PURPOSE
The standard of care for locally advanced rectal cancer in North America is neoadjuvant pelvic chemoradiation with fluorouracil (5FUCRT). Neoadjuvant chemotherapy with fluorouracil and oxaliplatin (FOLFOX) is an alternative that may spare patients the morbidity of radiation. Understanding the relative patient experiences with these options is necessary to inform treatment decisions.
METHODS
PROSPECT was a multicenter, unblinded, noninferiority, randomized trial of neoadjuvant FOLFOX versus 5FUCRT, which enrolled adults with rectal cancer clinically staged as T2N+, cT3N–, or cT3N+ who were candidates for sphincter-sparing surgery. Neoadjuvant FOLFOX was given in six cycles over 12 weeks, followed by surgery. Neoadjuvant 5FUCRT was delivered in 28 fractions over 5.5 weeks, followed by surgery. Adjuvant chemotherapy was suggested but not mandated in both groups. Enrolled patients were asked to provide patient-reported outcomes (PROs) at baseline, during neoadjuvant treatment, and at 12 months after surgery. PROs included 14 symptoms from the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Additional PRO instruments measured bowel, bladder, sexual function, and health-related quality of life (HRQL).
RESULTS
From June 2012 to December 2018, 1,194 patients were randomly assigned, 1,128 initiated treatment, and 940 contributed PRO-CTCAE data (493 FOLFOX; 447 5FUCRT). During neoadjuvant treatment, patients reported significantly lower rates of diarrhea and better overall bowel function with FOLFOX while anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting were lower with 5FUCRT (all multiplicity adjusted P < .05). At 12 months after surgery, patients randomly assigned to FOLFOX reported significantly lower rates of fatigue and neuropathy and better sexual function versus 5FUCRT (all multiplicity adjusted P < .05). Neither bladder function nor HRQL differed between groups at any time point.
CONCLUSION
For patients with locally advanced rectal cancer choosing between neoadjuvant FOLFOX and 5FUCRT, the distinctive PRO profiles inform treatment selection and shared decision making.
INTRODUCTION
The benefits of cancer therapies often come at the expense of adverse events, causing symptoms that impair functioning and quality of life.1-3 A goal of cancer clinical research and care delivery is to optimize the benefit-to-risk ratio of therapies and to inform patients and clinicians about short-term and long-term symptomatic adverse events so they can make informed decisions.4
CONTEXT
Key Objective
In the neoadjuvant treatment of locally advanced rectal cancer, do patient-reported outcomes differ between chemotherapy with fluorouracil and oxaliplatin (FOLFOX) versus pelvic chemoradiation with fluorouracil (5FUCRT)?
Knowledge Generated
During neoadjuvant treatment, patients receiving FOLFOX experienced less diarrhea and better bowel function, whereas those receiving 5FUCRT had less anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting. In contrast, at 6, 12, and 18 months after treatment, patients receiving FOLFOX had less fatigue and neuropathy and better sexual function, compared with 5FUCRT. Overall quality of life remained similar between treatment groups during and after treatment.
Relevance (A.H. Ko)
-
As treatment paradigms evolve for locally advanced rectal cancer, the quality of life and toxicity data from this large PROSPECT trial—as reported directly by patients—can help inform decision-making and counseling patients in deciding between neoadjuvant FOLFOX or fluorouracil-based chemoradiation.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Guidance from international regulatory authorities and professional consensus statements has emphasized the importance of incorporating the patient experience in the assessment of cancer treatment tolerability, through the systematic evaluation of patient-reported outcomes (PROs).5-9 To enable patient self-reporting of symptomatic adverse events in cancer trials, the National Cancer Institute (NCI) developed the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).10 When there are treatment options with similar clinical benefits, a comparative understanding of adverse event profiles, including time course and severity levels, may particularly inform individual treatment choices.
Since 1990, management for patients with locally advanced rectal cancer has included pelvic chemoradiation with fluorouracil (5FUCRT) administered in 28 fractions over 5.5 weeks to reduce the risk of pelvic recurrence, an outcome with high morbidity,11 with neoadjuvant 5FUCRT demonstrating better local control and tolerability than adjuvant 5FUCRT.12 Yet, 5FUCRT is associated with both short-term and long-term AEs, particularly impaired bowel and sexual function.13,14
The PROSPECT (N1048) randomized trial was designed to investigate whether neoadjuvant chemotherapy with fluorouracil and oxaliplatin (FOLFOX), with chemoradiation reserved only for the subset of patients intolerant of chemotherapy or whose tumors do not respond well to chemotherapy (<20% radiographic response), is noninferior to 5FUCRT for patients with clinical stage cT2N+, cT3N–, and cT3N+ tumors. As described by Schrag et al15 in a manuscript reporting the primary clinical efficacy outcomes of the PROSPECT trial, neoadjuvant FOLFOX was found to be noninferior to neoadjuvant 5FUCRT for the primary end point of disease-free survival, and there were no significant differences between treatment groups for secondary efficacy outcomes including overall survival, local recurrence, complete resection rate, and pathologic complete response. To understand the comparative patient experiences with treatments in the PROSPECT trial, we evaluated PRO-CTCAE and measures of health-related quality of life (HRQL) between those assigned to neoadjuvant FOLFOX versus neoadjuvant 5FUCRT before sphincter-sparing surgery.
METHODS
Trial Design and Patients
PROSPECT (North Central Cancer Treatment Group [now part of the Alliance for Clinical Trials in Oncology] N1048; ClinicalTrials.gov identifier: NCT01515787) was a multicenter, unblinded, randomized controlled noninferiority trial comparing neoadjuvant FOLFOX with selective use of pelvic chemoradiation (intervention) to neoadjuvant 5FUCRT (control) conducted in the United States, Canada, and Switzerland. Eligible patients were adults with previously untreated locally advanced rectal cancer, clinically staged as cT2N+, cT3N–, or cT3N+ on the basis of the American Joint Committee on Cancer seventh edition of the tumor node metastasis staging system. In addition, to be eligible, the patient's surgeon had to determine that neoadjuvant 5FUCRT followed by sphincter-sparing surgery was the indicated standard treatment. Random assignment was 1:1 using dynamic allocation with stratification by Eastern Cooperative Oncology Group performance status score (0-1 v 2). The trial Protocol (online only) was approved by the NCI central institutional review board and/or institutional review board at each participating institution. All patients provided written informed consent before enrolling.
Patients in the 5FUCRT group received neoadjuvant pelvic chemoradiation in 28 daily fractions accompanied by sensitizing chemotherapy with either fluorouracil or capecitabine over a 6-week period, followed by surgery. Patients in the FOLFOX group received six cycles of FOLFOX without radiation every 2 weeks over a 12-week period, followed by pelvic imaging and rectal endoscopy. If the primary tumor decreased in size by ≥20% compared with baseline, surgery was performed. Otherwise, patients received neoadjuvant 5FUCRT. Patients unable to complete at least five cycles of FOLFOX were also prescribed neoadjuvant 5FUCRT. In both groups, adjuvant treatment was also suggested but not mandated. Specifically, for the 5FUCRT group, eight cycles of adjuvant FOLFOX or capecitabine with oxaliplatin (CAPOX) were suggested, and for the FOLFOX group, an additional six cycles of FOLFOX or CAPOX were suggested.
End Points
PROSPECT was designed as a noninferiority trial, with the primary end point of disease-free survival. Prespecified secondary end points included overall survival, surgical and pathologic outcomes, and PROs, the focus of this manuscript. Prespecified PRO end points included the PRO-CTCAE and additional PRO measures of bowel, bladder, and sexual function, as well as overall HRQL, with the purpose to characterize the patient experience between treatment groups.
The PRO-CTCAE is a validated library of patient-reported items that measure 78 distinct symptomatic adverse events.10,16 For each adverse event, up to three individual attribute items assess frequency, severity, and interference with usual or daily activities over the past 7 days. Scores for each attribute can be reported individually or can be combined into a single composite score ranging from 0-3 for each symptom via an established algorithm.17 Higher individual or composite scores indicate worse magnitude for a given symptom. PRO-CTCAE items salient in a given clinical trial are selected and assembled into a survey on the basis of previous evidence and/or mechanism of action.18,19 In the PROSPECT trial, the investigators selected 14 PRO-CTCAE symptoms on the basis of evidence from previous clinical trials and patient representative input. The selected symptoms were anxiety, appetite loss, constipation, depression, diarrhea, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, pain, and vomiting.2,13
All patients in PROSPECT enrolled in the United States or Canada who spoke English or Spanish were asked to complete PRO-CTCAE self-report items at baseline, weekly during neoadjuvant treatment for up to 12 weeks, and then at 6, 12, and 18 months after surgery (patients enrolled to PROSPECT in Switzerland were not included in PRO collection because language translations and technology access were not feasible in Switzerland at the time of trial initiation).20 As described previously, patients could complete these items remotely between visits either by web, smart device, or automated telephone system.20,21 Each patient received an electronic message by email or telephone weekly at a time of their preference to remind them to complete the survey. After 24 and 48 hours, additional reminder messages were sent to noncompleters, and after 72 hours, a central coordinator contacted patients who still had not completed the survey to collect responses. The primary PRO-CTCAE analyses were prespecified as the time points encompassing neoadjuvant treatment and 12 months after surgery, with additional supporting time points at 6 and 18 months.
Additional PROs were assessed via the Memorial Sloan Kettering Bowel Function Instrument22; one question about bladder function each from the Prostate Health-Related QOL questionnaire23 and the International Prostate Symptom Score (IPSS)24; the International Index of Erectile Function (male patients only)25; the Female Sexual Function Index (female patients only)26; and the EuroQoL EQ-5D-5L for overall HRQL.27,28 These additional instruments were offered to approximately the first 500 patients enrolled in the United States or Canada via paper booklet at baseline, the end of neoadjuvant treatment (approximately 1-2 weeks before surgery), and at 12 and 24 months after surgery.
Statistics
Details of the trial design, random assignment, and statistical power are described in the Protocol and statistical analysis plan.
For each PRO-CTCAE symptomatic adverse event, a composite score was computed.17 The proportion of patients with each PRO-CTCAE composite score level for each of the 14 symptoms was tabulated at baseline. During neoadjuvant therapy, the maximum composite score, adjusting for baseline symptoms, was tabulated per patient.29,30 At 12 months after surgery, PRO-CTCAE scores per patient were cross-sectionally tabulated and adjusted for baseline. Maximum neoadjuvant and cross-sectional 12-month composite scores were primarily dichotomized as 0 versus ≥1 (absent v present) and supplemented with dichotomization at ≤2 versus 3 and were compared between groups using Chi squared tests. To account for multiplicity, Hochberg's step-up procedure31 was applied across 28 comparisons (14 PRO-CTCAE symptoms assessed at two time points). All patients who initiated protocol treatment as randomly assigned (per-protocol population) and who completed baseline and at least one postbaseline PRO-CTCAE were included in analyses. Previous analyses were reported on completion rates (92.0% patient completion rate for PRO-CTCAE surveys) and reasons for nonresponse.20 In this analysis, patient nonresponse was assumed to be missing at random.
For the additional supporting time points at 6 and 18 months after surgery, similar supplemental analyses were conducted. The 6-month time point encompassed the period of optional administration of adjuvant chemotherapy and therefore was conducted both including and excluding patients still receiving active chemotherapy at that time point. Supplemental analyses also considered individual PRO-CTCAE attribute item scores representing frequency, severity, and interference.
For the additional PRO end points, a linear combination of parameters from a general linear mixed model was used to estimate and compare mean changes from baseline between groups at each time point. Each model included all available data from all time points from all patients who initiated protocol treatment as randomly assigned and who completed baseline and at least one postbaseline assessment. Fixed effects included treatment group, time point, and treatment group by time point interaction. Repeated observations by patient were modeled using compound symmetric correlation structure over time. For the comparisons of bowel (MSKCC Bowel Function Instrument Aggregate Global Score), bladder (bladder emptying question from the IPSS), sexual function (International Index of Erectile Function Total Score for males and Female Sexual Function Index Total Score for females), and overall HRQL (EQ-5D-5L Index) at 12 months, P < .01 was considered statistically significant using a Bonferroni adjustment. Similar to PRO-CTCAE analysis, patient nonresponse was assumed to be missing at random. General linear models produce unbiased estimates under this missing data assumption.
Statistical testing was two-sided and carried out in SAS v9.4 (SAS Institute Inc, Cary, NC) or R v4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) on the study database frozen on December 15, 2022. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies.
RESULTS
Participants
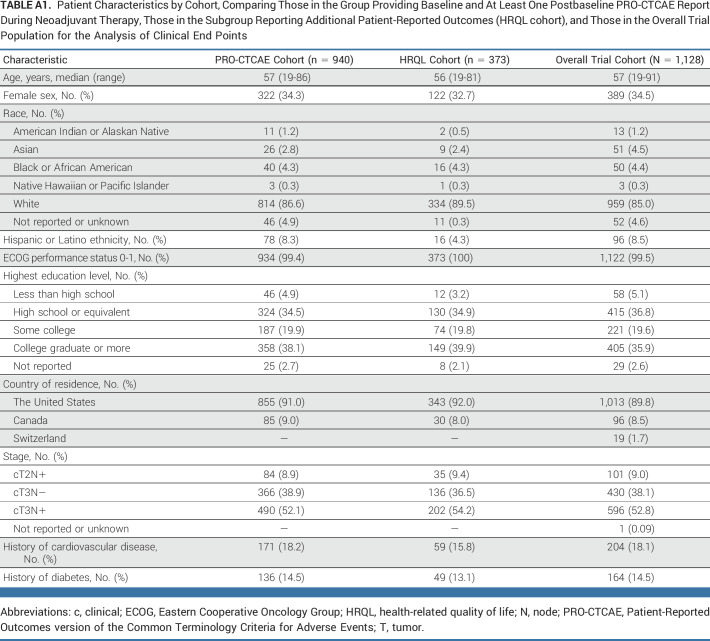
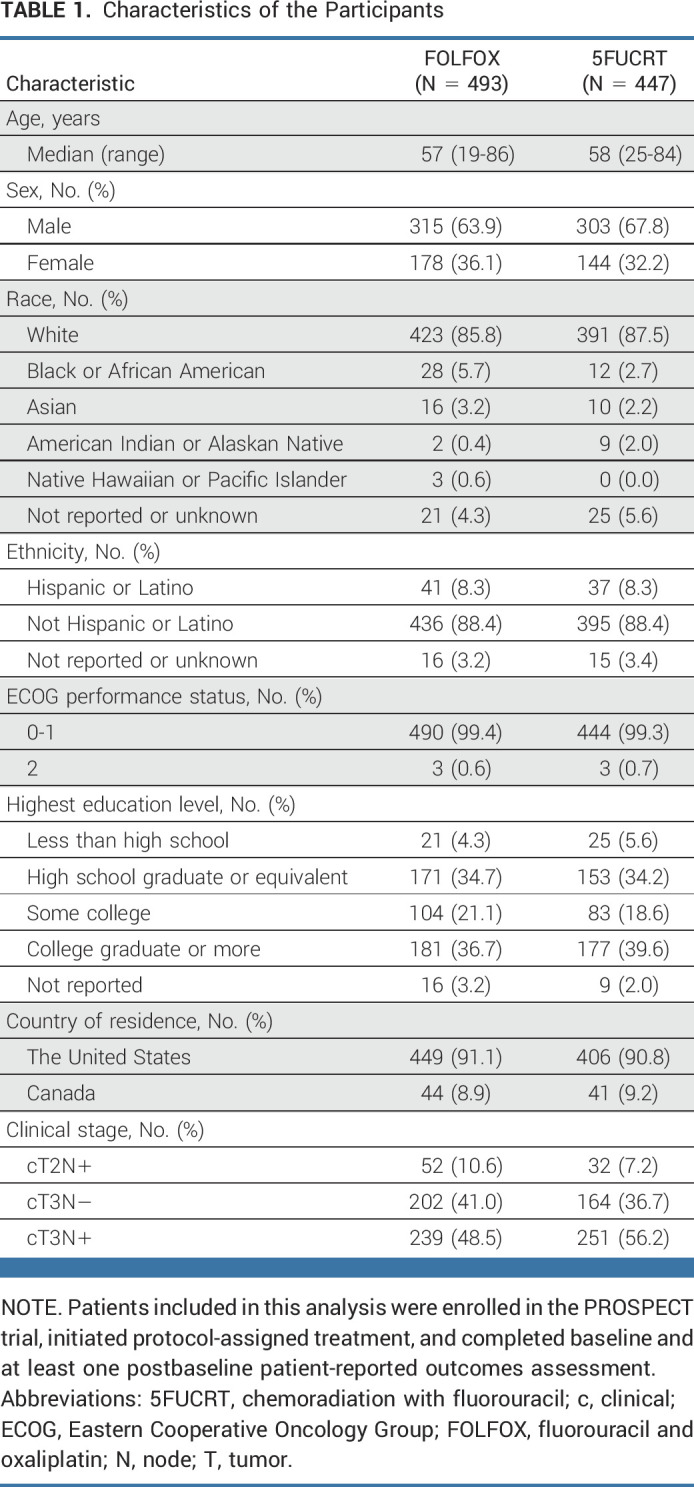
From June 2012 to December 2018, 1,194 patients were randomly assigned, 1,128 initiated protocol-assigned treatment, and 940 completed baseline and at least one postbaseline PRO-CTCAE assessment (493 assigned to FOLFOX; 447 to 5FUCRT; Fig 1). The median age was 57 (range, 19-86) years, with 124 of 940 (13.2%) younger than 45 years, 322 of 940 (34.3%) female, 814 of 940 (86.6%) White, and 370 of 940 (39.4%) with high school education or less (Table 1). Baseline clinical and demographic characteristics were similar among those completing PRO-CTCAE and the additional PRO measures of HRQL with the overall per-protocol trial population (Appendix Table A1, online only). Baseline symptoms were frequent and similar between treatment groups, with more than half of patients reporting fatigue and diarrhea and approximately one quarter reporting appetite loss, constipation, depression, dyspnea, nausea, neuropathy, and pain at enrollment (Appendix Fig A2, online only).
FIG 1.
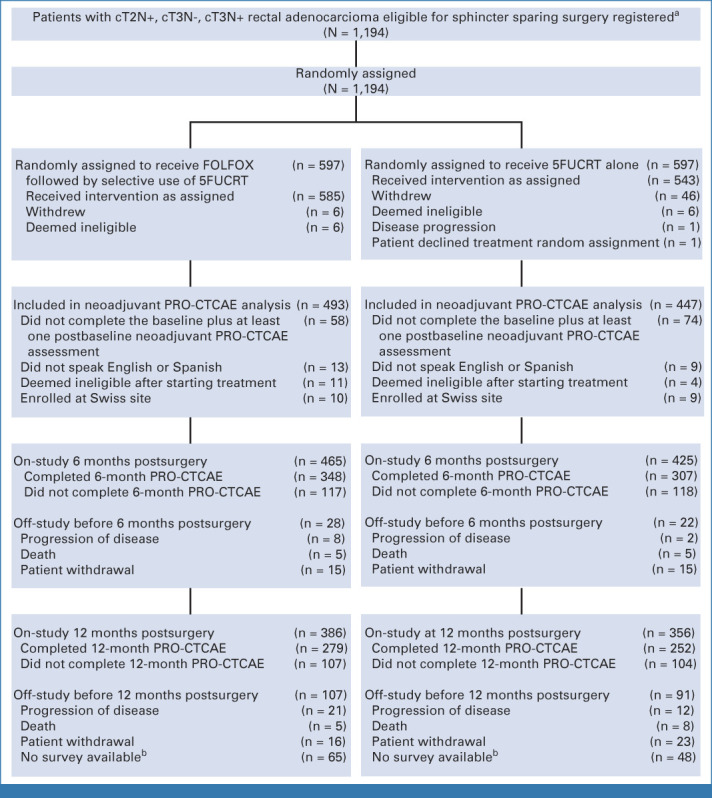
CONSORT diagram, patient enrollment and outcomes. aSites were not required to provide screening logs during recruitment; therefore, the number of patients assessed for eligibility is not available. bData at 12-month postsurgery time point is missing in these patients because the software system for electronic PRO-CTCAE data collection was discontinued in September 2019. 5FUCRT, chemoradiation with fluorouracil; c, clinical; FOLFOX, fluorouracil and oxaliplatin; N, node; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; T, tumor.
TABLE 1.
Characteristics of the Participants
Treatments Received
In the FOLFOX group, during neoadjuvant treatment, 555 of 585 (94.9%) patients received at least five cycles of FOLFOX, and 53 of 585 (9.1%) went on to receive neoadjuvant chemoradiation. In the 5FUCRT group, 515 of 543 (95.0%) received the full planned dose of 50.4 Gy radiation. In the FOLFOX group, 535 of 585 (91.4%) and in the 5FUCRT 510 of 543 (93.9%) completed surgery. In the FOLFOX group, 438 of 585 (74.8%) received adjuvant chemotherapy, of which 352 of 438 (80.4%) was FOLFOX with a median of six cycles (range, 1-12), whereas in the 5FUCRT group, 423 of 543 (77.9%) received adjuvant chemotherapy, of which 281 of 423 (66.4%) was FOLFOX with a median of eight cycles (range, 1-12). In the FOLFOX group, 1.4% (8 of 585) received postoperative radiation.
PROs
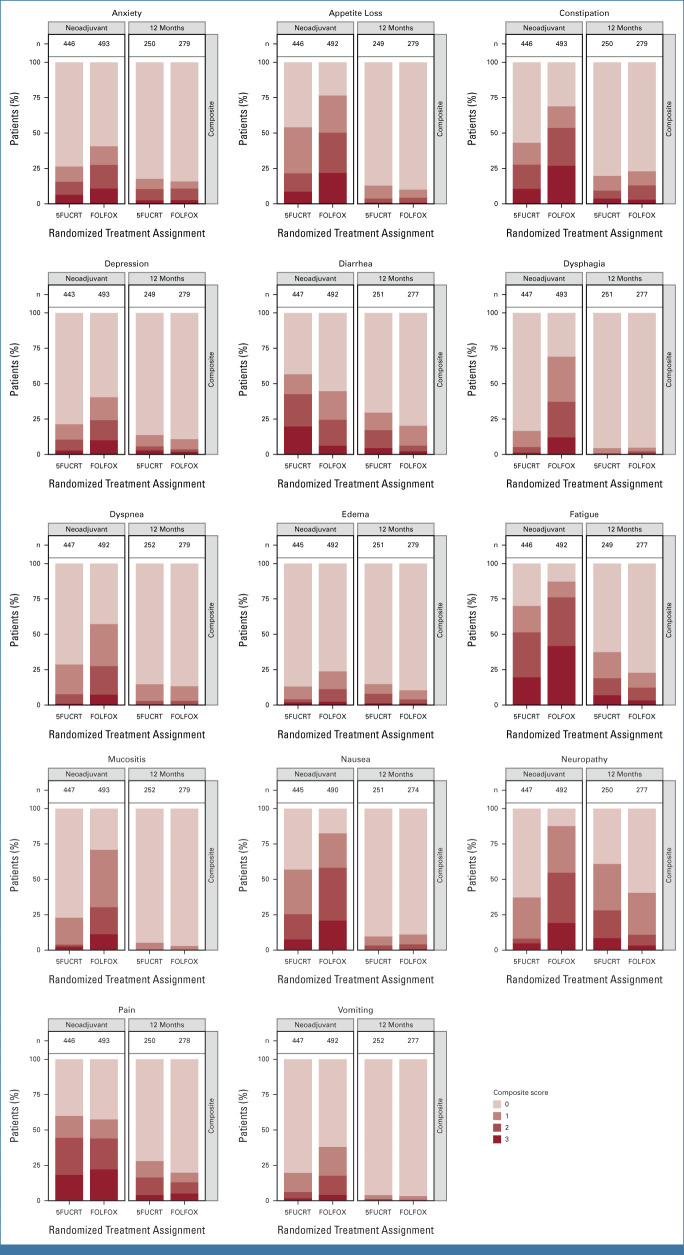
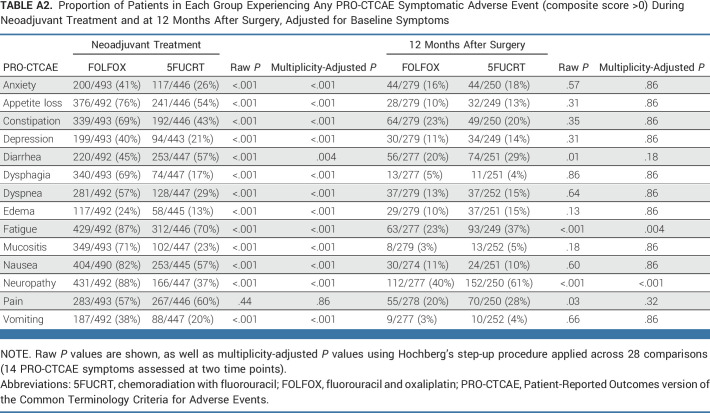
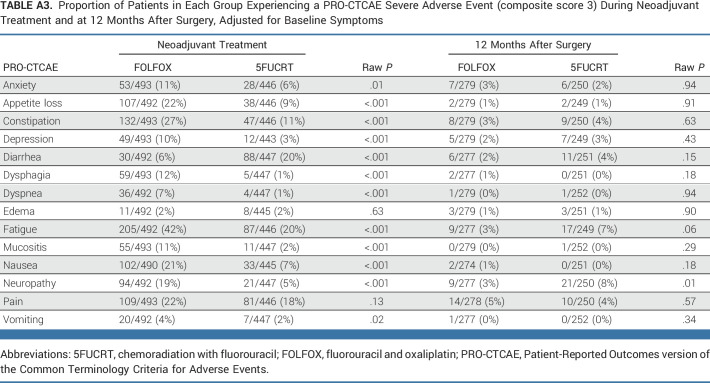
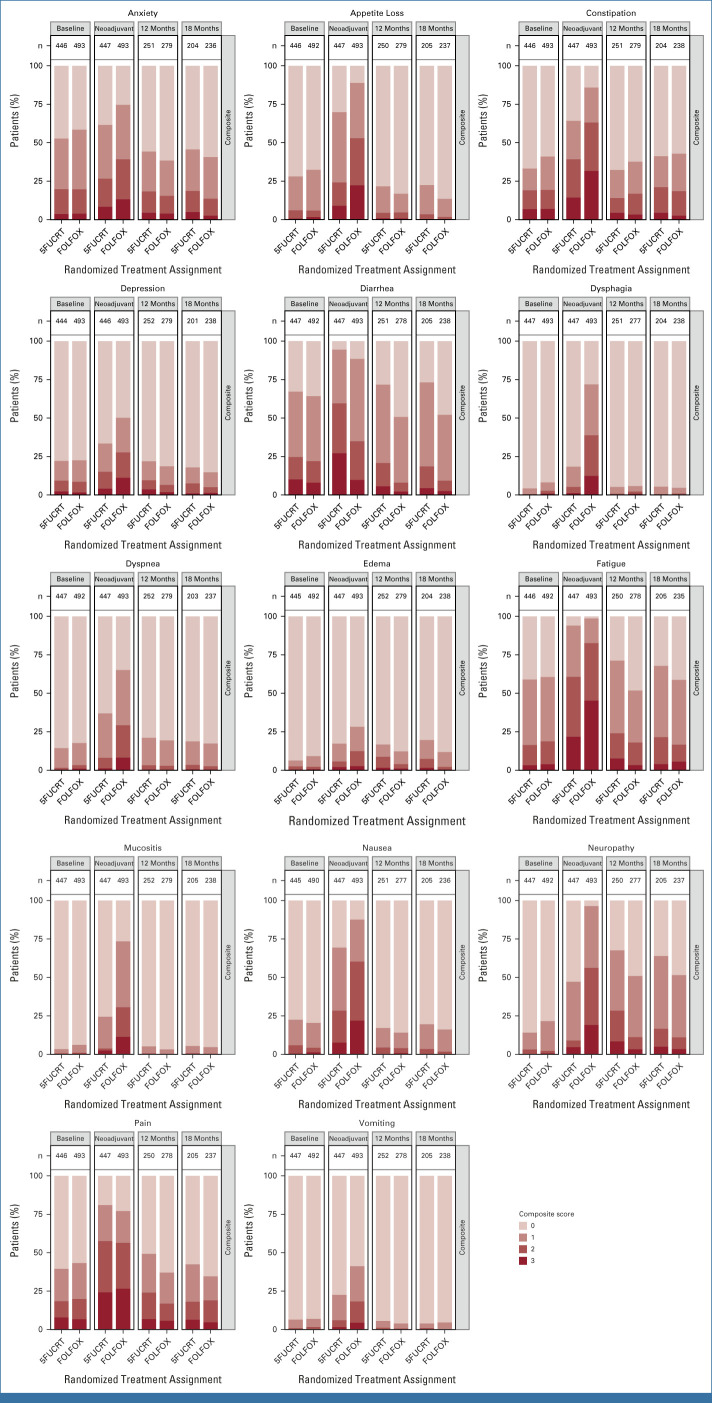
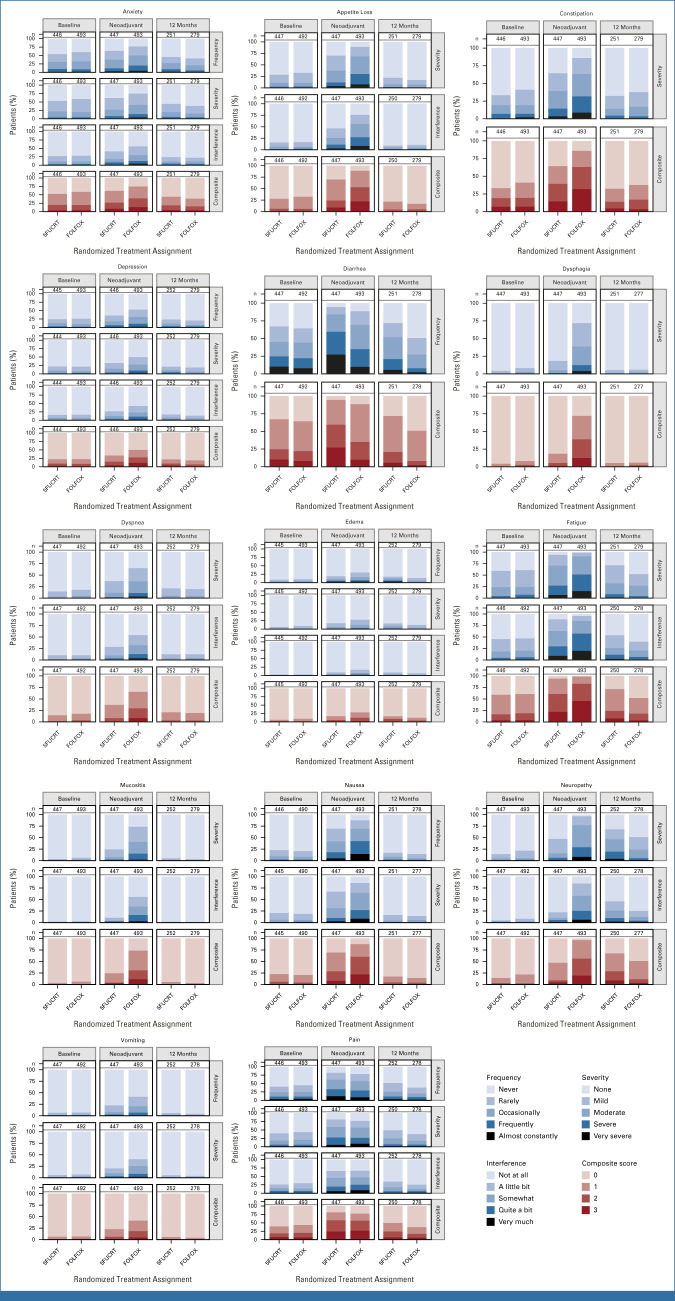
During neoadjuvant treatment, patients in the FOLFOX group reported significantly lower rates of diarrhea while rates of anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting were significantly lower with 5FUCRT (all multiplicity adjusted P < .05; Fig 2; Appendix Table A2, online only). Severe (composite score 3) symptoms during neoadjuvant treatment with FOLFOX reported by at least 15% of patients included appetite loss (107 of 492, 21.7%), constipation (132 of 493, 26.8%), fatigue (205 of 492, 41.7%), nausea (102 of 490, 20.8%), neuropathy (94 of 492, 19.1%), and pain (109 of 493, 22.1%) and with 5FUCRT included diarrhea (88 of 447, 19.7%), fatigue (87 of 446, 19.5%), and pain (81 of 446, 18.2%; Appendix Table A3, online only).
FIG 2.
PRO-CTCAE composite score distributions for each symptom during the neoadjuvant treatment period and 12 months after surgery, adjusted for baseline symptoms. Higher scores (represented by darker colors) are worse. The lightest color shading represents a score of zero. Column labels (n) show the number of subjects with an observed score. 5FUCRT, chemoradiation with fluorouracil; FOLFOX, fluorouracil and oxaliplatin; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events.
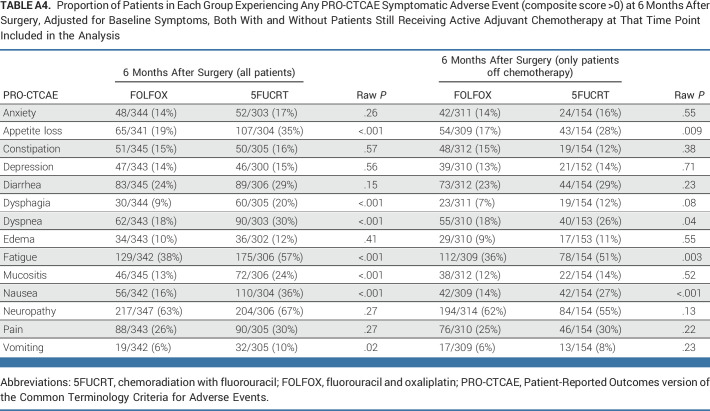
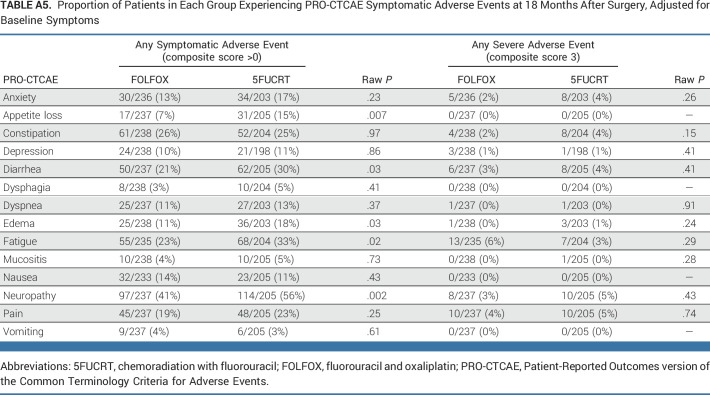
At 12 months after surgery, symptom rates were significantly lower with FOLFOX for fatigue and neuropathy (both multiplicity adjusted P < .05; Fig 2; Appendix Fig A1, online only). At this time point, there were no symptoms reported as severe (composite score 3) by at least 15% of patients in either group (Appendix Table A3). Differences between groups were similar 6 months after surgery (Appendix Table A4, online only), at which time 186 of 655 (28.4%) patients who completed PRO-CTCAE assessments were still receiving chemotherapy (33 of 348 [9.5%] FOLFOX; 153 of 307 [49.8%] 5FUCRT). Differences between groups were also similar at 18 months after surgery in a post hoc analysis (Appendix Fig A1; Appendix Table A5, online only).
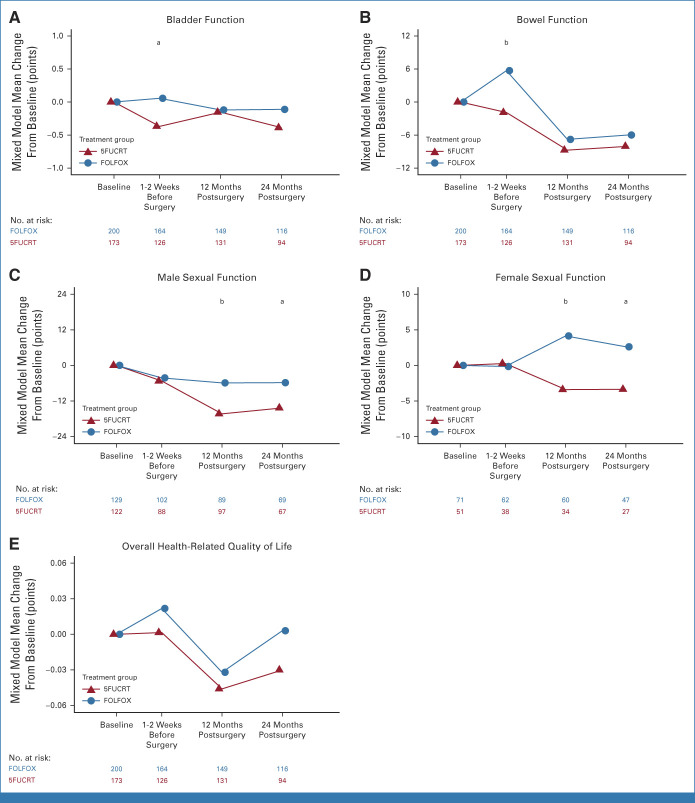
Additional PROs elicited from 373 patients (200 FOLFOX; 173 5FUCRT) demonstrated significantly better preoperative bowel function with FOLFOX compared with 5FUCRT and better sexual function for both males and females 12 months after surgery (raw P < .01 and multiplicity adjusted P < .05 at 12 months; Fig 3; Appendix Fig A3, online only; Data Supplement [Table], online only). No significant differences in bladder function or overall HRQL were detected at any time point after adjustment for multiple comparisons.
FIG 3.
Mean changes from baseline between groups during neoadjuvant treatment (1-2 weeks before surgery) and at 12 and 24 months after surgery for (A) bladder function (bladder emptying question from the International Prostate Symptom Score, administered both to males and females); (B) bowel function (Memorial Sloan Kettering Bowel Function Instrument Aggregate Global Score); (C) male sexual function (International Index of Erectile Function Total Score, administered to males only); (D) female sexual function (Female Sexual Function Index Total Score, administered to females only); and (E) overall health-related quality of life (EuroQoL EQ-5D-5L Index). Positive values represent improvement compared with baseline for all scales. Means were estimated using a general linear mixed model for each scale. Between-group differences with raw P < .05 are demarcated with a and P < .01 with b. The results for all domain subscales are shown in the Data Supplement. aBetween-group difference raw P < .05. bBetween-group difference raw P < .01 (12 months only: multiplicity-adjusted P < .05). 5FUCRT, chemoradiation with fluorouracil; FOLFOX, fluorouracil and oxaliplatin.
In an evaluation of patient-reported severity, frequency, and activity interference of each symptom, in the FOLFOX group, 15% or more patients reported high (score ≥3) severity for appetite loss, constipation, fatigue, mucositis, neuropathy, and pain; high frequency for anxiety, diarrhea, nausea, and pain; and high interference due to fatigue, mucositis, neuropathy, and pain during neoadjuvant treatment (Appendix Fig A2). In the 5FUCRT group, 15% or more patients reported high severity for fatigue and pain; high frequency for diarrhea and pain; and high interference due to fatigue and pain during neoadjuvant treatment. At 12 months, the only high-level symptom attribute reported by at least 15% of patients was high diarrhea frequency in the 5FUCRT group.
DISCUSSION
In a randomized clinical trial in patients with locally advanced rectal cancer, compared with the current standard of neoadjuvant 5FUCRT, patients receiving neoadjuvant FOLFOX followed by selected use of chemoradiation experienced significantly lower rates of diarrhea and better overall bowel function but higher rates of anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting. Once treatment was complete (12 months after surgery), most acute treatment-related symptomatic adverse events had resolved in both groups, although significantly higher rates of fatigue, neuropathy, and sexual dysfunction persisted among patients assigned to receive 5FUCRT. These findings were similar at 18 months. Despite these differences in symptoms and functioning between groups, overall HRQL did not significantly differ between groups at any time point.
Given the noninferiority between FOLFOX and 5FUCRT for disease-free survival and the similarity of other efficacy end points as described by Schrag et al,15 these findings can inform decision making by patients and their clinicians. For those concerned about acute impairments in bowel function during presurgical treatment, or fatigue and sexual function a year or more after surgery, FOLFOX may be preferable. For side effects beyond bowel function, 5FUCRT treatment resulted in fewer presurgical side effects and may remain preferable for some patients. Notably, the neoadjuvant treatment period for patients assigned to FOLFOX was nearly twice as long as 5FUCRT, which may explain the greater number of symptoms reported by this group.
Neuropathy has been highlighted previously as a concerning symptom associated with oxaliplatin in the FOLFOX regimen.32 In the PROSPECT trial, more patients initially experienced neuropathy during neoadjuvant chemotherapy with FOLFOX than with 5FUCRT, but this pattern reversed after treatment was completed, with more patients subsequently experiencing neuropathy at 12 and at 18 months in the 5FUCRT group. Given that most patients who receive neoadjuvant FOLFOX are subsequently administered fewer cycles of adjuvant oxaliplatin-based chemotherapy compared with those who receive neoadjuvant 5FUCRT (median of six v eight adjuvant cycles),15 the 12 and 18 month findings may be related to the timing and intensity of chemotherapy proximate to that time point or could reflect an enduring toxicity imbalance in a small number of patients favoring neoadjuvant FOLFOX.
Although no significant differences were seen between groups for overall HRQL, the instrument used in this trial, the EuroQoL EQ-5D-5L, only elicits information about mobility, self-care, daily activities, anxiety/depression, and pain toward the tabulation of its overall score27,28 and as such may have been insensitive to detecting the impact of the differential symptom burden observed between groups in this trial. Therefore, this metric may not reflect nuances of the overall patient experience with these treatments. Nonetheless, the EQ-5D-5L is a widely used instrument in clinical research, and the findings provide reassurance that there were not significant differences in functional impairment between treatment groups.
This trial demonstrates the value of PROs to characterize how patients feel and function, especially in a noninferiority trial. Few previous rectal cancer trials have included longitudinal PROs.33,34 Although PROs have been cited as a key component of understanding cancer treatment tolerability7 and are encouraged by international regulatory authorities,5,6,9 most drug development programs still do not adequately incorporate PRO end points.35 Patient-reported adverse events, such as measured in this trial with the PRO-CTCAE, can particularly illustrate the impact of treatments on patients.9,10,16 As the landscape for treatments in this disease evolves in the future, it will be informative to employ rigorous evaluation of PROs to understand the patient experience. As reported previously,20 PRO-CTCAE completion rates were high in this trial (>90%) because of rigorous data collection including multiple modes for PRO completion by patients and telephone backup calls to noncompleters.
In fact, this trial is a landmark for the NCI's PRO-CTCAE. Although now used widely in oncology drug development, to our knowledge, PROSPECT was the first large trial into which the PRO-CTCAE was embedded. The US Food and Drug Administration and other international regulatory authorities now encourage assessment of patient-reported symptomatic adverse events in cancer drug development.9,10
Since the PROSPECT trial was conceived and began recruitment in 2012, a variety of other strategies for management of rectal cancer have emerged. For example, total neoadjuvant therapy with 5FUCRT and chemotherapy followed by nonoperative management has potential to spare some patients the morbidity of surgery although this is offset by the need for close surveillance and high rates of recurrence in others.36 Other approaches include immunotherapy alone as a curative strategy for the subset of patients with mismatch repair-deficient rectal cancer.37 It is plausible that these approaches further decrease adverse symptoms and improve HRQL but there are not yet prospective trials comparing these strategies to 5FUCRT. A previous trial compared neoadjuvant short course radiation followed by chemotherapy to neoadjuvant 5FUCRT followed by adjuvant chemotherapy in a higher risk population of patients with rectal cancer than those recruited to PROSPECT and found that long-term quality of life outcomes were similar.34
This study has several limitations. When the trial was designed, clinicians were concerned that omission of radiation could compromise clinical outcomes, and therefore, patients with high-risk features such as T4 tumors or distal tumors requiring an abdominoperineal resection were excluded. Accordingly, these findings pertain to patients with moderate risk locally advanced tumors who were candidates for sphincter-sparing surgery at presentation. A prespecified set of symptoms was assessed, and there may have been other salient symptoms in this population, although there was a free-text option for patients to write in additional symptoms, which was previously examined in a pooled analysis.38 Although sexual function was assessed, reproductive outcomes were not analyzed, which may be important to patients of child-bearing age. Other domains such as patient preference (eg, time on treatment, convenience of treatment) also were not included. Not all enrolled patients were included in the PRO collection, although most provided data for PRO-CTCAE (940 of 1,128 [83.3%]) and a smaller prespecified subset for HRQL (373 of 604 [61.8%]). Baseline characteristics were similar between those completing PROs in these two subgroups and the overall per-protocol trial population. Nonetheless, there may have been informative missing data because of patients who dropped out or did not complete PROs, although PRO completion rates were generally high in this trial.20 The overall trial population was predominantly White and based in the United States and Canada and therefore does not reflect the diversity of patients facing treatment decisions for locally advanced rectal cancer. Future studies should focus on populations that have been underrepresented in clinical trials.
In conclusion, during neoadjuvant treatment, patients treated with FOLFOX experienced less diarrhea and better bowel function than those treated with 5FUCRT but had a higher burden of other symptoms including fatigue, anxiety, nausea, and neuropathy. After a year or more following surgery, patients assigned to FOLFOX reported significantly lower rates of fatigue and neuropathy and better sexual function compared with those assigned to 5FUCRT. By this time, rates of severe symptoms were low in both groups and there were no discernible differences in overall HRQL. For patients with locally advanced rectal cancer choosing between neoadjuvant chemotherapy and chemoradiation, the distinctive PRO profiles inform treatment selection and shared decision making.
APPENDIX
TABLE A1.
Patient Characteristics by Cohort, Comparing Those in the Group Providing Baseline and At Least One Postbaseline PRO-CTCAE Report During Neoadjuvant Therapy, Those in the Subgroup Reporting Additional Patient-Reported Outcomes (HRQL cohort), and Those in the Overall Trial Population for the Analysis of Clinical End Points
TABLE A2.
Proportion of Patients in Each Group Experiencing Any PRO-CTCAE Symptomatic Adverse Event (composite score >0) During Neoadjuvant Treatment and at 12 Months After Surgery, Adjusted for Baseline Symptoms
TABLE A3.
Proportion of Patients in Each Group Experiencing a PRO-CTCAE Severe Adverse Event (composite score 3) During Neoadjuvant Treatment and at 12 Months After Surgery, Adjusted for Baseline Symptoms
TABLE A4.
Proportion of Patients in Each Group Experiencing Any PRO-CTCAE Symptomatic Adverse Event (composite score >0) at 6 Months After Surgery, Adjusted for Baseline Symptoms, Both With and Without Patients Still Receiving Active Adjuvant Chemotherapy at That Time Point Included in the Analysis
TABLE A5.
Proportion of Patients in Each Group Experiencing PRO-CTCAE Symptomatic Adverse Events at 18 Months After Surgery, Adjusted for Baseline Symptoms
FIG A1.
PRO-CTCAE composite score distributions at baseline, during neoadjuvant treatment (unadjusted for baseline scores), and 12 and 18 months after surgery (unadjusted for baseline scores). Higher scores indicate worse magnitude of a given symptom. Column labels (n) show the number of patients with an observed score. 5FUCRT, chemoradiation with fluorouracil; FOLFOX, fluorouracil and oxaliplatin; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events.
FIG A2.
PRO-CTCAE individual attribute item score distributions for symptoms at baseline, during neoadjuvant treatment (unadjusted for baseline symptoms), and 12 months after surgery (unadjusted for baseline symptoms). Higher scores indicate worse magnitude of a given symptom. Column labels (n) show the number of patients with an observed score. 5FUCRT, chemoradiation with fluorouracil; FOLFOX, fluorouracil and oxaliplatin; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events
FIG A3.
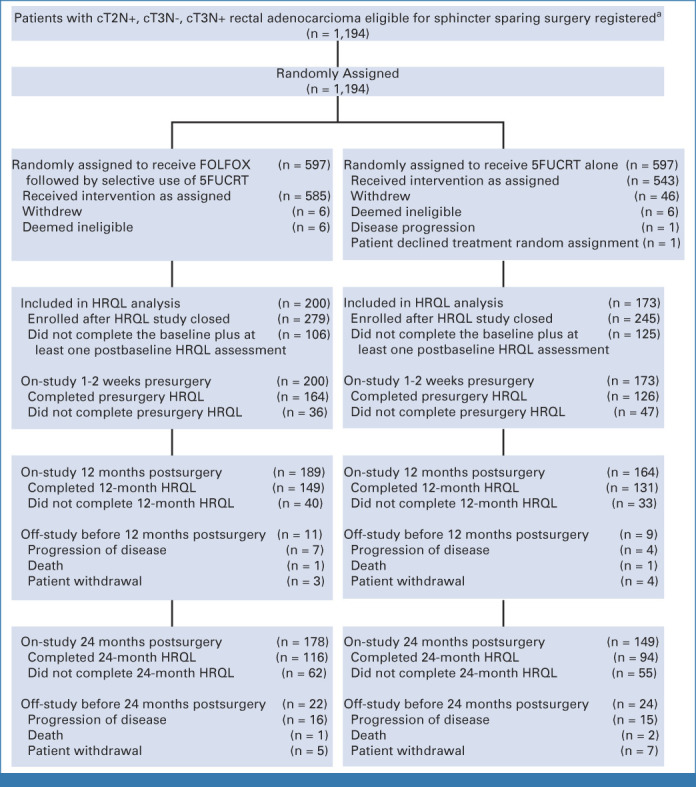
CONSORT diagram for HRQL measures evaluating bowel, bladder, sexual function, and overall HRQL. aSites were not required to provide screening logs during recruitment; therefore, the number of patients assessed for eligibility is not available. c, clinical; HRQL, health-related quality of life; N, node; T, tumor.
Ethan Basch
Consulting or Advisory Role: Sivan Innovation, Navigating Cancer, AstraZeneca, Resilience Care
Other Relationship: Centers for Medicare and Medicaid Services, National Cancer Institute, American Society of Clinical Oncology, JAMA—Journal of the American Medical Association, Patient-Centered OUtcomes Research Institute (PCORI)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/427875
Harvey Mamon
Honoraria: UpToDate
Consulting or Advisory Role: Merck Sharp & Dohme
Martin Weiser
Consulting or Advisory Role: Precisca
Patents, Royalties, Other Intellectual Property: UpToDate Section Editor
Leonard Saltz
Consulting or Advisory Role: Genor BioPharma
Marc Gollub
Stock and Other Ownership Interests: Pfizer
George Chang
Consulting or Advisory Role: Medicaroid
Gita Thanarajasingam
Research Funding: Seagen (Inst)
Benjamin Musher
Consulting or Advisory Role: Exelixis
Speakers' Bureau: Ipsen
Research Funding: Lokon Pharma, Merck
Expert Testimony: AstraZeneca
Thomas George
Consulting or Advisory Role: Tempus, BillionToOne, Pfizer/Array
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Lilly (Inst), Bayer (Inst), Incyte (Inst), Ipsen (Inst), Seagen (Inst), Genentech (Inst), Astellas Pharma (Inst), BioMed Valley Discoveries (Inst), GlaxoSmithKline (Inst), Amgen (Inst), OncoC4 (Inst), BillionToOne (Inst), Jounce Therapeutics (Inst), Elicio Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/321938
Alan Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Exelixis, BridgeBio Pharma, Bayer Health, Gilead Sciences, Exact Sciences, Bristol Myers Squibb Foundation/Janssen, Pfizer
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Jeffrey Farma
Stock and Other Ownership Interests: Genmab
Expert Testimony: Legal firms
Eileen O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: AstraZeneca, Autem Medical, Eisai, Exelixis, Genentech/Roche, Helio Health, Incyte, Ipsen, Merck, QED Therapeutics, Yiviva, Novartis, Boehringer Ingelheim
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Arcus Ventures (Inst), BioNTech (Inst), Bristol Myers Squibb (Inst), Helsinn Healthcare (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Yiviva (Inst)
Uncompensated Relationships: Thetis Pharma
Jeffrey A. Meyerhardt
Honoraria: Merck
Research Funding: Boston Biomedical (Inst)
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Regeneron, Hoosier Cancer Research Network, Kronos Bio, Mirati THERAPEUTICS
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Deborah Schrag
Stock and Other Ownership Interests: Merck
Consulting or Advisory Role: JAMA—Journal of the American Medical Association
Research Funding: AACR (Inst), GRAIL (Inst)
Patents, Royalties, Other Intellectual Property: PRISSMM model is trademarked and curation tools are available to academic medical centers and government under creative commons license
Other Relationship: JAMA—Journal of the American Medical Association
No other potential conflicts of interest were reported.
Listen to the podcast by Drs Basch and Schrag at https://sites.libsyn.com/58526/JCO
PRIOR PRESENTATION
Presented in part at the 2023 ASCO annual meeting, Chicago, IL, June 4, 2023.
SUPPORT
Supported by National Cancer Institute awards HHSN261200800063C, U01CA233046, UG1CA189823, U10CA180821, U10CA180882, U10CA180863, U10CA180820, U10CA180868, and U10CA180888 (https://acknowledgments.alliancefound.org).
CLINICAL TRIAL INFORMATION
NCT01515787 (PROSPECT)
E.B. and A.C.D. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Ethan Basch, Amylou C. Dueck, Sandra A. Mitchell, Leonard Saltz, Lauren Rogak, Lori Minasian, Andrea Denicoff, Qian Shi, Deborah Schrag
Financial support: Deborah Schrag
Administrative support: Lauren Rogak, Qian Shi, Deborah Schrag
Provision of study materials or patients: Lauren Rogak, George Chang, Thomas George, Deborah Schrag
Collection and assembly of data: Ethan Basch, Amylou C. Dueck, Sandra A. Mitchell, Lauren Rogak, Brian Colgrove, George Chang, Benjamin Musher, Alan Venook, Qian Shi, Deborah Schrag
Data analysis and interpretation: Ethan Basch, Amylou C. Dueck, Sandra A. Mitchell, Harvey Mamon, Martin Weiser, Lauren Rogak, Brenda Ginos, Gina L. Mazza, Lori Minasian, Gita Thanarajasingam, Thomas George, Alan Venook, Jeffrey Farma, Jeffrey A. Meyerhardt, Qian Shi, Deborah Schrag
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ethan Basch
Consulting or Advisory Role: Sivan Innovation, Navigating Cancer, AstraZeneca, Resilience Care
Other Relationship: Centers for Medicare and Medicaid Services, National Cancer Institute, American Society of Clinical Oncology, JAMA—Journal of the American Medical Association, Patient-Centered OUtcomes Research Institute (PCORI)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/427875
Harvey Mamon
Honoraria: UpToDate
Consulting or Advisory Role: Merck Sharp & Dohme
Martin Weiser
Consulting or Advisory Role: Precisca
Patents, Royalties, Other Intellectual Property: UpToDate Section Editor
Leonard Saltz
Consulting or Advisory Role: Genor BioPharma
Marc Gollub
Stock and Other Ownership Interests: Pfizer
George Chang
Consulting or Advisory Role: Medicaroid
Gita Thanarajasingam
Research Funding: Seagen (Inst)
Benjamin Musher
Consulting or Advisory Role: Exelixis
Speakers' Bureau: Ipsen
Research Funding: Lokon Pharma, Merck
Expert Testimony: AstraZeneca
Thomas George
Consulting or Advisory Role: Tempus, BillionToOne, Pfizer/Array
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Lilly (Inst), Bayer (Inst), Incyte (Inst), Ipsen (Inst), Seagen (Inst), Genentech (Inst), Astellas Pharma (Inst), BioMed Valley Discoveries (Inst), GlaxoSmithKline (Inst), Amgen (Inst), OncoC4 (Inst), BillionToOne (Inst), Jounce Therapeutics (Inst), Elicio Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/321938
Alan Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Exelixis, BridgeBio Pharma, Bayer Health, Gilead Sciences, Exact Sciences, Bristol Myers Squibb Foundation/Janssen, Pfizer
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Jeffrey Farma
Stock and Other Ownership Interests: Genmab
Expert Testimony: Legal firms
Eileen O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: AstraZeneca, Autem Medical, Eisai, Exelixis, Genentech/Roche, Helio Health, Incyte, Ipsen, Merck, QED Therapeutics, Yiviva, Novartis, Boehringer Ingelheim
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Arcus Ventures (Inst), BioNTech (Inst), Bristol Myers Squibb (Inst), Helsinn Healthcare (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Yiviva (Inst)
Uncompensated Relationships: Thetis Pharma
Jeffrey A. Meyerhardt
Honoraria: Merck
Research Funding: Boston Biomedical (Inst)
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Regeneron, Hoosier Cancer Research Network, Kronos Bio, Mirati THERAPEUTICS
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Deborah Schrag
Stock and Other Ownership Interests: Merck
Consulting or Advisory Role: JAMA—Journal of the American Medical Association
Research Funding: AACR (Inst), GRAIL (Inst)
Patents, Royalties, Other Intellectual Property: PRISSMM model is trademarked and curation tools are available to academic medical centers and government under creative commons license
Other Relationship: JAMA—Journal of the American Medical Association
No other potential conflicts of interest were reported.
REFERENCES
- 1. Henry DH, Viswanathan HN, Elkin EP, et al. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 2. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carlotto A, Hogsett VL, Maiorini EM, et al. The economic burden of toxicities associated with cancer treatment: Review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics. 2013;31:753–766. doi: 10.1007/s40273-013-0081-2. [DOI] [PubMed] [Google Scholar]
- 4. Hershman D, Calhoun E, Zapert K, et al. Patients' perceptions of physician-patient discussions and adverse events with cancer therapy. Arch Drug Inf. 2008;1:70–78. doi: 10.1111/j.1753-5174.2008.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food & Drug Administration, Oncology Center of Excellence, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research https://www.fda.gov/media/149994/download Guidance document: Core patient-reported outcomes in cancer clinical trials, draft guidance for industry, 2021.
- 6.Committee for Medicinal Products For Human Use (CHMP) Reflection paper on the regulatory guidance for the use of health related quality of life (HRQL) measures in the evaluation of medicinal products, 2005. Doc. Ref. EMEA/CHMP/EWP/139391/2004. 2005. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation_en.pdf.
- 7.Friends of Cancer Research https://friendsofcancerresearch.org/wp-content/uploads/Comparative-Tolerability-Whitepaper_FINAL.pdf White paper: Broadening the definition of tolerability in cancer clinical trials to better measure the patient experience, 2018.
- 8. Banerjee AK, Okun S, Edwards IR, et al. Patient-reported outcome measures in safety event reporting: PROSPER Consortium guidance. Drug Saf. 2013;36:1129–1149. doi: 10.1007/s40264-013-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kluetz PG, Kanapuru B, Lemery S, et al. Informing the tolerability of cancer treatments using patient-reported outcome measures: Summary of an FDA and Critical Path Institute Workshop. Value Health. 2018;21:742–747. doi: 10.1016/j.jval.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute https://healthcaredelivery.cancer.gov/pro-ctcae Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events.
- 11. NIH Consensus Conference: Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 12. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 13. Downing A, Glaser AW, Finan PJ, et al. Functional outcomes and health-related quality of life after curative treatment for rectal cancer: A population-level study in England. Int J Radiat Oncol Biol Phys. 2019;103:1132–1142. doi: 10.1016/j.ijrobp.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 14. Kumar AR, Sanford NN. Toxicity management in the era of changing treatment paradigms for locally advanced rectal cancer. Curr Colorectal Cancer Rep. 2022;18:55–59. doi: 10.1007/s11888-022-00478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrag D, Shi Q, Weiser MR, et al. Chemotherapy with selective use of chemoradiation versus chemoradiation for neoadjuvant treatment of locally advanced rectal cancer New Engl J Med (in press) [Google Scholar]
- 16. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1:1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basch E, Becker C, Rogak LJ, et al. Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Clin Trials. 2021;18:104–114. doi: 10.1177/1740774520975120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basch E, Pugh SL, Dueck AC, et al. Feasibility of patient reporting of symptomatic adverse events via the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) in a chemoradiotherapy cooperative group multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2017;98:409–418. doi: 10.1016/j.ijrobp.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basch E, Dueck AC. Patient-reported outcome measurement in drug discovery: A tool to improve accuracy and completeness of efficacy and safety data. Expert Opin Drug Discov. 2016;11:753–758. doi: 10.1080/17460441.2016.1193148. [DOI] [PubMed] [Google Scholar]
- 20. Basch E, Dueck AC, Rogak LJ, et al. Feasibility of implementing the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events in a multicenter trial: NCCTG N1048. J Clin Oncol. 2018;36:3120–3125. doi: 10.1200/JCO.2018.78.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett AV, Dueck AC, Mitchell SA, et al. Mode equivalence and acceptability of tablet computer-interactive voice response system-and paper-based administration of the U.S. National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Health Qual Life Outcomes. 2016;14:24. doi: 10.1186/s12955-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–1365. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 23. Befort CA, Zelefsky MJ, Scardino PT, et al. A measure of health-related quality of life among patients with localized prostate cancer: Results from ongoing scale development. Clin Prostate Cancer. 2005;4:100–108. doi: 10.3816/cgc.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 24. Barry MJ, Fowler FJ, O'leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 25. Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 26. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 27. Johnson JA, Coons SJ, Ergo A, et al. Valuation of EuroQOL (EQ-5D) health states in an adult US sample. Pharmacoeconomics. 1998;13:421–433. doi: 10.2165/00019053-199813040-00005. [DOI] [PubMed] [Google Scholar]
- 28. Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making. 2006;26:401–409. doi: 10.1177/0272989X06290496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dueck AC, Scher HI, Bennett AV, et al. Assessment of adverse events from the patient perspective in a phase 3 metastatic castration-resistant prostate cancer clinical trial. JAMA Oncol. 2020;6:e193332. doi: 10.1001/jamaoncol.2019.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basch E, Rogak LJ, Dueck AC. Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther. 2016;38:821–830. doi: 10.1016/j.clinthera.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 32. Ganz PA, Hays RD, Spritzer KL, et al. Health-related quality of life outcomes after neoadjuvant chemoradiotherapy for rectal cancer in NRG Oncology/NSABP R-04. Cancer. 2022;128:3233–3242. doi: 10.1002/cncr.34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kosmala R, Fokas E, Flentje M, et al. Quality of life in rectal cancer patients with or without oxaliplatin in the randomised CAO/ARO/AIO-04 phase 3 trial. Eur J Cancer. 2021;144:281–290. doi: 10.1016/j.ejca.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 34. Dijkstra EA, Hospers GAP, Kranenbarg EM, et al. Quality of life and late toxicity after short-course radiotherapy followed by chemotherapy or chemoradiotherapy for locally advanced rectal cancer—The RAPIDO trial. Radiother Oncol. 2022;171:69–76. doi: 10.1016/j.radonc.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 35. Gnanasakthy A, Norcross L, Clark M, et al. A review of patient-reported outcome considerations in oncologic drugs advisory committee meetings (2016-2021) JCO Oncol Pract. 2023;19:e745–e762. doi: 10.1200/OP.22.00774. [DOI] [PubMed] [Google Scholar]
- 36. Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5:e185896. doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung AE, Shoenbill K, Mitchell SA, et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) J Am Med Inform Assoc. 2019;26:276–285. doi: 10.1093/jamia/ocy169. [DOI] [PMC free article] [PubMed] [Google Scholar]