Abstract
Background/Aims
Various endoscopic submucosal dissection (ESD) methods for gastric tumors have been tried. However, no studies have yet compared results according to the ESD method for gastric body tumors using a dual knife. The objective of this study was to compare outcomes of two ESD methods for gastric body tumors the pocket-creation method and conventional method.
Methods
Patients who underwent ESD for a gastric body tumor were retrospectively reviewed. Patients were divided into two groups according to the ESD method the conventional method (group I) and pocket-creation method (group II). Characteristics of patients and tumors, hospitalization period, incidence of complications, resection margin status, incidence of surgical operation, procedure time, and laboratory findings were investigated.
Results
Of the total of 100 patients, 52 belonged to group I and 48 to group II. All tumors were successfully resected en bloc. Resection margin involvement was found in six (11.5%) of group I and six (12.5%) of group II. Complications were observed in seven (13.5%; major complication five, minor two) of group I and eight (16.7%; major two, minor six) of group II. There were no significant differences in ESD outcomes such as hospitalization period, incidence of complications, resection margin status, incidence of surgical operation, procedure time, or inflammatory response after ESD between the two groups.
Conclusions
Both methods are suitable for treating gastric body tumors with adequate treatment success rates and comparable complication rates.
Keywords: Endoscopy, Endoscopic submucosal dissection, Stomach neoplasms, Pocket-creation method
INTRODUCTION
Endoscopic submucosal dissection (ESD) is a minimally invasive therapy that allows en bloc resection of superficial gastrointestinal neoplasms regardless of the size or location of the tumor.1,2 Currently, it is widely used for the treatment of early esophageal and colorectal tumors as well as gastric tumor.3,4
The ESD procedure for gastric body tumor is relatively difficult. During submucosal dissection of a tumor located in the gastric body, bleeding frequently occurs due to abundant blood vessels. Penetrating vessels in the submucosal layer can be more easily detected and coagulated when needle-type knives are used than when insulated tip knives are used.5,6 Therefore needle-type knives such as dual knives and flush knives are widely used in ESD for tumors located in the gastric body.
During ESD for gastric tumor using a needle-type knife, the maintenance of tissue tension and good submucosal exposure during submucosal dissection are the most important factors for a successful ESD. When using a conventional method, submucosal dissection is mainly performed in a distal to proximal direction.7 With this method, the traction force is made by gravity and tension of the un-incised mucosa. On the other hand, when using a pocket-creation method, the ESD is mainly performed in a proximal to distal direction.8 With this method, a submucosal pocket under the lesion is formed after a small mucosal incision and the tip of the endoscope is inserted into the pocket to perform submucosal dissection. This method has advantages in that the view is stable and the operative field is well recognized.
For lesions in specific locations on the stomach, a specific approach may be useful. For example, only a proximal to distal approach is possible for lesions on the greater curvature (GC) side of the antrum. However, both approach methods, the pocket-creation method and the conventional method, can be used for gastric body tumors.
There have been studies comparing the pocket-creation method and the conventional method for esophageal or colon ESD.9-11 However, no studies have yet reported which ESD method is superior for gastric body tumors. Therefore, the objective of this study was to compare the results of two ESD methods, the pocket-creation method and the conventional method, for removing gastric body tumors using a dual knife.
MATERIALS AND METHODS
1. Subjects
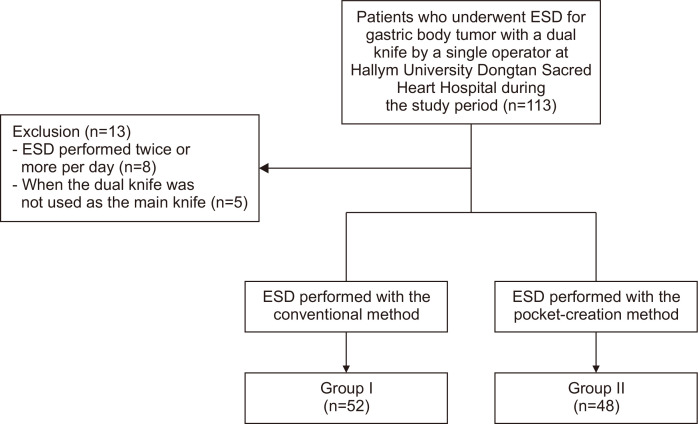
Patients who had undergone an ESD due to a gastric tumor at Hallym University Dongtan Sacred Heart Hospital (Hwaseong, Korea) from September 2018 to June 2021 were retrospectively reviewed (Fig. 1). To be eligible for inclusion, the tumor had to be located in the gastric body portion and the procedure had to be performed by a specialist (S.P.L.) with more than 10 years of ESD experience. Only ESD procedures using dual knives were included in the study, but other types of knives could be added if necessary. The procedure was performed according to expanded indications proposed by “Japan Clinical Oncology Group”.12,13
Fig. 1.
Flowchart of the study.
ESD, endoscopic submucosal dissection.
If there were small lesions that could be removed by endoscopic mucosal resection (EMR) (or polypectomy) other than lesions for which ESD was performed, ESD and EMR were performed sequentially at once. EMR was considered to have little effect on procedure time or complications, so a case where ESD and EMR were performed together was also included in the study. When the ESD procedure was performed more than twice within one day or when the dual knife was not used as the main knife, cases were excluded from this study. Patient’s sex, age, underlying disease, medications, tumor size, tumor location, tumor morphology, histologic findings, en bloc resection, resection margin status, complications, procedure time, incidence of surgical operation, and approach methods were investigated by reviewing endoscopic images, chart records, and histologic findings. C-reactive protein level and white blood cell count before and after the procedure, the length of hospitalization, and the maximum body temperature during hospitalization were also investigated.
This study was approved by the Institutional Review Board of Hallym University School of Medicine (IRB number: HDT 2021-05-001-001). After obtaining IRB approval, this study was registered in Clinical Research Information Service with CRISID of KCT0006402.
2. Endoscopic procedure
An EG-760R, 760Z, or 600WR endoscope with a 7000 endoscopic system (Fujifilm Co., Tokyo, Japan) with an ST Hood short-type (DH-28GR, Fujifilm Co.) attached was used. Standard upper endoscopy was performed after using lidocaine spray (XylocaineⓇ, AstraZeneca, Sweden) for local anesthesia according to the European Society of Gastrointestinal Endoscopy guideline. Midazolam (Dormicum; Bukwang Pharma., Seoul, Korea) with or without propofol (Fresofol MCT, 150 mg/15 mL/A; Fresenius-Kabi Korea, Seoul, Korea) for procedural sedation was administered just before starting the procedure. Carbon dioxide insufflation was used during the ESD. Precutting and submucosal dissection were carried out using a dual knife (KD-650L; Olympus, Tokyo, Japan) after injecting saline and hyaluronate solution in a standard fashion by one endoscopist and two nurses. During the procedure, IT-2 knife (KD-611L, Olympus) was selectively used if needed. However, the main knife used for the procedure was a dual knife, and most of the lesions were dissected using the dual knife. Resected specimen was pinned on cork without tension with needles immediately after removal and fixed into formalin.
We used a high-frequency generator (Erbe Elektromedizin, Tübingen, Germany) for cutting and coagulation. Bleeding during ESD was controlled using hemostatic forceps (CoagrasperⓇ, Olympus). ESD was performed by only one experienced endoscopic specialist (S.P.L.). Owing to the invasive nature and potential risks, all patients provided written consent before the procedure.
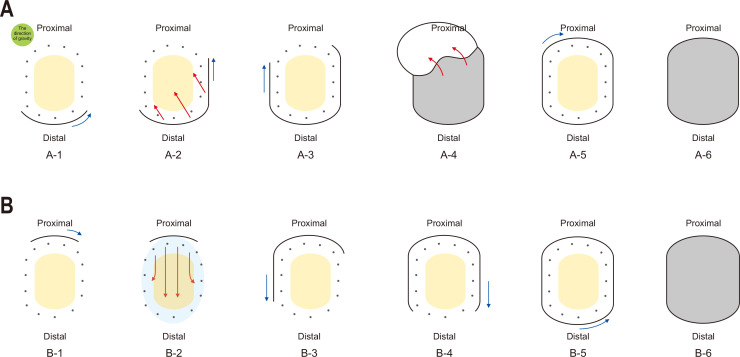
ESD in our unit was performed with the conventional method (Figs 2A, 3A, 3B) or the pocket-creation method (Figs 2B, 3C, 3D). In general, the conventional method was used before the operator went to a short-term training in Japan, and the pocket-creation method was mainly used after that. Those underwent ESD with the conventional method were assigned into group I. Those who underwent ESD with the pocket-creation method were assigned into group II. A detailed description of each method is shown below.
Fig. 2.
Schematic diagram of two endoscopic submucosal dissection methods for gastric body tumors using a dual knife. (A) Distal to proximal approach with the conventional method. A-1. Mucosal incision is performed in the distal part of the lesion. The incision is generally performed in retroflexion of the scope. A-2. Mucosal flaps are created through submucosal dissection along the direction of gravity. A-3. Mucosal incisions of both lateral parts are performed. In both lateral mucosal incisions, the order of incision is selected in consideration of the direction of gravity. A-4. Sufficient mucosal flaps are created through submucosal dissection. A-5. When submucosal dissection is almost complete, a mucosal incision of the proximal part is made last. A-6. After the tumor is completely isolated, an artificial ulcer remains. (B) Proximal to distal approach by the pocket-creation method. B-1. Mucosal incision is performed in the proximal side of the lesion. B-2. After creating a pocket from the proximal part, submucosal dissection is performed toward the distal side of the lesion. During submucosal dissection, the tip of the endoscope is inserted in the pocket. B-3, 4, and 5. When the submucosal dissection is almost complete, mucosal incision of the remaining part is performed. B-6. After the tumor is completely isolated, an artificial ulcer remains. Yellow oval, gastric tumor; dark gray dots, circumferential thermal marking; blue arrow, direction of mucosal incision; red arrow, direction of submucosal dissection; sky blue oval, range of submucosal dissection; gray oval, artificial ulceration.
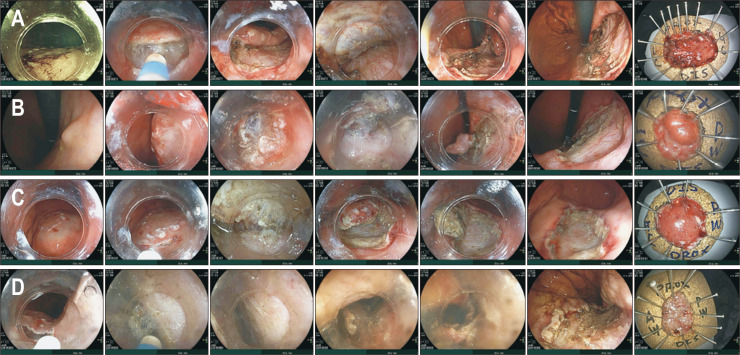
Fig. 3.
Endoscopic submucosal dissection (ESD) cases and their results. (A, B) Tumors were resected by the conventional method. (C, D) They were removed by the pocket-creation method. The four lesions were resected en bloc and the resection margin was clear. No complications occurred after ESD. Other details for each case are as follows. (A) This IIc+IIa tumor located at the posterior wall side of the mid-body was lifted by sodium hyaluronate injection into the submucosal layer. Submucosal dissection was then performed with the conventional method using a dual knife. The final histologic result was mucosal adenocarcinoma, 18-mm tumor size, and 37-mm specimen size (case No. 86). (B) This tumor was located at the postero-lesser curvature side of the high body. ESD with a dual knife was performed using the conventional method. The histopathological finding was adenoma with low-grade dysplasia (LGD), 13-mm tumor size, and 17-mm specimen size (case No. 80). (C) This IIc tumor was located at the postero-greater curvature side of the lower body. ESD with a dual knife was performed using the pocket-creation method. The histopathological finding was adenoma with LGD, 22-mm tumor size, and 27-mm specimen size (case No. 79). (D). This IIb tumor was located at the lesser curvature side of the high body. ESD with a dual knife was performed using the pocket-creation method. The histopathological finding was adenoma with LGD, 28-mm tumor size, and 35-mm specimen size (case No. 91).
3. Distal to proximal approach with the conventional method (group I)
In this study, “the conventional method” refers to the ESD method that has been mainly used to remove gastric body tumors using a dual knife (Fig. 2A). When using this method, the maintenance of tissue tension and good submucosal exposure during submucosal dissection are the most important factors for a successful ESD. With the conventional method, the traction force was made by tension of un-incised mucosa and gravity. Therefore, precutting was performed only in the distal part of the lesion. When precutting of the distal part of the lesion was complete and sufficient mucosal flaps were created through submucosal dissection, mucosal incisions of both lateral parts were performed. In both lateral mucosal incisions, the order of incision was selected considering the direction of gravity. When submucosal dissection was almost complete, mucosal incision of the proximal part was made last.
4. Proximal to distal approach with the pocket-creation method (group II)
With the pocket-creation method, precutting was performed in the proximal side of the lesion (Fig. 2B). After creating a pocket from the proximal part, submucosal dissection was performed toward the distal side of the lesion. During submucosal dissection, the tip of the endoscope was inserted into the pocket. When the submucosal dissection was almost complete, mucosal incision of the remaining part was performed.
5. ESD outcomes and complications
En bloc resection was defined as a resection of the targeted lesion in a single piece and no remaining mucosal tattoo remaining on the specimen.14 Piecemeal resection was regarded as separation of the final specimen into two or more pieces. The resection margin status including the horizontal and vertical margins was investigated through pathological reports.
Bowel perforation, post-ESD bleeding, post-ESD electro-coagulation syndrome (PEECS), suspicious PEECS, pneumonia, and fever of unknown origin were classified as ESD complications. Macroscopic perforation was defined as a gross defect noted by an endoscopist during ESD, with extraluminal organs, fatty tissues, or space visible through the lesion. Microscopic perforation was regarded as a bowel perforation detected by free air that was visible on computed tomography scan or abdominal X-ray after the procedure. Post-ESD bleeding was defined as ESD-related bleeding that required transfusion or endoscopic intervention. PEECS was defined as a condition characterized by abdominal tenderness or rebound tenderness with fever (≥37.8°C) and/or leukocytosis (≥10,000 cells/μL) without definite evidence of perforation.15,16 Suspicious PEECS was defined if PEECS was clinically suspected but the criteria of PEECS were not clearly met. Bowel perforation, post-ESD bleeding, and PEECS were classified as “major complications.” Suspicious PEECS, pneumonia, and fever of unknown origin were categorized as “minor complications.”
6. Tumor characteristics
The location of the body tumor was divided into high-, mid-, and lower-body along the long axis of the stomach, and the anterior wall side, posterior wall side, GC side, and lesser curvature side along the transverse axis.17 Tumors were morphologically classified as type I (protruded), type IIa (superficial elevated), type IIb (flat), type IIc (superficial depressed), and type III (excavated) according to the Japanese classification of gastric carcinoma.17 Tumors with two or more components were recorded in order of surface area occupied. Combination type tumors were finally classified according to their main morphology.
The size of the resected specimen and the tumor itself were investigated through pathological reports. The length of the long axis measured by the pathologist was defined as the size of the specimen or tumor. The final histologic results were also evaluated by reviewing pathologic reports. They were categorized into adenoma with low- or high-grade dysplasia, mucosal cancer, submucosal cancer, cancer with invasion into the proper muscle layer (muscularis propria cancer), hyperplastic polyp, inflammatory polyp, neuroendocrine tumor, and no tumor (i.e., normal gastric mucosa).
7. Statistical analysis
Differences between groups were evaluated using the Student t-test or Mann-Whitney U test for continuous data and the chi-square test or Fisher exact test for categorical data, as appropriate. Continuous variables were summarized as mean±standard deviation and categorical data were given as frequencies (%). Statistical analyses were performed via the SPSS version 19.0 (IBM Corp., Armonk, NY, USA) program. p<0.05 was considered statistically significant.
RESULTS
1. Patients and tumors
A total of 100 ESD cases (52 in group I and 48 in group II) were investigated, of which 69 were males (Table 1). Final pathological results were adenoma in 65, adenocarcinoma in 29, hyperplastic polyp in four, and neuroendocrine tumor in two patients. In two cases, ESD was performed on the previous EMR or ESD site because of tumor recurrence. Other baseline characteristics of patients and tumors are shown in Table 1.
Table 1.
Baseline Characteristics of the Patients and Their Tumors
| Variable | Group I (n=52) | Group II (n=48) | p-value |
|---|---|---|---|
| Age, yr* | 64.52±10.95 | 66.63±12.48 | 0.374 |
| ≥60 yr | 37 (71.2) | 33 (68.8) | 0.830 |
| Male sex | 37 (71.2) | 32 (66.7) | 0.670 |
| Comorbidity | 35 (67.3) | 30 (62.5) | 0.677 |
| Diabetes | 12 (23.1) | 11 (22.9) | 1.000 |
| Hypertension | 26 (50.0) | 20 (41.7) | 0.429 |
| Dyslipidemia | 19 (36.5) | 14 (29.2) | 0.525 |
| Ischemic heart disease and/or arrhythmia | 11 (21.2) | 8 (16.7) | 0.618 |
| Stroke | 3 (5.8) | 3 (6.3) | 1.000 |
| Liver cirrhosis or chronic liver disease | 8 (15.4) | 4 (8.3) | 0.362 |
| Chronic kidney disease | 5 (9.6) | 1 (2.1) | 0.207 |
| COPD or asthma | 1 (1.9) | 1 (2.1) | 1.000 |
| History of cancer | 5 (9.6) | 5 (10.4) | 1.000 |
| Antithrombotic agents | 15 (28.8) | 12 (25.0) | 0.822 |
| Anti-platelet drug | 14 (26.9) | 11 (22.9) | 0.279 |
| Aspirin/clopidogrel/aspirin+clopidogrel/cilostazol | 5/3/6/0 | 4/5/1/1 | |
| Anti-coagulation drug | 2 (3.8) | 2 (4.2) | 0.797 |
| Warfarin/NOAC | 0/2 | 1/1 | |
| Tumor location, longitudinal axis | 0.329 | ||
| High body | 7 (13.5) | 12 (25.0) | |
| Mid body | 12 (23.1) | 8 (16.7) | |
| Lower body | 33 (63.5) | 28 (58.3) | |
| Tumor location, transverse axis | 0.007* | ||
| Lesser curvature | 21 (40.4) | 14 (29.2) | |
| Greater curvature | 6 (11.5) | 20 (41.7) | |
| Anterior wall | 7 (13.5) | 3 (6.3) | |
| Posterior wall | 18 (34.6) | 11 (22.9) | |
| Recurrent case after ESD (or EMR) | 1 (2.1) | 1 (2.1) | 1.000 |
| Status post subtotal gastrectomy | 2 (3.8) | 0 | 0.496 |
| Tumor morphology | 0.303 | ||
| Type 0-IIa | 15 (28.8) | 22 (45.8) | |
| IIa/IIa+IIb/IIa+IIc/IIa+III/IIa+Is | 13/1/1/0/0 | 18/0/2/1/1 | |
| Type 0-IIb | 27 (51.9) | 18 (37.5) | |
| IIb/IIb+IIa/IIb+IIc | 21/2/4 | 12/0/6 | |
| Type 0-IIc | 6 (11.5) | 1 (2.1) | |
| IIc/IIc+IIa/IIc+IIb | 2/2/2 | 1/0/0 | |
| Type 0-I | 4 (7.7) | 7 (14.6) | |
| Is/Is+IIa | 3/1 | 5/2 | |
| Histopathologic type | 0.213 | ||
| Adenoma | 31 (59.6) | 34 (70.8) | |
| LGD/HGD | 24/7 | 31/3 | |
| Adenocarcinoma | 18 (34.6) | 11 (22.9) | |
| Mucosal cancer/SM cancer/invading PM | 16/2/0 | 7/4/0 | |
| Hyperplastic polyp | 2 (3.8) | 2 (4.2) | |
| Neuroendocrine tumor | 1 (1.9) | 1 (2.1) |
Data are presented as mean±SD or number (%). Continuous variable was analyzed using a Student t-test. All other data were analyzed using a chi-square test or Fisher exact test. Subjects underwent endoscopic submucosal dissection (ESD) with the conventional method were assigned into group I, and subjects who underwent ESD with the pocket-creation method were group II.
COPD, chronic obstructive pulmonary disease; NOAC, non-vitamin K antagonist oral anticoagulant; EMR, endoscopic mucosal resection; LGD, low-grade dysplasia; HGD, high-grade dysplasia; SM cancer, submucosal cancer; PM, muscularis propria.
*Statistically significant, p<0.05.
There were no statistically significant differences in age, sex, underlying diseases, history of cancer, medication history, tumor morphology, or histopathologic type of the tumor between the two groups. However, there was a significant difference in tumor location (p=0.007) between the two groups. Tumors located on the lesser curvature side were more common in group I (40.4%) than in group II (29.2%) while tumors on the GC side were more common in group II (41.7%) than in group I (11.5%).
2. ESD outcomes and complications
All tumors were successfully resected en bloc. Resection margin involvement was found in six (11.5%) of group I and six (12.5%) of group II. Post-ESD complications were observed in seven (13.5%; major complication five, minor complication two) of group I and eight (16.7%; major complication two, minor complication six) of group II (Table 2). Surgery was necessary for seven cases; involvement of the horizontal resection margin in two, involvement of the vertical resection margin in one, and resection margin free, but deep submucosal invasion (≥500 µm) in four cases. The two patients with a positive horizontal margin underwent surgery, because the histological result was cancer and the patient chose surgery rather than additional ESD. There was no statistically significant difference in the incidence of complications, procedure time, tumor size, specimen size, or resection margin status between the two groups. There was no significant difference in the incidence of surgery, hospitalization period, maximum body temperature, C-reactive protein levels, or white blood cell counts between the two groups. Although EMR (or snare polypectomy) was concurrently performed in 14 cases, it showed no significant difference between the two groups (eight in group I and six in group II).
Table 2.
Endoscopic Submucosal Dissection (ESD) Procedures and Post-ESD Outcomes
| Variable | Group I (n=52) | Group II (n=48) | p-value |
|---|---|---|---|
| Procedure time, min | 63.77±29.25 | 73.46±40.84 | 0.179 |
| ≥90 min | 7 (13.5) | 13 (21.7) | 0.132 |
| Additional EMR or polypectomy | 8 (15.4) | 6 (12.5) | 0.777 |
| Tumor size, cm | 12.50±7.15 | 13.67±9.67 | 0.497 |
| Specimen size, mm | 25.62±5.58 | 26.58±9.30 | 0.534 |
| ≥30 mm | 14 (26.9) | 17 (35.4) | 0.393 |
| En bloc resection | 52 (100) | 48 (100) | 1.000 |
| Positive resection margin | 6 (11.5) | 6 (12.5) | 1.000 |
| Horizontal margin | 5 (9.6) | 5 (10.4) | |
| Vertical margin | 1 (1.9) | 1(2.1) | |
| Post-ESD complications | 7 (13.5) | 8 (16.7) | 0.781 |
| Major complications | 5 (9.6) | 2 (4.2) | 0.439 |
| Macroscopic perforation | 1 (1.9) | 0 | 1.000 |
| Microscopic perforation | 0 | 0 | |
| Post-ESD bleeding | 3 (5.8) | 0 | 0.244 |
| PEECS | 1 (1.9) | 2 (4.2) | 0.606 |
| Minor complications | 2 (3.8) | 6 (12.5) | 0.149 |
| Suspicious PEECS | 1 (1.9) | 4 (8.3) | 0.192 |
| Pneumonia | 1 (1.9) | 0 | 1.000 |
| Fever of unknown origin | 0 | 2 (4.2) | 0.228 |
| Operation* | 3 (5.8) | 4 (8.3) | 0.833 |
| The reason for the operation | |||
| Margin involvement | 1 (1.9) | 2 (4.2) | |
| Horizontal margin/vertical margin | 1/0 | 1/1 | |
| Margin free, but deep submucosal layer invasion | 2 (3.8) | 2 (4.2) | |
| Laboratory findings | |||
| On admission | |||
| Leukocyte count, ×103/µL | 6.19±1.93 | 6.67±1.93 | 0.223 |
| C-reactive protein, mg/dL | 2.18±3.70 | 1.74±2.80 | 0.497 |
| On days 1–3 after ESD (the highest level) | |||
| Leukocyte count, ×103/µL | 8.44±2.57 | 9.02±2.83 | 0.292 |
| C-reactive protein, mg/dL† | 9.18±14.56 | 15.02±27.64 | 0.203 |
| ≥5 mg/dL | 15 (28.8) | 19 (39.6) | 0.295 |
| Total hospital stays, day | 3.54±0.98 | 3.31±0.66 | 0.176 |
| ≥4 day | 19 (36.5) | 12 (25.0) | 0.280 |
| Maximum body temperature during hospitalization, ºC | 37.16±0.34 | 37.26±0.34 | 0.169 |
Data are presented as mean±SD or number (%). Continuous variables were analyzed using a Student t-test or Mann-Whitney U test. All other data were analyzed using a chi-square test or Fisher exact test. Subjects underwent ESD with the conventional method were assigned into group I, and subjects who underwent ESD with the pocket-creation method were group II.
EMR, endoscopic mucosal resection; PEECS, post-ESD electro-coagulation syndrome.
*When the patients underwent surgery due to residual cancer, ESD failure, or complication, it was defined as “operation”; †C-reactive protein after ESD was not measured in two subjects.
3. Risk factors for post-ESD complications and clinical course of patients
Tumor size, specimen size, tumor location, tumor morphology, presence of cancer, sex, age, and use of antithrombotic agent were not associated with the risk of complications. The presence or absence of underlying disease did not increase the risk of complications. However, when looking at each underlying disease in detail, cardiovascular disease was the only one that had a significant association with the development of complications (p<0.001) (Table 3). Patients with complications showed significantly increased length of hospital stay than those without complications (p<0.001).
Table 3.
Comparison between Patients with Complications and Those without
| Variable | Complication group (n=15) | No complication group (n=85) | p-value |
|---|---|---|---|
| Age, yr | 66.73±13.57 | 65.32±11.41 | 0.708 |
| ≥60 yr | 11 (73.3) | 59 (69.4) | 1.000 |
| Male sex | 11 (73.3) | 58 (68.2) | 0.772 |
| Comorbidity | 12 (80.0) | 53 (62.4) | 0.247 |
| Diabetes | 3 (20.0) | 20 (23.5) | 1.000 |
| Hypertension | 7 (46.7) | 39 (45.9) | 1.000 |
| Dyslipidemia | 8 (53.3) | 25 (29.4) | 0.081 |
| Cardiovascular disease | 9 (60.0) | 10 (11.8) | <0.001* |
| Cerebrovascular disease | 1 (6.7) | 5 (5.9) | 1.000 |
| Liver cirrhosis or chronic liver disease | 3 (20.0) | 9 (10.6) | 0.383 |
| Chronic kidney disease | 2 (13.3) | 4 (4.7) | 0.220 |
| COPD or asthma | 0 | 2 (2.4) | 1.000 |
| Antithrombotic agents | 6 (40.0) | 21 (24.7) | 0.224 |
| Anti-platelet drug | 5 (33.3) | 20 (23.5) | 0.114 |
| Anti-coagulation drug | 1 (6.7) | 3 (3.5) | 0.484 |
| Additional EMR or polypectomy | 3 (20.0) | 11 (12.9) | 0.437 |
| Recurrent case after ESD (or EMR) | 0 | 2 (2.4) | 1.000 |
| Status post subtotal gastrectomy | 0 | 2 (2.4) | 1.000 |
| Procedure time, min | 59.40±27.12 | 70.01±36.62 | 0.200 |
| ≥90 min | 3 (20.0) | 17 (20.0) | 1.000 |
| Tumor size, mm | 11.73±6.14 | 13.29±8.78 | 0.406 |
| Specimen size, mm | 24.27±3.73 | 26.40±8.04 | 0.108 |
| ≥30 mm | 2 (13.3) | 29 (34.1) | 0.138 |
| Tumor morphology | |||
| Lesions including IIc or III | 4 (26.7) | 17 (20.0) | 0.512 |
| Tumor location | |||
| High body/mid body/lower body | 3/4/8 | 16/16/53 | 0.733 |
| LC/GC/AW/PW | 5/3/2/5 | 30/23/8/24 | 0.894 |
| Final pathology | |||
| Adenocarcinoma | 2 (13.3) | 27 (31.8) | 0.219 |
| Total hospital stays, day | 4.80±1.21 | 3.19±0.45 | <0.001* |
| ≥4 day | 15 (100) | 16 (18.8) | <0.001* |
Data are presented as mean±SD or number (%). Continuous variables were analyzed using a Student t-test or Mann-Whitney U test. All other data were analyzed using a chi-square test or Fisher exact test.
COPD, chronic obstructive pulmonary disease; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; LC, lesser curvature; GC, greater curvature; AW, anterior wall; PW, posterior wall.
*Statistically significant, p<0.05.
There were a total of 15 post-ESD complications, including five cases with suspicious PEECS, three with PEECS, three with post-ESD bleeding, two with fever of unknown origin, one with bowel perforation, and one with pneumonia. Post-ESD bleeding and bowel perforation occurred only in group I. Complications related to inflammation or infection such as PEECS and fever of unknown origin occurred mainly in group II. Among patients whose blood cultures were done, no microbial growth occurred (Table 4). Surgery associated with complications was not required for all cases. No procedure-related deaths occurred.
Table 4.
Characteristics and Clinical Course of the Patients with Complication
| Complication | Case No. |
Group | Age, yr/sex | Tumor | Clinical course | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor location |
Size of specimen, mm |
Tumor morphology |
Tumor histology |
Fever onset, hr after ESD |
Fever duration, hr |
Fasting period, day |
Hospital stays, day |
CRP, next day after ESD, mg/dL |
Maximum body temperature, ºC |
Surgery related with complication |
Blood culture |
|||||
| Macroscopic perforation | 70 | I | 54/M | MB, GC | 31 | IIc+IIb | Mucosal cancer | 15 | 2 | 3 | 7 | 41.0 | 37.7 | No | No growth | |
| Post-ESD bleeding | 6 | I | 49/M | MB, LC | 28 | IIa | Hyperplastic polyp | No fever | No fever | 3 | 7 | <1.0 | 36.8 | No | Not checked | |
| 93 | I | 70/F | LB, LC | 21 | IIb+IIc | Adenoma, HGD | No fever | No fever | 2 | 4 | 15.2 | 37.3 | No | Not checked | ||
| 99 | I | 54/M | LB, AW | 25 | IIb | Adenoma, LGD | No fever | No fever | 4 | 7 | 3.7 | 37.2 | No | Not checked | ||
| PEECS | 69 | I | 64/M | LB, PW | 25 | IIa | Adenoma, HGD | 18 | 4 | 2 | 4 | 46.5 | 38.0 | No | No growth | |
| 25 | II | 69/M | LB, PW | 18 | IIa | Adenoma, LGD | 12 | 6 | 2 | 4 | 18.9 | 37.8 | No | No growth | ||
| 36 | II | 38/M | HB, PW | 25 | Is+IIa | Adenoma, LGD | 18 | 12 | 2 | 4 | 48.3 | 37.9 | No | No growth | ||
| Suspicious PEECS | 67 | I | 64/M | LB, PW | 25 | IIa | Adenoma, HGD | 15 | 12 | 2 | 4 | 46.5 | 38.2 | No | No growth | |
| 21 | II | 63/M | LB, GC | 22 | IIa+IIc | Adenoma, LGD | 18 | 12 | 2 | 4 | 84.1 | 38.0 | No | No growth | ||
| 45 | II | 83/F | MB, LC | 24 | IIa+Is | Adenoma, LGD | 20 | 4 | 3 | 5 | 113.1 | 37.7 | No | No growth | ||
| 46 | II | 83/F | HB, GC | 27 | IIa | Submucosal cancer | 12 | 8 | 3 | 5 | 112.6 | 37.7 | No | No growth | ||
| 65 | II | 79/M | MB, LC | 19 | IIb+IIc | Adenoma, LGD | 18 | 24 | 2 | 5 | 12.0 | 37.8 | No | No growth | ||
| Pneumonia | 28 | I | 80/M | LB, AW | 22 | IIa | Adenoma, HGD | 15 | 4 | 2 | 4 | 35.9 | 38.1 | No | No growth | |
| Fever of unknown origin | 85 | II | 70/M | HB, LC | 22 | IIa | Adenoma, LGD | 36 | 1 | 1 | 4 | 1.8 | 37.9 | No | No growth | |
| 100 | II | 81/F | LB, PW | 30 | IIb | Adenoma, LGD | 8 | 18 | 2 | 4 | 2.5 | 37.6 | No | No growth | ||
ESD, endoscopic submucosal dissection; CRP, C-reactive protein; PEECS, post-ESD electro-coagulation syndrome; M, male; F, female; MB, mid body; GC, greater curvature; LC, lesser curvature; LB, lower body; AW, anterior wall; PW, posterior wall; HB, high body; HGD, high-grade dysplasia; LGD, low-grade dysplasia.
DISCUSSION
In general, gastric ESD with a dual knife may require different approaches or methods depending on the location of the tumor lesion. For tumors located in the gastric cardia, a proximal to distal approach is useful, although there might be disagreements. Tumors of the GC side of the antrum are also generally removed with a proximal to distal approach. However, for lesions located in the gastric body, both approaches, a proximal to distal approach and a distal to proximal approach, can be used. In the case of using the pocket-creation method or tunneling method, the ESD for gastric body tumor was mainly performed in a proximal to distal direction. On the other hand, in the case of using the conventional method, the dissection was mainly performed in the distal to proximal direction.
Our study showed that both conventional and pocket-creation methods were useful for ESD of gastric body tumors. There were no significant differences in ESD outcomes between the two methods used in our study. There was no significant difference in hospitalization period, incidence of complications, resection margin status, incidence of surgical operation, procedure time, or inflammatory response after ESD between the two groups. Treatment outcomes were excellent in both groups. Therefore, the ESD method for gastric body tumor can be selected according to the preference and skill level of the operator.
This study is the first study to find out whether there is a difference in ESD outcome depending on the method of removing a tumor located in the gastric body with a dual knife. Recently, a comparative study between the tunneling method and the conventional method for gastric ESD was published. It showed that the tunneling method had a shorter procedure time and a lower incidence of perforation than the conventional method.18 However, that study had a disadvantage of not considering the location of the gastric tumor. Although the tunneling method is technically similar to the pocket-creation method, they show many differences in details such as the starting point of mucosal incision, the direction of the dissection, and the extent of the dissection.
All complications in our study improved with conservative treatment. There was no complications-associated surgery or procedure-related death. However, post-ESD complications were associated with prolonged hospitalization. In addition, regardless of the history of antithrombotic use, cardiovascular disease was significantly associated with the development of complications. Therefore, more careful attention during and after ESD are required for patients with cardiovascular diseases to reduce complication risk and shorten their hospital stay.
This study has some limitations. First, the biggest drawback of this study was that it was not randomized because it was a retrospective study. Since the approach of ESD was selected according to various circumstances, there might be a selection bias. In our study, the pocket-creation method was more frequently selected for lesions on the GC side of the body, while the conventional method was more likely to be selected for lesions on the lesser curvature side of the body. This might be because, among gastric body tumors, lesions at a specific location are easier to treat with a specific approach. Second, the number of subjects was not large enough. Our study showed that post-ESD fever was more common in group II while bleeding was more common in group I, although the difference between the two was not statistically significant. The pocket-creation method is advantageous for checking blood vessels and hemostasis because it is easier to secure a field of view than the conventional method. However, its risk of micro-perforation or coagulation syndrome might be greater because it is difficult to fix the endoscope in some cases. Since the dual knife does not have an insulation tip, if the patient's cooperation is poor during the procedure, the risk of perforation may increase. In the future, if the number of subjects is sufficiently increased and each complication is analyzed separately, a significant difference in the results of the two methods might be identified.
In conclusion, ESD outcomes of the two methods were not different from each other. The pocket-creation method for gastric body tumors does not reduce the rate of incomplete tumor resection, complications, or inflammatory response after ESD compared to conventional methods. Both methods are suitable for treating gastric body tumors because they have adequate treatment success rates with comparable complication rates. Although additional large-scale study is needed to confirm our study results, this study might serve as a cornerstone for conducting future prospective studies.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by Korean Gastrointestinal Endoscopy Research Foundation, 2022 (Project Number: 6H220301001S000100).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: S.P.L. Data acquisition: H.J.J., S.H.K., J.G.L. Data analysis and interpretation: S.P.L. Drafting of the manuscript: S.P.L. Critical revision of the manuscript for important intellectual content: H.J.J., S.H.K., J.G.L. Statistical analysis: S.P.L. Administrative, technical, or material support; study supervision: S.P.L. Approval of final manuscript: all authors.
REFERENCES
- 1.Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 2.Nishizawa T, Yahagi N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver. 2018;12:119–124. doi: 10.5009/gnl17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S67–70. doi: 10.1016/S1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 5.Ikezawa N, Tanaka S, Toyonaga T. Novel strategy using pocket creation method to reduce intraoperative bleeding in gastric endoscopic submucosal dissection. Dig Endosc. 2020;32:e136–e137. doi: 10.1111/den.13765. [DOI] [PubMed] [Google Scholar]
- 6.Chai NL, Li HK, Linghu EQ, et al. Consensus on the digestive endoscopic tunnel technique. World J Gastroenterol. 2019;25:744–776. doi: 10.3748/wjg.v25.i7.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe S, Wu SY, Ego M, et al. Efficacy of current traction techniques for endoscopic submucosal dissection. Gut Liver. 2020;14:673–684. doi: 10.5009/gnl19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura Y, Hayashi Y, Lefor AK, Osawa H, Yamamoto H. The pocket-creation method of ESD for gastric neoplasms. Gastrointest Endosc. 2016;83:457–458. doi: 10.1016/j.gie.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 9.Yamashina T, Nemoto D, Hayashi Y, et al. Prospective randomized trial comparing the pocket-creation method and conventional method of colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:368–379. doi: 10.1016/j.gie.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Gong J, Chen T, Tan Y, Liu D. Pocket-creation method improves efficacy of colorectal endoscopic submucosal dissection: a system review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1241–1246. doi: 10.1097/MEG.0000000000001864. [DOI] [PubMed] [Google Scholar]
- 11.Gong J, Zhou BY, Liang CB, et al. Comparison between tunneling and standard endoscopic submucosal dissection for treatment of large esophageal superficial neoplasm. Acta Gastroenterol Belg. 2019;82:469–474. [PubMed] [Google Scholar]
- 12.Takizawa K, Takashima A, Kimura A, et al. A phase II clinical trial of endoscopic submucosal dissection for early gastric cancer of undifferentiated type: Japan Clinical Oncology Group study JCOG1009/1010. Jpn J Clin Oncol. 2013;43:87–91. doi: 10.1093/jjco/hys189. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association, author. Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. 2020;53:142–166. doi: 10.5946/ce.2020.032.6802de303eba453f80d3588cebae2bb5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy. 2013;45:714–717. doi: 10.1055/s-0033-1344555. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Cheoi KS, Chung H, et al. Clinical features and predictive factors of coagulation syndrome after endoscopic submucosal dissection for early gastric neoplasm. Gastric Cancer. 2012;15:83–90. doi: 10.1007/s10120-011-0073-x. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association, author. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 18.Ojima T, Takifuji K, Nakamura M, et al. Endoscopic submucosal tunnel dissection versus conventional endoscopic submucosal dissection for early gastric cancers: outcomes of 799 consecutive cases in a single institution. Surg Endosc. 2020;34:5625–5631. doi: 10.1007/s00464-020-07849-1. [DOI] [PubMed] [Google Scholar]