Abstract
A growing body of evidence has demonstrated an intricate association between inflammatory bowel disease (IBD) and neurodegenerative conditions, expanding beyond previous foci of comorbidities between IBD and mood disorders. These new discoveries stem from an improved understanding of the gut-microbiome-brain axis: specifically, the ability of the intestinal microbiota to modulate inflammation and regulate neuromodulatory compounds. Clinical retrospective studies incorporating large sample sizes and population-based cohorts have demonstrated and confirmed the relevance of IBD and chronic neurodegeneration in clinical medicine. In this review, we expound upon the current knowledge on the gut-microbiome-brain axis, highlighting several plausible mechanisms linking IBD with neurodegeneration. We also summarize the known associations between IBD with Parkinson disease, Alzheimer disease, vascular dementia and ischemic stroke, and multiple sclerosis in a clinical context. Finally, we discuss the implications of an improved understanding of the gut-microbiome-brain axis in preventing, diagnosing, and managing neurodegeneration among IBD and non-IBD patients.
Keywords: Inflammatory bowel diseases, Dementia, Parkinson disease, Multiple sclerosis, Gut-brain axis
INTRODUCTION
Inflammatory bowel diseases (IBD) are characterized by pathologic immune activation against microbiome dysbiosis in a genetically susceptible individual.1 Manifesting mainly as either ulcerative colitis (UC) or Crohn’s disease (CD), both present as chronic relapsing and remitting gastrointestinal and systemic inflammation. The two diseases demonstrate distinct phenotypes, encompassing different patterns of gastrointestinal involvement, depths of inflammation, impact on surrounding organs, in addition to endoscopic, histologic, and radiographic characteristics.
Recently, increasing evidence suggests an association between chronic inflammatory disorders and neurodegeneration. This was demonstrated by the ARIC (Atherosclerosis Risk in Communities) study, which established that systematic inflammation was associated with increased cognitive decline among patients followed for up to 20 years.2 Furthermore, patients with IBD or rheumatologic diseases have higher incidence of multiple sclerosis (MS) and vice versa.3-5 While epidemiologic and clinical evidence are robust, pathophysiology remains uncertain.
Until recently, research on brain disorders among IBD patients has focused primarily on depression and anxiety, two highly prevalent comorbid mental illnesses linked with worse IBD clinical outcomes.6-8 Two research publications from 2018 and 2019 examined and found positive correlation between IBD and development of Parkinson disease (PD), prompting investigation between IBD and chronic neurodegeneration beyond mood disorders.9,10 Our team subsequently investigated the risk of dementia among IBD patients using the Taiwan National Health Insurance Research Database and found increased risks for both overall dementia and Alzheimer disease (AD), and both diagnosed at younger ages among IBD patients compared to matched controls.11 These associations were supported by subsequent findings from the United States,12 while studies from Korea and Denmark reported significant but less dramatic risks,13,14 and the U.K. Biobank study reported lack of association.15
Several plausible mechanisms link IBD with neurodegeneration, including genetic factors, gut microbiome dysbiosis, and environmental factors which may affect both the enteric nervous system (ENS) and central nervous system (CNS).4,5 Moreover, there is an increased risk of vascular dementia given the high thromboembolic risk in IBD patients.16 This paper reviews the current literature to present the incidence, pathophysiology, and risk factors of neurodegenerative diseases in IBD. We also discuss the clinical impact of these associations and implications for the management of neurodegenerative diseases in IBD patients.
GUT-BRAIN AXIS
The gut-brain axis consists of the CNS, ENS, autonomic nervous system, and the hypothalamic pituitary adrenal pathway.17 Through these connections, the gut-brain axis plays an essential role in the crosstalk between the gastrointestinal system and the CNS. There are many pathways of communication between the gut and the brain including neuronal, immune, endocrine, and metabolic methods of communication.18 This gut-brain bidirectional communication system has mostly been explored to understand the pathophysiology of irritable bowel syndrome patients, although more recent literature has begun to explore its role in IBD patients.19
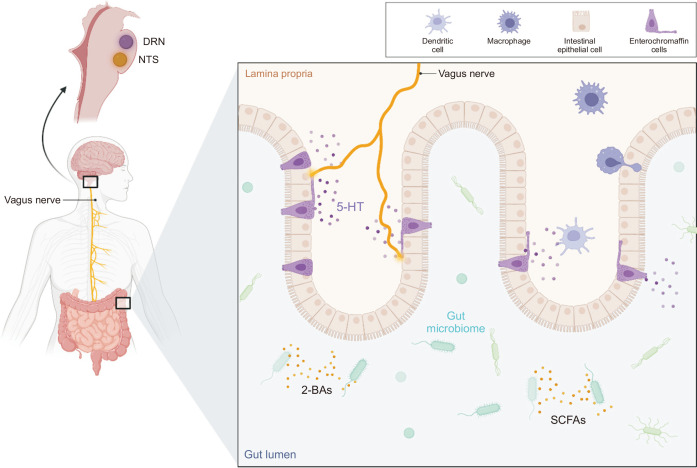
The balance of microbial diversity and richness influences neurons and neurotransmitters of the ENS including dopamine, serotonin (5-HT), and catecholamines.20 These neuropeptides induce vasodilation, leukocyte migration, and plasma extravasation to regulate colonic inflammation. They also function as signaling molecules of the autonomic nervous system, thus serving as neuromodulators and neurohormones to mediate between the nervous system and the gut.21 Disturbance of normal intestinal flora in IBD patients induce a pro-inflammatory effect in the intestines that is relayed to the nervous system (Fig. 1). Spore-forming bacteria in the gut produce short-chain fatty acids (SCFAs) and secondary bile acids that stimulate 5-HT production and secretion from the enterochromaffin cells (ECCs) of the gut epithelium. ECCs have neuropod-like extensions that allow them to communicate with the vagal afferent fibers, which ultimately project to the nucleus tractus solitarius in the brainstem.19,22 On the other hand, central regulation of the intestine is also present. The autonomic nervous system can activate ECCs to release 5-HT into the gut. ECCs have more than 90% of the body’s 5-HT and are uptaken by 5-HT receptors of the intestinal system.19 Cholinergic signals from the vagal efferent fibers can also exert anti-inflammatory effects by inhibiting cytokine release from macrophages.22
Fig. 1.
The gut microbiome produces molecules such as SCFAs and 2-BAs that simulate enterochromaffin cells to release 5-HTs. 5-HTs communicate via the vagus nerve, traveling to the nucleus tractus solitarius in the brainstem. The autonomic nervous system also communicates via the vagus nerve and can activate enterochromaffin cells to release 5-HT into the gut.
SCFAs, short-chain fatty acids; 2-BAs, secondary bile acids; 5-HTs, serotonins; DRN, dorsal raphe nucleus; NTS, nucleus tractus solitarii.
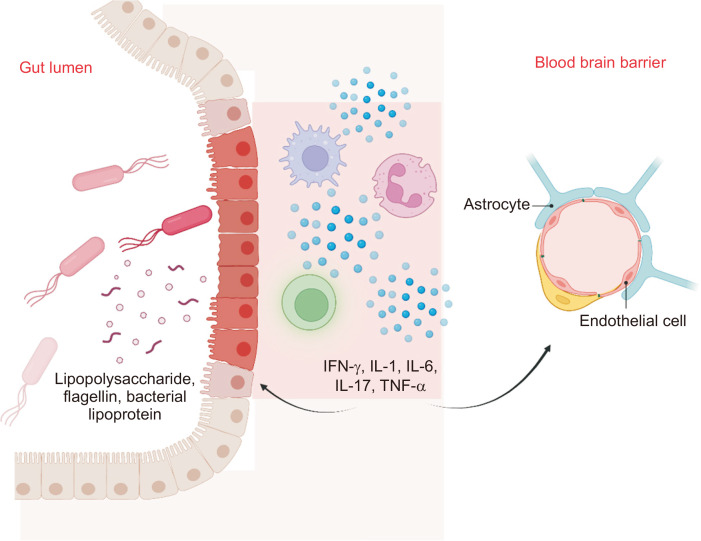
Immune and endocrine pathways of communication are also important components of the gut-brain axis. Stress such as local infection, food antigens, and dysbiosis will trigger intestinal inflammation as microbial cell wall components such as lipopolysaccharide (LPS), flagellin, and bacterial lipoprotein come into contact with the Toll-like receptors of immune cells (Fig. 2).17 Activated immune cells release pro-inflammatory cytokines and modulators such as interferon gamma, interleukin (IL)-1, IL-6, IL-17, and tumor necrosis factor alpha (TNF-α). These pro-inflammatory mediators disturb epithelial tight junctions, both increasing intestinal permeability and disrupting the blood-brain barrier (BBB).22 The “leaky gut” and compromised BBB allows for translocation of systemic immune cells and cytokines to travel from the gut to the brain.22 Moreover, intestinal inflammation from stressors triggers activation of the hypothalamic pituitary adrenal axis, increasing release of glucocorticoids. Glucocorticoids affect intestinal function and immune activity, which may help restore intestinal homeostasis. However, the balance is nuanced given multiple factors. Blunting of the hypothalamic pituitary adrenal axis with microbiota deficiency and dampening of the corticotropin release hormone (CRF) gene associated with chronic colitis may counteract the release of glucocorticoids.22,23
Fig. 2.
Microbial components such as lipopolysaccharide, flagellin, and bacterial lipoprotein encounter immune cells and activate them. The activated immune cells release pro-inflammatory cytokines and modulators such as IFN-γ, IL-1, IL-6, IL-17, and TNF-α, which alters the gut epithelium and blood-brain barrier, leading to increased intestinal and blood-brain barrier permeability.
IFN-γ, interferon gamma; IL, interleukin; TNF-α, tumor necrosis factor alpha.
Lastly, microbe derived neuroactive molecules such as microbial metabolites play a key role in the gut-brain communication. SCFAs, which regulate intestinal motility, secretion, and production of 5-HT as mentioned above, are produced by fermentation of dietary fiber by microbes in the gut.17,19 Bile acids, which are synthesized and conjugated from cholesterol in the liver, are metabolized by gut microbes. Primary bile acids are deconjugated and dehydroxylyzed into secondary bile acids.17 Secondary bile acids bind to farnesoid X receptor and G protein-coupled receptor 5, which are expressed in ECCs, intrinsic and extrinsic nerves, as well as immune cells.24 They have many different roles in gut homeostasis including glucose metabolism, 5-HT production, and immune response modulation.19,25,26 The gut microbiome also produce tryptophan metabolites such as 5-HT, kynurenine, and ligands for aryl hydrocarbon receptor.17,21 Increased production of kynurenine associated with various local stress and dysbiosis can cross the BBB and exert neuroinflammatory effects.19 Taken together, several physiologic mechanisms serve to relay intestinal inflammation reflecting IBD to the CNS. Focused discussions of the gut-brain-axis’ role in specific neurodegenerative diseases are described below.
PARKINSON DISEASE
Increased risk of PD in IBD patients has been reported. Several of these studies revealed significantly increased incidence of IBD before and after the PD diagnosis, suggesting a bidirectional relationship.27-29 These findings were also supported by research utilizing several population-based health databases including the Taiwanese, Korean, Sweden, and Danish databases.9,29-31 Specifically, higher risk of developing PD were identified among IBD patients who were older (>65 years) and those who did not undergo treatment with anti-TNF-α agents or azathioprine. There were no differences between men and women.27
Several theories have been speculated to explain the relationship between IBD and PD. Genetic overlap in the pathogenesis of IBD and PD has been one. Specifically, mutations in the LRRK2 gene, the most identified genetic cause of PD, are also associated with an increased risk of CD.32 The study of Hui et al.33 demonstrated that a variant of this gene increased risk of CD in PD patients, while another haplotype of the gene was likely protective. LRRK2 plays a role in α-synuclein phosphorylation and is expressed in neurons, glial cells, and immune cells; therefore, LRRK2 mutations may induce α-synuclein accumulation in the gut that spreads to the brain, leading to neurodengeration.34,35 Increased LRRK2 activity is also associated with both gastrointestinal and systemic inflammation, reflecting CD development and disease activity. Other than the LRRK2 gene, six other loci were strongly associated between IBD and PD, with the strongest pleiotropic associations found between CD and PD.36
Another potential mechanism for the pathogenic mechanism of PD among IBD patients is the Braak hypothesis, which states that accumulation of α-synuclein in the gastrointestinal tract from gastrointestinal inflammation is slowly transported to the CNS via the vagus nerve.36,37 This was supported by studies using mouse models, which showed that when α-synuclein preformed fibrils were injected to the intestine, there was spread of phosphorylated α-synuclein in the brain with associated loss of dopaminergic neurons.38 Moreover, when vagotomy was performed, spread of phosphorylated α-synuclein in the brain was prevented and cognitive deficits were not observed.38 Clinically, patients who underwent truncal vagotomy were suggested to have a lower risk of developing PD, further supporting the spread of α-synuclein via the vagus nerve.39
One theory to explain similar pathologic inflammation characteristic of IBD and PD is the transference of intestinal inflammation to the brain. Inflammation in IBD is characterized by increased activity of CD4 T cells, mainly Th1 and Th17 cells and attenuated activity of Treg cells which have suppressive effects on inflammation.36 The Treg cells also have anti-inflammatory effects in the CNS, and hence, decreased Treg activity in IBD may contribute to increased CNS inflammation seen in PD.40 IBD and PD are also both characterized by increased intestinal permeability, which in combination with the systemic inflammation in IBD and PD have been associated with BBB dysfunction and enhanced brain inflammation.36,41 Increased intestinal permeability results in increased passage of bacteria and bacterial products such as LPS into the bloodstream, which in turn activates immune cells. LPS and activated cytokines may enter the BBB to induce CNS inflammation. LPS has also been associated with degeneration of dopaminergic neurons.36 Furthermore, gut dysbiosis and inadequate signaling and metabolites from the gut microbiome is associated with enhanced neuro-inflammation and dysregulation of dopamine in IBD and PD patients. Altered gut microbiome and especially SCFA producing bacteria are found in both IBD and PD patients. SCFAs are known to have anti-inflammatory effects by regulating oxidative stress in the colonic mucosa and its decrease has been associated with accumulation of α-synuclein.32,36
Literature providing evidence of association between IBD and PD continues to increase, providing mechanistic possibilities linking the two seemingly unrelated diseases. The shared common theme of pathogenesis is the bidirectional spread of inflammation between the gut and brain, which is secondary to genetic polymorphisms, increased intestinal barrier permeability, and gut dysbiosis. Early exposure to anti-TNF therapy has been shown to reduce incidence of PD in IBD patients,10,27 supporting that unchecked systemic inflammation is pathologic for both diseases. Clinician awareness of the association between IBD and PD may enable earlier diagnoses, leading to improved medical outcomes.
ALZHEIMER DISEASE
In recent years, several studies have reported increased risk of dementia among IBD patients.11,12,42,43 Our team first showed IBD patients were diagnosed with dementia at an earlier age compared to matched controls (mean age 76 vs 83) and risk was elevated with increased chronicity of IBD diagnosis.11 Numerous studies demonstrated an increased risk of dementia in IBD patients regardless of age, while one retrospective study revealed specifically increased risk of dementia in IBD patients aged 60 to 70 years old.42-44 Of note, incidence of dementia before IBD diagnosis and comorbidity rate of dementia in IBD patients were not significantly different from those without IBD, suggesting unidirectional relationship,45 which may reflect the differences in age of onset for IBD and dementia. While most studies demonstrated no significant differences in dementia risk between CD and UC patients, some reported mixed results.14,44,46
While majority of studies demonstrate positive associations between IBD and dementia, few recent studies failed to or only identified minimal association.14,15,47 Specifically, Sun et al.15 are following IBD and non-IBD patients for a mean of 11.58 years and demonstrated no significant difference in the hazard ratio of dementia. They also studied the brain structures via brain magnetic resonance imaging and found no statistical difference in anatomic and tissue-specific volumes. Two-sample Mendelian randomization utilizing large-scale genome-wide association studies by Guo et al.48 surprisingly showed a decreased risk of Alzheimer’s disease in genetically-determined IBD. A third report from Denmark showed minimal association, which the authors explained was likely due to increased healthcare contact.14 These results show that the association is likely complex and cannot be explained by genetics or healthcare policies alone. Taken together, existing meta-analyses demonstrate positive unidirectional relationship between IBD and dementia, with most studies reporting the hazard ratio to be greater than 1 and less than 2.42,43
Neurocognitive degeneration and AD among IBD patients may reflect changes in the gut microbiome. The exact pathogenic process remains clandestine, but one hypothesis is that gut dysbiosis and increased intestinal permeability in IBD leads to increased neuronal inflammation from transference of intestinal inflammation.45,49 One potential way is via the vagus nerve, which serves as a liaison between the ENS and the CNS. Sun et al.50 showed that beta-amyloid injected in the intestinal tract of mice was found in the vagus nerve and brain one year later and accompanied concomitant cognitive dysfunction. Hence, chronic inflammation in IBD may lead to beta-amyloid plaque formation in the intestinal tract that spreads to the brain via the vagus nerve.49 Studies of decreased beta-amyloid and Tau lesions following vagotomy and restoration of impaired memory further support this hypothesis.51
Another possibility linking IBD and dementia is intestinal microbiota dysbiosis, a prominent feature of IBD.45,52 Both IBD and AD patients were found to have decreased microbiome diversity.49 The gut microbiome is capable of producing anti-inflammatory metabolites such as SCFAs, certain bile acids including tauroursodeoxycholic acid, and ligands for aryl hydrocarbon receptor, which are capable of crossing the BBB to modulate inflammation in the CNS.49,53 Decreases in the production of these beneficial metabolites are secondary to diminished microorganisms responsible for their production often associated with decreased microbiome diversity. Conversely, several studies have also noted that chronic systematic inflammation in IBD accompanies the production of neurotoxic metabolites which in turn promote inflammation in the CNS via activation of microglia and astrocytes. These intestinal metabolites include kynurenine and certain bile acids, and inflammatory biopolymer substances such as LPS and enterotoxins. The biopolymer substances in particular damage the intestinal lining and the resulting “leaky gut” potentiates the capacity of neurotoxic metabolites to migrate from the gut lumen into circulation and possibly the CNS.17,49,53,54
Although the exact mechanisms of pathogenesis of AD in IBD patients remain unclear, several studies have suggested clinical implications in IBD patients in respect to prevention of AD. The study of Kim et al.13 showed that female sex and age ≥65 years experienced increased AD risk, while those living in an urban area had decreased risk. Dementia screening strategies in IBD patients, and especially those harboring other risks, are recommended. Furthermore, control of IBD activity likely protects against inflammation-associated neurodegeneration.12 Finally, healthy lifestyle behavior including exercise, maintaining a healthy weight and diet, and cessation of tobacco and alcohol may improve IBD and prevent chronic neurodegeneration.55,56 Microbiome-based research on dementia and AD potentiate the future development of novel diagnostic and therapeutic modalities for dementia through manipulation of the intestinal flora.49
VASCULAR DEMENTIA AND ISCHEMIC STROKE
Current literature suggests association between vascular dementia and IBD given overall 2- to 3-fold increased risk of venous thromboembolisms (VTEs) among IBD patients, which is an established independent risk factor for thromboembolic events.16,57-59 Although the pathogenesis is multifactorial,60-62 recent focus on “vascular hypothesis” suggests micro- and macrovascular endothelial dysfunction secondary to elevated inflammatory cytokines in IBD leads to increased VTE risk.60,61 While most thromboembolic events are deep vein thromboses or pulmonary embolisms, several cases have also described cerebral arterial infarction in IBD patients.63,64
Among IBD patients, higher risk for VTEs is associated with active and severe disease, corticosteroids use, extensive colonic involvement, hospitalization, surgery, and pregnancy.59,65,66 Given these additional risk factors, IBD patients hospitalized for flare should be initiated on deep vein thromboses prophylaxis medications unless otherwise contraindicated.16 Use of prophylactic anticoagulation has been deemed safe and the risk of gastrointestinal bleeding is not higher among those receiving anticoagulation.67 For postoperative patients, including those who underwent colorectal surgery, standard prophylactic dosages of anticoagulation may be inadequate, and higher doses with longer period may be more beneficial.68 Managing other known risk factors by maintaining the state of remission and avoiding use of steroids, hormone replacement therapy, immobilization, and smoking also help prevent VTEs.16 Lastly, although outpatient use of prophylactic anticoagulation has been associated with decreased lifetime risk of VTEs, its use is not recommended due to lack of cost-effectiveness.69
MULTIPLE SCLEROSIS
The association of MS, a demyelinating disorder of the CNS, and IBD has been well established. While difficult to ascertain which condition precedes the other, there is higher prevalence of MS among IBD patients and vice versa. Specifically, meta-analyses have reported relative risk of >1.5 for the comorbidities.4,5 Interestingly, patients with both MS and IBD were found to have milder neurological course compared to patients with only MS. Zéphir et al.70 showed MS-IBD patients had lower Expanded Disability Status Scale compared to MS-only patients and a lower proportion transitioned from relapsing-remitting MS to secondary-progressive MS after a median of 12 years of disease follow-up.
Many hypotheses attempt to explain the correlation between MS and IBD. Both are classified as immune-mediated inflammatory diseases.71 MS patients have reduction in the phyla Bacteroidetes and Firmicutes, which are associated with production of anti-inflammatory SCFA.71 Other studies have elucidated dysbiosis relating to the development of experimental autoimmune encephalomyelitis (EAE), the murine equivalent of MS.72 Increased intestinal permeability associated with increased pro-inflammatory Th1 and Th17 cellular responses and reduced anti-inflammatory Treg activity precedes and worsens with development of EAE.73-75 Conversely, germ-free or antibiotic-treated mice with altered microbiome exhibited attenuation of EAE disease severity corresponding to normalization of Th1 and Th17, while the regulatory effect of Treg was heightened leading to increased release of IL-10 and IL-13.76,77 Taken together, the gut microbiome and its metabolism have a clear role in the pathogenesis and severity of EAE/MS.
Beyond the gut-microbiome-brain axis, shared genetic and environmental risk factors are found in patients with MS and IBD. Environmental variables include vitamin D deficiency, smoking, cold climate, and high socioeconomic factors.4 Several experimental studies have shown that vitamin D is required for the normal development of T cells, and supplementation has a protective effect against MS and IBD through suppressing Th1 autoimmune responses while activating anti-inflammatory Treg cells.78,79 Genetically, three single‐nucleotide polymorphisms were found to be associated with IBD and MS. Greater correlation was found between MS and UC patients (commonly harboring the single‐nucleotide polymorphism rs116555563) than between MS and CD patients (commonly harboring the single‐nucleotide polymorphisms rs13428812, rs9977672). However, a causal relationship could not be established.80 In IBD patients with concomitant MS, anti-TNF agents are avoided given concerns of drug-induced demyelination.81 Several case reports have shown development of MS after treatment with anti-TNF agents.82,83 Clinical studies reported 1.43 to 2 times increased risk of MS in IBD patients who underwent anti-TNF therapy compared to IBD patients who did not.84,85 Although the exact mechanism of drug-induced demyelination is unclear, one hypothesis is that anti-TNF agents stop the apoptosis of autoreactive T cells, which subsequently cross the BBB to induce demyelination. Moreover, anti-TNFs cannot cross the BBB and thus may lead to paradoxical increased TNF-α level in the CNS promoting an inflammatory environment.85 On the other hand, TNF-α can also have anti-inflammatory effects by promoting Treg cells. Hence, another theory is anti-TNF agents may systemically induce autoimmunity by decreasing TNF-α associated upregulation of Treg cells.85
The use of sphingosine-1-phosphate (S1P) receptor modulators for treatment of coexisting IBD and MS is an area of ongoing research.86-88 S1P is a lysophospholipid signaling molecule that regulates the immune system via S1P1, S1P4, and S1P5 receptors. Ozanimod, a selective S1P receptor modulator that specifically binds to S1P1 and S1P5 receptors, has been approved for the treatment of relapsing forms of MS,86 and was recently approved for UC.87,88 For moderately to severely active CD, the phase 2 trial has shown clinical, endoscopic, and histologic improvements and phase 3 trials are in investigation.87 By decreasing lymphocyte activation via internalization of S1P receptors, S1P receptor modulators may specifically be considered for management UC and possibly CD in patients with coexisting MS.88
CONCLUSION
There continues to be a growing interest in the role of the gut-brain axis in IBD and neurodegenerative diseases. Current literature supports bidirectional associations between PD and MS with IBD, whereas increased risk of dementia and AD among IBD patients is more established with emerging evidence. Increased risk of VTEs associated with chronic inflammation accompanying IBD may contribute to the unidirectional association between IBD patients and dementia via heightened risk for vascular dementia.
While the phenotypes, clinical management and sequelae differ greatly among these chronic neurodegenerative diseases, several shared etiologic mechanisms are affiliated with IBD. Chronic systemic inflammation drives increased neuro-inflammation and higher permeability of the BBB. Intestinal microbial dysbiosis, a characteristic feature of IBD relapse, reflects decreased production of anti-inflammatory metabolites and potential increase in microbial-derived neurotoxic and neuromodulatory molecules. Gastrointestinal inflammation accompanying “leaky gut” increases the potential of these molecules to enter systemic circulation to eventually penetrate the CNS. Inflammatory signaling may also travel from the intestines to the CNS via the vagus nerve. Finally, shared genetic and environmental risk factors may contribute to both neurodegeneration and development of IBD, several of which have been described. These mechanisms may also play role in other brain disorders, including psychiatric manifestations.89,90
This review summarizes current knowledge regarding the relationship between IBD and neurodegenerative diseases. Existing research to-date rely largely upon animal models or observational studies from population-based cohorts, of which conclusions drawn from the former may not be directly applicable to humans, and the latter may contain biases and confounders. Large prospective observational studies will enhance the strength of available evidence; however, an increased mechanistic understanding of the gut-microbiome-brain axis and pathogenic mechanisms are needed to generate novel clinical diagnostics and therapeutics.
In conclusion, great strides have been made to study the role of the gut-brain axis in neurodegeneration and brain disorders. Existing evidence supports bidirectional crosstalk between the CNS and the ENS. Further experimental and epidemiological studies on IBD and less studied neurodegenerative diseases such as Lewy body dementia, frontotemporal dementia, and alcohol-related dementia may add to the current foundation of knowledge of the gut-brain axis. Moreover, clinical studies that are controlled and prospective would enhance current understanding of the precise methodology of the bidirectional crosstalk. Specifically, future research focusing on discriminating the contributions of each possible mechanistic pathway to neurodegenerative disorders from the gastrointestinal tract may lay the foundation for the development of novel diagnostics and therapeutics for the management of chronic neurodegeneration.
Funding Statement
ACKNOWLEDGEMENTS The study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 110-2628-B-075-009) and Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program 108-V-B-045. B.Z. receives research support from the Zumberge Individual Award of the University of Southern California. The funding sources had no role in any process of our study.
The authors thank I-Fan Hu for his friendship and support.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 2.Walker KA, Gottesman RF, Wu A, et al. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC study. Neurology. 2019;92:e1256–e1267. doi: 10.1212/WNL.0000000000007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Aviña-Zubieta JA, Bernstein CN, et al. Risk of multiple sclerosis among users of antitumor necrosis factor alpha in four Canadian provinces: a population-based study. Neurology. 2023;100:e558–e567. doi: 10.1212/WNL.0000000000201472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Wan J, Wang M, Zhang Y, Wu K, Yang F. Multiple sclerosis and inflammatory bowel disease: a systematic review and meta-analysis. Ann Clin Transl Neurol. 2022;9:132–140. doi: 10.1002/acn3.51495.bc4c1f1f24954e6eaf5f3c1ac66a7b46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosmidou M, Katsanos AH, Katsanos KH, et al. Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J Neurol. 2017;264:254–259. doi: 10.1007/s00415-016-8340-8. [DOI] [PubMed] [Google Scholar]
- 6.Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359–370. doi: 10.1016/S2468-1253(21)00014-5. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19:717–726. doi: 10.1038/s41575-022-00634-6. [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Graff LA, Fisk JD, Patten SB, Bernstein CN. The relationship between symptoms of depression and anxiety and disease activity in IBD over time. Inflamm Bowel Dis. 2021;27:1285–1293. doi: 10.1093/ibd/izaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson's disease: a Danish nationwide cohort study 1977-2014. Gut. 2019;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 10.Peter I, Dubinsky M, Bressman S, et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, Wang HE, Bai YM, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70:85–91. doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal M, Alkhayyat M, Abou Saleh M, et al. Alzheimer disease occurs more frequently in patients with inflammatory bowel disease: insight from a nationwide study. J Clin Gastroenterol. 2023;57:501–507. doi: 10.1097/MCG.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 13.Kim GH, Lee YC, Kim TJ, et al. Risk of neurodegenerative diseases in patients with inflammatory bowel disease: a nationwide population-based cohort study. J Crohns Colitis. 2022;16:436–443. doi: 10.1093/ecco-jcc/jjab162. [DOI] [PubMed] [Google Scholar]
- 14.Rønnow Sand J, Troelsen FS, Horváth-Puhó E, Henderson VW, Sørensen HT, Erichsen R. Risk of dementia in patients with inflammatory bowel disease: a Danish population-based study. Aliment Pharmacol Ther. 2022;56:831–843. doi: 10.1111/apt.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Geng J, Chen X, et al. Association between inflammatory bowel disease and dementia: a longitudinal cohort study. Inflamm Bowel Dis. 2022;28:1520–1526. doi: 10.1093/ibd/izab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol. 2014;20:13863–13878. doi: 10.3748/wjg.v20.i38.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer EA, Nance K, Chen S. The gut-brain axis. Annu Rev Med. 2022;73:439–453. doi: 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- 18.Ancona A, Petito C, Iavarone I, et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig Liver Dis. 2021;53:298–305. doi: 10.1016/j.dld.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittal R, Debs LH, Patel AP, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol. 2017;232:2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günther C, Rothhammer V, Karow M, Neurath M, Winner B. The gut-brain axis in inflammatory bowel disease-current and future perspectives. Int J Mol Sci. 2021;22:8870. doi: 10.3390/ijms22168870.300785d169d54e3f95440b7eb01f63ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 23.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Ní Dhonnabháín R, Xiao Q, O'Malley D. Aberrant gut-to-brain signaling in irritable bowel syndrome: the role of bile acids. Front Endocrinol (Lausanne) 2021;12:745190. doi: 10.3389/fendo.2021.745190.f804cf6ac8d94c29b9e189ba22695d2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banfi D, Moro E, Bosi A, et al. Impact of microbial metabolites on microbiota-gut-brain axis in inflammatory bowel disease. Int J Mol Sci. 2021;22:1623. doi: 10.3390/ijms22041623.bcd6b0cc13ef48a0a0a12c5aa02ffab6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao L, Liu Q, Luo M, Xiong L. Gut microbiota-derived metabolites in irritable bowel syndrome. Front Cell Infect Microbiol. 2021;11:729346. doi: 10.3389/fcimb.2021.729346.b1d18af4988f4d0f89e16248f66132f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Yuan M, Liu Y, et al. Association between inflammatory bowel diseases and Parkinson's disease: systematic review and meta-analysis. Neural Regen Res. 2022;17:344–353. doi: 10.4103/1673-5374.317981.fc237ed25a3b4a22a30f6fb485c4279e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu F, Li C, Gong J, Zhu W, Gu L, Li N. The risk of Parkinson's disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2019;51:38–42. doi: 10.1016/j.dld.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Weimers P, Halfvarson J, Sachs MC, et al. Inflammatory bowel disease and Parkinson's disease: a nationwide swedish cohort study. Inflamm Bowel Dis. 2019;25:111–123. doi: 10.1093/ibd/izy190. [DOI] [PubMed] [Google Scholar]
- 30.Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between Parkinson's disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm Bowel Dis. 2016;22:1049–1055. doi: 10.1097/MIB.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Kim J, Chun J, et al. Patients with inflammatory bowel disease are at an increased risk of Parkinson's disease: a South Korean nationwide population-based study. J Clin Med. 2019;8:1191. doi: 10.3390/jcm8081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudek T. Inflammatory bowel diseases and Parkinson's disease. J Parkinsons Dis. 2019;9:S331–S344. doi: 10.3233/JPD-191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui KY, Fernandez-Hernandez H, Hu J, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med. 2018;10:eaai7795. doi: 10.1126/scitranslmed.aai7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HS, Lobbestael E, Vermeire S, Sabino J, Cleynen I. Inflammatory bowel disease and Parkinson's disease: common pathophysiological links. Gut. 2021;70:408–417. doi: 10.1136/gutjnl-2020-322429. [DOI] [PubMed] [Google Scholar]
- 35.Cabezudo D, Baekelandt V, Lobbestael E. Multiple-hit hypothesis in Parkinson's disease: LRRK2 and inflammation. Front Neurosci. 2020;14:376. doi: 10.3389/fnins.2020.00376.4d231066900b4e11b5967a425add9ae5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Chen Y, Jiang L, et al. Intestinal inflammation and Parkinson's disease. Aging Dis. 2021;12:2052–2068. doi: 10.14336/AD.2021.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Álvarez-Luquín DD, Arce-Sillas A, Leyva-Hernández J, et al. Regulatory impairment in untreated Parkinson's disease is not restricted to Tregs: other regulatory populations are also involved. J Neuroinflammation. 2019;16:212. doi: 10.1186/s12974-019-1606-1.d47ae9cb624d4a43b2122a6968eca91d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson's disease: correlations with inflammatory cytokines in serum. PLoS One. 2012;7:e47387. doi: 10.1371/journal.pone.0047387.ab5856f4667041cbacf1f2b0b93c6f31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang MN, Shi YD, Jiang HY. The risk of dementia in patients with inflammatory bowel disease: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:769–775. doi: 10.1007/s00384-022-04131-9. [DOI] [PubMed] [Google Scholar]
- 43.Liu M, Li D, Hong X, Sun Z. Increased risk for dementia in patients with inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Front Neurol. 2022;13:813266. doi: 10.3389/fneur.2022.813266.5c37e91a7b80431588e6a6691a1e8a61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingel R, Bohlken J, Kostev K. Association between inflammatory bowel disease and dementia: a retrospective cohort study. J Alzheimers Dis. 2021;80:1471–1478. doi: 10.3233/JAD-210103. [DOI] [PubMed] [Google Scholar]
- 45.Fousekis FS, Katsanos AH, Kourtis G, et al. Inflammatory bowel disease and patients with mental disorders: what do we know? J Clin Med Res. 2021;13:466–473. doi: 10.14740/jocmr4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuin M, De Giorgio R, Capatti E, Boschetti E, Zuliani G. Inflammatory bowel disease as a new risk factor for dementia. Aging Clin Exp Res. 2022;34:1725–1728. doi: 10.1007/s40520-022-02076-1. [DOI] [PubMed] [Google Scholar]
- 47.Vadstrup K, Alulis S, Borsi A, et al. Extraintestinal manifestations and other comorbidities in ulcerative colitis and crohn disease: a Danish nationwide registry study 2003-2016. Crohns Colitis 360. 2020;2:otaa070. doi: 10.1093/crocol/otaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo X, Chong L, Zhang X, Li R. Letter to the editor: genetically determined IBD is associated with decreased risk of Alzheimer's disease: a Mendelian randomisation study. Gut. 2022;71:1688–1689. doi: 10.1136/gutjnl-2021-325869. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Zhang X, Du H. Inflammatory bowel disease: a potential pathogenic factor of Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110610. doi: 10.1016/j.pnpbp.2022.110610. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Sommerville NR, Liu JY, et al. Intra-gastrointestinal amyloid-β1-42 oligomers perturb enteric function and induce Alzheimer's disease pathology. J Physiol. 2020;598:4209–4223. doi: 10.1113/JP279919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C, Zhou Y, Wang H, et al. Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer's disease. EMBO J. 2021;40:e106320. doi: 10.15252/embj.2020106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia W, Rajani C, Kaddurah-Daouk R, Li H. Expert insights: the potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer's disease and hepatic encephalopathy. Med Res Rev. 2020;40:1496–1507. doi: 10.1002/med.21653. [DOI] [PubMed] [Google Scholar]
- 54.Mulak A. Bile acids as key modulators of the brain-gut-microbiota axis in Alzheimer's disease. J Alzheimers Dis. 2021;84:461–477. doi: 10.3233/JAD-210608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu W, Tan L, Wang HF, et al. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 56.Serrano-Pozo A, Growdon JH. Is Alzheimer's disease risk modifiable? J Alzheimers Dis. 2019;67:795–819. doi: 10.3233/JAD181028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuhara H, Steinmaus C, Corley D, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953–962. doi: 10.1111/apt.12294. [DOI] [PubMed] [Google Scholar]
- 58.Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014;8:469–479. doi: 10.1016/j.crohns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cainzos-Achirica M, Glassner K, Zawahir HS, et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2020;76:2895–2905. doi: 10.1016/j.jacc.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 61.Papa A, Scaldaferri F, Danese S, et al. Vascular involvement in inflammatory bowel disease: pathogenesis and clinical aspects. Dig Dis. 2008;26:149–155. doi: 10.1159/000116773. [DOI] [PubMed] [Google Scholar]
- 62.Giannotta M, Tapete G, Emmi G, Silvestri E, Milla M. Thrombosis in inflammatory bowel diseases: what's the link? Thromb J. 2015;13:14. doi: 10.1186/s12959-015-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johns DR. Cerebrovascular complications of inflammatory bowel disease. Am J Gastroenterol. 1991;86:367–370. [PubMed] [Google Scholar]
- 64.Katsanos AH, Kosmidou M, Giannopoulos S, et al. Cerebral arterial infarction in inflammatory bowel diseases. Eur J Intern Med. 2014;25:37–44. doi: 10.1016/j.ejim.2013.08.702. [DOI] [PubMed] [Google Scholar]
- 65.Kappelman MD, Horvath-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60:937–943. doi: 10.1136/gut.2010.228585. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 67.Ra G, Thanabalan R, Ratneswaran S, Nguyen GC. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7:e479–e485. doi: 10.1016/j.crohns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Scarpa M, Pilon F, Pengo V, et al. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World J Surg. 2010;34:1629–1636. doi: 10.1007/s00268-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen GC, Sharma S. Feasibility of venous thromboembolism prophylaxis during inflammatory bowel disease flares in the outpatient setting: a decision analysis. Inflamm Bowel Dis. 2013;19:2182–2189. doi: 10.1097/MIB.0b013e31829c01ef. [DOI] [PubMed] [Google Scholar]
- 70.Zéphir H, Gower-Rousseau C, Salleron J, et al. Milder multiple sclerosis course in patients with concomitant inflammatory bowel disease. Mult Scler. 2014;20:1135–1139. doi: 10.1177/1352458513515081. [DOI] [PubMed] [Google Scholar]
- 71.Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown J, Quattrochi B, Everett C, Hong BY, Cervantes J. Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Mult Scler. 2021;27:807–811. doi: 10.1177/1352458520928301. [DOI] [PubMed] [Google Scholar]
- 73.Buscarinu MC, Romano S, Mechelli R, et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. 2018;15:68–74. doi: 10.1007/s13311-017-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alkhawajah MM, Caminero AB, Freeman HJ, Oger JJ. Multiple sclerosis and inflammatory bowel diseases: what we know and what we would need to know! Mult Scler. 2013;19:259–265. doi: 10.1177/1352458512461393. [DOI] [PubMed] [Google Scholar]
- 75.Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014;9:e106335. doi: 10.1371/journal.pone.0106335.24b7572cb6cf4f0790a348176ab2c54a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleck AK, Schuppan D, Wiendl H, Klotz L. Gut-CNS-axis as possibility to modulate inflammatory disease activity-implications for multiple sclerosis. Int J Mol Sci. 2017;18:1526. doi: 10.3390/ijms18071526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 78.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–64. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 79.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–106. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, Musco H, Simpson-Yap S, et al. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat Commun. 2021;12:5641. doi: 10.1038/s41467-021-25768-0.2ec0b36ae4d245beadcad12099f9f93a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gandhi S, Zelman S, De Armas RE, et al. Natural history of new-onset inflammatory bowel disease among patients with multiple sclerosis. Inflamm Bowel Dis. 2022;28:1614–1617. doi: 10.1093/ibd/izac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pfueller CF, Seipelt E, Zipp F, Paul F. Multiple sclerosis following etanercept treatment for ankylosing spondylitis. Scand J Rheumatol. 2008;37:397–399. doi: 10.1080/03009740802136164. [DOI] [PubMed] [Google Scholar]
- 83.Casella G, Tontini GE, Bassotti G, et al. Neurological disorders and inflammatory bowel diseases. World J Gastroenterol. 2014;20:8764–8782. doi: 10.3748/wjg.v20.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avasarala J, Guduru Z, McLouth CJ, et al. Use of anti-TNF-α therapy in Crohn's disease is associated with increased incidence of multiple sclerosis. Mult Scler Relat Disord. 2021;51:102942. doi: 10.1016/j.msard.2021.102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferro JM, Oliveira Santos M. Neurology of inflammatory bowel disease. J Neurol Sci. 2021;424:117426. doi: 10.1016/j.jns.2021.117426. [DOI] [PubMed] [Google Scholar]
- 86.Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. 2017;16:495–503. doi: 10.1016/j.autrev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Feagan BG, Sandborn WJ, Danese S, et al. Ozanimod induction therapy for patients with moderate to severe Crohn's disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5:819–828. doi: 10.1016/S2468-1253(20)30188-6. [DOI] [PubMed] [Google Scholar]
- 88.Sandborn WJ, Feagan BG, D'Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385:1280–1291. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 89.Zhang B, Wang HE, Bai YM, et al. Bidirectional association between inflammatory bowel disease and depression among patients and their unaffected siblings. J Gastroenterol Hepatol. 2022;37:1307–1315. doi: 10.1111/jgh.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sung KY, Zhang B, Wang HE, et al. Schizophrenia and risk of new-onset inflammatory bowel disease: a nationwide longitudinal study. Aliment Pharmacol Ther. 2022;55:1192–1201. doi: 10.1111/apt.16856. [DOI] [PubMed] [Google Scholar]