There was evidence of a disproportionately high burden of severe symptoms among Black and Hispanic patients without a corresponding level of previous uterine-sparing treatments attempted.
OBJECTIVE:
To evaluate whether greater symptom severity can explain higher hysterectomy rates among premenopausal non-Hispanic Black compared with White patients in the U.S. South rather than potential overtreatment of Black patients.
METHODS:
Using electronic health record data from 1,703 patients who underwent hysterectomy in a large health care system in the U.S. South between 2014 and 2017, we assessed symptom severity to account for differences in hysterectomy rates for noncancerous conditions among premenopausal non-Hispanic Black, non-Hispanic White, and Hispanic patients. We used Poisson generalized linear mixed modeling to estimate symptom severity (greater than the 75th percentile on composite symptom severity scores of bleeding, bulk, or pelvic pain) as a function of race–ethnicity. We calculated prevalence ratios (PRs). We controlled for factors both contra-indicating and contributing to hysterectomy.
RESULTS:
The overall median age of non-Hispanic White (n=1,050), non-Hispanic Black (n=565), and Hispanic (n=158) patients was 40 years. The White and Black patients were mostly insured (insured greater than 95%), whereas the Hispanic patients were often uninsured (insured 58.9%). White and Black patients were mostly treated outside academic medical centers (nonmedical center: 63.7% and 58.4%, respectively); the opposite was true for Hispanic patients (nonmedical center: 34.2%). Black patients had higher bleeding severity scores compared with Hispanic and White patients (median 8, 7, and 4 respectively) and higher bulk scores (median 3, 1, and 0, respectively), but pain scores differed (median 3, 5, and 4, respectively). Black and Hispanic patients were disproportionately likely to have severe symptoms documented on two or more symptoms (referent: not severe on any symptoms) (adjusted PR [Black vs White] 3.02, 95% CI 2.29–3.99; adjusted PR [Hispanic vs White] 2.61, 95% CI 1.78–3.83). Although Black and Hispanic patients were more likely to experience severe symptoms, we found no racial and ethnic differences in the number of alternative treatments attempted before hysterectomy.
CONCLUSION:
We did not find evidence of overtreatment of Black patients. Our findings suggest potential undertreatment of Black and Hispanic patients with uterine-sparing alternatives earlier in their disease progression.
Rates of hysterectomy before menopause remain disproportionately high among U.S. Black populations and in U.S. territories such as Puerto Rico.1–6 Hysterectomy, an effective treatment for gynecologic conditions such as uterine leiomyomas (fibroids) and dysmenorrhea,7 is also sterilizing. Overtreatment with sterilizing procedures remains a concern among marginalized communities, given state-sponsored eugenics programs in the 20th century and the even longer history of gynecologic abuse of Black women.8–10 However, in the past 20 years, few quantitative studies have investigated potentially racialized overtreatment with hysterectomy.11–14 Research has focused on clinical outcomes or surgical route rather than treatment decision making.15–17
One barrier to investigating overtreatment with hysterectomy is the difficulty of defining “overtreatment.” Recommendations for premenopausal hysterectomy decision making center on impairment of patient quality of life from symptoms such as pelvic pain, heavy or unexpected uterine bleeding, or pressure from large leiomyomas.7 Unfortunately, neither quality-of-life measures nor symptom severity are captured in the insurance claims databases often used in health services research.18
To overcome these methodologic challenges, we compared patient symptom severity across race–ethnicity categories in a case series of patients who underwent hysterectomy. If Black patients were being overtreated with hysterectomy, we would expect to observe lower symptom severity among Black patients than non-Hispanic White patients. Using electronic health record (EHR) data from 1,703 patients treated in a large health care system in the U.S. South19 between 2014 and 2017,20 we evaluated symptom severity as a potential mechanism for differences in hysterectomy rates among premenopausal non-Hispanic Black, non-Hispanic White, and Hispanic patients.
METHODS
We identified eligible patients using a health care system “data warehouse” that leverages structured EHR data, such as self-reported race–ethnicity, procedure and diagnostic codes, age, and hospital, among other fields. We supplemented these eligibility data with other data-warehouse–derived data, such as laboratory values and prescriptions. Further, professional abstractors collected data from surgical notes as well as imaging and other records related to patients' gynecologic symptoms for at least 180 days before patients' surgeries.21–23 They did not collect data on patient race or ethnicity, nor was this information in the REDCap abstraction tool or EHR tabs reviewed. For quality control, 100 records were randomly selected for independent re-abstraction.20 This study was approved by the University of North Carolina IRB (17-2728.)
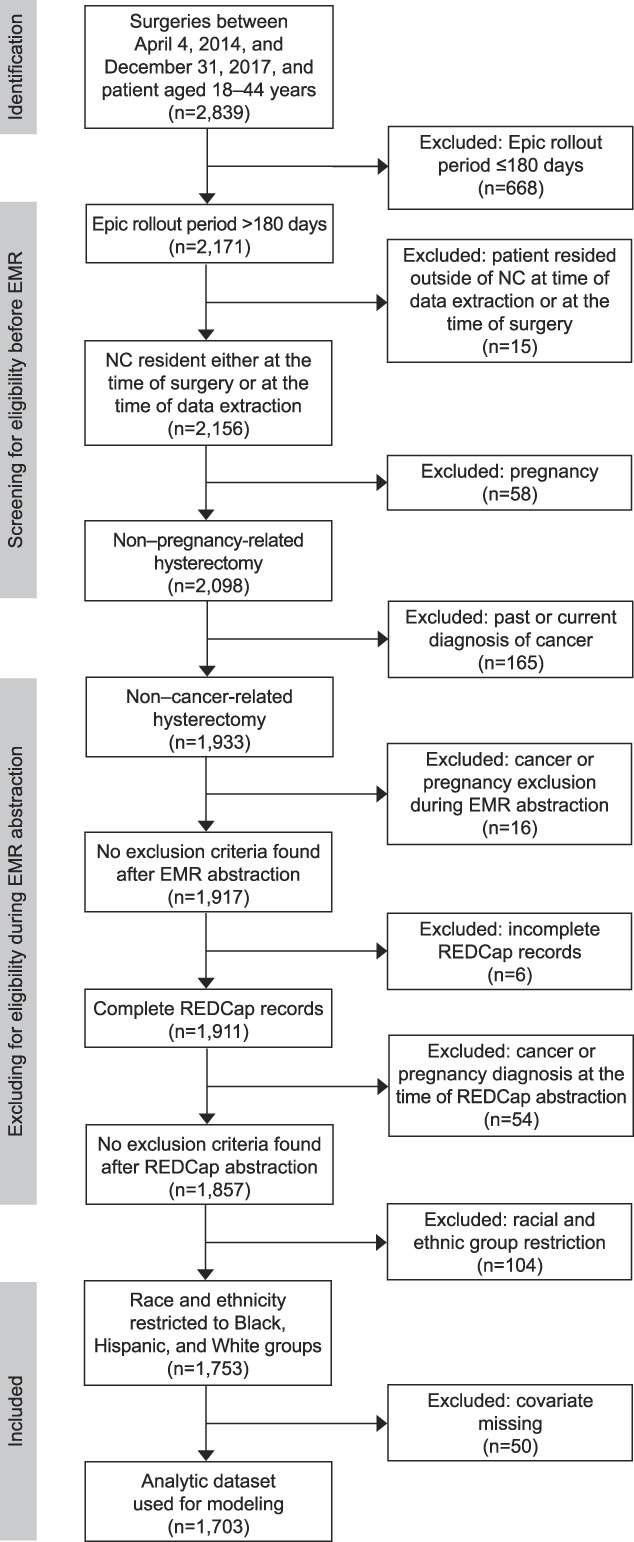
We assembled a study population to meet the following eligibility criteria: North Carolina residents aged 18–44 years who underwent hysterectomy for noncancerous, non–pregnancy-related indications between October 2, 2014, and December 31, 2017, at 1 of 10 hospitals in a large health system in the U.S. South that had used the health system's EHR platform for at least 180 days. To determine eligibility, we first queried the health system's structured EHR data (see Appendix 1, available online at http://links.lww.com/AOG/D190, for diagnostic codes used). Of 2,839 patients aged 18–44 years who underwent hysterectomy, 668 were ineligible because their hospital had been using the system's EHR for 180 days or less at the time of their hysterectomy, which complicates the collection of adequate presurgical data on symptom severity. Of the 2,171 remaining patients, 314 were excluded for ineligibility due to residence, evidence of cancer or pregnancy, or incomplete abstraction records (see Fig. 1). Of the 1,857 patients remaining, we excluded 104 patients who were not non-Hispanic White, non-Hispanic Black, or Hispanic or Latina (race–ethnicity described in more detail below). Finally, 50 patients were excluded because of missing covariate data on body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) (n=37), previous laparotomy (n=10), or both (n=3). The final analytic sample consisted of 1,703 patients.
Fig. 1. Flow chart showing inclusion and exclusion criteria to obtain the analytic data set for the population of patients who underwent hysterectomy, aged 18–44 years. NC, North Carolina; EMR, electronic medical record.
Robinson. Racial and Ethnic Differences in Hysterectomy. Obstet Gynecol 2023.
The independent variable of interest was three-level race–ethnicity: non-Hispanic Black; Hispanic of any race; and non-Hispanic White, the referent group. This composite race–ethnicity variable was derived from two EHR variables that are legally required to be self-reported24: six-level race (White, Black, Asian, Native, Other, Refused or Unknown) and dichotomous Hispanic ethnicity. Any patient classified as Hispanic on the ethnicity variable was coded as Hispanic. Where patients had multiple values for the EHR's race variable (always White plus another non-White value), we classified them as the non-White value in our collapsed race–ethnicity variable to err on the side of being more inclusive of the experience of non-White patients, who are often underrepresented in gynecologic research.25 Further, we conceptualized these U.S. racial and ethnic categories as social constructs that reflect differential exposure to systemic and institutional racism as well as other social determinants of health, especially for any deviations from Whiteness.26,27 We restricted multivariable analyses to non-Hispanic Black, Hispanic, and non-Hispanic White patients because of nonconvergence of models due to small sample sizes for other racial and ethnic groups.
We scored each patient on severity of their gynecologic symptoms on three domains: bulk, vaginal bleeding, and pelvic pain.2–6 The construction and validation of the severity scores are described in detail elsewhere.20 Our dichotomous outcomes were Yes/No to being in the top 25th percentile of bulk, pelvic pain, or bleeding symptom severity scores. Because symptom data from EHR notes are often missing, we focused on the high end of the score range. Although low scores may miscategorize missing data as low symptom severity, high scores are likely to be a specific indicator of high symptom severity. We also fit two “combined severity” models for being in the highest 25th percentile 1) for any of the three constructs or 2) two of the three constructs, each compared with not being in top 25th percentile on any construct.
We controlled for other factors that influence the decision to perform hysterectomy, either those that are relatively contra-indicating against hysterectomy or those that would support the decision to perform hysterectomy (contributing). We expected contra-indicating factors to be associated with greater symptom severity at the time of hysterectomy, whereas contributing factors would be associated with lower symptom severity. Relatively contra-indicating factors include younger age, high BMI (eg, higher than 40),7,8 comorbidities (Charlson Comorbidity Index based on the hysterectomy's hospital-billed diagnostic codes), and previous abdominal surgeries (Yes/No). Previous abdominal surgery (Yes/No) was defined as laparotomy for gynecologic indication and other abdominal surgery (excluding cesarean deliveries, due to high prevalence [greater than 30.0%]). The contributing indications were ovarian cysts, pelvic masses, number of alternative treatments attempted before hysterectomy (zero, one, two or more), and previous completed pregnancies. Presence of ovarian cysts (Yes/Absent from the record) or cervical dysplasia (Yes/Absent) was obtained from EHR progress and preoperative notes. Previous treatments considered were oral contraceptives, vaginal ring, hormonal patch, oral progestins, Depo Provera, contraceptive implants (Implanon and Nexplanon), Lysteda (tranexamic acid), LupronDepot or gonadotropin-releasing hormone agonist, hormonal intrauterine device, uterine artery embolization, uterine ablation, myomectomy, hysteroscopy, laparoscopy for gynecologic indication, and laparotomy for gynecologic indication. Another contributing indication for surgery is achievement of desired childbearing. Because there was not a direct measure of this in the EHR, we examined number of previous deliveries (continuous) as a covariate in supplemental analyses. Because missingness was high on this variable (25.5%), we did not include it in the main analyses.
Descriptive statistics are presented by race and Hispanic ethnicity. Medians and interquartile ranges are reported for continuous variables, and frequencies and percentages are reported for categorical variables. Robust Poisson models, which allow for calculation of each outcome's prevalence ratio (PR), were fit for the combined symptom severity outcome (one or more vs zero, two or more vs zero) and for each of the symptom severity outcomes. The simplest models presented here included race–ethnicity, age at time of hysterectomy, a fixed effect for hospital type (academic medical center vs other community hospitals), and lead surgeon (the attending or billing surgeon of record; n=115) as a random effect to account for patient clinical mix (a priori test for inclusion: intraclass correlation greater than 75.0% total variance). The fully adjusted models included the following additional covariates: categorized BMI, cervical dysplasia, ovarian cyst or pelvic mass, previous laparotomy, previous treatment count, and Charlson Comorbidity Index.
We performed several supplemental analyses to understand possible contributors to racial differences in symptom differences. First, we ran five fully adjusted models among subsets of the data stratified by common diagnoses treated with hysterectomy, with the model outcomes chosen to correspond to symptom scores related to that diagnosis: leiomyoma diagnosis (bulk and bleeding severity outcomes), abnormal uterine bleeding or menorrhagia diagnosis (bleeding severity), chronic pelvic pain diagnosis (pain severity) and endometriosis diagnosis (pain). The diagnoses were considered present if any preoperative or progress notes listed them as a main indication for surgery or even as a relevant or suspected gynecologic diagnosis.
Second, in the full analytic sample, we added number of deliveries (variable has high missingness) as a covariate in fully adjusted models of each symptom severity score. Third, in another series of models of symptom severity scores, to assess modification of the race–ethnicity association with symptom threshold, we added interaction terms between race–ethnicity and the following contributing factors for hysterectomy: 1) ovarian cyst or pelvic mass and 2) cervical dysplasia (overall F-test α=0.20). Finally, we described the distribution of number of prior treatments by race–ethnicity and symptom severity. Specifically, we examined the distributions graphically and evaluated differences in the medians of race–ethnicity–specific distributions of number of prior treatments (Kruskal-Wallis tests, α=0.05). In addition, in adjusted regression models, we added interaction terms between race–ethnicity and prior treatments (F-test α=.20).
RESULTS
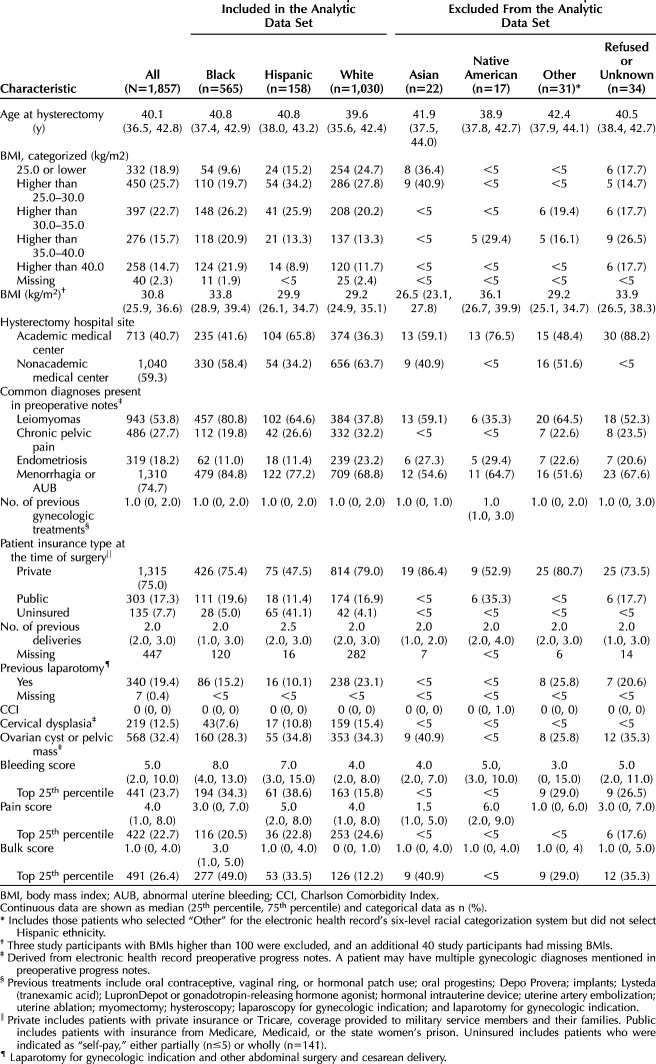
To contextualize the sample, Table 1 shows patient characteristics before the sample was restricted by race–ethnicity (N=1,857). This study analyzed data on the subsample (n=1,703) of non-Hispanic White (n=1,050), non-Hispanic Black (n=565), and Hispanic (n=158) patients. The median age at the time of surgery was 40 years. The Hispanic patients were most likely to be uninsured (41.1% vs less than 5.0% among White and Black patients). The Hispanic patient sample was mostly treated at academic medical centers (65.8%), whereas White and Black patients were more often treated at community hospitals in this sample (63.7% and 58.4%, respectively). Black patients in this sample had higher median bleeding severity scores (8) compared with their White (4) and Hispanic (7) counterparts. On the bulk score, the Black patient sample had higher median scores (3) than White (0) and Hispanic (1) patients. The pain severity median scores were as follows: Hispanic 5, White 4, Black 3. In this sample, the most commonly noted diagnoses for Black patients in the EHR progress or preoperative notes were menorrhagia (84.8%) and leiomyomas (80.8%). These diagnoses were also common among the Hispanic and White patients in this sample, who had menorrhagia noted 77.2% and 68.8% of the time, respectively, and leiomyomas noted 64.6% and 37.8% of the time, respectively.
Table 1.
Descriptive Characteristics of Patients Who Underwent Hysterectomy in 2014–2017, Aged 18–44 Years, by Racial and Ethnic Group Before Restriction of Data Sets to Hispanic, Black, and White Patients
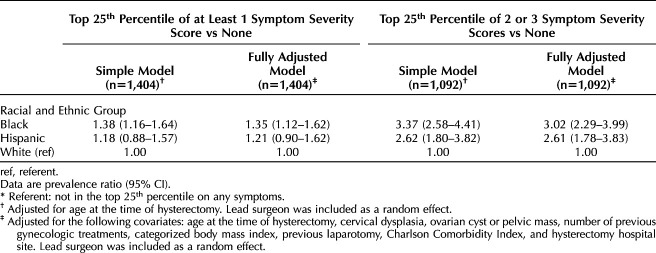
In analyses of overall symptom severity, we did not find evidence that young Black women were being overtreated with hysterectomy in this health system (Table 2). Black patients were more likely than White patients to have documentation of high symptom severity. For instance, in the fully adjusted model (Table 2), Black patients had 35.0% greater risk of being in the top 25.0% of at least one symptom construct. Black patients were disproportionately more likely to have severe symptoms documented on two or three symptoms (fully adjusted PR [Black vs White] 3.02, 95% CI 2.29–3.99) (Table 2). Results for Hispanic patients tended to be similar to those for Black patients but less pronounced. For instance, the fully adjusted association of Hispanic race–ethnicity with at least one severe symptom was 1.21 (95% CI 0.90–1.62) compared with White patients; the increased likelihood of two or more severe symptoms compared with White patients was more than double (fully adjusted PR [Hispanic vs White] 2.61, 95% CI 1.78–3.83).
Table 2.
Multivariable-Adjusted Models Calculating the Prevalence Ratios of Patients Being in the Top 25th Percentile of at Least One Symptom Severity Score or Two or Three Symptom Severity Scores*
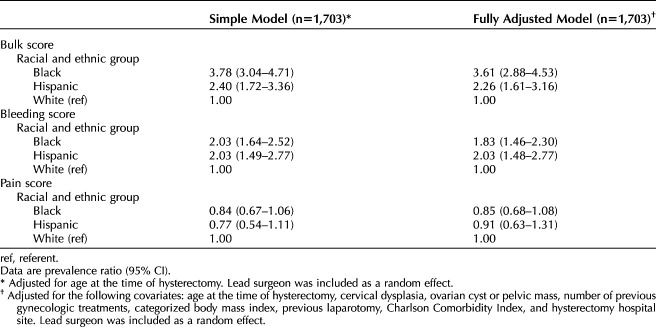
To more precisely characterize racial differences in symptom severity, we ran models separately for each of the three symptom constructs (Table 3). The models for severe bleeding and bulk were consistent with the models for overall composite severity (described above) but with more pronounced racial and ethnic differences. For instance, Black patients were almost twice as likely as White patients to be in the highest quartile of bleeding symptoms (fully adjusted PR 1.83, 95% CI 1.46–2.30; see Table 3) and more than three times as likely to be in the highest quartile of severe bulk (PR 3.61, 95% CI 2.88–4.53). Similarly, Hispanic patients were twice as likely to be in the highest quartiles of severe bleeding (fully adjusted PR 2.03, 95% CI 1.48, 2.77) or bulk (PR 2.26, 95% CI 1.61–3.16). For pain severity, patterns of racial and ethnic difference were markedly different than for bleeding and bulk. For pain, there were no statistically significant differences (α=.05) in likelihood of severe pain prevalence by race–ethnicity (Table 3).
Table 3.
Prevalence Ratios From Multivariable-Adjusted Models of Being in the Top 25th Percentile of Bulk, Bleeding, or Pain Severity Scores, Patients Aged 18–44 Years Who Underwent Hysterectomy, 2014–2017
First, in the five fully adjusted models stratified by four common diagnoses (leiomyomas, abnormal uterine bleeding or menorrhagia, chronic pelvic pain, and endometriosis), Black and Hispanic prevalence of high symptom severity compared with White patients was generally similar to that in analyses not stratified by diagnosis (Appendix 2, available online at http://links.lww.com/AOG/D190). Second, adding number of deliveries as a covariate did not substantially change estimates (Appendix 3, available online at http://links.lww.com/AOG/D190). Third, interactions with race–ethnicity and ovarian cyst or pelvic mass or cervical dysplasia were not statistically significant (Appendices 4 and 5, available online at http://links.lww.com/AOG/D190).
Overall, 30.0% of patients in the high symptom severity quartile had no documentation of previous uterine-sparing treatments. For bleeding, there was evidence that greater number of prior treatments was more associated with greater bleeding severity among White patients than among Black or Hispanic patients (P=.09; PR for two or more treatments 2.20 for White patients; PR 1.25 and 1.53 for Black and Hispanic patients, respectively). Notably, the prevalence of severe bleeding symptoms before hysterectomy was greater among Black patients with no documentation of previous treatments (48/120, 40.0%) than among White patients who had tried two or more previous treatments (86/309, 28.0%). For bulk and pain, there was little evidence of statistical modification of racial differences in symptom severity by number of prior treatments (F-test P>.20) (Appendix 6, available online at http://links.lww.com/AOG/D190).
DISCUSSION
We did not find evidence that premenopausal Black or Hispanic patients were systematically overtreated with hysterectomy for their gynecologic symptoms. Instead, our findings point to potential undertreatment of Black and Hispanic patients earlier in their disease processes. Undertreatment can involve multiple potential pathways, including patient–physician interactions, but also systemic health care barriers to diagnosis and treatment as well as patient preferences about treatment options.28–30 The majority of patients had a bleeding- or bulk-related primary indication for surgery. However, Black and Hispanic patients who underwent hysterectomy had more severe bulk and bleeding symptoms compared with their White counterparts. Despite greater symptom burden, Black and Hispanic patients had received a similar number of prior uterine-sparing treatments before hysterectomy as White patients. The fact that Black and Hispanic patients had worse symptoms but no evidence of escalated uterine-sparing treatments indicates potential undertreatment of severe bleeding and leiomyoma-related bulk in Black and Hispanic patients before progression to hysterectomy. This discrepancy is particularly notable for severe bleeding, which was the symptom most amenable to uterine-sparing treatments.
The similarity of results, particularly for bleeding, across the Black and Hispanic patients in this analysis casts doubt on the idea that innate biological factors specific to people of African ancestry drive Black patients' high prevalence of severe symptoms or faster disease progression.31 The reproductive-aged Hispanic population in the catchment area of this North Carolina study is distinct from the Black population: heavily first-generation immigrants from Mexico and Central America, as well as being culturally and genetically distinct from the Black population in this region.32 Instead, what is common between these populations may be systemic factors in the ways that these economically and socially marginalized populations are treated by health care systems and cultural factors shaped by environments in which marginalization is much more common than among White populations.
The results for pain were notably different from the results for the bleeding and bulk symptoms. These results may reflect a true racial and ethnic difference in the burden of gynecologic pain or treatment of gynecologic pain with hysterectomy. Alternatively, the results may reflect systematic racial and ethnic bias in pain appraisal and treatment by health care professionals in the United States.33–35 The symptom severity score for pain here relied on physician documentation and previous treatments administered, such as opioids prescribed for gynecologic pain or emergency department admissions.20 Previously, we have documented that barriers to diagnosis with pain-related conditions such as endometriosis are most pronounced for Black and Hispanic patients, especially patients with low socioeconomic status, in North Carolina.36 Finally, pain can co-occur with severe bulk and bleeding. Given a complex symptom profile, barriers to comprehensive diagnosis, and racialized understandings of gynecologic health,37,38 health care professionals may default to ascribing a constellation of symptoms in a Black or Hispanic patient to bulk or abnormal uterine bleeding–related symptoms rather than the gynecologic pain they may also be experiencing.
Anchoring this investigation to symptom severity rather than diagnoses is an important strength of this work. We used previously developed indices of symptom severity derived from free text in the EHR, in addition to diagnostic and procedure codes.20 For instance, 80% or more of the population of Black patients who underwent hysterectomy had diagnoses of dysmenorrhea or abnormal uterine bleeding associated with their surgeries. Differentiating the population by symptom severity rather than diagnosis allowed us to better differentiate potential disparity in surgery. Finally, we expanded on limited research on the gynecologic health of Hispanic populations in the U.S. South, a region with a growing population of first- and second-generation immigrants from Mexico and Central America.39
Our findings should be interpreted in the context of the following limitations. The case series design does not include those who were successful with less invasive treatments, which limits inference. Investigations of earlier symptom and treatment progression could clarify mechanisms by which racial and ethnic differences in symptom severity arise among patients who undergo hysterectomy. Further, health care–based study designs do not account for people who self-manage care or who never receive care despite burdensome disease. Additionally, our EHR data did not include reliable data on access to, or patient decisions to forgo, conservative alternative treatments. However, our use of EHR data is more inclusive of patients with no insurance and those with Medicaid insurance than most claims-based analyses. Another limitation of EHR data is gaps in documentation. Acknowledging that missing data could underestimate symptom severity, we focused on the quartile of greatest symptom severity, which we expect to be a highly specific measure of severe symptoms.20
In one large health care system in the U.S. South, a region with high rates of premenopausal hysterectomy,4,5,14,40 we found little evidence of overtreatment of non-Hispanic Black and Hispanic patients given their levels of symptom burden. However, there was evidence of a disproportionately high burden of severe symptoms among Black and Hispanic patients without a corresponding level of previous uterine-sparing treatments attempted. Undertreatment of initial symptoms may contribute to faster disease progression and arrival at greater symptom severity among Black and Hispanic patients before hysterectomy.41
Footnotes
Research reported in this publication was supported by the National Institute of Minority Health and Health Disparities of the National Institutes of Health under award number 1R01MD011680 (PI: Robinson). The project was also supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The funding bodies had no role in the study design, collection, analysis, or interpretation of data; writing of the report; or decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The database infrastructure partly used for this project was supported by the Cecil G. Sheps Center for Health Services Research and the CER Strategic Initiative of UNC's Clinical and Translational Science Award (UL1TR001111). We thank Martha Grace Cromeens, Tamara Watson, Kathy Borron, Madeline Llewyn, and Mekhala Dissanayake for assisting with medical record abstraction. We thank Loretta Fearrington and Samyuktha Nandhakumar for their help with data provisioning.
Each author has confirmed compliance with the journal's requirements for authorship.
Financial Disclosure Whitney R. Robinson receives research funding and support as a co-investigator (1R01AG077947-01) from the National Institute of Aging (NIA). She has received honoraria (<$1,000) for speaking at institutions such as the University of Wisconsin School of Medicine and Public Health, and she has received honoraria for serving on a standing study section for the National Institutes of Health (NIH). Erin T. Carey provides expert witness testimony for Southern Insurance, Beytin McLaughlin, and Wagstaff. She has received payment from BriannaCope, StockmanConnor, and McBridehall. She also has two provisional patents unrelated to this work:421/518 PCT; U.S. Provisional No. 63/322,886; DEC POA UNC 19-0083 U.S. 17/769,042 (Our Ref. 421/465 PCT/US). Evan R. Myers disclosed receiving payment from Merck for HPV vaccination and Moderna for CMV vaccination. He has received payment from AbbVie, Inc. and Hologic, Inc. (cervical cancer screening). Til Stürmer receives investigator-initiated research funding and support as Principal Investigator (R01AG056479) from the National Institute of Aging (NIA). He owns stock in Novartis and Roche and received payment from Novo Nordisk. The other authors did not report any potential conflicts of interest.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D191.
REFERENCES
- 1.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black–White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health 2009;99:300–7. doi: 10.2105/AJPH.2008.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss G, Noorhasan D, Schott LL, Powell L, Randolph JF, Jr., Johnston JM. Racial differences in women who have a hysterectomy for benign conditions. Womens Health Issues 2009;19:202–10. doi: 10.1016/j.whi.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer JR, Rao RS, Adams-Campbell LL, Rosenberg L. Correlates of hysterectomy among African-American women. Am J Epidemiol 1999;150:1309–15. doi: 10.1093/oxfordjournals.aje.a009962 [DOI] [PubMed] [Google Scholar]
- 4.Gartner DR, Delamater PL, Hummer RA, Lund JL, Pence BW, Robinson WR. Patterns of Black and White hysterectomy incidence among reproductive aged women. Health Serv Res 2021;56:847–53. doi: 10.1111/1475-6773.13633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gartner DR, Delamater PL, Hummer RA, Lund JL, Pence BW, Robinson WR. Integrating surveillance data to estimate race/ethnicity-specific hysterectomy inequalities among reproductive-aged women: who's at risk? Epidemiology 2020;31:385–92. doi: 10.1097/EDE.0000000000001171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson WR, Cheng MM, Howard AG, Carpenter WR, Brewster WR, Doll KM. For U.S. Black women, shift of hysterectomy to outpatient settings may have lagged behind White women: a claims-based analysis, 2011–2013. BMC Health Serv Res 2017;17:526. doi: 10.1186/s12913-017-2471-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choosing the route of hysterectomy for benign disease. Committee Opinion No. 701. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e155–9. doi: 10.1097/AOG.0000000000002112 [DOI] [PubMed] [Google Scholar]
- 8.Stern AM. STERILIZED in the name of public health. Am J Public Health 2005;95:1128–38. doi: 10.2105/AJPH.2004.041608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouche T, Rivard L. America's hidden history: the eugenics movement. Accessed August 29, 2022. https://www.nature.com/scitable/forums/genetics-generation/america-s-hidden-history-the-eugenics-movement-123919444/
- 10.BBC News. ICE whistleblower: nurse alleges “hysterectomies on immigrant women in US”. Accessed March 20, 2023. https://www.bbc.com/news/world-us-canada-54160638
- 11.Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol 2016;214:31–44. doi: 10.1016/j.ajog.2015.07.044 [DOI] [PubMed] [Google Scholar]
- 12.Lawson EH, Gibbons MM, Ingraham AM, Shekelle PG, Ko CY. Appropriateness criteria to assess variations in surgical procedure use in the United States. Arch Surg 2011;146:1433–40. doi: 10.1001/archsurg.2011.581 [DOI] [PubMed] [Google Scholar]
- 13.Corona LE, Swenson CW, Sheetz KH, Shelby G, Berger MB, Pearlman MD, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol 2015;212:304.e1–7. doi: 10.1016/j.ajog.2014.11.031 [DOI] [PubMed] [Google Scholar]
- 14.Harvey SV, Pfeiffer RM, Landy R, Wentzensen N, Clarke MA. Trends and predictors of hysterectomy prevalence among women in the United States. Am J Obstet Gynecol 227, 2022:482–3. doi: 10.1016/j.ajog.2022.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClurg A, Wong J, Louie M. The impact of race on hysterectomy for benign indications. Curr Opin Obstet Gynecol 2020;32:263–8. doi: 10.1097/GCO.0000000000000633 [DOI] [PubMed] [Google Scholar]
- 16.Dedden SJ, Geomini PM, Huirne JA, Bongers MY. Vaginal and Laparoscopic hysterectomy as an outpatient procedure: a systematic review - PubMed. Eur J Obstet Gynecol Reprod Biol 216;212-23. doi: 10.1016/j.ejogrb.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 17.Sandberg EM, Twijnstra ARH, Driessen SRC, Jansen FW. Total laparoscopic hysterectomy versus vaginal hysterectomy: a systematic review and meta-analysis. J Minimally Invasive Gynecol 2017;24:206–17.e22. doi: 10.1016/j.jmig.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 18.Goodin A, Delcher C, Valenzuela C, Wang X, Zhu Y, Roussos-Ross D, et al. The power and pitfalls of big data research in obstetrics and gynecology: a consumer's guide. Obstet Gynecol Surv 2017;72:669–82. doi: 10.1097/OGX.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Encyclopaedia Britannica. The South: definition, states, & history. Accessed March 24, 2023. https://www.britannica.com/place/the-South-region
- 20.Doll KM, Howard AG, Stürmer T, Carey T, Nicholson WK, Carey E, et al. Development of an algorithm to assess unmeasured symptom severity in gynecologic care. Am J Obstet Gynecol 2022;226:388.e1–11. doi: 10.1016/j.ajog.2021.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alson JG, Anderson LG, Robinson WR, Myers ER, Carey ET, Doll KM. CHC aim1-2 electronic medical record REDCap abstraction instrument. Accessed February 8, 2023. https://osf.io/fuwd5/
- 22.Alson JG, Anderson LG, Robinson WR, Myers ER, Carey ET, Doll KM. CHC aim 1-2 electronic medical record abstraction codebook. Accessed February 8, 2023. https://osf.io/6qfv5/
- 23.Alson JG, Anderson LG, Robinson WR, Myers ER, Carey ET, Doll KM. CHC aim 1-2 electronic medical record abstraction protocol. Accessed February 7, 2023. https://osf.io/fn45c/
- 24.General Assembly of North Carolina. An act to improve the collection and reporting of race and ethnicity data to public health officials and to the statewide data processor. Accessed August 29, 2022. http://www.ncleg.net/Sessions/2007/Bills/Senate/PDF/S4v5.pdf
- 25.Striving for diversity in research studies. N Engl J Med 2021;385:1429–30. doi: 10.1056/NEJMe2114651 [DOI] [PubMed] [Google Scholar]
- 26.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology 2014;25:473–84. doi: 10.1097/EDE.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. HealthAffairs July 2, 2020. doi: 10.1377/forefront.20200630.939347 [DOI]
- 28.Goldin Evans M, Broyles S, Frederiksen B, Long-acting reversible contraceptive utilization after policy change increasing device reimbursement to wholesale acquisition cost in Louisiana. Am J Obstet Gynecol 2019;221:128.e1–10. doi: 10.1016/j.ajog.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 29.Dehlendorf C, Park SY, Emeremni CA, Comer D, Vincett K, Borrero S. Racial/ethnic disparities in contraceptive use: variation by age and women's reproductive experiences. Am J Obstet Gynecol 2014;210:526.e1–9. doi: 10.1016/j.ajog.2014.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conspiracy beliefs about birth control: barriers to pregnancy prevention among African Americans of reproductive age. Health Educ Behav 32:474–87. doi: 10.1177/1090198105276220 [DOI] [PubMed] [Google Scholar]
- 31.Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, prevalence, and treatment of uterine fibroids: a survey of U.S. women. J Women's Health 2018;27:1359–67. doi: 10.1089/jwh.2018.7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.State Center for Health Statistics and Office of Minority Health and Health Disparities. North Carolina minority health facts: Hispanics/Latinos. Accessed August 31, 2022. https://schs.dph.ncdhhs.gov/schs/pdf/Hispanic_FS_WEB_080210.pdf
- 33.Mende-Siedlecki P, Qu-Lee J, Backer R, Van Bavel JJ. Perceptual contributions to racial bias in pain recognition. J Exp Psychol Gen 2019;148:863–89. doi: 10.1037/xge0000600 [DOI] [PubMed] [Google Scholar]
- 34.Meints SM, Cortes A, Morais CA, Edwards RR. Racial and ethnic differences in the experience and treatment of noncancer pain. Pain Manag 2019;9:317–34. doi: 10.2217/pmt-2018-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morden NE, Chyn D, Wood A, Meara E. Racial inequality in prescription opioid receipt — role of individual health systems. N Engl J Med 2021;385:342–51. doi: 10.1056/NEJMsa2034159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cromeens MG, Carey ET, Robinson WR, Knafl K, Thoyre S. Timing, delays and pathways to diagnosis of endometriosis: a scoping review protocol. BMJ Open 2021;11:e049390. doi: 10.1136/bmjopen-2021-049390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol 2010;202:514–21. doi: 10.1016/j.ajog.2010.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton M, Anachebe N, Lee R, Skanes H. Racial and ethnic disparities in reproductive health services and outcomes, 2020. Obstet Gynecol 2021;137:225–33. doi: 10.1097/AOG.0000000000004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schminkey DL, Liu X, Annan S, Sawin EM. Contributors to health inequities in rural Latinas of childbearing age: an integrative review using an ecological framework. SAGE Open 2019;9:215824401882307. doi: 10.1177/2158244018823077 [DOI] [Google Scholar]
- 40.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance-United States, 1994–1999. MMWR Morb Mortal Wkly Rep 2002;51:1–8. [Google Scholar]
- 41.Farland L, Horne A. Disparity in endometriosis diagnoses between racial/ethnic groups. BJOG 2019;126:1115–6. doi: 10.1111/1471-0528.15805 [DOI] [PMC free article] [PubMed] [Google Scholar]