Abstract
Background/Aims
There are limitations in treating ampullary adenomas with intraductal extension using conventional endoscopic modalities. Endoscopic intraductal radiofrequency ablation (ID-RFA) may be useful for treating intraductal (common bile duct [CBD] and/or pancreatic duct [PD]) extensions of ampullary adenomas, but long-term data are lacking. We thus evaluated the long-term outcomes of endoscopic ID-RFA for managing ampullary adenomas with intraductal extension.
Methods
Prospectively collected endoscopic ID-RFA database at Asan Medical Center was reviewed to identify consecutive patients with ampullary adenoma who underwent ID-RFA for intraductal extension between January 2018 and August 2021. Technical success, short-term and long-term clinical success, and adverse events were evaluated.
Results
A total of 29 patients (14 CBD, 1 PD, and 14 CBD and PD) were analyzed. All patients had undergone endoscopic snare papillectomy prior to ID-RFA. A median of one session of ID-RFA (range, 1 to 3) for residual or relapsed intraductal extension of ampullary adenoma were successfully performed (technical success=100%). Both biliary and pancreatic stenting were routinely performed after ID-RFA to prevent ductal stricture. After a median follow-up of 776 days (interquartile range, 470 to 984 days), the short-term and long-term clinical success rates were 93% and 76%, respectively. Seven patients experienced procedural adverse events and three patients developed ductal strictures.
Conclusions
Endoscopic ID-RFA showed good long-term outcomes in treating residual or relapsed ampullary adenomas with intraductal extension. Repeated ID-RFA may be considered as an option for managing recurrence. Further studies are needed to standardize the procedure.
Keywords: Radiofrequency ablation; Ampulla of Vater; Adenoma; Catheter ablation; Cholangiopancreatography, endoscopic retrograde
INTRODUCTION
Ampullary adenoma is a rare precancerous lesion, but its incidence has been increasing due to increases in cross-sectional imaging and upper gastrointestinal endoscopy.1-3 Considering their malignant potentials,4,5 ampullary adenomas are usually treated by complete primary surgical resection or endoscopic snare papillectomy (ESP).6 However, because surgical resection including pancreaticoduodenectomy entails high risks of morbidity and mortality,7-10 the use of ESP for treating ampullary adenoma has been recently increasing.11 Yet, ESP is prone to resulting in incomplete primary resection due to insufficient safety margin,12 and conventional endoscopic treatment of ampullary adenomas with intraductal extension to the common bile duct (CBD) or pancreatic duct (PD) is challenging.13-15 In such cases, endoscopic ablative therapy can be used as adjunctive to ESP.
Intraductal radiofrequency ablation (ID-RFA) has been developed for the ablation of intraductal tumors, and is often used in malignant biliary strictures for the purpose of palliation.16,17 Considering the favorable outcomes of ID-RFA in malignant biliary obstructions,16-20 endoscopic retrograde cholangiopancreatography (ERCP)-directed ID-RFA may be used as an adjunctive to ESP to eradicate intraductal lesions of ampullary neoplasms.
Several studies21-25 reported encouraging results in the efficacy and safety profile of ID-RFA for intraductal lesions of ampullary neoplasms after ESP. However, to date, only small-sized studies with short follow-up durations have been reported regarding the efficacy of ID-RFA for ampullary adenoma with intraductal extension. Therefore, we aimed to evaluate the long-term outcomes of ID-RFA for residual or relapsed intraductal lesions of ampullary adenoma by reviewing the experience gathered at a large-sized tertiary center.
MATERIALS AND METHODS
1. Patients
We reviewed the prospectively collected ERCP database at Asan Medical Center (Seoul, South Korea) to retrieve information on consecutive patients with ampullary adenoma who underwent ERCP-directed ID-RFA for management of residual or relapsed intraductal extension between January 2018 and August 2021. The inclusion criteria were as follows: (1) ampullary adenoma confirmed by histologic diagnosis; and (2) presence of intraductal lesion confirmed histologically by endoscopic intraductal biopsy during the same session of ESP or at follow-up duodenoscopy after ESP. The exclusion criteria were as follows: (1) age younger than 20 years, (2) pregnancy, or (3) absence of follow-up ERCP with surveillance biopsy after ID-RFA.
The presence of an intraductal extension of ampullary adenoma was first determined based on cross-sectional image (computed tomography and/or magnetic resonance image), ductography (cholangiogram and pancreatogram through ERCP), and visual inspection through the duodenoscopy. If ductography showed a filling defect or far distal stricture, intraductal involvement was suspected. Endoscopic ultrasound (EUS) was additionally performed to confirm the presence of intraductal involvement in selected patients with suspected intraductal involvement, but not confirmed in cross-sectional image, ductography, and visual inspection. In cases of intraductal lesions suspected to extend beyond 10 mm in length, EUS was also performed to assess whether the ablation zone could cover beyond the tumor margins. All patients with suspected intraductal involvement underwent endoscopic intraductal biopsy. The decision to perform ID-RFA for the intraductal extension of ampullary adenoma was made by the attending endoscopist based on clinical circumstances such as histologic result, comorbidities, and patients’ preferences.
2. Data collection
We collected data on the demographics, history of familial adenomatous polyposis, ERCP reports including the gross appearance of the ductal orifice, cholangiogram, pancreatogram, endoscopic procedure for the management of ampullary adenoma, pathologic reports of the ESP specimens and follow-up biopsy specimens after ID-RFA, and details of RFA procedures including the power of delivery energy, preset temperature, and delivery time. Medical records including vital sign, assessment of pain scale, and post-procedure laboratory test results were also reviewed to evaluate the procedural adverse events. The protocols of this study were approved by the Institutional Review Board of Asan Medical Center (IRB number: 2021-0030). All patients provided written informed consent before the procedure.
3. Definition of outcomes
The main outcomes evaluated in this study were as follows: (1) technical success, (2) short-term and long-term clinical success, (3) procedural adverse events, and (4) late adverse events. Technical success was defined as successful placement of the RFA probe in the CBD and/or PD, along with completion of ablation as determined by the observation of whitening change at ductal orifice during the procedure. The residual lesion was defined as the persistent intraductal lesion confirmed by first follow-up biopsy after one session of ID-RFA. Relapsed lesion was defined as the development of recurrent intraductal lesion confirmed by follow-up biopsy in cases in which no residual lesion was histologically confirmed by the first follow-up biopsy after ID-RFA. The short-term clinical success was defined as the disappearance of dysplasia confirmed in the first follow-up biopsy specimen after single session of ID-RFA. The long-term clinical success was defined as maintained no evidence of disease statuses after single session of ID-RFA, which was confirmed by serial follow-up biopsies. In this definition, long-term clinical failure included short-term clinical failure. Procedural adverse events were defined as any procedure-related adverse events that developed within 2 weeks after ID-RFA, including pancreatitis, cholangitis, bleeding, and perforation. Late adverse events were defined as those developing 2 weeks after ID-RFA, including CBD stricture and PD stricture.
4. Equipment and instruments
RFA catheters (ELRATM; STARmed, Co., Ltd., Goyang, Korea) were used to perform ID-RFA. The RFA catheter had a diameter of 7-F and a length of 175-cm, with bipolar probes consisting of 4-mm width and 11-mm length electrodes (Fig. 1A). The temperature sensor is built in the ablation zone of the probe and could monitor the temperature on the contact area during RFA. This temperature-controlled RFA probe was connected to an RFA generator (VIVA ComboTM; STARmed, Co., Ltd.) that had a function of temperature-controlled mode to turn on and off automatically according to the preset target temperature (80℃) (Fig. 1B). When the temperature rose above the preset temperature, the delivery of radiofrequency (RF) energy was automatically terminated to avoid overheating during the procedure.
Fig. 1.
Equipment for intraductal-radiofrequency ablation (RFA). (A) RFA catheter. (B) RFA generator. Images from STARmed Co., Ltd.
5. Procedure
All procedures were performed by two experienced endoscopists (S.S.L. and T.J.S.). During the procedure, all patients were maintained under conscious sedation with intravenous administration of midazolam or propofol and meperidine and were administered prophylactic antibiotics intravenously. Using a conventional side-viewing endoscope (TJF-260; Olympus, Tokyo, Japan), selective cannulation of CBD and PD was performed followed by the acquisition of cholangiogram and pancreatogram, respectively, to identify the extent of intraductal involvement. After cannulation, we inserted the temperature-controlled RFA catheter through the 0.035-inch guidewire (Jagwire; Boston Scientific, Natick, MA, USA) or 0.025-inch guidewire (Visiglide; Olympus). Additional procedures for the dilatation of orifice including endoscopic sphincterotomy or endoscopic papillary balloon dilatation were not routinely performed before the insertion of an RFA catheter into the duct. In cases of intraductal lesions in both CBD and PD, RFA was performed for both lesions in the same session. The electrode of the RFA probe was placed on the adenomatous lesion under fluoroscopic guidance or direct inspection of side-viewing duodenoscopy. Ablation was performed by an RFA generator with a preset electrode temperature of 80℃, electrical energy power of 7 W according to the protocols described in previous studies.26-28 The duration of RF energy delivery was chosen at the discretion of the endoscopist. The completion of RFA was determined as the whitening change in the ductal orifice during RF energy delivery. Concomitant endoscopic treatments with ID-RFA including argon plasma coagulation (APC) (APC 2; Erbe Elektromedizin, Tuebingen, Germany) and snare resection were performed at the discretion of endoscopists considering the grade of the adenoma and extent of the tumor. After ID-RFA, both plastic or metal biliary stent (Zimmon or Cotton-Leung Sof-Flex biliary stent; Cook Medical, Winston-Salem, NC, USA, or BONASTENT biliary; Standard Sci-Tech Inc., Seoul, Korea) and pancreatic stent (Zimmon or Greenen pancreatic stent; Cook Medical) were routinely inserted to prevent ductal strictures. The type and size of biliary and pancreatic stents were chosen at the discretion of the attending endoscopist (Table 1).
Table 1.
Details of the Intraductal-RFA Procedures
| Variable | Value (n=29) |
|---|---|
| Concomitant treatment | 15 (52) |
| Snare resection | 2 |
| Argon plasma coagulation | 8 |
| Snare resection+argon plasma coagulation | 5 |
| Duration of RFA delivery | |
| Common bile duct , sec | 120 (30–173) |
| Pancreatic duct, sec | 30 (20–60) |
| Stenting after RFA | |
| ERBD | 29 (100) |
| FCSEMS | 8 (28) |
| 6 cm long, 10 mm diameter | 4 |
| 6 cm long, 6 mm diameter | 4 |
| Plastic stent | 21 (72) |
| 7 cm long, 10-F diameter | 14 |
| 5 cm long, 10-F diameter | 7 |
| ERPD | 29 (100) |
| 5-F diameter* | 20 |
| 7-F diameter† | 9 |
Data are presented as number (%) or median (interquartile range).
RFA, radiofrequency ablation; ERBD, endoscopic retrograde biliary drainage; FCSEMS, fully covered self-expandable metal stent; ERPD, endoscopic retrograde pancreatic drainage.
*Length of pancreatic plastic stent: 3 cm (n=4), 5 cm (n=2), 7 cm (n=10), and 9 cm (n=4); †Length of pancreatic plastic stent: 7 cm (n=1), 9 cm (n=6), and 10 cm (n=2).
6. Follow-up
The first follow-up ERCP with biopsy sampling was performed at 1 to 2 months after ID-RFA. When no suspicious residual lesion was noted on follow-up endoscopy, CBD orifice and/or PD orifice of the RFA site was targeted for surveillance biopsy. Both biliary stent and pancreatic stent were removed at the first follow-up ERCP; thereafter, follow-up endoscopy with biopsy, laboratory test, and computed tomography scan were regularly performed every 6 months.
7. Statistical analysis
Results are presented as medians and interquartile ranges (IQRs) for continuous variables and numbers with percentages for categorical variables. IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
RESULTS
1. Patient characteristics
The flowchart of the management of ampullary adenoma with intraductal extension after ESP is shown in Fig. 2. A total of 33 consecutive patients meeting the inclusion criteria were identified in our database. After excluding four patients without follow-up biopsy sampling after ID-RFA, 29 patients (14 CBD, 1 PD, and 14 CBD and PD) were included in the analysis. The baseline characteristics of the patients before ID-RFA are presented in Table 2.
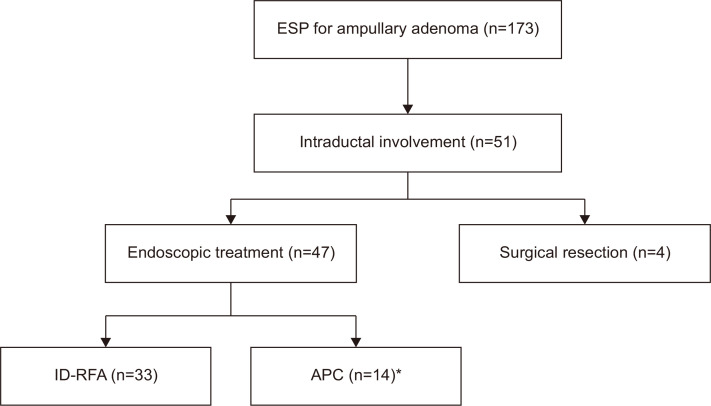
Fig. 2.
Flowchart of the management of ampullary adenoma with intraductal extension after endoscopic snare papillectomy (ESP).
ID-RFA, intraductal radiofrequency ablation; APC, argon plasma coagulation. *Only APC (n=10) and APC with snare resection (n=4).
Table 2.
Baseline Characteristics of the Patients before Intraductal-Radiofrequency Ablation
| Characteristic | Value (n=29) |
|---|---|
| Age, yr | 62 (50–71) |
| Sex | |
| Male | 23 (79) |
| Female | 6 (21) |
| Familial adenomatous polyposis | 5 (17) |
| Duct involvement | |
| Common bile duct | 14 (48) |
| Pancreatic duct | 1 (3) |
| Common bile duct+pancreatic duct | 14 (48) |
| Pathology on specimen of ESP | |
| TA-LGD | 17 (59) |
| TA-HGD | 3 (10) |
| TV-LGD | 4 (14) |
| TV-HGD | 5 (17) |
| Longest diameter of adenoma, mm | 14 (10–19) |
| Margin involvement on specimen of ESP, deep margin/latera margin | |
| Negative/negative | 16 (55) |
| Positive/negative | 6 (21) |
| Negative/positive | 2 (7) |
| Positive/positive | 1 (3) |
| Indeterminate* | 4 (14) |
| Detection of intraductal lesion | |
| Same session of ESP | 5 (17) |
| Remnant lesion after ESP | 14 (48) |
| Recurrent lesion after ESP | 10 (34) |
| Follow-up duration, day | 776 (470–984) |
Data are presented as median (interquartile range) or number (%).
ESP, endoscopic snare papillectomy; TA, tubular adenoma; TV, tubulovillous adenoma; LGD, low-grade dysplasia; HGD, high-grade dysplasia.
*Margin involvement could not be evaluated due to severe squeezing artifacts and cauterization artifacts of the resected specimens.
Of the study patients, all patients underwent ESP, cross-sectional imaging, and ductography through ERCP prior to the session of ID-RFA. EUS was performed to assess the presence and extent of intraductal involvement in four out of 29 patients, in whom the median length of intraductal involvement measured by EUS was 9 mm (range, 6 to 10 mm). Histologic diagnosis of the ESP specimen was tubular adenoma (TA) with low-grade dysplasia (LGD) in 17 patients (59%), TA with high-grade dysplasia (HGD) in three patients (10%), tubulovillous adenoma (TV) with LGD in four patients (14%), and TV with HGD in five patients (17%). According to the inclusion criteria, intraductal involvements were confirmed histologically in all cases. The histologic diagnosis of ESP specimen was concordant with that of intraductal specimen in 22 out of 29 patients. In the remaining seven patients, the worst histology was confirmed in ESP specimens rather than in intraductal specimens (TV-HGD on ESP specimen and TA-LGD on intraductal specimen [n=4]; TV-LGD on ESP specimen and TA-LGD on intraductal specimen [n=3]). Five patients had a history of familial adenomatous polyposis. The median follow-up duration was 776 days (IQR, 470 to 984 days). In five of 29 patients, intraductal extension of ampullary adenoma was directly observed via side-viewing duodenoscope just after ESP. In those patients, biopsy sampling at the intraductal lesion was carried out during the same session of ESP. The remaining 24 patients were histologically diagnosed with intraductal extension of ampullary adenoma during follow-up endoscopy after ESP.
2. Procedure of ID-RFA
The details of the ID-RFA procedure are summarized in Table 1, and the sequence of ID-RFA is shown in Fig. 3. The median interval between ESP and RFA was 174 days (IQR, 43 to 360 days). In all patients, RFA catheters were passed to the CBD and/or PD, and RFA probes were successfully placed in the target lesions under fluoroscopic guidance. During the delivery of RF energy, whitening changes of duct orifices were observed in all patients (technical success=100%). Fourteen (48%) patients underwent RFA alone, and the remaining 15 patients underwent concomitant endoscopic therapy (2 snare resection, 8 APC, 5 both snare resection and APC) to treat adenomatous lesions on the duodenal wall during the ID-RFA session. Among the 15 patients, 13 underwent concomitant therapy to treat nodular lesions located in the papillectomy scar (2 snare resection, 6 APC, 5 both snare resection and APC), and the remaining two patients underwent APC alone to ablate adenomatous lesion around the ductal orifice and not the intraductal lesion. The median duration of RF energy delivery was 120 seconds (IQR, 30 to 173 seconds) in the CBD and 30 seconds (IQR, 20 to 60 seconds) in the PD. Both biliary stents (8 metal stents, 21 plastic stents) and pancreatic stents were placed in all patients regardless of the target lesion of ducts.
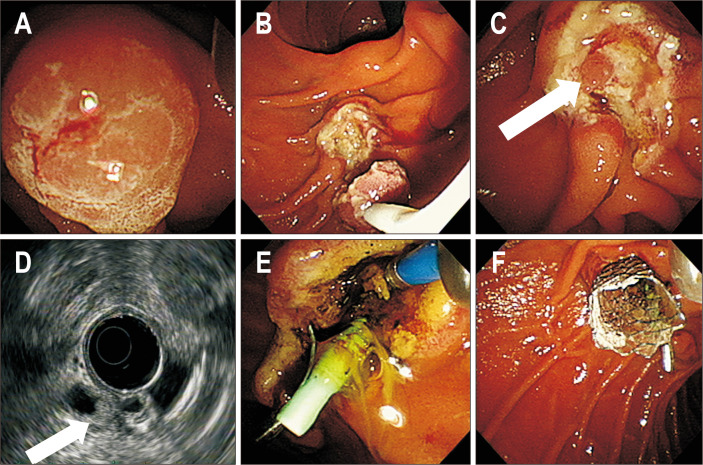
Fig. 3.
Intraductal-radiofrequency ablation procedure. (A) Endoscopic view showing ampullary tubular adenoma with high-grade dysplasia confirmed in a previous pathology examination. (B) Endoscopic image showing endoscopic snare papillectomy, which was performed for resection of the ampullary adenoma. (C) Endoscopic view showing nodular lesions in the common bile duct (CBD) orifice (white arrow). (D) Radial endoscopic ultrasound showing a 6-mm-sized echogenic mass in the far distal CBD (white arrow). (E) Endoscopic image showing whitening change of the CBD orifice during intraductal-radiofrequency ablation. (F) Endoscopic image showing placement of the metallic biliary stent and plastic pancreatic stent.
3. Clinical outcomes
The procedural outcomes are presented in Table 3 and Fig. 4. During follow-up, two patients (7%) showed persistent residual lesions in the first follow-up biopsy (short-term clinical success rate=93%); one was TA with HGD in both the CBD and PD, and the other was TV with HGD in both the CBD and PD. These two patients underwent a second session of RFA at 5 months and 3 months after the first RFA, and their final biopsy results were free of dysplasia.
Table 3.
Procedural Outcomes and Adverse Events of Intraductal-Radiofrequency Ablation
| Variable | Value (n=29) |
|---|---|
| Technical success | 29 (100) |
| Clinical success | |
| Short-term clinical success | 27 (93) |
| Long-term clinical success* | 22 (76) |
| Adverse event | |
| Early adverse event | 7 (24) |
| Pancreatitis | 6 |
| Bleeding | 1 |
| Late adverse event | 3 (10) |
| Common bile duct stricture | 2 |
| Pancreatic duct stricture | 1 |
Data are presented as number (%).
*Four patients and one patient underwent an additional one session and two sessions of intraductal-radiofrequency ablation, respectively.
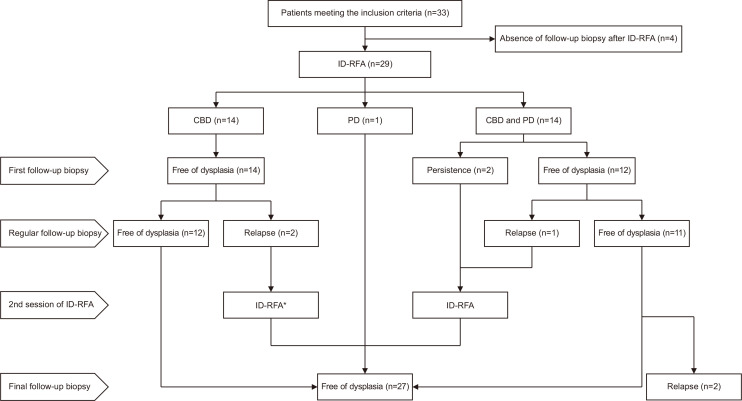
Fig. 4.
Flowchart of the outcomes of ID-RFA.
ID-RFA, intraductal-radiofrequency ablation; CBD, common bile duct; PD, pancreatic duct. *One patient underwent additional two sessions of ID-RFA.
Five patients developed recurrence at regular follow-up biopsies (long-term clinical success rate=76%). Among them, three patients underwent repeated ID-RFA for the management of relapses. They developed recurrence at 17 months, 10 months, and 22 months follow-up after the first ID-RFA, respectively; the former one patient with TA with LGD in both the CBD underwent two additional sessions of RFA, and the latter two patients (1 TA with LGD in the CBD, 1 TV with LGD in both CBD and PD) underwent additional one session of RFA for management of relapses. The last follow-up biopsy results of them were free of dysplasia after additional ID-RFA.
The remaining two patients with relapses had intraductal lesions in both CBD and PD (1 TA with LGD, 1 TV with LGD) simultaneously, and developed relapses at 21 months and 24 months follow-up after first ID-RFA, respectively. They had planned to undergo endoscopic local treatment for recurrent intraductal lesions after the end of the study.
4. Adverse events
Seven (24%) patients developed procedural adverse events, including six (21%) patients with mild acute pancreatitis and one (3%) patient with bleeding. All cases of mild acute pancreatitis occurred within 24 hours of ID-RFA and were completely resolved after conservative management. In one patient with both CBD and PD involvement of ampullary adenoma, minimal bleeding was detected during surveillance endoscopy 1 day after the first session of ID-RFA. Endoscopic hemostasis was successfully performed by endoscopic hemoclipping and epinephrine injection therapy.
Three patients (10%) developed late adverse events, which were ductal strictures (2 CBD stricture, 1 PD stricture). One patient developed a CBD stone at 2 months after ID-RFA. We performed ERCP with stone extraction, and a cholangiogram showed distal CBD stricture. Another patient presented with obstructive jaundice. Biliary stenting was performed in both patients, and recurrent biliary complications such as jaundice and cholangitis did not develop during follow-up. The other patient developed obstructive pancreatitis due to pancreatic ductal strictures at 68 months follow-up. Obstructive pancreatitis was resolved after pancreatic stent insertion, and no recurrent pancreatitis occurred during follow-up periods (Table 3).
DISCUSSION
In the present study, ID-RFA for residual or relapsed intraductal extension of ampullary adenoma after ESP had high technical and clinical success rates and an acceptable safety profile. To our knowledge, this study is the largest study with a longer follow-up duration evaluating the efficacy and safety of ID-RFA for ampullary adenoma with CBD and/or PD extension.
ESP has been the preferred modality for treating ampullary adenoma without intraductal extension as a less invasive procedure compared with surgical resection.12,14,29 A recent comparative study also reported that the long-term outcomes of ESP were comparable to those of surgical resection even in early-stage ampullary cancer.30 However, in the case of intraductal extension of ampullary adenoma, eradication of the lesion is challenging with conventional endoscopic therapies such as ESP and APC because they have limitations in approaching intraductal lesions. The risks of morbidity and mortality of surgery may also outweigh the benefits of clinical success by surgical resection in terms of managing intraductal lesions of ampullary adenoma.7-10 ID-RFA has been introduced for the ablation of intraductal lesions of biliary malignancy for palliation and showed high feasibility and acceptable safety profiles.16-20 ID-RFA is thus considered as a potential adjunctive modality for treating intraductal extension of ampullary adenoma, and some case series showed favorable results of the procedure.23-25
In terms of ductal strictures, the present study showed lower rates of ductal stricture (10%) than those in previous studies (36% and 15%).21,22 Unlike previous studies, we routinely inserted stent into both CBD and PD simultaneously in the same session of ID-RFA to prevent ductal strictures. In case of endoscopic retrograde biliary drainage placement, fully covered metal stents were placed into the CBD in eight of 29 patients. This might contribute to the prevention of ductal strictures.
The rate of mild pancreatitis (21%) as adverse events in our study was higher than that in a previous study (15%) that only included endobiliary adenoma remnants and not pancreatic remnants.22 The involvement of both CBD and PD accounted for the largest proportion (48%) of the cases in this study. This might have contributed to the higher risk of pancreatitis as early procedural adverse events. Of the six patients who developed mild pancreatitis within 24 hours of ID-RFA, three had ablative lesions in the CBD and the remaining three patients had ablative lesions in both CBD and PD. All occurrences of pancreatitis were mild and did not require any interventions. Pancreatic stenting is regarded to play a role in reducing the severity of pancreatitis. In addition, cholangitis also did not occur as an early adverse event in the present study. The placement of both biliary duct and PD can thus be regarded to prevent severe cholangitis and pancreatitis.
We evaluated the clinical success at the first follow-up (short-term clinical success) as well as at the serial follow-up (long-term clinical success) to evaluate the long-term outcomes of ID-RFA. In five of seven patients with long-term clinical failure, repeated ID-RFA (4 one additional session, 1 two additional sessions) were performed rather than surgical resection for management of relapses, resulting in free of dysplasia confirmed by last follow-up biopsies. This suggests that ID-RFA can be an optional modality for the management of relapses after ID-RFA in high surgical risk patients. Both patients with clinical failure after a single session of ID-RFA had HGD. These results are similar to the results of a previous study in which HGD of adenoma was identified as a significant factor associated with RFA failure.22 Considering that HGD in the intraductal area entails the risk of hidden malignancy, surgical resection seemed to be an appropriate therapeutic modality in patients with clinical failure of ablating HGD. However, both patients refused surgical resection and were treated with ID-RFA as an alternative modality.
The standard delivery time of RF energy needed for sufficient ablation while minimizing the risk of adverse events is yet to be determined. Previous studies reported varying median energy delivery times ranging from 30 to 120 seconds.21-25 In the present study, the median energy delivery time was 120 seconds in the CBD and 30 seconds in the PD. The delivery time was determined at the discretion of endoscopists and varied depending on clinical situations, including the tumor extent, degree of dysplasia, and the whitening change of the ductal orifice. To minimize the possibility of acute pancreatitis after the procedure, the duration of energy delivery was shorter in the PD than in the CBD.
In the two patients with clinical failure at the first follow-up biopsy, the delivery time of RF energy in the CBD was 60 seconds and 30 seconds. A previous study22 used shorter energy delivery times of 30 seconds, which could have contributed to the relatively low clinical success rate after a single session of the procedure (70%) compared with the present study outcomes (93%). However, we could not analyze the energy delivery time as a factor associated with clinical success due to the small number of cases with clinical failure at the first follow-up biopsy. Thus, a large-sized prospective study is needed to develop the standardized protocols of the procedure.
Our study has several limitations that mainly arise from its retrospective design. First, we did not measure the actual extent of proximal margin of intraductal lesion in all cases. Also, as we did not perform EUS or peroral cholangioscopy as a follow-up evaluation after ID-RFA, the proximal area of the ablated lesion could not be evaluated. Peroral cholangioscopy might be helpful for evaluating the proximal extent of lesions before ablation and remnant lesions proximal to the ablated area; however, because mucosal lesions in the far distal CBD are difficult to accurately observe even with peroral cholangioscopy, it is not certain whether peroral cholangioscopy will be of significant help in such cases. Second, 52% of the patients (15/29) underwent concomitant endoscopic therapy during ID-RFA. However, all concomitant therapies such as snare resection and APC were performed to treat adenomatous lesions located at the papillectomy scar or around the ductal orifice, not at intraductal lesion; thus, these therapies are not likely to have significantly affected the outcomes of ID-RFA. Third, although the endoscopists shared their therapeutic strategy for endoscopic treatment in this single center, the duration of RF energy delivery and types of stents inserted after ID-RFA varied depending on the clinical situation and the endoscopist’s preference. Thus, the outcomes may be different when using standardized procedure protocols. Therefore, large, randomized, prospective comparative studies are needed to standardize the procedure. Fourth, the long-term outcomes of ID-RFA were evaluated in this single-arm retrospective study. Further well-designed comparative studies are also needed to compare the clinical outcomes of ID-RFA with those of other modalities such as APC.
In conclusion, ID-RFA for intraductal extension of ampullary adenoma showed good long-term outcomes. Repeated ID-RFA may be considered as an optional modality for management of recurrence. Further large, randomized, prospective studies are needed to develop standardized procedure protocols for endoscopic ID-RFA for improving the outcomes of patients with ampullary adenoma with intraductal extension.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2020R1A2C2003604).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: S.S.L. Data acquisition: S.H.C., D.O., T.J.S., D.H.P., D.W.S., S.K.L., M.H.K., S.S.L. Data analysis and interpretation of data: S.H.C., D.O., T.J.S., D.H.P., D.W.S., S.K.L., M.H.K., S.S.L. Drafting of the manuscript: S.H.C. Critical revision of the manuscript for important intellectual content: S.S.L. Statistical analysis: S.H.C. Study supervision: S.S.L. Approval of final manuscript: all authors.
REFERENCES
- 1.Baron TH. Ampullary adenoma. Curr Treat Options Gastroenterol. 2008;11:96–102. doi: 10.1007/s11938-008-0021-y. [DOI] [PubMed] [Google Scholar]
- 2.Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: the expanding role of endoscopy. World J Gastrointest Endosc. 2011;3:241–247. doi: 10.4253/wjge.v3.i12.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker HL, Caldwell DW. Lesions of the ampulla of Vater. Surgery. 1947;21:523–531. [PubMed] [Google Scholar]
- 4.Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–382. doi: 10.3109/00365529609006414. [DOI] [PubMed] [Google Scholar]
- 5.Baczako K, Büchler M, Beger HG, Kirkpatrick CJ, Haferkamp O. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol. 1985;16:305–310. doi: 10.1016/S0046-8177(85)80018-6. [DOI] [PubMed] [Google Scholar]
- 6.Mendonça EQ, Bernardo WM, Moura EG, et al. Endoscopic versus surgical treatment of ampullary adenomas: a systematic review and meta-analysis. Clinics (Sao Paulo) 2016;71:28–35. doi: 10.6061/clinics/2016(01)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. doi: 10.1016/j.jamcollsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Di Giorgio A, Alfieri S, Rotondi F, et al. Pancreatoduodenectomy for tumors of Vater's ampulla: report on 94 consecutive patients. World J Surg. 2005;29:513–518. doi: 10.1007/s00268-004-7498-x. [DOI] [PubMed] [Google Scholar]
- 10.Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379–387. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 11.Kang SH, Kim KH, Kim TN, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol. 2017;17:69. doi: 10.1186/s12876-017-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoleon B, Gincul R, Ponchon T, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy. 2014;46:127–134. doi: 10.1055/s-0034-1364875. [DOI] [PubMed] [Google Scholar]
- 13.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–560. doi: 10.1016/j.gie.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Chathadi KV, Khashab MA, et al. ASGE Standards of Practice Committee, author. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773–781. doi: 10.1016/j.gie.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/S0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 16.Dolak W, Schreiber F, Schwaighofer H, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854–860. doi: 10.1007/s00464-013-3232-9. [DOI] [PubMed] [Google Scholar]
- 17.Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149–153. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Rustagi T, Jamidar PA. Intraductal radiofrequency ablation for management of malignant biliary obstruction. Dig Dis Sci. 2014;59:2635–2641. doi: 10.1007/s10620-014-3237-9. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Sánchez MV, Napoléon B. Review of endoscopic radiofrequency in biliopancreatic tumours with emphasis on clinical benefits, controversies and safety. World J Gastroenterol. 2016;22:8257–8270. doi: 10.3748/wjg.v22.i37.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laquière A, Boustière C, Leblanc S, Penaranda G, Désilets E, Prat F. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:1242–1248. doi: 10.1007/s00464-015-4322-7. [DOI] [PubMed] [Google Scholar]
- 21.Rustagi T, Irani S, Reddy DN, et al. Radiofrequency ablation for intraductal extension of ampullary neoplasms. Gastrointest Endosc. 2017;86:170–176. doi: 10.1016/j.gie.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Camus M, Napoléon B, Vienne A, et al. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: a multicenter prospective study. Gastrointest Endosc. 2018;88:511–518. doi: 10.1016/j.gie.2018.04.2332. [DOI] [PubMed] [Google Scholar]
- 23.Valente R, Urban O, Del Chiaro M, et al. ERCP-directed radiofrequency ablation of ampullary adenomas: a knife-sparing alternative in patients unfit for surgery. Endoscopy. 2015;47 Suppl 1 UCTN:E515–E516. doi: 10.1055/s-0034-1392866. [DOI] [PubMed] [Google Scholar]
- 24.Tian Q, Wang G, Zhang Y, et al. Endoscopic radiofrequency ablation combined with fully covered self-expandable metal stent for inoperable periampullary carcinoma in a liver transplant patient: a case report. Medicine (Baltimore) 2017;96:e5790. doi: 10.1097/MD.0000000000005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehendiratta V, Desilets DJ. Use of radiofrequency ablation probe for eradication of residual adenoma after ampullectomy. Gastrointest Endosc. 2015;81:1055–1056. doi: 10.1016/j.gie.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Lee YN, Jeong S, Choi HJ, et al. The safety of newly developed automatic temperature-controlled endobiliary radiofrequency ablation system for malignant biliary strictures: a prospective multicenter study. J Gastroenterol Hepatol. 2019;34:1454–1459. doi: 10.1111/jgh.14657. [DOI] [PubMed] [Google Scholar]
- 27.Cho JH, Lee KH, Kim JM, Kim YS, Lee DH, Jeong S. Safety and effectiveness of endobiliary radiofrequency ablation according to the different power and target temperature in a swine model. J Gastroenterol Hepatol. 2017;32:521–526. doi: 10.1111/jgh.13472. [DOI] [PubMed] [Google Scholar]
- 28.Cho JH, Jeong S, Kim EJ, Kim JM, Kim YS, Lee DH. Long-term results of temperature-controlled endobiliary radiofrequency ablation in a normal swine model. Gastrointest Endosc. 2018;87:1147–1150. doi: 10.1016/j.gie.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JS, So H, Oh D, et al. Long-term outcomes of endoscopic papillectomy for early-stage cancer in duodenal ampullary adenoma: comparison to surgical treatment. J Gastroenterol Hepatol. 2021;36:2315–2323. doi: 10.1111/jgh.15462. [DOI] [PubMed] [Google Scholar]