SUMMARY
OBJECTIVE:
Coronavirus disease 2019 emerges as a disease caused by severe acute respiratory syndrome coronavirus 2. It is a systemic disease associated with vascular inflammation and endothelial damage. In this study, we aimed to investigate whether vascular endothelial growth factor gene insertion/deletion polymorphism is associated with coronavirus disease 2019 in the Turkish population.
METHODS:
The study included 179 participants (79 patients with coronavirus disease 2019 and 100 controls). DNA isolation was made from peripheral blood, and then the polymerase chain reaction analysis was performed.
RESULTS:
When we analyze vascular endothelial growth factor gene insertion/deletion polymorphism in the study group, we found that the DD genotype and D allele were found to be statistically significantly different when compared to coronavirus disease 2019 patients with high vitamin D value (p=0.005 for DD genotype and p=0.006 for D allele) in the control group. In this high-level control group, when we analyze II+ID genotype versus DD, a statistically significant difference was also detected (p=0.007).
CONCLUSION:
As a result of the study, we found that DD genotype and D allele were associated with vitamin D level in Turkish patients with coronavirus disease 2019.
Keywords: Vascular endothelial growth factors, Vitamin D, Coronavirus disease 2019, Polymerase chain reaction
INTRODUCTION
Pathogens of the coronavirus family can infect both humans and animals. A novel coronavirus (nCoV) was recently identified, leading to severe pneumonia cases in the Chinese city of Wuhan at the end of 2019. With its rapid spread, it became a global threatening pandemic after causing an epidemic throughout China. The World Health Organization (WHO) designated the viral disease as COVID-19 (i.e., coronavirus disease 2019) in February 2020. The virus that causes COVID-19 infection has been renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to replace the previous name 2019-nCoV. The purpose of this review was to outline the three-phase clinicogenomic course of COVID-19 immune syndrome as identified in a recent bioinformatics study 1 .
Considering the pathogenesis of COVID-19, the infection starts with the binding of the virus to the angiotensin converting enzyme 2 (ACE 2) receptors expressed in various tissues, thus triggering an excessive immune response. Cytokine storm resulting from the overproduction of proinflammatory cytokines has been associated with severe progression of COVID-19 infection and organ damage. The pathophysiological mechanism of COVID-19 infection has not been fully elucidated, but the effects of genetic variations in genes in inflammation-related pathways on the course and severity of the infection are being investigated. In older adults and people with comorbidities, COVID-19 infection is more severe. COVID-19 infection is characterized by various symptoms such as fever, cough, shortness of breath, weakness, muscle aches, taste and smell disorders, diarrhea, and headache. The results of studies on genetic variations show differences between populations 2 . The vascular endothelial growth factor (VEGF) family and its receptors are key regulators of angiogenesis and barrier function. The VEGF family consists of VEGF-A, PlGF, VEGF-B, VEGF-C, VEGF-D, and VEGF-E. The researchers detected elevated plasma levels of VEGF-A in the serum of COVID-19 patients, which were found to be correlated with disease severity 3,4 . VEGF-B levels did not appear to be altered in COVID-19, while VEGF-D level, which promotes angiogenesis and lymphangiogenesis, was lower in COVID-19 compared to healthy control 5 . Kong et al. identified high VEGF-D as the most important indicator of disease severity in a small cohort of COVID-19 patients 6 . Further studies are needed to clarify these contrast findings and the potential mechanistic role of VEGF-D in COVID-19. Based on these findings, we aimed to investigate the relationship between VEGF gene insertion/deletion polymorphism and COVID-19 in the Turkish population.
METHODS
Study sample
This study included 179 participants (79 patients with COVID-19 and 100 controls). The study was conducted with 79 patients who were diagnosed with COVID-19 and 100 healthy controls in Samsun Education and Research Hospital between 2021 and 2022. The University of Samsun Health Science Clinical Ethical Committee approved the study (the ethical number is GOKA 2021/10/18). All the participants were informed about the study and signed the informed consent form. Patients’ information was obtained and white blood cells, neutrophils, lymphocytes, platelets, C-reactive protein (CRP) (mg/dL), D-dimer (ng/mL), vitamin D (ng/mL), monocytes, prothrombin time, activated partial thromboplastin time, international normalized ratio, ferritin, and procalcitonin (PCT) values were recorded.
DNA isolation and polymerase chain reaction methods
DNA isolation was performed from 179 samples belonging to the patient and control groups. It was carried out from 2 mL peripheral blood sample taken into a tube with EDTA. After DNA isolation, a polymerase chain reaction (PCR) was performed using primers. The promoter region of the vascular endothelial growth factor gene was amplified by PCR. For the reaction, 50 ng of DNA, 1 μM from each primer, 10 μM dNTP from each dNTP, 1.5 mM MgCl2, 0.2 units of Taq polymerase, and 10´ PCR buffer were placed into 25 μL total mixture. The PCR conditions were as follows: the initial denaturation was applied for 4 min at 94°, 35 cycles, and the denaturation was applied for 45 s at 95° and 45 s at 62°. Forward primers such as 5’-GCTGAGGATGGGGCTGACTAGGTA-3’ and reverse primers such as 5’-GTTT CTGACCTGGCTATTTCCAGG-3’ were used. Elongation was performed at 72° for 45 s, and the final extension was performed at 72° for 7 min. We visualized the amplification products on a 2.5% agarose gel. The amplified PCR product size of VEGF was the 228 bp fragments showing the D allele and the 221 bp fragments showing the I allele. To check the results, 20% of the randomly selected samples were reworked and a 100% match was found.
STRING analysis
STRING database annotates the functional interactions between the proteins in a cell. In this study, VEGF protein interactions were evaluated.
Statistical analysis
The SPSS 20 program was used in the statistical analysis of our study. Chi-square analysis was used to calculate genotype distributions and allele frequencies. The OpenEpi program was also used for genotype distributions and grouped genotype and allele comparisons. In the statistical analysis results, the p-value >0.05 was accepted as statistically significant.
RESULTS
The mean age of the control group was 56.44±19.08 years, and the mean age of the patients was 55.84±13.82 years. In the patient group, the rate of women was 55.7%, while the rate of men was 44.3%. In the control group, the rate of women was 32% and the rate of men was 17%. Table 1 represents the clinical characteristics of COVID-19 patients. When the control group with normal vitamin D value was compared with the patients (p=0.232), there was no statistical difference between genotype distributions and allele frequencies, but a statistically significant difference was found when compared with the control group with high vitamin D value (Table 2). The results of our study showed that the DD genotype and D allele were found to be statistically significantly different when compared to COVID-19 patients with high vitamin D value (p=0.005 for DD genotype and p=0.006 for D allele) in the control group. In this high-level control group, when we analyze II+ID genotype versus DD, a statistically significant difference was also detected (p=0.007) (Table 3).
Table 1. Clinical characteristics of coronavirus disease 2019 patients.
| Clinical findings of patients | Mean±SD |
|---|---|
| White blood cells | 9.48 (7.30) |
| Neutrophils | 6.67 (6.25) |
| Lymphocytes | 1.19 (0.73) |
| Platelets | 244.40 (101.68) |
| C-Reactive protein (mg/dL) | 81.03 (89.81) |
| D-dimer (ng/mL) | 2.65 (5.71) |
| Vitamine D (ng/mL) | 12.96 (6.26) |
| Monocytes | 0.51 (0.35) |
| Prothrombin time | 12.52 (1.54) |
| Activated partial thromboplastin time | 24.15 (7.64) |
| International normalized ratio | 1.10 (0.14) |
| Ferritin | 533.50 (570.36) |
| Procalcitonin (PCT) | 0.55 (2.01) |
| Services | 29 (36.7) |
| Intensive care units | 50 (63.3) |
Table 2. Genotype distribution and allele frequencies of coronavirus disease 2019 patients and control with high vitamin D level.
| Vascular endothelial growth factor insertion/deletion | COVID-19 group n=79 (%) | Control group n=100 (%) | χ2 | OR (95%CI) | p-value |
|---|---|---|---|---|---|
| Genotypes | |||||
| II | 9 (11.4) | 2 (4.1) | 0.005 | ||
| ID | 53 (67.1) | 45 (91.8) | |||
| DD | 17 (21.5) | 2 (4.1) | |||
| II+ID:DD | 62:17 | 47:2 | 7.275 | 0.157 | 0.007 |
| DD+ID:II | 70:9 | 47:2 | 2.058 | 0.333 | 0.151 |
| Alleles | |||||
| I | 71 | 49 | 0.6227 | 0.4925–1.352 | 0.4301 |
| D | 87 | 49 | |||
Bold values indicate that p<0.05 is statistically significant.
Table 3. The distribution of the genotypes and alleles in coronavirus disease 2019 and control groups.
| Vascular endothelial growth factor insertion/deletion | COVID-19 group n=79 (%) | Control group n=100 (%) | χ2 | OR (95%CI) | p-value |
|---|---|---|---|---|---|
| Genotypes | |||||
| II | 9 (11.4) | 17 (17) | 2.921 | 0.232 | |
| ID | 53 (67.1) | 71 (71) | |||
| DD | 17 (21.5) | 12 (12) | |||
| II+ID:DD | 63:17 | 88:12 | 2.814 | 0.5073 | 0.093 |
| DD+ID:II | 71:9 | 83:12 | 0.078 | 1.14 | 0.779 |
| Alleles | |||||
| I | 71 | 105 | 2.001 | 0.7409 | 0.157 |
| D | 87 | 95 |
STRING analysis
By analyzing the VEGF protein with the STRING database, we found the predicted functional partners of the protein as follows: vascular endothelial growth factor receptor 3 (FLT4), vascular endothelial growth factor receptor 2 (KDR), neuropilin 1, the membrane-bound isoform 1 (NRP1), vascular endothelial growth factor receptor 1 (FLT1), neuropilin 2 (NRP2), hypoxia-inducible factor 1-alpha (HIF1A), high-affinity nerve growth factor receptor (NTRK1), cadherin 5 (CDH5), fibronectin 1 (FN1), and fibroblast growth factor 2 (FGF2). The interaction network of these proteins is shown in Figure 1.
Figure 1. Interactions of vascular endothelial growth factor-A protein, according to STRING database predictions.
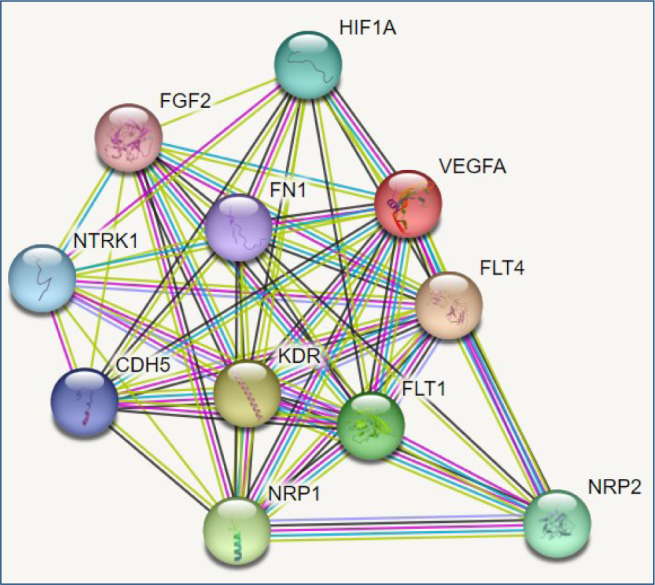
STRING analysis
By analyzing the VEGF protein with the STRING database, we found the predicted functional partners of the protein as follows: vascular endothelial growth factor receptor 3 (FLT4), vascular endothelial growth factor receptor 2 (KDR), neuropilin 1, the membrane-bound isoform 1 (NRP1), vascular endothelial growth factor receptor 1 (FLT1), neuropilin 2 (NRP2), hypoxia-inducible factor 1-alpha (HIF1A), high-affinity nerve growth factor receptor (NTRK1), cadherin 5 (CDH5), fibronectin 1 (FN1), and fibroblast growth factor 2 (FGF2). The interaction network of these proteins is shown in Figure 1.
DISCUSSION
COVID-19, caused by SARS-CoV-2, has become a persistent health emergency since its outbreak in late 2019 6 . Progression of COVID-19 often involves excessive pro-inflammatory cytokines and mediators 7 . VEGF, a key factor involved in vascular permeability and inflammation 8 , was found to be skyrocketed in the blood of COVID-19 patients and related to disease severity 9 .
Vitamin D regulates the immune modulatory mechanisms by decreasing the proinflammatory environment in vivo and increasing the secretion of anti-inflammatory cytokines. It has been reported that 25(OH)D deficiency, a physiologically quantifiable form of vitamin D, is strongly associated with unfavorable clinical outcomes. Finally, no adverse effects of using high doses of vitamin D in COVID-19 and other circumstances have been reported 10 .
Overall, melatonin is an intriguing compound, not unlike vitamin D, which is pleiotropic in activity and responsive to light-dark cycles. From a scientific perspective, melatonin acts as a powerful antioxidant that can cross the blood-brain barrier, inhibit inflammation, and interact with the gut microbiome. From a clinical point of view, melatonin imbalance may indicate “darkness deficiency” in much the same way that vitamin D may infer whether or not someone has a “light deficiency” 11 .
Soluble levels of a circulating form of the VEGF-A receptor are markedly increased in COVID-19 patients and correlated with disease severity 2-4 . Under normal conditions, this form is electrostatically bound to proteoglycans and thus sequestered 12 . Its elevated level could therefore theoretically be a result of damage. Its overexpression has been well shown to promote endothelial dysfunction, particularly during preeclampsia 13 . Dupont et al. reported that plasma levels (n=46) at admission to the ICU are associated with the need for mechanical ventilation, the need for vasopressor support, the development of severe acute kidney injury, and death 14 . However, unlike preeclampsia, its high levels in COVID-19 are clearly not accompanied by a decrease in PlGF 15,16 . This finding is apparently very consistent, as the rate remains low in pregnant women. It allows a good distinction between COVID-19 pneumonia and true preeclampsia and preeclampsia-like symptoms due to COVID-19 17 . VEGF plays a primary role in maintaining the growth, development, and maintenance of a healthy circulatory system, thereby ensuring normal angiogenesis 18 . They bind with VEGFR and activate the endothelial cell. Alveolar immune regulation is important and is maintained by the integrity of the endothelial barrier in lung tissue, which is crucial in patients affected by COVID-19 19 . Serum levels of VEGF have been found to be elevated in people affected by SARS-CoV-2. Based on these findings, we aimed to investigate whether VEGF insertion/deletion gene polymorphism was associated with COVID-19 and we found that the DD genotype and D allele was associated with vitamin D level in Turkish COVID-19 patients. In COVID-19, neutrophils, monocytes, and macrophages become hyperactivation, and as a result, it shows that it can lead to dysregulation in the inflammatory response and cytokine storm (Fernandes 2022) 20-22 . These changes have also been associated with an increase in various interleukins and VEGFs in COVID-19 patients. A large-scale study reports that vitamin D mechanisms also play an important role in these processes 23 .
CONCLUSION
In accordance with our results, it is previously reported that it has been hypothesized that sufficient vitamin D levels could prevent cytokine storm while promoting an adequate adaptive immune response in patients with COVID-19. In the literature, there are no studies about investigated relation between VEGF gene insertion/deletion polymorphism and COVID-19. Our results would provide important contributions to the literature. Genetic polymorphisms are very important in the field of medicine and will allow for the discovery of new treatment strategies and drugs as their mechanisms are understood.
ACKNOWLEDGMENTS
The authors would like to thank all participants for their time and excellent cooperation.
Footnotes
Funding: none.
REFERENCES
- 1.Turk C, Turk S, Malkan UY, Haznedaroglu IC. Three critical clinicobiological phases of the human SARS-associated coronavirus infections. Eur Rev Med Pharmacol Sci. 2020;24(16):8606–20. doi: 10.26355/eurrev_202008_22660. [DOI] [PubMed] [Google Scholar]
- 2.Turk C, Turk S, Malkan UY, Haznedaroglu IC, Sungnak W, Huang N, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–57. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smadja DM, Philippe A, Bory O, Gendron N, Beauvais A, Gruest M, et al. Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J Thromb Haemost. 2021;19(7):1823–30. doi: 10.1111/jth.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byzova TV. “Fishing” out the real VEGFs. Blood. 2016;128(19):2283–4. doi: 10.1182/blood-2016-09-737023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rader B, Scarpino SV, Nande A, Hill AL, Adlam B, Reiner RC, et al. Crowding and the shape of COVID-19 epidemics. Nat Med. 2020;26(12):1829–34. doi: 10.1038/s41591-020-1104-0. [DOI] [PubMed] [Google Scholar]
- 7.Choreño-Parra JA, Jiménez-Álvarez LA, Cruz-Lagunas A, Rodríguez-Reyna TS, Ramírez-Martínez G, Sandoval-Vega M, et al. Clinical and immunological factors that distinguish COVID-19 from pandemic Influenza A(H1N1) Front Immunol. 2021;12:593595. doi: 10.3389/fimmu.2021.593595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10(10):1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syed F, Li W, Relich RF, Russell PM, Zhang S, Zimmerman MK, et al. Excessive matrix metalloproteinase-1 and hyperactivation of endothelial cells occurred in COVID-19 patients and were associated with the severity of COVID-19. J Infect Dis. 2021;224(1):60–9. doi: 10.1093/infdis/jiab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292:198235. doi: 10.1016/j.virusres.2020.198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minich DM, Henning M, Darley C, Fahoum M, Schuler CB, Frame J. Is Melatonin the “Next Vitamin D”?: a review of emerging science, clinical uses, safety, and dietary supplements. Nutrients. 2022;14(19):3934. doi: 10.3390/nu14193934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E. Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res. 2011;108(9):1063–70. doi: 10.1161/CIRCRESAHA.110.239665. [DOI] [PubMed] [Google Scholar]
- 13.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont V, Kanagaratnam L, Goury A, Poitevin G, Bard M, Julien G, et al. Excess soluble fms-like tyrosine kinase 1 correlates with endothelial dysfunction and organ failure in critically ill coronavirus disease 2019 patients. Clin Infect Dis. 2021;72(10):1834–7. doi: 10.1093/cid/ciaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardini V, Carrer A, Casati M, Contro E, Vergani P, Gambacorti-Passerini C. Increased sFLT-1/PlGF ratio in COVID-19: a novel link to angiotensin II-mediated endothelial dysfunction. Am J Hematol. 2020;95((8)):E188–91. doi: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negro A, Fama A, Penna D, Belloni L, Zerbini A, Giuri PG. SFLT-1 levels in COVID-19 patients: association with outcome and thrombosis. Am J Hematol. 2021;96((2)):E41–3. doi: 10.1002/ajh.26037. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127((11)):1374–80. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggiero D, Dalmasso C, Nutile T, Sorice R, Dionisi L, Aversano M, et al. Genetics of VEGF serum variation in human isolated populations of cilento: importance of VEGF polymorphisms. PLoS One. 2011;6((2)):e16982. doi: 10.1371/journal.pone.0016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fajgenbaum DC, June CH, Cytokine storm N. Cytokine storm. N Engl J Med. 2020;383(23):2255–73. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;7(6):998–1002. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


