Abstract
The endoplasmic reticulum (ER) response mechanism to cellular stress is mediated by the unfolded protein response/ER-associated degradation (UPR/ERAD) pathway. A viral infection can trigger ER stress and engage some transcription factors, depending on the host cell and virus type, activating or inhibiting autophagy. The relationship between ER response and autophagy in rabies has not been investigated yet. In the present study, the mouse brain was infected with street rabies virus (SRABV). Total RNA was extracted from the brains of animals, and cDNA was synthesized. Next, real-time PCR assay was performed using specific primers. The expression of hypoxanthine–guanine phosphoribosyltransferase (Hprt), CCAAT/enhancer binding protein homologous protein (CHOP), apoptosis signal-regulating kinase 1 (ASK1), activating transcription factor 6 (ATF6), and caspase 3 (CASP3) genes was also investigated. Based on the results, SRABV caused significant changes in the mRNA expression of ATF6, CHOP, and ASK1 genes in the brains of infected mice in the control group (group V). Treatment of infected cells with the pIRES-EGFP-Beclin-1 vector and rapamycin caused changes in nearly most of the parameters. However, alterations in CASP3 gene expression were only observed when the vector and the virus were simultaneously injected into the cells. Overall, protection and autophagy against cell death induced by SRABV infection can be achieved by activating the ER stress pathway, followed by a marked increase in the expression of ATF6, CHOP, ASK1, and CASP3 genes.
Keywords: Autophagy, Unfolded protein response, Street rabies virus, Host cell, Transcription factors
Introduction
The endoplasmic reticulum (ER) is an important cellular organelle in eukaryotic cells. ER dysfunction may occur under cellular stress conditions, due to which ER loses its ability to modulate protein folding, resulting in the accumulation of unfolded and misfolded proteins in the lumen and imbalance in calcium homeostasis. The ER response mechanism to stress is mediated by the unfolded protein response/ER-associated degradation (UPR/ERAD) pathway, which prevents protein translation and then activates the transcription of genes, thereby enhancing the folding potency of ER proteins.
Activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1α (IRE1α), as two important molecules, mediate separate signaling pathways to provide an allied response to ER stress with activation of UPR (Ron and Walter 2007). However, binding of ER sensors to binding immunoglobulin protein (BiP) and its continuous activation can lead to its interaction with tumor necrosis factor receptor-associated factor 2 (TRAF2) and result in the regular and programmed destruction of cells (apoptosis), which requires the coordinated interaction and activation of two pathways. The interaction with TRAF2 results in the activation of apoptosis signal-regulating kinase 1 (ASK1) and ultimately causes autophagy (Adams et al. 2019). Autophagy is a highly conserved process of degradation, which occurs at low levels in many normal eukaryotic cellular events or inhibits the survival of pathogens in infected macrophages (Ghavami et al. 2015). Beclin-1 is an important 52-kDa protein, which functions as a molecular signal in neurodegeneration, tumorigenesis, and autophagic cell death. The rabies virus is a negative-sense RNA virus with a 12-kb genome, which belongs to the Rhabdoviridae family and the genus Lyssavirus. The present study aimed to survey the effects of ER stress caused by SRABV, a pathogenic wild-type strain of rabies, for the first time, to determine how it differs from the pathogenic laboratory and vaccine strains.
Materials and methods
The animals used in this study were maintained according to the guidelines for animal care by the Institutional Animal Ethics Committee of Pasteur Institute of Iran (certificate No.: IR.PII. REC.1395.49). The accession number of SRABV strain used in this study is KX148186 in the GenBank (Hosseini Heydarabadi et al. 2020). A total of 70 male NMRI mice, aged 21 days, with a mean weight of 16 ± 2 g, were examined in the current study. The mice were divided into seven groups. The specifications and amounts of the virus, vectors, or drugs injected to each group are presented in Table 1. All materials were injected via unilateral intracerebroventricular (ICV) injections. The direct fluorescent antibody (DFA) test was performed in all groups, except for R and T groups and comparisons with the positive and negative controls confirmed SRABV infection in mice. Tissue sections were collected from the brains of malad (sick), paralysis, and prostration (moments before death) mice in each group, as described in the World Health Organization laboratory techniques in rabies protocol (World Health Organization, Rupprecht et al. 2018). Tissue sections were fixed for 30 minutes in cold acetone and staining was carried out with adding an anti-rabies nucleocapsid polyclonal antibody, conjugated with fluorescein isothiocyanate (FITC, BioRad, Canada) for 30–60 minutes in 37 °C. Then, tissue sections were washed in phosphate-buffered saline pH 7.4 for 5 minutes. A known positive mouse brain sample (from a CVS-11 infected mouse as the positive control) and a 3-week-old normal mouse brain sample (as the negative control) from the WHO Collaborating Center for Reference and Research on Rabies were used in this study. Finally, the stained tissue sections were examined under a UV microscope (40 × magnification).
Table 1.
The seven groups of mice evaluated in this study. A total of 70 male NMRI mice, aged 21 days, with a mean weight of 16 ± 2 g, were divided into seven groups
| Group | V | R | T | A | B | D | E |
|---|---|---|---|---|---|---|---|
| Description | SRABV (LD50 8.398) | Rapamycin 40 μg/μL | 3-Methyladenine 400 nM | Injection of 3 µg pIRES-EGFP-Beclin1 vector, 12 h before SRABV | Simultaneous injection of 3 µg of vector and SRABV | Simultaneous injection of 3 µg of vector and SRABV and rapamycin | Simultaneous injection of 3 µg of vector and SRABV and 3-methyladenine |
Total RNA was extracted from the mouse brain tissue, using an All-In-One DNA/RNA/Protein Miniprep Kit (Bio Basic Inc., Markham, Ontario, Canada). Next, cDNA was synthesized (AddScript cDNA Synthesis Kit, ADDBio Inc., Korea). Following reverse transcription, a Rotor-Gene Real-Time qPCR system was used to evaluate the expression of candidate genes (Qiagen™, Germany) (Table 2). The Genevestigator program (NEBION, Switzerland) was also used to select hypoxanthine–guanine phosphoribosyltransferase (Hprt) for normalizing the target genes. The relative quantitative ΔΔCT method was applied to evaluate the expression of target genes. To control for the false discovery rate, the Benjamini–Hochberg procedure was carried out (Benjamini and Hochberg 1995). To plot differences between the groups and control treatment, the ggplot2 package was used (Villanueva and Chen 2019). Statistical analysis was performed in R software (v4.1.1, 2021, R Core Team).
Table 2.
Primers were used throughout this study
| Gene | Primer | Product size | Used for RT-PCR |
|---|---|---|---|
| Hprt | Forward: 5′ CTCAACTTTAACTGGAAAGAATGT 3′ | 99 bp | 33 |
| Reverse: 5′ GGGCTGTACTGCTTAACC 3′ | |||
| CHOP | Forward: CCAGGAAACGAAGAGGAAGAATCA | This study | |
| Reverse: ATGTGCGTGTGACCTCTGTTG | |||
| ASK-1 | Forward: GCCATGTTCAAGGTGGGGA | This study | |
| Reverse: CTCCGCCGACATGGACTCTG | |||
| ATF-6 | Forward: GACAACCAGAAAGACAGTTACAGCTA | This study | |
| Reverse: AGGTGGAGGCATATAAAGCAATGG | |||
| Casp3 | Forward: TGGACTCTGGGATCTATCTGGAC | This study | |
| Reverse: TCCGTACCAGAGCGAGATGAC |
Results and discussion
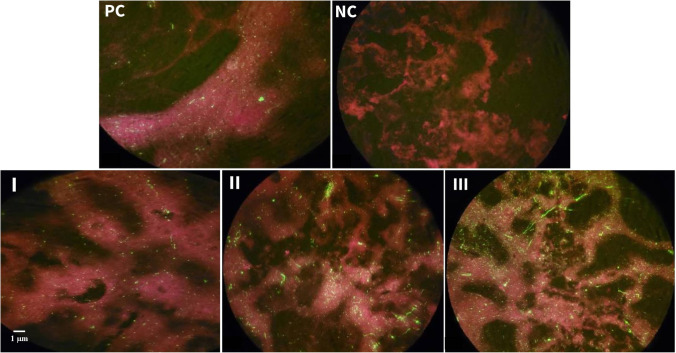
DFA test and comparisons with positive and negative controls confirmed SRABV infection in mice with condition of malad, paralysis, and prostration. Moreover, the examination of stained tissue slides under UV microscopy showed that in all groups, except R and T groups, and all stages of rabies disease (malad, paralysis, prostration) the Negri bodies were visible (Fig. 1-I, II, III). The Negri bodies were found as round or oval green fluorescent inclusions within the cytoplasm of rabies infected cells.
Fig. 1.
Fluorescent antibody test. The background color is red because Evans blue was added as counterstain for immunofluorescence staining with FITC labeled antibodies. Positive control (PC), Negri bodies are visible as bright green spots abundantly. This indicates infection with SRABV. Negative control (NC), no fluorescent spot indicating the absence of rabies infection. Green fluorescent spot appears increasingly in samples of the brain cells of the malad (I), paralysis (II), and prostration (III) mice. It indicates the presence of rabies infection in the tested mice groups
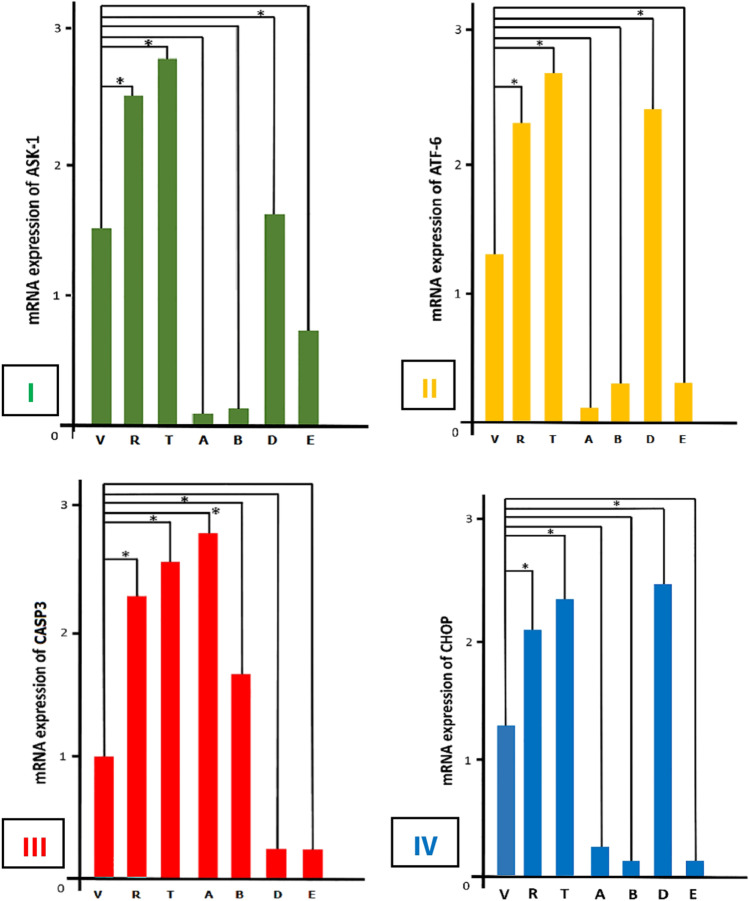
Based on the present results, during SRABV infection, all parameters, including the expression of ASK1, ATF6, and CHOP genes, except CASP3, increased in the control group, which consisted of mice only receiving the virus. Moreover, the effects of both activator and inhibitor drugs of autophagy were compared with the control group. The results indicated significant differences in the expression of ASK1, ATF6, CHOP, and CASP3 genes when rapamycin and 3-methyladenine (3-MA) were administered. Changes in these four parameters were incremental and significant in both rapamycin (R) and 3-MA (T) groups (Table 1). Comparison of the relative mRNA expression of ASK1 gene in the brains of tested mice only indicated a significant change in group D (p < 0.05) (Fig. 2-I). In this study, group D included mice receiving a virus, a pIRES-EGFP-Beclin-1 vector, and rapamycin simultaneously. Additionally, the ATF6 mRNA expression was examined, and a significant difference was observed in group D (p < 0.05) (Fig. 2-II).
Fig. 2.
(I) Comparison of relative mRNA expression of ASK-1 in the brain of 7 groups of mice. Changes in rapamycin (autophagy stimulant) and 3-MA (autophagy inhibitor) groups were significant compared to the control (SRABV) group (*, p < 0.05). Only in group D, mRNA expression of Ask-1 gene showed significant change (*, p < 0.05). Group V: street rabies virus. Group R: rapamycin. Group T: 3-methyladenine. Group A: pIRES-EGFP-beclin1 vector in vivo transfection was 12 h before virus injection. Group B: pIRES-EGFP-beclin1 vector and virus injection was simultaneously. Group D: virus, pIRES-EGFP-beclin1 vector, and rapamycin injected together. Group E: virus, pIRES-EGFP-beclin1 vector, and 3-methyladenine injected together. (II) Comparison of relative mRNA expression of ATF-6 in all groups. The changes in rapamycin and 3-MA groups have increased compared to the control group (SRABV) (*, p < 0.05). Also, mRNA expression of the ATF-6 gene in group D showed significant changes (*, p < 0.05). (III) Comparison of relative mRNA expression of CASP3 in tested groups. As expected, changes in rapamycin and 3-MA groups were increased significantly compared with the control (SRABV) group (*, p < 0.05). mRNA expression of the CASP3 gene in groups A and B showed significant changes (*, p < 0.05). (IV) Comparison of relative mRNA expression of CHOP in all groups of mice. Significant incremental changes were observed in rapamycin and 3-MA groups compared with the control (SRABV) group (*, p < 0.05). Meanwhile, the study of CHOP mRNA expression in group D showed significant changes (*, p < 0.05)
The analysis of CASP3 gene expression revealed a significant difference between group A and group B (p < 0.05) (Fig. 2-III). In this study, group A received pIRES-EGFP-Beclin-1 vector at 12 hours before SRABV inoculation, whereas group B simultaneously received the vector and the virus. According to Fig. 2-IV, significant changes were observed in CHOP gene expression in group D (p < 0.05); nevertheless, the CHOP gene expression in this group was similar to that of group R and group T. Since the current study is the first examination of the effects of SRABV on the mouse nervous system cells in the laboratory, it is important to compare the present results with those of previous studies on other viruses.
The results indicated the high expression of ASK1 mRNA in group D. This finding was predictable, because SRABV, a pathogenic virus, activates autophagy to preserve neurons and consequently, increases viral replication. Therefore, SRABV activates the IRE1α pathway, and an increase in ASK1 gene expression causes autophagy. Besides, a significant increase was observed in the mRNA expression of ATF6 gene in group D. Overall, the presence of abnormal proteins leads to the production of GRP78, which activates ATF6 gene and stimulates UPR in response to ER stress. Previous or simultaneous injection of pIRES-EGFP-Beclin-1 and SRABV did not cause any significant differences in response to ER stress. The injection of both pIRES-EGFP-Beclin-1 and SRABV increased CASP3 gene expression; significant changes were only observed in group A and group B. Expectedly, the increase in CASP3 gene expression led to apoptosis.
Considering the changes in CHOP gene expression, they were only significant in group D. This might be due to the activation of IRE1α pathway or abnormal protein production during SRABV infection, resulting in the increased expression of ATF6 gene. It was concluded that SRABV infection can increase the level of abnormal proteins and ER stress, which may lead to the activation of UPR/ERAD pathway. Although the activation of this pathway initially prolongs cell survival through autophagy, it causes apoptosis and cell death as the infection progresses. Further detailed research is required to investigate the mechanism of ER stress during wild-type rabies infection.
Abbreviations
- ATF6
Activating transcription factor 6
- AMPK
Adenosine monophosphate-activated protein kinase
- ASK1
Apoptosis signal-regulating kinase 1
- BiP
Binding immunoglobulin protein
- CASP3
Caspase 3
- CHOP
CCAAT/enhancer binding protein homologous protein
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- DFA
Direct fluorescent antibody test
- GRP78
Glucose-regulated protein 78
- Hprt
Hypoxanthine-guanine phosphoribosyltransferase
- IRE1α
Inositol-requiring enzyme 1α
- ROS
Reactive oxygen species
- qPCR
Real-time/quantitative PCR
- SRABV
Street rabies virus
- 3-MA
3-Methyladenine
- TRAF2
Tumor necrosis factor receptor-associated factor 2
- UPR
Unfolded protein response
Author contribution
FS was responsible for study conceptualization, funding acquisition, project administration, and writing, reviewing, and editing the manuscript; SP was responsible for the molecular experiments, data curation, and writing the original draft of the manuscript; KB contributed to the conceptualization and supervision of the project; SG performed the statistical analysis; RS plotted the tables and charts; MSK developed the methodology of the study and animal experiments; and MF arranged the references and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was financially supported by a grant (No. 564) from Pasteur Institute of Iran (Tehran, Iran).
Data Availability
Data will be made available if requested.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Some of the results presented here were first published in a preprint (Poorghobadi et al. 2022).
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shima Poorghobadi, Email: shimapoorghobadi@gmail.com.
Kazem Baesi, Email: kbaesi@gmail.com.
Safoora Gharibzadeh, Email: safoora.gharibzadeh@gmail.com.
Reza Shirzad, Email: shirzadreza07@gmail.com.
Mohammad S. Khosravy, Email: mskhosravy@gmail.com
Maryam Fazeli, Email: fazelim9@gmail.com.
Farzaneh Sheikholeslami, Email: f_sheikh@pasteur.ac.ir.
References
- Adams CJ, Kopp MC, Larburu N, et al. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR Statist Soc: Series B (methodological) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Ghavami S, Cunnigton RH, Gupta S, Yeganeh B, Filomeno KL, et al. Autophagy is a regulator of TGF-β1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis. 2015;6(3):e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini Heydarabadi F, Baesi K, Bashar R, Fazeli M, Sheikholeslami F. A phylogenetic study of new rabies virus strains in different regions of Iran. Virus Genes. 2020;56(3):361–368. doi: 10.1007/s11262-020-01752-6. [DOI] [PubMed] [Google Scholar]
- Poorghobadi S, Baesi k, Gharibzadeh S, et al (2022) Autophagy and unfolded protein response induction: crosstalk between street rabies virus and host. Research Square. 10.21203/rs.3.rs-1549416/v1 [DOI] [PMC free article] [PubMed]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Villanueva RA, Chen ZJ. ggplot2: elegant graphics for data analysis (2nd ed.) Meas: Interdisciplinary Research and Perspectives. 2019;17(3):160–167. doi: 10.1080/15366367.2019.1565254. [DOI] [Google Scholar]
- World Health Organization, Rupprecht CE, Fooks AR, Abela-Ridder B et al (2018) Laboratory techniques in rabies, volume 1, 5th ed. World Health Organization. https://apps.who.int/iris/handle/10665/310836.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available if requested.