SUMMARY
OBJECTIVE:
Seborrheic dermatitis is a common papulosquamous skin disease with unknown pathogenesis. The aim of our study was to determine the serum level of 25-hydroxy vitamin D in patients with seborrheic dermatitis SD.
METHODS:
A total of 53 patients and 60 healthy controls were included in the study. Serum vitamin D, calcium, phosphorus, and parathormone levels were measured in the patient and control groups, and a comparison was made between the two groups regarding these parameters.
RESULTS:
Severe vitamin D deficiency was more frequent among patients with seborrheic dermatitisSD compared to controls (52.8 vs. 25.8%, p=0.003). In patients with severe vitamin D deficiency, seborrheic dermatitis SD was detected more frequently at an early age (p=0048) and in women (p=0.015). No correlation was found between the seborrheic dermatitis skin involvement site and vitamin D level.
CONCLUSION:
The fact that vitamin D levels decreased in patients with seborrheic dermatitis SD and patients with severe vitamin D deficiency develop seborrheic dermatitis SD earlier suggests that the low levels of vitamin D are related to seborrheic dermatitis.
Keywords: Seborrheic dermatitis, Vitamin D, Calcium, Phosphorus, Parathormone
INTRODUCTION
Seborrheic dermatitis (SD) is encountered with a frequency of 3–5% (2.35–11.3) in the society. It is a chronic inflammatory disease that is more common, especially in men 1 . Scalp, eyebrows, nasolabial region, ear, sternal region, and flexor regions are frequently involved. It is frequently observed in infants in the first years of life, in pubertal age when sebaceous gland activity increases, and in advanced ages 2,3 . The most accused ones in its etiopathogenesis are microbial factors such as seborrhea, malassezia, and demodex, androgenic hormones, immunological disorders, drugs, hereditary factors, and stress 1,4-6 . Skin diseases, such as psoriasis, atopic dermatitis, acne, and rosacea, are also frequently associated with SD 4 . The etiopathogenesis is not fully known. It is considered an abnormal focal inflammatory immune response to Malassezia species and their metabolites 1-3 . Moreover, it has been reported that the lesions exacerbate in winter and undergo remission in summer with the curative effect of UV rays 6 .
A total of 95% of active vitamin D (vit D) is formed in the skin, especially after exposure to UVB (290–320 nm) rays. A very small amount of it is taken with the diet 7 . Vit D is known as the basic hormone that plays a key role in calcium (Ca)-phosphate (P) metabolism and shows its effects on the intestines, kidneys, and musculoskeletal system 8 . Apart from Ca and bone metabolism, vit D plays a role in cell proliferation, differentiation, regulation of hormone secretion, and immunological functions 9,10-12 .
Vit D, which is found in cells such as keratinocytes, mast cells, melanocytes, fibroblasts, the immune system, and many tissues, shows its effect through its nuclear receptors (VDR) 8,13,14 . VDR is mostly expressed in the skin 14,15 .
The effect of vit D has been shown in many systemic diseases, including skin diseases 10,15-18 . It is used in the treatment of various skin diseases such as psoriasis, vitiligo, and morphea alopecia areata, in which vit D deficiency is detected 12-14 . Vit D supplements or natural sunlight were shown to be beneficial in SD 19 .
Studies have been conducted to investigate vit D levels in patients with the assumption that vit D deficiency may play a role in the development of the disease due to its effects on the inflammatory process and immune response in SD etiopathogenesis 20-23 . There are few studies on this subject in our country 24,25 . For this reason, we wanted to investigate vit D levels in patients with SD.
METHODS
This study was planned as a single-center case-control study. A total of 53 volunteer patients over the age of 18 years who were diagnosed with SD in our outpatient clinic and 60 healthy controls were included in the study. Patients’ age, gender, age of disease onset, disease duration, lesion localization, smoking status and alcohol use, subjective stress history, and presence of additional skin and systemic diseases were collected and recorded. Pregnant women, those with systemic disease and neoplastic disease, those who have received systemic and topical drug therapy in the last 3 months, and those who have used drugs containing vit D and Ca were not included in the study. Routine biochemistry tests were performed, and vit D, Ca, P, and parathormone (PTH) levels were measured in patients’ sera. Vit D and PTH values were measured using Simens Atellica immunoassay autoanalyzer, and routine biochemistry and Ca and P levels were measured using Simens Atellica IM/CIH biochemistry autoanalyzer. The study was carried out between November and May.
The control groups were selected from healthy people who came for general control purposes. Before the study, permission was obtained from the local ethics committee (ethics committee no. 26/0172022; date and number E1-22-2347). The study was conducted according to good clinical practices and Helsinki Declaration. Consent was obtained from the patient and control groups. The informed consent form was obtained from the patients.
In the patient group, the diagnosis of SD was made based on the clinical findings. The lesion localization was differentiated.
Serum vit D levels were compared between the patient and control groups.
Vit D levels were evaluated according to 2 units (ng/mL and nmol/L). Vit D levels <20 ng/mL were identified as severe deficiency, 20–30 ng/mL as mild-moderate deficiency (moderate), and 150> ng/mL as toxicity risk. Similarly, vit D levels <50 nmol/L were identified as severe deficiency, 50–75 nmol/L as mild-moderate deficiency (moderate), and >375 nmol/L as toxicity risk.
Ca, P, and PTH levels were compared between the patient and control groups (the normal Ca values were accepted to be 8.7–10.4 mg/dL, the normal P-values were accepted to be 2.4–5.1 mg/dL, and the normal PTH values were accepted to be 18.4–80.1 ng/L). The Ca, P, and PTH levels were compared between patients with and without severe vit D deficiency.
Duration of the disease: It was divided into three groups, namely, 1–5 years, 6–10 years, and more than 10 years.
A comparison was made between those with or without severe vit D deficiency in terms of age, gender, age of onset, duration, area of involvement, subjective stress history, smoking status, alcohol use, and presence of additional systemic and skin diseases. The relationship between disease localization and duration of the disease and vit D levels was evaluated. Involvement areas were grouped as scalp, forehead, face, nasolabial, chin, eyebrows, ear, and mid-chest.
Statistical method
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 21.0 for Windows (SPSS, Inc., Chicago, USA) package program. While descriptive values were expressed as numbers (n) and percentage (%) for categorical values, they were expressed as the mean (standard deviation, SD) if normally distributed and as the median (interquartile range, IQR) if not normally distributed. Pearson chi-square and Fisher’s exact tests were used to compare categorical variables. Whether the continuous variables fit the normal distribution or not was evaluated with Kolmogorov-Smirnov and Shapiro-Wilk tests, and comparisons were made using Student’s t-test for numerical variables that fit normal distribution and Mann-Whitney U test for numerical variables that did not fit normal distribution. The statistical significance level was accepted as p<0.05 in all comparisons.
While calculating the sample size in our study, it was predicted that the frequency of severe vit D deficiency would be 53% in patients with SD and 26% in the control group. Thus, the total (patient+control) number of participants required for an effect size of 0.28 was calculated as 108 (strength 80%, type 1 error 5%, and df=1). Sample size calculation was done using G-power.
RESULTS
The median age of the patients was 28 years (IQR: 25–37.5). The median age of the controls was 32 years (IQR: 26–47.3). The patient and control groups were similar in terms of age (p=0.14). In the patient group, 58.5% were males and 41.5% were females (male/female ratio 1:4). In the control group, 48.3% were males and 51.7% were females. The patient and control groups were similar in terms of gender (p=0.28).
The duration of the disease was 1–5 years in 77.4% of the patients, 6–10 years in 20.8% of the patients, and more than 10 years in 1.9% of the patients. The median age of onset of the disease was 24 years (IQR: 21–32).
Figure 1 shows the distribution of vit D deficiencies in the patient and control groups. The presence of severe vit D deficiency was significantly more frequent in the patient group than in the control group (p=0.003) (Figure 1). There was no relationship between vit D levels and disease duration or disease localization.
Figure 1. Presence of severe vitamin Dvit D deficiency in the patient and control groups.
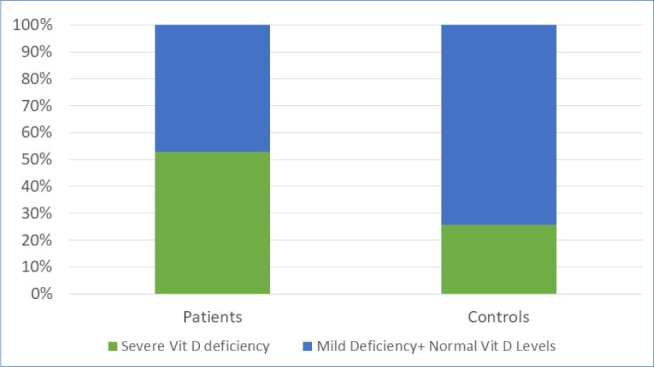
Patients with severe vit D deficiency were younger than patients with mild vit D deficiency and normal vit D levels (p=0.048). Additionally, female gender was more frequent in patients with severe vit D deficiency (57.1% in the severe vit D deficiency group vs. 24% in the mild deficiency+normal vit D level group) (p=0.015). In the control group, no statistically significant association was observed between vit D levels and age or sex (p-values=0.94 and 0.25, respectively). All characteristics of patients with and without severe vit D deficiency, disease localization, and rates are summarized in Table 1.
Table 1. All characteristics of patients with and without severe vitamin D deficiency, disease localization, and rates.
| Patients with severe vit D deficiency | Patients without severe vit D deficiency | p-value | |
|---|---|---|---|
| Age, average (standard deviation) | 28.9 (9.7) | 35.2 (13.1) | 0.048 |
| Sex, n (%) | |||
| Female | 16 (57.1) | 6 (24) | 0.015 |
| Male | 12 (42.9) | 19 (76) | |
| Disease duration, years, median (IQR) | 3 (1–5) | 3 (1.7–8.5) | 0.34 |
| Age of onset, median (IQR) | 23 (18.9–28.4) | 26 (22–34.5) | 0.14 |
| Number of sites involved, median (IQR) | 1.5 (1–3.8) | 2 (1–2.5) | 0.85 |
| Scalp involvement, n (%) | |||
| Yes | 26 (92.9) | 22 (88) | 0.55 |
| No | 2 (7.1) | 3(12) | |
| Facial involvement, n (%) | |||
| Yes | 8 (28.6) | 6 (24) | 0.71 |
| No | 20 (71.4) | 19 (76) | |
| Nasolabial involvement, n (%) | |||
| Yes | 3 (10.7) | 1 (4) | 0.36 |
| No | 25 (89.3) | 24 (96) | |
| Eyebrow involvement, n (%) | |||
| Yes | 6 (21.4) | 5 (20) | 0.89 |
| No | 22 (78.6) | 20 (80%) | |
| Ear involvement, n (%) | |||
| Yes | 6 (21.4) | 5 (20) | 0.89 |
| No | 22 (78.6) | 20 (80%) | |
| Chin involvement, n (%) | |||
| Yes | 2 (7.1) | 2 (8) | 0.91 |
| No | 26 (92.9) | 23 (92) | |
| Chest involvement, n (%) | |||
| Yes | 6 (21.4) | 4 (16) | 0.61 |
| No | 22 (78.6) | 21 (84) | |
| Smoking status, n (%) | |||
| Yes | 7 (25) | 3 (12) | 0.23 |
| No | 21 (75) | 22 (88) | |
| Alcohol use, n (%) | |||
| Yes | 3 (10.7) | 2 (8) | 0.73 |
| No | 25 (89.3) | 23 (92) | |
| Additional systemic disease, n (%) | |||
| Yes | 10 (35.7) | 7 (28) | 0.55 |
| No | 18 (64.3) | 18 (72) | |
| Additional skin disease, n (%) | |||
| Yes | 1 (3.6) | 5(20) | 0.089 |
| None | 27 (96.4) | 20 (80%) | |
| Subjective stress, n (%) | |||
| Yes | 17 (60.7) | 14 (56) | 0.73 |
| No | 11 (39.3) | 11 (44) | |
When calcium, phosphorus, and PTH levels in the patient and control groups were compared, phosphorus was found to be significantly lower in the patient group (p=0.013) (Table 2).
Table 2. Comparison of calcium, phosphorus, and parathormone levels in the patient and control groups.
| Patient group | Control group | p-value | |
|---|---|---|---|
| Calcium (mg/dL), average (standard deviation) (n=8.7–10.4) | 9.61 (0.76) | 9.69 (0.61) | 0.52 |
| Phosphorus (mg/dL), average (standard deviation) (n=2.4–5.1) | 3.47 (0.52) | 3.75 (0.54) | 0.013 |
| Parathormone (ng/L), median (IQR) (n=18.4–80.1) | 35.7 (28–55.3) | 40.5 (32.5–54.5) | 0.63 |
Patients with and without severe vit D deficiency were similar in terms of serum levels of Ca, P, and PTH (Table 3).
Table 3. Comparison of calcium, phosphorus, or parathormone levels in patients with and without severe vitamin D deficiency.
| Those with severe vit D deficiency | Those without severe vit D deficiency | p-value | |
|---|---|---|---|
| Calcium (mg/dL), average (standard deviation) | 9.61 (0.79) | 9.8 (0.59) | 0.75 |
| Phosphorus (mg/dL), average (standard deviation | 3.63 (0.57) | 3.66 (0.54) | 0.72 |
| Parathormone (ng/L), median (IQR) | 39.9 (30.8–53) | 36.7 (28.5–56.8) | 0.87 |
DISCUSSION
SD is a common inflammatory disease that can last for years with relapses and remissions. As the disease is chronic and has no definite treatment, it negatively affects the quality of life of patients. Various studies have been conducted on the etiology of the disease, and many theories have been proposed. However, no definite conclusion has been reached 2,4 .
In various studies, increased CD16+expression in NK cells, activation of complement systems, and increased inflammatory interleukins in the skin of patients with SD were observed 25,26 . In addition to this, various studies have shown that Malassezia-type yeasts, which play a role in the etiopathogenesis of SD, increase the release of inflammatory cytokines such as IL-6, IL-8, and TNF-α from keratinocytes 3,27,28 . This suggests that there is increased subclinical inflammation in SD 27 .
Several studies have shown the effect of vit D on many inflammatory and autoimmune skin diseases such as psoriasis, atopic dermatitis, polymorphous light eruption, systemic lupus erythematosus, vitiligo, and alopecia areata 15-17 . In these studies, vit D levels were generally found to be low 29-31 . In these diseases in which vit D deficiency is detected, it is recommended to keep the vit D level normal 16,25 . In the etiopathogenesis of SD, vit D deficiency may have a role in the development of the disease due to its effects on the inflammatory process and immune response 11 . Moreover, the fact that SD shows spontaneous improvement in summer and benefit from vit D preparations used in the treatment brought up the relationship between vit D and disease, and SD and vit D levels were investigated 20-25 . In these studies, the questions “Is remission achieved in SD by eliminating vit D deficiency?” and “What role does vit D play in skin diseases?” were tried to be answered.
The data in studies on the use of topical vit D analogs in the treatment of SD are still contradictory. While beneficial results are obtained in some studies, others are not found to be effective enough 32-37 .
In 2013, Dimitriova et al. conducted the first study on vit D levels in SD. They found that optimal vit D levels could not be determined in any of the patients, and vit D was found to be deficient or insufficient in all patients. Moreover, no significant difference was found between male and female genders. In 2017, the same authors examined remission in their study by giving vit D supplements to patients with SD and found that their attacks decreased 19,20 .
On the contrary, Rahimi et al. found that vit D levels were significantly lower in the study group, which included 118 patients and 171 controls, compared to the control group. This difference was found to be more pronounced in patients with facial involvement compared to scalp involvement. The authors found that the vit D level was lower in patients with severe SD in the scalp than in the scalp involvement and reported that vit D was inversely proportional to the severity of the disease 21 . Sobhan et al. found that the vit D level of the patients with SD was significantly lower than the controls, but no correlation was found with the severity of SD in this study 22 . In a retrospective study conducted in Turkey, vit D levels were found to be lower in patients with SD compared to references 24 . Inan et al. investigated vit D levels in patients with telogen effluvium and SD and found low vit D levels in the presence of SD and both acute and chronic telogen effluvium, but they could not detect a significant relationship between vit D levels only in patients with SD 25 .
Scalp involvement is normally around 50–70% in SD 1,2,6 . In a study by Byung et al., they reported that sebaceous gland activity increased in 20 and 30 years of life as well as in adolescents. They stated that this may be due to the intense number and activity of sebaceous glands in the scalp, thus facilitating the development of SD 38 . In our study, scalp involvement was also higher.
Similar to other studies, Gray et al. reported that half (52%) of the patients with SD had vit D deficiency. These authors also stated that the lesions are mostly located on the scalp, and like some researchers, they reported that the severity of the disease in the scalp is associated with higher and lower serum vit D levels and that it is seen at younger ages such as the third decade 23 . In this study, we found vit D levels in patients with SD to be severely low at a rate of 52.8%, similar to previous studies. It was also lower in young female patients.
The known function of vit D is to regulate Ca and P metabolism. It achieves this effect by increasing the absorption of both Ca and P in the intestine; vit D decreases PTH secretion and increases bone resorption and Ca and magnesium resorption from the kidneys 39 . While there is a negative correlation between PTH phosphate levels, vit D has a stimulating effect on both Ca and P homeostasis. When the vit D level decreases, Ca absorption decreases, PTH increases, and urinary excretion of P increases. PTH increases due to hypocalcemia and hypophosphatemia, but there may be cases where it does not 40 . However, the reason for this is not known exactly. In their studies, Aydın et al. found PTH elevation only in 6.3% of the patients with low vit D 39 .
In this study, besides the vit D level, Ca, P, and PTH levels were also examined in the patient and control groups. In the analysis, PTH increase was not detected in any of the patients with low vit D levels. There was no difference between Ca levels, but P levels were found to be significantly lower in the patient group. No pathology was found in any of the patients to decrease blood P. Therefore, it was found that low P could be due to vit D deficiency or incidental. It was concluded that P may have another unknown effect on the pathogenesis of SD, and further research is needed on this subject. Furthermore, there was no difference between Ca, P, and PTH values in comparison of those with and without severe vit D levels.
The limitations of the study can be summarized as the small number of participants, not specifying the severity scale of SD and skin type, not performing questioning of the lifestyle and clothing style, sun exposure history, and diet questioning, which could be potential sources of bias and uncertainty.
CONCLUSION
Recently, vit D has been extensively studied by researchers not only for its role in Ca metabolism but also for its immunoregulatory, antiproliferative, and differentiation-controlling properties. The obtained data show that vit D is promising to researchers. In this study, vit D levels were found to be low in patients with SD. The fact that vit D levels decreased in patients with SD and patients with severe vit D deficiency develop SD earlier suggests that the low levels of vit D are related to SD.
Footnotes
Funding: none.
REFERENCES
- 1.Gupta AK, Richardson M, Paquet M. Systematic review of oral treatments for seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2014;28(1):16–26. doi: 10.1111/jdv.12197. [DOI] [PubMed] [Google Scholar]
- 2.Kaçar SD, Özuğuz P. Seboreik dermatitte güncel yaklaşım. Kocatepe Med J. 2016;17(2):72–6. [Google Scholar]
- 3.Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):343–51. doi: 10.1016/j.clindermatol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Aksoy M, Arıca DA. Seboreik dermatit. Turkiye Klinikleri J Dermatol. 2016;26(2):90–100. [Google Scholar]
- 5.Zander N, Sommer R, Schäfer I, Reinert R, Kirsten N, Zyriax BC, et al. Epidemiology and dermatological comorbidity of seborrhoeic dermatitis: population-based study in 161 269 employees. Br J Dermatol. 2019;181(4):743–48. doi: 10.1111/bjd.17826. [DOI] [PubMed] [Google Scholar]
- 6.Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2) doi: 10.13188/2373-1044.1000019. doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–92. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umar M, Sastry KS, Al Ali F, Al-Khulaifi M, Wang E, Chouchane AI. Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol Physiol. 2018;31(2):74–86. doi: 10.1159/000485132. [DOI] [PubMed] [Google Scholar]
- 9.Birlea SA, Costin GE, Norris DA. New insights on therapy with vitamin D analogs targeting the intracellular pathways that control repigmentation in human vitiligo. Med Res Rev. 2009;29(3):514–46. doi: 10.1002/med.20146. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13(1):3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kıdır M. D vitamininin, immün sistem, deri ve kanser ile ilişkisi. S.D.Ü. Tıp Fak. Derg. 2013;20(4):158–61. [Google Scholar]
- 13.Mostafa WZ, Hegazy RA. Vitamin D and the skin: Focus on a complex relationship: a review. J Adv Res. 2015;6(6):793–804. doi: 10.1016/j.jare.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eller ABP, Ejzenberg D, Monteleone PAA, Soares JM, Baracat EC. Vitamin D and in vitro fertilization: a systematic review. J Assist Reprod Genet. 2023 doi: 10.1007/s10815-023-02767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wat H, Dytoc M. Off-label uses of topical vitamin D in dermatology: a systematic review. J Cutan Med Surg. 2014;18(2):91–108. doi: 10.2310/7750.2013.13109. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Triviño FJ, Arias-Santiago S, Gilaberte-Calzada Y. Vitamin D and the skin: a review for dermatologists. Actas Dermosifiliogr (Engl Ed). 2019;110(4):262–72. doi: 10.1016/j.ad.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Kechichian E, Ezzedine K. Vitamin D and the skin: an update for dermatologists. Am J Clin Dermatol. 2018;19(2):223–35. doi: 10.1007/s40257-017-0323-8. [DOI] [PubMed] [Google Scholar]
- 18.Wadhwa B, Relhan V, Goel K, Kochhar AM, Garg VK. Vitamin D and skin diseases: a review. Indian J Dermatol Venereol Leprol. 2015;81(4):344–55. doi: 10.4103/0378-6323.159928. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrova J. The effect of vitamin D supplementation on recurrences of seborrheic dermatitis. Int J Curr Adv Res. 2017;6(3):2446–8. doi: 10.24327/ijcar.2017.2448.0025. [DOI] [Google Scholar]
- 20.Dimitrova J. Study of the level of 25-hydroxyvitamin D in patients with seborrheic dermatitis. Scripta Sci Med. 2013;45(1):75–8. doi: 10.14748/ssm.v45i1.345. [DOI] [Google Scholar]
- 21.Rahimi S, Nemati N, Shafaei-Tonekaboni SS. Serum levels of 25-hydroxyvitamin d in patients with seborrheic dermatitis: a case-control study. Dermatol Res Pract. 2021;2021:6623271. doi: 10.1155/2021/6623271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobhan M, Nazari F, Mohammadi Y. Correlation between seborrheic dermatitis and metabolic syndrome in patients referred to sina hospital of Hamadan. Avicenna J Clin Med. 2020;27(1):13–20. doi: 10.29252/ajcm.27.1.13. [DOI] [Google Scholar]
- 23.Reyes RGT, Veyra MLA. Prevalence of vitamin D insufficiency and deficiency among seborrheic dermatitis patients: a crosssectional study at Makati Medical Center. J Phil Dermatol Soc. 2021:24–28. ISSN 2094-201X. [Google Scholar]
- 24.Çiftçi ND. Vitamini düzeylerinin deri hastalıkları üzerine etkisinin retrospektif değerlendirilmesi. Kocaeli Med J. 2018;7(3):47–54. [Google Scholar]
- 25.İnan Dogan E, Ozkesıcı Kurt B. Vitamin D levels in patients with seborrheic dermatitis and telogen effluvium. Firat Med J. 2021;26(4):206–10. [Google Scholar]
- 26.Elgash M, Dlova N, Ogunleye T, Taylor SC. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18(1):24–7. [PubMed] [Google Scholar]
- 27.Bakardzhiev I, Argirova A. New Insights into the etiopathogenesis of seborrheic dermatitis. Clin Res Dermatol Open Access. 2017;4(1):1–5. [Google Scholar]
- 28.Thomas DS, Ingham E, Bojar RA, Holland KT. In vitro modulation of human keratinocyte pro- and anti-inflammatory cytokine production by the capsule of Malassezia species. FEMS Immunol Med Microbiol. 2008;54(2):203–14. doi: 10.1111/j.1574-695X.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 29.Gisondi P, Rossini M, Cesare A, Idolazzi L, Farina S, Beltrami G, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166(3):505–10. doi: 10.1111/j.1365-2133.2011.10699.x. [DOI] [PubMed] [Google Scholar]
- 30.Varikasuvu SR, Aloori S, Varshney S, Bhongir AV. Decreased circulatory levels of Vitamin D in Vitiligo: a meta-analysis. An Bras Dermatol. 2021;96(3):284–94. doi: 10.1016/j.abd.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borzutzky A, Camargo CA. Role of vitamin D in the pathogenesis and treatment of atopic dermatitis. Expert Rev Clin Immunol. 2013;9(8):751–60. doi: 10.1586/1744666X.2013.816493. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama J. Four cases of sebopsoriasis or seborrheic dermatitis of the face and scalp successfully treated with 1a-24 (R)-dihydroxycholecalciferol (tacalcitol) cream. Eur J Dermatol. 2000;10(7):528–32. [PubMed] [Google Scholar]
- 33.Berth-Jones J, Adnitt PI. Topical calcipotriol is not effective in facial seborrhoeic dermatitis. J Dermatolog Treat. 2001;12(3):179. doi: 10.1080/09546630152608339. [DOI] [PubMed] [Google Scholar]
- 34.Basak PY, Ergin S. Comparative effects of calcipotriol and betamethasone 17-valerate solution in the treatment of seborrhoeic dermatitis of the scalp. J Eur Acad Dermatol Venereol. 2001;15(1):86–8. doi: 10.1046/j.1468-3083.2001.00193-9.x. [DOI] [PubMed] [Google Scholar]
- 35.Kowalzick L, Schlehaider U. An open pilot study of topical calcipotriol in seborrhoeic eczema. J Dermatol Treatment. 2009;9(1):49–51. [Google Scholar]
- 36.Tadaki T, Kato T, Tagami H. Topical active vitamin D3analogue, 1,24-dihydroxycholecalciferol, an effective new treatment for facial seborrhoeic dermatitis. J Dermatol Treatment. 2009;7(3):139–41. [Google Scholar]
- 37.Yap FB. The role of combination calcipotriol plus betamethasone dipropionate gel in the treatment of moderate-to-severe scalp seborrhoeic dermatitis. Sultan Qaboos Univ Med J. 2018;18(4):e520-3. doi: 10.18295/squmj.2018.18.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ro BI, Dawson TL. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J Investig Dermatol Symp Proc. 2005;10(3):194–7. doi: 10.1111/j.1087-0024.2005.10104.x. [DOI] [PubMed] [Google Scholar]
- 39.Aydın T. Düşük serum D vitamin düzeylerine parathormon yanıtını etkileyen faktörler. ACU Sağlık Bil Derg. 2020;11(4):706–10. [Google Scholar]
- 40.Zhang S, Miller DD, Li W. Non-musculoskeletal benefits of vitamin d beyond the musculoskeletal system. Int J Mol Sci. 2021;22(4):2128. doi: 10.3390/ijms22042128. [DOI] [PMC free article] [PubMed] [Google Scholar]


