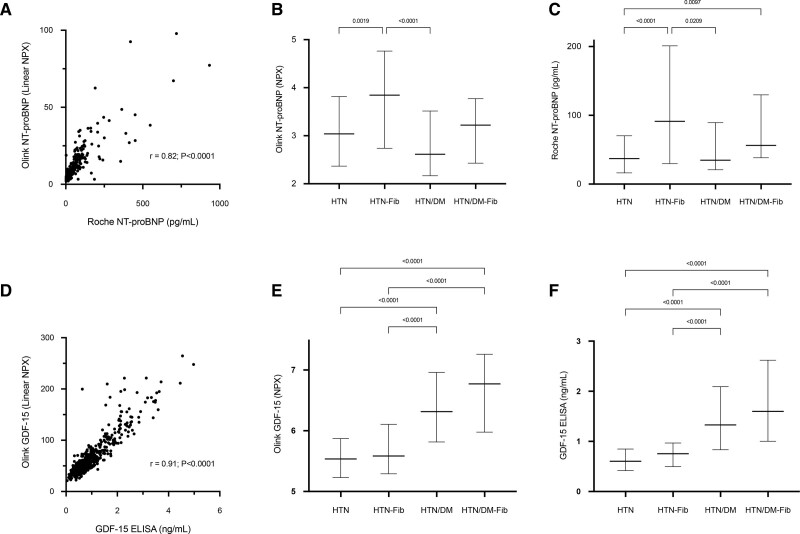
The Core Analysis module in the IPA characterizes key biological/pathological processes represented by differentially expressed proteins. Increased biological activities of inflammatory response and immune cell trafficking were strongly associated with replacement myocardial fibrosis in those with HTN/DM HTN/DM (Figure 3; Table S4).
Figure 3.
Ingenuity pathway analysis (IPA) of biological and disease processes associated with replacement myocardial fibrosis in patients with hypertension (HTN) with and without diabetes. Increased biological functions in inflammatory response and immune cell trafficking were associated those with HTN and type 2 diabetes (HTN/T2DM; A and B). Z scores of <−2 or >2 were considered significantly inhibited or activated, respectively.
DISCUSSION
Despite being well matched in age and sex and with similar LV mass and SBP, patients with HTN/DM demonstrated adverse cardiac remodeling compared to patients with hypertension: increased concentricity, worse global wall stress and multidirectional strain; and increased myocardial fibrosis. In patients with HTN/DM, GDF-15 was independently associated with replacement fibrosis and ECV. In patients with hypertension alone, NT-proBNP was independently associated with replacement fibrosis and ECV. IPA suggested activation in inflammatory response and immune cell trafficking in patients with HTN/DM and myocardial fibrosis.
In previous studies, patients with HTN/DM had the highest LV mass and greatest amount of myocardial fibrosis compared to patients with either hypertension or DM alone.7,15,16 These studies included patients who had elevated SBP or died of heart failure and other cardiovascular causes, suggesting more advanced disease. In contrast, our study consisted of asymptomatic patients free from overt cardiovascular diseases, with a mean SBP of 130 mmHg. This difference in population characteristics partly accounts for the smaller magnitude of differences observed in CMR markers of cardiac remodeling. Despite this, it is noteworthy to highlight about 25% of patients with HTN/DM and normal LV mass had myocardial fibrosis compared to just 8.5% of those with hypertension. In combination with the contemporary studies, we would suggest diabetes accelerates adverse cardiac remodeling in already at-risk patients with hypertension, beyond the hemodynamic consequences of elevated blood pressure.
Left atrial enlargement typically occurs as a late manifestation of increased left ventricular filling pressures. A previous study conducted on participants from the UK biobank demonstrated reduced atrial volumes in those with diabetes.17 Moreover, despite having the worst diastolic function among individuals with HTN/DM compared with those with hypertension and diabetes alone, there was no associated increase in left atrial size.18 These findings, along with other observations in our study, suggest that the identification of reduced left atrial volumes in our study likely indicates an earlier stage of cardiac remodeling related to diabetes.
The hypertrophic growth of cardiomyocytes is the primary response by which the heart reduces unit wall stress due to pressure overload from elevated blood pressure. Cardiomyocyte apoptosis occurs as a consequence of the maladaptive processes mediating the transition from compensated to decompensated LVH.19 Increasingly, the roles of inflammation and immune activation have also been implicated as important mediators of fibrosis.20,21 Our study accords with current knowledge. In our study, IPA demonstrated that increased biological activities of inflammatory response and immune activation were strongly associated with myocardial fibrosis in those with HTN/DM.
Distinct protein signatures of myocardial fibrosis were identified in patients with HTN/DM and hypertension. Natriuretic peptides (BNP and NT-proBNP) are released from the ventricles as a result of increased myocardial stretch and diastolic wall stress.22 In accord with this, we have previously demonstrated an independent association between NT-proBNP concentrations and myocardial fibrosis in patients with hypertension.9 Under cellular stress and tissue injury, GDF-15 (a member of the TGF [transforming growth factor]-β family) is secreted by many cell types including macrophages, endothelial and vascular cells; and cardiomyocytes. Recent evidence supports the role of GDF-15 on the activation of metabolic pathways and the association with apoptosis, fibrosis, inflammation, and adverse cardiac remodeling.23 Identifying strong upregulation of GDF-15 in our patients with HTN/DM and myocardial fibrosis (replacement and diffuse) provided further validation for the study. Future studies will be needed to investigate the role of these novel proteins in cardiac health and remodeling.
Clinical Implications
Myocardial fibrosis is associated with worse outcomes in patients with hypertension,3 with and without DM (Figure S2). There is increasing interest to target the myocardial interstitium to improve diagnosis, risk stratification and to discover novel therapies.24–26 As demonstrated in the study, myocardial fibrosis is a heterogeneous pathology resulting from cardiac disease-specific biology. HTN/DM is associated with accelerated adverse cardiac remodeling and this observation endorses the importance to consider early risk stratification by imaging and biomarker profiling in these patients, possibly even before they have developed LVH. Furthermore, an understanding of the differences in proteomic signatures provides insights for a personalized approach to selecting effective pharmacological agents. Whether patients with HTN/DM derive incremental anti-fibrotic benefits from therapies targeting inflammation/immune activation warrant further investigations.27 Our study suggests in addition to conventional circulating markers of collagen turnover, proteins of immune activation are complementary markers to detect myocardial fibrosis.
Study Limitations
The cross-sectional study does not permit determination of causal relationships between LV mass, concentricity, and myocardial fibrosis. Statistical adjustment may not account for all confounding variables, particularly for unknown or unmeasured confounders. We acknowledge the inherent limitations of a targeted proteomics technology may have excluded other promising candidates and the circulating protein signatures may not fully reflect relevant intracellular mechanisms associated with myocardial fibrosis. We did not have a validation cohort to verify the proteins identified in the study. Despite these limitations, the findings of the unique protein signatures and biological activities will provide important pathophysiological insights. In addition, a number of the proteins identified here were consistent with other studies, supporting the study validity.
Conclusions
Diabetes is associated with adverse cardiac remodeling in patients with hypertension. The novel proteomic signatures and associated biological activities of increased immune activation/inflammatory response may partly explain these observations. The study has implications on risk stratification and novel targeted therapies that should be investigated further.
ARTICLE INFORMATION
Acknowledgments
The authors thank the radiographers at the National Heart Center Singapore for their assistance in the study.
Sources of Funding
The study was supported by the Ministry of Health and National Medical Research Council.
Disclosures
None.
Supplemental Material
Tables S1–S4
Figures S1 and S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CMR
- cardiovascular magnetic resonance
- CVD
- cardiovascular disease
- ECV
- extracellular volume
- EDV
- LV end-diastolic volume
- HTN/DM
- hypertension and diabetes
- LGE
- late gadolinium enhancement
- LVH
- left ventricular hypertrophy
- RI
- remodeling index
- SBP
- systolic blood pressure
T.-T. Le and C.W.L. Chin contributed equally.
For Sources of Funding and Disclosures, see page 552.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.123.015051.
Contributor Information
Chee Jian Pua, Email: pua.chee.jian@nhcs.com.sg.
Germaine Loo, Email: loo.germaine@gmail.com.
Michelle Kui, Email: michelle.kui.s.l@singhealth.com.sg.
Wai Lun Moy, Email: wailunmoy@hotmail.com.
An-An Hii, Email: hii.an.an@nhcs.com.sg.
Vivian Lee, Email: mdclchr@nus.edu.sg.
Chee-Tang Chin, Email: cchin03m@gmail.com.
Jennifer A. Bryant, Email: jenbryant210@gmail.com.
Desiree-Faye Toh, Email: desiree.faye.t.k@nhcs.com.sg.
Chi-Hang Lee, Email: mdclchr@nus.edu.sg.
Stuart A. Cook, Email: stuart.cook@duke-nus.edu.sg.
A. Mark Richards, Email: mark.richards@nus.edu.sg.
Thu-Thao Le, Email: gmsltt@nus.edu.sg.
REFERENCES
- 1.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn W, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart - structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7 [DOI] [PubMed] [Google Scholar]
- 2.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer NR, Le TT, Kui MSL, Tang HC, Chin CT, Phua SK, Bryant JA, Pua CJ, Ang B, Toh DF, et al. Markers of focal and diffuse nonischemic myocardial fibrosis are associated with adverse cardiac remodeling and prognosis in patients with hypertension: the REMODEL study. Hypertension. 2022;79:1804–1813. doi: 10.1161/HYPERTENSIONAHA.122.19225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5:543–551. doi: 10.1016/j.jchf.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 5.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792 [DOI] [PubMed] [Google Scholar]
- 6.Sahai A, Ganguly PK. Congestive heart failure in diabetes with hypertension may be due to uncoupling of the atrial natriuretic peptide receptor-effector system in the kidney basolateral membrane. Am Heart J. 1991;122:164–170. doi: 10.1016/0002-8703(91)90774-c [DOI] [PubMed] [Google Scholar]
- 7.Grossman E, Messerli FH. Diabetic and hypertensive heart disease. Ann Intern Med. 1996;125:304–310. doi: 10.7326/0003-4819-125-4-199608150-00009 [DOI] [PubMed] [Google Scholar]
- 8.Smith JG, Gerszten RE. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation. 2017;135:1651–1664. doi: 10.1161/CIRCULATIONAHA.116.025446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh VJ, Le TT, Bryant J, Wong JI, Su B, Lee CH, Pua CJ, Sim CPY, Ang B, Aw TC, et al. Novel index of maladaptive myocardial remodeling in hypertension. Circ Cardiovasc Imaging. 2017;10:e006840. doi: 10.1161/CIRCIMAGING.117.006840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le TT, Tan RS, Deyn MD, Goh EPC, Han Y, Leong BR, Cook SA, Chin CWL. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson. 2016;18:21. doi: 10.1186/s12968-016-0236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai J, Bryant JA, Le TT, Su B, de Marvao A, O’Regan DP, Cook SA, Chin CWL. Fractal analysis of left ventricular trabeculations is associated with impaired myocardial deformation in healthy Chinese. J Cardiovasc Magn Reson. 2017;19:102. doi: 10.1186/s12968-017-0413-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:1–19. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le TT, Lim V, Ibrahim R, Teo MT, Bryant J, Ang B, Su B, Aw TC, Lee CH, Bax J, et al. The remodelling index risk stratifies patients with hypertensive left ventricular hypertrophy. Eur Heart J Cardiovasc Imaging. 2021;22:670–679. doi: 10.1093/ehjci/jeaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Factor SM, Minase T, Sonnenblick EH. Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am Heart J. 1980;99:446–458. doi: 10.1016/0002-8703(80)90379-8 [DOI] [PubMed] [Google Scholar]
- 16.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848 [DOI] [PubMed] [Google Scholar]
- 17.Jensen MT, Fung K, Aung N, Sanghvi MM, Chadalavada S, Paiva JM, Khanji MY, de Knegt MC, Lukaschuk E, Lee AM, et al. Changes in cardiac morphology and function in individuals with diabetes mellitus: the UK Biobank Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2019;12:e009476. doi: 10.1161/CIRCIMAGING.119.009476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo C, Jin Z, Homma S, Rundek T, Eikind MSV, Sacco RL, Di Tullio MR. Effects of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur J Heart Fail. 2010;12:454–461. doi: 10.1093/eurjhf/hfq022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González A, Fortuño MA, Querejeta R, Ravassa S, López B, López N, Díez J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–562. doi: 10.1016/s0008-6363(03)00498-x [DOI] [PubMed] [Google Scholar]
- 20.González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–1706. doi: 10.1016/j.jacc.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 21.Shinde AV, Frangogiannis NG. Mechanisms of fibroblast activation in the remodeling myocardium. Curr Pathobiol Rep. 2017;5:145–152. doi: 10.1007/s40139-017-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, Hasin Y, Biasucci LM, Giannitsis E, Lindahl B, et al. ; Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the study group on biomarkers in cardiology of the ESC working group on acute cardiac care. Eur Heart J. 2012;33:2001–2006. doi: 10.1093/eurheartj/ehq509 [DOI] [PubMed] [Google Scholar]
- 23.Wesseling M, Poel JHC, Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail. 2020;7:1488–1501. doi: 10.1002/ehf2.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin CWL. Targeting the myocardium in hypertensive left ventricular hypertrophy. Expert Rev Cardiovasc Ther. 2017;15:653–655. doi: 10.1080/14779072.2017.1361320 [DOI] [PubMed] [Google Scholar]
- 25.Gyöngyösi M, Winkler J, Ramos I, Do QT, Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail. 2017;19:177–191. doi: 10.1002/ejhf.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis GA, Dodd S, Naish JH, Selvanayagam JB, Dweck MR, Miller CA. Considerations for clinical trials targeting the myocardial interstitium. JACC Cardiovasc Imaging. 2019;12:2319–2331. doi: 10.1016/j.jcmg.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 27.Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021;128:1451–1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]