Abstract
McCune-Albright syndrome (MAS) is a rare genetic disease affecting multiple organs, including endocrine tissues. This endocrinopathy is sometimes responsible for infertility, as it may induce an independent functioning of the ovaries leading to anovulatory cycles. This case report describes the infertility journey of a 22-year-old female who had early puberty and irregular periods with high estrogen and progesterone levels, low FSH and LH (on day 3 of her menstrual cycle), and a multi-cystic right ovary. She received several infertility treatments: initially in vitro oocyte maturation (IVM) followed by cyst transvaginal ultrasound-guided aspiration, all unsuccessful. A right hemi-ovariectomy was performed that eventually restored regular cycles and made it possible to perform ovarian stimulation (OS) and in vitro fertilization (IVF). Live birth was obtained after the first embryo transfer.
Keywords: McCune-Albright syndrome, Infertility, ART, In vitro maturation IVM
Introduction
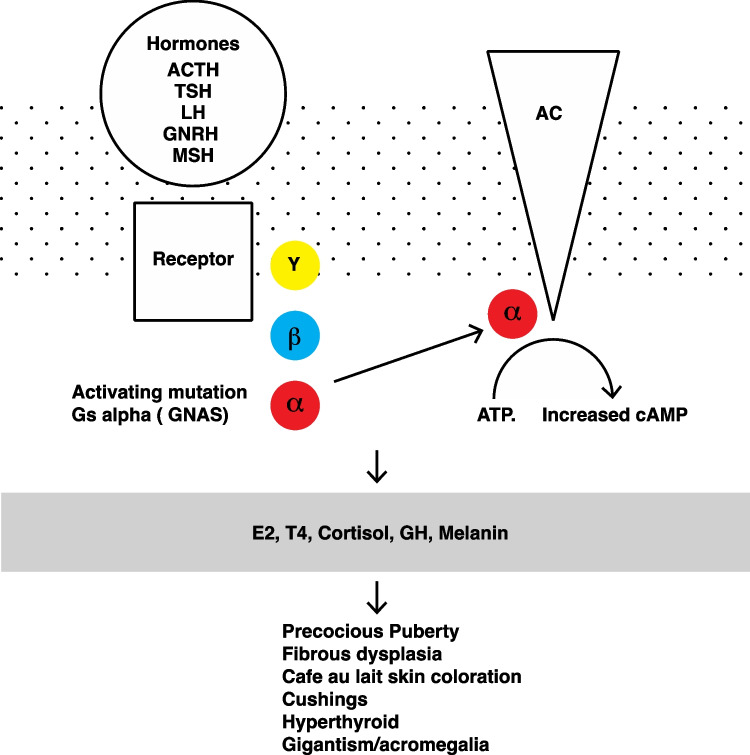
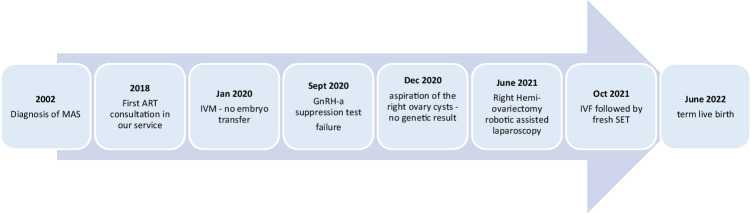
McCune-Albright syndrome (MAS) is a rare genetic non-heritable disease affecting the bones, skin, and endocrine tissues. Aside from precocious puberty, this endocrinopathy may also cause infertility [1]. This disorder can cause infertility, as it may induce an independent functioning of the ovaries through an insufficient hypothalamo-pituitary retro-control that may lead to anovulatory cycles. A representation of the multiple physiological implications is summarized in Fig. 1. In the literature [2–7], several strategies have been reported to treat infertility in women with McCune-Albright syndrome. The first step is often to restore regular cycles via medical treatment (aromatase inhibitor administration) or surgical treatment (cystectomy or whole ovariectomy). The goal of these medical strategies is to either obtain spontaneous pregnancies or perform ART when possible and needed. Our case report describes the infertility journey of a 22-year-old female with ovarian dysfunction due to MAS. Several medical treatments were carried out (Fig. 2) that also included, for the first time, an in vitro maturation (IVM).
Fig. 1.
The summary of the multiple implications in the McCune-Albright syndrome
Fig. 2.
Proposed infertility treatment timeline
Epidemiology
McCune-Albright syndrome is a rare disease, as its prevalence is estimated between 1 and 9/1,000,000 [1]. This syndrome is more common in girls [1, 8]. MAS is defined by a clinical triad including bone fibrous dysplasia, “cafe au lait” skin coloration, and precocious puberty. Other pathologies derived from an autonomous functioning of various endocrine glands such as hyperthyroidism due to thyroid autonomy [9], acromegaly, hypercorticism, or renal phosphate wasting are sometimes associated with this syndrome [1]. Some of these manifestations such as acromegaly are found particularly in adults [10].
Physiology
This syndrome is caused by an activating somatic mutation of the GNAS (for guanine nucleotide-binding protein, alpha stimulating) gene, located on chromosome 20 that induces an unregulated activity (gain in function) of the adenylate cyclase system [11, 12]. GNAS gene codes for the α subunit of G-protein-coupled receptors (GPCRs), also known as 7-transmembrane-domain receptors involved in cell signaling. GNAS gene somatic mutation, in the case of MAS, results in a mosaic distribution of cells bearing constitutively active adenylate cyclase [12]. GPCRs are very common in the endocrine system, as they represent the membrane receptors for LH, TSH, GRH, and ACTH (Fig. 1). The mutations have a mosaic arrangement and occur after meiosis at a relatively early stage of development of the embryo and explain the phenotypic variability and also the difficulty of detection in certain tissues, respectively [13, 14].
Clinical findings
MAS is characterized by great clinical heterogeneity. Polyostotic fibrous dysplasia is the most common symptom. Associated hypophosphatemia must be systematically screened for, as it is a risk factor for small size or rickets in children in the absence of treatment [15]. The “café au lait” macules (CALM) are typically present at birth but become more apparent with age. The macules typically either occur or respect the midline of the body, arranging themselves along the lines of migration cells during development [16, 17]. CALM are not specific to MAS, and they can be associated with differential diagnoses such as neurofibromatosis type 1 (NF), Legius syndrome, or Watson syndrome [18]. The spots in MAS have jagged borders (“coast of Maine”), whereas those in NF are smooth (“coast of California”) [1].
The most common endocrinopathy observed is precocious puberty, typically peripheral, FSH/LH independent, defined by menarche and breast development occurring before the age of 8 in girls [1]. Thus, the GnRH-agonist test does not suppress estradiol secretion [19]. The pelvic ultrasound found one or more ovarian cysts uni- or bilateral, often hemorrhagic, heterogeneous, and hyperechoic [20]. ACTH-independent Cushing’s syndrome usually occurs during the first year of life. The sur-renal glands present a larger volume in CT scans, containing one or several self-sufficient secreting nodules, bilateral [21]. Hyperthyroidism occurs later during childhood and is also associated with thyroid nodules [22].
Diagnosis
The diagnosis for MAS is essentially clinical (pubertal development before the age of 8 years in girls or 9 years in boys, cutaneous hyperpigmentation), along with bone X-rays showing fibrous dysplasia. Genetic testing is not used in routine practice. A negative result does not exclude the disease due to the mosaic distribution of the GNAS gene [23]. Genetic testing on bone tissue or endocrine tissue is not recommended if a clinical diagnosis has already been established.
Treatment
Patients must be referred to a pediatric endocrinologist for appropriate specialized therapeutic management in a reference center [23]. Therapeutic care of early puberty is mainly based on aromatase inhibitors, which are blocking the conversion of testosterone into estradiol [24]. In the case of voluminous ovarian cysts, ultrasound-guided aspiration may sometimes prove to be necessary. It is recommended to perform the cystectomy, although surgery may decrease ovarian reserve and therefore fertility, while the risk of recurrence remains high [23, 25].
Case report
We report the case of a 22-year-old patient with known MAS treated in our academic ART center in Foch Hospital, Suresnes, France. The patient’s McCune-Albright syndrome diagnosis had been established during childhood when she was presented with the classic clinical triad: early puberty (she had her first period at age 6), multifocal bone dysplasia on MRI, and bilateral “café au lait” spots. The genetic testing of GNAS gene mutations was performed at that time but remained inconclusive. Androgenic treatment was initiated at age 6, and she had regular specialized follow-up visits. She had a surgical history of right ovarian cystectomy at the age of 19. The patient and her male partner consulted our center for primary infertility in May 2018, as they had been trying to conceive for 15 months without success. Clinical examination exhibited a small size (150 cm) with a corresponding weight of 42 kg (BMI 18.6). The clinical revealed an oligo-anovulation, with menstrual cycles ranging from 40 to 120 days associated with diffuse pelvic pain. Hormonal blood tests performed on day 3 of her menstrual cycle revealed abnormally high E2 levels (E2 1.053 pg/mL) along with low LH (0.7 UI/L) and FSH (0.9 UI/L) and high progesterone levels (1.32 ng/mL). Serum levels for anti-Mullerian hormone (AMH) and TSH were 2.8 ng/mL and 3.6 mUI/L, respectively. A pelvic ultrasound and MRI showed a small-sized left ovary and a large multi-cystic right ovary. Hysteroscopy and hysterosalpingography were reported to be normal.
Patient care
First step of treatment: in vitro oocyte maturation (IVM) attempt
Our center’s multidisciplinary consultation team first offered to perform ART using the oocyte in vitro maturation (IVM) technique. It was our team’s understanding that her right ovary’s hormonal secretion was autonomous and independent from the hypothalamo-pituitary axis, so no ovarian stimulation (OS) was considered possible. No ovarian suppression test was performed at this point. Although IVM outcomes are still debatable and inferior to those of in vivo maturation, this technique was considered to be a reasonable alternative given the impossibility of performing OS with gonadotrophins before invasive surgery.
Continuous treatment with 2-mg estrogen (E2) BID was started on the first day of menstruation (Provames 2 mg, Sanofi Aventis, Paris, France). The first ultrasound control on day 22 using a 5.0–9.0-MHz multifrequency transvaginal probe (Voluson™ S8 system, GE Healthcare) showed a right ovary with four follicles of 20 mm and six small follicles and a left ovary with one follicle of 14 mm and one of 10 mm. Endometrial thickness was measured at 7 mm. Ovulation was induced by a choriogonadotropin alfa injection (Ovitrelle®. Merck Pharmaceuticals) on day 25, followed by egg retrieval 36 h later using a 19-gauge needle (Cook Medical) linked to a Cook Medical pump with a negative pressure at 80 mmHg.
Concerning ART laboratory technique, a total of 5 oocyte-cumulus complexes (OCC) were retrieved and placed for maturation in a specific medium (MediCult IVM System, Cooper Surgical, France) supplemented with 20% decomplemented patient serum and hMG (Menopur; Ferring Pharmaceutical, France) at a concentration of 10 IU/mL. OCC were incubated at 37 °C, 6.5% CO2, and 5.0% O2 in the humid atmosphere. After 27 h in culture, cumulus cells were removed with hyaluronidase (Vitrolife, France) to assess oocytes’ maturity: Only 1 was mature, and 3 were at the vesical germinal (VG) stage. The mature oocyte was micro-injected with the partner’s sperm. Immature oocytes were replaced in the IVM medium for one more day, but no additional mature oocyte was obtained. A total fertilization failure and no embryo development were observed.
Second step of treatment: GnRh-agonist suppression test and genetic characterization assay
After the IVM attempt failed, our multidisciplinary team reassessed the situation and decided to perform a gonadotropin-releasing agonist hormone (GnRH-a) suppression test; so therefore, the patient received an injection of triptorelin (Decapeptyl® 11.25 mg) in September 2020. The purpose was to validate which ovary is autonomous and independent from the hypothalamo-pituitary axis. We were looking to genetically confirm the diagnosis of GNAS (for guanine nucleotide-binding protein, alpha stimulating) mutation gene by performing polymerase chain reaction (PCR) amplification on the right ovary cystic fluid and potentially, if confirmed, propose a surgical treatment targeting a confirmed pathologic ovarian tissue. The goal for this would be to reinstate the proper control of the hypothalamo-pituitary axis and, in turn, the patient’s fertility. The outcome was as expected, with an appropriate GnRH-a suppressed behavior of the left ovary and an apparently non-suppressed right ovary which remained multi-cystic, with an identical ultrasound aspect as prior. The hormonal blood workup on the third day of her cycle showed similar results to those obtained in January: FSH 0.9 UI/L, LH 0.1 UI/L, E2 172 pg/mL, and PG 1.76 ng/mL. In December 2020, she underwent a transvaginal ultrasound-guided aspiration of the right ovarian cysts without hormonal treatment. The cystic liquid was sent to a molecular biology and genetics laboratory in Necker Academic Hospital. After PCR amplification, unfortunately, no mutation was found on a panel of genes corresponding to the MAS spectrum, on the samples, despite a large quantity of cyst fluid. Another pelvic ultrasound a month later showed still a dystrophic right ovary with several cysts.
Third step of treatment: right hemi-ovariectomy by robotic-assisted laparoscopy
In June 2021, our multidisciplinary team proposed to surgically remove part of the multi-cystic right ovary by robotic-assisted laparoscopy, and this, for multiple reasons. The main hypothesis, based on available literature [2, 4–6, 20], for her infertility, was that the independent right ovary function was slowing down the hypothalamo-pituitary axis, resulting, among other things, in anovulatory cycles. Moreover, the appropriate response of the left ovary to the GnRH-agonist suppression test suggested good chances of recovering a functional axis after a potential surgery. Intensifying diffuse chronic pelvic pain provided another reason to perform the surgery. After extended patient information and the consent signature, the surgery was performed in June 2021 without any complications. The pathological analysis of the surgical specimen confirmed multiple right ovarian cysts without any malignant characteristics and a peritoneal fluid deprived of malignant elements. The histopathological examination findings were non-conclusive for MAS.
In July 2021, 1 month after surgery, she started to experience spontaneous regular menses for the first time in her life. The pelvic ultrasound performed after surgery showed a right ovary with one 9-mm follicle and a left ovary with one dominant follicle measuring 16 mm and five smaller ones measuring approximately 9 mm. This was a first since we never found it during all the examinations performed prior to a functioning left ovary.
Fourth attempt at ART using gonadotropin for OS
In August 2021, the patient’s menstrual cycle was still regular, and the pelvic ultrasound was similar to the one performed a month before. The hormonal workup on the 3rd day of her cycle showed hormonal values that had returned to normal with FSH 6.5 UI/L, LH 2.4 UI/L, E2 40 pg/mL, and PG 0.61 ng/mL, with a post-operative AMH of 1.2 ng/mL.
In September 2021, we decided to start a new OS treatment followed by ART, as per our routine protocols. For OS, individually set doses of hormones were used: 600 IU/day of FSH in a GnRH-antagonist protocol, without pre-treatment.
The development of ovarian follicles monitored by transvaginal ultrasonography and the hormonal blood work results are summarized in Table 1.
Table 1.
Blood and ultrasound monitoring of our patient’s ovarian stimulation for intra-cytoplasmic sperm injection (ICSI)
| Ovarian stimulation follow-up | Before OS, 3rd day of natural cycle | 8th day of OS | 10th day of OS | 11th day of OS |
|---|---|---|---|---|
| Luteinizing hormone (mUI/L) | 0.5 | 1.6 | 0.8 | 0.5 |
| Estradiol (pg/mL) | 32 | 1199 | 2583 | 3541 |
| Progesterone (ng/mL) | < 0.5 | 0.5 | 0.86 | 1.22 |
| Right ovary follicles (size in mm number) | 2–63 | 2–63 | 2–62, 7–92 | 2–65, 7–90,101 |
| Left ovary follicles (size in mm number) | 2–69, 131 | 2–63, 7–92, 111,121,132, 144,161 | 2–61, 7–92, 111, 122, 132, 151, 161, 171, 192, 202 | 2–63, 7–93, 112, 121, 151, 161, 171, 183, 204 |
| Endometrium thickness (mm) | 5 | 7 | 7 | 7.7 |
Ovulation was triggered on the 11th day of OS with GnRh-agonist with 0.2 mg of triptorelin (Decapeptyl® 0.1 mg), and transvaginal ultrasound-guided oocyte retrieval was performed 36 h after, with a double-lumen Cook® needle.
A total of 8 oocytes were retrieved, among which 6 were in metaphase II. Intra-cytoplasmic sperm injection (ICSI) was performed for fertilization. Embryo culture was performed in microdrops of culture media (One Step®, Sage, Cooper Surgical, France) under oil (Ovoil®, Vitrolife, France) at 37 °C and pH between 7.2 and 7.4. We obtained 5 fertilized oocytes and 6 cleaved embryos on day 2. A single embryo, harboring 4 cells with typical cleavage and no fragmentation, was transferred 48 h after the oocyte retrieval using a soft catheter (Sure View®, Wallace, Cooper surgical, France) under transabdominal ultrasound guidance. Among the five surplus embryos, four reached the blastocyst stage and were frozen on day 5 (B5CB, B4BB, B4BC, and B3BA according to Gardner classification) [25].
Daily oral estrogen and vaginal plus subcutaneous progesterone were administered for 2 months (period of the expected lutheo-placental shift, after 8 weeks of gestation). Blood hCG test was positive 12 days after the embryo transfer. The first pregnancy ultrasound was performed after 2 months and showed an intrauterine pregnancy with a single embryo with heart activity. The patient had a full-term delivery of a healthy baby girl a few months later.
Discussion
The evolution and the clinical presentation of MAS associated with early puberty have been well described and reported; however, this is not the case for ovarian function during adulthood [13]. Understanding the gonadotropic profile of MAS patients is fundamental in selecting adequate treatment. Two groups of patients can be distinguished: those with hypogonadotropic hypergonadism and those with hypergonadotropic hypergonadism. Initially, there is an autonomous activation of the ovarian tissue itself without the involvement of the hypothalamic-pituitary axis, which leads to ovarian cysts and estrogen hypersecretion. Later, the hypothalamic-pituitary axis may be activated, resulting, for example, in central precocious puberty. GnRh-analogs are found to be beneficial in that second case [19, 26].
Thus, a variable degree of ovarian autonomy can persist in the adulthood of patients with MAS. In some cases, the gonadotropic function seems to override ovarian autonomy allowing normal regulation of the menstrual cycle and unassisted conception. That is why cases of natural conception have been reported and probably involved women with minor ovarian autonomy [3, 4, 27–29]. In other cases, like our patient, the persistent partial ovarian autonomy leads to irregular menstruation, anovulation, and cyst formation [2, 4–6, 20, 30]. Thus, the mechanism of infertility is related to anovulatory cycles resulting from autonomous ovarian activity [20].
What is particular in our case is the difference in ovarian response to the GnRH-analog administration, depending on the side of the ovary. The result was an appropriate behavior of the left ovary but no response from the right ovary. The right ovary, therefore, seemed to have an independent peripheral secretion. In addition, our patient presented spanio-menorrhea and ovarian cysts on the right side, which reinforce this idea.
Considering that, the decision to partial right ovariectomy was taken with the aim of blocking this ovarian autonomic secretion and restoring ovarian function. Regarding the literature, there are 4 reports of women with frequent ovarian activation who underwent total unilateral ovariectomy in attempts to improve contralateral ovarian function and fertility [2, 4, 5, 30]. Lavoué et al. reported a 33-year-old woman in whom ovulation restoration was observed after unilateral right ovariectomy [30]. The particularity of this case was the GNAS mutation found on both ovaries, but only the right ovary presented cyst formations. The level of mutation reported in the left ovary was much lower than in the right one, suggesting that the minimal number of mutant cells seems to be necessary to result in autonomous function [30].
Thus, GnRH-analog administration may help to identify those patients who might benefit from unilateral ovariectomy. Regarding our case report, partial hemi-ovariectomy is a new approach in cases of women with an autonomous secretion from a single ovary. It allows good chances of recovering a functional axis and so to avoid the development of new cysts, to obtain oocytes, and to improve the quality of life by reducing pelvic pain. Moreover, this surgery is less invasive than the ovariectomy itself.
This is the first known case report that performed the IVM technique in women with MAS. The rationale of the technique was to collect immature oocytes due to the impossibility of stimulating the patient’s ovaries before invasive surgery. Finally, one mature oocyte was obtained that unfortunately did not fertilize with the partner’s sperm. Although in our case this method was not successful, it should be considered in the management of patients with MAS. IVM could appear as an alternative to surgery, especially in cases where both ovaries are affected, and a bilateral ovariectomy would induce premature ovarian failure.
The prevalence rate of infertility among MAS females is difficult to evaluate due to its rarity. A recent retrospective cohort study conducted on 39 patients investigated the gynecologic symptoms and fertility of women with MAS. These authors reported that 43% of patients experienced infertility, among which two patients had iatrogenic premature ovarian failure due to surgical resection of cysts [27]. Moreover, endocrine-related diseases such as hyperprolactinemia, thyroid dysfunction, or Cushing’s syndrome can decrease women’s fertility [1, 3].
Available data on ART outcomes in women with MAS are insufficient. We have found in the literature four MAS females diagnosed with infertility that performed ART. One benefited from an ovariectomy followed by ART [5], one performing intrauterine insemination (IUI) (29), and two performing direct ART [3, 6].
Chung et al. [14] reported one case of an infertile woman of 29 years old suffering from MAS since childhood, with an autonomous secretion of the left ovary. Finally, live birth was obtained after the 2nd embryo transfer. The authors concluded that ART was the ideal solution to treat infertility in this patient. The success, in this case, could also be due to the prior use of an aromatase inhibitor (Letrozole®) that permitted to reverse the pituitary suppression and to restore ovulation. Considering this report, aromatase inhibitor followed by ART could be considered an interesting strategy, but more research is needed to conclude on its efficacy and indication. We could also speculate that aromatase is overexpressed in granulosa cells of MAS patients due to constitutive FSH signaling resulting in supraphysiological intrafollicular E2 concentrations [20]. Only one case in the literature reported ART in ovarian autonomization without pre-treatment or surgery [10]. But in this case, the plasma estradiol was slightly elevated at 81 pg/mL (normal follicular range 27 to 156 pg/mL), and gonadotropin levels were not very low: LH 1.6 IU/L (normal range 2.4 to 12.6 IU/L) and FSH 3.3 IU/L (normal 3.5 to 12.5 IU/L). Finally, the patient with MAS who benefited from intrauterine insemination had regular menstrual cycles, and the choice of this method was due to a male cause of infertility and not an autonomous pathologic ovarian secretion [7].
In terms of obstetrics, no data specifies the existence of a potential high risk of miscarriages or stillbirths, as well as the presence of the mutation on miscarriage samples, oocytes, or embryos [3]. In the literature, early miscarriages [4, 7] and one stillbirth [5] were reported among 3 female patients. In the previously mentioned study including 39 patients, four pregnancies (18%) resulted in spontaneous abortion and one (4%) resulted in fetal demise due to placental abruption at 27 weeks of gestation [27].
Furthermore, our case confirms that the GNAS genetic mutation is not always found in MAS patients. The diagnosis should not be overlooked if neither the blood test nor the aspiration of follicular fluid cannot identify the mutation. Some cases reported mistaken ovariectomy for suspicion of ovarian tumors in young girls who were actually suffering from MAS [31, 32]. This surgery can lead to potential infertility, in particular when both ovaries are involved. This is also the reasoning for favoring a very conservative approach in order to avoid the risk of repeated surgery, in particular in young girls [33].
Conclusion
It is difficult for healthcare professionals to handle McCune-Albright syndrome and its consequences on female fertility, mainly due to its rarity and lack of genetic diagnosis confirmation. We report a few failed infertility treatment attempts (IVM, cyst aspiration), and then a successful pregnancy was achieved through regular OS followed by ART after a surgical right hemi-ovariectomy that restored the functionality of the hypothalamo-pituitary axis. Partial hemi-ovariectomy represents a new approach as it has not been described in literature before. The delay between surgery and ART was short in order to avoid new cyst development and loss of hypothalamo-pituitary axis retro-control. Although not ideal, we share our experience of treating MAS-related infertility in the hope of helping other infertility specialists.
Author contribution
P Pirtea: conception, data collection and interpretation, writing of the case report, corresponding author.
E Heggarty: literature review and critical revising of the article.
E Hagege: editing and critical revising of the article.
C Tran: literature review, critical revising of the article.
D De Ziegler: critical review of the manuscript.
C Farabet: literature review and data collection.
M Filali: data collection.
M Poulain: critical revising of the article.
J-M Ayoubi: surgeon, critical revising of the article.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: In this article, HEGGARTY was incorrectly written as Haggerty.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/11/2023
A Correction to this paper has been published: 10.1007/s10815-023-02885-x
References
- 1.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanson P, Salenave S, Young J. Ovarian dysfunction by activating mutation of GS alpha: McCune-Albright syndrome as a model. Ann Endocrinol (Paris) 2010;71(3):210–213. doi: 10.1016/j.ando.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Agopiantz M, Sorlin A, Vabres P, Leheup B, Carmignac V, Malaplate-Armand C, et al. Fertility in McCune Albright syndrome female: a case study focusing on AMH as a marker of ovarian dysfunction and a literature review. J Gynecol Obstet Hum Reprod. 2021;50(9):102171. doi: 10.1016/j.jogoh.2021.102171. [DOI] [PubMed] [Google Scholar]
- 4.Laven JS, Lumbroso S, Sultan C, Fauser BC. Management of infertility in a patient presenting with ovarian dysfunction and McCune-Albright syndrome. J Clin Endocrinol Metab. 2004;89(3):1076–1078. doi: 10.1210/jc.2003-031245. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier N, Paris F, Fontana S, Delotte J, Gaspari L, Ferrari P, et al. Postpubertal persistent hyperestrogenemia in McCune-Albright syndrome: unilateral oophorectomy improved fertility but detected an unexpected borderline epithelial ovarian tumor. J Pediatr Adolesc Gynecol. 2015;28(6):e169–e172. doi: 10.1016/j.jpag.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Chung RK, Mancuso AC, Kresowik JD. Treatment of primary infertility in McCune-Albright syndrome: a case report of a successful in vitro fertilization cycle. F S Rep. 2021;2(3):352–356. doi: 10.1016/j.xfre.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obuobie K, Mullik V, Jones C, John R, Rees AE, Davies JS, et al. McCune-Albright syndrome: growth hormone dynamics in pregnancy. J Clin Endocrinol Metab. 2001;86(6):2456–2458. doi: 10.1210/jcem.86.6.7609. [DOI] [PubMed] [Google Scholar]
- 8.Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lair-Milan F, Blevec GL, Carel JC, Chaussain JL, Adamsbaum C. Thyroid sonographic abnormalities in McCune-Albright syndrome. Pediatr Radiol. 1996;26(6):424–426. doi: 10.1007/BF01387320. [DOI] [PubMed] [Google Scholar]
- 10.Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, et al. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993;123(4):509–518. doi: 10.1016/S0022-3476(05)80943-6. [DOI] [PubMed] [Google Scholar]
- 11.Rodbell M. The role of GTP-binding proteins in signal transduction: from the sublimely simple to the conceptually complex. Curr Top Cell Regul. 1992;32:1–47. doi: 10.1016/B978-0-12-152832-4.50003-3. [DOI] [PubMed] [Google Scholar]
- 12.Neubig RR. Membrane organization in G-protein mechanisms. Faseb j. 1994;8(12):939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 13.Chapurlat RD, Orcel P. Fibrous dysplasia of bone and McCune-Albright syndrome. Best Pract Res Clin Rheumatol. 2008;22(1):55–69. doi: 10.1016/j.berh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet. 1986;29(4):321–324. doi: 10.1111/j.1399-0004.1986.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 15.Benhamou J, Gensburger D, Messiaen C, Chapurlat R. Prognostic factors from an epidemiologic evaluation of fibrous dysplasia of bone in a modern cohort: the FRANCEDYS study. J Bone Miner Res. 2016;31(12):2167–2172. doi: 10.1002/jbmr.2894. [DOI] [PubMed] [Google Scholar]
- 16.Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14(1):139. doi: 10.1186/s13023-019-1102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 18.Anderson S. Café au lait macules and associated genetic syndromes. J Pediatr Health Care. 2020;34(1):71–81. doi: 10.1016/j.pedhc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Mishra A, Purvar R, Malik S, Agarwal K, Gera R, Sridhar S. McCune Albright syndrome from gynaecological perspective. J Obstet Gynaecol India. 2016;66(Suppl 2):672–674. doi: 10.1007/s13224-016-0864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laven JS, Lumbroso S, Sultan C, Fauser BC. Dynamics of ovarian function in an adult woman with McCune–Albright syndrome. J Clin Endocrinol Metab. 2001;86(6):2625–2630. doi: 10.1210/jcem.86.6.7595. [DOI] [PubMed] [Google Scholar]
- 21.Kirk JM, Brain CE, Carson DJ, Hyde JC, Grant DB. Cushing’s syndrome caused by nodular adrenal hyperplasia in children with McCune-Albright syndrome. J Pediatr. 1999;134(6):789–792. doi: 10.1016/S0022-3476(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 22.Feuillan PP, Shawker T, Rose SR, Jones J, Jeevanram RK, Nisula BC. Thyroid abnormalities in the McCune-Albright syndrome: ultrasonography and hormonal studies. J Clin Endocrinol Metab. 1990;71(6):1596–1601. doi: 10.1210/jcem-71-6-1596. [DOI] [PubMed] [Google Scholar]
- 23.Haute Autorité de Santé HAS . Dysplasie et syndrome de McCune-Albright. Saint-Denis La Plaine: Guide maladie chronique; 2022. [Google Scholar]
- 24.Feuillan P, Calis K, Hill S, Shawker T, Robey PG, Collins MT. Letrozole treatment of precocious puberty in girls with the McCune-Albright syndrome: a pilot study. J Clin Endocrinol Metab. 2007;92(6):2100–2106. doi: 10.1210/jc.2006-2350. [DOI] [PubMed] [Google Scholar]
- 25.Schoolcraft WB, Gardner DK, Lane M, Schlenker T, Hamilton F, Meldrum DR. Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril. 1999;72(4):604–609. doi: 10.1016/S0015-0282(99)00311-8. [DOI] [PubMed] [Google Scholar]
- 26.Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71(5):1251–1258. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 27.Boyce AM, Casey RK, Ovejero Crespo D, Murdock CM, Estrada A, Guthrie LC, et al. Gynecologic and reproductive outcomes in fibrous dysplasia/McCune-Albright syndrome. Orphanet J Rare Dis. 2019;14(1):90. doi: 10.1186/s13023-019-1057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88(9):4413–4417. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- 29.Lee PA, Van Dop C, Migeon CJ. McCune-Albright syndrome. Long-term follow-up. Jama. 1986;256(21):2980–2984. doi: 10.1001/jama.1986.03380210076028. [DOI] [PubMed] [Google Scholar]
- 30.Lavoué V, Morcel K, Bouchard P, Sultan C, Massart C, Grall JY, et al. Restoration of ovulation after unilateral ovariectomy in a woman with McCune-Albright syndrome: a case report. Eur J Endocrinol. 2008;158(1):131–134. doi: 10.1530/EJE-07-0482. [DOI] [PubMed] [Google Scholar]
- 31.Nabhan ZM, West KW, Eugster EA. Oophorectomy in McCune-Albright syndrome: a case of mistaken identity. J Pediatr Surg. 2007;42(9):1578–1583. doi: 10.1016/j.jpedsurg.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Guan H-Y, Wang L, Zhang W. Mistaken oophorectomy in an adolescent with McCune-Albright syndrome: a case report and literature review. Reprod Dev Med. 2018;2(4):252–255. doi: 10.4103/2096-2924.249887. [DOI] [Google Scholar]
- 33.Chae HS, Rheu CH. Precocious pseudopuberty due to an autonomous ovarian follicular cyst: case report with a review of literatures. BMC Res Notes. 2013;6:319. doi: 10.1186/1756-0500-6-319. [DOI] [PMC free article] [PubMed] [Google Scholar]