Abstract
The endometrium is a dynamic tissue that undergoes extensive remodeling during the menstrual cycle and further gets modified during pregnancy. Different kinds of stem cells are reported in the endometrium. These include epithelial stem cells, endometrial mesenchymal stem cells, side population stem cells, and very small embryonic-like stem cells. Stem cells are also reported in the placenta which includes trophoblast stem cells, side population trophoblast stem cells, and placental mesenchymal stem cells. The endometrial and placental stem cells play a pivotal role in endometrial remodeling and placental vasculogenesis during pregnancy. The dysregulation of stem cell function is reported in various pregnancy complications like preeclampsia, fetal growth restriction, and preterm birth. However, the mechanisms by which it does so are yet elusive. Herein, we review the current knowledge of the different type of stem cells involved in pregnancy initiation and also highlight how their improper functionality leads to pathological pregnancy.
Graphical Abstract
Keywords: Endometrial stem cells, Placental stem cells, Trophoblast stem cell, Placental mesenchymal stem cells, Pathological pregnancy
Introduction
Embryo implantation involves an intricate series of events that creates the connection between maternal and embryonic tissues [1]. After fertilization, the zygote goes through multiple stages of division and morphogenesis to form a blastocyst 4–5 days post-fertilization. A blastocyst is composed of two different cell lineages: the outer specialized trophectodermal epithelium and the inner cell mass [2]. At implantation, the free-floating mature blastocyst attaches to the receptive endometrium and invades the stroma to establish the placenta. Implantation requires synchrony between the embryo and the endometrium. Hence, successful implantation demands a competent embryo, receptive endometrium, and embryo-endometrium synchrony [1, 3].
At the time of apposition, the blastocyst differentiates into the inner cell mass (embryo) and the outer trophectoderm (TE), which forms the placenta (Fig. 1). After embryo implantation, the trophectoderm proliferates to form the cytotrophoblast shell. The cells of the cytotrophoblastic shell can differentiate into either extravillous trophoblast (EVT) or syncytiotrophoblast (Fig. 1), depending on the signals they receive [4]. EVT facilitates the placental anchoring to the uterine wall and also remodels maternal spiral arterioles (Fig. 1) to provide plenty of blood supply to the growing fetus [4]. Thus, the spatial-temporal regulation of trophoblast differentiation and invasion is indispensable for successful placentation. Failure of blastocyst adhesion and implantation leads to pregnancy failure and recurrent pregnancy losses [5]. Impaired trophoblast invasion reinforces common pregnancy disorders affecting both mother and fetus, such as pre-eclampsia and/or intrauterine growth restriction [6].
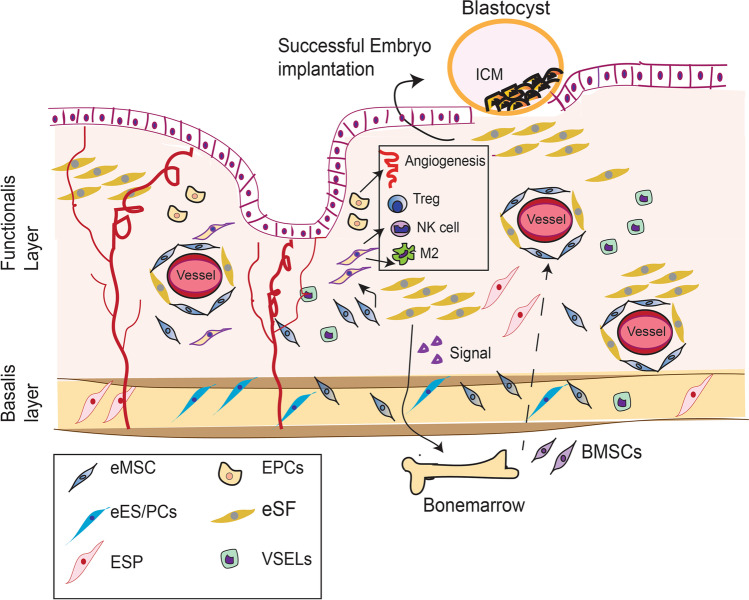
Fig. 1.
Depicts how endometrial stem cells contribute in successful pregnancy. In response to eSCs-derived factors, BM-MSCs migrate to the human endometrium and contribute to the stem cell pool. They differentiate into eSFs and maintain immunosuppressive environment by triggering anti-inflammatory T-regulatory (Treg) or alternatively activated M2 macrophage polarization. EPCs and eSFs also regulate angiogenesis and vasculogenesis, critical for successful embryo implantation. eES participates in endometrial epithelial regeneration after each menstrual cycle, which makes endometrium susceptible for embryo implantation
Both embryo and endometrium adopt different strategies for successful implantation. In order to adhere to the luminal epithelium of the endometrium, blastocyst exploits their receptor to interact with the oligosaccharide ligands l-selectin on endometrial epithelia. It has been reported that the embryo secretes extracellular vesicle (EV)-bound miRNAs which target the endometrial genome to support apposition [1]. Embryo implantation is associated with endometrial stromal cell decidualization, which also occurs after ovulation during the menstrual cycle [7, 8]. Ovarian steroids regulated decidualization in the endometrium where elongated fibroblast-like endometrial stromal cells (eSCs) differentiate into a more rounded and extremely specialized secretory epithelioid cell type [1, 9]. This event is also accompanied by uterine natural killer cell influx and vascular remodeling. Disrupted endometrial decidualization is associated with recurrent implantation failure (RIF) and pregnancy losses [10, 11].
Although a plethora of cellular events in embryo-endometrium cross-talk has been identified by multiple research groups, a comprehensive understanding of the molecular mechanism underpinning embryo implantation is far from clear. Different studies speculated on the role of embryonic and adult stem cells in the regulation of endometrium regeneration and placental function.
The human placenta and endometrium undergo rapid growth and differentiation throughout the gestation period, suggesting the involvement of placental and uterine stem cells in the regulation of successful pregnancy. The endometrial MSCs and endometrial epithelial progenitor cells aid in endometrial regeneration, and trophoblast stem cells and placental MSCs play a pivotal role in placental development. Different growth factors and extracellular matrix components maintain the stemness of these cells [12].
The purpose of this review is to convey/organize our current understanding of how stem cells contribute to successful embryo implantation and placentation. We further review how their malfunction triggers pathological pregnancy, and how these stem cells can be utilized to rectify pregnancy-related disorders. For the sake of simplicity, most of the article focuses on human system unless otherwise stated.
Types of stem cells in the endometrium and placenta
To date, four different kinds of stem cells are identified in the endometrium. These include epithelial stem cells, endometrial mesenchymal stem cells, side population stem cells, and very small embryonic-like stem cells [13–15] (Fig. 1). Among these, the endometrial mesenchymal stem cells are well characterized in endometrial remodeling during the menstrual cycle [16]. However, their specific role in the process of embryo implantation and pregnancy initiation is unknown. Since these events take place in the most inaccessible part of the mammalian body, the major restriction of these studies is the inaccessibility of the experimental model. Hence, most studies are conducted ex situ, and the interpretation is based on in vitro assays.
Endometrial stem cells (eSCs)
The endometrium is composed of an upper functionalis layer and an underlying basalis layer. During menstruation or parturition, the upper functionalis layer is sloughed off, the basalis layer remains intact and is thought to be a reservoir of the adult stem, progenitor cells to support cyclic regeneration of the endometrial functionalis layer [17]. Although recent studies have identified multiple lineage-restricted stem/progenitor populations located within the functionalis layer as well [18], initially, it was believed that a single type of adult stem cells presents in the endometrium with the colony-forming capacity [19, 20]. Later on, multiple studies reported the existence of various subpopulations of stem cells in the epithelial or stromal compartment of the endometrium [21–24].
Endometrial mesenchymal stem cells (eMSCs)
Origin
Schwab and colleagues first identified human endometrium-resident large colony-forming cells (0.02% of total stroma), which were assumed to be MSC-like cells participating in the stroma and vascular system regeneration [19]. The source of endometrial mesenchymal stem cells (eMSCs) is controversial. Few groups of researchers claimed residual fetal stem cells as eMSCs’ endogenous source [25], whereas others identified bone marrow as the potential source of eMSCs. The latter group reported that embryo implantation induces the migration of MSCs from bone marrow, which contributes to decidual stroma formation [26].
Markers
Perivascular markers CD146 and PDGF-Rβ are considered to be the key markers for isolating large colony-forming unit (CFU) from the endometrium perivascular niche. These cells express MSC-specific surface receptors (CD29+, CD44+, CD73+, and CD90+; ~ 20% expressed CD105+ and CD146+; and CD31−, CD34−, and CD45−) and possess mesenchymal lineage differentiation capacity (21, [12]. However, they do not express mesenchyme precursor cell marker Stro-1 [12]. Interestingly, CD146+ and PDGF-Rβ+ cells can be isolated not only from the functionalis and basalis layer but also from the menstrual blood [21]. In addition, with these markers, the sushi domain containing 2 proteins (SUS2D) is also considered an eMSCs marker and is now commonly used for isolating eMSCs from human endometrial tissue [13]. W5C5+ cells which are located in the perivascular location in both basal and functional layers of the endometrium, showed MSC-like differentiation potential and high clonogenicity, suggesting W5C5’s capacity to serve as the possible eMSCs marker [14].
Despite the identification of many eMSCs markers, there is controversy regarding their specificity, since many eMSCs’ markers are also expressed by stromal fibroblasts [15]. Hence, more extensive studies are required to isolate pure eMSCs with high clonogenicity.
Regulation of stemness
The interplay between Notch and Wnt signaling pathways regulates eMSCs’ stemness and activities [16]. The Notch signaling pathway is well-defined in the regulation of tissue homeostasis and maintenance of somatic stem cells [17]. Different Notch family members such as Notch1-4 which are expressed across the menstrual cycle in human endometrium [19], as well as augmented Notch signal activation in eMSCs [20], suggest their role in maintaining eMSCs’ functionality. Interestingly, Notch deactivation is associated with endometriosis and defective decidualization [20, 21]. Whether the loss of Notch in the eMSCs contributes to the defective decidualization or it is a cell-autonomous effect is yet to be dissected.
Similar to Notch signaling Wnt/β-Catenin pathway is also known to be involved in the self-renewal and maintenance of somatic stem cells [22]. During menstruation, endometrial cells regulate eMSCs’ functionality through activation of the Wnt/β-Catenin signaling pathway [23]. In support of the interdependency between the Notch and Wnt pathway, studies identified that the inhibition of Notch signaling triggers β-Catenin downregulation, whereas WNT activity gets upregulated in eMSCs upon Notch activation [16]. Hence, all of the reports collectively indicate that both of the Notch and WNT signaling regulate eMSCs’ stemness and functionality. The essential role of Wnt genes has been identified in uterine biogenesis [24] and endometrial cancers [25]. However, their role in the maintenance of eMSCs stemness and subsequent endometrium’s functionality, during embryo implantation, has not been well deciphered.
Functions
eMSCs are postulated to participate in the regeneration of both epithelial and stromal cell compartments through the process of reversible mesenchymal to epithelial (MET/EMT) transition [26, 27]. According to the report of Blanks and Brosens [28], the inflammatory response caused by the sudden fall of progesterone level before menstruation employs eMSCs at the endometrium to initiate tissue regeneration. Interestingly, eMSCs can differentiate into endometrial stromal fibroblasts which decidualized in vitro under the influence of estradiol and progesterone [29, 30]. However, some studies identified endometrium-resident epithelial progenitor cells which co-express epithelial marker E-cadherin and mesenchymal markers N-cadherin and vimentin, suggesting an EMT/MET process in their development. Such hybrid stem cells are reported in a wide variety of cancers [31]. Whether the cells expressing both epithelial and mesenchymal markers are differentiated eMSCs or a separate population of hybrid stem cells needs to be investigated. Additionally, the menstrual blood-derived MSCs express significantly higher levels of genes specific for vasculogenesis, angiogenesis, proliferation, and migration as compared to MSCs derived from bone marrow [32], suggesting the tissue-specific functionality of MSCs.
There is no direct evidence so far demonstrating the role of eMSCs in endometrium regeneration in vivo. However, exogenously delivered MSCs do aid in improving endometrial injury and increase endometrial thickness leading to pregnancy in women with Asherman’s syndrome [33, 34]. But, there is limited evidence supporting the involvement of eMSCs in endometrial regeneration using animal models [35, 36].
It will be imperative to demonstrate the regeneration potential of the eMSCs using animal studies and human trails to justify their clinical use for fertility treatment.
Endometrial epithelial stem/progenitor cells (eES/PCs)
Origin
The regenerative capacity of the endometrium mainly the glandular epithelium is also assigned to stem/progenitor cells occupying the basalis layer of the tissue. The glands in the functionalis zone of the endometrium grow vertically from the horizontal, branching networks formed by the basalis glands [37]. Post menstruation, under the influence of rising estrogen, the cells of the glands in the basalis layer proliferate to regenerate the glands in the functionalis zone [38]. This capacity of gland renewal is thought to be by the eES/PCs. Studies have identified epithelial progenitor cells as label-retaining cells in the mouse endometrium [39] and clonogenic populations in EpCAM+ epithelial cell preparation from the whole human uterus. These cells are localized in the basalis region of the human uterus and are characterized by the nuclear AXIN2, SOX9, and β-catenin markers. While these markers are reported in the basalis region of the mouse and human uterus, lineage tracing tools have identified bipotent stem cells residing at the intersection zone between luminal and glandular epithelial compartments [40]. Irrespective of the eES/PCs’ residential site and their ability to initiate daughter cell proliferation to reinstate homeostasis of damaged tissue, these cells are quiescent and rarely proliferate, which deviates from the primary property of stem cells’ self-renewal and proliferation criteria.
Markers
The epithelial stem/progenitor cells (eES/PCs) are not a single entity but appear to be a heterogeneous population of cells with different markers. Alternately, these cells could be a part of a hierarchy that is being dissected [18]. In general, the N-cad-positive and SSEA1-negative cells in the basalis region with nuclear SOX9/Axin2 are thought to be the primitive eES/PCs. Another population is the SSEA1+ N-Cad cells in the intersection zone between luminal and glandular epithelial compartments having clonogenic properties.
Regulation of stemness
To maintain life-long tissue homeostasis, eES/PCs must possess self-renewal and differentiation capacity. Wnt/β-catenin signaling pathways are considered to be the master regulator of specification, maintenance, and activation of epithelial stem cells in most of the tissues like the intestine and mammary glands [41]. Emerging evidence suggests that the Wnt signaling also controls self-renewal capacity of endometrial eES/PCs [42]. Human endometrium expresses Wnt2, Wnt3, Wnt4, Wnt5, Wnt7a, and Wnt7b in both proliferative and secretory stages of the menstrual cycles [43, 44]. Additionally, Wnt receptor and coreceptors FZD6 and LRP6, as well as downstream effectors (DKK1, DVL-1, GSK3β, and β-catenin), are also expressed during the menstrual cycle [43]. Wnt ligand binding to cognate Frizzled receptors triggers the activation of downstream signaling cascade and cytoplasmic accumulation of β-catenin, where they interact with Tcf/Lef family of DNA-binding proteins. Hence β-catenin and Tcf/Lef combinatorially function as a transcriptional cofactor to stimulate the transcription of Wnt target genes. Interestingly, overexpression of β-catenin is reported in epithelial cells in cases of endometrial hyperplasia and endometrial cancer [25]. This study further showed that mice with loss of HOXA10 develop endometrial epithelial cell expansion which label strongly for the eES/PCs marker Sox9 [25]. Thus, it appears that HOXA10 is required for the differentiation of eES/PCs to mature epithelial cells.
Function
The luminal epithelial SSEA-1+SOX9+ cells are thought to participate in endometrial epithelial regeneration which occurs in a piecemeal fashion during every menstrual cycle. The regenerative functionality of eES/PCs is established in a mouse xenograft model, where single-cell suspensions of epithelial and stromal cells transplanted under the kidney capsule generate an endometrial-like structure [45]. Intriguingly, these structures are responsive to cyclical exogenous estradiol and progesterone. These cells regenerate the luminal and glandular epithelium [39] which are known to have distinct molecular and functional properties.
Endometrial side population stem cells (eSPs)
Origin
Endometrial side population stem cells (eSPs) are a well-characterized endometrial adult stem cell population which is thought to participate in endometrial regeneration. Kiyoko and colleagues first identified the existence of eSPs in normal human endometrium [46] with a percentage of 0.01–3 in the population of live endometrial cells. Freshly isolated eSPs can differentiate into several mesodermal lineages such as adipocytes and osteocytes, when they are expanded under hypoxic conditions [47, 48], indicating their multipotency. Though it has been proposed that eSPs exist in the basalis layer of endometrium, even distribution of ABCG2 expressing eSPs was identified in both the functionalis and basalis layers of the endometrium [49] in proximity to eMSCs.
Markers
ABCG2/Bcrp1, an ATP-binding cassette transporter protein, is the marker of eSP phenotype [50]. ESPs also express epithelial cell marker EMA and MSCs’ markers CD105 and CD146 [51]. eSPs are negative for CD9, CD13, CD45, and CD 34 [46].
Regulation of stemness
The regulation of self-renewal and growth of eSP cells has not been well deciphered to date. Emerging evidence from other systems has revealed the role of Oct4 or Sox2 in self-renewal and expansion of side population stem cells with the tumor formation capacity. For example, expressions of Oct4, Sox2, and Nanog are significantly higher in SP cells derived from non-small cell lung cancers (NSCLCs), though only Sox2 plays an indispensable role in the maintenance of stemness, whose expression is regulated through EGFR, Src, and Akt signaling pathway [52]. On the contrary, another study established the role of Oct4 in the maintenance of stem cell properties of the ovarian cancer SP cells, through OCT4/JAK/STAT signaling pathway [53]. Hence, studies are required to confirm the role of Oct4, Sox2, and Nanog in the maintenance of eSP cells’ stemness.
Function
eSPs have the potential to differentiate into endometrial gland-like structures expressing CD9 and E-cadherin (epithelial markers) as well as CD13 (stromal marker) expressing fibroblast, after long culturing on feeder cells [46]. This supports their role as the endometrial epithelial and stromal stem/progenitor cells. Additionally, estradiol and progesterone treatment induce morphologic and functional changes in eSPs resembling decidualization, which is characteristic of secretory endometrial stromal cells [51, 54]. Interestingly, xenotransplantation study revealed that freshly isolated eSPs can reconstruct the entire endometrium tissues containing glandular structures, stromal tissue, and blood vessels [49]. However, no genetic analysis or lineage tracing experiments in vivo have been done to validate this observation.
Very small embryonic like stem cells (VSELs)
Origin
Pluripotent very small embryonic like stem cells (VSELs) have been primarily identified in bone-marrow (~ 0.01% of bone-marrow mono nuclear cell) as non-hematopoietic stem cells with the capacity to differentiate into three germ layers like endoderm (inner layer), ectoderm (outer layer), and mesoderm (middle layer). This small-sized (3–5 μm) diploid cells have large nuclei with open chromatin structure for Oct-4 and Nanog promoter [55]. Studies identified their existence in both mouse uterine endometrium and myometrium [56]. VSELs can also be detected in higher numbers in the brain, kidneys, muscles, pancreas, human testes [57], ovaries [58], and cord blood [59, 60] tissue. Primordial germ cells are proposed to be the developmental origin of VSELs, which are placed in the developing organs through embryogenesis [61]. Importantly, they are thought to generate hematopoietic stem cells (HSCs) and MSCs in the hematopoietic [62] and reproductive system [63]. VSELs share many embryonic stem cells like features, namely, chromatin structure, cell size, or nuclear to cytoplasm ratio [55]. Studies identified their tissue migratory capacity in response to tissue/organ injury [64].
Markers
Like embryonic stem (ES) cells, VSELs do not express MSC-specific markers CD90, CD105, CD29, and also negative for MHC-1 and HLADR [54, 65]. They express pluripotent stem cell–specific markers, such as stage-specific antigen A-1 (SSEA-1), nuclear Oct-4, Nanog, and Rex-1 and Rif-1 telomerase protein [64]. They can also express germ cell markers like Mvh, Stella, Fragilis, Nobox, and Hdac-6 [66].
Regulation of stemness
VSELs express ES cell–specific proteins Oct-4, Nanog, and Rex-1. Oct4, a member of POU family transcription factor, plays a central role to maintain a pluripotent state. Oct4, Sox2, and Nanog cooperatively activate both protein-coding genes and noncoding RNAs essential for self-renewal and pluripotency [67, 68]. Hence, it can be predicted that they also play a crucial role in the maintenance of VSELs’ pluripotency like mouse or human ES cells.
Function
In general, VSELs are thought to serve as a reservoir for adult stem cells, which under stress conditions migrate to the injury site to maintain tissue-homeostasis [63]. Similar to the other adult tissues, VSELs participate in uterine homeostasis with the help of tissue-resident progenitor cells. The expected role of VSELs is to regenerate endometrium by proliferating and differentiating into epithelial cells lining the endometrial lumen and glands and myometrial cells in assistance of MSCs, which provide a favorable microenvironment to the uterine stem cells [56]. However, lineage tracing experiments in vivo have been done to validate this observation.
Endothelial progenitor cells (EPCs)
Origin
EPCs, as a population of bone marrow cells, were first isolated by Asahara and colleagues from human peripheral blood [69]. They established the capacity of the EPCs’ to differentiate into mature endothelium with neovascularization capacity [69]. Later on, several other groups supported this notion and define these cells as bone-marrow–derived circulating mononuclear cells with the ability to differentiate into mature endothelial cells [70]. EPCs can be traced inside the vasculature and stroma of the endometrium and myometrium after inducing the ovulation cycle in mice [71].
Markers
EPCs express antigens like CD34, kinase insert domain receptor (KDR), and the extracellular domain of vascular endothelial growth factor receptor (VEGFR), which are signatures of both early hematopoietic stem cells and mature endothelial cells [72, 73].
Regulation of stemness
Multiple in vivo and in vitro studies confirmed the involvement of VEGFs in EPCs’ differentiation during the vascular repair process in human [74] and mouse model [75]. Li et al. demonstrated that VEGF-induced EPC differentiation is mediated through the upregulation of gap junction protein Connexin 43 [76], which regulates EPCs’ proliferation, migration, and angiogenesis [77, 78]. However, there is no specific study present that investigates endometrial EPCs and the factors that regulate their stemness and differentiation potential.
Function
EPCs are proposed to play a critical role in the maturation, regulation, and maintenance of vasculature during pregnancy. In the reproductive cycle, endometrial vasculature grows in preparation for implantation starting in the proliferative phase and continuing in the secretory phase [79]. Elongation and intussusception of existing small vessels are thought to control this vasculature growth process. EPCs are proposed to participate in the physiological endometrial angiogenesis process in mice [80]. Uterine vasculature remodeling is an important step of placentation, mediated by the invasion of interstitial and endovascular trophoblast through maternal uterine spiral arteries. Hence, it can be assumed that the circulating EPCs might have a role in restoring maternal spiral artery endothelium which gets damaged during the trophoblast invasion. However, this process slightly differs in mice and humans, so deciphering the mechanism in the mice model may not be directly translatable to humans.
Sugawara and colleagues tested 20 pregnant women to identify circulating EPCs in their peripheral blood [81]. Interestingly, they noticed that EPCs’ colony-forming capacity is augmented with the increasing gestational age due to increasing estradiol concentration. Estrogen is thought to induce EPCs’ mobilization from the bone marrow [82] and support vasculogenesis of the pregnant mouse decidua [83]. However, there is a controversy over the relation between EPC number and gestational age. A study by Matsubara and colleagues identified a lower number of EPCs with increasing gestational age [84]. These controversial reports suggest that the method of measuring EPCs cell count should be standardized for different cross-sectional studies. However, no lineage tracing or genetic studies have been conducted to determine if EPCs are indeed involved in vascular remodeling of the endometrium during the menstrual cycle and in pregnancy.
Placental stem cells
Placenta is the temporary fetal organ formed from the blastocyst after implantation. This organ supplies oxygen and nutrients to the fetus and removes waste products. Despite its importance in pregnancy, to date, knowledge of stem cells that contribute to this organ has not been well deciphered. Trophectoderm, the outer layer of the blastocyst, generates the trophoblast of the placenta. The cells of the Trophectoderm differentiate into syncytiotrophoblasts, cytotrophoblasts, and the extravillous trophoblasts [4] (Fig. 2a,b). Three types of stem cells have been identified in the placenta: (1) Trophoblast stem cells, (2) side population trophoblast stem cells (SpTSCs), (3) mesenchymal stem cells [85, 86].
Fig. 2.
a depicts the path of human trophoblast differentiation. Trophoblast stem cells (TSCs) are originated from the trophectoderm (TE). As the blastocyst implants itself in the endometrium, it differentiates into cytotrophoblasts (inner), which further differentiate into either extravillous trophoblast (EVT) or syncytiotrophoblast (outer), depending on the signals they receive. These cells maintain the interactions between the fetus and the mother. b depicts the post-implantation placenta, where placental villus showing the three major trophoblast populations
Trophoblast stem cells (TSCs)
Origin
Trophoblast stem cells (TSCs) are the self-renewing multipotent stem cells of the placenta. A large section of researcher considers the mitotically active polar trophectoderm (TE) as the immediate precursor of self-renewing TSCs [87]. Inner cell mass–derived EGF and FGF4 signal supports their survival and proliferation [88]. Studies identified that the fibroblast growth factor 4 (FGF4) and embryonic fibroblast-conditioned medium (contain TGF-β and activin-A) are essential for the survival of mouse blastocyst-derived TSCs [89]. These factors trigger continued undifferentiated proliferation of TSCs without differentiating in to the committed placental trophoblast lineages [87].
Although other than the human, mouse, and the rhesus macaque placenta, the location and phenotype of TSCs are not well-recognized, it is presumed that TSCs exist at the early stage of placental development when trophoblast growth is maximum [89]. In the mouse, trophectoderm has been identified as the immediate precursor of self-renewing TSCs from which the mature trophoblast lineages emerge [87]. In human placenta, all of the mature trophoblast lineages are thought to be derived from stem cell–like villous cytotrophoblast. However, many studies argued the multipotent nature of villous cytotrophoblast and demanded the existence of another sub-population of cytotrophoblast having the syncytiotrophoblast or extravillous trophoblast differentiation capacity. Researchers have also identified another form of trophoblast that is derived from embryonic stem cells. Their transcriptome signature is intermediate between that of TE and that of villous trophoblast [90]. Whether this resembles the TCS population is yet not clear.
Markers
The expression of TSCs markers vary with the type of primates. TSCs derived from rhesus macaque blastocysts express mouse TSCs’ markers such as chorionic gonadotropin (CG), KRT7, CD9, POU5F1, and EOMES. However, the primate TSCs do not express CDX2, indicating their function as the partially differentiated committed progenitor cells [91, 92]. In humans, the source of TSCs has not been identified so far. Moreover, lack of trophoblast-specific markers restricts to define trophoblast cell fate in in vitro cell lines [93]. Cytokeratin 7 (KRT7), HLA-G, and human chorionic gonadotropin (hCG) are commonly used trophoblast markers, though their specificity is questionable. Several transcription factors such as CDX2 and EOMES belonging to mouse TSCs network are also served as TSCs markers; however, their function in human has not been well established [93].
Regulation of stemness
TSCs’ self-renewal and differentiation are manifested by alteration of their cellular morphology, transcription factors or protein markers. TSCs necessitate various transcription factors and external signals (FGF4, INHBA/NODAL/TGFB1) for self-renewal [94] multi-potency. Genetic modification of the mouse indicated that the caudal-related transcription factor Cdx2 is directly involved in regulating trophoblast (TR) emergence, and CDX2 expression is lost with the TSC differentiation. Cdx2−/− mouse conceptuses lost their implanting capacity, supporting its direct role in embryo implantation [95]. Indeed, multiple studies revealed that the CDX2 is required for the trophoblast lineage establishment but may not be necessary for initial trophectoderm specification [96]. Interestingly, CDX2 expression beyond the early morula stage is dependent upon the Tead4 gene. Either Cdx2−/− or Tead4−/− blastocysts cannot serve as a potential source of TSCs [97]. The T-box gene Eomes is also crucial for trophectoderm to trophoblast transition, as evidenced by arrested blastocyst-like stage in Eomes−/− mice with the TSC-lacked conceptuses [94]. Another TEAD4 regulated gene Gata3 is also assumed to maintain TSC stemness independent of CDX2 [98]. In TSCs, another transcription factor TCFAP2C in combination with EOMES and SMARCA4 bind to the promoter of genes viz. Bmp4, Esrrb, Klf5, Lifr, Stat3, and Zfp42, which are known to be involved in embryonic stem cell self-renewal. This indicates that the embryonic stem cell and TSCs maintain their stemness and pluripotency by some common mechanisms [96]. Ets2 gene expression is also imperative for proper mouse embryo development, silencing of which prevents extraembryonic ectoderm (ExE) development [99] and induces abnormal trophoblast differentiation [100] resulting in embryonic lethality by day 8.5. Elf5, a second ETS-domain transcription factor, also directs crucial events in the early placental development. It is presumed that Elf5, Cdx2, and Eomes form a positive feedback loop that buttresses the early stages of TSCs commitment [101].
Functions
TSCs generate the epithelial components of the placenta as they differentiate into either syncytiotrophoblasts or extravillous trophoblasts [102]. Syncytiotrophoblasts cover the surface of the placental villi which resides within the intervillous space and establish the nutrient transport surface through the placenta [103], thereby directly regulating the maternal-fetal gas or nutrient exchange. EVTs’ function varies, based on their localization. Interstitial EVTs which invade deeply to the maternal decidua anchor placental villi to the maternal decidua. Whereas endovascular EVTs which replace maternal endothelium in the spiral arteries promote adequate blood flow in the intervillous space. This is to prevent hypoxia and oxidative stress–related damage as seen in pathological pregnancies like preeclampsia which has inadequate spiral artery remodeling [104–106].
Side population Trophoblast stem cells (SpTSCs)
Origin
SpTSCs, as a candidate human TSCs population, are considered to be 1.44% of the total live trophoblasts. These cells can be isolated by the Hoechst side-population technique from first-trimester placentae [107]. They have differentiation capacity and resemble cytotrophoblasts at both the transcriptomic or methylome level.
Markers
The protein encoding genes precisely upregulated in SpTSCs compared to other trophoblast cells are considered to be the SpTSC-specific markers. These are extracellular matrix proteins collagen 6A3, collagen 6A2, laminin A2, and V-CAM-1 [107]. However, no specific markers for these cells have been reported to enable sorting from tissues.
Regulation of stemness
SpTSCs are transcriptomically discrete placental stem cell population, which express many similar kinds of pluripotent and trophoblast-like genes [107]. SpTSCs’ differentiation potential is also similar with that of TSCs. Interestingly, the genes like ELF5 and CDX2, which regulate the TSCs’ stemness, are also expressed by SpTSCs; however, the expression level differs from each other. Other than these genes, a number of genes like Elf3/5, Oct-6, Wnt5A, and Igfbp1 which are involved in maintenance of stem cell niche for other tissue and embryo development are also upregulated in SpTSCs, though their specific function in SpTSCs’ stemness maintenance still remains elusive [107].
Functions
spTSCs are a candidate human TSCs population with similar TSCs like differentiation capacity and functionality. Interestingly, SpTSCs can be isolated from the placenta at any stage of gestation period, and their percentage is significantly reduced in the placentae of growth-restricted fetuses. These observations signify there is a correlation between trophoblast progenitor number and fetal growth restriction [86, 107]. However, precise functions in placental biology have not been determined.
Placental mesenchymal stem cells (PMSCs)
Origin
Placental mesenchymal stem cells (PMSCs) are formed by trophectoderm [108] and can be expanded in large quantities in chorionic villi [109]. In vitro studies have shown that PMSCs can be easily isolated from term placentae by enzymatic digestion and culture expanded with specific stem cell medium. Though placental tissue is considered as a rich source of MSCs, the exact anatomic location of the niche has not been identified. Like adipose, bone marrow and other familiar tissue sources, PMSCs can be differentiated into derivatives of the mesenchymal cell lineage such as osteocytes, adipocytes, myocytes, and chondrocytes [110, 111].
Markers
Like MSCs form other tissues, PMSCs express specific cell surface markers (STRO-1, 3G5, CD106, CD105, a-SMA, CD146, CD49a) and negative for hematopoietic stem cell markers (CD117, CD34) and endothelial markers (CD34, vWF) [109]. However, further research is necessary to detect unique PMSCs’ surface markers to identify their specific microenvironment in the chorionic villi of placental tissue.
Regulation of stemness
PMSCs express the embryonic stem cell markers: Nanog, Sox2, Rex-1, and Oct4. These transcription factors are also expressed by MSCs and essential for maintaining their multipotency [112]. Hence, the self-renewal and differentiation of PMSCs are regulated in a similar manner like other adult stem cells.
Functions
PMSCs present in the perivascular niche of the placenta throughout the gestation period. Since in the placenta, bone marrow-derived endothelial progenitor cells are absent at the early stage of pregnancy, placental MSCs are thought to be the contributor to placental vasculogenesis. It is postulated that the placental MSCs differentiate into hemangiogenic stem cells, which further differentiate into CD34+ endothelial cell cords and gradually form a connecting network of higher-order vascular structures [113, 114]. Though there is no direct evidence showing MSCs’ involvement in the placental angiogenesis process, in vitro, the chorionic villus MSCs’ capacity to differentiate into both endothelial and vascular smooth muscle cells [115, 116]. Hence, it has been speculated that MSCs play an important role in the branching and non-branching angiogenesis process via the release of proangiogenic factors like transforming growth factor beta (TGFβ) and vascular endothelial growth factor-A (VEGF-A) [117] to stimulate endothelial growth [117, 118].
In addition, PMSCs prevent endothelial cell apoptosis through the release of insulin-like growth factor 1 (IGF-1) and secreted frizzled-related protein 1 (SFRP1) [119, 120]. Therefore, MSCs contribute to placental development by forming a vascular network, hence support optimal nutrient exchange. However, direct evidence for the same in vivo is lacking.
Stem cells of the feto-maternal interface in pathological conditions
As stem cells play essential role in successful pregnancy, impairment of their functionality may lead to pathological pregnancies (Table 1). Hence, studying the role of stem cells in normal and pathological pregnancy is important to understand the causes and pathobiology of pregnancy-related disorders.
Table 1.
Function of stem cells in pregnancy regulation
| Type of stem cells | Physiological Function | Pathological function | Ref. |
|---|---|---|---|
| eMSCs | Angiogenesis, regeneration of both epithelial and stromal cell compartments | Development of Endometriosis, recurrent miscarriage | [26, 124, 129] |
| eES/PCs | Regeneration of the luminal and glandular epithelium | Establishment of Endometriosis related endometriotic lesions | Mice [45, 132] |
| eSPs | Reconstruction of the entire endometrium tissues with glandular structures, stromal tissue, and blood vessels | ESPs may involve in generating endometriotic lesions | [49, 152] |
| VSELs | Regenerate epithelial cells lining the endometrial lumen and glands | Not well deciphered | [56] |
| EPCs | Uterine vasculature remodeling | Preeclampsia may be developed due to abnormal EPCs’ function | [79, 141] |
| TSCs | Provides nutrients to the embryo | abnormal development and function of trophoblasts leads to Preeclampsia | [153, 154] |
| SpTSCs | TSCs like differentiation capacity and functionality | [86] | |
| PMSCs | Involved in placental angiogenesis process | Induce fetal growth restriction | [113, 155] |
Implantation failure
Among the many other maternal factors, endometrial abnormalities are one of the common causes of implantation failure [121]. Endometrial stem cells play a pivotal role in regenerating endometrial glands and luminal epithelium as well as stromal decidualization. Hence, it can be implied that eSCs’ abnormalities generate defective endometrial receptivity [122]. eMSCs derived from non-RIF and RIF patient showed differential expression of receptivity markers —integrin (ITG) β1, Rac1, HoxA11, ITGβ3, and Noggin [123]. Dysregulation of eMSCs secreted factors particularly IL33 and LIF is reported in women with idiopathic recurrent miscarriage [124, 125]. eMSCs also showed different clonogenic capacity in obese women with infertility [126], suggesting the impact of their impaired functionality on pregnancy outcome. Other than eSCs, placental stem cells also responsible for miscarriage, though extensive comparative study between eMSCs and placental stem cells is lacking to establish association. Studies identified that the reduced proliferation of cytotrophoblast population during early gestational period causes TSCs’ deficiency, resulting in abnormal placentation and result in miscarriage [127].
Endometriosis
Endometriosis is growth of endometrium outside the uterine cavity, which affects ~ 17% of reproductive-aged women and ~ 50% of infertile females [128]. Of the many theories, it is also believed that endometriosis is due to accumulation of dysregulated eSCs at the ectopic sites. Improper endometrial differentiation of bone marrow MSCs, which are thought to be a potential source of endometrial progenitor stem cells, also may lead to the ectopic endometrial lesions [129]. Experimental evidences also indicate the contribution of extrauterine has a significantly higher migratory, proliferative, and invasive properties compare to these properties accomplished by eutopic endometrium-derived eMSCs [129]. Some experimental study attributed endometriosis to the EPC-mediated vascularization [130]. Further study depicted the contribution of bone marrow–derived MSCs in vasculature of endometriosis [131]. eES/Eps also participate in developing endometriosis by their shedding into pelvic cavity during retrograde menstruation, which causes ectopic endometriosis lesions [132].
While all these studies do suggest point towards an involvement of stem cell populations in endometriosis development and/or its pathogenesis, the precise roles of different kinds of stem cells in endometriosis are yet not clear.
Asherman syndrome/thin endometrium
In Asherman’s syndrome (AS), scar tissue forms inside the uterus causing thin endometrium which is incapable of embryo implantation. This endometrium consists of inactive glands and poor vascularized stroma. In healthy endometrium, endometrial epithelial layer regenerates without forming scar following menstruation or parturition. However, in AS, due to the damage in basal layer or myometrium, endometrial functional layer fails to regenerate. Since eSCs are involved in endometrial regeneration process, reduced number of eMSCs or EPCs in the damaged stroma is considered as the principal cause of this disorder [133]. Consequently, their role can be investigated for the disorders of endometrial regeneration in AS. Although the specific role of eSCs has not been investigated till now, studies identified that the bone marrow–derived stem cell transplantation enhances fertility in a mouse model of AS [36, 128, 134]. Transplantation intrauterine administration of eMSCs aids in increasing endometrial thickness in humans [135]. Future study on the mechanism of eSCs’ function in occurrence of endometrial disorder would be beneficial to invent cell-based therapeutic for the treatment of thin endometrium.
Endometrial cancer
Endometrial cancer is the most frequent gynecologic cancer [136] in women worldwide. The treatment is limited and based upon surgical intervention, which is total hysterectomy and bilateral adnexal removal for locally restricted endometrial cancer [137]. Studies have suggested that small population of endometrial stromal cells behave like cancer stem cells in women with endometrial cancers. They regulate tumor growth, invasion, and metastasis. Studies identified the two signaling pathways MYC and NF-κβ, which are responsible for maintenance and survival of eSCs [138]. Among the eSCs, the role of MSCs in cancer progression has been studied well. eMSCs secrete cytokines specially IL-6 and IL-8 as well as immunosuppressive molecule programmed death ligands PD-L1 that are thought to be responsible for tumor progression. In addition, there is a recent study which has shown that loss of HOXA10 in the mouse endometrium leads to endometrial hyperplasia that progresses to grade 1 adenocarcinoma [25]. In this model, the epithelial stem cell maker SOX9 is highly upregulated and detected in many cells of the glandular epithelium implying that endometrial cancer might be due to extensive proliferation and/or failure of differentiation of epithelial stem cells [25]. Hence, understanding the detailed molecular mechanism of eSCs-mediated cancer progression is pivotal for developing anti-cancer drug.
Pre-eclampsia
Preeclampsia is a pregnancy-related disorder, manifested by high blood pressure, proteinuria, and damage of other organ systems, most often the liver and kidneys. Dysfunctional maternal vascular endothelium with shallow trophoblast invasion leading to inadequate spiral artery remodeling is considered to be the probable cause of pre-eclampsia [139]. This is associated with placental dysfunctions, oxidative damage, and inflammation in the feto-maternal interface [140].
A study has shown that the number of circulating EPCs decreased, and the rate of EPC senescence increased in women with preeclampsia [141]. Since EPCs are involved in vascular remodeling [142], it is possible that along with invasion defect, the endothelial dysfunction due to altered EPCs may also be an additional factor causing preeclampsia [143]. However, this hypothesis is yet not rigorously tested. Nevertheless, reduced levels of angiogenesis markers, sFlt-1:PLGF imbalance are reported in preeclamptic pregnancies as compared to normal pregnancies [144, 145] suggestive of vascular cell dysfunction. It will be imperative to determine if this is due to defective EPCs.
Conclusion and future perspective
The presence of endometrial and placental stem cells and their demonstrated role in different mouse models suggest that they are involved in successful pregnancy establishment. Endometrial mesenchymal stem cells, epithelial stem/progenitor cells, and endometrial side population stem cells are the major populations of endometrial stem cells to aid in endometrial regeneration. The altered functionality of endometrial stem cells is observed in endometrial disorders like thin endometrium, endometriosis, endometrial cancer, and others. Like in the case of endometrium, the placenta also has three major populations of stem cells viz. trophoblast stem cells, side population trophoblast stem cells, and placental mesenchymal stem cells. In addition, there are endothelial stem cells whose numbers are reported to be altered in pregnancy. Though the function of most placental stem cells remains elusive, their perturbed differentiation and functionality has been observed in pathological pregnancies.
Stem cells isolated from different tissue sources have been tested pre-clinically or clinically to treat infertility. Among various stem cells, MSC therapies have been particularly proven beneficial in infertile women for their immunomodulatory and tissue-regenerative properties. For example, autologous bone marrow and endometrial MSC transplantation successfully recover uterine fertility by regenerating endometrium in a subset of Asherman’s syndrome patients [146]. As PMSCs also showed a promising role in angiogenesis induction in an animal model of myocardial infarction [147], it is tempting to postulate that PMSCs may aid in enhancing angiogenesis in the feto-maternal interface for a healthy pregnancy. In the therapeutic context, it is important to be borne in mind that there is differential expression of procoagulant tissue factor (TF/CD142) in stem cells derived from various sources including the placenta and decidua [148, 149]. The high expression of TF/CD142 in decidual stromal cells protects the mother and fetus from extensive internal bleeding [149]; it might lead to thromboembolic events in patients [150, 151], and hemocompatibility testing should be evaluated while using perinatally derived MSCs for therapeutic purposes [151].
The therapeutic application of placental and endometrium stem cells would be more efficacious if their molecular and functional role in normal and pathologic pregnancy can be dissected. Hence, special emphasis should be made on identifying cell-specific markers to achieve a pure and high-quality stem cell population.
Funding
The manuscript bears the NIRRCH ID:REV/1502/02-2023. DM lab and is supported by grants from the Indian Council of Medical Research (ICMR, Govt of India). JG is a recipient of the DBT/Wellcome Trust India Alliance Early Career Fellowship
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jayeeta Giri, Email: jgiri.stem@gmail.com.
Deepak Modi, Email: deepaknmodi@yahoo.com, Email: modid@nirrh.res.in.
References
- 1.Ashary N, Tiwari A, Modi D. Embryo implantation: war in times of love. Endocrinol. 2018;159(2):1188–1198. doi: 10.1210/en.2017-03082. [DOI] [PubMed] [Google Scholar]
- 2.Marikawa Y, Alarcon VB. Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol Reprod Dev. 2009;76(11):1019–1032. doi: 10.1002/mrd.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godbole G, Modi D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol. 2010;85(2):130–139. doi: 10.1016/j.jri.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. Part I: what do we know about formative placental development following implantation? Placenta. 2012;33(5):327–334. doi: 10.1016/j.placenta.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Kimber SJ, Spanswick C. Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol. 2000;11(2):77–92. doi: 10.1006/scdb.2000.0154. [DOI] [PubMed] [Google Scholar]
- 6.Barrientos G, et al. Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat. Mol Hum Reprod. 2017;23(7):509–519. doi: 10.1093/molehr/gax024. [DOI] [PubMed] [Google Scholar]
- 7.Okada H, Tsuzuki T, Murata H. Decidualization of the human endometrium. Reproductive medicine and biology. 2018;17(3):220–227. doi: 10.1002/rmb2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75(3):341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 9.Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95(4):1278–1283. doi: 10.1016/j.fertnstert.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Sfakianoudis K, et al. The role of uterine natural killer cells on recurrent miscarriage and recurrent implantation failure: from pathophysiology to treatment. Biomed. 2021;9(10):1425. doi: 10.3390/biomedicines9101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ticconi C, Di Simone N, Campagnolo L, Fazleabas A. Clinical consequences of defective decidualization. Tissue Cell. 2021;72:101586. doi: 10.1016/j.tice.2021.101586. [DOI] [PubMed] [Google Scholar]
- 12.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorsek Sparovec T, et al. The fate of human SUSD2+ endometrial mesenchymal stem cells during decidualization. Stem Cell Res. 2022;60:102671. doi: 10.1016/j.scr.2022.102671. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H, Anwar SS, Buhring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21(10):2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- 15.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Chan RWS, Ng EHY, Yeung WSB. The role of Notch signaling in endometrial mesenchymal stromal/stem-like cells maintenance. Commun Biol. 2022;5(1):1064. doi: 10.1038/s42003-022-04044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 18.Cousins FL, Pandoy R, Jin S, Gargett CE. The elusive endometrial epithelial stem/progenitor cells. Front Cell Dev Biol. 2021;9:640319. doi: 10.3389/fcell.2021.640319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su RW, et al. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015;100(3):E433–E442. doi: 10.1210/jc.2014-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moldovan GE, et al. Notch effector recombination signal binding protein for immunoglobulin kappa J signaling is required for the initiation of endometrial stromal cell decidualizationdagger. Biol Reprod. 2022;107(4):977–983. doi: 10.1093/biolre/ioac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev Rep. 2014;10(4):512–525. doi: 10.1007/s12015-014-9515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu S, Chan RWS, Li T, Ng EHY, Yeung WSB. Correction to: Understanding the regulatory mechanisms of endometrial cells on activities of endometrial mesenchymal stem-like cells during menstruation. Stem Cell Res Ther. 2022;13(1):199. doi: 10.1186/s13287-022-02871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deutscher E, Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol. 2007;307(2):227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra A, Ganguli N, Majumdar SS, Modi D. Loss of HOXA10 causes endometrial hyperplasia progressing to endometrial cancer. J Mol Endocrinol. 2022;69(3):431–444. doi: 10.1530/JME-22-0051. [DOI] [PubMed] [Google Scholar]
- 26.Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update. 2019;25(1):114–133. doi: 10.1093/humupd/dmy035. [DOI] [PubMed] [Google Scholar]
- 27.Kirkwood PM, et al. Single-cell RNA sequencing and lineage tracing confirm mesenchyme to epithelial transformation (MET) contributes to repair of the endometrium at menstruation. eLife. 2022;11:e77663. doi: 10.7554/eLife.77663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanks AM, Brosens JJ. Meaningful menstruation: cyclic renewal of the endometrium is key to reproductive success. BioEssays. 2013;35(5):412. doi: 10.1002/bies.201300022. [DOI] [PubMed] [Google Scholar]
- 29.Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich D, et al. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum Reprod. 2014;29(9):1895–1905. doi: 10.1093/humrep/deu159. [DOI] [PubMed] [Google Scholar]
- 31.Sahoo S, Ashraf B, Duddu AS, Biddle A, Jolly MK. Interconnected high-dimensional landscapes of epithelial-mesenchymal plasticity and stemness in cancer. Clin Exp Metastasis. 2022;39(2):279–290. doi: 10.1007/s10585-021-10139-2. [DOI] [PubMed] [Google Scholar]
- 32.Santos RA, et al. Intrinsic angiogenic potential and migration capacity of human mesenchymal stromal cells derived from menstrual blood and bone marrow. Int J Mol Sci. 2020;21(24):9563. doi: 10.3390/ijms21249563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4(1):43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tandulwadkar S, Mishra S, Gupta S. Successful application of combined autologous bone marrow-derived stem cells and platelet-rich plasma in a case of severe Asherman syndrome and subsequent in vitro fertilization conception. J Hum Reprod Sci. 2021;14(4):446–449. doi: 10.4103/jhrs.jhrs_138_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, et al. Intrauterine infusion of human platelet-rich plasma improves endometrial regeneration and pregnancy outcomes in a murine model of Asherman's syndrome. Front Physiol. 2020;11:105. doi: 10.3389/fphys.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9(5):e96662. doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi M, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24(4):102258. doi: 10.1016/j.isci.2021.102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98(1):11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci U S A. 2019;116(14):6848–6857. doi: 10.1073/pnas.1814597116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen HP, Sprung CN, Gargett CE. Differential expression of Wnt signaling molecules between pre- and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinol. 2012;153(6):2870–2883. doi: 10.1210/en.2011-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tulac S, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88(8):3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 44.Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL. Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer. 1997;75(8):1131–1136. doi: 10.1038/bjc.1997.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda H, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci U S A. 2007;104(6):1925–1930. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato K, et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22(5):1214–1223. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- 47.Cervello I, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5(6):e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cervello I, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6(6):e21221. doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda H, et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5(4):e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou S, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji S, et al. Side population cells contribute to the genesis of human endometrium. Fertil Steril. 2008;90(4 Suppl):1528–1537. doi: 10.1016/j.fertnstert.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Singh S, et al. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruan Z, Yang X, Cheng W. OCT4 accelerates tumorigenesis through activating JAK/STAT signaling in ovarian cancer side population cells. Cancer Manag Res. 2019;11:389–399. doi: 10.2147/CMAR.S180418. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Zuba-Surma EK, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12(1):292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36(6):742–751. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhartiya D, Singh P, Sharma D, Kaushik A. Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Rev Rep. 2022;18(5):1718–1727. doi: 10.1007/s12015-021-10243-6. [DOI] [PubMed] [Google Scholar]
- 57.Ratajczak MZ, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 58.Zuba-Surma EK, et al. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44(5):865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hess DC, et al. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186(2):134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 61.Ratajczak MZ, Ratajczak J, Kucia M. Very small embryonic-like stem cells (VSELs) Circ Res. 2019;124(2):208–210. doi: 10.1161/CIRCRESAHA.118.314287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taichman RS, et al. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19(10):1557–1570. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhartiya D, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016;23(1):41–76. doi: 10.1093/humupd/dmw030. [DOI] [PubMed] [Google Scholar]
- 64.Kucia M, et al. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20(1):18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 65.Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331(1):125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 66.Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev. 2009;130(1-2):58–66. doi: 10.1016/j.mad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Card DA, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28(20):6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. Rna. 2010;16(2):324–337. doi: 10.1261/rna.1441510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 70.Shi Q, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92(2):362–367. [PubMed] [Google Scholar]
- 71.Masuda H, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 72.Fina L, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75(12):2417–2426. [PubMed] [Google Scholar]
- 73.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 74.Kalka C, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 75.Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci U S A. 2002;99(18):11951–11956. doi: 10.1073/pnas.182215799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L, et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017;8(1):237. doi: 10.1186/s13287-017-0684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang HH, et al. Reduction of connexin43 in human endothelial progenitor cells impairs the angiogenic potential. Angiogenesis. 2013;16(3):553–560. doi: 10.1007/s10456-013-9335-z. [DOI] [PubMed] [Google Scholar]
- 78.Behrens J, Kameritsch P, Wallner S, Pohl U, Pogoda K. The carboxyl tail of Cx43 augments p38 mediated cell migration in a gap junction-independent manner. Eur J Cell Biol. 2010;89(11):828–838. doi: 10.1016/j.ejcb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Gargett CE, Rogers PA. Human endometrial angiogenesis. Reprod. 2001;121(2):181–186. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- 80.Asahara T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 81.Sugawara J, et al. Circulating endothelial progenitor cells during human pregnancy. J Clin Endocrinol Metab. 2005;90(3):1845–1848. doi: 10.1210/jc.2004-0541. [DOI] [PubMed] [Google Scholar]
- 82.Robb AO, Mills NL, Newby DE, Denison FC. Endothelial progenitor cells in pregnancy. Reprod. 2007;133(1):1–9. doi: 10.1530/REP-06-0219. [DOI] [PubMed] [Google Scholar]
- 83.Tal R, Dong D, Shaikh S, Mamillapalli R, Taylor HS. Bone-marrow-derived endothelial progenitor cells contribute to vasculogenesis of pregnant mouse uterusdagger. Biol Reprod. 2019;100(5):1228–1237. doi: 10.1093/biolre/ioy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsubara K, Abe E, Matsubara Y, Kameda K, Ito M. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2006;56(2):79–85. doi: 10.1111/j.1600-0897.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 85.James JL, Srinivasan S, Alexander M, Chamley LW. Can we fix it? Evaluating the potential of placental stem cells for the treatment of pregnancy disorders. Placenta. 2014;35(2):77–84. doi: 10.1016/j.placenta.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Gamage TK, et al. Side-population trophoblasts exhibit the differentiation potential of a trophoblast stem cell population, persist to term, and are reduced in fetal growth restriction. Stem Cell Rev Rep. 2020;16(4):764–775. doi: 10.1007/s12015-020-09991-8. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282(5396):2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 88.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 89.Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol. 2004;275(1):158–169. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 90.Roberts RM, Ezashi T, Sheridan MA, Yang Y. Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol Reprod. 2018;99(1):212–224. doi: 10.1093/biolre/ioy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vandevoort CA, Thirkill TL, Douglas GC. Blastocyst-derived trophoblast stem cells from the rhesus monkey. Stem Cells Dev. 2007;16(5):779–788. doi: 10.1089/scd.2007.0020. [DOI] [PubMed] [Google Scholar]
- 92.Douglas GC, CA VV, Kumar P, Chang TC, Golos TG. Trophoblast stem cells: models for investigating trophectoderm differentiation and placental development. Endocr Rev. 2009;30(3):228–240. doi: 10.1210/er.2009-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee CQ, et al. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem cell reports. 2016;6(2):257–272. doi: 10.1016/j.stemcr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kidder BL, Palmer S. Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Res. 2010;20(4):458–472. doi: 10.1101/gr.101469.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386(6620):84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 96.Roberts RM, Fisher SJ. Trophoblast stem cells. Biol Reprod. 2011;84(3):412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Ralston A, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137(3):395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 99.Georgiades P, Rossant J. Ets2 is necessary in trophoblast for normal embryonic anteroposterior axis development. Development. 2006;133(6):1059–1068. doi: 10.1242/dev.02277. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto H, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12(9):1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng RK, et al. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat Cell Biol. 2008;10(11):1280–1290. doi: 10.1038/ncb1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okae H, et al. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22(1):50–63. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 103.Kidima WB. Syncytiotrophoblast functions and fetal growth restriction during placental malaria: updates and implication for future interventions. Biomed Res Int. 2015;2015:451735. doi: 10.1155/2015/451735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang CW, Parast MM. Human trophoblast stem cells: Real or not real? Placenta. 2017;60(Suppl 1):S57–S60. doi: 10.1016/j.placenta.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 106.Sato Y, Fujiwara H, Konishi I. Mechanism of maternal vascular remodeling during human pregnancy. Reprod Med Biol. 2012;11(1):27–36. doi: 10.1007/s12522-011-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.James JL, et al. Isolation and characterisation of a novel trophoblast side-population from first trimester placentae. Reprod. 2015;150(5):449–462. doi: 10.1530/REP-14-0646. [DOI] [PubMed] [Google Scholar]
- 108.Ryan JM, Pettit AR, Guillot PV, Chan JK, Fisk NM. Unravelling the pluripotency paradox in fetal and placental mesenchymal stem cells: Oct-4 expression and the case of The Emperor's New Clothes. Stem Cell Rev Rep. 2013;9(4):408–421. doi: 10.1007/s12015-011-9336-5. [DOI] [PubMed] [Google Scholar]
- 109.Castrechini NM, et al. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31(3):203–212. doi: 10.1016/j.placenta.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Fukuchi Y, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 111.Wulf GG, et al. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10(7-8):1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- 112.Matic I, et al. Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Maced J Med Sci. 2016;4(1):9–16. doi: 10.3889/oamjms.2016.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Demir R, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25(6):560–572. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 114.Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat. 1989;136(3):190–203. doi: 10.1159/000146886. [DOI] [PubMed] [Google Scholar]
- 115.Meraviglia V, et al. Human chorionic villus mesenchymal stromal cells reveal strong endothelial conversion properties. Differ. 2012;83(5):260–270. doi: 10.1016/j.diff.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 117.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 118.Shohara R, et al. Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy. 2012;14(10):1171–1181. doi: 10.3109/14653249.2012.706705. [DOI] [PubMed] [Google Scholar]
- 119.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Timeva T, Shterev A, Kyurkchiev S. Recurrent implantation failure: the role of the endometrium. J Reprod Infertil. 2014;15(4):173–183. [PMC free article] [PubMed] [Google Scholar]
- 122.Al-Lamee H, et al. The role of endometrial stem/progenitor cells in recurrent reproductive failure. J Pers Med. 2022;12(5):775. doi: 10.3390/jpm12050775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esmaeilzadeh S, Mohammadi A, Mahdinejad N, Ghofrani F, Ghasemzadeh-Hasankolaei M. Receptivity markers in endometrial mesenchymal stem cells of recurrent implantation failure and non-recurrent implantation failure women: a pilot study. J Obstet Gynaecol Res. 2020;46(8):1393–1402. doi: 10.1111/jog.14340. [DOI] [PubMed] [Google Scholar]
- 124.Karaer A, Cigremis Y, Celik E, Urhan Gonullu R. Prokineticin 1 and leukemia inhibitory factor mRNA expression in the endometrium of women with idiopathic recurrent pregnancy loss. Fertil Steril. 2014;102(4):1091–1095. doi: 10.1016/j.fertnstert.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Salker MS, et al. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One. 2012;7(12):e52252. doi: 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murakami K, et al. Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure--a pilot study. PLoS One. 2013;8(12):e82582. doi: 10.1371/journal.pone.0082582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knofler M, et al. Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol Life Sci. 2019;76(18):3479–3496. doi: 10.1007/s00018-019-03104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mishra A, Galvankar M, Vaidya S, Chaudhari U, Modi D. Mouse model for endometriosis is characterized by proliferation and inflammation but not epithelial-to-mesenchymal transition and fibrosis. J Biosci. 2020;45:1–5. [PubMed] [Google Scholar]
- 129.Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33(5):333–340. doi: 10.1055/s-0035-1564609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Becker CM, et al. Circulating endothelial progenitor cells are up-regulated in a mouse model of endometriosis. Am J Pathol. 2011;178(4):1782–1791. doi: 10.1016/j.ajpath.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 132.Cousins FL, Gargett CE. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:27–38. doi: 10.1016/j.bpobgyn.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 133.Gao Y, Wu G, Xu Y, Zhao D, Zheng L. Stem cell-based therapy for Asherman syndrome: promises and challenges. Cell Transplant. 2021;30:9636897211020734. doi: 10.1177/09636897211020734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mishra A, Galvankar M, Singh N, Modi D. Spatial and temporal changes in the expression of steroid hormone receptors in mouse model of endometriosis. J Assist Reprod Genet. 2020;37(5):1069–1081. doi: 10.1007/s10815-020-01725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome. J Hum Reprod Sci. 2011;4(1):49–52. [PMC free article] [PubMed] [Google Scholar]
- 136.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 137.Obermair A, et al. Improved surgical safety after laparoscopic compared to open surgery for apparent early stage endometrial cancer: results from a randomised controlled trial. Eur J Cancer. 2012;48(8):1147–1153. doi: 10.1016/j.ejca.2012.02.055. [DOI] [PubMed] [Google Scholar]
- 138.Banz-Jansen C, Helweg LP, Kaltschmidt B. Endometrial cancer stem cells: where do we stand and where should we go? Int J Mol Sci. 2022;23(6):3412. doi: 10.3390/ijms23063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCarthy AL, Woolfson RG, Raju SK, Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol. 1993;168(4):1323–1330. doi: 10.1016/0002-9378(93)90389-z. [DOI] [PubMed] [Google Scholar]
- 140.Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. 2018;19(5):1496. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sugawara J, et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90(9):5329–5332. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- 142.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58(2):148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 143.Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci. 2007;12:2383–2394. doi: 10.2741/2240. [DOI] [PubMed] [Google Scholar]
- 144.Atakul T. Serum levels of angiogenic factors distinguish between women with preeclampsia and normotensive pregnant women but not severity of preeclampsia in an obstetric center in Turkey. Med Sci Monit. 2019;25:6935–6942. doi: 10.12659/MSM.915092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luppi P, et al. Maternal circulating CD34+VEGFR-2+ and CD133+VEGFR-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci. 2010;17(7):643–652. doi: 10.1177/1933719110366164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santamaria X, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 147.Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020;77(2):253–265. doi: 10.1007/s00018-019-03268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moll G, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 149.Moll G, et al. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev. 2015;24(19):2269–2279. doi: 10.1089/scd.2015.0120. [DOI] [PubMed] [Google Scholar]
- 150.Moll G, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30(7):1565–1574. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 151.Moll G, Ankrum JA, Olson SD, Nolta JA. Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl Med. 2022;11(1):2–13. doi: 10.1093/stcltm/szab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masuda H, et al. Endometrial side population cells: potential adult stem/progenitor cells in endometrium. Biol Reprod. 2015;93(4):84. doi: 10.1095/biolreprod.115.131490. [DOI] [PubMed] [Google Scholar]
- 153.Bansal AS, et al. Mechanism of human chorionic gonadotrophin-mediated immunomodulation in pregnancy. Expert Rev Clin Immunol. 2012;8(8):747–753. doi: 10.1586/eci.12.77. [DOI] [PubMed] [Google Scholar]
- 154.Horii M, et al. Modeling preeclampsia using human induced pluripotent stem cells. Sci Rep. 2021;11(1):5877. doi: 10.1038/s41598-021-85230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Umapathy A, et al. Mesenchymal stem/stromal cells from the placentae of growth restricted pregnancies are poor stimulators of angiogenesis. Stem Cell Rev Rep. 2020;16(3):557–568. doi: 10.1007/s12015-020-09959-8. [DOI] [PubMed] [Google Scholar]