Abstract
Most marine invertebrate larvae either obligately feed or depend on maternally provided reserves during planktonic development. A small number of species have the capacity to do both, in a mode of development known as facultative planktotrophy. We describe facultative feeding in a larva from the Oregon coast, and identify it as being an undescribed species in the genus Amphiodia, which we refer to as Amphiodia sp. opaque. We quantified the effects of food on larval and juvenile quality by culturing larvae, collected as embryos, with and without microalgal food at 15°C. The resulting juveniles were monitored under conditions of starvation. A cohort of juveniles of larvae caught as plankton was subjected to the same starvation treatment for comparison with our laboratory‐reared larvae. We observed benefits to offspring that received food: larvae provided with microalgae developed more quickly and metamorphosed at higher rates. Furthermore, juveniles resulting from fed larvae were larger and were able to avoid starvation for longer after metamorphosis. Our results varied across two experimental years, suggesting that provisions provided by parents vary between populations and years. Juveniles from planktonic larvae exhibited sizes not statistically different from larvae cultured in the absence of food, but died from starvation more quickly.
Keywords: development, facultative feeding, larva, larval nutrition strategies, ophiuroid
We describe an unusual brittle star larva that represents an intermediate evolutionary stage between feeding and nonfeeding larvae. Larvae of this species are capable of feeding, but can forego food and make a juvenile. Offspring that ate as larvae had shortened development times, higher percent metamorphosis, larger size, and longer juvenile survival times.

1. INTRODUCTION
The evolutionary transition in developmental mode from feeding (planktotrophic) to nonfeeding (lecithotrophic) larvae is widespread across marine invertebrate taxa, and significantly impacts number and size of offspring (Collin & Moran, 2018; Marshall et al., 2012; Strathmann, 1978, 1985). Lineages with feeding larvae have given rise to those with nonfeeding larvae in many marine taxa, including in closely related species (e.g., Byrne, 2006; Collin, 2004; Jeffery et al., 2003; Keever & Hart, 2008; Krug et al., 2015; Pappalardo et al., 2014; Waeschenbach et al., 2012), sometimes with great frequency, for example, at least 15 times in living echinoids (Emlet, 1990). These contrasting patterns signify an evolutionary trade‐off between parental investment per offspring and fecundity: feeding larvae develop from smaller eggs that are produced in far greater numbers than those of related species with nonfeeding development (Strathmann, 1985). The limited provisions of small eggs require planktotrophic larvae to acquire materials through exogenous food to complete metamorphosis. The larger, more lipid‐rich eggs of lecithotrophic larvae contain sufficient material for larvae to create a juvenile without feeding.
An intermediate pattern known as facultative planktotrophy (Chia, 1974; Emlet, 1986; Vance, 1973a), where larvae are not only capable of feeding in the plankton but can also complete metamorphosis without food, is a rare but persistent mode of development that has been observed at least eight times across several marine invertebrate taxa (Table 1; see also Allen & Pernet, 2007). Facultative planktotrophy is often considered an intermediate mode of development in the evolutionary transition between feeding and nonfeeding larvae, but whether or not intermediate modes can maximize reproductive success over evolutionary timescales is still up for debate (Christiansen & Fenchel, 1979; Levitan, 2000; McEdward, 1997; Vance, 1973a, 1973b). Larvae with facultative planktotrophy provide an opportunity to test the effects of larval feeding on several aspects of early life history, including planktonic duration, percent metamorphosis, juvenile size, and energetic reserves in juveniles.
TABLE 1.
Effects of larval feeding on development of facultative planktotrophs.
| Taxa | Development time | Larval survivorship | Juvenile size | References |
|---|---|---|---|---|
| Echinodermata: Echinoidea | ||||
| Clypeaster rosaceus | = | = | + | Allen et al. (2006), Emlet (1986) |
| Brisaster latifrons | NM | NM | + | Hart (1996) |
| Echinodermata: Ophiuroidea | ||||
| Amphiodia sp. opaque | − | + | + | This study |
| Macrophiothrix rhabdota | − | + | + | Allen and Podolsky (2007) |
| Mollusca: Gastropoda | ||||
| Adalaria proxima | − | = | NM | Kempf and Todd (1989) |
| Conus pennaceus | = | = | NM | Perron (1981) |
| Phestilla sibogae | = | + | + | Kempf and Hadfield (1985), Miller (1993) |
| Annelida: Polychaeta | ||||
| Streblospio benedicti | NM | NM | NM | Pernet and McArthur (2006) |
| Arthropoda: Copepoda | ||||
| Tisbe sp. | − | + | + | Gangur and Marshall (2020) |
Note: Larval and juvenile metrics are represented as increasing (+), decreasing (−), equal (=), or not measured (NM) because of larval feeding.
Planktonic duration, the time interval between introduction of embryos or larvae to the water column and metamorphosis in the benthos, varies widely across developmental modes of planktonic larvae and affects the composition and distribution of benthic adult populations (Becker et al., 2007; Shanks, 2009; Strathmann, 1985). For feeding larvae, the accumulation of materials necessary for metamorphosis can take weeks to months, whereas nonfeeding larvae can settle in the benthos in hours to days after release (Strathmann, 1987). The larval phase is the dominant dispersal stage for benthic marine invertebrates, and larvae that spend weeks in the plankton tend to disperse further than those that spend only hours in the water column before settlement (Shanks, 2009). Realized dispersal distances are modulated by a complex set of temporal, physical, and behavioral factors, but greater interchange of genetic propagules via feeding larvae tends to lead to greater genetic connectivity between benthic adult populations (reviewed in Cowen & Sponaugle, 2009; Shanks, 2009). The increased dispersal potential of feeding larvae influences biogeography: in cone snails, echinoids, and cowries, species with feeding planktonic larvae have larger geographic ranges when compared to species with nonfeeding development (Emlet, 1995; Kohn & Perron, 1994; Paulay & Meyer, 2006); and in fossil gastropods, there is evidence that planktotrophic development can positively influence geographical distribution and species longevity (Hansen, 1978, 1980; Jablonski, 1986).
Planktonic duration can be mediated by factors both environmental and taxon‐specific, but extended planktonic duration generally increases the likelihood of larval mortality prior to metamorphosis (Rumrill, 1990). Low food availability (Miner et al., 2005; Rendleman et al., 2018; Sewell et al., 2004; Strathmann et al., 1992) and low temperature (O'Connor et al., 2007) can slow larval development in echinoids, as well as facultative planktotrophs from some taxa (Allen & Podolsky, 2007; Paulay et al., 1985). Larvae with longer development times relative to conspecifics experienced lower survivorship in two fishes (Hare & Cowen, 1997; Meekan & Fortier, 1996) and increased mortality from predation in a number of invertebrate species (Cowen & Sponaugle, 2009; Rumrill, 1990). Long planktonic durations also expose larvae to the risk of advection away from suitable habitat for settlement (Pineda et al., 2010), although long‐lived larvae in coastal habitats may not always disperse widely (Shanks, 2009). In these ways, planktonic duration can be tied to the percentage of larvae in a cohort that survive through metamorphosis, hereafter referred to as percent metamorphosis.
Metamorphosis does not promise a new beginning: embryonic and larval experiences can be expressed latently in juvenile and adult quality (Emlet & Sadro, 2006; Pechenik, 2006). Food limitation and prolonged planktonic duration have been shown to negatively influence juvenile size, growth, and survival (reviewed in Pechenik, 2018). In facultative planktotrophs, larval feeding resulted in larger juveniles in echinoderms (Allen & Podolsky, 2007; Emlet, 1986; Hart, 1996) and gastropods (Kempf & Hadfield, 1985; Miller, 1993). Juvenile size is an important life history characteristic because larger juveniles tend to exhibit higher survival, growth, reproduction, and longevity across several species (Marshall et al., 2018).
Here, we present evidence for a facultatively planktotrophic larva of a brittle star, Amphiodia sp. opaque (Echinodermata: Ophiuroidea), a previously unknown species that occurs in the northeastern Pacific from Oregon to British Columbia. There is limited documentation of developmental mode within the Ophiuroidea (ca. 12% of ophiuroid species, N. Nakata, unpublished data), but the following developmental modes are known: planktotrophy via an eight‐armed ophiopluteus, facultative planktotrophy, lecithotrophy via a reduced pluteus or vitellaria larva that can be pelagic or demersal, and brooding (Byrne & Selvakumaraswamy, 2002; Hendler, 1991). There are many unresolved relationships within families, but widespread occurrence of developmental diversity suggests frequent transitions from feeding to nonfeeding larvae (Allen & Podolsky, 2007; Lessios & Hendler, 2022; O'Hara et al., 2019). This study utilizes larvae of intermediate mode of development to assess the effect of larval feeding on planktonic duration, percent metamorphosis, juvenile size, and juvenile energetic reserves. Because our experimental individuals were collected as embryos from the plankton and the adults remain unknown (in Oregon), we used DNA barcoding to identify our animals and to reveal their relationship to other ophiuroids in the northeast Pacific.
2. MATERIALS AND METHODS
2.1. Molecular identification of embryos and larvae
To investigate species identity of the embryonic morphotypes, we froze one embryo from each year's cohort of larvae collected for experimentation (2019–2021, see section 2.2) at −20°C in a small volume of seawater. We compared the resulting sequences with those of approximately a dozen larvae of the same morphotype collected over the last decade, as well as with sequences for adults and larvae of other amphiurids from the northeast Pacific. Genomic DNA was extracted with the chelex‐based InstaGene™ Matrix (Bio‐Rad) and fragments of cytochrome c oxidase subunit I (COI) were amplified and sequenced using the primers jgLCO1490 and jgHCO2198 (Geller et al., 2013). PCR amplification reactions were performed in a 20 μL total reaction volume that included 11.4 μL nuclease‐free water, 4 μL 5X Green Buffer, 0.4 μL dNTP 10 mM, 0.2 μL GoTaq Polymerase (Promega), and 1 μL each of forward and reverse primers. PCR conditions were as follows: initial step 95°C for 2 min, followed by 34 cycles of denaturation at 95°C for 40 s, annealing at 45 or 48°C for 40 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 2 min. PCR products were cleaned up using the Wizard SV Gel and PCR Clean up System (Promega) prior to Sanger sequencing (Sequetech). Barcode sequences were compared to GenBank (www.ncbi.nlm.nih.gov/genbank/), BOLD (Ratnasingham & Hebert, 2007), and our unpublished dataset of ophiuroid sequences using the BLAST function in Geneious Prime (https://www.geneious.com). We aligned COI sequences of Amphiurid spp. of the northeast Pacific (Table 2) using the Geneious MAFFT plug‐in (Katoh & Standley, 2013) created a maximum likelihood tree using the PhyML plug‐in with 100 bootstraps and based on the HKY85 model (Guindon et al., 2010; Hasegawa et al., 1985).
TABLE 2.
Collection and accession data for specimens included in tree (Figure 2).
| Species ID | Specimen code | Life stage | Collection location, State/Province | Accession number |
|---|---|---|---|---|
| Amphichondrius granulatus | Agran1 | Adult | Catalina Is., CA | OOPH005-18 |
| Agran2 | Adult | Catalina Is., CA | OOPH006-18 | |
| Amphipholis pugetana | Ampu1 | Adult | Cape Arago, OR | OOPH003-18 |
| Ampu2 | Adult | Cape Arago, OR | OOPH004-18 | |
| Or43‐12913 | Larva | Charleston, OR | OLAB015-22 | |
| Or811‐12115 | Larva | Charleston, OR | OLAB016-22 | |
| Amphiodia occidentalis | Amoc2 | Adult | Charleston, OR | OOPH002-18 |
| AoMc1a | Adult | Charleston, OR | OOPH020-22 | |
| Amoc1 | Adult | Charleston, OR | OOPH001-18 | |
| AoMc2a | Adult | Charleston, OR | OOPH022-22 | |
| Amphiodia sp. sensu Emlet (2006) | FHLAmphioegg1 | Egg | Orcas Island, WA | OLAB045-22 |
| OrR7 | Larva | Charleston, OR | OLAB031-22 | |
| Or8P‐121812 | Larva | Charleston, OR | OLAB033-22 | |
| MMB17 | Adult | Charleston, OR | OOPH032-22 | |
| Amphiodia sp. opaque | BFHL 4167 | Adult | Boundary Bay, WA | BBPS564-19 |
| Orop3a | Larva | Charleston, OR | OLAB001-22 | |
| Opq4 | Larva | Charleston, OR | OLAB006-22 | |
| Oop1 | Larva | Charleston, OR | OLAB007-22 | |
| QHAK‐00565 | Larva | Quadra Island, BC | QHAK711-21 | |
| Opq2 | Larva | Charleston, OR | OLAB004-22 | |
| opaque 11‐7‐18 | Larva | Charleston, OR | OOPH029-22 | |
| Opq1 FHL | Larva | Friday Harbor, WA | OLAB047-22 | |
| Amphiodia urtica | Or988 | Juvenile | Charleston, OR | OLAB028-22 |
| A.urt adt 4‐23‐19 | Adult | Cape Arago, OR | OOPH026-22 | |
| DISA835‐19 | Adult | Los Angeles, CA | DISA835-19 | |
| HM542069 | Adult | Bamfield, BC | *HM542069 | |
| urtica 11‐5‐18 | Larva | Charleston, OR | OOPH027-22 | |
| CCDB‐31778 B01 | Adult | Dana Point, CA | ECHCA108-18 | |
| KU495782 | Adult | Queen Charlotte Is., BC | *KU495782 |
2.2. Larval cultures and data collection
Adults of Amphiodia sp. opaque have not yet been found in Oregon. We obtained late blastulae, hatched gastrulae, and larvae of Amphiodia sp. from plankton tows collected with 130‐μm mesh net in the Coos Bay estuary (Oregon) approximately 3 km from the entrance to the Pacific Ocean (Charleston Marina: 43°21.2′ N, 124°20′ W). We collected plankton daily, approximately 1 h before high tide and examined our catch within 2 h using a stereomicroscope. On several occasions, the bright orange embryos characteristic of Amphiodia sp. opaque (Figure 1a,b) were abundant enough to obtain sufficient material for experimentation. In 2020, we collected approximately 120 blastulae or gastrulae, ~1 day post spawn (dps), on February 23 and 100 more of similar stage on February 24. Because of their similar stages of development, embryos from each day represented separate spawning events 1 day apart. In 2021, we collected 240 embryos of similar stage on March 7. In 2019, no embryos were found, but we collected approximately 80 ophioplutei on January 28 and 29. These larvae had either formed juvenile rudiments or did so within a week of capture and were analyzed for juvenile size and starvation time as a “wild” treatment. The ophiuroid hydrocoele rudiment wraps around the larval esophagus and marks the cessation of larval feeding; therefore, we considered larvae with rudiments to have completed or nearly completed larval feeding in their natural environment prior to their collection and monitoring in the laboratory. Wild ophioplutei and rudiment‐stage larvae were kept in filtered sea water (FSW) for the remainder of their development. We did not manipulate microalgal food or estimate planktonic duration for larvae collected in 2019 because we could not determine their prior history in the plankton.
FIGURE 1.

Amphiodia sp. opaque (a) fertilized egg, (b) unhatched blastula, (c) gastrula, (d) early pluteus 3 days post spawn (dps), (e) reduced pluteus 6 dps from no‐food treatment, with mouth (m) and empty stomach (st), (f) reduced pluteus 6 dps from food treatment, with algal food visible in the stomach (arrowheads), (g) pluteus at 10 dps from no‐food treatment, with three pairs of arms: anterolateral (al), postoral (po), and posterolateral (pl), (h) pluteus 10 dps from food treatment, with juvenile rudiment (r), (i) juvenile from no‐food treatment with disk diameter (dd), and (j) juvenile from food treatment. Scale bars are 100 μm; same scale for (a–f) and for (g–j).
2.3. Feeding experiments
For experimental manipulations of microalgal food, we kept embryos (collected in 2020 or 2021) in FSW until just before the formation of the mouth, approximately 2 days after collection. At this point, we haphazardly divided early plutei into replicate finger bowls each with 10 larvae per 30 mL (2020) or 17 larvae per 51 mL (2021) for a standard density of 0.3 larva per mL. We randomly assigned bowls to “food” or “no‐food” treatments and kept them in an incubator at 15°C. We fed larvae in the food treatment a tripartite microalgal diet composed of two parts by volume of Rhodomonas lens, to one part each of Dunaliella tertiolecta and Isochrysis galbana at a combined concentration of 5000 cells per mL. Larvae in the no‐food treatment were kept in FSW alone. Small flakes of cetyl alcohol were added to all cultures to prevent larvae from perishing in the air–water interface.
We collected data on stages of larval development every 2 or 3 days when FSW and microalgal food were refreshed. We categorized larvae visually as (1) ophiopluteus, (2) rudiment‐stage larva, or (3) juvenile (Figure 1). Developmental stages were defined as follows: ophioplutei had up to six larval arms and an open mouth; rudiment‐stage larvae had a pair of posterolateral arms, the right anterolateral arm and a well‐developed rudiment that occluded the larval mouth; and juveniles lacked larval arms including the larval skeleton and ciliary band, and all locomotion was by their podia. Planktonic duration was defined as the number of days from hatching—estimated as 1 day prior to the blastula stage in which they were collected—until they were scored as juveniles. We defined percent metamorphosis as the total number of juveniles produced relative to the initial number of larvae from a given bowl. When we found new juveniles, we removed them from the larval culture bowl and put each individual in its own 35 mm Petri dish with FSW. We kept juveniles in FSW without food at 15°C, observing them every 2–3 days when FSW was changed. A juvenile was considered dead when podia did not move, even following water agitation and the juvenile did not hold onto the dish bottom.
2.4. Analysis
We conducted all statistical analyses in the R environment v3.6.0 (R Core Team, 2022), and visualized plots with the package “ggplot2” (Wickham, 2016).
For each statistical test to follow, aside from the survival analysis of juveniles, we considered the finger bowl to be the experimental unit. We calculated the percent of larvae that successfully metamorphosed into juveniles (hereafter percent metamorphosis), and mean values for planktonic duration, juvenile size, and juvenile starvation time for each bowl. We calculated survival statistics in two ways: by bowl (Figure 6b) or pooled within treatments and years in survival analysis (Figure 7).
FIGURE 6.
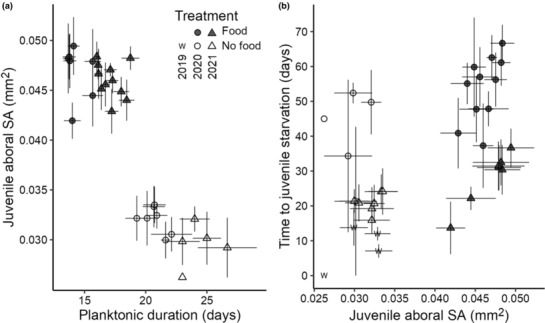
Scatterplots of (a) planktonic duration by juvenile size, and (b) juvenile aboral surface area (SA) by time to juvenile starvation. Points are mean values for replicate finger bowls (larval culture containers) and bars are standard error. Treatment is coded by color (food: dark gray, no‐food: white, wild: “w”) and experimental year by shape (2020: circle, 2021: triangle, wild: “w”).
FIGURE 7.

Kaplan–Meier survival curves for Amphiodia juveniles according to larval food treatment and year. No juveniles were censored as they were followed until the time of the event, death. Lowercase letters below survival curves indicate significantly different pairwise comparisons.
To quantify the effect of larval food on percent metamorphosis, we compared between treatments, keeping years separate. As values for percent metamorphosis were not normally distributed and did not have equal variances (Shapiro–Wilk, p = .015; Levene's test, p = .004), we used a nonparametric Kruskal–Wallis test, followed by a Dunn's test for pairwise comparisons.
We used aboral surface area of the disk as our value for juvenile size. We measured disk diameter (dd, Figure 1i), the length from one arm tip through the center of the disk to the opposite interradius, using the ocular micrometer on the dissecting microscope (dh, Figure 1i), measured to the nearest 20 μm. Using the disk diameter as the height of a regular pentagon (h, Equation 1), we solved for the length of a single side (a) in order to calculate the area of the pentagonal juvenile disc (A, Equation 2).
| (1) |
| (2) |
The data for juvenile aboral surface area did not satisfy the assumption of normality (Shapiro–Wilk, 2020 p = .001, 2021 p = .006), but variances were not significantly different (Levene's test, 2020 p = .97, 2021 p = .09). To determine if the presence of microalgal food had an effect on juvenile size, we compared aboral surface area across treatments using a nonparametric Kruskal–Wallis test, followed by a post hoc Dunn's test.
To determine whether larvae that developed more quickly also produced larger juveniles, we modeled the association between planktonic duration and juvenile aboral surface area using a set of generalized linear models (GLMs) with a Gaussian family, including combinations of potential covariates including treatment and year.
To examine the relationship between juvenile size and survival, we modeled the association between juvenile aboral surface area and time to starvation using a set of GLMs and combinations of covariates treatment, year, and their interaction. As we predict time to juvenile starvation represents a waiting time, we used GLMs with a gamma distribution. We assessed model fit using Akaike's and Bayesian Information Criteria (Table S4), with the lowest value indicating the best fit.
To assess differences in juvenile survival time between treatments and years, we used survival analysis. The survival time data violated the assumptions of parametric statistics, rendering traditional tests inappropriate for their analysis. Such data can be analyzed using nonparametric survival analysis (Kleinbaum & Klein, 2012; Moore, 2016), a collection of statistical procedures for which the outcome variable of interest is time until an event occurs. Juvenile starvation times were analyzed using survival analysis in the R package “survival” (Therneau, 2020) and visualized with the package “survminer” (Kassambara et al., 2020).
Most survival analyses must contend with censoring, which occurs when we have some information about individual survival time, but we do not know the survival time exactly. Our data on time to juvenile starvation are “interval‐censored,” because counts were done at intervals when water changes happened every 2 or 3 days. The exact time of metamorphosis or death is unknown and can only be placed within a specified window of time. Censoring can also occur when an individual withdraws from the study prior to the time of the event. In our analysis of juvenile starvation, the event of interest was death, and no juveniles were censored as all were followed until the time interval of the event. Survival curves for juvenile death probabilities were compared using a nonparametric log‐rank test.
3. RESULTS
3.1. Species identity and occurrence of larvae
The opaque orange larvae studied here belong to an undescribed species of Amphiodia that occurs at least from southern Oregon to British Columbia. On many occasions since March 2003, we have collected eggs, unhatched and hatched embryos, and larvae from Oregon plankton that we assigned, by color and morphology to Amphiodia sp. opaque. These samples were collected between January and April, but on one occasion, the embryos were collected in November (2018). We also collected these larvae in the Salish Sea (Friday Harbor, Washington) in August of 2019 and September of 2020. We have generated COI‐barcodes for more than 15 specimens collected from 2013 to 2019 that we assigned to Amphiodia sp. opaque, and their sequences are 98+ % similar (Figure 2). During the times we collected these embryos and larvae, we never found similar orange embryos or larvae that had different barcodes and were other species.
FIGURE 2.

Maximum likelihood tree of COI sequences from adult and larval Amphiurid spp. in the northeastern Pacific, constructed using the PhyML plug‐in in Geneious. Sequences from GenBank (*) and BOLD are labeled with accession number (and see Table 2). Development modes are indicated on the right, with characteristic larvae depicted. Bootstrap values are shown next to nodes.
The sequences of our larvae are a close match (>98% pairwise nucleotide similarity) to that of a juvenile amphiurid specimen (2.5 mm disk diameter) collected from Boundary Bay WA (BOLD specimen record: BBPS564‐19). The juvenile is clearly a member of the genus Amphiodia, because it has the appropriate pattern of three similarly sized oral papillae per jaw. In BOLD, we found another close match (>99%) to a larva with the same morphology from Hyacinthe Bay, Quadra Island, British Columbia (BOLD specimen record: QHAK711‐21, Figure 2).
A neighbor‐joining tree of COI sequences from larvae and adults of other amphiurid species known from the NE Pacific shows Amphiodia sp. opaque as the sister species to A. urtica which has obligately feeding larvae (Schiff & Bergen, 1996, N. Nakata, unpublished data; Figure 2). Together these clades are sister to a species complex of A. occidentalis (R. Emlet, unpublished data).
3.2. Larval developmental mode
Larvae of Amphiodia sp. opaque develop from eggs of moderate size (140 μm) and are facultative planktotrophs (Figure 1): larvae can feed but do not require food for metamorphosis into juveniles. While percent metamorphosis did differ between years, some larvae developed into juveniles in the absence of food. We observed larvae from unfed culture with empty stomachs, and those from fed cultures with stomachs full of microalgal food (Figure 1e,f). Furthermore, aspects of larval and juvenile performance differed according to larval food treatment (Table 3).
TABLE 3.
Summary of traits for larval and juvenile Amphiodia sp. opaque.
| Year | Treatment | Total larvae/no. replicate bowls | Planktonic duration (days) | Juv., n | Percent metamorphosis | Juv. aboral surface area (mm2) | Time to starvation (days) |
|---|---|---|---|---|---|---|---|
| 2019 | Wild | 80/7 | – | 46 | – | 0.032 ± 0.001 | 0–6–38 |
| 2020 | Food | 110/11 | 15–17–21 | 88 | 80 ± 3 | 0.046 ± 0.001 | 2–59–104 |
| No‐food | 110/11 | 20–23.5–30 | 16 | 14 ± 6 | 0.030 ± 0.001 | 0–48.5–76 | |
| 2021 | Food | 120/7 | 13–13–19 | 54 | 45 ± 4 | 0.047 ± 0.001 | 2–23–65 |
| No‐food | 120/7 | 15–22–33 | 64 | 53 ± 3 | 0.032 ± 0.001 | 2–18.5–54 |
Note: Larvae were raised with and without microalgal food in 2020 and 2021 and collected as late‐stage plutei in 2019. Planktonic duration is given for all larvae that completed metamorphosis. Planktonic duration and time to juvenile starvation are time data and are listed as minimum, median, and maximum. Percent metamorphosis and juvenile aboral surface area are given as means ± standard error for replicate bowls and for individuals, respectively.
3.3. Effects of larval food on developmental timing
Fed larvae reached metamorphosis more quickly than unfed larvae (Figure 3). Planktonic duration differed between years but was about a week shorter for fed larvae than for larvae raised in FSW (2020 median 17 vs. 23.5 days; 2021 median 13 vs. 22 days; see Table 3).
FIGURE 3.

Cumulative sum of juveniles over time by treatment and year: (a) 2020 and (b) 2021. Each year had the same initial number of larvae in each treatment (2020 n = 110 per treatment, 2021 n = 120). Final time points represent date of discovery of the last juvenile in that treatment.
3.4. Effects of larval food on percent metamorphosis
The effect of treatment on the percent of larvae that completed metamorphosis differed significantly in 2020 but not in 2021 (across all treatments and years: Kruskal–Wallis test: χ 2 = 29.25, df = 3, p < .001; Figure 4). Pairwise comparisons show a significant difference between treatments in 2020 (Dunn's test: adj. p < .001, Table S1), when food had a strong effect: 80% of fed larvae created a juvenile whereas only 15% of unfed larvae were able to do so (Figure 4). By contrast, in 2021, slightly more juveniles resulted from cultures without larval food (food: 45%, no‐food: 53%), and percent metamorphosis was not statistically different between treatments (p = .44).
FIGURE 4.

Bar plots of percent metamorphosis (= no. juveniles/initial no. larvae) averaged across experimental bowls for years 2020 (initial larvae n = 220/22 bowls) and 2021 (n = 240/14 bowls). Error bars represent standard error (SE). Lowercase letters above bars represent significant differences in pairwise comparisons.
3.5. Effects of larval food on juvenile size
Juvenile sizes measured as aboral surface area were significantly different between treatments in both years (Kruskal–Wallis test: χ 2 = 26.149, df = 4, p < .0001). Pairwise comparisons showed that juveniles from fed larvae were larger than juveniles from unfed larvae in both years. Juveniles from wild larvae were not significantly different in size from juveniles from the no‐food treatment (Dunn's test, Table S2; Figure 5).
FIGURE 5.

Boxplot of juvenile aboral surface area (SA) at metamorphosis by food treatment, pooled across years. Lowercase letters above boxplots represent significant differences in pairwise comparisons.
We modeled the association between planktonic duration and juvenile aboral surface area (Figure 6a) using a series of GLMs and found that the best fit model included only treatment as a covariate (AIC = −288.3, Table S3).
3.6. Effects of larval food on juvenile survival
The survival time in juveniles that received food as larvae was longer compared to those that did not receive food, but survival times varied between years (2020 median 59 days [n = 88 vs. 48.5 days, n = 16; 2021 median 23 days, n = 54 vs. 18.5 days, n = 64, Table 3]). Juveniles that resulted from wild‐caught larvae had relatively short survival times under starvation conditions (median 6 days, n = 80).
Bigger juveniles tended to survive longer, and juveniles from fed larvae had greater surface areas than those from larvae that did not receive food (Figure 6b). Juvenile aboral surface area was positively associated with days to juvenile starvation and the best‐fit model included an interaction between treatment and year (GLM, AIC 1672.1; Table S4). Treatment (food, no‐food) and experimental year (2020, 2021) were both significant covariates (p = .02 and p < .001, respectively).
The survival curves for each treatment and year were significantly different from each other (log‐rank test, p < .0001) and were significantly different in each pairwise comparison (p < .0001; Figure 7).
4. DISCUSSION
4.1. Species identity
Through DNA barcoding of the COI gene, we found a single species‐level match (>98% pairwise nucleotide similarity) to our Amphiodia larvae in a juvenile specimen from Boundary Bay, WA (BBPS564‐19, provided by G. Paulay), which was difficult to assign to species from its morphology. The specimen was small (2.5 mm disk diameter) and the adult characteristics necessary for identification may have been absent or reduced, as they often are in small specimens (Stöhr, 2005). The specimen had three oral papillae of equal size and spacing, as is characteristic of Amphiodia. Its oral shields are pentagonal in shape, and the arm spines are tapered to a point like in A. urtica, but the radial shields are approximately two times as long as they are wide, the dorsal arm plates are oblong and the ventral arms plates are squarish like in A. occidentalis (Lambert & Austin, 2007). Furthermore, molecular data for COI delineates this specimen from adults of A. occidentalis or A. urtica (Figure 2). Rather than signifying a new species, the lack of a molecular match with morphological identification may be due to the limited genetic sampling of the genus Amphiodia (8 of 34 species, N. Nakata, unpublished data) and of amphiurids of the northeast Pacific (5 of 12 species, N. Nakata, unpublished data).
4.2. Larval morphology and developmental mode
Using feeding assays, we confirmed that Amphiodia sp. opaque is a facultative planktotroph. Amphiodia sp. opaque has eggs of moderate size (140 μm), which is consistent with other ophiuroids with abbreviated development (Hendler, 1991). Larvae of this species developed into juveniles in the absence of microalgal food but were still capable of planktonic feeding. Percent metamorphosis varied across treatments and study years, but 15%–53% of larvae given no microalgal food successfully completed metamorphosis, supporting our diagnosis of facultative planktotrophy.
This developmental mode cannot be diagnosed from larval morphology alone, because larvae retain the structures necessary for feeding. Larvae must be cultured in the presence and absence of microalgal food to determine developmental mode. We did suspect facultative planktotrophy of this larva because it was opaque orange in color and has a reduced pluteus morphology (Hendler, 1975), as determined by comparison with a sympatric congener A. urtica, which has a transparent, planktotrophic larva with eight arms. The posterolateral arms are reduced in length relative to those of A. urtica, and the posterodorsal arms are highly reduced or absent, resulting in six larval arms instead of eight. Larvae of Amphiodia sp. opaque have other features that are associated with evolutionary transitions in developmental pattern, including an egg of intermediate size (ca. 140 μm) and intermediate planktonic duration.
Facultative planktotrophy has been observed in six other marine invertebrates (Table 1). Amphiodia sp. opaque is the second facultative planktotroph to be described from the Ophiuroidea and the first from the family Amphiuridae. Only one ophiuroid was previously known to have this developmental pattern: Macrophiothrix rhabdota (Ophiotrichidae) develops via an 8‐arm pluteus. The family Ophiotrichidae diverged from Amphiuridae approximately 200 Mya (O'Hara et al., 2017), meaning Amphiodia sp. opaque represents an independent evolution of the facultative planktotroph phenotype. Food‐limited growth is common for larvae of benthic invertebrates (Paulay et al., 1985), and facultative planktotrophy may have evolved as a bet‐hedging strategy to increase the number of resulting juveniles in regions or spawning times when planktonic food availability is low.
4.3. Development time
Fed larvae developed more quickly than larvae that did not receive food. Larvae in the food treatment completed metamorphosis in fewer days than their starved peers (Figure 2). This is consistent with echinoids with feeding larvae, which are known to take longer to form their rudiment in no‐ and low‐food conditions (Miner et al., 2005; Sewell et al., 2004; Strathmann et al., 1992).
Compared to congenerics of contrasting development modes, Amphiodia sp. opaque has an intermediate planktonic duration (medians of 13–17 days when fed). The planktotroph Amphiodia urtica has a median planktonic duration of 20 days (n = 10 larvae collected at different times, N. Nakata, unpublished data) and a congeneric, pelagic, direct‐developer may be planktonic for 8 days at 15°C (Emlet, 2006).
The availability of food for larvae does not always decrease time to metamorphosis in facultative planktotrophs, For example, there was no difference between fed and unfed treatments in the echinoid Clypeaster rosaceus or the gastropods Conus pennaceus and Phestilla sibogae (Emlet, 1986; Kempf & Hadfield, 1985; Perron, 1981). In the ophiuroid Macrophiothtrix rhabdota, planktonic duration was decreased in the presence of food (Allen & Podolsky, 2007). Shorter planktonic interval may limit mortality from predation in the plankton (Rumrill, 1990) and may reduce capacity for dispersal in species with shorter developmental times (Hendler, 1991; Shanks, 2009).
We interpret larval developmental times as dependent on the accumulation of energetic reserves necessary for metamorphosis and juvenile life, but other factors may have contributed to planktonic durations observed in this study. Little is known about cues for competency or settlement in ophiuroids (Hendler, 1991; Hodin et al., 2015). Furthermore, ophiuroids are known to be capable of undergoing metamorphosis in the plankton and continuing to ride ocean currents as juveniles (Hendler et al., 1999), indicating that some species may not require benthic cues to begin metamorphosis once sufficient food has been consumed in the plankton.
4.4. Percent metamorphosis
The effect of larval feeding on the proportion of larvae that completed metamorphosis was different across years for Amphiodia sp. opaque. In 2020, food had a strong effect on the proportion of larvae able to complete metamorphosis, as was true for the other facultatively planktotrophic ophiuroid (Allen & Podolsky, 2007). In 2021, percent metamorphosis was not significantly different between treatments and slightly more juveniles resulted from the no‐food treatment (Figures 2 and 3). Among facultative planktotrophs, larval feeding did not affect percent metamorphosis (referred to as larval survival) in an echinoid (Emlet, 1986) and two gastropods (Kempf & Todd, 1989; Perron, 1981).
It is possible that the observed variation between cohorts resulted from differences in culture conditions between years, or from intrapopulation or interannual variation in parental investment and developmental regimes. As all embryos originated from the plankton, we are unable to determine if differences between cohorts reflect variation in maternal provisioning due to nutrition or genetic variation between populations.
4.5. Juvenile size
We observed that fed larvae of Amphiodia sp. opaque produced juveniles that were ca. 50% larger in surface area than those of starved larvae (Table 3; Figure 5). Increased juvenile size as a consequence of larval feeding has been observed in other facultative planktotrophs (Allen & Podolsky, 2007; Emlet, 1986; Hart, 1996; Kempf & Hadfield, 1985; Miller, 1993). Juveniles of Amphiodia sp. opaque from the fed treatment were slightly smaller than those of a sympatric congener with feeding larvae, A. urtica (juvenile aboral surface area 0.047 mm2, n = 18; N. Nakata, unpublished data).
Advanced larvae collected from wild plankton (2019) produced juveniles that were not significantly different in size from those from the experimental no‐food treatment. This suggests that these larvae had limited opportunity to feed in their natural environment (and they were not fed in the laboratory). Even low‐food conditions can lead to smaller juveniles in a calyptraeid gastropod (Chiu et al., 2007, 2008). Evidence from a barnacle has shown that even food deprivation during a portion of the larval life can lead to smaller juveniles with the early stages being the most important (Emlet & Sadro, 2006).
4.6. Juvenile survival
Juveniles of fed larvae lived longer than those from larvae raised without food, suggesting that they gained greater energetic reserves due to larval feeding. However, juvenile survival times differed among treatments and experimental years (Figure 7). Juveniles from both treatments in 2021 had shorter survival times than juveniles from either treatment in 2020. Interestingly, the juveniles that resulted from wild‐caught larvae from 2019 fared the poorest of all. They were no different in size from juveniles of laboratory‐reared larvae that received no food (Figure 5), but they died from starvation more quickly (Figures 6b and 7). This may have been the result of variation in egg composition or size, but whether that was due to intraspecific genetic variation, local environmental deficiencies that lowered maternal condition, or some combination was not determined.
5. CONCLUSION
In this study, we tested the influence of larval feeding in an unidentified facultatively planktotrophic larva, Amphiodia sp. opaque. We know this animal only from its larva. We utilized phylogenetic analysis in an attempt to determine species identification and to compare it with closely related ophiuroids from the NE Pacific. Nevertheless, we were able to conduct a series of experiments that showed clear benefits of larval feeding across multiple life history characters: larvae that received food developed more quickly, experienced higher rates of metamorphosis, and produced larger juveniles that evaded starvation conditions for longer than those of larvae that received no food.
AUTHOR CONTRIBUTIONS
Nicole N. Nakata: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (lead); project administration (lead); resources (supporting); writing – original draft (lead). Richard B. Emlet: Conceptualization (supporting); funding acquisition (equal); resources (lead); writing – review and editing (lead).
FUNDING INFORMATION
William R. Sistrom Memorial Fellowship of the University of Oregon, Charles Lambert Memorial Fellowship of the Friday Harbor Laboratories (partial funding), and NSF grant OCE 1950520 to R. Emlet and M. Watts.
CONFLICT OF INTEREST STATEMENT
These authors declare no conflict of interest.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We thank William R. Sistrom Memorial Fellowship of the University of Oregon and Charles Lambert Memorial Fellowship of the Friday Harbor Laboratories for partial funding of this project. This research was also supported by NSF grant OCE 1950520 to R. Emlet and M. Watts.
Nakata, N. N. , & Emlet, R. B. (2023). Having cake and eating too: The benefits of an intermediate larval form in a brittle star Amphiodia sp. opaque (Ophiuroidea). Ecology and Evolution, 13, e10298. 10.1002/ece3.10298
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in BOLD at boldsystems.org, or GenBank at https://www.ncbi.nlm.nih.gov, under the reference numbers listed in Table 2.
REFERENCES
- Allen, J. D. , & Pernet, B. (2007). Intermediate modes of larval development: Bridging the gap between planktotrophy and lecithotrophy: Intermediate larval forms. Evolution & Development, 9(6), 643–653. 10.1111/j.1525-142X.2007.00202.x [DOI] [PubMed] [Google Scholar]
- Allen, J. D. , & Podolsky, R. D. (2007). Uncommon diversity in developmental mode and larval form in the genus Macrophiothrix (Echinodermata: Ophiuroidea). Marine Biology, 151(1), 85–97. 10.1007/s00227-006-0470-6 [DOI] [Google Scholar]
- Allen, J. D. , Zakas, C. , & Podolsky, R. D. (2006). Effects of egg size reduction and larval feeding on juvenile quality for a species with facultative‐feeding development. Journal of Experimental Marine Biology and Ecology, 331(2), 186–197. 10.1016/j.jembe.2005.10.020 [DOI] [Google Scholar]
- Becker, B. J. , Levin, L. A. , Fodrie, F. J. , & McMillan, P. A. (2007). Complex larval connectivity patterns among marine invertebrate populations. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3267–3272. 10.1073/pnas.0611651104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, D. A. , Cavanaugh, M. , Clark, K. , Karsch‐Mizrachi, I. , Lipman, D. J. , Ostell, J. , & Sayers, E. W. (2017). GenBank. Nucleic Acids Research, 45(D1), D37–D42. 10.1093/nar/gkw1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M. (2006). Life history diversity and evolution in the Asterinidae. Integrative and Comparative Biology, 46(3), 243–254. 10.1093/icb/icj033 [DOI] [PubMed] [Google Scholar]
- Byrne, M. , & Selvakumaraswamy, P. (2002). Phylum Echinodermata: Ophiuroidea. In Young C. M. (Ed.), Atlas of marine invertebrate larvae. Academic Press. [Google Scholar]
- Chia, F.‐S. (1974). Classification and adaptive significance of developmental patterns in marine invertebrates. Thalassia Jugoslavica, 10, 121–130. [Google Scholar]
- Chiu, J. M. Y. , Ng, T. Y. T. , Wang, W. X. , Thiyagarajan, V. , & Qian, P. Y. (2007). Latent effects of larval food limitation on filtration rate, carbon assimilation and growth in juvenile gastropod Crepidula onyx . Marine Ecology Progress Series, 343, 173–182. 10.3354/meps06928 [DOI] [Google Scholar]
- Chiu, J. M. Y. , Wang, H. , Thiyagarajan, V. , & Qian, P. Y. (2008). Differential timing of larval starvation effects on filtration rate and growth in juvenile Crepidula onyx . Marine Biology, 154(1), 91–98. 10.1007/s00227-007-0902-y [DOI] [Google Scholar]
- Christiansen, F. B. , & Fenchel, T. M. (1979). Evolution of marine invertebrate reproductive patterns. Theoretical Population Biology, 16(3), 267–282. 10.1016/0040-5809(79)90017-0 [DOI] [PubMed] [Google Scholar]
- Collin, R. (2004). Phylogenetic effects, the loss of complex characters, and the evolution of development in calyptraeid gastropods. Evolution, 58(7), 1488–1502. 10.1111/j.0014-3820.2004.tb01729.x [DOI] [PubMed] [Google Scholar]
- Collin, R. , & Moran, A. (2018). Evolutionary transitions in mode of development. In Carrier T. J., Reitzel A. M., & Heyland A. (Eds.), Evolutionary ecology of marine invertebrate larvae (pp. 50–66). Oxford University Press. [Google Scholar]
- Cowen, R. K. , & Sponaugle, S. (2009). Larval dispersal and marine population connectivity. Annual Review of Marine Science, 1(1), 443–466. 10.1146/annurev.marine.010908.163757 [DOI] [PubMed] [Google Scholar]
- Emlet, R. B. (1986). Facultative planktotrophy in the tropical echinoid Clypeaster rosaceus (Linnaeus) and a comparison with obligate planktotrophy in Clypeaster subdepressus (gray) (Clypeasteroida: Echinoidea). Journal of Experimental Marine Biology and Ecology, 95, 183–202. [Google Scholar]
- Emlet, R. B. (1990). World patterns of developmental mode in echinoid echinoderms. Advances in Invertebrate Reproduction, 5, 329–334. [Google Scholar]
- Emlet, R. B. (1995). Developmental mode and species geographic range in regular sea urchins (Echinodermata: Echinoidea). Evolution, 49(3), 476–489. 10.1111/j.1558-5646.1995.tb02280.x [DOI] [PubMed] [Google Scholar]
- Emlet, R. B. (2006). Direct development of the brittle star Amphiodia occidentalis (Ophiuroidea, Amphiuridae) from the northeastern Pacific Ocean. Invertebrate Biology, 125(2), 154–171. 10.1111/j.1744-7410.2006.00049.x [DOI] [Google Scholar]
- Emlet, R. B. , & Sadro, S. S. (2006). Linking stages of life history: How larval quality translates into juvenile performance for an intertidal barnacle (Balanus glandula). Integrative and Comparative Biology, 46(3), 334–346. 10.1093/icb/icj023 [DOI] [PubMed] [Google Scholar]
- Gangur, A. N. , & Marshall, D. J. (2020). Facultative feeding in a marine copepod: Effects of larval food and temperature on performance. Marine Ecology Progress Series, 652, 33–47. 10.3354/meps13470 [DOI] [Google Scholar]
- Geller, J. , Meyer, C. , Parker, M. , & Hawk, H. (2013). Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all‐taxa biotic surveys. Molecular Ecology Resources, 13(5), 851–861. 10.1111/1755-0998.12138 [DOI] [PubMed] [Google Scholar]
- Guindon, S. , Dufayard, J.‐F. , Lefort, V. , Anisimova, M. , Hordijk, W. , & Gascuel, O. (2010). New algorithms and Methods to estimate maximum‐likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59(3), 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Hansen, T. A. (1978). Larval dispersal and species longevity in lower tertiary gastropods. Science, 199(4331), 885–887. 10.1126/science.199.4331.885 [DOI] [PubMed] [Google Scholar]
- Hansen, T. A. (1980). Influence of larval dispersal and geographic distribution on specie longevity in neogastropods. Paleobiology, 6(2), 193–207. [Google Scholar]
- Hare, J. A. , & Cowen, R. K. (1997). Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae). Ecology, 78(8), 2415–2431. 10.1890/0012-9658(1997)078[2415:SGDASO]2.0.CO;2 [DOI] [Google Scholar]
- Hart, M. W. (1996). Evolutionary loss of larval feeding: Development, form and function in a facultatively feeding larva, Brisaster latifrons . Evolution, 50(1), 174–187. 10.1111/j.1558-5646.1996.tb04484.x [DOI] [PubMed] [Google Scholar]
- Hasegawa, M. , Kishino, H. , & Yano, T. (1985). Dating of the human‐ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22, 160–174. [DOI] [PubMed] [Google Scholar]
- Hendler, G. (1975). Adaptational significance of the patterns of Ophiuroid development. American Zoologist, 15(3), 691–715. 10.1093/icb/15.3.691 [DOI] [Google Scholar]
- Hendler, G. (1991). Echinodermata: Ophiuroidea. In Giese A. C., Pearse J. S., & Pearse V. B. (Eds.), Reproduction of marine invertebrates: Vol. VI (pp. 355–511). Boxwood Press. [Google Scholar]
- Hendler, G. , Baldwin, C. C. , Smith, D. G. , & Thacker, C. E. (1999). Planktonic dispersal of juvenile brittle stars (Echinodermata: Ophiuroidea) on a Caribbean reef. Bulletin of Marine Science, 65(1), 6. [Google Scholar]
- Hodin, J. , Ferner, M. C. , Ng, G. , Lowe, C. J. , & Gaylord, B. (2015). Rethinking competence in marine life cycles: Ontogenetic changes in the settlement response of sand dollar larvae exposed to turbulence. Royal Society Open Science, 2(6), 150114. 10.1098/rsos.150114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, D. (1986). Background and mass extinctions: The alternation of macroevolutionary regimes. Science, 231(4734), 129–133. 10.1126/science.231.4734.129 [DOI] [PubMed] [Google Scholar]
- Jeffery, C. H. , Emlet, R. B. , & Littlewood, D. T. J. (2003). Phylogeny and evolution of developmental mode in temnopleurid echinoids. Molecular Phylogenetics and Evolution, 28(1), 99–118. 10.1016/S1055-7903(03)00030-7 [DOI] [PubMed] [Google Scholar]
- Kassambara, A. , Kosinski, M. , & Biecek, P. (2020). survminer: Drawing survival curves using “ggplot2” (R package version 0.4.7). https://CRAN.R‐project.org/package=survminer
- Katoh, K. , & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30(4), 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keever, C. C. , & Hart, M. W. (2008). Something for nothing? Reconstruction of ancestral character states in asterinid sea star development: Ancestral states in asterinids. Evolution & Development, 10(1), 62–73. 10.1111/j.1525-142X.2008.00214.x [DOI] [PubMed] [Google Scholar]
- Kempf, S. C. , & Hadfield, M. G. (1985). Planktotrophy by the lecithotrophic larvae of a nudibranch, Phestilla sibogae (Gastropoda). The Biological Bulletin, 169(1), 119–130. 10.2307/1541392 [DOI] [Google Scholar]
- Kempf, S. C. , & Todd, C. D. (1989). Feeding potential in the Lecithotrophic larvae of Adalaria proxima and Tritonia hombergi: An evolutionary perspective. Journal of the Marine Biological Association of the United Kingdom, 69(3), 659–682. 10.1017/S0025315400031052 [DOI] [Google Scholar]
- Kleinbaum, D. G. , & Klein, M. (2012). Introduction to survival analysis. Springer New York. 10.1007/978-1-4419-6646-9_1 [DOI] [Google Scholar]
- Kohn, A. J. , & Perron, F. E. (1994). Life history and biogeography: Patterns in Conus. Oxford University Press. [Google Scholar]
- Krug, P. J. , Vendetti, J. E. , Ellingson, R. A. , Trowbridge, C. D. , Hirano, Y. M. , Trathen, D. Y. , Rodriguez, A. K. , Swennen, C. , Wilson, N. G. , & Valdés, Á. A. (2015). Species selection favors dispersive life histories in sea slugs, but higher per‐offspring investment drives shifts to short‐lived larvae. Systematic Biology, 64(6), 983–999. 10.1093/sysbio/syv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, P. , & Austin, W. C. (2007). Brittle stars, sea urchins and feather stars of British Columbia, Southeast Alaska and Puget Sound. Royal BC Museum Handbook. [Google Scholar]
- Lessios, H. A. , & Hendler, G. (2022). Mitochondrial phylogeny of the brittle star genus Ophioderma . Scientific Reports, 12(1), 5304. 10.1038/s41598-022-08944-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan, D. R. (2000). Optimal egg size in marine invertebrates: Theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. The American Naturalist, 156(2), 18–192. [DOI] [PubMed] [Google Scholar]
- Marshall, D. J. , Krug, P. J. , Kupriyanova, E. K. , Byrne, M. , & Emlet, R. B. (2012). The biogeography of marine invertebrate life histories. Annual Review of Ecology, Evolution, and Systematics, 43(1), 97–114. 10.1146/annurev-ecolsys-102710-145004 [DOI] [Google Scholar]
- Marshall, D. J. , McAlister, J. S. , & Reitzel, A. M. (2018). Evolutionary ecology of parental investment and larval diversity. In Carrier T. J., Reitzel A. M., & Heyland A. (Eds.), Evolutionary ecology of marine invertebrate larvae. Oxford University Press. 10.1093/oso/9780198786962.003.0003 [DOI] [Google Scholar]
- McEdward, L. R. (1997). Reproductive strategies of marine benthic invertebrates revisited: Facultative feeding by Planktotrophic larvae. The American Naturalist, 150(1), 48–72. 10.1086/286056 [DOI] [PubMed] [Google Scholar]
- Meekan, M. G. , & Fortier, L. (1996). Selection for fast growth during the larval life of Atlantic cod Gadus morhua on the Scotian Shelf. Marine Ecology Progress Series, 137, 25–37. 10.3354/meps137025 [DOI] [Google Scholar]
- Miller, S. E. (1993). Larval period and its influence on post‐larval life history: Comparison of lecithotrophy and facultative planktotrophy in the aeolid nudibranch Phestilla sibogae . Marine Biology, 117(4), 635–645. [Google Scholar]
- Miner, B. G. , McEdward, L. A. , & McEdward, L. R. (2005). The relationship between egg size and the duration of the facultative feeding period in marine invertebrate larvae. Journal of Experimental Marine Biology and Ecology, 321(2), 135–144. 10.1016/j.jembe.2005.01.008 [DOI] [Google Scholar]
- Moore, D. F. (2016). Applied survival analysis using R (1st ed.). Springer International Publishing. 10.1007/978-3-319-31245-3_1 [DOI] [Google Scholar]
- O'Connor, M. I. , Bruno, J. F. , Gaines, S. D. , Halpern, B. S. , Lester, S. E. , Kinlan, B. P. , & Weiss, J. M. (2007). Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proceedings of the National Academy of Sciences of the United States of America, 104(4), 1266–1271. 10.1073/pnas.0603422104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara, T. D. , Hugall, A. F. , Cisternas, P. A. , Boissin, E. , Bribiesca‐Contreras, G. , Sellanes, J. , Paulay, G. , & Byrne, M. (2019). Phylogenomics, life history and morphological evolution of ophiocomid brittlestars. Molecular Phylogenetics and Evolution, 130, 67–80. 10.1016/j.ympev.2018.10.003 [DOI] [PubMed] [Google Scholar]
- O'Hara, T. D. , Hugall, A. F. , Thuy, B. , Stöhr, S. , & Martynov, A. V. (2017). Restructuring higher taxonomy using broad‐scale phylogenomics: The living Ophiuroidea. Molecular Phylogenetics and Evolution, 107, 415–430. 10.1016/j.ympev.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Pappalardo, P. , Rodríguez‐Serrano, E. , & Fernández, M. (2014). Correlated evolution between mode of larval development and habitat in muricid gastropods. PLoS One, 9(4), e94104. 10.1371/journal.pone.0094104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulay, G. , Boring, L. , & Strathmann, R. R. (1985). Food limited growth and development of larvae: Experiments with natural sea water. Journal of Experimental Marine Biology and Ecology, 93(1–2), 1–10. 10.1016/0022-0981(85)90145-5 [DOI] [Google Scholar]
- Paulay, G. , & Meyer, C. (2006). Dispersal and divergence across the greatest ocean region: Do larvae matter? Integrative and Comparative Biology, 46, 269–281. [DOI] [PubMed] [Google Scholar]
- Pechenik, J. A. (2006). Larval experience and latent effects—Metamorphosis is not a new beginning. Integrative and Comparative Biology, 46(3), 323–333. 10.1093/icb/icj028 [DOI] [PubMed] [Google Scholar]
- Pechenik, J. A. (2018). Latent effects: Surprising consequences of embryonic and larval experience on life after metamorphosis. In Carrier T. J., Reitzel A. M., & Heyland A. (Eds.), Evolutionary ecology of marine invertebrate larvae (Vol. 1). Oxford University Press. 10.1093/oso/9780198786962.003.0014 [DOI] [Google Scholar]
- Pernet, B. , & McArthur, L. (2006). Feeding by larvae of two different developmental modes in Streblospio benedicti (Polychaeta: Spionidae). Marine Biology, 149(4), 803–811. 10.1007/s00227-006-0266-8 [DOI] [Google Scholar]
- Perron, F. E. (1981). Larval growth and metamorphosis of Conus (Gastropoda: Toxoglossa) in Hawaii. Pacific Science, 35(1), 25–38. [Google Scholar]
- Pineda, J. , Porri, F. , Starczak, V. , & Blythe, J. (2010). Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. Journal of Experimental Marine Biology and Ecology, 392(1–2), 9–21. 10.1016/j.jembe.2010.04.008 [DOI] [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R‐project.org/ [Google Scholar]
- Ratnasingham, S. , & Hebert, P. D. N. (2007). BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molecular Ecology Notes, 7, 355–364. 10.1111/j.1471-8286.2006.01678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendleman, A. J. , Rodriguez, J. A. , Ohanian, A. , & Pace, D. A. (2018). More than morphology: Differences in food ration drive physiological plasticity in echinoid larvae. Journal of Experimental Marine Biology and Ecology, 501, 1–15. 10.1016/j.jembe.2017.12.018 [DOI] [Google Scholar]
- Rumrill, S. S. (1990). Natural mortality of marine invertebrate larvae. Ophelia, 32(1–2), 163–198. [Google Scholar]
- Schiff, K. C. , & Bergen, M. (1996). Impact of wastewater on reproduction of Amphiodia urtica . Southern California Coastal Water Research Project, 1994–1995, 78–84.
- Sewell, M. A. , Cameron, M. J. , & McArdle, B. H. (2004). Developmental plasticity in larval development in the echinometrid sea urchin Evechinus chloroticus with varying food ration. Journal of Experimental Marine Biology and Ecology, 309(2), 219–237. 10.1016/j.jembe.2004.03.016 [DOI] [Google Scholar]
- Shanks, A. L. (2009). Pelagic larval duration and dispersal distance revisited. The Biological Bulletin, 216(3), 373–385. 10.1086/BBLv216n3p373 [DOI] [PubMed] [Google Scholar]
- Stöhr, S. (2005). Who's who among baby brittle stars (Echinodermata: Ophiuroidea): Postmetamorphic development of some North Atlantic forms. Zoological Journal of the Linnean Society, 143(4), 543–576. 10.1111/j.1096-3642.2005.00155.x [DOI] [Google Scholar]
- Strathmann, M. (1987). Reproduction and development of marine invertebrates of the Northern Pacific Coast. University of Washington Press. [Google Scholar]
- Strathmann, R. R. (1978). The evolution and loss of feeding larval stages of marine invertebrates. Evolution, 32(4), 894–906. 10.2307/2407502 [DOI] [PubMed] [Google Scholar]
- Strathmann, R. R. (1985). Feeding and nonfeeding larval development and life‐history evolution in marine invertebrates. Annual Review of Ecology and Systematics, 16, 339–361. [Google Scholar]
- Strathmann, R. R. , Fenaux, L. , & Strathmann, M. F. (1992). Heterochronic developmental plasticity in larval sea urchins and its implications for evolution of nonfeeding larvae. Evolution, 46(4), 972–986. 10.1111/j.1558-5646.1992.tb00613.x [DOI] [PubMed] [Google Scholar]
- Therneau, T. M. (2020). A package for survival analysis in R (R package version 3.1‐12). http://CRAN.R‐project.org/package=survival
- Vance, R. R. (1973a). On reproductive strategies in marine benthic invertebrates. The American Naturalist, 107(955), 339–352. [DOI] [PubMed] [Google Scholar]
- Vance, R. R. (1973b). More on reproductive strategies in marine benthic invertebrates. The American Naturalist, 107(955), 353–361. [DOI] [PubMed] [Google Scholar]
- Waeschenbach, A. , Taylor, P. D. , & Littlewood, D. T. J. (2012). A molecular phylogeny of bryozoans. Molecular Phylogenetics and Evolution, 62(2), 718–735. 10.1016/j.ympev.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer‐Verlag. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The data that support the findings of this study are available in BOLD at boldsystems.org, or GenBank at https://www.ncbi.nlm.nih.gov, under the reference numbers listed in Table 2.


