PURPOSE
Compared with people living without HIV (PWOH), people living with HIV (PWH) and cancer have traditionally been excluded from immune checkpoint inhibitor (ICI) trials. Furthermore, there is a paucity of real-world data on the use of ICIs in PWH and cancer.
METHODS
This retrospective study included PWH treated with anti–PD-1- or anti–PD-L1-based therapies for advanced cancers. Kaplan-Meier method was used to estimate overall survival (OS) and progression-free survival (PFS). Objective response rates (ORRs) were measured per RECIST 1.1 or other tumor-specific criteria, whenever feasible. Restricted mean survival time (RMST) was used to compare OS and PFS between matched PWH and PWOH with metastatic NSCLC (mNSCLC).
RESULTS
Among 390 PWH, median age was 58 years, 85% (n = 331) were males, 36% (n = 138) were Black; 70% (n = 274) received anti–PD-1/anti–PD-L1 monotherapy. Most common cancers were NSCLC (28%, n = 111), hepatocellular carcinoma ([HCC]; 11%, n = 44), and head and neck squamous cell carcinoma (HNSCC; 10%, n = 39). Seventy percent (152/216) had CD4+ T cell counts ≥200 cells/µL, and 94% (179/190) had HIV viral load <400 copies/mL. Twenty percent (79/390) had any grade immune-related adverse events (irAEs) and 7.7% (30/390) had grade ≥3 irAEs. ORRs were 69% (nonmelanoma skin cancer), 31% (NSCLC), 16% (HCC), and 11% (HNSCC). In the matched mNSCLC cohort (61 PWH v 110 PWOH), 20% (12/61) PWH and 22% (24/110) PWOH had irAEs. Adjusted 42-month RMST difference was –0.06 months (95% CI, –5.49 to 5.37; P = .98) for PFS and 2.23 months (95% CI, –4.02 to 8.48; P = .48) for OS.
CONCLUSION
Among PWH, ICIs demonstrated differential activity across cancer types with no excess toxicity. Safety and activity of ICIs were similar between matched cohorts of PWH and PWOH with mNSCLC.
CATCH-IT consortium study by @talalzarif1 @aminnassarMD @adlib_elio @thenasheffect et al demonstrates #ICI safety and activity across cancer types in people with #HIV. Also, similar outcomes in a matched cohort of people with and without HIV with metNSCL.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have successfully shifted the treatment paradigm in oncology and received regulatory approval for a broad spectrum of tumor types.1-6 People living with HIV (PWH) remain at higher risk than people living without HIV (PWOH) for developing various cancers where ICIs are the standard of care, including lung cancer, the second leading cause of cancer deaths in PWH.7-10
CONTEXT
Key Objective
Determine safety and activity of immune checkpoint inhibitors (ICIs) among people living with HIV (PWH) and cancer.
Knowledge Generated
Three hundred and ninety PWH received ICIs while on antiretroviral therapy (ART), including 30% PWH with baseline CD4+ T-cell counts <200 cells/µL. ICIs were deemed safe and had differential activity across tumor types. Among PWH with non–small-cell lung cancer (NSCLC), clinical outcomes were not generally influenced by CD4+ T-cell counts or ART regimens. In a subset of PWH with metastatic NSCLC, the safety and activity of ICIs were comparable with a matched cohort of people living without HIV after matching for relevant clinical variables at the same institution.
Relevance (G.K. Schwartz)
-
General concerns have persisted on the safety of ICIs in patients with cancer and HIV. This study should reassure physicians that the use of ICIs is safe and effective in this patient population, even for those on ART.*
*Relevance section written by JCO Associate Editor Gary K. Schwartz, MD, FASCO.
Since PWH may have dysfunctional immune systems, there have been safety and efficacy concerns of including them in clinical trials with ICIs. Consequently, these studies have either entirely excluded PWH or limited their participation to specific inclusion criteria such as baseline CD4+ T-cell counts ≥350 cells/μL, HIV viral load (VL) <400 copies/mL, and adherence to antiretroviral therapy (ART).11 However, recent clinical trials and retrospective studies that included PWH demonstrated that ICIs were active and safe for PWH, although these observations were hampered by small sample sizes and heterogeneous tumor types.11-21
Given the potential benefit of ICIs in PWH and cancer,22 larger real-world cohorts are needed to address the existing knowledge gaps, guide clinical decision making, and increase therapeutic opportunities for PWH. Herein, we implement a grassroots effort and assemble a real-world international cohort of PWH who received ICIs for advanced cancers. We evaluate the safety and activity of ICIs in PWH with cancer. Additionally, we compare the clinical outcomes for metastatic NSCLC (mNSCLC) among a subset of PWH who were matched to a cohort of PWOH.
METHODS
Study Design
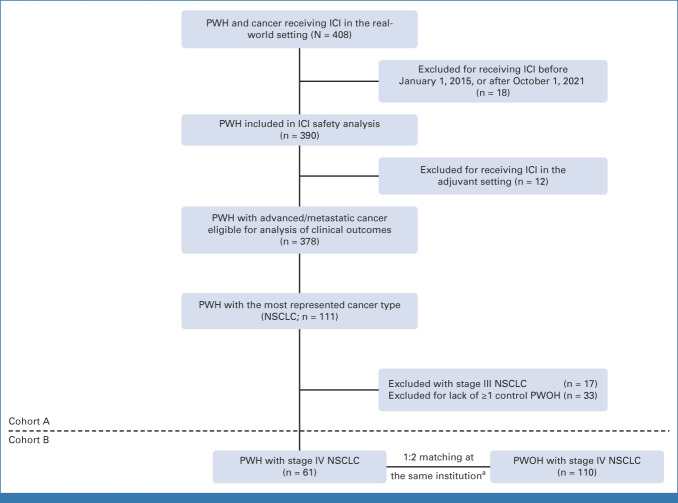
This study is based on an analysis of a retrospective, multicenter database of the Cancer Therapy using Checkpoint inhibitors in PWH-International (CATCH-IT) consortium. Data from 33 participating institutions across the United States, Europe, and Australia were obtained and are currently housed and maintained at the Dana-Farber Cancer Institute (DFCI; Data Supplement [Fig 1], online only). This study is approved by the institutional review boards at DFCI and participating centers per institutional policy and the Declaration of Helsinki. Since this was a retrospective study and involved deidentified information, individual patient consent was waived at the respective institutions.
Cohort A: Inclusion Criteria
Medical charts for PWH who received ICIs and had a diagnosis of HIV (International Classification of Diseases-10 codes Z21 or B20) were manually curated. PWH were included in the overall cohort A if they (1) had a laboratory-confirmed diagnosis of HIV, (2) had a biopsy-proven solid or hematologic malignancy, and (3) received ≥1 dose of ICI-based therapy between January 1, 2015, and October 1, 2021 (date of database lock). Baseline CD4+ T-cell counts, CD4:CD8 T-cell ratios, Hepatitis B virus, Hepatitis C virus, and HIV VLs were collected up to 3 months before ICI initiation. ICI regimens included anti–PD-1 or anti–PD-L1 monotherapy or in combination with anti–cytotoxic T-lymphocyte–associated antigen-4 (anti–CTLA-4) or other anticancer therapies (ie, chemotherapy or targeted agents such as tyrosine kinase inhibitors, antibody-drug conjugates, and antiangiogenic therapies). On the basis of institutional documentation, all PWH included in this study had received treatment in the real-world setting.
Cohort B: Matching Criteria for PWOH With Stage IV NSCLC
To evaluate the impact of HIV diagnosis on clinical outcomes, cohort B was assembled. PWH and PWOH with mNSCLC were matched at each participating institution in a 1:2 or 1:1 ratio, depending on the feasibility of finding appropriately matched PWOH. Matching was performed for all of the following variables: (1) 10-year age groups (41-50, 51-60, 61-70, and 71-80 years), (2) sex, (3) class of ICI, (4) use of concurrent chemotherapy (yes/no), and (5) number of lines of systemic therapy before ICI initiation. To detect a hazard ratio of 1.64 on progression or death using a two-sided alpha of .05 and a power of 80%, the probability of a progression-free survival (PFS) event was assumed to be equal to 0.8 and a required sample size of 180 (60 PWH:120 PWOH) was calculated. Accordingly, cohort B included a subset of 61 PWH with mNSCLC from cohort A and 110 matched PWOH across 15 institutions. A flowchart of the study is represented in Figure 1.
FIG 1.
Flowchart of the study design. aMatched variables are sex, age group, ICI class, use of chemotherapy, and number of lines of prior systemic therapy. ICI, immune checkpoint inhibitor; NSCLC, non–small-cell lung cancer; PWH, people living with HIV; PWOH, people living without HIV.
Clinical Outcomes and Toxicity Profiles
The primary end point of this study was overall survival (OS), defined as the date of ICI initiation to death or censored at the date of last follow-up. The secondary end points were (1) immune-related adverse events (irAEs), graded per the Common Terminology Criteria for Adverse Events version 5.0, and (2) PFS, the time from ICI initiation to radiologic or clinical disease progression, death, or censored on the date of last follow-up. Objective response rates (ORRs) were measured either by the clinical investigator or whenever possible per the RECIST 1.1 criteria for solid tumors and the Lugano classification for Hodgkin lymphoma and non-Hodgkin lymphoma. The AIDS clinical trials group criteria for ORR and staging were used for Kaposi sarcoma (KS).23
Statistical Analysis
Cohort A
The cumulative incidence rates (CIRs) of irAEs at 24 weeks after ICI initiation were calculated after considering ICI discontinuation (ie, secondary to progression or other reasons) as a competing risk. Gray's test was used to compare the CIRs of irAEs between the groups stratified by baseline CD4+ T-cell counts and CD4:CD8 ratios, respectively. CD4+ T-cell counts and HIV VL at baseline and any time after the last dose of ICI.
For analyses of clinical outcomes (OS, PFS, and ORR), PWH with advanced-stage cancers (locally advanced/metastatic) were included and those receiving adjuvant or neoadjuvant ICI therapy were excluded (Fig 1). OS and PFS were estimated by the Kaplan-Meier method, and ORRs were calculated and presented as percentages for each cancer type with ≥10 PWH who were response evaluable. Since hepatocellular carcinoma (HCC) was the second most represented cancer type, PWH were stratified by Child-Pugh (CP) score subgroups, and comparisons were performed by log-rank tests for OS and PFS and the Fisher's exact test for ORR.
Cohort B
OS and PFS were calculated using the Kaplan-Meier method. Since there was evidence of nonproportionality on the basis of the log-minus-log plots, restricted mean survival time (RMST) was used instead of the Cox proportional hazards model to compare time-to-event outcomes between the two groups. RMST is the area under the survival curve corresponding to the average event-free time up to a prespecified time point.24,25 In our analysis, RMST was determined by integrating the Kaplan-Meier curves up to 42 months after ICI initiation because of the limited number of PWH or PWOH beyond that time point (n < 5). To further estimate the effect of HIV status on RMST, a linear regression model was fitted for OS and PFS using RMST as the outcome and HIV infection as a predictor after adjusting for race, Eastern Cooperative Oncology Group performance status (ECOG-PS), histology, smoking status, and PD-L1 expression.
RESULTS
Characteristics of PWH Receiving ICIs in Cohort A
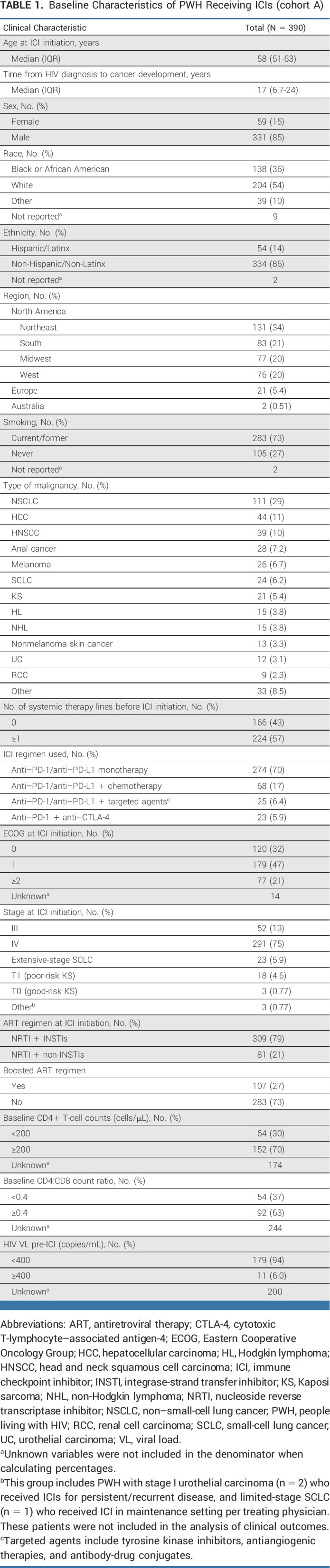
In total, 390 PWH treated with ICIs for different cancer types were included in cohort A (Data Supplement [Table 1 and Fig 2]). The median age in cohort A was 58 years (IQR, 51-63), and 85% (331/390) were males. Black/African American (race) and Hispanic/Latinx (ethnicity) PWH constituted 36% (138/381) and 14% (54/378) of the cohort, respectively. All PWH received ≥1 dose of ICI, with 70% (274/390) receiving anti–PD-1/anti–PD-L1 monotherapy (Table 1). Overall, 70% (152/216) had a CD4+ T-cell count ≥200 cells/µL, while 63% (92/146) had CD4:CD8 ratio ≥0.4. HIV was well controlled (HIV VL <400 copies/mL) in 94% (179/190). All PWH were on ART before ICI initiation, with 79% (309/390) on an integrase-strand transfer inhibitor (INSTI) as part of their ART regimen (Table 1).
TABLE 1.
Baseline Characteristics of PWH Receiving ICIs (cohort A)
Safety and Impact of ICIs on HIV VL and CD4+ T Cells in Cohort A
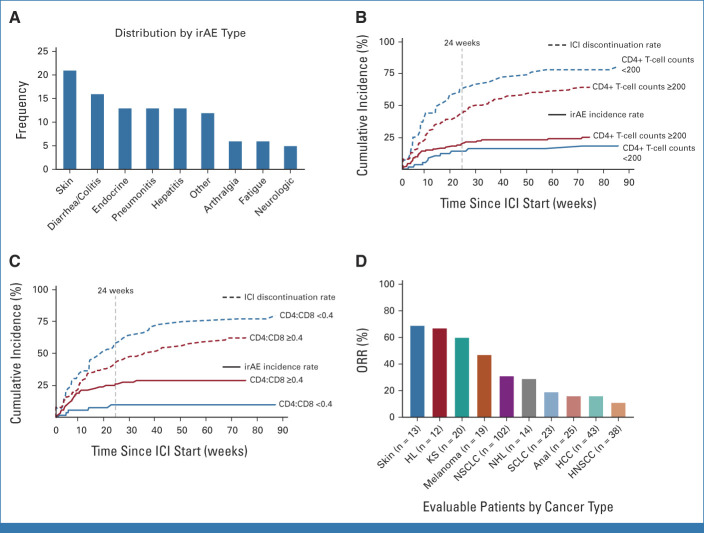
The incidence of irAEs of any grade was 20% (79/390). The distribution of irAEs is shown in Figure 2A and the Data Supplement (Table 2). The median time of irAE onset was 8.1 weeks in 91% (72/79) PWH with available data, and the 24-week CIR of any grade irAEs was 15% (95% CI, 12 to 19). All 20 PWH with available HIV VL at irAE onset had undetectable HIV VL. Overall, grade ≥3 irAEs occurred in 7.7% (30/390) PWH, and 7.4% (29/390) required hospitalization. The most common grade ≥3 irAEs were colitis/diarrhea or pneumonitis, each occurring in 1.5% (6/390) of PWH. At the time of grade ≥3 irAEs, CD4+ T-cell counts were available for 47% (14/30) PWH with a median of 286 (IQR, 244-344) cells/µL. Steroids were administered in 49% (39/79) PWH with irAEs, where 54% (21/39) received high-dose glucocorticoids (≥1 mg/kg). Only one person required additional immunosuppression with mycophenolate mofetil. The main reasons for treatment discontinuation were tumor progression or death from cancer in 70% (233/334) and irAEs in 12% (39/334) PWH. Death was attributed to ICIs in one person presenting with a grade 5 pneumonitis 16 days after receiving one dose of nivolumab for SCLC.
FIG 2.
Safety profiles and activity of ICIs among PWH. (A) irAEs by type; cumulative incidence of irAEs among (B) CD4+ T-cell counts and (C) CD4:CD8 ratio subgroups; and (D) ORR across top 10 cancer types. HCC, hepatocellular carcinoma; HL, Hodgkin lymphoma; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; KS, Kaposi sarcoma; NHL, non-Hodgkin lymphoma; NSCLC, non–small-cell lung cancer; ORR, objective response rate; PWH, people living with HIV; SCLC, small-cell lung cancer.
Among 274 PWH who received ICI monotherapy, 55 (20%) developed any grade irAEs, and 22 (8.0%) developed grade ≥3 irAE. In patients who received ICI with chemotherapy, 19% (13/68) developed any grade irAEs and 5.9% (4/68) developed grade ≥3 irAEs. Any grade irAEs and grade ≥3 irAEs were identified in 8.0% (2/25) and 4.0% (1/25) of patients treated with ICI plus targeted agents, respectively; and 39% (9/23) and 13% (3/23) with dual ICI therapy (anti–PD-1 + anti–CTLA-4), respectively (Data Supplement [Table 2]).
Among PWH with baseline CD4+ T-cell counts <200 cells/µL (n = 64), any grade irAEs occurred in 16% (10/64), with 7.8% (5/64) being grade ≥3. The most common irAEs were pneumonitis and endocrine irAEs, occurring in 4.7% (n = 3) each. Among PWH with CD4+ T-cell counts ≥200 cells/µL (n = 152), irAEs occurred in 24% (37/152), with 9.9% (15/152) being grade ≥3. Cutaneous irAEs were the most common, occurring in 6.6% (10/152) PWH (Data Supplement [Table 2]). Additionally, the incidence of any grade irAEs was 9.3% (5/54) and 30% (28/92) in PWH with CD4:CD8 ratio <0.4 and ≥0.4, respectively (Data Supplement [Table 2]). The difference in the CIR of any grade irAEs was not statistically significant between PWH with CD4+ T-cell counts <200 cells/µL versus ≥200 cells/µL (24-week CIR; 16% [95% CI, 7 to 26] v 20% [95% CI, 13 to 27]; Gray's test: P = .22; Fig 2B); however, it was significantly lower among PWH with baseline CD4:CD8 ratio <0.4 versus ≥0.4 (CIR, 10% [95% CI, 4 to 20] v 26% [95% CI, 17 to 36]; Gray's test: P = .01; Fig 2C) after accounting for the competing risk of treatment discontinuation.
For PWH with data at baseline and after ICI initiation, there were no significant changes in CD4+ T-cell counts (n = 74; P = .19) or HIV VL (n = 107; P = .97) during treatment (Data Supplement [Fig 3]). Furthermore, since data on the use of anti–CTLA-4 among PWH is scarce, we tracked the evolution of the HIV VL among 43% (10/23) PWH who received the combination of nivolumab and ipilimumab and had ≥1 HIV VL datapoint before or after ICI initiation. In this subset, 70% (7/10) patients had undetectable VL throughout treatment, whereas three PWH had transient elevations in HIV VL peaking at 63 copies/mL (Data Supplement [Fig 4]).
Finally, six PWH had an active opportunistic infection treated at ICI initiation which did not worsen during treatment (Data Supplement [Table 3]). Only one patient with HCC developed herpes zoster during ICI treatment.
Clinical Outcomes of PWH in Cohort A
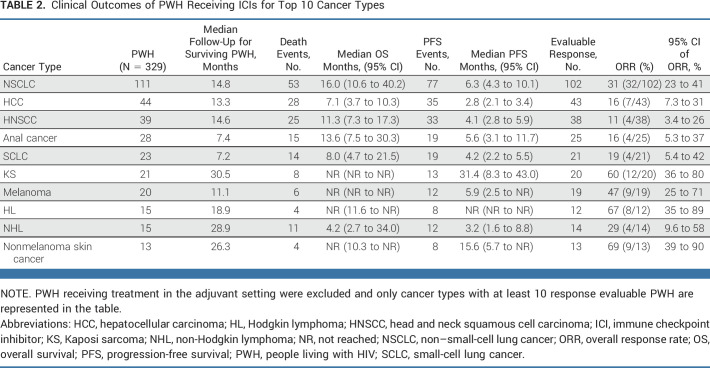
The most represented cancer type was NSCLC (28%; n = 111), with 51% (56/111) PWH receiving ICI in ≥second-line setting and 36% (40/111) on combination ICI and chemotherapy. The ORR of PWH with NSCLC was 31% (Table 2; Fig 2D), with ORR = 38% versus 25% in the first-line versus ≥second-line settings (P = .063; Data Supplement [Table 4]). Among 67 PWH with NSCLC and available baseline CD4+ T-cell counts data, the median CD4+ T-cell counts was 314 cells/µL (IQR, 206-472; Data Supplement [Table 5]), whereas, among 57 PWH with available VL, 96% (n = 55) had VL <400 copies/mL. There was no significant difference in OS (P = .88) or PFS (P = .72) between PWH with NSCLC presenting with baseline CD4+ T-cell counts ≥200 versus <200 cells/µL (Data Supplement [Fig 5]). Additionally, there were no significant differences in OS (P = .90) or PFS (P = .44) among PWH with NSCLC receiving INSTI (n = 83) or not (n = 28; Data Supplement [Fig 6]). The ORRs in these subgroups were 33% (25/76) versus 27% (7/26), respectively (P = .63).
TABLE 2.
Clinical Outcomes of PWH Receiving ICIs for Top 10 Cancer Types
The second most represented cancer type in cohort A was locally advanced or metastatic HCC (Data Supplement [Table 6]), which included 44 PWH: 75% (33/44) received nivolumab, and 25% (11/44) received atezolizumab ± bevacizumab. ICI treatment was used in the first-line setting in 55% (24/44) of PWH. At baseline, 61% (26/43) presented with CP A (CP-A), 77% (34/44) had Barcelona Clinic Liver Criteria (BCLC) stage C, and 46% (20/44) had an ECOG-PS of 0. Prolonged OS (P = .02) and PFS (P < .001) were observed for PWH with CP-A versus CP-B (Data Supplement [Fig 7 and Table 7]). The ORR of CP-A and CP-B subgroups were 24% (95% CI, 10 to 46) and 6.3% (95% CI, 0.33 to 32), respectively (P = .13).
For the rest of cohort A, the tumor types with the highest ORR to ICI therapy were nonmelanoma skin cancer (ORR, 69%; n = 9/13), KS (ORR, 60%; n = 12/20), and melanoma (ORR, 47%; n = 9/19; Table 2; Fig 2D). By contrast, PWH with HNSCC had the lowest ORR of 11% (n = 4/38). Furthermore, PWH with the three most represented cancer types (NSCLC, HCC, and HNSCC) treated with ICIs in the first-line setting had numerically higher ORRs versus ≥second-line setting (Data Supplement [Table 4]).
Clinical Outcomes and Toxicity Profiles: Matched Cohort B
Since NSCLC was the most represented cancer type and a powered analysis was feasible, we next sought to better characterize the safety and activity of ICIs in PWH compared with a matched control group of PWOH as a proof of principle. We matched 61 PWH to 110 PWOH treated with ICIs for mNSCLC in cohort B. Notably, the PWH cohort was over-represented with Black individuals and those with a positive PD-L1 tumor proportion score (TPS; Table 3). Comparing PWH versus PWOH and after controlling for race, ECOG-PS, histology, smoking status, and PD-L1 TPS, the adjusted RMST difference within 42 months was 2.23 months (95% CI, –4.0 to 8.5; P = .48) for OS and –0.06 months (95% CI, –5.5 to 5.4; P = .98) for PFS (Fig 3 and Data Supplement [Tables 8 and 9]). In addition, the 24-month OS rates were 42.3% for PWH versus 41.5% for PWOH, whereas the 24-month PFS rates were 17.8% PWH versus 18.4% PWOH. The ORR was similar between both groups (28% PWH v 36% PWOH; P = .31).
TABLE 3.
Baseline Demographic and Clinical Characteristics of PWH With mNSCLC by HIV Status (cohort B)
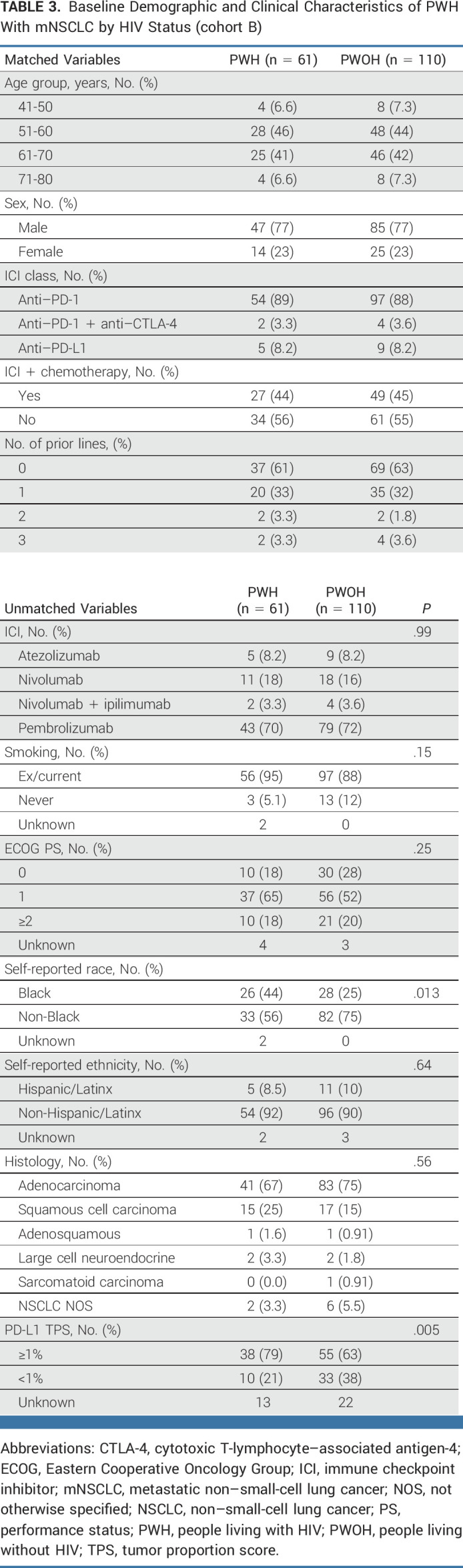
FIG 3.
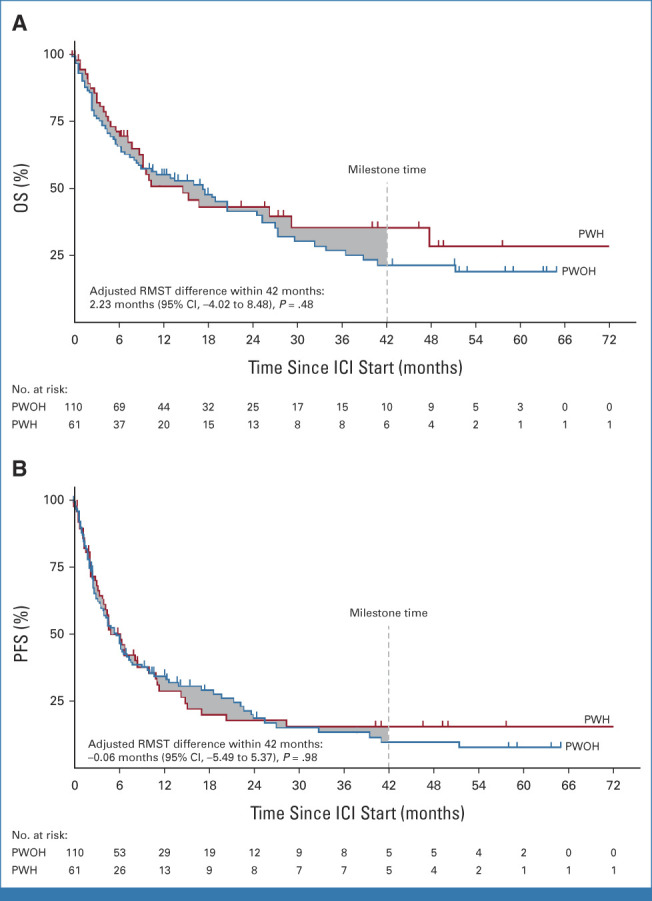
Kaplan-Meier analysis of (A) OS and (B) PFS between PWH and PWOH with mNSCLC. The gray area corresponds to the difference in the areas under the survival curves or the RMST up to 42 months after ICI initiation (milestone time) where the number of PWH or PWOH is ≥5. ICI, immune checkpoint inhibitor; mNSCLC, metastatic non–small-cell lung cancer; OS, overall survival; PFS, progression-free survival; PWH, people living with HIV; PWOH, people living without HIV; RMST, restricted mean survival time.
The incidence of irAEs was comparable among matched PWH and PWOH, where any grade irAEs occurred in 20% (12/61) versus 22% (24/110), respectively. Additionally, grade ≥3 irAEs occurred in 12% (7/61) PWH versus 9.1% (10/110) PWOH, and systemic steroids were required for 9.8% (6/61) and 15% (16/110) of PWH and PWOH, respectively (Data Supplement [Table 10]).
DISCUSSION
Although recent advocacy efforts have successfully increased the enrollment of PWH in clinical trials,26-28 PWH have been historically excluded from the majority of the trials, including those with ICIs.6,29-35
In this large real-world cohort of PWH receiving ICIs for various cancer types, we build on prior efforts and demonstrate that PWH have no excess or unexpected treatment-related immune toxicities compared with historical and matched controls without HIV. In addition, our study adds a breadth of safety data to recently reported clinical trials of ICIs in PWH that had considerably smaller sample sizes.19-21 In our cohort, we overcame some of these limitations by including PWH with >10 distinct cancer types, baseline CD4+ T-cell counts <200 cells/μL (30%; n = 64/216), baseline HIV VL ≥400 (5.8%; n = 11/190), or opportunistic infections (n = 6). Our data also support the extension of the safety signal to treatment regimens that remain unexplored in prospective studies, such as the combination of ICIs with chemotherapy or targeted agents. Furthermore, we show that CD4+ T-cell counts remained stable with modest changes in HIV VL below the clinical significance threshold (<400 copies/mL) even when PWH received the combination of anti–PD-1 and anti–CTLA-4 therapy. These data provide real-world evidence and support the findings of the currently ongoing AIDS Malignancy Consortium 095 clinical trial where there was only a modest increase in plasma HIV RNA in PWH receiving the combination of nivolumab and ipilimumab.36
Notably, our sample size allowed for examining the rate of irAEs in PWH with CD4+ T-cell counts <200 cells/μL, a subset that is also poorly represented in prior ICI-based trials, yet constituted 30% of our cohort.19,20 We showed that the 24-month incidence rate of irAEs of any grade in this group is comparable with PWH with CD4+ T-cell counts >200 cells/μL. Similar findings have been reported by Odeny et al,17 where the effect of baseline CD4+ T-cell counts on the incidence of treatment-emergent adverse events was not modified by HIV status. These data further endorse the need to abrogate arbitrary CD4 cutoffs when using ICIs in the appropriate setting for the treatment of PWH and subsequently reduce barriers to ICI access on the basis of their favorable benefit-to-risk profiles. Furthermore, we showed that when accounting for the competing risk of treatment discontinuation, PWH with CD4:CD8 >0.4 had higher incidence of irAEs, which may be driven by the presence of abundant circulating CD4+ T cell counts relative to CD8+ T cells, as has been shown by Lozano et al37 among patients developing severe irAEs after ICI treatment. Nevertheless, validation of these findings in ongoing ICI clinical trials among PWH is warranted.
Correlative efforts from Chaudhary et al38 recently demonstrated that PWH have higher levels of circulating dysfunctional T cells and are associated with cancer development and progression, even when on ART with viral suppression. However, it is unclear whether these findings may have an impact on outcomes of PWH to immune-modulating agents compared with PWOH. In our robustly matched cohort of PWH, who were on ART, and PWOH with mNSCLC, we showed that both groups had comparable OS, PFS, and ORRs. Similarly, ORRs across other cancers followed the general trends seen in PWOH, wherein skin, melanoma, and NSCLC had higher ORRs than other tumor types.4,6,32,34,39 Despite that, the heterogeneity in response to ICI treatment and the modifying effect of HIV infection on clinical outcomes in different tumors warrant further exploration through prospective studies with robust translational correlatives.40
Overall, these results add to the growing body of evidence supporting the use of ICIs among PWH to enhance their inclusion in ICI clinical studies. Therefore, similar to our matched cohort analysis in mNSCLC, future studies comparing large, matched cohorts of PWH and other cancer types are warranted for formal comparisons with the general population.
There are several limitations to this retrospective study. First, we were unable to monitor trends in HIV VL to assess the impact of ICIs on the HIV reservoir.41 This is partially explained by the infrequent VL testing by oncologists in a real-world setting, highlighting the importance of a multidisciplinary approach that includes infectious disease specialists for this unique patient population. Second, missing data on some tumor-specific biomarkers (ie, PD-L1 status and tumor mutational burden) might have influenced our findings, particularly in the matched mNSCLC cohort. Third, response evaluations were a mixture of objective response assessments (ie, RECIST v1.1) and investigator-based evaluations, which may affect the categorization in a subset of PWH in our cohort into the appropriate response groups. Finally, the lower incidence of irAEs may be mainly due to the treatment of PWH outside of clinical trials, treatment discontinuation because of progression, and suboptimal capture of events. Additionally, PWH with missing baseline CD4+ T-cell counts and CD4:CD8 ratio were excluded from subset analyses of irAEs, which might have introduced bias to our analyses. Nonetheless, this is unlikely to affect reporting of higher-grade irAEs (grade ≥3) that usually influence the course of treatment.
The unique strength of our study lies in the broad and diverse data of this one-of-a-kind international registry within the CATCH-IT consortium that included PWH treated with ICIs for several cancer types. Our registry offers a unique opportunity for additional real-world subanalyses of clinical outcomes of PWH receiving ICIs that could guide oncologists treating this unique population as we await results from ongoing ICI clinical trials.42,43
ACKNOWLEDGMENT
The authors thank patient advocate Mr David Palm who has provided relevant insights related to HIV and cancer management from a patient perspective. The authors are also grateful to all the participating PWH and their families whose contributions have made this study possible. Finally, the authors thank the CATCH-IT consortium investigators and staff members at the participating centers who have contributed to our work.
Elio Adib
Employment: Amgen
Tarek H. Mouhieddine
Consulting or Advisory Role: Legend Biotech
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, Aveo
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Arjun Mittra
Stock and Other Ownership Interests: Merck
Dwight H. Owen
Research Funding: Bristol Myers Squibb (Inst), Palobiofarma (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Onc.AI (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Genentech
Robert A. Baiocchi
Consulting or Advisory Role: Viracta Therapeutics, Prelude Therapeutics, Atara Biotherapeutics
Research Funding: Prelude Therapeutics, Esanex, Codiak Biosciences
Christopher Dittus
Research Funding: Seagen, AstraZeneca, Genentech
Patents, Royalties, Other Intellectual Property: Royalty payment from Springer Publishing for a medical textbook publication
Nazli Dizman
Consulting or Advisory Role: Vivreon Biosciences
Noha Abdel-Wahab
Honoraria: ChemoCentryx
Consulting or Advisory Role: ChemoCentryx
Adi Diab
Honoraria: Array BioPharma
Consulting or Advisory Role: Nektar, CureVac, Celgene, Idera, Memgen Therapeutics, Regeneron, CytomX Therapeutics, Pfizer (Inst)
Research Funding: Nektar (Inst), Idera (Inst), Celgene (Inst), Pfizer (Inst), Apexigen (Inst), Lytix Biopharma (Inst)
Travel, Accommodations, Expenses: Nektar
Chul Kim
Consulting or Advisory Role: Janssen, Novartis, PierianDx, AstraZeneca, Sanofi, Diffusion Pharmaceuticals, Mirati Therapeutics, Jazz Pharmaceuticals, Arcus Biosciences, Daiichi Sankyo, Eisai
Research Funding: AstraZeneca, Novartis, Karyopharm Therapeutics, Bristol Myers Squibb, Regeneron, Debiopharm Group, Janssen, Genentech/Roche, Daiichi Sankyo
Neil J. Shah
Honoraria: MJH Life Sciences, MedNet, LLC
Consulting or Advisory Role: Merck Sharp & Dohme Corp, Exelixis, Graticule
Research Funding: Aravive (Inst), Exelixis
Travel, Accommodations, Expenses: Merck
Anwaar Saeed
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis, Pfizer, Five Prime Therapeutics, Daiichi Sankyo/Astra Zeneca
Research Funding: AstraZeneca/MedImmune (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Merck Sharp & Dohme (Inst), Five Prime Therapeutics (Inst), Astellas Pharma (Inst), Actuate Therapeutics (Inst), Seagen (Inst), Daiichi Sankyo/UCB Japan (Inst), KAHR Medical (Inst), Innovent Biologics (Inst)
Melissa G. Lechner
Patents, Royalties, Other Intellectual Property: Patent US09746480B2 Human myeloid derived suppressor cell cancer markers
Alexandra Drakaki
Employment: Athos Therapeutics, Attica Sciences, Dyania Health
Leadership: Athos Therapeutics, Attica Sciences
Stock and Other Ownership Interests: Urogen Pharma, Alimera Sciences, Kyn Therapeutics, Athos Therapeutics, Attica Sciences, Dyania Health, Moderna Therapeutics, Proteas Bioanalytics
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Radmetrix, Seagen, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, Dyania Health, Aveo
Research Funding: Kite/Gilead, AstraZeneca (Inst), Genentech/Roche (Inst), BMS (Inst), Merck Sharp & Dohme (Inst), Jounce Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Seattle Genetics/Astellas (Inst), Immunomedics/Gilead (Inst)
Patents, Royalties, Other Intellectual Property: My significant other has several patents with UCLA, Harvard Medical School, Athos Therapeutics, Proteas Bioanalytics, and ATTICA Sciences
Travel, Accommodations, Expenses: Lilly, AstraZeneca, Seagen
Javier Baena
Honoraria: AstraZeneca Spain, Bristol Myers Squibb/Pfizer
Consulting or Advisory Role: AstraZeneca Spain, Roche
Expert Testimony: Roche
Travel, Accommodations, Expenses: MSD
Michael A. Morse
Honoraria: Genentech/Roche, Novartis, Sanofi, Lexicon, Ipsen, Bayer, Taiho Pharmaceutical, Boehringer Ingelheim, Eisai, Merck, Exelixis, AstraZeneca/Daiichi Sankyo, Servier, Tersera, QED Therapeutics
Speakers' Bureau: Genentech/Roche, Taiho Pharmaceutical, Ipsen, Exelixis, Eisai, Servier, AstraZeneca
Research Funding: Precision Biologics (Inst), Bristol Myers Squibb (Inst), Onyx (Inst), Eisai (Inst), Lexicon (Inst), MedImmune (Inst), Advanced Accelerator Applications (Inst), AlphaVax (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Vaccines against antigens involved in therapy resistance and methods of using same, patent number: 9956276 Assignee Duke University, pharmaceutical product, medical food or dietary supplement for preventing cancer and inflammatory diseases, Publication number: 20170246136, Applicant: OliVentures, Inc, Inventors: Carlos María Peña Díaz, Guillermo Muñoz Fernández, Michael Morse, Compositions and methods for modulating and redirecting immune responses, Publication number: 20170015758, Inventors: Scott A. Hammond, Michael A. Morse, Takuya Osada, Herbert Kim Lyerly
Alessio Cortellini
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, MSD Oncology, OncoC4, IQvia
Speakers' Bureau: AstraZeneca, Eisai
David J. Pinato
Honoraria: Roche/Genentech, Bristol Myers Squibb, Da Volterra, Avammune, Mursla Bio, Starpharma, Lift Biosciences
Consulting or Advisory Role: Eisai, Mina Therapeutics, Roche, H3 Biomedicine, Da Volterra, AstraZeneca, Ipsen
Speakers' Bureau: Bayer, ViiV Healthcare, Falk Pharma, Roche
Research Funding: MSD Oncology (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Bayer, MSD Oncology
Other Relationship: Wiley
Evan Hall
Research Funding: Treatment Technologies and Insights (Inst), Neoleukin Therapeutics (Inst), ImCheck therapeutics (Inst), Nektar (Inst), Replimune (Inst), NiKang Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1368769
Nathan Bahary
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Incyte
Ankit Mangla
Honoraria: Targeted Oncology
Consulting or Advisory Role: SpringWorks Therapeutics
Research Funding: Nektar, Tracon Pharma, SpringWorks Therapeutics, Regeneron
Parminder Singh
Honoraria: Curio Science, Medpage/ASCO
Consulting or Advisory Role: Janssen Oncology, Seattle Genetics/Astellas (Inst), EMD Serono, Aveo, Bayer
Research Funding: EMD Serono (Inst)
Uncompensated Relationships: Seagen
Ryan W. Dobbs
Stock and Other Ownership Interests: Pfizer, United Health Group, Bristol Myers Squibb/Sanofi, Merck, Lilly, Medtronic, Johnson & Johnson/Janssen
Consulting or Advisory Role: Boston Scientific
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1392783
Pauline Funchain
Consulting or Advisory Role: Eisai, Novartis, GigaGen
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Taiho Oncology (Inst)
Georgina V. Long
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Agenus, Amgen, Array BioPharma, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion Biotech, Hexal, Highlight Therapeutics, Innovent Biologics, Merck Sharp & Dohme, Novartis, OncoSec, PHMR, Pierre Fabre, Provectus, QBiotics, Regeneron, AstraZeneca
Alexander M. Menzies
Consulting or Advisory Role: MSD Oncology, Novartis, Pierre Fabre, Bristol Myers Squibb, Roche, QBiotics
Carlo Genova
Honoraria: AstraZeneca, Amgen, Bristol Myers Squibb, Merck, Roche, Sanofi, Takeda
Consulting or Advisory Role: AstraZeneca, Amgen, Bristol Myers Squibb, Roche, Sanofi
Research Funding: Bristol Myers Squibb (Inst)
Sonam Puri
Honoraria: Aptitude Health (Inst), IntegrityCE
Consulting or Advisory Role: AstraZeneca (Inst), G1 Therapeutics (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Dava Oncology
Vaia Florou
Consulting or Advisory Role: Incyte, Deciphera
Maristella Saponara
Honoraria: Sanofi, Sun Pharma, BMS, MSD, Novartis, Pierre Fabre
Paola Queirolo
Consulting or Advisory Role: Roche/Genentech, Novartis, MSD, Bristol Myers Squibb, Pierre Fabre, Sanofi, Sun Pharma Advanced Research Company, Merck Serono
Travel, Accommodations, Expenses: MSD Oncology, Sanofi/Regeneron
Alfredo Addeo
Consulting or Advisory Role: Roche, AstraZeneca/MedImmune, Bristol Myers Squibb Foundation, MSD Oncology, Pfizer, Novartis, Astellas Pharma, Amgen, Lilly
Speakers' Bureau: Lilly, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Takeda
Melissa Bersanelli
Consulting or Advisory Role: Novartis, Pfizer, Ipsen, Sanofi, Merck
Speakers' Bureau: Bristol Myers Squibb, Novartis, Pfizer, AstraZeneca, Pierre Fabre, Ipsen
Research Funding: Seqirus (Inst), Roche (Inst), Pfizer (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Copyright transfer for scientific materials by Sciclone Pharmaceuticals (Cina), Copyright transfer for scientific materials by IPSEN, Copyright transfer for scientific materials by Pfizer, Copyright transfer for scientific materials by Sanofi, Copyright transfer for scientific materials by Pierre Fabre, Copyright transfer for scientific materials by MSD
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Pierre Fabre, Pfizer/EMD Serono, Ipsen, MSD Oncology
Wanling Xie
Consulting or Advisory Role: Convergent Therapeutics
Erin G. Reid
Employment: EpicentRx
Leadership: EpicentRx
Stock and Other Ownership Interests: EpicentRx
Research Funding: ADC Therapeutics (Inst), Aptose Biosciences (Inst), Millenium Pharamceuticals (Inst), Xencor (Inst)
Patents, Royalties, Other Intellectual Property: Husband hold multiple patents related to lymphedema management, oncolytic viral therapy
Open Payments Link: https://openpaymentsdata.cms.gov/physician/86601
Douglas B. Johnson
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Iovance Biotherapeutics, Catalyst Pharmaceuticals, Oncosec, Pfizer, Mosaic ImmunoEngineering, Targovax, Mallinckrodt
Research Funding: Incyte, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy
Ramya Ramaswami
Research Funding: Celgene (Inst), Genentech (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Merck Serono (Inst)
Mark Bower
Honoraria: ViiV Healthcare, Gilead Sciences, Bristol Myers Squibb, MSD, Janssen, EUSA Pharma
Brinda Emu
Stock and Other Ownership Interests: Bristol Myers Squibb Foundation
Consulting or Advisory Role: Theratechnologies
Thomas U. Marron
Consulting or Advisory Role: Regeneron, Boehringer Ingelheim, AstraZeneca, DBV Technologies, Celldex, Surface Oncology, NGM Biopharmaceuticals, Glenmark
Research Funding: Regeneron (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: Patent on the combined use of IL4 blockade and PD-1 blockade (Inst), patient on the neoadjuvant use of cemiplimab for the treatment of HCC
Toni K. Choueiri
Stock and Other Ownership Interests: Precede Bio, Osel, Tempest Therapeutics, Pionyr, Curesponse
Honoraria: Honoraria and Advisory/Consultancy are same
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, Alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), Tracon Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genetech (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Ipsen (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Roche (Inst), Calithera Biosciences (Inst), NiKang Therapeutics (Inst), Arcus Biosciences (Inst), Aveo (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy (Inst), International Patent Application No. PCT/US2018/12209, titled PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response (Inst), ctDNA Technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Heron, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Kathryn Lurain
Research Funding: Merck (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Miltenyi Biotec (Inst), Janssen Oncology (Inst), Bristol Myers Squibb/Celgene (Inst)
Guru P. Sonpavde
Employment: Myriad Genetics
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Janssen, Bristol Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seagen, Gilead Sciences, Scholar Rock, G1 Therapeutics, Loxo/Lilly, Infinity Pharmaceuticals, Syapse, Lucence, Vial
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape, Gilead Sciences, Seagen, Natera, Exelixis, Informação Brasileira de Oncologia
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), QED Therapeutics (Inst), Bristol Myers Squibb (Inst), Predicine (Inst), EMD Serono (Inst), Jazz Pharmaceuticals (Inst), GeneCentric (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Astellas Pharma, QED Therapeutics, Elsevier, Mereo BioPharma, G1 Therapeutics
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds, p. 3682
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, Chicago, IL, June 3-7, 2022; and the Society for Immunotherapy of Cancer Annual Meeting, Boston, MA, November 8-12, 2022.
T.E.Z., A.H.N., and E.A., contributed equally as first authors. G.P.S. and A.R.N. contributed equally as senior authors to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Talal El Zarif, Amin H. Nassar, Elio Adib, Nazli Dizman, Vishal Shah, Parminder Singh, Alexander M. Menzies, Dame Idossa, Elad Sharon, Mark Bower, Thomas U. Marron, Toni K. Choueiri, Lindsey R. Baden, Kathryn Lurain, Guru P. Sonpavde, Abdul Rafeh Naqash
Financial support: Abdul Rafeh Naqash
Administrative support: Alexandra Drakaki, Dory Freeman, Elizabeth Y. Chiao, Elad Sharon, Thomas U. Marron, Toni K. Choueiri, Lindsey R. Baden, Guru P. Sonpavde
Provision of study materials or patients: Arjun Mittra, Robert A. Baiocchi, Noha Abdel-Wahab, Batool Abu Ghazal, Anwaar Saeed, Melissa G. Lechner, Alexandra Drakaki, Michael A. Morse, Alessio Cortellini, David J. Pinato, Alessia Dalla Pria, Vishal Shah, Frank Aboubakar Nana, Carlo Genova, Sonam Puri, Vaia Florou, Paola Queirolo, Giuseppe Lamberti, Dory Freeman, Erin G. Reid, Elad Sharon, Ramya Ramaswami, Mark Bower, Brinda Emu, Thomas U. Marron, Toni K. Choueiri, Kathryn Lurain, Guru P. Sonpavde, Abdul Rafeh Naqash
Collection and assembly of data: Talal El Zarif, Amin H. Nassar, Elio Adib, Bailey G. Fitzgerald, Tarek H. Mouhieddine, Paul G. Rubinstein, Taylor Nonato, Rana R. McKay, Mingjia Li, Arjun Mittra, Dwight H. Owen, Robert A. Baiocchi, Michael Lorentsen, Christopher Dittus, Nazli Dizman, Adewunmi Falohun, Noha Abdel-Wahab, Adi Diab, Anand Bankapur, Alexandra Reed, Chul Kim, Aakriti Arora, Neil J. Shah, Edward El-Am, Elie Kozaily, Wassim Abdallah, Ahmad Al-Hader, Batool Abu Ghazal, Anwaar Saeed, Claire Drolen, Melissa G. Lechner, Alexandra Drakaki, Javier Baena, Caroline A. Nebhan, Tarek Haykal, Michael A. Morse, Alessio Cortellini, David J. Pinato, Alessia Dalla Pria, Evan Hall, Veli Bakalov, Nathan Bahary, Aarthi Rajkumar, Ankit Mangla, Vishal Shah, Frank Aboubakar Nana, Nerea Lopetegui-Lia, Danai Dima, Ryan W. Dobbs, Pauline Funchain, Rabia Saleem, Rachel Woodford, Georgina V. Long, Alexander M. Menzies, Carlo Genova, Giulia Barletta, Sonam Puri, Vaia Florou, Dame Idossa, Maristella Saponara, Paola Queirolo, Giuseppe Lamberti, Melissa Bersanelli, Dory Freeman, Elad Sharon, Douglas B. Johnson, Ramya Ramaswami, Mark Bower, Thomas U. Marron, Kathryn Lurain, Guru P. Sonpavde, Abdul Rafeh Naqash
Data analysis and interpretation: Talal El Zarif, Amin H. Nassar, Elio Adib, Jiaming Huang, Mingjia Li, Arjun Mittra, Dwight H. Owen, Christopher Dittus, Nazli Dizman, Noha Abdel-Wahab, Chul Kim, Neil J. Shah, Claire Drolen, Melissa G. Lechner, Alexandra Drakaki, Michael A. Morse, Alessia Dalla Pria, Vishal Shah, Rabia Saleem, Alexander M. Menzies, Dame Idossa, Alfredo Addeo, Melissa Bersanelli, Wanling Xie, Erin G. Reid, Elizabeth Y. Chiao, Elad Sharon, Douglas B. Johnson, Ramya Ramaswami, Mark Bower, Brinda Emu, Thomas U. Marron, Toni K. Choueiri, Lindsey R. Baden, Kathryn Lurain, Guru P. Sonpavde, Abdul Rafeh Naqash
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Activity of Immune Checkpoint Inhibitors in People Living With HIV and Cancer: A Real-World Report From the Cancer Therapy Using Checkpoint Inhibitors in People Living With HIV-International (CATCH-IT) Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elio Adib
Employment: Amgen
Tarek H. Mouhieddine
Consulting or Advisory Role: Legend Biotech
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, Aveo
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Arjun Mittra
Stock and Other Ownership Interests: Merck
Dwight H. Owen
Research Funding: Bristol Myers Squibb (Inst), Palobiofarma (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Onc.AI (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Genentech
Robert A. Baiocchi
Consulting or Advisory Role: Viracta Therapeutics, Prelude Therapeutics, Atara Biotherapeutics
Research Funding: Prelude Therapeutics, Esanex, Codiak Biosciences
Christopher Dittus
Research Funding: Seagen, AstraZeneca, Genentech
Patents, Royalties, Other Intellectual Property: Royalty payment from Springer Publishing for a medical textbook publication
Nazli Dizman
Consulting or Advisory Role: Vivreon Biosciences
Noha Abdel-Wahab
Honoraria: ChemoCentryx
Consulting or Advisory Role: ChemoCentryx
Adi Diab
Honoraria: Array BioPharma
Consulting or Advisory Role: Nektar, CureVac, Celgene, Idera, Memgen Therapeutics, Regeneron, CytomX Therapeutics, Pfizer (Inst)
Research Funding: Nektar (Inst), Idera (Inst), Celgene (Inst), Pfizer (Inst), Apexigen (Inst), Lytix Biopharma (Inst)
Travel, Accommodations, Expenses: Nektar
Chul Kim
Consulting or Advisory Role: Janssen, Novartis, PierianDx, AstraZeneca, Sanofi, Diffusion Pharmaceuticals, Mirati Therapeutics, Jazz Pharmaceuticals, Arcus Biosciences, Daiichi Sankyo, Eisai
Research Funding: AstraZeneca, Novartis, Karyopharm Therapeutics, Bristol Myers Squibb, Regeneron, Debiopharm Group, Janssen, Genentech/Roche, Daiichi Sankyo
Neil J. Shah
Honoraria: MJH Life Sciences, MedNet, LLC
Consulting or Advisory Role: Merck Sharp & Dohme Corp, Exelixis, Graticule
Research Funding: Aravive (Inst), Exelixis
Travel, Accommodations, Expenses: Merck
Anwaar Saeed
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis, Pfizer, Five Prime Therapeutics, Daiichi Sankyo/Astra Zeneca
Research Funding: AstraZeneca/MedImmune (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Merck Sharp & Dohme (Inst), Five Prime Therapeutics (Inst), Astellas Pharma (Inst), Actuate Therapeutics (Inst), Seagen (Inst), Daiichi Sankyo/UCB Japan (Inst), KAHR Medical (Inst), Innovent Biologics (Inst)
Melissa G. Lechner
Patents, Royalties, Other Intellectual Property: Patent US09746480B2 Human myeloid derived suppressor cell cancer markers
Alexandra Drakaki
Employment: Athos Therapeutics, Attica Sciences, Dyania Health
Leadership: Athos Therapeutics, Attica Sciences
Stock and Other Ownership Interests: Urogen Pharma, Alimera Sciences, Kyn Therapeutics, Athos Therapeutics, Attica Sciences, Dyania Health, Moderna Therapeutics, Proteas Bioanalytics
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Radmetrix, Seagen, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, Dyania Health, Aveo
Research Funding: Kite/Gilead, AstraZeneca (Inst), Genentech/Roche (Inst), BMS (Inst), Merck Sharp & Dohme (Inst), Jounce Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Seattle Genetics/Astellas (Inst), Immunomedics/Gilead (Inst)
Patents, Royalties, Other Intellectual Property: My significant other has several patents with UCLA, Harvard Medical School, Athos Therapeutics, Proteas Bioanalytics, and ATTICA Sciences
Travel, Accommodations, Expenses: Lilly, AstraZeneca, Seagen
Javier Baena
Honoraria: AstraZeneca Spain, Bristol Myers Squibb/Pfizer
Consulting or Advisory Role: AstraZeneca Spain, Roche
Expert Testimony: Roche
Travel, Accommodations, Expenses: MSD
Michael A. Morse
Honoraria: Genentech/Roche, Novartis, Sanofi, Lexicon, Ipsen, Bayer, Taiho Pharmaceutical, Boehringer Ingelheim, Eisai, Merck, Exelixis, AstraZeneca/Daiichi Sankyo, Servier, Tersera, QED Therapeutics
Speakers' Bureau: Genentech/Roche, Taiho Pharmaceutical, Ipsen, Exelixis, Eisai, Servier, AstraZeneca
Research Funding: Precision Biologics (Inst), Bristol Myers Squibb (Inst), Onyx (Inst), Eisai (Inst), Lexicon (Inst), MedImmune (Inst), Advanced Accelerator Applications (Inst), AlphaVax (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Vaccines against antigens involved in therapy resistance and methods of using same, patent number: 9956276 Assignee Duke University, pharmaceutical product, medical food or dietary supplement for preventing cancer and inflammatory diseases, Publication number: 20170246136, Applicant: OliVentures, Inc, Inventors: Carlos María Peña Díaz, Guillermo Muñoz Fernández, Michael Morse, Compositions and methods for modulating and redirecting immune responses, Publication number: 20170015758, Inventors: Scott A. Hammond, Michael A. Morse, Takuya Osada, Herbert Kim Lyerly
Alessio Cortellini
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, MSD Oncology, OncoC4, IQvia
Speakers' Bureau: AstraZeneca, Eisai
David J. Pinato
Honoraria: Roche/Genentech, Bristol Myers Squibb, Da Volterra, Avammune, Mursla Bio, Starpharma, Lift Biosciences
Consulting or Advisory Role: Eisai, Mina Therapeutics, Roche, H3 Biomedicine, Da Volterra, AstraZeneca, Ipsen
Speakers' Bureau: Bayer, ViiV Healthcare, Falk Pharma, Roche
Research Funding: MSD Oncology (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Bayer, MSD Oncology
Other Relationship: Wiley
Evan Hall
Research Funding: Treatment Technologies and Insights (Inst), Neoleukin Therapeutics (Inst), ImCheck therapeutics (Inst), Nektar (Inst), Replimune (Inst), NiKang Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1368769
Nathan Bahary
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Incyte
Ankit Mangla
Honoraria: Targeted Oncology
Consulting or Advisory Role: SpringWorks Therapeutics
Research Funding: Nektar, Tracon Pharma, SpringWorks Therapeutics, Regeneron
Parminder Singh
Honoraria: Curio Science, Medpage/ASCO
Consulting or Advisory Role: Janssen Oncology, Seattle Genetics/Astellas (Inst), EMD Serono, Aveo, Bayer
Research Funding: EMD Serono (Inst)
Uncompensated Relationships: Seagen
Ryan W. Dobbs
Stock and Other Ownership Interests: Pfizer, United Health Group, Bristol Myers Squibb/Sanofi, Merck, Lilly, Medtronic, Johnson & Johnson/Janssen
Consulting or Advisory Role: Boston Scientific
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1392783
Pauline Funchain
Consulting or Advisory Role: Eisai, Novartis, GigaGen
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Taiho Oncology (Inst)
Georgina V. Long
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Agenus, Amgen, Array BioPharma, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion Biotech, Hexal, Highlight Therapeutics, Innovent Biologics, Merck Sharp & Dohme, Novartis, OncoSec, PHMR, Pierre Fabre, Provectus, QBiotics, Regeneron, AstraZeneca
Alexander M. Menzies
Consulting or Advisory Role: MSD Oncology, Novartis, Pierre Fabre, Bristol Myers Squibb, Roche, QBiotics
Carlo Genova
Honoraria: AstraZeneca, Amgen, Bristol Myers Squibb, Merck, Roche, Sanofi, Takeda
Consulting or Advisory Role: AstraZeneca, Amgen, Bristol Myers Squibb, Roche, Sanofi
Research Funding: Bristol Myers Squibb (Inst)
Sonam Puri
Honoraria: Aptitude Health (Inst), IntegrityCE
Consulting or Advisory Role: AstraZeneca (Inst), G1 Therapeutics (Inst), Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Dava Oncology
Vaia Florou
Consulting or Advisory Role: Incyte, Deciphera
Maristella Saponara
Honoraria: Sanofi, Sun Pharma, BMS, MSD, Novartis, Pierre Fabre
Paola Queirolo
Consulting or Advisory Role: Roche/Genentech, Novartis, MSD, Bristol Myers Squibb, Pierre Fabre, Sanofi, Sun Pharma Advanced Research Company, Merck Serono
Travel, Accommodations, Expenses: MSD Oncology, Sanofi/Regeneron
Alfredo Addeo
Consulting or Advisory Role: Roche, AstraZeneca/MedImmune, Bristol Myers Squibb Foundation, MSD Oncology, Pfizer, Novartis, Astellas Pharma, Amgen, Lilly
Speakers' Bureau: Lilly, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Takeda
Melissa Bersanelli
Consulting or Advisory Role: Novartis, Pfizer, Ipsen, Sanofi, Merck
Speakers' Bureau: Bristol Myers Squibb, Novartis, Pfizer, AstraZeneca, Pierre Fabre, Ipsen
Research Funding: Seqirus (Inst), Roche (Inst), Pfizer (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Copyright transfer for scientific materials by Sciclone Pharmaceuticals (Cina), Copyright transfer for scientific materials by IPSEN, Copyright transfer for scientific materials by Pfizer, Copyright transfer for scientific materials by Sanofi, Copyright transfer for scientific materials by Pierre Fabre, Copyright transfer for scientific materials by MSD
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Pierre Fabre, Pfizer/EMD Serono, Ipsen, MSD Oncology
Wanling Xie
Consulting or Advisory Role: Convergent Therapeutics
Erin G. Reid
Employment: EpicentRx
Leadership: EpicentRx
Stock and Other Ownership Interests: EpicentRx
Research Funding: ADC Therapeutics (Inst), Aptose Biosciences (Inst), Millenium Pharamceuticals (Inst), Xencor (Inst)
Patents, Royalties, Other Intellectual Property: Husband hold multiple patents related to lymphedema management, oncolytic viral therapy
Open Payments Link: https://openpaymentsdata.cms.gov/physician/86601
Douglas B. Johnson
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Iovance Biotherapeutics, Catalyst Pharmaceuticals, Oncosec, Pfizer, Mosaic ImmunoEngineering, Targovax, Mallinckrodt
Research Funding: Incyte, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy
Ramya Ramaswami
Research Funding: Celgene (Inst), Genentech (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Merck Serono (Inst)
Mark Bower
Honoraria: ViiV Healthcare, Gilead Sciences, Bristol Myers Squibb, MSD, Janssen, EUSA Pharma
Brinda Emu
Stock and Other Ownership Interests: Bristol Myers Squibb Foundation
Consulting or Advisory Role: Theratechnologies
Thomas U. Marron
Consulting or Advisory Role: Regeneron, Boehringer Ingelheim, AstraZeneca, DBV Technologies, Celldex, Surface Oncology, NGM Biopharmaceuticals, Glenmark
Research Funding: Regeneron (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: Patent on the combined use of IL4 blockade and PD-1 blockade (Inst), patient on the neoadjuvant use of cemiplimab for the treatment of HCC
Toni K. Choueiri
Stock and Other Ownership Interests: Precede Bio, Osel, Tempest Therapeutics, Pionyr, Curesponse
Honoraria: Honoraria and Advisory/Consultancy are same
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, Alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), Tracon Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genetech (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Ipsen (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Roche (Inst), Calithera Biosciences (Inst), NiKang Therapeutics (Inst), Arcus Biosciences (Inst), Aveo (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy (Inst), International Patent Application No. PCT/US2018/12209, titled PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response (Inst), ctDNA Technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Heron, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Kathryn Lurain
Research Funding: Merck (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Miltenyi Biotec (Inst), Janssen Oncology (Inst), Bristol Myers Squibb/Celgene (Inst)
Guru P. Sonpavde
Employment: Myriad Genetics
Honoraria: UpToDate
Consulting or Advisory Role: Genentech, Merck, Janssen, Bristol Myers Squibb, Exelixis, EMD Serono, Astellas Pharma, Bicycle Therapeutics, Pfizer, Seagen, Gilead Sciences, Scholar Rock, G1 Therapeutics, Loxo/Lilly, Infinity Pharmaceuticals, Syapse, Lucence, Vial
Speakers' Bureau: Physicans' Education Resource, Onclive, Research to Practice, Medscape, Gilead Sciences, Seagen, Natera, Exelixis, Informação Brasileira de Oncologia
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), QED Therapeutics (Inst), Bristol Myers Squibb (Inst), Predicine (Inst), EMD Serono (Inst), Jazz Pharmaceuticals (Inst), GeneCentric (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Astellas Pharma, QED Therapeutics, Elsevier, Mereo BioPharma, G1 Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1. Ribas A, Wolchok JD. Combining cancer immunotherapy and targeted therapy. Curr Opin Immunol. 2013;25:291–296. doi: 10.1016/j.coi.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:446–453. doi: 10.1016/S1470-2045(17)30104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 5. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 6. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 7. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378:1029–1041. doi: 10.1056/NEJMra1615896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haas CB, Engels EA, Horner M-J, et al. Trends and risk of lung cancer among people living with HIV in the USA: A population-based registry linkage study. Lancet HIV. 2022;9:e700–e708. doi: 10.1016/S2352-3018(22)00219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lurain K, Ramaswami R, Yarchoan R, et al. Anti-PD-1 and anti-PD-L1 monoclonal antibodies in people living with HIV and cancer. Curr HIV/AIDS Rep. 2020;17:547–556. doi: 10.1007/s11904-020-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horner M-J, Shiels MS, Pfeiffer RM, et al. Deaths attributable to cancer in the US human immunodeficiency virus population during 2001–2015. Clin Infect Dis. 2021;72:e224–e231. doi: 10.1093/cid/ciaa1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vora KB, Ricciuti B, Awad MM. Exclusion of patients living with HIV from cancer immune checkpoint inhibitor trials. Sci Rep. 2021;11:6637. doi: 10.1038/s41598-021-86081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah NJ, Al-Shbool G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7:353. doi: 10.1186/s40425-019-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: A systematic review. JAMA Oncol. 2019;5:1049–1054. doi: 10.1001/jamaoncol.2018.6737. [DOI] [PubMed] [Google Scholar]
- 14. Ostios-Garcia L, Faig J, Leonardi GC, et al. Safety and efficacy of PD-1 inhibitors among HIV-positive patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:1037–1042. doi: 10.1016/j.jtho.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 15. Spano JP, Veyri M, Gobert A, et al. Immunotherapy for cancer in people living with HIV: Safety with an efficacy signal from the series in real life experience. AIDS. 2019;33:F13–F19. doi: 10.1097/QAD.0000000000002298. [DOI] [PubMed] [Google Scholar]
- 16. Lavole A, Guihot A, Veyri M, et al. PD-1 blockade in HIV-infected patients with lung cancer: A new challenge or already a strategy? Ann Oncol. 2018;29:1065–1066. doi: 10.1093/annonc/mdx817. [DOI] [PubMed] [Google Scholar]
- 17. Odeny TA, Lurain K, Strauss J, et al. Effect of CD4+ T cell count on treatment-emergent adverse events among patients with and without HIV receiving immunotherapy for advanced cancer. J Immunother Cancer. 2022;10:e005128. doi: 10.1136/jitc-2022-005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lurain K, Ramaswami R, Mangusan R, et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin’s lymphoma. J Immunother Cancer. 2021;9:e002097. doi: 10.1136/jitc-2020-002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uldrick TS, Goncalves PH, Abdul-Hay M, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer—A phase 1 study. JAMA Oncol. 2019;5:1332–1339. doi: 10.1001/jamaoncol.2019.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez-Cao M, Moran T, Dalmau J, et al. Assessment of the feasibility and safety of durvalumab for treatment of solid tumors in patients with HIV-1 infection: The phase 2 DURVAST study. JAMA Oncol. 2020;6:1063–1067. doi: 10.1001/jamaoncol.2020.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavole A, Mazieres J, Schneider S, et al. Assessment of nivolumab in HIV-Infected patients with advanced non-small cell lung cancer after prior chemotherapy. The IFCT-1602 CHIVA2 phase 2 clinical trial. Lung Cancer. 2021;158:146–150. doi: 10.1016/j.lungcan.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 22. Abbar B, Baron M, Katlama C, et al. Immune checkpoint inhibitors in people living with HIV: What about anti-HIV effects? AIDS. 2020;34:167–175. doi: 10.1097/QAD.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 23. Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immune deficiency syndrome: A proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7:1201–1207. doi: 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 24. McCaw ZR, Yin G, Wei L-J. Using the restricted mean survival time difference as an alternative to the hazard ratio for analyzing clinical cardiovascular studies. Circulation. 2019;140:1366–1368. doi: 10.1161/CIRCULATIONAHA.119.040680. [DOI] [PubMed] [Google Scholar]
- 25. Uno H, Wittes J, Fu H, et al. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med. 2015;163:127–134. doi: 10.7326/M14-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reuss JE, Stern D, Foster JC, et al. Assessment of cancer therapy evaluation Program advocacy and inclusion rates of people living with HIV in anti–PD1/PDL1 clinical trials. JAMA Netw Open. 2020;3:e2027110. doi: 10.1001/jamanetworkopen.2020.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology–Friends of Cancer research HIV Working Group. J Clin Oncol. 2017;35:3774–3780. doi: 10.1200/JCO.2017.73.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.https://www.fda.gov/media/127712/download Enhancing the diversity of clinical trial populations—Eligibility criteria, enrollment practices, and trial designs guidance for industry.
- 29. Marabelle A, Cassier PA, Fakih M, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: Results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol Hepatol. 2022;7:446–454. doi: 10.1016/S2468-1253(21)00382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 31. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 33. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 34. Grob JJ, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629) J Clin Oncol. 2020;38:2916–2925. doi: 10.1200/JCO.19.03054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 36. Rasmussen TA, Rajdev L, Rhodes A, et al. Impact of anti-PD-1 and anti-CTLA-4 on the human immunodeficiency virus (HIV) reservoir in people living with HIV with cancer on antiretroviral therapy: The AIDS malignancy consortium 095 study. Clin Infect Dis. 2021;73:e1973–e1981. doi: 10.1093/cid/ciaa1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lozano AX, Chaudhuri AA, Nene A, et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med. 2022;28:353–362. doi: 10.1038/s41591-021-01623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaudhary O, Trotta D, Wang K, et al. Patients with HIV-associated cancers have evidence of increased T cell dysfunction and exhaustion prior to cancer diagnosis. J Immunother Cancer. 2022;10:e004564. doi: 10.1136/jitc-2022-004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delyon J, Biard L, Renaud M, et al. PD-1 blockade with pembrolizumab in classic or endemic Kaposi's sarcoma: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2022;23:491–500. doi: 10.1016/S1470-2045(22)00097-3. [DOI] [PubMed] [Google Scholar]
- 40. Evans VA, van der Sluis RM, Solomon A, et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS. 2018;32:1491–1497. doi: 10.1097/QAD.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uldrick TS, Adams SV, Fromentin R, et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci Transl Med. 2022;14:eabl3836. doi: 10.1126/scitranslmed.abl3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.https://clinicaltrials.gov/ct2/show/NCT02408861 Nivolumab and ipilimumab in treating patients with HIV associated relapsed or refractory classical Hodgkin lymphoma or solid tumors that are metastatic or cannot be removed by surgery.
- 43.https://clinicaltrials.gov/ct2/show/NCT02595866 Testing the addition of an experimental medication MK-3475 (pembrolizumab) to usual anti-retroviral medications in patients with HIV and cancer.