Background and Aims:
Analyzing the interplay among serum HBV DNA, HBsAg, anti-HBs, and alanine aminotransferase (ALT) during nucleic-acid polymer (NAP)-based therapy for chronic hepatitis B provides a unique opportunity to identify kinetic patterns associated with functional cure.
Methods:
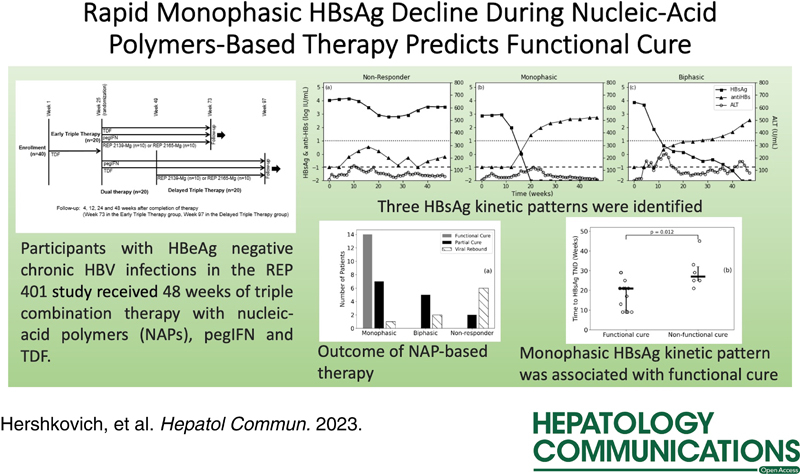
All participants with HBeAg-negative chronic HBV infection in the REP 401 study (NCT02565719) first received 24 weeks of tenofovir-disoproxil-fumarate (TDF) monotherapy. The early triple therapy group (n = 20) next received 48 weeks of TDF+pegylated interferon-α2a (pegIFN)+NAPs. In contrast, the delayed triple therapy group (n = 20) next received 24 weeks of TDF+pegIFN before 48 weeks of triple therapy. Three participants discontinued treatment and were excluded. Functional cure (HBsAg and HBV DNA not detectable with normal ALT) was assessed at 48 weeks post-treatment. Different kinetic phases were defined by at least a 2-fold change in slope. A single-phase decline was categorized as monophasic, and 2-phase declines were categorized as biphasic.
Results:
Fourteen (35%) participants achieved a functional cure. HBV DNA remained below or near undetectable for all participants by the end of TDF monotherapy and during subsequent combination therapies. Three HBsAg kinetic patterns were found in both the early and delayed groups, nonresponders (n = 4 and n = 4), monophasic (n = 11 and n = 11), and biphasic (n = 4 and n = 3), respectively. All participants who achieved a functional cure had a monophasic HBsAg kinetic pattern during triple therapy. Among participants with a monophasic HBsAg decline, those who had a functional cure had a shorter median time to HBsAg loss of 21 (interquartile range=11) weeks compared with those who did not achieve functional cure [median: 27 (7) weeks] (p = 0.012).
Conclusions:
Functional cure was associated with a rapid monophasic HBsAg decline during NAP-based therapy. A nonmonophasic HBsAg kinetic pattern had a 100% negative predictive value (NPV) for a functional cure.
INTRODUCTION
Chronic HBV infection is a hepatotropic infection, which affects >250 million people worldwide.1,2 Chronic HBV can cause hepatic fibrosis, cirrhosis, and liver cancer resulting in >820,000 deaths annually.3 Currently approved therapies with nucleos(t)ide analogs (NUCs) are highly effective in suppressing HBV viremia to clinically undetectable levels by inhibiting HBV reverse transcriptase.4 While NUCs can inhibit liver disease progression and might decrease the risk of developing hepatocellular carcinoma,5 they have limited efficacy in reducing HBsAg, suppressing HBV genomic recycling, that is, intracellular replenishment of the covalently closed circular DNA4 or eliminating integrated HBV DNA.6–10 These limited antiviral effects often result in a relapse in hepatitis B viremia upon discontinuation of NUCs.11 Because HBsAg loss (defined as undetectable HBsAg with current assays) is associated with an improvement in liver-related outcomes12 including a further decrease in hepatocellular carcinoma risk compared with HBV DNA suppression without HBsAg loss,13 the suppression of both HBV DNA and HBsAg along with normal alanine aminotransferase (ALT) level (termed functional cure) is considered a desirable clinical endpoint.14 However, the HBsAg loss rate with NUCs is <10% after many years of therapy.15
Circulating HBsAg, which is primarily comprised of noninfectious subviral particles (SVPs), prevents HBV-specific host immune responses from efficiently targeting HBV infection.16–18 HBsAg loss provides the basis for a functional cure.19 Of the current investigational drugs in development for chronic HBV infection,20 nucleic-acid polymers (NAPs) are the only agents that have been shown to efficiently target the assembly and secretion of SVPs and inhibit HBsAg production, both in mechanistic studies21–23 and human trials.24 The most recent combination study (REP 401) with NAPs, tenofovir-disoproxil-fumarate (TDF), and pegylated interferon-α2a (pegIFN) achieved high rates of HBsAg loss during therapy (60%), high rates of host-mediated transaminase flares, and high rates of functional cure (39%) after completion of triple therapy.25
Establishing predictive markers for functional cure has been hampered by the low rates of functional cure with approved agents but is an important part of the developing clinical landscape for establishing optimized combination therapy regimes. The goals of this study were to provide the first kinetic characterization of HBV DNA, HBsAg, anti-HBs, and ALT during treatment arms with TDF alone, TDF+pegIFN, and triple therapy with TDF+pegIFN+NAPs and to assess for kinetic patterns associated with functional cure in the REP 401 study.
METHODS
REP 401 study25
Forty chronic HBV-infected, HBeAg-negative, mostly genotype D participants received 24 weeks of TDF monotherapy. At the end of TDF monotherapy, 20 participants were randomly assigned to an early NAPs treatment group and 20 to a delayed NAPs treatment group. The early group received 48 weeks of triple therapy, consisting of TDF+pegIFN+NAPs. The delayed group received 24 weeks of TDF+pegIFN (dual therapy) and was then treated with NAPs triple therapy for 48 weeks. Within each treatment arm, 10 participants were randomly assigned to receive REP 2139-Mg and 10 participants to receive REP 2165-Mg. REP 2165-Mg is a bioequivalent variant of REP 2139-Mg, which is designed to degrade faster and, thus, have reduced organ accumulation.26 The baseline characteristics for all participants are shown in Table 1.25 As a result of the randomization process, there was no significant difference between the baseline characteristics of the 2 groups. After completing 48 weeks of triple therapy, patients were then followed for 48 weeks in the absence of all therapy. The study design with the disposition of participants from the initiation of the study to the end of follow-up is shown in Supplemental Figure S1 (http://links.lww.com/HC9/A365).
TABLE 1.
Baseline characteristics before monotherapy in the REP 401 study25
| Parameters | Delayed triple group | Early triple group |
|---|---|---|
| Participants | 20 | 20 |
| Documented history of infection before therapy [median (range)] (y) | 9 (0.1–19) | 7 (0.25–26) |
| Previous therapy | ||
| NUCs | 0 | 1 |
| pegIFN | 0 | 0 |
| Age [median (range)] | 40.5 (22–53) | 40.5 (23–52) |
| Males [n (%)] | 17 (85) | 16 (80) |
| HBV genotype [n (%)] | ||
| A | 1 (5) | 2 (10) |
| D | 19 (95) | 18 (90) |
| Fibroscan [n (%)] | ||
| ≤7 kPa | 12 (60) | 10 (50) |
| >7–9 kPa | 4 (20) | 7 (35) |
| >9–11 kPa | 0 (0) | 2 (10) |
| >11 kPa | 4 (20) | 1 (5) |
| Virologic baseline [median (IQR)] | ||
| HBV DNA (log10 IU/mL) | 4.62 (2.07) | 5.00 (1.19) |
| HBsAg (log10 IU/mL) | 3.97 (0.67) | 3.94 (0.36) |
| Anti-HBs (mIU/mL) | 0 (0.42) | 0 (0.13) |
| ALT [median (range)] (U/L) | 49 (21–302) | 56.5 (2–276) |
| Normal ALT ( <50 U/L) [n (%)] | 11 (55) | 8 (40) |
| Platelets [median (range)] (×106/mL) | 175 (148–260) | 168.5 (124–331) |
| Liver median stiffness [median (range)] (kPa) | 6.65 (4.3–14.1) | 7.3 (4.4–19.6) |
Note: The delayed triple therapy group (called the control group in the REP 401 study) consists of participants treated with TDF alone and then dual therapy with TDF+pegIFN followed by triple therapy with TDF+pegIFN+NAPs (termed delayed triple). The early triple therapy (called the experimental group in the REP 401 study) group consists of participants treated with TDF alone, followed by triple therapy with TDF+pegIFN+NAPs (termed early triple).
Abbreviations: ALT, alanine aminotransferase; IQR, interquartile range; NAP, nucleic-acid polymer; NUC, nucleos(t)ide analog; pegIFN, pegylated interferon-α2a; TDF, tenofovir-disoproxil-fumarate.
The REP 401 study was conducted at 3 sites in Chişinău, Republic of Moldova; 2 sites at the Toma Ciorbă Infectious Diseases Hospital and a third site at the Republican Clinical Hospital. For complete enrollment criteria, see http://replicor.com/wp-content/uploads/2020/03/REP-401-Protocol.pdf. Written informed consent at enrollment was provided by all participants. The study protocol was compliant with current guidelines on Good Clinical Practice, with the Declaration of Helsinki and Istanbul, and was approved by the National Ethics Committee and National Medicines Agency of the Republic of Moldova (Approval Numbers 42_02.09.2015 and A07PS01Rg02-5856_05.08.2015 respectively).
HBV DNA, HBsAg, anti-HBs, and ALT measurements
During TDF monotherapy, HBV DNA [Abbott Realtime; lower level of quantification (LLoQ) 10 IU/mL, target not detected (TND) 1 IU/mL], serum HBsAg (Abbot Architect; LLoQ 0.05 IU/mL, TND 0.01 IU/mL), anti-HBs (Abbot Architect; TND 0.1 mIU/mL), and ALT were measured at 1, 10, 18, and 24 weeks. During dual and triple therapies, measurements were made weekly. All participants received follow-up evaluations and testing at 4, 12, 24, and 48 weeks after the end of therapy (week 72 for the early NAPs group and week 96 for the delayed group).
Kinetic and statistical analyses
Three participants (“02-010” and “03-016” from the delayed group and “03-020” from the early group) discontinued treatment before the end of the study and were, thus, excluded from the dual therapy, and early and delayed triple therapy analyses. Two additional patients (02-053 and 02-050) who had missing HBV DNA data points during TDF monotherapy were excluded from the kinetic analysis of the TDF treatment phase. The kinetics of each marker (HBV DNA, HBsAg, anti-HBs, and ALT) were assessed during each stage of treatment for participants in both the early and delayed NAPs treatment groups. Both groups underwent an identical initial stage of TDF monotherapy, so n = 35 participants were included in the analysis of the first phase of therapy. Since only the delayed treatment group received 24 weeks of TDF+pegIFN dual therapy, this treatment phase was analyzed separately. Data obtained during the triple therapy phase were analyzed separately for the early and delayed NAPs treatment groups, and then were combined for further analysis.
For the purposes of plotting and statistical analyses, each marker was right censored to the boundaries of the detection limit of the assay before being placed on a log10 scale. HBsAg values <0.05 IU/mL were right censored to 0.01 IU/mL, anti-HBs values of <0.1 mIU/mL were right censored to 0.1 mIU/mL, HBV DNA<LLoQ was right censored to 10 IU/mL, and HBV DNA TND was right censored to 1 IU/mL.
The kinetics of HBV DNA and HBsAg during treatment were grouped into decline patterns. As previously done,27–29 monophasic responses were characterized by a single linear decline until reaching LLoQ or TND (whichever was first detected), whereas biphasic responses were characterized by at least a 2-fold change in slope from the first phase of decline before reaching LLoQ or TND. Nonresponders (NR) were defined as demonstrating a decline from baseline <1.5 log10 IU/mL. For HBV DNA, we defined an additional flat partial response kinetic pattern where the decline was >1.5 log10 but plateaued rather than trending toward TND. The slopes for each distinct decline phase were calculated with linear regression.
A high upper limit of normal (ULN) of ALT = 50 U/L was used for the REP 401 study, which was performed in a Moldovan population as previously described.25 ALT (in U/L) responses were analyzed including the magnitude of the peak value, the time delay from initiation of dual or triple therapy until the peak was achieved, the peak value as fold increase above pretreatment levels, and the peak value as fold increase above ULN. Anti-HBs seroconversion was defined as the time when titer antibody values reached ≥10 mIU/mL.
All kinetic analysis was performed with R and Python, version 3.9 with Scipy, version 1.7.3. Unless otherwise specified, variables are summarized as the median and interquartile range (IQR), linear correlations were calculated with Spearman R, and sample medians were compared with the Mann-Whitney U test or Kruskal-Wallis H test when >2 samples were being compared. p values ≤0.05 were noted as being statistically significant.
RESULTS
HBV DNA decline in TDF monotherapy
Three distinct HBV DNA patterns were observed during 24 weeks of TDF monotherapy (Figure 1 and Supplemental Figures S2 and S3, http://links.lww.com/HC9/A365). All 35 participants experienced a rapid first phase of HBV DNA decline with a median slope of 0.35 (IQR: 0.11) log IU/week. Five participants experienced a monophasic decline in HBV DNA with a continuous downward slope (n = 4) or reduction below LLoQ (n = 1) (Figure 1A). Thirty participants had a second viral decline phase, including 19 who showed a biphasic pattern (Figure 1B) and 11 who had a flat partial response (Figure 1C). There was no difference in HBV DNA at baseline between participants with monophasic, biphasic, and flat partial response decline patterns (p = 0.62). The biphasic HBV DNA patterns were characterized by an initial decline followed by a second slower decline with a median slope of 0.08 (IQR: 0.03) log IU/week. In the 12 participants with FPRs, the second phase was a plateau of HBV DNA at a median 1.0 (IQR: 0) log IU/mL. At the end of TDF monotherapy, 5 of the 35 participants had HBV DNA TND, 26 of the 35 (74%) of participants were at least <LLoQ, and 9 (26%) had quantifiable HBV DNA (Table 2). HBV DNA remained below or near LLoQ for all of the 37 (including the 2 patients (02-053 and 02-050) who had missing HBV DNA data points during TDF monotherapy for kinetic analysis) participants studied during subsequent combination therapy through the end of triple treatment (EOT) (Table 2). There were no associations between patients’ baseline characteristics and HBV DNA kinetic patterns.
FIGURE 1.
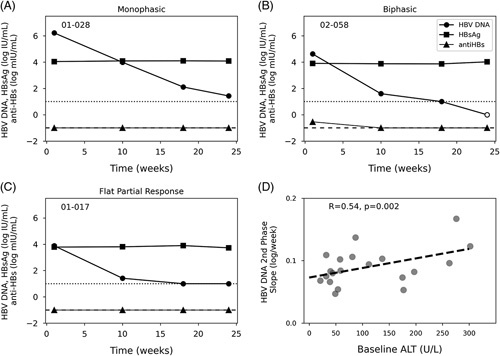
Representative HBV DNA kinetic patterns in patients treated with TDF monotherapy. Representative serum HBV DNA kinetic patterns monotherapy (A), biphasic (B), and flat partial response (C). Dotted and dashed horizontal lines represent the HBV DNA LLoQ (10 IU/mL) and anti-HBs detection limit (0.1 mIU/mL), respectively. Empty circles represent undetectable HBV DNA. (D) Correlation between second-phase HBV DNA decline and baseline ALT level for participants experiencing a biphasic HBV DNA decline. All 37 participants’ responses are available in Supplemental Figures S2 and S3 (http://links.lww.com/HC9/A365). Abbreviations: ALT, alanine aminotransferase; LLoQ, lower level of quantification; TDF, tenofovir-disoproxil-fumarate.
TABLE 2.
Virological baselines before each of the 3 segments of treatment
| Parameters | Dual therapy (N = 18) | Delayed triple (N = 18) | Early triple (N = 19) |
|---|---|---|---|
| Virology [median (IQR)] | |||
| HBsAg (log10 IU/mL) | 3.93 (0.38) | 3.86 (0.61) | 3.89 (0.40) |
| ALT (U/L) | 39.5 (16) | 71.5 (47.8) | 35 (22.5) |
| HBV DNA (log10 IU/mL) | 1.0 (0.86) | 1.0 (1.03) | 1.0 (0.17) |
| HBV DNA<LLoQa [n (%)] | 11 (61) | 13 (72) | 14 (74) |
| HBV DNA TND [n (%)] | 5 (28) | 8 (44) | 4 (21) |
| HBV DNA QF>10 IU/mL [n (%)] | 7 (38) | 5 (28) | 5 (26) |
| HBsAg TND (%) | 0 | 0 | 0 |
| Anti-HBs TND [n (%)] | 14 (78) | 13 (72) | 16 (84) |
Note: The dual therapy group underwent 24 weeks of TDF+pegIFN and then transitions to 48 weeks of TDF+pegIFN+NAPs, called delayed triple therapy. The immediate triple therapy group underwent 48 weeks of TDF+pegIFN+NAPs triple therapy. HBV DNA LLoQ, 10 IU/mL; HBsAg TND, 0.1 IU/mL; QF; and anti-HBs TND, 0.1 mIU/mL.
Less than LLoQ count includes participants with HBV DNA TND.
Abbreviations: ALT, alanine aminotransferase; IQR, interquartile range; LLoQ, lower level of quantification; NAP, nucleic-acid polymer; pegIFN, pegylated interferon-α2a; QF, quantifiable; TDF, tenofovir-disoproxil-fumarate; TND, target not detected.
The median baseline ALT level before TDF monotherapy was 54 (IQR: 56) U/L. The initial HBV DNA decline was not correlated with baseline ALT, but the second-phase HBV DNA decline for biphasic HBV DNA responses correlated with baseline ALT (R = 0.53, p = 0.002, Figure 1D), suggesting that the rate of turnover of infected hepatocytes at baseline may play a role in HBV DNA kinetics during TDF monotherapy.
Dual therapy with TDF and pegIFN
The delayed group then underwent 24 weeks of TDF+pegIFN dual therapy. Since baseline HBV DNA at the initiation of the dual therapy was <LLoQ for 11/18 participants and remained <1.5 log10 IU/mL (17/18 participants) throughout this phase, and during subsequent triple therapy (delayed triple therapy) (Table 2), HBV DNA levels were not further characterized after the TDF monotherapy phase.
Only 1/18 participants had an HBsAg decline during dual therapy (Figure 2A) while in the remaining patients it remained at pretreatment levels (Figure 2 B-D). Seventeen patients began dual therapy with undetectable anti-HBs, and the remaining participant was anti-HBs positive with a titer <10 mIU/mL. None of the 17 anti-HBs negative patients achieved anti-HBs seroconversion with dual therapy including a single outlier (Patient 02-001) who had a monophasic decline of HBsAg after a delay of 8 weeks (Figure 2).
FIGURE 2.
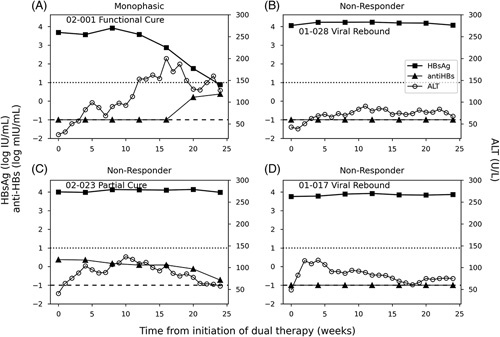
Representative figures of HBsAg kinetics (and ALT flares) during dual therapy. (A) Participant 01-028 represents the 17 HBsAg nonresponders. (B) Participant 02-001 who was the only one who experienced HBsAg monophasic decline during dual therapy and achieved functional cure (HBV DNA not detected, HBsAg < lower level of quantification, and normal ALT after follow-up). Dotted and dashed horizontal lines represent the anti-HBs seroconversion threshold (10 mIU/mL) and anti-HBs target not detected (0.1 mIU/mL), respectively. (C) Participant 02-023 represents those HBsAg nonresponders who had partial cure (HBV DNA ≤ 2000 IU/mL, normal ALT) after triple therapy. (D) Participant 01-017 represents those HBsAg nonresponders who had virological rebound (HBV DNA >2000 IU/mL) after triple therapy. Abbreviations: ALT, alanine aminotransferase.
The median ALT value at the start of dual therapy was 40 (IQR: 16) U/L. During dual therapy, ALT kinetics involved transient increases of varying magnitudes with a peak and a subsequent decline in all participants (Figure 2). The magnitude of ALT peak values varied and was not associated with HBV DNA or HBsAg kinetic parameters. The time at which the peak ALT level occurred during dual therapy was significantly (p = 0.0017) later [median: 16 (IQR: 10) weeks] for patients who subsequently achieved functional cure after triple therapy compared with those who did not subsequently reach functional cure after triple therapy [median: 6 (IQR: 6) weeks] (Figure 3).
FIGURE 3.
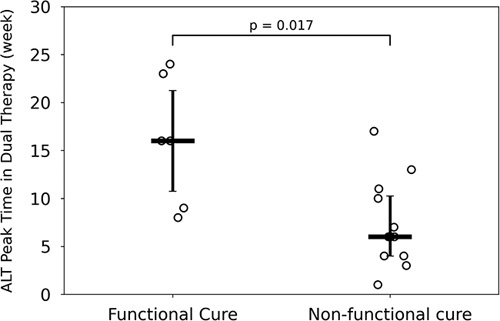
Therapy outcome versus ALT peak time in dual therapy. ALT peak time during dual therapy is associated with treatment outcomes at follow-up after triple therapy. Peak time is measured from the beginning of dual therapy. Horizontal and vertical lines represent the median and interquartile range, respectively. Abbreviation: ALT, alanine aminotransferase.
Early triple therapy kinetics
The 19 participants in the “Early Triple Therapy” group underwent 48 weeks of TDF+pegIFN+NAPs triple therapy immediately following TDF monotherapy (Supplemental Figure S4, http://links.lww.com/HC9/A365). Baseline HBV DNA at the initiation of early triple therapy was <LLoQ for 14/19 participants and <1.5 log10 IU/mL (16/19 participants) throughout this phase (Table 2), so HBV DNA levels were not further characterized.
Three HBsAg kinetic patterns were identified during early triple therapy. Four participants were NR (<1.5 log10 IU/mL reduction from baseline) (Figure 4A), 11 had a monophasic decline (Figure 4B), and 4 had a biphasic decline (Figure 4C). The median rate of HBsAg decline in monophasic patients was 0.52 (IQR: 0.26) log IU/mL/week, with all patients reaching LLoQ [at median: 18 (IQR: 8) weeks]. The median initial phase slope in biphasic participants was 0.29 (IQR: 0.18) log IU/mL/week, which was slower (p = 0.02) than in the monophasic participants (Supplemental Table S1, http://links.lww.com/HC9/A365). In patients with a biphasic HBsAg decline, the median slope of the second-phase decline of 0.02 (IQR: 0.02) log IU/week was slower than the first phase with only 1 patient reaching <LLoQ at EOT (Figure 4C). No difference was observed in kinetic patterns between participants receiving either REP 2139-Mg or REP 2165-Mg, nor were associations observed between kinetic patterns and the participants’ age or known duration of HBV infection.
FIGURE 4.
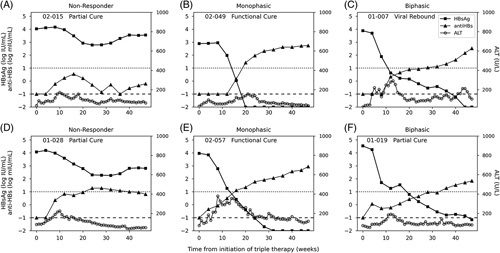
Representative participants based on HBsAg response kinetic patterns during 48-week combination therapy with TDF, pegIFN, and NAPs. The participant-ID of each representative figure, along with the treatment outcome for that participant at the end of follow-up, is listed at the top left corner of each respective graph [functional cure (HBsAg<LLoQ, HBV DNA not detected and normal ALT), partial cure (HBV DNA≤2000 IU/mL, normal ALT), and VR = virological rebound (HBV DNA>2000 IU/mL)]. Participants of early triple therapy are shown in the top row and delayed triple therapy in the bottom row. The dotted horizontal line represents anti-HBs seroconversion (10 mIU/mL). The dashed horizontal line represents anti-HBs TND (0.1 mIU/mL). HBsAg seroconversion threshold or TND (0.01 IU/mL) is the lower boundary of the figure (ie, −2 log IU/mL. All 37 participants’ responses are available in Supplemental Figures S4 and S6 (http://links.lww.com/HC9/A365). Abbreviations: ALT, alanine aminotransferase; LLoQ, lower level of quantification; NAP, nucleic-acid polymer; pegIFN, pegylated interferon-α2a; TDF, tenofovir-disoproxil-fumarate; TND, target not detected.
Anti-HBs were undetectable in 16/19 participants at the beginning of early triple therapy (Table 2). In 10/11 participants with a monophasic decline, anti-HBs seroconversion occurred after a median of 14 (IQR: 5) weeks and had a median anti-HBs increase slope of 0.19 (IQR: 0.05) log mIU/week (Figure 4B). Only 2/4 patients with a biphasic decline in HBsAg (Figure 4C) and none of the NR participants (Figure 4A) achieved anti-HBs seroconversion by EOT (Figure 4A).
The median ALT level at the start of early triple therapy was 35 (IQR: 23) U/L. All participants had a transient increase in ALT to a median 7.2 (IQR: 5.4]-fold peak above ULN at week 12 (IQR: 8.5) of triple therapy. Peak ALT levels were followed by a median decline to 1.5 (IQR: 1.3)-fold above ULN at EOT. In most participants (8/12), ALT peak time was achieved earlier than anti-HBs seroconversion time [median: 2.5 (IQR: 7.8) weeks], and the timing of the 2 events was significantly correlated (R = 0.65, p = 0.02) for participants undergoing early triple therapy (Supplemental Figure S5, http://links.lww.com/HC9/A365). There was no difference in ALT peaks/kinetic parameters between REP 2139-Mg and REP 2165-Mg.
Delayed triple therapy kinetics
During 48 weeks of delayed triple therapy, 3 HBsAg kinetic patterns were identified (Supplemental Figure S6, http://links.lww.com/HC9/A365). Four participants were NR (Figure 4D), 11 had a monophasic decline (Figure 4E), and 3 had a biphasic decline (Figure 4F). The median slope of the HBsAg decline in monophasic HBsAg responses was 0.29 (IQR: 0.14) log IU/mL/week (Supplemental Table S1, http://links.lww.com/HC9/A365) with 9 of the 11 participants reaching LLoQ by median week 20 (IQR: 4). The HBsAg decline following the introduction of NAPs was delayed in patients experiencing a biphasic decline [median: 4 (IQR: 0) weeks] relative to the patients experiencing a monophasic decline [median: 0 (IQR: 2) weeks, p = 0.003]. The median initial rate of decline in biphasic HBsAg responses was 0.66 (IQR: 0.10) log IU/mL/week followed by a slower second-phase decline with slope 0.08 (IQR: 0.01) log IU/week with only 2 patients reaching the LLoQ at EOT. No difference was observed in HBsAg kinetic patterns between participants receiving either REP 2139-Mg or REP 2165-Mg, nor were associations observed between kinetic patterns and the participants’ age or known duration of HBV infection.
Anti-HBs was not detected in 13/18 (72%) participants at baseline. Slightly delayed (relative to early NAPs therapy) (median: 17, IQR: 4 weeks) anti-HBs seroconversion occurred in 10/11 patients with an HBsAg monophasic decline with a median anti-HBs increase slope of 0.15 (IQR: 0.02) log mIU/week. Only 2/3 patients experiencing a biphasic decline in HBsAg and 1/4 of the HBsAg NR participants achieved anti-HBs seroconversion by EOT (Figure 4D).
The median ALT in the delayed triple therapy group started at a baseline value of 72 (IQR: 48) U/L. All participants experienced transient increases in ALT to a median 4.1 (IQR: 3.4)-fold peak above ULN at week 16 (IQR: 14) of triple therapy. Peaks were followed by a decline to a median 1.2 (IQR: 1.1)-fold above ULN at EOT. There was no difference in ALT peaks/kinetic parameters between REP 2139-Mg and REP 2165-Mg.
Combined triple therapy kinetics
Due to ALT kinetics during dual therapy, the baseline ALT values were on average 1.8 times higher before initiation of delayed triple therapy (Table 2) (p < 0.001) relative to ALT levels before starting early triple therapy. ALT level peaks in the early triple therapy group were, on average, 1.8 times higher compared with the delayed group (p = 0.02). ALT elevation at baseline (before the introduction of TDF) was not correlated with the magnitude of ALT flares during triple therapy.
While participants with monophasic HBsAg decline under early triple had ∼2-fold faster (p = 0.02) decline slope compared with participants with monophasic under delayed triple, the overall distributions of parameters such as HBsAg response delay, HBsAg first and second decline slopes in monophasic and biphasic participants, and HBsAg baseline values were not significantly different between the early and delayed triple therapy groups (Supplemental Figure S7 and Table S1, http://links.lww.com/HC9/A365). Anti-HBs seroconversion occurred with similar frequency between both groups, with 12 and 13 total participants experiencing anti-HBs seroconversion in the early and delayed triple therapy groups, respectively. In both groups, the anti-HBs seroconversion was divided into exactly 10 participants with monophasic HBsAg declines and 2 with biphasic HBsAg declines [with one additional HBsAg NR in the delayed group who had anti-HBs seroconversion (Figure 4D)].
Due to their similarities in HBsAg kinetics, the groups were analyzed together for treatment outcomes. The distribution of outcomes for each of the HBsAg kinetic patterns during triple therapy is shown in Figure 5A. All participants who achieved functional cure after the follow-up measurements had a monophasic kinetic pattern during triple therapy. The positive predictive value (PPV) for a monophasic HBsAg decline correlation with functional cure was 64%, and the negative predictive value (NPV) was 100% (sensitivity 100%, specificity 65%). In addition, for patients with a monophasic response, HBsAg reached TND significantly sooner [median: 21 (IQR: 11) weeks vs. median 27 (IQR: 7) weeks, p = 0.012] in patients who reached a functional cure compared with those who did not achieve functional cure (Figure 5B).
FIGURE 5.
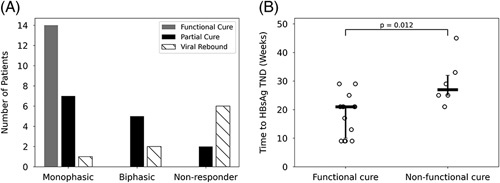
HBsAg kinetic patterns and outcome of therapy. (A) Distribution of therapy outcomes for each HBsAg kinetic pattern. (B) Time to HBsAg TND (0.1 IU/mL) for a functional cure and nonfunctional cure participants with monophasic HBsAg decline. The median and interquartile range are marked as horizontal and vertical lines, respectively. Abbreviation: TND, target not detected.
Anti-HBs seroconversion occurred in 25/37 (68%) participants. Of the 22 participants with monophasic HBsAg responses during triple therapy, 20 (91%) achieved anti-HBs seroconversion. Four of the 7 (57%) participants with biphasic HBsAg responses with triple therapy achieved anti-HBs seroconversion, and only 1 of the 8 (13%) participants who were HBsAg NR with triple therapy achieved anti-HBs seroconversion. Of the 14 participants that achieved functional cure, only 1 participant (02-024) did not achieve anti-HBs seroconversion during therapy but did so at 24 weeks of follow-up. Achieving anti-HBs seroconversion during triple therapy had a 52% PPV and a 92% NPV (sensitivity 93%, specificity 48%) for predicting a functional cure.
DISCUSSION
The goal of novel therapies in development for the treatment of chronic HBV infection is to achieve a functional cure, defined as the absence of detectable HBV DNA and HBsAg in the blood and normal ALT level.30 Establishing high rates of functional cure will likely require a combination of agents, which control viral replication, clear HBsAg from the circulation and liver, and stimulate immune function.31 HBsAg loss during therapy is an essential step toward achieving functional cure13 although functional cure also likely requires reactivation of the adaptive immune response made senescent from exposure to long-term antigen load.32,33 Identifying kinetic patterns associated with a functional cure is integral to developing and optimizing new treatment strategies for chronic HBV.
Three different kinetic HBsAg responses were observed with NAP-based triple therapy: nonresponse (<1.5 log10 IU/mL decline from baseline) in 8/37 participants, monophasic HBsAg decline in 22/37 participants, and biphasic HBsAg decline in 7/37 participants. All participants achieving functional cure experienced a monophasic decline in HBsAg during NAP-based triple therapy, and the presence of a monophasic decline had a PPV of 64% and an NPV of 100% predictive for a functional cure. In addition, the time to HBsAg loss following the introduction of NAP-based triple therapy was significantly faster in participants achieving a functional cure. The pattern of the findings with NAP-based triple therapy is consistent with data from pegIFN monotherapy, where strong and rapid declines in HBsAg by week 24 were associated with HBsAg loss after completion of therapy.34,35
HBsAg turnover is a rapid process, with a published half-life ranging from 1 to 6 days.36,37 NAPs block replenishment of circulating HBsAg by blocking the assembly and secretion of HBV SVPs and drive the reduction and elimination of intrahepatic HBsAg via enhanced intracellular degradation of unassembled HBsAg.22,26,38,39 The rapid monophasic decline of HBsAg observed with NAP-based triple therapy likely largely reflects the suppression of HBsAg replenishment.
HBV infection confounds the immune response by producing a very large excess of SVP that floods the blood with HBsAg protein.40 HBsAg has an inhibitory effect on both innate and adaptive immune functions and is the primary driver of chronically active HBV infection by persistent suppression of immune control.40 A monophasic decline in HBsAg to undetectable levels could remove immune inhibition caused by HBsAg, allowing for the most efficient effect of immunotherapy used in combination (in the case of the current study, pegIFN) to potentially restore immune control over HBV and establish a functional cure. The rates of HBsAg monophasic decline, HBsAg loss, functional cure, and anti-HBs seroconversion were similar between the early and delayed triple therapy groups, suggesting that priming with 24 weeks of dual TDF+pegIFN therapy in the delayed group did not provide a noticeable benefit in patients who subsequently received 48 weeks of NAP-based triple therapy.
In the current study, 25/37 patients achieved anti-HBs seroconversion during triple therapy, which had a PPV of 52% and an NPV of 92% for predicting a functional cure. It is important to note that anti-HBs seroconversion occurred in 4 participants [2 NR (02-018 who achieved anti-HBs seroconversion after triple stopped) and 01-028), 1 monophasic (01-017), 1 biphasic (01-019)] who experienced viral rebound after triple therapy stopped and anti-HBs > 10 mIU/mL was still followed by the re-emergence of viral replication (partial cure).41 The findings indicate that anti-HBs seroconversion could happen during triple therapy or even after treatment cessation without fully suppressing HBV DNA and/or HBsAg production, reminiscent of a recent phase II clinical study in chronic HBV patients treated with therapeutic vaccine BRII-179.42
Liver enzyme elevations during therapy with well-controlled viremia might serve as indicators of host-mediated immune clearance of infected hepatocytes that do not alter liver function but universally improve virologic and liver function outcomes. Previous analyses had indicated that the highly prevalent elevations in ALT levels during NAP-based therapy in the REP 401 study were similarly host-mediated and predicted functional cure when they occurred concomitantly with HBsAg decline to <10 IU/mL during therapy.41 Interestingly, we observed in this analysis that early-onset ALT flares during dual therapy (TDF+pegIFN) in the delayed group were associated with viral rebound, whereas flares were delayed in participants achieving functional cure (Figure 3). In patients with delayed ALT flares during dual therapy, there may be a distinct immune reactivity that is less easily activated but has a greater impact on clearing infected hepatocytes. This remains to be further investigated since outcomes could not be determined clearly from dual therapy alone in this study.
Our study is limited by the fact that the REP 401 study had a restricted HBV genotype (mostly D), mostly male participants, and lacked patients with cirrhosis. The inclusion of an NUC+REP 2139-Mg treatment arm in future studies will be required to examine the virologic response in the absence of pegIFN. It will be important to confirm the predictive effects of the rapid HBsAg declines in the presence of pegIFN with other immunotherapies, such as thymosin α1 or therapeutic vaccines, and in other HBV genotypes.
In summary, rapid monophasic HBsAg decline during NAP-based combination therapy may signal highly efficient targeting of SVP production and predict the achievement of a functional cure.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
REP 401 clinical trial design by Andrew Vaillant and Michel Bazinet. REP 401 trial conduct and data collection was supervised by Victor Pântea and Gheorge Placinta with assistance from Michel Bazinet and Andrew Vaillant. Data curation by Andrew Vaillant. Data modeling and analysis by Leeor Herschkovich and Louis Shekhtman and supervised by Harel Dahari. Manuscription preparation by Leeor Herschkovich with assistance from Louis Shekhtman, Scott J. Cotler, Andrew Vaillant, and Harel Dahari.
FUNDING INFORMATION
The REP 401 clinical trial was supported by Replicor Inc. The current study was supported by NIH grants R01AI144112 and R01AI146917. The funders had no role in the study design, and analysis, the decision to publish, or preparation of the manuscript.
CONFLICTS OF INTEREST
Michel Bazinet and Andrew Vaillant are employees and shareholders of Replicor Inc. and inventors of patents assigned to Replicor. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine aminotransferase; EOT, end of treatment; HBV, hepatitis B virus; HBsAg, hepatitis B virus surface antigen; IQR, interquartile range; LLoQ, lower level of quantification; NAP, nucleic-acid polymer; NPV, negative predictive value; NR, nonresponders; NUC, nucleos(t)ide analog; pegIFN, pegylated interferon α2a; QF, quantifiable; SVP, subviral particle; TDF, tenofovir-disoproxil-fumarate; TND, target not detected; ULN, upper limit of normal.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Leeor Hershkovich, Email: lhershkovich@luc.edu.
Louis Shekhtman, Email: lsheks@gmail.com.
Michel Bazinet, Email: mbazinet@replicor.com.
Victor Pântea, Email: victorpantea@mail.ru.
Gheorge Placinta, Email: gheorgheplacinta@yahoo.com.
Scott J. Cotler, Email: scotler@luc.edu.
Andrew Vaillant, Email: availlant@replicor.com.
Harel Dahari, Email: hdahari@luc.edu.
REFERENCES
- 1. World Health Organization. Interim guidance for country validation of viral hepatitis elimination. Licence: CC BY-NC-SA 3.0 IGO; 2021.
- 2. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Hepatitis B. Key Facts; Accessed May, 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b .
- 4. Pierra Rouviere C, Dousson CB, Tavis JE. HBV replication inhibitors. Antiviral Res. 2020;179:104815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Liu X, Dang Z, Yu L, Jiang Y, Wang X, et al. Nucleos(t)ide analogues for reducing hepatocellular carcinoma in chronic hepatitis B patients: a systematic review and meta-analysis. Gut Liver. 2020;14:232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcellin P, Buti M, Krastev Z, de Man RA, Zeuzem S, Lou L, et al. Kinetics of hepatitis B surface antigen loss in patients with HBeAg-positive chronic hepatitis B treated with tenofovir disoproxil fumarate. J Hepatol. 2014;61:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Striki A, Manolakopoulos S, Deutsch M, Kourikou A, Kontos G, Kranidioti H, et al. Hepatitis B s antigen kinetics during treatment with nucleos(t)ides analogues in patients with hepatitis B e antigen-negative chronic hepatitis B. Liver Int. 2017;37:1642–1650. [DOI] [PubMed] [Google Scholar]
- 8. Zoutendijk R, Hansen BE, van Vuuren AJ, Boucher CA, Janssen HL. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss. J Infect Dis. 2011;204:415–418. [DOI] [PubMed] [Google Scholar]
- 9. Lai CL, Wong D, Ip P, Kopaniszen M, Seto WK, Fung J, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66:275–281. [DOI] [PubMed] [Google Scholar]
- 10. Chow N, Wong D, Lai CL, Mak LY, Fung J, Ma HT, et al. Effect of antiviral treatment on hepatitis B virus integration and hepatocyte clonal expansion. Clin Infect Dis. 2023;76:e801–e809. [DOI] [PubMed] [Google Scholar]
- 11. Dandri M, Petersen J. cccDNA maintenance in chronic hepatitis B—targeting the matrix of viral replication. Infect Drug Resist. 2020;13:3873–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yip TC, Wong VW, Lai MS, Lai JC, Hui VW, Liang LY, et al. Risk of hepatic decompensation but not hepatocellular carcinoma decreases over time in patients with hepatitis B surface antigen loss. J Hepatol. 2023;78:524–533. [DOI] [PubMed] [Google Scholar]
- 13. Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B Surface antigen and long-term clinical outcomes of patients with chronic hepatitis b virus infection: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:463–472. [DOI] [PubMed] [Google Scholar]
- 14. Liang LY, Wong VW, Wong GL, Yip TC. Moving toward hepatitis B virus functional cure - the impact of on-treatment kinetics of serum viral markers. Clin Mol Hepatol. 2023;29:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim WR. Emerging therapies toward a functional cure for hepatitis B virus infection. Gastroenterol Hepatol (N Y). 2018;14:439–442. [PMC free article] [PubMed] [Google Scholar]
- 16. Fang Z, Li J, Yu X, Zhang D, Ren G, Shi B, et al. Polarization of monocytic myeloid-derived suppressor cells by hepatitis B surface antigen is mediated via ERK/IL-6/STAT3 signaling feedback and restrains the activation of T cells in chronic hepatitis B virus infection. J Immunol. 2015;195:4873–4883. [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Ghosh A, Ayithan N, Romani S, Khanam A, Park JJ, et al. Circulating serum HBsAg level is a biomarker for HBV-specific T and B cell responses in chronic hepatitis B patients. Sci Rep. 2020;10:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebosse F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. 2017;66:897–909. [DOI] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 20. Hepatitis B Foundation. Drug watch; Accessed May, 2023. https://www.hepb.org/treatment-and-management/drug-watch/ .
- 21. Blanchet M, Sinnathamby V, Vaillant A, Labonte P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells. Antiviral Res. 2019;164:97–105. [DOI] [PubMed] [Google Scholar]
- 22. Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonte P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res. 2020;183:104853. [DOI] [PubMed] [Google Scholar]
- 23. Boulon R, Angelo L, Blanchet M, Vaillant A, Labonté P. PH-dependent intercation of NAPs with the HSP40 chaperon DnaJB12. Hepatology. 2021;74(suppl):512A. [Google Scholar]
- 24. Bazinet M, Anderson M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, et al. HBsAg isoform dynamics during NAP-based therapy of HBeAg-negative chronic HBV and HBV/HDV infection. Hepatol Commun. 2022;6:1870–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon Alfa-2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology. 2020;158:2180–2194. [DOI] [PubMed] [Google Scholar]
- 26. Roehl I, Seiffert S, Brikh C, Quinet J, Jamard C, Dorfler N, et al. Nucleic acid polymers with accelerated plasma and tissue clearance for chronic hepatitis B therapy. Mol Ther Nucleic Acids. 2017;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Etzion O, Hamid S, Lurie Y, Gane EJ, Yardeni D, Duehren S, et al. Treatment of chronic hepatitis D with peginterferon lambda—the phase 2 LIMT-1 clinical trial. Hepatology. 2023;77:2093–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lhomme S, DebRoy S, Kamar N, Abravanel F, Metsu D, Marion O, et al. Plasma hepatitis E virus kinetics in solid organ transplant patients receiving ribavirin. Viruses. 2019;11:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guedj J, Dahari H, Pohl RT, Ferenci P, Perelson AS. Understanding silibinin’s modes of action against HCV using viral kinetic modeling. J Hepatol. 2012;56:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66:1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol. 2020;73:409–422. [DOI] [PubMed] [Google Scholar]
- 32. Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest. 2018;128:4588–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, et al. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–461. [DOI] [PubMed] [Google Scholar]
- 35. De Ridder F, Sonneveld MJ, Lenz O, Janssen HLA, Talloen W, Hansen BE. Mean HBsAg decline at week 24 of PEG-IFN-based treatment predicts subsequent rate of HBsAg clearance—suggesting a valuable endpoint for early development HBV trials. J Viral Hepat. 2021;28:1563–1569. [DOI] [PubMed] [Google Scholar]
- 36. Shekhtman L, Cotler SJ, Hershkovich L, Uprichard SL, Bazinet M, Pantea V, et al. Modelling hepatitis D virus RNA and HBsAg dynamics during nucleic acid polymer monotherapy suggest rapid turnover of HBsAg. Sci Rep. 2020;10:7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loomba R, Decaris M, Li KW, Shankaran M, Mohammed H, Matthews M, et al. Discovery of half-life of circulating hepatitis B surface antigen in patients with chronic hepatitis B infection using heavy water labeling. Clin Infect Dis. 2019;69:542–545. [DOI] [PubMed] [Google Scholar]
- 38. Noordeen F, Scougall CA, Grosse A, Qiao Q, Ajilian BB, Reaiche-Miller G, et al. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS ONE. 2015;10:e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinet J, Jamard C, Burtin M, Lemasson M, Guerret S, Sureau C, et al. Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks. Hepatology. 2018;67:2127–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaillant A. HBsAg, Subviral particles, and their clearance in establishing a functional cure of chronic hepatitis B virus infection. ACS Infect Dis. 2021;7:1351–1368. [DOI] [PubMed] [Google Scholar]
- 41. Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Benefit of transaminase elevations in establishing functional cure of HBV infection during nap-based combination therapy. J Viral Hepat. 2021;28:817–825. [DOI] [PubMed] [Google Scholar]
- 42. Ma H, Lim TH, Leerapun A, Weltman M, Jia J, Lim YS, et al. Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep. 2021;3:100361. [DOI] [PMC free article] [PubMed] [Google Scholar]


