Abstract
Purpose
Currently, owing to the limitations of high-throughput sequencing depth and the allele dropout caused by the whole-genome amplification, detection of chromosomal variants in embryos with CNVs <5 Mb is unsatisfactory at the single-cell level using only conventional sequencing methods. Therefore, here we aimed to use a strategy of preimplantation genetic testing for monogenic (PGT-M) to compensate for the shortcomings of conventional sequencing methods. The purpose of this study is to report the effectiveness of haplotype linkage analysis by karyomapping for preimplantation diagnosis microdeletion diseases.
Methods
Six couples carrying chromosomal microdeletions associated with X-linked ichthyosis were recruited, and all couples entered the PGT process. Multiple displacement amplification (MDA) method was used to amplify the whole-genome DNA of trophectoderm cells. Then karyomapping based on single nucleotide polymorphism (SNP) was used for haplotype linkage analysis to detect alleles carrying microdeletions, and CNVs of embryos were identified to determine euploid identity. Amniotic fluid tests were performed in the second trimester to verify the PGT-M results.
Results
All couples were tested for chromosomal microdeletions, with deletion fragments ranging in size from 1.60 to 1.73 Mb, and one partner in each couple did not carry the microdeletion. Three couples successfully underwent PGT-M assisted conception and obtained healthy live births.
Conclusion
This study shows that haplotype linkage analysis by karyomapping could effectively detect the carrier status of embryos with microdeletions at the single-cell level. This approach may be applied to the preimplantation diagnosis of various chromosomal microvariation diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02812-0.
Keywords: PGT-M, Haplotype linkage analysis, Karyomapping, Microdeletions, X-linked ichthyosis
Introduction
X-linked ichthyosis (MIM #308100) is caused by a loss-of-function mutation in the gene encoding the microsomal enzyme steroid sulfatase (STS) [1–3]. Insufficient activity of STS leads to hyperkeratosis and impaired permeability of the skin [4]. Histopathological manifestations include compact hyperkeratosis and mild acanthosis with a normal granular layer [5]. The global incidence of X-linked ichthyosis is estimated to be between 1:2000 and 1:6000 births [6].
In this disease, abnormal skin manifestations usually appear within 1 year of birth, with 15–20% cases showing symptoms at birth. Typical clinical manifestations include widespread, dark brown, larger polygonal scales, and generalized dryness. Usually, these symptoms are not resolved with age [7], and most patients to date have not received effective clinical treatment. X-linked ichthyosis is a recessive genetic disease and female carriers rarely exhibit a cutaneous phenotype, most likely because the region of the X chromosome where the STS gene is located escapes X-inactivation [8–10]. Affected males present with generalized or neonatal peeling at birth or shortly thereafter, and in some severe cases, may develop into “collodion babies” [11, 12]. In addition to abnormal skin appearance, patients with X-linked ichthyosis may have other characteristics, such as severe neuropsychiatric diseases, including recurrent epilepsy, attention deficit hyperactivity disorder, and autism [13]. Abnormalities such as corneal clouding, cryptorchidism, and testicular cancer have also been observed [14–16].
It has been reported that up to 90% cases of X-linked ichthyosis are caused by complete deletion of the STS gene [17–19], while others are caused by point mutations or partial deletions of STS. Additional deletions in the flanking regions usually accompany STS deletion, resulting in a change in the copy number of Xp22.31; this is due to its location on the distal tip of the short arm of the X chromosome. At this time, patients may also develop some manifestations of adjacent gene syndromes, such as short stature, mental retardation, hypogonadism, or loss of smell [7, 20–22]. Differences in the size of chromosomal microdeletions provide an explanation for the phenotypic differences in different families.
Abnormal copy number variants (CNVs) of Xp22.31 were detected mainly by genetic examinations of ichthyosis patients in families or routine examinations of female aborted tissues, but some of them were detected via blood tests of couples. According to the American College of Medical Genetics and Genomics (ACMG) classification, the absence of this region is classified as pathogenic. In an attempt to obtain a healthy infant, couples visited to our hospital for genetic counseling and were advised to use preimplantation genetic testing for monogenic defects (PGT-M) to assist in pregnancy. PGT-M can prevent fetuses from having known genetic diseases. For X-linked recessive genetic diseases, performing specific DNA diagnosis presents advantages over sex determination: non-pathogenic healthy male embryos are not discarded and female carriers can be identified and potentially used for transfer according to the wishes of the patient and the center’s policies [23].
PCR-based whole-genome amplification (WGA) techniques are prone to preferential and non-specific amplification, resulting in allele dropout (ADO). Multiple displacement amplification (MDA) can effectively reduce the risk of ADO and is commonly used in single-cell genomic studies [24, 25]. Currently, chromosomal microarray analysis or copy number variation sequencing (CNV-seq) is used to detect CNVs in individuals. However, at the single-cell level (the analysis of which is limited by the depth of high-throughput sequencing and unbalanced genome-wide amplification), microvariants with copy numbers <5 Mb are prone to underdiagnosis and a marked detection effect is not observed even if the sequencing depth is increased. Therefore, we used method of haplotype linkage analysis by karyomapping to determine whether the embryos had microdeletion variants. In addition, genome-wide aneuploidy testing of embryos from patients undergoing in vitro fertilization (IVF) has been shown to improve the rate of sustained pregnancy [26, 27]. In karyomapping, genome-wide single nucleotide polymorphism (SNP) analysis also supports the detection of aneuploidy in embryos [28].
In the present work, we studied six couples with Xp22.31 microdeletions (containing STS). The strategy reported herein could help identify whether embryos had inherited microdeletion fragments from their parents and could also detect whether embryos were diploid.
Materials and methods
Patients
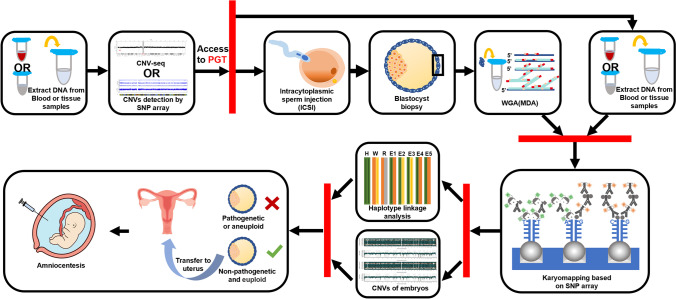
From 2019 to 2022, six couples were recruited in our study for Xp22.31 microdeletions associated with X-linked ichthyosis, at the Reproductive Medicine Center of the First Affiliated Hospital of Zhengzhou University. Inclusion criteria were that one partner in each couple undergoing assisted reproductive technology carried the Xp22.31 microdeletion including the STS gene and its flanking regions, excluding patients with point mutations in the STS gene or complete or partial deletion of the STS gene, and excluding patients in which both couples carried the Xp22.31 microdeletion. All included families had abnormal CNVs associated with X-linked ichthyosis, and siblings or grandparents of embryos were selected as references for this experiment. All couples received assisted reproductive technology (ART), and each couple included an unaffected partner. The subject was approved by the Internal Review Board of The First Affiliated Hospital of Zhengzhou University (Ethic no. 2022-KY-1542) and written informed consent was obtained from all patients. The workflow is shown in Fig. 1.
Fig. 1.
Workflow diagram for detecting carrier status of embryonic microdeletions using the PGT-M strategy
CNV detection and genetic counseling
To confirm the presence of CNVs in the genomes of couples and references, we extracted DNA using the QIAamp DNA Blood Mini Kit (Qiagen, Dusseldorf, Germany) or QIAamp DNA Mini Kit (Qiagen). Subsequently, we obtained CNV results for the sample DNA using the HumanCytoSNP-12 bead chip (Illumina, San Diego, CA, USA) or a CNV-seq platform based on high-throughput sequencing. According to the ACMG guidelines, we evaluated the pathogenicity and performed genetic counseling. PGT-M was recommended for all families to assist in pregnancy.
ART procedure
All couples underwent ART according to the standard procedures. The long-term GnRH agonist protocol was used to superovulate women in the luteal phase, and mature oocytes were fertilized using intracytoplasmic sperm injection (ICSI). Normal fertilized embryos were cultured in vitro for 5–6 days to the blastocyst stage according to the standard sequence, and laser-mediated trophectoderm (TE) cell biopsy was performed. Three to five biopsied cells were removed, washed, and collected in Eppendorf tubes filled with PBS. The blastocysts were vitrified and stored frozen in liquid nitrogen until genetic results were obtained.
WGA of blastocyst cells
Because the amount of DNA in biopsied cells is limited, the genetic testing of blastocyst cells relies on WGA. According to the manufacturer’s protocol (Repli-G Single Cell Kit, Qiagen), we performed WGA on biopsied cells using the MDA method [29], which amplifies genomic DNA from the picogram to nanogram level.
SNP array
SNP array was used to process the genomic DNA of couples, references, and embryos. The purification steps were performed according to the Karyomapping Array Manual, including DNA fragmentation, precipitation, resuspension, hybridization, washing, extension, and staining. Subsequently, the extracted and purified DNA samples (400 ng) were overlaid on a HumanKaryomap-12 bead chip (Illumina). The chip could scan more than 300,000 SNP sites. We used an iScan SQ microarray scanner (Illumina) to scan the chip and imported the data into the BlueFuse Multi software (Illumina) for analysis. Genotypes in the SNP array are represented by the following four values: AA, AB, BB, or NC, where A indicates adenine (A) and thymine (T), B indicates guanine (G) and cytosine (C), and NC indicates no call.
Haplotype linkage analyses
We performed linkage analyses of informative SNPs data from couples and references and established a haplotype associated with the disease. Next, microdeletion and flanking regions were designed as analysis regions, and the selected flanking regions were 1.0 Mb upstream and 1.0 Mb downstream of microdeletion. According to the SNP data of the biopsied TE cells, haplotype linkage analyses were performed to identify the microdeletion carrier status of embryos. When performing linkage analyses, we chose the male genotype as homozygous AA or BB, the female genotype as heterozygous AB, and the reference genotype as homozygous AA or BB.
Aneuploidy detection of embryos
The large amount of raw SNP data obtained from karyomapping allowed the detection of genome-wide CNVs in embryos, which allowed for the detection of aneuploidy in embryos. This method determines whether an embryo is diploid by visualizing the B-allele frequency plots and logarithm R ratio plots.
Frozen embryo transfer and prenatal diagnosis
Non-pathogenic euploid blastocyst was thawed and transferred to the uterus. Approximately 35 days after transfer, ultrasound was performed to check if clinical pregnancy had been achieved. Amniocentesis was performed at week 16–24 of clinical gestation to verify the results of gene detection.
Results
CNV results for couples and references
Six couples who received ART due to Xp22.31 microdeletions associated with X-linked ichthyosis in the Reproductive Medicine Center of the First Affiliated Hospital of Zhengzhou University from 2019 to 2022 were included. In all couples, the affected partners were women; men did not have an abnormal copy number of Xp22.31. Sizes of X chromosomal microdeletions of all included women ranged from 1.60 to 1.73 Mb (Table 1). The references for two families were hemizygous mutants, which were X-linked ichthyosis patients (cases 1 and 6). The references for three families were heterozygous affected, which were microdeletion carriers (cases 2, 4, and 5). The reference for the remaining family was a healthy child, who was the biological daughter of the couple and had no relevant copy number deletion variation (case 3).
Table 1.
Characteristics of the recruited couples
| Case ID | Female age | Female karyotype | Male age | Male karyotype | Affected partner | Microdeletion assessed | Genes involveda | ACMG | Reference source | Reference’s carrying state | Cycle | No. of retrieved oocytes | No. of MII oocytes | No. of biopsied blastocysts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | 46,XX | 34 | 46,XY | Female | del(X)(p22.31)(6488721-8097511)*1 | STS, PNPLA4, PUDP, VCX | P | Female’s father | Sick | 1 | 21 | 19 | 8 |
| 2 | 26 | 46,XX | 28 | 46,XY | Female | del(X)(p22.31)(6420555-8147809)*1 | STS, PNPLA4, PUDP, VCX, VCX2, VCX3A | P | Female’s mother | Mutant-carrying | 1 | 15 | 13 | 6 |
| 3 | 34 | 46,XX | 31 | 46,XY | Female | del(X)(p22.31)(6453050-8131442)*1 | STS, PNPLA4, PUDP, VCX, VCX3A | P | Couple’s daughter | Normal | 1 | 7 | 5 | 2 |
| 4 | 29 | 46,XX | 32 | 46,XY | Female | del(X)(p22.31)(6516735-8131442)*1 | STS, PNPLA4, PUDP, VCX | P | Aborted fetus | Mutant-carrying | 2 | 10 | 9 | 7 |
| 15 | 14 | 9 | ||||||||||||
| 5 | 26 | 46,XX | 25 | 46,XY | Female | del(X)(p22.31)(6527147-8131442)*1 | STS, PNPLA4, PUDP, VCX | P | Aborted fetus | Mutant-carrying | 1 | 12 | 10 | 3 |
| 6 | 30 | 46,XX | 32 | 46,XY | Female | del(X)(p22.31)(6460000-8140000)*1 | STS, PNPLA4, PUDP, VCX, VCX2 | P | Aborted fetus | Sick | 2 | 7 | 6 | 2 |
| 13 | 10 | 3 |
aGenes involved: data from DECIPHER database
ACMG, the American College of Medical Genetics and Genomics
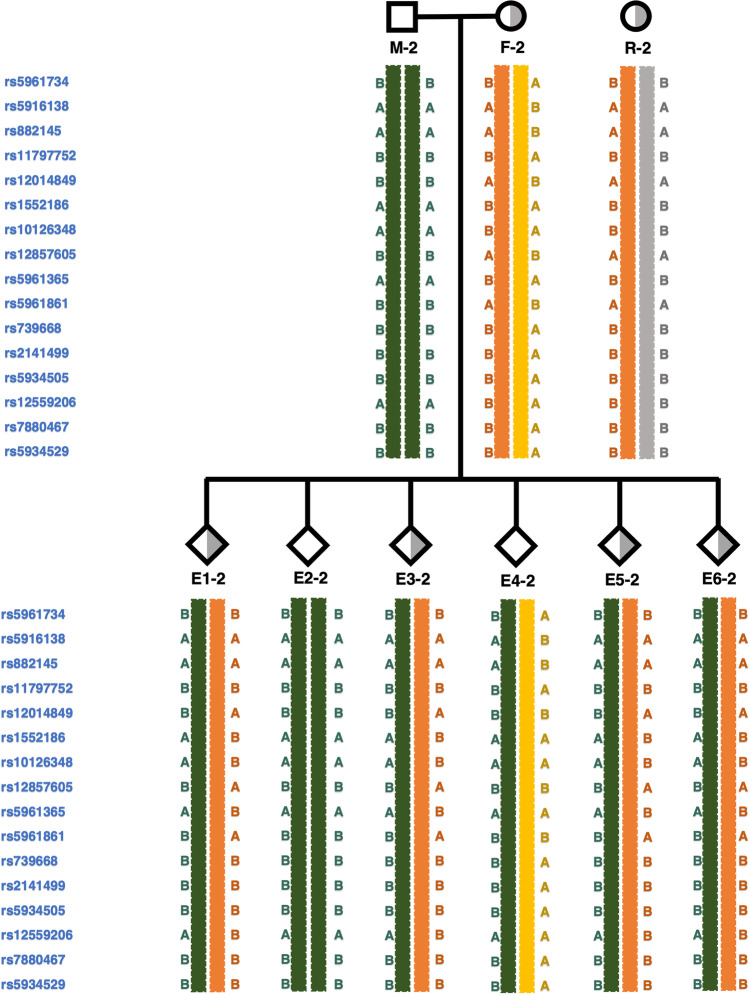
Taking case 2 as an example, both the woman and her mother had a heterozygous deletion of Xp22.31. The variant fragment of the woman was del(X) (p22.31) (6420555-8147809) *1 (Fig. 2a) and the variant fragment of her mother was del (X) (p22.31) (6516735-8131442) *1 (Fig. 2b).
Fig. 2.
Results of copy number variations (CNVs) of the female and her mother in case 2. a CNV result of the female from CNV-seq platform. b CNV result of the female’s mother from SNP microarray platform
Characteristics of couples and WGA
The characteristics of the recruited couples are shown in Table 1. Forty embryos were biopsied from eight PGT-M cycles in six couples. There were two cycles in cases 4 and 6. The average number of blastocyst biopsies per cycle was five. The success rate of MDA amplification in the TE cells was 100% (40/40).
Informative SNP sites and linkage analyses
The average number of informative SNP sites in all six cases was 52.33, and these informative SNP sites are shown in Table 2 and Supplementary Tables 1–5. Haplotype linkage analysis showed that 52.5% (21/40) of embryos carried Xp22.31 microdeletions, and 47.5% (19/40) of embryos were not variant carriers. Notably, the proportion of embryos carrying Xp22.31 microdeletions but presumed not to be pathogenic was 30.0% (12/40). The carrier status of all embryos is listed in Table 3.
Table 2.
The informative SNP sites on the flanks and haplotype linkage analysis of case 2
| Probe ID | Chr | Pos | M-2 | F-2 | R-2 | E1-2 | E2-2 | E3-2 | E4-2 | E5-2 | E6-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs5961734 | 23 | 5462278 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs5916138 | 23 | 5467127 | A A | A B | A A | A A | B B | A A | A B | A A | A A |
| rs882145 | 23 | 5587394 | A A | A B | A A | A A | B B | A A | A B | A A | A A |
| rs11797752 | 23 | 5588984 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs12014849 | 23 | 5629949 | B B | A B | A A | A B | B B | A B | B B | A B | A B |
| rs1552186 | 23 | 5698640 | A A | B A | B B | B A | A A | B A | A A | B A | B A |
| rs10126348 | 23 | 5710628 | A A | B A | B B | B A | A A | B A | A A | B A | B A |
| rs12857605 | 23 | 5714338 | B B | A B | A A | A B | B B | A B | B B | A B | A B |
| rs5961365 | 23 | 5719981 | A A | B A | B B | B A | A A | B A | A A | B A | B A |
| rs5961861 | 23 | 5728176 | B B | A B | A A | A B | B B | A B | B B | A B | A B |
| rs739668 | 23 | 8873607 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs2141499 | 23 | 8896510 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs5934505 | 23 | 8913826 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs12559206 | 23 | 8937050 | A A | B A | B B | B A | A A | B A | A A | B A | B A |
| rs7880467 | 23 | 9097367 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
| rs5934529 | 23 | 9100240 | B B | B A | B B | B B | A A | B B | A B | B B | B B |
Table 3.
Pathogenic carrier status and genome-wide CNVs of embryos
| Case | Cycle | Embryo ID | Embryo days | No. of biopsy cells | CNVs | Carrier status | Recommendation |
|---|---|---|---|---|---|---|---|
| 1 | 1 | E1-1 | D5 | 3-5 | 46,XN | Carrier, high risk | Not transferred |
| 1 | E2-1 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 1 | E3-1 | D6 | 3-4 | 46,XN | Carrier, low risk | Transferred | |
| 1 | E4-1 | D6 | 3-5 | 46,XN | WT | Priority transferred | |
| 1 | E5-1 | D6 | 3-4 | 47,XN,+16 | Carrier, high risk | Not transferred | |
| 1 | E6-1 | D6 | 3-4 | 47,XN,+18 | Carrier, low risk | Not transferred | |
| 1 | E7-1 | D6 | 3-4 | 45,XN,−17 | Carrier, high risk | Not transferred | |
| 1 | E8-1 | D6 | 3-4 | 47,XN,del(6) (q24.1-q27),+16 | Carrier, high risk | Not transferred | |
| 2 | 1 | E1-2 | D5 | 3-5 | 46,XN,+Xq | Carrier, low risk | Not transferred |
| 1 | E2-2 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 1 | E3-2 | D5 | 3-5 | 46,XN | Carrier, low risk | Transferred | |
| 1 | E4-2 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 1 | E5-2 | D5 | 3-5 | 46,XN | Carrier, low risk | Transferred | |
| 1 | E6-2 | D6 | 3-5 | 46,XN | Carrier, low risk | Transferred | |
| 3 | 1 | E1-3 | D6 | 3-5 | 46,XN | WT | Priority transferred |
| 1 | E2-3 | D6 | 3-4 | 46,XN | Carrier, high risk | Not transferred | |
| 4 | 1 | E1-4 | D5 | 3-4 | 45,XN,−22 | WT | Not transferred |
| 1 | E2-4 | D5 | 3-4 | 47,XN,+11 | Carrier, low risk | Not transferred | |
| 1 | E3-4 | D6 | 3-4 | 46,XN,del(5) (q33.1-q35.3) | Carrier, high risk | Not transferred | |
| 1 | E4-4 | D6 | 3-5 | 46,XN | Carrier, high risk | Not transferred | |
| 1 | E5-4 | D6 | 3-4 | 46,XN | WT | Priority transferred | |
| 1 | E6-4 | D6 | 3-5 | 46,XN | WT | Priority transferred | |
| 1 | E7-4 | D6 | 3-4 | 47,XN,del(14) (q12-qter),+16 | Carrier, high risk | Not transferred | |
| 2 | E8-4 | D5 | 3-5 | 46,XN,del(10) (pter-p12.1) | WT | Not transferred | |
| 2 | E9-4 | D5 | 3-5 | 45,XN,−7 | WT | Not transferred | |
| 2 | E10-4 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 2 | E11-4 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 2 | E12-4 | D5 | 3-5 | 46,XN | WT | Priority transferred | |
| 2 | E13-4 | D5 | 3-5 | 47,XN,+15 | Carrier, low risk | Not transferred | |
| 2 | E14-4 | D5 | 3-5 | 46,XN | Carrier, low risk | Transferred | |
| 2 | E15-4 | D6 | 3-5 | 46,XN,del(3) (q24-qter) | WT | Not transferred | |
| 2 | E16-4 | D6 | 3-5 | 46,XN | Carrier, high risk | Not transferred | |
| 5 | 1 | E1-5 | D5 | 3-4 | 47,XN,+1 | Carrier, low risk | Not transferred |
| 1 | E2-5 | D5 | 3-4 | 46,XN | WT | Priority transferred | |
| 1 | E3-5 | D5 | 3-4 | 46,XN | WT | Priority transferred | |
| 6 | 1 | E1-6 | D5 | 3-4 | 46,XN | Carrier, low risk | Transferred |
| 1 | E2-6 | D6 | 3-5 | 48,XN,+1,+3 | WT | Not transferred | |
| 2 | E3-6 | D5 | 3-4 | 46,XN | WT | Priority transferred | |
| 2 | E4-6 | D6 | 3-5 | 41,XO,−10,−14,−16,−20 | WT | Not transferred | |
| 2 | E5-6 | D6 | 3-5 | 46,XN | Carrier, low risk | Transferred |
Taking case 2 as an example, Xp22.31 microdeletions existed in both the woman and her mother, the woman’s mother served as a reference. A total of 16 informative SNP sites were available (Fig. 3 and Table 2). Since the woman carried an X chromosomal microdeletion fragment, the SNP genotypes in this region were homozygous and the woman could not provide maternal informative SNPs to the embryo. Therefore, the informative SNPs we screened only come from the flanking regions. In the linkage analysis of embryo 1, we found the first informative SNP site located in rs5961734, where embryo 1 was B/B, the woman was A/B, the man was B/B, and the woman’s mother was B/B. The woman’s mother had two B alleles, hence the woman inherited the B allele from her mother and the A allele from her father. Since both the woman and her mother were variant carriers, but the woman’s father was normal, it was speculated that the B allele inherited by the woman from her mother was pathogenic. The man could only pass on his exclusive B allele to his offspring; Embryo 1 inherited the non-pathogenic B allele from the man and the pathogenic B allele from the woman. Therefore, the B/B allele of embryo 1 was presumed to be a mutant at the locus rs5961734. We used the same method to infer the remaining SNP sites.
Fig. 3.
Pedigree diagram and haplotype linkage analysis in case 2. M-2, male of case 2, unaffected; F-2, female of case 2, affected; R-2, reference of case 2, affected, female’s mother; E, embryo. The probe ID on the left displays the informative SNPs within 1.0 Mb upstream and 1.0 Mb downstream of microdeletion region, and there are no available informative SNP sites in the microdeletion region itself. Orange stripes indicate pathogenic haplotypes
The haplotype linkage analysis results for all embryos in case 2 are shown in Fig. 3. There were six embryos available for testing; E1-2, E3-2, E5-2, and E6-2 were maternal-variant carriers and were presumed not to be pathogenic, and E2-2 and E4-2 were non-maternal-variant carriers and were also considered to be non-pathogenic.
Embryonic aneuploidy detection
We detected aneuploidy in 40 embryos using the SNP raw data. The results showed that the proportion of euploid embryos reached 60.0% (24/40) and the proportion of aneuploid embryos reached 40.0% (16/40). The results of the genome-wide CNVs for all embryos are shown in Table 3.
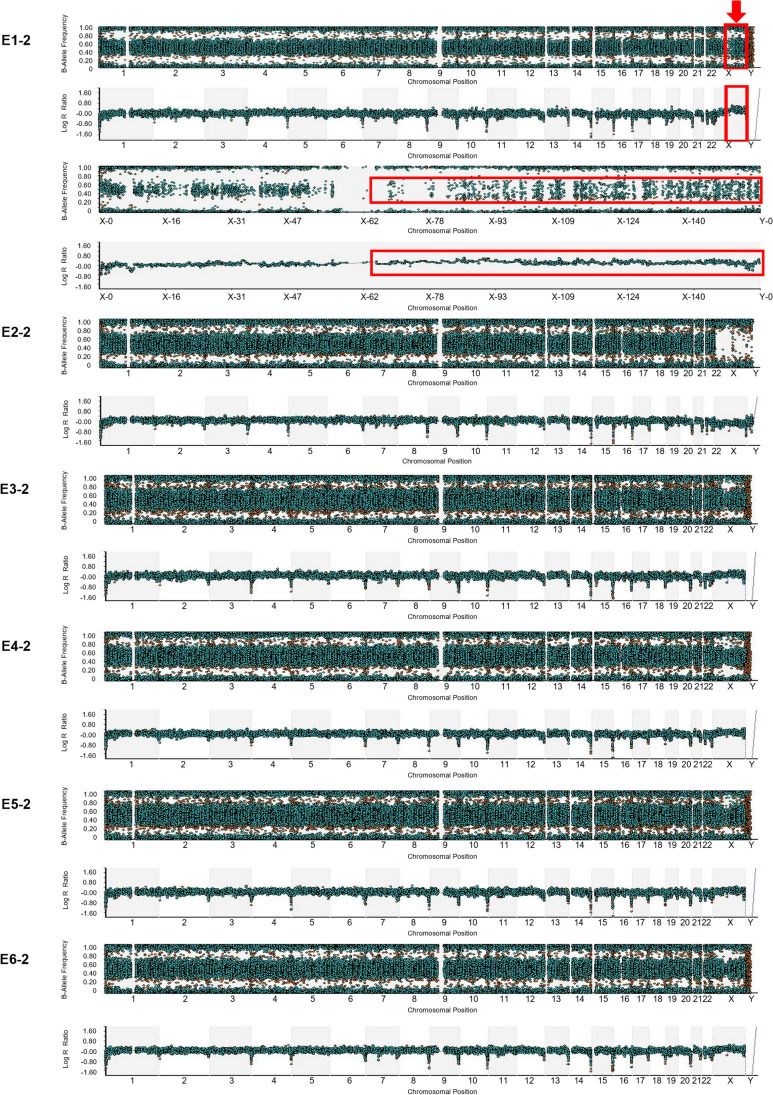
Taking case 2 as an example, embryo 1 exhibited abnormal CNVs, logarithm R ratio plots showed that the long arm of the X chromosome was obviously shifted, and the B-allele frequency plots of this region were scattered compared with other regions (Fig. 4, E1-2), suggesting that the copy number of the long arm of the X chromosome was >2. That is, the embryo E1-2 was aneuploid and could not be transferred. With regard to the other five embryos, embryos E2-2, E3-2, E4-2, E5-2, and E6-2 were identified to be euploid, based on B-allele frequency plots and logarithm R ratio plots (Fig. 4, E2-2, E3-2, E4-2, E5-2, and E6-2).
Fig. 4.
Results of CNV detection of all six embryos in case 2. The results of the B-allele frequency plots and logarithm R ratio plots are presented under strict criteria. The obtained CNV results utilized the raw data of karyomapping microarray. (E1-2) There are AAA, BBB, BAA, and BBA alleles in the long arm region of X chromosome. Compared with the diploid karyotype of other regions, the B-allele frequency plot of the long arm region of X chromosome is scattered. Logarithm R ratio plots show that the long arm region of X chromosome is obviously shifted. (E2-2) This is a normal male embryo. (E3-2, E4-2, E5-2, E6-2) These are normal female embryos
Frozen embryo transfer and validation of results
Through haplotype linkage analyses and aneuploidy detection, a total of 13 euploid embryos without Xp22.31 microdeletions were identified, which could be transferred preferentially. In addition, seven embryos were identified as euploid heterozygotes with Xp22.31 microdeletions, but they were not pathogenic, and hence they could also be transferred. The average number of transferable embryos per PGT-M cycle was 2.5 (Table 3).
All couples underwent frozen embryo transfer, and one embryo was transferred each time. In case 1, the woman received two embryo transfers: the first one did not lead to a clinical pregnancy, but after the second transfer, the woman gave birth to a baby boy with a normal phenotype. However, she refused amniocentesis in the second trimester because she was worried about the risk of pregnancy caused by it. In case 2, the woman underwent an embryo transfer and achieved clinical pregnancy. She underwent amniocentesis in the second trimester of pregnancy, confirming that the fetus did not carry Xp22.31 microdeletions, and successfully gave birth to a healthy baby girl. In case 3, the woman underwent an embryo transfer and became pregnant, but the embryo stopped developing at week 14, and the aborted tissues did not undergo gene analysis. In case 4, the woman underwent two embryo transfers in the first cycle, but neither of them achieved clinical pregnancy. In the second cycle, the woman underwent an embryo transfer and achieved clinical pregnancy, but the embryo stopped developing at week 7 of pregnancy, and the aborted tissues did not undergo gene analysis. In case 5, the woman received an embryo transfer but did not achieve clinical pregnancy. In case 6, the woman did not undergo embryo transfer in the first cycle because the couple did not want to transfer a female embryo carrying a microdeletion variation. Subsequently, an embryo without microdeletion was transferred to this woman during the second cycle, she successfully achieved clinical pregnancy and gave birth to a healthy baby girl, and unfortunately she did not undergo an amniocentesis test.
Discussion
X-linked ichthyosis, primarily caused by the deletion of the STS gene and its flanking regions on the X chromosome, has one of the highest incidences of chromosomal deletions among all genetic disorders [17]. The incidence rates of X-linked ichthyosis are higher in men than in women. Currently, there are few studies on the treatment of X-linked ichthyosis. Some women undergo induced abortion due to presence of pathogenic chromosomal microdeletions in the fetus, which can be physically and emotionally devastating for patients. PGT-M provides a new strategy that can effectively block the vertical transmission of known pathogenic copy number variations with a resolution of <5 Mb.
PGT-M currently uses haplotype linkage analysis by SNP construction to diagnose whether embryos carry pathogenic loci, and the potential mechanisms associated with chromosome microdeletions allow the design of PGT strategies for assisted reproductive conception. Therefore, we used this strategy to achieve PGT-M-assisted pregnancies in patients with chromosomal microdeletion disorders. In the present study, using haplotype linkage analysis by karyomapping, we were able to correctly determine which blastocysts had received chromosomes carrying microdeletions and effectively identify the parental origin of each chromosome locus. Karyomapping makes PGT-M highly accurate and fast and can detect not only unbalanced forms of chromosomal rearrangements but also de novo deletions [30–32]. Moreover, growing evidence suggests that the drastic decline in female IVF success is caused primarily by embryonic aneuploidy [33–35], yet our strategy can identify aneuploidy of embryonic chromosomes based on raw genomic SNP data, thus effectively reducing miscarriage due to embryonic aneuploidy status.
To the best of our knowledge, there have been no reports of using a haplotype linkage analysis strategy by karyomapping to diagnose Xp22.31 microdeletions associated with X-linked ichthyosis. This approach has several advantages. First, using haplotype linkage analysis by karyomapping, the missed diagnosis of microdeletions <5 Mb caused by direct sequencing at the single-cell level can be avoided, in addition to avoiding the risk of ADO. Second, karyomapping based on SNP arrays can simultaneously detect the variant-carrying status and aneuploidy of embryos. Third, WGA of embryonic trophoblast cells using MDA allows human DNA to be amplified from a few picograms to nanograms at the single-cell level. Moreover, the MDA reaction is performed at room temperature and is simple and convenient to operate [36], thus facilitating the detection of SNPs.
Nevertheless, the present study has some limitations. Genome-wide SNP analysis allows karyomapping to support CNVs, which can detect trisomies and monosomies [28]. However, not all aneuploidy events can be detected. NGS technology can provide high-throughput sequencing data rapidly and easily, and complete confirmation of euploidy requires NGS analysis. NGS has now been demonstrated to be reliable for application in PGT-M [37].
Because there is only one X chromosome in the male genome, the haplotype of the X chromosome inherited by the offspring of the male is certain. Thus, we can select the normal offspring of the couple as a reference. More importantly, in the case of an X-linked recessive disorder, embryos from both carriers (female) and non-carriers (male and female) can be transferred. Carrier status is used to classify the priority of embryos, and we give priority to non-carriers. The identity of the carrier will not be made public unless the couple makes it clear that they do not want to transfer the female embryo of the carrier [38].
In conclusion, the current study further confirms that haplotype linkage analysis by karyomapping is a highly effective and feasible method for detection of chromosomal microdeletion in preimplantation embryos, which compensates for the deficiency of the conventional sequencing methods in detecting chromosomal microvariation at the single-cell level. In cases of Xp22.31 microdeletion associated with X-linked ichthyosis, this strategy has successfully resulted in healthy live births, and the advancement of the technique could remarkably improve its clinical applicability. Importantly, this approach may be successfully applied to the preimplantation diagnosis of all chromosomal microvariation diseases, thus avoiding the vertical transmission of pathogenic diseases.
Supplementary information
(DOCX 80 kb)
Acknowledgements
We thank the families who participated and everyone involved in the research for their contributions.
Author contribution
Y.S. and H.S. designed the study; H.J. and W.S. performed embryo culture and biopsy; H.S. collected the data; W.N., X.B., and H.L. analyzed the data; C.C. reviewed the data; J.Y. drafted the manuscript; and H.S. revised the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China for the National Key R&D Program of China (2019YFA0110900).
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
This study was approved by the Internal Review Board of The First Affiliated Hospital of Zhengzhou University (Ethic no. 2022-KY-1542).
Consent to participate
All couples signed informed consent forms for ICSI treatment, PGT, cryopreservation, thawing, and embryo transfer.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingya Yang and Hao Shi contributed equally to this work.
References
- 1.Jöbsis AC, De Groot WP, Tigges AJ, De Bruijn HW, Rijken Y, Meijer AE, et al. X-linked ichthyosis and X-linked placental sulfatase deficiency: a disease entity. Histochemical observations. Am J Pathol. 1980;99(2):279–289. [PMC free article] [PubMed] [Google Scholar]
- 2.Mohandas T, Shapiro LJ, Sparkes RS, Sparkes MC. Regional assignment of the steroid sulfatase-X-linked ichthyosis locus: implications for a noninactivated region on the short arm of human X chromosome. Proc Natl Acad Sci. 1979;76(11):5779–5783. doi: 10.1073/pnas.76.11.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppe G, Marinković-Ilsen A, Rijken Y, De Groot WP, Jöbsis AC. X-linked icthyosis. A sulphatase deficiency. Arch Dis Child. 1978;53(10):803–806. doi: 10.1136/adc.53.10.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster D, France JT, Shapiro LJ, Weiss R. X-linked ichthyosis due to steroid-sulphatase deficiency. Lancet (London, England). 1978;1(8055):8070–8072. doi: 10.1016/s0140-6736(78)90005-3. [DOI] [PubMed] [Google Scholar]
- 5.Pinkova B, Buckova H, Borska R, Fajkusova L. Types of congenital nonsyndromic ichthyoses. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(4):357–365. doi: 10.5507/bp.2020.050. [DOI] [PubMed] [Google Scholar]
- 6.Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Sorèze 2009. J Am Acad of Dermatol. 2010;63(4):607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Crane JS, Paller AS. X-linked ichthyosis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 8.Thauvin-Robinet C, Lambert D, Vaillant G, Caillier P, Donzel A, Cusin V, et al. X-linked recessive ichthyosis in a girl: strategy for identifying the causal mechanism. Br J Dermatol. 2005;152(1):191–193. doi: 10.1111/j.1365-2133.2005.06367.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagtzaam IF, Stegmann AP, Steijlen PM, Herbergs J, Van Lent-Albrechts JA, Van Geel M, et al. Clinically manifest X-linked recessive ichthyosis in a female due to a homozygous interstitial 1·6-Mb deletion of Xp22.31. Br J Dermatol. 2012;166(4):905–907. doi: 10.1111/j.1365-2133.2011.10685.x. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM, Williams ML, Choi EH, Feingold KR. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis. Biochim Biophys Acta. 2014;1841(3):353–361. doi: 10.1016/j.bbalip.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10(6):351–364. doi: 10.2165/11311070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Oji V, Preil ML, Kleinow B, Wehr G, Fischer J, Hennies HC, et al. S1 guidelines for the diagnosis and treatment of ichthyoses - update. J Dtsch Dermatol Ges. 2017;15(10):1053–1065. doi: 10.1111/ddg.13340. [DOI] [PubMed] [Google Scholar]
- 13.Traupe H. Revealing the mysteries of X-linked recessive ichthyosis. Br J Dermatol. 2018;179(4):821–822. doi: 10.1111/bjd.16821. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62(3):480–485. doi: 10.1016/j.jaad.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Ingordo V, D’Andria G, Gentile C, Decuzzi M, Mascia E, Naldi L. X-linked ichthyosis in southern Italy. J Am Acad Dermatol. 2003;49(5):962–963. doi: 10.1016/S0190-9622(03)00768-0. [DOI] [PubMed] [Google Scholar]
- 16.Lykkesfeldt G, Høyer H, Lykkesfeldt AE, Skakkebaek NE. Steroid sulphatase deficiency associated with testis cancer. Lancet (London, England). 1983;2(8365-66):1456. doi: 10.1016/S0140-6736(83)90801-2. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Martín A, González-Sarmiento R, De Unamuno P. X-linked ichthyosis: an update. Br J Dermatol. 1999;141(4):617–627. doi: 10.1046/j.1365-2133.1999.03098.x. [DOI] [PubMed] [Google Scholar]
- 18.Cuevas-Covarrubias SA, Kofman-Alfaro SH, Maya-Núñez G, Díaz-Zagoya JC, Orozco OE. X-linked ichthyosis in Mexico: high frequency of deletions in the steroid sulfatase encoding gene. Am J Med Genet. 1997;72(4):415–416. doi: 10.1002/(SICI)1096-8628(19971112)72:4<415::AID-AJMG8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Saeki H, Kuwata S, Nakagawa H, Shimada S, Tamaki K, Ishibashi Y. Deletion pattern of the steroid sulphatase gene in Japanese patients with X-linked ichthyosis. Br J Dermatol. 1998;139(1):96–98. doi: 10.1046/j.1365-2133.1998.02320.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohyama A, Nakano H, Imanishi Y, Seto T, Tsuruta D, Fukai K. A novel missense mutation of the STS gene in two siblings with X-linked ichthyosis, complicated by short stature, bone density reduction, epilepsy, and cryptorchidism. Clin Exp Dermatol. 2019;44(1):78–79. doi: 10.1111/ced.13741. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy S, Kapoor S, Yadav S. Nephrotic syndrome with X-linked ichthyosis, Kallmann Syndrome and unilateral renal agenesis. Indian Pediatr. 2007;44(4):301–303. [PubMed] [Google Scholar]
- 22.Diociaiuti A, Angioni A, Pisaneschi E, Alesi V, Zambruno G, Novelli A, et al. X-linked ichthyosis: clinical and molecular findings in 35 Italian patients. Exp Dermatol. 2019;28(10):1156–1163. doi: 10.1111/exd.13667. [DOI] [PubMed] [Google Scholar]
- 23.De Rycke M, Berckmoes V. Preimplantation genetic testing for monogenic disorders. Genes (Basel). 2020;11(8):871. doi: 10.3390/genes11080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendez P, Fang LT, Jablons DM, Kim IJ. Systematic comparison of two whole-genome amplification methods for targeted next-generation sequencing using frozen and FFPE normal and cancer tissues. Sci Rep. 2017;7(1):4055. doi: 10.1038/s41598-017-04419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 26.Dahdouh EM, Balayla J, García-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod Biomed Online. 2015;30(3):281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Scott RT, Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100(3):624–630. doi: 10.1016/j.fertnstert.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Sabria-Back J, Monteagudo-Sánchez A, Sánchez-Delgado M, Ferguson-Smith AC, Gómez O, Pertierra Cartada A, et al. Preimplantation genetic testing for a chr14q32 microdeletion in a family with Kagami-Ogata syndrome and Temple syndrome. J Med Genet. 2022;59(3):253–261. doi: 10.1136/jmedgenet-2020-107433. [DOI] [PubMed] [Google Scholar]
- 29.Spits C, Le Caignec C, De Rycke M, Van Haute L, Van Steirteghem A, Liebaers I, et al. Whole-genome multiple displacement amplification from single cells. Nat Protoc. 2006;1(4):1965–1970. doi: 10.1038/nprot.2006.326. [DOI] [PubMed] [Google Scholar]
- 30.Natesan SA, Bladon AJ, Coskun S, Qubbaj W, Prates R, Munne S, et al. Genome-wide karyomapping accurately identifies the inheritance of single-gene defects in human preimplantation embryos in vitro. Genet Med. 2014;16(11):838–845. doi: 10.1038/gim.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giménez C, Sarasa J, Arjona C, Vilamajó E, Martínez-Pasarell O, Wheeler K, et al. Karyomapping allows preimplantation genetic diagnosis of a de-novo deletion undetectable using conventional PGD technology. Reprod Biomed Online. 2015;31(6):770–775. doi: 10.1016/j.rbmo.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Beyer CE, Lewis A, Willats E, Mullen J. Preimplantation genetic testing using Karyomapping for a paternally inherited reciprocal translocation: a case study. J Assist Reprod Genet. 2019;36(5):951–963. doi: 10.1007/s10815-019-01413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23(12):2806–2812. doi: 10.1093/humrep/den217. [DOI] [PubMed] [Google Scholar]
- 34.Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–1703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 35.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–63.e1. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Yan J, Feng J, Hosono S, Sommer SS. Assessment of multiple displacement amplification in molecular epidemiology. Biotechniques. 2004;37(1):136-8–140-3. doi: 10.2144/04371DD04. [DOI] [PubMed] [Google Scholar]
- 37.Treff NR, Fedick A, Tao X, Devkota B, Taylor D, Scott RT., Jr Evaluation of targeted next-generation sequencing-based preimplantation genetic diagnosis of monogenic disease. Fertil Steril. 2013;99(5):1377–84.e6. doi: 10.1016/j.fertnstert.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Dimitriadou E, Melotte C, Debrock S, Esteki MZ, Dierickx K, Voet T, et al. Principles guiding embryo selection following genome-wide haplotyping of preimplantation embryos. Hum Reprod. 2017;32(3):687–697. doi: 10.1093/humrep/dex011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 80 kb)
Data Availability Statement
The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request.