Abstract
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation is followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors’ suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.
People with HIV (PWH) have an increased lifetime risk of developing certain cancers, even when HIV is well-controlled with antiretroviral therapy. Despite the tremendous advancements in HIV and cancer care over the past several decades, PWH have lower cancer-related survival compared with the general population. Treating HIV-associated cancers requires a multidisciplinary team to manage concurrent opportunistic infections, potential drug-drug interactions, and the co-occurrence of more than one cancer in the same patient. Many factors may lead PWH to receive inappropriate dose adjustments, exclusion from emerging therapies and clinical trials, or no cancer therapy at all. In general, PWH should receive the same standard, full-dose cancer therapy used in the general population unless there are data for specific cancer regimens in PWH. Agents targeting PD-1 and PD-L1 have US Food and Drug Administration (FDA)–approved indications in many HIV-associated cancers, including Hodgkin lymphoma, cervical cancer, head and neck cancer, hepatocellular carcinoma, and non–small-cell lung cancer; however, PWH were excluded from all clinical trials that led to FDA approval of these agents. Several prospective studies and an international retrospective study of PWH with advanced cancer have shown anti–PD-(L)-1 agents to be safe and effective across expected cancer types and CD4+ T-cell counts, supporting their use in PWH for FDA-approved indications. Learning from the experience in anti–PD-(L)-1 agents, future cancer clinical trials should include and seek to actively enroll PWH, so that they have equal and timely access to emerging cancer therapies.
CASE PRESENTATION
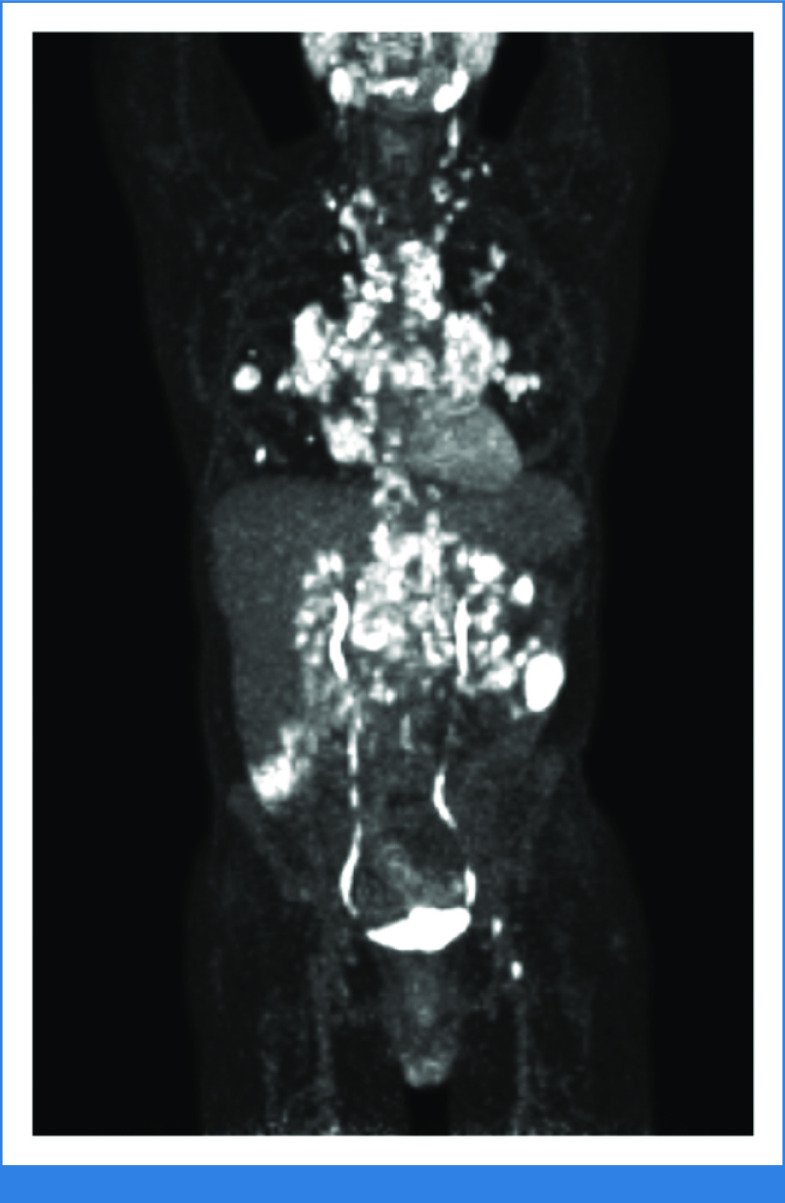
A 28-year-old cisgender man was diagnosed with HIV at a mobile testing clinic. He was referred to an HIV specialist and immediately started on bictegravir/emtricitabine/tenofovir alafenamide (B/FTC/TAF). Before starting B/FTC/TAF, his HIV viral load was 520,000 copies/mL and his CD4+ T-cell count was 350 cells/µL. Opportunistic infection (OI) screening revealed secondary syphilis, and he received penicillin. Three months later, he presented to his HIV specialist with fevers, night sweats, fatigue, and bilateral neck swelling. He was adherent to B/FTC/TAF with an undetectable HIV viral load and a CD4+ T-cell count of 300 cells/µL. Cervical lymph node biopsy revealed Epstein-Barr virus (EBV)–positive mixed cellularity classical Hodgkin lymphoma (HL). Positron emission tomography-computed tomography (PET-CT) showed 18-fluorodeoxyglucose (18FDG)–avid lymph nodes in the cervical, mediastinal, and retroperitoneal chains along with 18FDG-avid masses within the lung parenchyma consistent with stage IV disease. He received six cycles of brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A + AVD) with a complete response on end-of-treatment PET-CT. The disease relapsed 13 months later, and he received second-line chemotherapy followed by autologous hematopoietic stem-cell transplant with a complete response. The patient experienced fevers, night sweats, and fatigue along with new shortness of breath 6 months later, with PET-CT showing widespread 18FDG-avid disease (Fig 1). Supraclavicular lymph node biopsy again revealed relapsed HL. His HIV was well-controlled on B/FTC/TAF with an undetectable HIV viral load and CD4+ T-cell count of 110 cells/µL. He was referred to you for consideration of anti–PD-1 therapy for relapsed HL.
FIG 1.
Positron emission tomography-computed tomography at the time of second relapse of HIV-associated Hodgkin lymphoma showing widespread 18-fluorodeoxyglucose-avid disease in the neck, mediastinum, lungs, porta hepatis, mesentery, retroperitoneum, left peritoneum, and left inguinal region.
CLINICAL CHALLENGES IN EVALUATION AND TREATMENT
In the current era of effective and widely available antiretroviral therapy (ART), cancer is one of the most common causes of morbidity and mortality in people with HIV (PWH) worldwide.1 The lifetime risk of developing certain cancers is significantly increased in PWH compared with the general population. For example, the lifetime risk of developing HL in PWH is 5- to 20-fold greater than in the general population, and the period of highest risk of HL diagnosis is in the months after ART initiation.2 HIV causes T-cell loss and dysfunction, B-cell dysfunction, chronic inflammation, and cytokine disarray resulting in decreased immunosurveillance and proliferation of oncogenic viruses and cancer cells. ART cannot completely reverse these effects, so even people with well-controlled HIV remain at increased risk of cancer. HIV-associated cancers can generally be categorized by how strongly their incidence is associated with CD4+ lymphopenia. Kaposi sarcoma, primary central nervous system lymphoma, and other B-cell non-Hodgkin lymphomas (NHL) tend to occur at higher levels of immunosuppression and lower CD4+ T-cell counts, whereas HL, cervical cancer, anogenital cancers, head and neck cancers, hepatocellular carcinoma, lung cancer, and nonmelanoma skin cancers tend to occur in less immunocompromised patients with preserved CD4+ T-cell counts.1 A diagnosis of any of these cancers should prompt HIV testing if a patient's HIV status is unknown. Recent immunosuppression and prolonged HIV viremia are associated with an increased risk of NHL, whereas a low historic CD4+ nadir and an extended proportion of time when the CD4+ T-cell count is <200 cells/µL are associated with an increased risk of HPV-associated cancers. As PWH are living longer with near-normal life expectancies, they may also develop incidental cancers that are common in the general population. The historic categories of AIDS-defining and non–AIDS-defining cancers have become obsolete in the era of ART and evolving cancer therapy. AIDS-defining includes outdated nomenclature for NHL, excludes cancers with strong associations with CD4+ lymphopenia, and was originally meant to identify patients who should be tested for HIV. The widespread use of ART has shifted the epidemiology of HIV-associated cancers. The incidence rate of cancers associated with more advanced immunosuppression, such as Kaposi sarcoma and primary central nervous system lymphoma, have drastically decreased since the beginning of the HIV pandemic, but cancers associated with less immunosuppression, such as HL and anal cancer, have increased over time.3 With the important exception of lung cancer, many HIV-associated cancers have strong associations with oncogenic viruses: EBV, human papillomavirus (HPV), hepatitis B and C viruses (HBV and HCV, respectively), Merkel cell polyomavirus, and Kaposi sarcoma herpesvirus (also known as human herpesvirus 8). The association with these viruses is greater in PWH than in the general population. HIV-associated HL is associated with EBV almost 100% of the time.
Despite the tremendous advancements in HIV and cancer care over the past several decades, PWH have lower cancer-related survival compared with the general population.4 PWH tend to present with more advanced-stage disease and have tumors with more high-risk features. In HIV-associated HL, B symptoms and extranodal sites of disease are more common.2 Concurrent OIs, avoiding drug-drug interactions (DDI), and the co-occurrence of more than one cancer in the same patient can significantly complicate cancer treatment, particularly in patients with severe immunosuppression, highlighting the need for multidisciplinary care. Within the health care system, patients are stigmatized because of their HIV status and as well as their sexual orientation, gender identity, race, immigration status, and socioeconomic status. In addition, most oncologists do not receive formal training in HIV-associated cancers. These factors may lead to PWH receiving inappropriate dose reductions, exclusion from emerging therapies and clinical trials, or no cancer therapy at all.5
SUMMARY OF THE RELEVANT LITERATURE
At least for certain cancers that occur at higher CD4+ T-cell counts, including HL, there is evidence that when PWH receive the same treatment as the general population, cancer outcomes are the same.6-8 PWH should generally receive the standard therapy for their cancer and HIV infection itself is not a reason to alter therapy or adjust treatment doses as recommended by National Comprehensive Cancer Network guidelines for cancer in PWH.9 There have been a few studies evaluating first-line treatment of HIV-associated HL. SWOG S0816 evaluated PET-adapted therapy with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in stage III-IV HL and included a small number of PWH. This multicenter phase II study showed this treatment approach was feasible in PWH and had similar outcomes to HIV-negative participants. The phase II AIDS Malignancy Consortium (AMC) study 085 evaluated A + AVD in stage II-IV HIV-associated HL and showed comparable safety and efficacy outcomes to the ECHELON-1 study of A + AVD in stage III-IV HIV-negative HL. The 2-year progression-free survival was 85% in AMC 085 compared with 82% in ECHELON-1. Rates of neutropenia and neuropathy were higher in AMC 085 compared with ECHELON-1 but remained manageable. Most impressive were the notable increases in the participants' CD4+ and CD8+ T-cell counts during therapy.10 Two enrolled participants were later excluded because their ART regimen included the CYP3A4 inhibitor, ritonavir, an exclusion criterion, underscoring the need for communication between oncology and HIV providers.
Despite recommendations from the American Society of Clinical Oncology and US Food and Drug Administration (FDA) and efforts of the National Cancer Institute (NCI), PWH are too frequently unnecessarily excluded from the majority of cancer clinical trials.11,12 In regard to anti–PD-(L)1 agents, PWH were excluded from all trials that led to FDA approval of these agents, although PWH are at higher risk for the cancers for which these agents are approved, including HL, cervical cancer, head and neck cancer, non–small-cell lung cancer, and hepatocellular carcinoma.13 Anti–PD-1 agents, nivolumab and pembrolizumab, are FDA-approved for relapsed or refractory HL, with overall response rates of 65%-70%.14,15
Anti–PD-(L)1 agents are attractive agents for HIV-associated cancers as they are T-cell-sparing and allow for immune reconstitution after HIV or prior cancer therapy. Immune reconstitution with ART is integral to cancer control in PWH, and there are reports of cancer resolution with ART alone. HIV viremia after cancer therapy is associated with increased mortality, emphasizing the importance of continuing ART alongside cancer therapy.16 Anti–PD-(L)1 agents have a beneficial effect on the underlying HIV as they have shown to induce HIV latency reversal and in combination with other interventions may reduce the HIV reservoir.17 Anti–PD-(L)1 agents may also be particularly effective in HIV-associated cancers as HIV causes upregulation of PD-1 expression in both HIV-specific CD4+ and CD8+ T cells and in T cells specific for viral antigens or tumor neoantigens. These HIV-related effects are not completely reversed by ART alone.13 Mounting evidence, which includes two prospective clinical trials, a systematic review, and most recently an international retrospective study of 390 PWH and advanced cancer (in the accompanying article by El Zarif et al18), convincingly shows that anti–PD-(L)1 agents are safe in patients with well-controlled HIV.19-21 Comparisons between people with and without HIV receiving anti–PD-(L)1 therapies with CD4+ T-cell counts <350 cells/µL have not shown significant differences in rates of immune-mediated adverse events.22 There are also data to support the safety of anti–PD-(L)1 agents with chronic infections frequently seen in PWH. For example, there are data for the safe use of these agents in people with HBV receiving concurrent suppressive HBV therapy as well as in people with untreated HCV.13 One important safety consideration, however, is concurrent M tuberculosis infection in PWH, as anti–PD-(L)1 agents have been associated with primary infection or reactivation of M tuberculosis.23
HIV-related CD4+ T-cell lymphopenia has raised concerns about the efficacy of anti–PD-(L)1 agents in PWH. However, the prospective and retrospective studies of these agents in PWH have shown efficacy across a range of expected cancer types (eg, non–small-cell lung cancer, head and neck cancer, and hepatocellular carcinoma). In a matched cohort of patients with metastatic non–small-cell lung cancer, El Zarif et al18 showed that cancer outcomes were similar between people with and without HIV across CD4+ T-cell counts, including <200 cells/µL. There is a notable lack of data specific to the efficacy of anti–PD-(L)1 agents in HIV-associated HL. In PWH and cancer, low CD4+ T-cell counts may be related to HIV but also related to prior cancer therapies and do not reflect ongoing immunosuppression from HIV. It should be noted that many patients with relapsed or refractory cancers who do not have HIV also have low CD4+ T-cell counts related to prior cancer therapy.
APPROACH TO MANAGEMENT
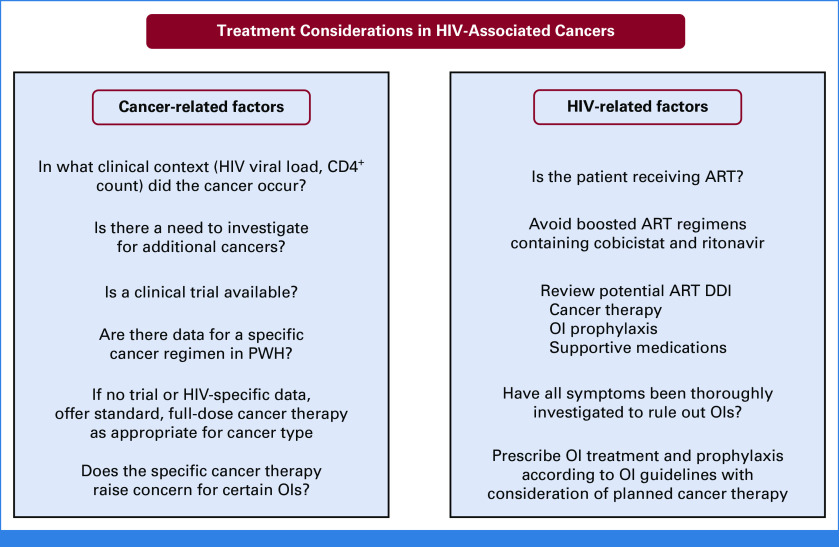
As with all patients with cancer, PWH should be offered a clinical trial if one is available (Fig 2). The AMC runs clinical trials specifically for PWH with sites in the United States and countries in sub-Saharan Africa and South America. It is also imperative that clinical investigators broaden eligibility criteria for the inclusion of PWH. Notably, the NCI Cancer Therapy Evaluation Program has an initiative to include PWH in all NCI-sponsored trials unless there is a compelling scientific reason to exclude them.24 If no clinical trial is available, I evaluate whether there are data in PWH for the patient's cancer type. Evidence specific to PWH is generally scarce, but there are data for certain chemotherapy regimens, particularly for HIV-associated lymphomas.10,25 There is also specific evidence supporting the safety and feasibility of autologous and allogeneic hematopoietic stem-cell transplant in PWH.26 If there are no data specific to PWH for the patient's cancer type, I proceed with standard therapy. Per guideline recommendations, I do not take HIV status into account when determining regimen or dosing.9 I consider standard indications for dose-adjustments such as performance status, organ function, and DDI, but avoid doing so when the intent of treatment is cure.
FIG 2.
Treatment of HIV-associated cancers should consider both cancer- and HIV-related factors to determine the treatment plan. The treatment team should be multidisciplinary and include an oncologist, HIV specialist, pharmacist, and social worker. Although these treatment considerations are important at the time of diagnosis and choosing a cancer treatment plan, the health care team should remain vigilant for the development of OIs and continue to review potential DDI throughout cancer treatment. ART, antiretroviral therapy; DDI, drug-drug interactions; OI, opportunistic infections; PWH, people with HIV.
For FDA-approved indications, I routinely give anti–PD-(L)1 agents to PWH without regard to CD4+ T-cell count or timing of the initiation of ART, including patients with detectable HIV viremia. Before initiation of anti–PD-(L)1 therapy, I document HBV and HCV status and ensure patients with chronic HBV are receiving suppressive therapy with tenofovir and emtricitabine or lamivudine and closely monitor liver enzymes and function tests in patients with HBV and untreated HCV throughout treatment. I assess M tuberculosis testing and treatment history, and perform chest imaging in patients with a positive interferon-gamma release assay to evaluate for active infection. I treat all patients with active and latent M tuberculosis infection, carefully considering DDI, to prevent M tuberculosis–related immune reconstitution inflammatory syndrome. M tuberculosis treatment is also important because TNF-alpha inhibitors that are used in rare cases of refractory immune-mediated adverse events are associated with reactivation and severe infection of M tuberculosis.
For relapsed or refractory HIV-associated cancers, AMC 095 is an ongoing phase 1 trial of nivolumab in combination with ipilimumab, a CTLA-4 inhibitor, for advanced HIV-associated cancers, including an HL cohort (ClinicalTrials.gov identifier: NCT02408861). The NCI intramural program in Bethesda, Maryland, also has an ongoing phase I trial of nivolumab in combination with the immunomodulatory agent, pomalidomide, for advanced virus-associated cancers, which includes relapsed and refractory EBV-associated HL (ClinicalTrials.gov identifier: NCT04902443). When clinical trials are not available, I treat relapsed or refractory HIV-associated HL with either of the FDA-approved anti–PD-1 agents, pembrolizumab or nivolumab, in concert with my HIV and pharmacy colleagues. Given the increased risk of HL in PWH, the importance of anti–PD-(L)1 agents in HL treatment, and the safety data of these agents in other HIV-associated cancers, it is imperative that future studies of anti–PD-(L)1 agents include PWH.
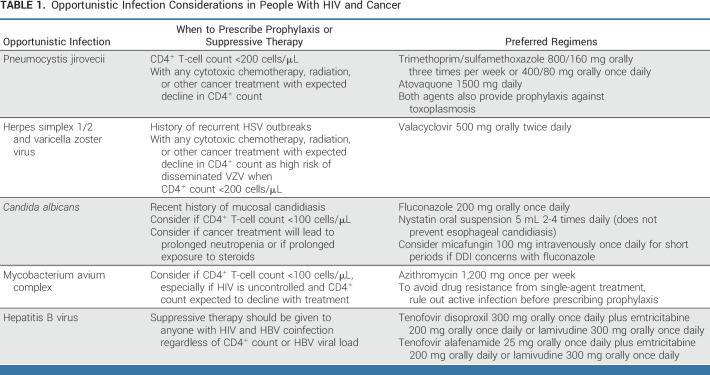
When evaluating PWH with a new cancer diagnosis or at the time of cancer relapse, it is important to understand a patient's current immune status. Did the cancer occur in the setting of controlled or uncontrolled HIV and at what level of immunosuppression? Answering these questions provides an understanding of the biological and clinical scenario in which the cancer occurred and guides the workup for OIs as well as other cancers. Cancer should not be assumed to be the single cause of a patient's symptoms or laboratory and radiologic abnormalities. Particularly for patients with CD4+ T-cell counts <200 cells/µL, a detailed investigation for OIs is required. For example, in a patient with shortness of breath and lung metastases, pneumocystis pneumonia should be ruled out with sputum evaluation and potentially bronchoscopy. OI screening will be guided by the level of immunosuppression and the endemic pathogens of where a patient has lived or traveled in consultation with an HIV specialist (Table 1). Interpreting imaging in PWH, especially those with uncontrolled or recently uncontrolled HIV, can be difficult. For example, in HIV-associated NHL, interim FDG-PET has an excellent negative but poor positive predictive value.25 Follicular hyperplasia is a benign cause of lymphadenopathy that can confound cancer staging and response to therapy. Certain OIs such as M avium complex can cause PET-avid lymphadenopathy as well as organ infiltration. Patients with advanced HIV may have multiple cancers, especially patients with severe immunosuppression. In these patients, additional tissue biopsies can be helpful to exclude the presence of other conditions even after a confirmed diagnosis of cancer. Identifying additional cancers or OIs should not necessarily dissuade clinicians from seeking aggressive cancer treatment options.
TABLE 1.
Opportunistic Infection Considerations in People With HIV and Cancer
A multidisciplinary and holistic approach is needed to care for PWH and cancer. Oncologists should engage with an HIV specialist and a pharmacist with both oncology and HIV expertise. DDI need to be carefully considered, especially when treating with small molecule inhibitors, but are less of a concern when using monoclonal antibodies. I take a considerable role in HIV treatment and monitoring while patients are undergoing cancer therapy. Many HIV specialists do not have the expertise to know the expected level and duration of immunosuppression from a particular cancer regimen or the expected toxicities requiring supportive medications that could have potential DDI. Thus, it is important for oncologists to play a leading and coordinating role in the care of PWH and cancer. In addition, social work involvement is essential to assist patients with reliable transportation or address issues with food security, housing, and the social support needed when undergoing cancer therapy. Consulting with an endocrine specialist may be helpful when caring for patients who are transgender.
All PWH should be on ART throughout the course of cancer management, and there is almost no clinical scenario in which I discontinue ART. However, I sometimes do change the ART regimen depending on the cancer treatment plan, presence of OIs, and need for supportive medications. So called boosted ART regimens include either ritonavir or cobicistat, which inhibit the CYP3A4 pathway and help maintain therapeutic levels of protease inhibitors or certain integrase inhibitors in the regimen. Ritonavir and cobicistat have major DDI with many anticancer therapies. I generally avoid boosted regimens even when they do not interact with the cancer therapy because they interact with many drug classes commonly used for infection prophylaxis, such as azoles used for candida prophylaxis, and supportive medications, such as neurokinin-1 receptor antagonists. Currently, most patients can receive integrase strand inhibitor–based ART regimens that have few DDI. In the few patients who have resistant HIV and require a boosted ART regimen for HIV suppression, I work with a pharmacist to dose-modify the interacting cancer agent or find alternative cancer treatment. I try to avoid this whenever possible, particularly when the cancer treatment is curative. As another consideration, in patients receiving nephrotoxic treatments, such as cisplatin, I try to avoid ART regimens that contain tenofovir, which is also nephrotoxic. Any changes in ART must be done in consultation with an HIV specialist as there may be reasons for specific ART regimens, such as HIV resistance or concurrent HBV, that would preclude certain drug changes.
It is important to monitor CD4+ T-cell count and HIV viral load at baseline and at 8- to 12-week intervals throughout treatment to ensure patients are adhering to ART therapy and receiving appropriate infection prophylaxis on the basis of the level of immunosuppression. It is important to work with a pharmacist to evaluate for DDI with cancer therapies and supportive medications when choosing infection prophylaxis. In patients with a detectable HIV viral load, I work with an HIV specialist to send a resistance panel in case the ART regimen needs to be adjusted for resistance or DDI. OI guidelines are useful to guide treatment; however, there are almost no data in PWH and cancer. Therefore, clinical judgment on the part of the oncologist is required. Because CD4+ T-cell counts decrease with many cytotoxic cancer therapies and radiation therapy, I prescribe either trimethoprim/sulfamethoxazole or atovaquone for pneumocystis pneumonia prophylaxis and valacyclovir for herpes simplex 1/2 and varicella zoster prophylaxis in the majority of patients, even in those with normal CD4+ T-cell counts at the start of treatment. In patients with CD4+ T-cell counts <100 cells/µL, I strongly consider M avium complex prophylaxis with azithromycin once per week. Prolonged neutropenia and the use of steroids also increase the risk of certain infections, such as candida, and may require antifungal prophylaxis. In most circumstances, I do not prescribe antifungal prophylaxis with fluconazole because of its considerable DDI.
The management of HIV-associated cancers can be complex and requires a multidisciplinary team that works actively to deliver health care that is free of bias and avoids stigmatizing patients. Cancer incidence is likely to increase in the population of PWH as they live longer, and oncologists need to be prepared to care for them and offer clinical trials and emerging cancer therapies. The prospective and real-world evidence that anti–PD-(L)1 agents are safe and can be effective in PWH should provide confidence to oncologists to offer these therapies to PWH for FDA-approved indications. For the patient in the case presentation, I recommended standard therapy with anti–PD-1 inhibitor, pembrolizumab 200 mg intravenously once every 3 weeks, with testing to rule out HBV and HCV and M tuberculosis before starting treatment. Although his CD4+ T-cell count was low, his shortness of breath was likely due to HL involvement in the lungs, so sputum testing was sufficient to rule out Pneumocystis jirovecii. I started trimethoprim/sulfamethoxazole 800/160 mg orally three times per week for pneumocystis prophylaxis and valacyclovir orally 500 mg twice daily for herpes simplex virus 1/2 and varicella prophylaxis. After four cycles of pembrolizumab, he had resolution of his fatigue, B symptoms, and shortness of breath, and PET-CT showed a complete response. At this time, his CD4+ T-cell count had improved to 240 cell/µL, and his HIV viral load remained undetectable on B/FTC/TAF. He continued pembrolizumab and I referred him for allogeneic hematopoietic stem-cell transplant. As PWH were excluded from prior anti–PD-(L)1 therapy trials, their access to these agents has been significantly delayed, although anti–PD-(L)1 therapies are approved in many cancers that occur at higher rates in PWH. We now have robust data supporting the use of these agents in PWH, and future clinical trials should include and seek to actively enroll PWH, so they have equal and timely access to emerging cancer therapies.
ACKNOWLEDGMENT
The author thanks Drs Ramya Ramaswami, Thomas Uldrick, and Robert Yarchoan for their review of the manuscript.
Kathryn Lurain
Research Funding: Merck (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Miltenyi Biotec (Inst), Janssen Oncology (Inst), Bristol Myers Squibb/Celgene (Inst).
No other potential conflicts of interest were reported.
See accompanying Article, p. 3712
SUPPORT
Supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treating Cancer in People With HIV
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to http://www.asco.org/rwc or https://ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathryn Lurain
Research Funding: Merck (Inst), EMD Serono (Inst), CTI BioPharma Corp (Inst), Miltenyi Biotec (Inst), Janssen Oncology (Inst), Bristol Myers Squibb/Celgene (Inst).
No other potential conflicts of interest were reported.
REFERENCES
- 1. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378:1029–1041. doi: 10.1056/NEJMra1615896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uldrick TS, Little RF. How I treat classical Hodgkin lymphoma in patients infected with human immunodeficiency virus. Blood. 2015;125:1226–1235. doi: 10.1182/blood-2014-08-551598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez-Ramirez RU, Shiels MS, Dubrow R, et al. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV. 2017;4:e495–e504. doi: 10.1016/S2352-3018(17)30125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coghill AE, Shiels MS, Suneja G, et al. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33:2376–2383. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suneja G, Shiels MS, Angulo R, et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol. 2014;32:2344–2350. doi: 10.1200/JCO.2013.54.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30:4111–4116. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated burkitt lymphoma. J Clin Oncol. 2020;38:2519–2529. doi: 10.1200/JCO.20.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danilov AV, Li H, Press OW, et al. Feasibility of interim positron emission tomography (PET)-adapted therapy in HIV-positive patients with advanced Hodgkin lymphoma (HL): A sub-analysis of SWOG S0816 phase 2 trial. Leuk Lymphoma. 2017;58:461–465. doi: 10.1080/10428194.2016.1201573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reid E, Suneja G, Ambinder RF, et al. Cancer in people living with HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018;16:986–1017. doi: 10.6004/jnccn.2018.0066. [DOI] [PubMed] [Google Scholar]
- 10. Rubinstein PG, Moore PC, Bimali M, et al. Safety and efficacy of brentuximab vedotin in combination with AVD in stage II-IV HIV-associated classical Hodgkin lymphoma: Results of the phase 2 study, AMC 085. Blood. 2019;134:130. [Google Scholar]
- 11. Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. J Clin Oncol. 2017;35:3774–3780. doi: 10.1200/JCO.2017.73.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration: Cancer clinical trial eligibility criteria: Patients with HIV, Hepatitis B Virus, or Hepatitis C Virus infections: Guidance for industry. July 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-patients-hiv-hepatitis-b-virus-or-hepatitis-c-virus. [Google Scholar]
- 13. Lurain K, Ramaswami R, Yarchoan R, et al. Anti-PD-1 and anti-PD-L1 monoclonal antibodies in people living with HIV and cancer. Curr HIV/AIDS Rep. 2020;17:547–556. doi: 10.1007/s11904-020-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gopal S, Patel MR, Yanik EL, et al. Association of early HIV viremia with mortality after HIV-associated lymphoma. AIDS. 2013;27:2365–2373. doi: 10.1097/QAD.0b013e3283635232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uldrick TS, Adams SV, Fromentin R, et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci Transl Med. 2022;14:eabl3836. doi: 10.1126/scitranslmed.abl3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El Zarif T, Nassar AH, Adib E, et al. Safety and activity of immune checkpoint inhibitors in people living with HIV and cancer: A real-world report from the Cancer Therapy using Checkpoint inhibitors in people living with HIV-International (CATCH-IT) consortium. J Clin Oncol. 2023;41:3712–3723. doi: 10.1200/JCO.22.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uldrick TS, Goncalves PH, Abdul-Hay M, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-A phase 1 study. JAMA Oncol. 2019;5:1332–1339. doi: 10.1001/jamaoncol.2019.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez-Cao M, Moran T, Dalmau J, et al. Assessment of the feasibility and safety of durvalumab for treatment of Solid tumors in patients with HIV-1 infection: The phase 2 DURVAST study. JAMA Oncol. 2020;6:1063–1067. doi: 10.1001/jamaoncol.2020.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: A systematic review. JAMA Oncol. 2019;5:1049–1054. doi: 10.1001/jamaoncol.2018.6737. [DOI] [PubMed] [Google Scholar]
- 22. Odeny TA, Lurain K, Strauss J, et al. Effect of CD4+ T cell count on treatment-emergent adverse events among patients with and without HIV receiving immunotherapy for advanced cancer. J ImmunoTherapy Cancer. 2022;10:e005128. doi: 10.1136/jitc-2022-005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langan EA, Graetz V, Allerheiligen J, et al. Immune checkpoint inhibitors and tuberculosis: An old disease in a new context. Lancet Oncol. 2020;21:e55–e65. doi: 10.1016/S1470-2045(19)30674-6. [DOI] [PubMed] [Google Scholar]
- 24. Reuss JE, Stern D, Foster JC, et al. Assessment of cancer therapy evaluation program advocacy and inclusion rates of people living with HIV in anti-PD1/PDL1 clinical trials. JAMA Netw Open. 2020;3:e2027110. doi: 10.1001/jamanetworkopen.2020.27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ambinder RF, Capoferri AA, Durand CM. Haemopoietic cell transplantation in patients living with HIV. Lancet HIV. 2020;7:e652–e660. doi: 10.1016/S2352-3018(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]