Abstract
Objective
To evaluate the distribution of chromosomal abnormalities in a recurrent pregnancy loss (RPL) cohort and explore the associations between chromosomal abnormalities and clinical characteristics.
Method
Over a 5-year period, fresh products of conception (POC) from women with RPL were analyzed by single-nucleotide polymorphism (SNP) array at our hospital. After obtaining the information on clinical characteristics, we investigated the associations between the causative chromosomal abnormalities and clinical characteristics by the chi-squared test or Fisher’s exact test and logistic regression.
Results
A total of 2383 cases were enrolled. Overall, 56.9% (1355/2383) were identified with causative chromosomal abnormalities, of which 92.1% (1248/1355) were numerical abnormalities, 7.5% (102/1355) were structural variants, and 0.4% (5/1355) were loss of heterozygosity (LOH). The risk of numerical abnormalities was increased in women with maternal age ≥ 35 years (OR, 1.71; 95% CI, 1.41–2.07), gestational age at pregnancy loss ≤ 12 weeks (OR, 2.78; 95% CI, 1.79–4.33), less number of previous pregnancy losses (twice: OR, 2.32; 95% CI, 1.84–2.94; 3 times: OR, 1.59; 95% CI, 1.23–2.05, respectively), and pregnancy with a female fetus (OR, 1.37; 95% CI, 1.15–1.62). The OR of pregnancy loss with recurrent abnormal CMA was 4.00 (95% CI: 1.87–8.58, P < 0.001) and the adjusted OR was 5.05 (95% CI: 2.00–12.72, P = 0.001). However, the mode of conception was not associated with the incidence of numerical abnormality. No association was noted between structural variants and clinical characteristics.
Conclusion
Chromosomal abnormality was the leading cause of RPL. Numerical chromosome abnormality was more likely to occur in cases with advanced maternal age, an earlier gestational age, fewer previous pregnancy losses, and pregnancy with a female fetus.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-023-02816-w.
Keywords: Chromosomal abnormality, RPL, Products of conception, SNP-array
Introduction
Recurrent pregnancy loss (RPL) is defined as the loss of two or more pregnancies before 20 to 24 weeks of gestation, according to the American Society of Reproductive Medicine (ASRM) [1] and the European Society of Human Reproduction and Embryology (ESHRE) [2]. Epidemiological studies have revealed that it affects about 1–5% of reproductive-age couples [3]. The underlying chromosomal abnormalities account for 25.4–60.4% [4–9] of etiologies for RPL, and the ESHRE guidelines strongly recommended the use of chromosomal microarray analysis (CMA) testing as first-line genetic analysis of the product of conception (POC) [2].
Compared with traditional karyotyping, CMA, including array comparative genomic hybridization (array-CGH) and single-nucleotide polymorphism array hybridization (SNP-array), can detect genome-wide copy number variations (CNVs) with the uncultured cell of POC at a shorter turnaround time, in which SNP-array can detect triploidy, loss of heterozygosity (LOH), and uniparental disomy (UPD) at the same time.
The association between chromosomal abnormalities and clinical characteristics in RPL has been investigated in several studies to help genetic counselling [4, 6, 7, 9, 10]. An increased rate of chromosomal numerical aberrations has been identified in women with advanced maternal age [4], the timing of pregnancy loss [11, 12], and previous conceptions with chromosomal aberration [10, 13]. The conclusions on the relationship between the number of previous pregnancy losses and chromosomal abnormalities have been inconsistent in different studies [6, 9, 14]. It was also unclear whether the incidence of chromosomal abnormality for RPL varied among different modes of conception or fetal gender. [15–17]. In addition, few studies investigated the relationship between structural variants and clinical characteristics.
We performed a retrospective cohort study to explore the associations between various clinical characteristics and the chromosome anomaly detected by SNP-array in RPL cases from our center.
Materials and methods
This study was approved by Ethics Committee, Shanghai First Maternity and Infant Hospital. The results of SNP-array offered for POC from women with RPL referred to Shanghai First Maternity and Infant Hospital between February 2016 and May 2020 were reviewed and analyzed retrospectively. All patients signed the informed consent for genetic testing for POC. Demographic and baseline clinical data such as maternal age, gestational age, the number of previous pregnancy losses, the mode of conception, and CMA results for previous pregnancy loss were collected by the electronic medical record, and fetal gender was obtained from laboratory records of our center. RPL was defined as the loss of two or more pregnancies before 24 weeks of gestation. Cases with failed SNP-array due to maternal-cell contamination (MCC) or missing gestational age were excluded.
All chorionic villus tissue was carefully separated from fresh POC specimens by a dissecting microscope, and the B allele frequency plot was further used to estimate MCC [18].
DNA was extracted using QIAamp DNA Blood and Tissue kits (Qiagen, Dusseldorf, Germany). According to the manufacturer’s protocol, genomic DNA was fragmented, labeled, and hybridized to CytoScan Optima arrays. Affymetrix CytoScan Optima was used to identify chromosomal abnormalities of the DNA of chorionic villus tissue in this study. Affymetrix CytoScan Optima array contains 148,000 oligonucleotide probes and 18,000 SNP probes that can be used for genotyping. The building of the human genome assembly was based on GRCH37. Regions of microdeletion, microduplication, and LOH were displayed at a threshold of 1 megabase pair (Mb), 2 Mb, and 20 Mb, respectively.
Chromosomal abnormalities identified on SNP-array were categorized into numerical anomalies, structural variants, and LOH. Numerical abnormalities included aneuploidy and polyploidy in the study. Structural variants detected on SNP-array in the study included large CNVs (≥ 10 Mb) and microscopic imbalances (< 10 Mb) [6, 19]. which are generally unable to be detected by traditional karyotyping.
The CNVs were classified into pathogenic, likely pathogenic, variants of uncertain significance (VOUS), likely benign, and benign types as per American College of Medical Genetics and Genomics (ACMG) standards and guidelines. A causative structural variant was primarily determined by its ACMG classification [20] and whether the CNVs were reported to be related to pregnancy loss by previous large sample studies. Both experienced clinicians and laboratory technologists will determine the results that are difficult to judge. We defined causative numerical chromosomal abnormality when it was reported to be associated with an increased risk of pregnancy loss. For example, all the autosomal trisomies and XXY conceptions are associated with pregnancy loss, which we classified into “causative abnormality.” However, cases with 47XXX and 47XYY usually have a relatively good prognosis [21, 22] and have not been identified among spontaneous miscarriage or stillbirth, which we did not define as “causative” [23]. We analyzed and classified all samples into two groups: (1) causative and diagnostic numerical and structural variants, (2) non-diagnostic numerical and structural variants (uncertain whether causative of pregnancy loss) or variants undetected, and then investigated the associations between causative and diagnostic numerical and structural variants and clinical characteristics.
All analyses were performed in SPSS 25.0. Number and proportion were used to describe the distribution of chromosomal abnormality, and chi-square tests or Fisher’s exact tests were conducted to compare the differences among different characteristics. Logistic regression was used to evaluate the risk of chromosomal abnormality. A difference was deemed statistically significant if the P-value was < 0.05.
Results
From Feb 2016 to May 2020, 2602 products of the conception (POC) from RPL were received and analyzed by SNP-array; 1 case was excluded due to contamination by maternal DNA, and 218 cases were excluded due to the missing gestational age. The remaining 2383 cases were included in the final analysis. The number of previous pregnancies was as follows: twice in 1215 cases, three times in 739 cases, and ≥ 4 times in 429 cases. The mean gestational age at the time of pregnancy loss and BMI were 8.66 ± 1.91 weeks and 22.00 ± 2.89 kg/m2, respectively. Fetal gender was recorded for 2330 cases and included 1130 males and 1200 females. A total of 1513 women conceived naturally, 205 by in vitro fertilization (IVF) and 27 by intracytoplasmic sperm injection (ICSI). And the mean age of women conceived via naturally, IVF, and ICSI was 31.81 years (range 21–45 years), 34.88 years (range 24–44 years), and 36.11 years (range 26–45 years), respectively. A total of 120 women had more than one POC sample of consecutive pregnancy loss, of which 32 with twice pregnancy losses, 48 with three times pregnancy losses, and 40 with four times pregnancy losses.
Chromosome numerical abnormalities and structural variants on SNP-array
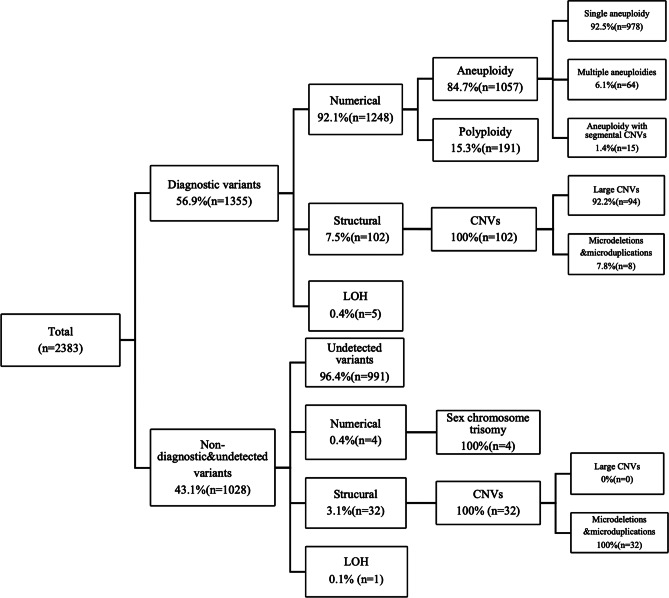
A total of 58.4% (1392/2383) were detected to be with chromosomal abnormalities on SNP-array, of which 89.9% (1252/1392) were numerical chromosome abnormalities, 9.6% (134/1392) were structural variants, and 0.4% (6/1392) were LOH. Overall, after careful evaluation, 56.9% (1355/2383) of the cases were identified to be with diagnostic chromosomal abnormalities, and 1.6% (37/2383) of the cases were considered to be with non-diagnostic variants. The type and number of chromosomal abnormalities and groupings are summarized in Fig. 1.
Fig. 1.
Incidence and spectrum of chromosome variants in POC specimens and grouping. CNVs, copy number variations; LOH, loss of heterozygosity
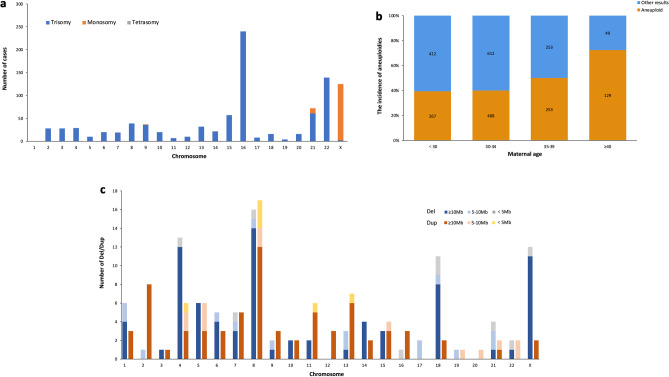
Among cases with diagnostic variants, 92.1% (1248/1355) cases had numerical chromosomal abnormalities, of which there were 84.7% (1057/1248) for aneuploidies and 15.3% (191/1248) for polyploidy. As the most abnormal finding, aneuploidy includes 92.5% (978/1057) single aneuploidy, 6.1% (64/1057) multiple aneuploidies, and 1.4% (15/1057) aneuploidy with segmental CNV. As shown in Fig. 2a, single aneuploidy was observed in all chromosomes except chromosome 1. Most of the single aneuploidies were trisomy (86.1%, 842/978), and the remainders were monosomy (13.8%, 135/978) and tetrasomy (0.1%, 1/978). Trisomy 16 was the most common (28.5%, 240/842), followed by trisomy 22 (16.5%, 139/842) and trisomy 21 (7.2%, 61/842). Monosomic cases were limited to chromosome X in 123 cases, chromosome 21 in 11 cases, and chromosome 9 in 1 case. Only one tetrasomy case was observed, located at chromosome 14. Figure 2b shows the relationship between aneuploidies and maternal age; the frequency of aneuploidy increased with maternal age and was highest when maternal age was over 35.
Fig. 2.
Frequency of causative chromosomal variants detected in RPL cases. a Distribution of single chromosomal aneuploidies. b The incidence of aneuploidies in cases with different maternal ages. c Distribution of causative CNVs detected by SNP-array. CNV, copy number variation; Del, deletion; Dup, duplication; RPL, recurrent pregnancy loss
7.5% (102/1355) of cases carried structural variants on SNP-array, including 92.2% (94/102) large CNVs and 7.8% (8/102) pathogenic microdeletions and microduplications. 87.3% (89/102) cases with large CNVs or deletion coupled with duplication may indicate the underlying balanced parental translocation. Among these, 74 cases were with known parental (48 cases) or only maternal karyotypes (26 cases), and 13.5% (10/74) of cases confirmed with either maternal (7 cases) or paternal (3 cases) balanced translocation.
Figure 2c shows the distribution and size of 191 causative CNV in 102 cases, including 40.8% (78/191) deletions (≥ 10 Mb), 36.6% (70/191) duplications (≥ 10 Mb), 12.6% (24/191) microdeletions (< 10 Mb), and 9.9% (19/191) microduplications (< 10 Mb). CNVs were observed in all chromosomes. Both the deletion and duplication of large CNVs occur most frequently in chromosome 8 (17.9%, 14/78; 17.1%, 12/70, respectively), while microdeletion occurred mostly in chromosomes 18 and 21 (12.5%, 3/24; 12.5%, 3/24, respectively), and microduplication in chromosome 8 (26.3%, 5/19). 0.4% (5/1355) were with genome-wide LOH.
Cases with non-diagnostic variants or no variants detected were identified in 43.1% (1028/2383) of the cases. Of these, 96.4% (991/1028) could not detect any variants, 0.4% (4/1028) were with sex chromosome trisomy (three 47, XYY; and one 47, XXX), 3.1% (32/1028) were with microdeletions and microduplications, and 0.1% (1/1028) were with LOH located on chromosomal 14 and with a size of 52.858 Mb (Supplementary Table S1).
The association between clinical characteristics and chromosomal abnormalities
Table 1 displays the proportion of diagnostic chromosomal anomalies among different clinical features.
Table 1.
The correlations between clinical characteristics and chromosomal variants
| Clinical characteristics | Sample number | Numeral abnormalities | Structural variants | |
|---|---|---|---|---|
| The number of pregnancy losses (times) | 2 | 1215 | 712 (58.6%) | 50 (4.1%) |
| 3 | 739 | 367 (49.7%) | 34 (4.6%) | |
| ≥ 4 | 429 | 169 (39.4%) | 18 (4.2%) | |
| P | < 0.001 | 0.872 | ||
| Gestational age (week) | ≤ 12 | 2278 | 1212 (53.2%) | 98 (4.3%) |
| > 12 | 105 | 36 (34.3%) | 4 (3.8%) | |
| P | < 0.001 | 0.998 | ||
| Maternal age (years) | < 35 | 1699 | 823 (48.4%) | 86 (5.1%) |
| ≥ 35 | 684 | 425 (62.1%) | 16 (2.3%) | |
| P | < 0.001 | 0.003 | ||
| Fetal gender | Male | 1130 | 554 (49.0%) | 48 (4.2%) |
| Female | 1200 | 674 (56.2%) | 49 (4.1%) | |
| P | < 0.001 | 0.843 | ||
| Mode of conception | Natural | 1513 | 806 (53.3%) | 52 (3.4%) |
| IVF | 205 | 99 (48.3%) | 4 (2.0%) | |
| ICSI | 27 | 16 (59.3%) | 1 (3.7%) | |
| P | 0.323 | 0.454 | ||
| The CMA of previous pregnancy loss | Normal | 58 | 21 (36.2%) | 1 (1.7%) |
| Abnormal | 62 | 40 (64.5%) | 4 (6.5%) | |
| P | 0.002 | 0.402 |
The proportion of numerical chromosome abnormalities decreased significantly with the increased number of pregnancy losses (P < 0.001), with 58.6% for women with two pregnancy losses, 49.7% for three pregnancy losses, and 39.4% for ≥ 4 pregnancy losses. The rate of numerical chromosome abnormalities was significantly higher in women with maternal age ≥ 35 years (62.1%) than in those < 35 years (48.4%, P < 0.001). The numerical abnormality rate in women with gestational age ≤ 12 weeks (53.2%) was much higher than in those > 12 weeks (34.3%, P < 0.001). Also, the loss of a female fetus (56.2%) had a higher numerical abnormalities rate than that of a male fetus (49.0%, P < 0.001). In addition, in 120 RPL cases with two CMA results, the numerical abnormality rate in those with abnormal CMA in previous pregnancy loss (64.5%) was higher than in those with normal CMA (36.2%, P = 0.002). However, the proportion of numerical abnormalities was not different among different modes of conception (P = 0.323).
The proportion of structural variants was higher in women with maternal age < 35 years (5.1%) than in those ≥ 35 years (2.3%, P = 0.003). No association was observed between structural variants and other clinical features, such as times of pregnancy loss (P = 0.872), different gestational ages (P = 0.998), fetal gender (P = 0.843), abnormal CMA results or not in previous pregnancy loss (P = 0.402), and different modes of conception (P = 0.454).
Table 2 shows the results from multiple logistic analyses. Compared with the corresponding comparison groups, the risk of numerical aberration was increased in women with advanced age (≥ 35 years, OR, 1.71; 95% CI, 1.41–2.07), an earlier gestational age (≤ 12 weeks, OR, 2.78; 95% CI, 1.79–4.33), less number of previous pregnancy loss (twice: OR, 2.32; 95% CI, 1.84–2.94; 3 times: OR, 1.59; 95% CI, 1.23–2.05, respectively), and female fetus (OR, 1.37; 95% CI, 1.15–1.62). No association was observed between the above clinical factors and structural variants.
Table 2.
Multiple logistic regression analyses of clinical risk characteristics for chromosomal variants
| Variable | n | Numerical aberration | Structural variants | ||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Maternal age (years) | |||||
| < 35 (reference) | 1662 | ||||
| ≥ 35 | 668 | 1.71 (1.41–2.07) | < 0.001 | 0.65 (0.37–1.13) | 0.128 |
| Gestational age (week) | |||||
| ≤ 12 | 2229 | 2.78 (1.79–4.33) | < 0.001 | 1.65 (0.58–4.63) | 0.345 |
| > 12(reference) | 101 | ||||
| The number of pregnancy losses (times) | |||||
| 2 | 1192 | 2.32 (1.84–2.94) | < 0.001 | 1.61 (0.90–2.90) | 0.112 |
| 3 | 722 | 1.59 (1.23–2.05) | < 0.001 | 1.41 (0.76–2.63) | 0.280 |
| ≥ 4(reference) | 416 | ||||
| Fetal gender | |||||
| Male (reference) | 1130 | ||||
| Female | 1200 | 1.37 (1.15–1.62) | < 0.001 | 1.12 (0.74–1.71) | 0.576 |
Among 120 women with abnormal CMA in previous pregnancy loss, 36.7% (44/120) were with chromosomal abnormalities in both miscarriages, of which 68.2% (30/44) were with recurrent aneuploidy, 2.3% (1/44) with recurrent triploidy, and 29.5% (13/44) with combinations of different types of variants. For women who had pregnancy loss with an abnormal CMA, the proportion of their subsequent pregnancy loss with an abnormal CMA is higher than that with a normal CMA (71.0% vs. 29.0%). Binary logistic regression showed that the OR of pregnancy loss with recurrent abnormal CMA is 4.00 (95% CI:1.87–8.58); after adjusting maternal age, gestational age, the number of previous pregnancy losses, and fetal gender, the OR was 5.05 (95% CI: 2.00–12.72).
Discussion
The genetic factor is the leading cause of pregnancy loss. Our study showed that the proportion of chromosomal abnormalities in RPL women was 58.4%, and aneuploidy was the most frequently observed abnormality on SNP-array, consistent with the previous studies [4, 6]. In this study, we analyzed the association between causative variants and clinical characteristics of RPL based on the largest sample size of its kind as far as we know and relatively comprehensive clinical features, which was more focused and may be more helpful in clinical counseling.
The incidence of chromosomal numerical abnormalities increased with maternal age due to the precocious separation of sister chromatids or pre-division and reverse segregation of oocytes [24]. A higher rate of numerical abnormalities was identified in RPL at an earlier gestational age, which was consistent with the previous studies [8, 12]. Our study showed that the more number of previous pregnancy losses that RPL women experienced, the less likely they were to have current pregnancy loss related to numerical abnormalities and more prone to normal chromosomes. For women with multiple pregnancy losses, a chromosomal normal pregnancy loss may be a predictor for subsequent pregnancy loss [7] and may indicate an underlying permanent unfavorable maternal factor [25], such as single gene disorders [26–28] which are involved in embryonic development and may lead to fetal death, and increase the risk of women with the normal chromosome of a subsequent pregnancy loss.
The study also identified that females might be more susceptible to RPL than males, consistent with previous studies [29, 30]. The specific mechanism of the higher incidence of female fetuses is still unknown. One possible reason was that we could not differentiate cases with male 45XO from female 45XO, which may overestimate the proportion of the female fetal loss, and this might also be the explanation for numerical chromosomal abnormalities that were more frequently observed in females than in males. The other possible reason was X-chromosome inactivation. Since one of the female fetus’ two copies of the X-chromosome was inactivated randomly during embryogenesis (X-chromosome inactivation), the expression of genes from the maternal X-chromosome in male embryos may support a more stable development during early embryogenesis and the mutation of X-chromosome is more prone to benefit males [31], as compared with female embryos [32]. More researches are needed to be conducted to verify the association between fetal gender and numerical chromosomal abnormalities and clarify the specific mechanism.
Our study showed that previous loss with chromosomal abnormalities might increase the recurrent risk, particularly the recurrent risk of numeral chromosome abnormalities, consistent with prior studies [10, 33, 34]. It has been reported that maternal germline mosaicism, maternal skewed X-chromosome inactivated [35, 36], and mutation of methylenetetrahydrofolate reductase gene [37] were possible mechanisms.
We did not find any association between structural variants with maternal age, the number of pregnancy losses, gestational age, or fetal gender for the RPL cohort, which was consistent with previous studies [12, 38, 39]. However, structural variants were found to happen more frequently in younger pregnancies, which may be associated with a balanced parental translocation. Our data showed that IVF or ICSI did not increase the proportion of chromosome aberration of RPL. It is still controversial whether there is an association between chromosomal abnormalities and the mode of conception. Several studies reported that the ratio of chromosomal abnormalities slightly increased in ICSI pregnancies [40, 41]. However, it has been unclear whether the difference was related to the ICSI technique itself or some etiologies of male infertility, such as morphologically abnormal spermatozoa, which was thought to be associated with a higher risk of chromosomal abnormalities for offspring [42].
Several limitations should be acknowledged: firstly, it is a retrospective study; information on fetal gender, mode of conception, and the results of chromosomal analysis for previous pregnancy loss were incomplete, especially since the sample size of women convinced by IVF and ICSI is limited, and we lack the information on the indication for IVF/ICSI, which may lead to biased on the association between above clinical characteristics and chromosomal abnormalities. Secondly, though SNP-based CMA can suggest part of the presence of contaminating maternal DNA [18], according to our result, the female/male ratio is 1.06, in accordance with the conclusion that total female mortality during pregnancy slightly exceeds male mortality [43]. We could not accurately exclude cases with MCC. We did not substantially perform a quantitative fluorescent-polymerase chain reaction based on short tandem repeat marker analysis. It was costly to patients and difficult to acquire maternal peripheral blood in the routine clinical environment. The limitations of interpretation were indicated in the report when a level interfered with the interpretation of fetal results. Thirdly, due to the limited number of SNP probes in the study, the resolution of genomic abnormalities is limited, and we may not detect all diagnostic CNVs. Finally, SNP-array in the study could only provide genetic information on the current pregnancy loss. The genetic etiology for RPL could only be partly investigated due to the lack of genetic information for previous pregnancy loss in the study, which would warrant a well-designed prospective study in the near future.
In conclusion, chromosomal abnormality is the leading cause of pregnancy loss. The incidence of chromosome abnormality was associated with maternal age, the number of previous pregnancy losses, gestational age, and fetal gender, and it may be more prone to be observed in women with previous chromosomal abnormally pregnancy loss and may not be related to the mode of conception. More researches are needed to be carried out to clarify the mechanism of chromosomal abnormalities in RPL and to explore the application of next-generation sequence [27, 44, 45] in investigating other underlying genetic factors for RPL with unknown reasons.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients in our study, physicians who recorded the clinical data, and center members who supported us.
Funding
The study was supported by the National Key Research and Development Program of China (2022YFC2704700, 2022YFC2704703).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
The study was reviewed and approved by the Ethical Committee of Shanghai First Maternity and Infant Hospital (KS2203).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Practice Committee of the American Society for Reproductive M Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Bender Atik R, Christiansen OB, RPL EGGo et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):4. doi: 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai R, Regan L. Recurrent miscarriage. The Lancet. 2006;368(9535):601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 4.Grande M, Borrell A, Garcia-Posada R, et al. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27(10):3109–3117. doi: 10.1093/humrep/des251. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104(4):784–788. doi: 10.1097/01.AOG.0000137832.86727.e2. [DOI] [PubMed] [Google Scholar]
- 6.Sheng YR, Hou SY, Hu WT, et al. Characterization of copy-number variations and possible candidate genes in recurrent pregnancy losses. Genes (Basel). 2021;12(2):141. [DOI] [PMC free article] [PubMed]
- 7.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–304. doi: 10.1016/s0015-0282(99)00495-1. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, Li K, Li R, et al. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front Genet. 2021;12:667697. doi: 10.3389/fgene.2021.667697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein M, Svirsky R, Reches A, Yaron Y. Does the number of previous miscarriages influence the incidence of chromosomal aberrations in spontaneous pregnancy loss? J Matern Fetal Neonatal Med. 2017;30(24):2956–2960. doi: 10.1080/14767058.2016.1269317. [DOI] [PubMed] [Google Scholar]
- 10.Nikitina TV, Sazhenova EA, Zhigalina DI, Tolmacheva EN, Sukhanova NN, Lebedev IN. Karyotype evaluation of repeated abortions in primary and secondary recurrent pregnancy loss. J Assist Reprod Genet. 2020;37(3):517–525. doi: 10.1007/s10815-020-01703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am J Med Genet A. 2016;170(10):2671–2680. doi: 10.1002/ajmg.a.37795. [DOI] [PubMed] [Google Scholar]
- 12.Qu S, Wang L, Cai A, et al. Exploring the cause of early miscarriage with SNP-array analysis and karyotyping. J Matern Fetal Neonatal Med. 2019;32(1):1–10. doi: 10.1080/14767058.2017.1367379. [DOI] [PubMed] [Google Scholar]
- 13.Munne S, Sandalinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn. 2004;24(8):638–643. doi: 10.1002/pd.957. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–304. doi: 10.1016/s0015-0282(99)00495-1. [DOI] [PubMed] [Google Scholar]
- 15.Ma S, Philipp T, Zhao Y, Stetten G, Robinson WP, Kalousek D. Frequency of chromosomal abnormalities in spontaneous abortions derived from intracytoplasmic sperm injection compared with those from in vitro fertilization. Fertil Steril. 2006;85(1):236–239. doi: 10.1016/j.fertnstert.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Lathi RB, Milki AA. Rate of aneuploidy in miscarriages following in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2004;81(5):1270–1272. doi: 10.1016/j.fertnstert.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Causio F, Fischetto R, Sarcina E, Geusa S, Tartagni M. Chromosome analysis of spontaneous abortions after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) Europ J Obstet Gynecol Reprod Biol. 2002;105(1):44–48. doi: 10.1016/s0301-2115(02)00151-3. [DOI] [PubMed] [Google Scholar]
- 18.Sahoo T, Dzidic N, Strecker MN, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19(1):83–89. doi: 10.1038/gim.2016.69. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Li Y, Chen Y, et al. Systematic analysis of copy-number variations associated with early pregnancy loss. Ultrasound Obstet Gynecol. 2020;55(1):96–104. doi: 10.1002/uog.20412. [DOI] [PubMed] [Google Scholar]
- 20.Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) Genet Med. 2020;22(2):245–257. doi: 10.1038/s41436-019-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47, XXX) Orphanet J Rare Dis. 2010;5:8. doi: 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardsley MZ, Kowal K, Levy C, et al. 47, XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163(4):1085–1094. doi: 10.1016/j.jpeds.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassold TJ, Jacobs PA. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- 24.Gruhn JR, Zielinska AP, Shukla V, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365(6460):1466–1469. doi: 10.1126/science.aav7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner RJM, Amor DJ. Gardner and Sutherland’s chromosome abnormalities and genetic counseling. 5th ed. Oxford University Press; 2018. p. 781.
- 26.Qiao Y, Wen J, Tang F, et al. Whole exome sequencing in recurrent early pregnancy loss. Mol Hum Reprod. 2016;22(5):364–372. doi: 10.1093/molehr/gaw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Chai H, Zhou Q, et al. Exome sequencing analysis on products of conception: a cohort study to evaluate clinical utility and genetic etiology for pregnancy loss. Genet Med. 2021;23(3):435–442. doi: 10.1038/s41436-020-01008-6. [DOI] [PubMed] [Google Scholar]
- 28.Gourhant L, Bocher O, De Saint ML, et al. Whole exome sequencing, a hypothesis-free approach to investigate recurrent early miscarriage. Reprod Biomed Online. 2021;42(4):789–798. doi: 10.1016/j.rbmo.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Del Fabro A, Driul L, Anis O, et al. Fetal gender ratio in recurrent miscarriages. Int J Womens Health. 2011;3:213–217. doi: 10.2147/IJWH.S20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng HH, Ou CY, Tsai CC, et al. Chromosome distribution of early miscarriages with present or absent embryos: female predominance. J Assist Reprod Genet. 2014;31(8):1059–1064. doi: 10.1007/s10815-014-0261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patten MM. The X chromosome favors males under sexually antagonistic selection. Evolution. 2019;73(1):84–91. doi: 10.1111/evo.13646. [DOI] [PubMed] [Google Scholar]
- 32.Evdokimova VN, Nikitina TV, Lebedev IN, Sukhanova NN, Nazarenko SA. Sex ratio in early embryonal mortality in man. Ontogenez. 2000;31(4):251–257. [PubMed] [Google Scholar]
- 33.Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27(8):2297–2303. doi: 10.1093/humrep/des179. [DOI] [PubMed] [Google Scholar]
- 34.Finley J, Hay S, Oldzej J, et al. The genomic basis of sporadic and recurrent pregnancy loss: a comprehensive in-depth analysis of 24,900 miscarriages. Reprod Biomed Online. 2022;45(1):125–134. doi: 10.1016/j.rbmo.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Sangha KK, Stephenson MD, Brown CJ, Robinson WP. Extremely skewed X-chromosome inactivation is increased in women with recurrent spontaneous abortion. Am J Hum Genet. 1999;65(3):913–917. doi: 10.1086/302552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beever CL, Stephenson MD, Penaherrera MS, et al. Skewed X-chromosome inactivation is associated with trisomy in women ascertained on the basis of recurrent spontaneous abortion or chromosomally abnormal pregnancies. Am J Hum Genet. 2003;72(2):399–407. doi: 10.1086/346119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Park SY, Choi JW, et al. Association between MTHFR 1298A>C polymorphism and spontaneous abortion with fetal chromosomal aneuploidy. Am J Reprod Immunol. 2011;66(4):252–258. doi: 10.1111/j.1600-0897.2011.00996.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Cheng Q, Meng L, et al. Clinical application of SNP array analysis in first-trimester pregnancy loss: a prospective study. Clin Genet. 2017;91(6):849–858. doi: 10.1111/cge.12926. [DOI] [PubMed] [Google Scholar]
- 39.Fan L, Wu J, Wu Y, et al. Analysis of chromosomal copy number in first-trimester pregnancy loss using next-generation sequencing. Front Genet. 2020;11:545856. doi: 10.3389/fgene.2020.545856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belva F, Bonduelle M, Buysse A, et al. Chromosomal abnormalities after ICSI in relation to semen parameters: results in 1114 fetuses and 1391 neonates from a single center. Hum Reprod. 2020;35(9):2149–2162. doi: 10.1093/humrep/deaa162. [DOI] [PubMed] [Google Scholar]
- 41.Samli H, Solak M, Imirzalioglu N, Beyatli Y, Simsek S, Kahraman S. Fetal chromosomal analysis of pregnancies following intracytoplasmic sperm injection with amniotic tissue culture. Prenat Diagn. 2003;23(10):847–850. doi: 10.1002/pd.706. [DOI] [PubMed] [Google Scholar]
- 42.Kim JW, Lee WS, Yoon TK, et al. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153. doi: 10.1186/1471-2350-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orzack SH, Stubblefield JW, Akmaev VR, et al. The human sex ratio from conception to birth. Proc Natl Acad Sci U S A. 2015;112(16):E2102–E2111. doi: 10.1073/pnas.1416546112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang D, Wang Y, Ji X, et al. Clinical application of whole-genome low-coverage next-generation sequencing to detect and characterize balanced chromosomal translocations. Clin Genet. 2017;91(4):605–610. doi: 10.1111/cge.12844. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Bartanus J, Liang D, et al. Characterization of chromosomal abnormalities in pregnancy losses reveals critical genes and loci for human early development. Hum Mutat. 2017;38(6):669–677. doi: 10.1002/humu.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.