Abstract
Purpose
Recurrent pregnancy loss (RPL) is affecting 1–4% of women who conceive approximately, and no cause could be found in more than 50% of women suffering from RPL. Inherited thrombophilias have got increasing attention in women with unexplained RPL, so we aim to explore the relationship among these most common thrombophilic polymorphisms and RPL through a literature review and meta-analysis.
Methods
Observational studies from PubMed, Embase, Cochrane, and Web of Science from 1997 to 7 April 2022 were searched. For each genetic variant, a fixed or random-effect model was used according to the heterogeneity test to calculate pooled ORs and 95% CIs for both dominant and recessive genetic models. Egger’s line regression test was used to assess publication bias. The quality of the included articles was assessed by the Newcastle Ottawa scale.
Results
A total of 124 articles comprising 17,278 RPL patients and 16,021 controls were included. Results showed that hyperhomocysteinemia (MTHFR) C677T (dominant model: OR, 1.43; 95% CI, 1.25–1.64; recessive model: OR, 1.60; 95% CI, 1.36–1.87), MTHFR A1298C (dominant model: OR, 1.66; 95% CI, 1.26–2.18; recessive model: OR, 1.79; 95% CI, 1.42–2.26), PAI-1 4G/5G (dominant model: OR, 1.67; 95% CI, 1.36–2.06; recessive model: OR, 1.80; 95% CI, 1.39–2.32), angiotensin-converting enzyme I/D (OR, 1.23; 95% CI, 1.00–1.53), Factor XIII V34L (OR, 1.38; 95% CI, 1.02–1.87), and β-fibrinogen-455G/A (OR, 1.60; 95% CI, 1.02–2.51) were significantly associated with RPL.
Conclusion
This study provides potentially useful clinical markers to evaluate the risk of RPL or to help unexplained RPL patients identify possible causes, which may allow for targeted treatment.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-023-02823-x.
Keywords: Meta-analysis, Recurrent pregnancy loss, Thrombophilic gene polymorphisms, Clinical marker
Introduction
Although the definition of recurrent pregnancy loss (RPL) is highly controversial in several studies, it is generally accepted that RPL is the spontaneous end of two or more clinically recognized pregnancies, as defined by Guidelines from the American Society for Reproductive Medicine [1]. It is estimated that approximately 1–4% of women who conceive are affected by RPL and the rates may be underestimated due to inconsistencies in definition and classification [2, 3]. The underlying triggers vary among women, but several common risk factors are identified including maternal age, health, genetic abnormalities, environmental, and lifestyle factors [4]. However, more than 50% of women suffering from RPL have no clearly identifiable etiology [1, 5, 6].
Inherited thrombophilia, which increases the risk of forming venous or arterial thromboembolism, could be one of the suspected causes for women with unexplained RPL. The normal growth and development of the fetus depends on adequate blood supply in the placental circulation. The persistent abnormal hypercoagulable states in women with inherited thrombophilias, and the hemostatic balance shifting towards hypercoagulability physiologically in pregnant women, lead to a tendency to form blood clots and, therefore, disrupt this blood supply and impede the growth and development of embryos [7]. Several related genetic risk factors have been discovered including coagulation factors (Factor II, Factor V, Factor XIII, and fibrinogenβ), defects of the fibrinolytic system (PAI-1), hyperhomocysteinemia (MTHFR), and angiotensin-converting enzyme (ACE). When the genes that encode these factors are mutated, the risk of thromboembolism is greatly increased. Yet the results of studies researching the association between inherited thrombophilias and RPL were discrepant. Liu et al. had done a thorough and comprehensive assessment on the relationship between RPL and the two common polymorphisms factor V Leiden and G20210A mutation of the prothrombin gene recently [8], but there is still no consensus on other polymorphisms, including MTHFR C677T, MTHFR A1298C, PAI-1 4G/5G, ACE I/D, Factor V R2, Factor XIII V34L, and β-fibrinogen-455G/A. Specific information about these polymorphisms is shown in Table 1. In this case, integrating data from similar research for these polymorphisms would be necessary.
Table 1.
Specific information about thrombophilic gene polymorphisms
| Gene | Variant | Minor allele | Consequence | Studies | Cases | Controls |
|---|---|---|---|---|---|---|
| MTHFR | C677T | T | Elevated plasma homocysteine levels | 85 | 11,823 | 11,776 |
| MTHFR | A1298C | C | Elevated plasma homocysteine levels | 40 | 7121 | 6739 |
| PAI-1 | 4G/5G | 4G | Elevated plasma PAI-1 levels | 34 | 5294 | 4254 |
| ACE | I/D | Deletion | Higher serum ACE levels | 26 | 4284 | 2992 |
| Factor V | R2/HR2 | G | Poor response to activated protein C | 13 | 1771 | 1172 |
| Factor XIII | G103T | T | Interferes with fibrin cross-linking and regulation of fibrinolysis | 15 | 1282 | 1093 |
| β-Fibrinogen | G455A | A | Elevated the plasma fibrinogen levels | 12 | 1258 | 891 |
The aim of this study was to perform a literature review and meta-analysis to determine the association between patients diagnosed with RPL and common thrombophilic polymorphisms.
Materials and methods
Information sources, search strategy
This meta-analysis was conducted by two authors independently. We screened studies published from 1997 to 7 April 2022 without any restriction of countries from databases PubMed, Embase, Cochrane, and Web of Science. The major search terms used in the strategy include RPL, MTHFR C677T, MTHFR A1298C, PAI-1 4G/5G, ACE I/D, Factor V R2, Factor XIII V34L, and β-fibrinogen-455G/A. More detailed search terms are listed in Supplemental Data 1. Moreover, references of relative reviews and meta-analyses (other sources) were manually checked to ensure all the eligible studies. Only studies published in English and Chinese were included. We contacted authors via e-mail if needed. The protocol for this review was registered in advance on International Prospective Register of Systematic Reviews with ID CRD42022327937.
Eligibility criteria
Inclusion criteria were as follows: (1) observational studies (cohort or case–control study) searching the relationship between RPL and genetic polymorphisms; (2) at least two groups where one was diagnosed with RPL and the other was healthy population; (3) genotypes involved in the candidate genes; (4) study population only women were included; (5) reliable genetic method to detect genotype; (6) sufficient information of genotyping to calculate ORs and the corresponding 95% CIs. Literature would be excluded in the following cases: (1) reviews, letters, case reports, or abstracts; (2) cases with anatomic, chromosomal, hormonal, autoimmune, infectious, or other known causes. Only one would be included if there were several studies from the same population with the same distribution of genotypes.
Exposure and outcomes
The key exposure variable was the presence of hereditary thrombophilia, including MTHFR, PAI-1, ACE, Factor V, Factor XIII, and β-fibrinogen mutation. C677T and A1298C are the two most common polymorphisms of MTHFR, with C > T substitution at nucleotide 677 and A > C substitution at nucleotide 1298, respectively. The PAI-1 4G/5G mutation results in a common guanosine insertion/deletion 675-bp upstream from the start site of translation. The most studied ACE I/D polymorphism results from an Alu element insertion or deletion in intron 16 of the ACE gene. A novel complex haplotype called R2 with an A to G transition at nucleotide 4070 in exon 13 of the gene is one of the exposures here. Factor XIII Val34Leu is a common polymorphism where a G-to-T transition (FXIII G103T) is in exon 2 of the gene encoding for FXIIIA. Fibrinogen variants were studied here because the synthesis of the fibrinogen β-chain is considered to be the rate-limiting step in the fibrinogen biosynthesis, especially G–455A substitution in the 5-flanking region.
The main outcome was RPL, including early RPL and late RPL. Early RPL was defined as pregnancy losses before the 12th week of gestation, while late RPL was defined as pregnancy losses after the 12th week of gestation.
Quality assessment
The quality of the included articles was assessed by the Newcastle Ottawa scale by two authors from the following eight major criteria: adequate determination of cases, representativeness of the cases, selection of controls, determination of controls, comparability of cases and controls, ascertainment of exposure, same method of ascertainment for cases and controls, and non-response rate [9]. With the maximum score of 9, the higher the score, the better the quality. Studies with low scores (< 5) will be excluded.
Data extraction
For the articles finally included, the following data were abstracted: the first author’s name, publication year, geographic region, sample size, distribution of genotypes, mean age of the population, method of genotyping, and definition of RPL. Any divarication was assessed again by two reviewers or by consulting a third author.
Statistical analysis
The effect magnitude to measure the association between thrombophilic gene polymorphism and RPL was odds ratios (ORs) with 95% CIs. For each genetic variant, a fixed or random-effect model was used according to the heterogeneity test to calculate pooled ORs and 95% CIs for both dominant and recessive genetic models. For a pair of alleles A and a, where A is the major allele and a is the minor allele, the dominant model refers to Aa + aa vs. AA, and the recessive model refers to AA + Aa vs. aa. To qualify the effect of heterogeneity, we used I2 statistic which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (I2 > 50% implied a high degree of heterogeneity, and I2 < 50% implied a low degree of heterogeneity) [10]. Publication bias was assessed by Egger’s line regression test [11]. Trim and fill method was used in models with significant publication bias to evaluate the robustness of results by correcting for bias [12]. Sensitivity analysis was performed by excluding each included study and repeating the meta-analysis to assess the stability of the results. In addition, in view of the defining differences of RPL and widely distributed geographical regions, we performed the subgroup analyses according to the number of pregnancy loss (2 or more pregnancy losses, 3 or more pregnancy losses), gestational age at pregnancy loss (early RPL, late RPL), and ethnicity (Caucasian, non-Caucasian). P < 0.05 was considered statistically significant for all the analyses. All the analyses were conducted using R programming language (R-4.1.3).
Results
Study selection and characteristics
The specific process of literature screening is shown in the flow diagram of articles included in the meta-analysis (Fig. 1). A total of 124 articles published from 1997 to 2022 were included in this meta-analysis. The detailed information of the 124 included articles is shown in Table 2. In total, 120 were in English and 4 were in Chinese. Of the studies included here, 123 were case–control studies, and only one was a cohort study. These studies were conducted in 37 countries worldwide, with the population in 98 studies being Caucasian and in 26 studies being non-Caucasian. After the quality assessment, all studies were rated as having medium or high quality, with scores ranging from 5 to 9. The results of meta-analyses and subgroup analyses are presented in Table 3 and Table 4, respectively.
Fig. 1.
Flow diagram of articles included in the meta-analysis
Table 2.
The detailed information of included articles
| MTHFR (C677T) | |||||||||
| Author | Year | Country | Genotype frequency(C/C:C/T:T/T) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| W L Nelen [13] | 1997 | Netherlands | 77:79:29 | 48:59:6 | NA | NA | 2 or more spontaneous consecutive miscarriages before 17 weeks of gestation from the same partner | PCR–RFLP | 8 |
| I Quere [14] | 1998 | France | 28:52:20 | 32:54:14 | NA | NA | Recurrent early miscarriages of unknown cause (≥ 3 consecutive episodes) | PCR–RFLP | 6 |
| Z R Holmes [15] | 1999 | UK | 71:47:11 | 31:30:6 | NA | NA | At least 3 consecutive miscarriages before 12 weeks of gestation | PCR–RFLP | 6 |
| A Lissak [16] | 1999 | Israel | 17:20:4 | 7:7:4 | NA | NA | ≥ 2 consecutive first-trimester spontaneous abortions or a total of ≥ 3 first-trimester spontaneous abortions | PCR–RFLP | 6 |
| R P Murphy [17] | 2000 | Ireland | 18:19:3 | 214:270:56 | 32 | 25 | At least 2 previous and unexplained events at any point during pregnancy | PCR–RFLP | 6 |
| M L Wramsby [18] | 2000 | Sweden | 27:32:3 | 27:35:7 | NA | NA | At least 3 spontaneous consecutive miscarriages | PCR–RFLP | 6 |
| R Pihusch [19] | 2001 | Germany | 41:47:14 | 55:61:12 | 35 | 32 | 2 or more unexplained consecutive abortions at 25 weeks of gestation | PCR–RFLP | 6 |
| A Dilley [20] | 2002 | USA | 27:25:7 | 39:45:9 | 36 | 33 | 3 or more fetal losses, regardless of trimester of loss or previous live birth, or any late loss | PCR–RFLP | 8 |
| Gertrud Unfried [21] | 2002 | Austria | 64:46:23 | 46:24:4 | 32 | 56 | At least 3 spontaneous, consecutive miscarriages before 20 weeks of gestation | PCR–RFLP | 7 |
| Wang YW [22] | 2002 | China | 13:33:16 | 43:53:23 | 28.23 | 28.7 | 2 or more abortions with unexplained causes | PCR–RFLP | 8 |
| K S D Kumar [23] | 2003 | India | 18:6:0 | 22:2:0 | 26.1 | NA | 3 or more consecutive pregnancy losses at less than 22 weeks of gestation | PCR-SSCP | 8 |
| Maria Hohlagschwandtner [24] | 2003 | Austria | 72:52:21 | 53:41:7 | 32 | 56 | 3 or more consecutive spontaneous miscarriages before 20 weeks of gestation | PCR-ASO | 7 |
| T Buchholz [25] | 2003 | Germany | 74:87:22 | 55:61:11 | 35 | 32.8 | At least 2 unexplained consecutive spontaneous miscarriages before 25 weeks of gestation | PCR–RFLP | 7 |
| Hans-Ulrich Pauer [26] | 2003 | Germany | 28:32:9 | 64:51:15 | NA | NA | 2 or more consecutive miscarriages | PCR–RFLP | 6 |
| Aiko Makino [27] | 2004 | Japan | 33:42:10 | 29:32:15 | 31 | 30 | 2 or more unexplained first-trimester recurrent embryonal losses (before 10 weeks of gestation) | PCR–RFLP | 7 |
| Wang XP [28] | 2004 | China | 49:78:20 | 43:34:5 | 27.7 | 32 | 3 or more consecutive pregnancy losses before 20 weeks of gestation | PCR–RFLP | 6 |
| Li XM [29] | 2004 | China | 16:32:9 | 25:20:5 | 28.97 | 27.86 | ≥ 2 spontaneous abortions | PCR–RFLP | 6 |
| Li-xue Guan [30] | 2005 | China | 13:59:55 | 19:73:25 | 27 | 26 | At least 3 spontaneous abortions | PCR–RFLP | 7 |
| Gen Kobashi [31] | 2005 | Japan | 15:20:3 | 67:82:25 | NA | 29.8 | 2 or more consecutive spontaneous with unexplained etiological causes | PCR–RFLP | 6 |
| Song LY [32] | 2005 | China | 36:2:12 | 40:12:4 | NA | NA | Unexplained repeated spontaneous abortions | PCR–RFLP | 6 |
| Egle Couto [33] | 2005 | Brazil | 29:47:12 | 53:26:9 | NA | NA | Recurrent spontaneous abortions | PCR–RFLP | 8 |
| Carolyn B Coulam [34] | 2006 | USA | 58:80:12 | 11:9:0 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| N Mtiraoui [35] | 2006 | Tunisia | 92:47:61 | 156:30:14 | 28.68 | 28.24 | 3 or more consecutive RPLs at 5–30 weeks of gestation | PCR–RFLP | 8 |
| Dong, S.Q [36] | 2006 | China | 2:14:20 | 12:27:18 | 29.58 | 27.04 | 3 or more spontaneous abortions | PCR–RFLP | 8 |
| Alexandros Sotiriadis [37] | 2007 | Greece | 24:61:12 | 32:57:13 | 32.2 | 32.2 | 3 or more consecutive miscarriages with the same partner in < 15 weeks of gestation | PCR–RFLP | 8 |
| Venkatesan Vettriselvi [38] | 2008 | India | 86:15:3 | 98:19:3 | NA | NA | 3 or more spontaneous consecutive miscarriages less than 20 weeks of gestation | PCR–RFLP | 6 |
| Arijit Biswas [39] | 2008 | India | 74:11:0 | 23:8:0 | 27.9 | 26 | With spontaneous recurrent abortions (mean number of recurrent abortions, 3) | PCR–RFLP | 6 |
| Bettina Toth [40] | 2008 | Germany | 71:68:12 | 68:70:19 | 33.2 | 45.2 | 2 or three and more consecutive miscarriages | PCR–RFLP | 6 |
| Rupak Mukhopadhyay [41] | 2009 | India | 75:6:3 | 78:2:0 | NA | NA | 2 or more than two pregnancy losses | PCR–RFLP | 8 |
| Vinukonda Govindaiah [42] | 2009 | USA | 111:25:4 | 112:28:0 | NA | NA | 3 or more unexplained recurrent pregnancy losses | PCR–RFLP | 7 |
| C Ciacci [43] | 2009 | Italy | 16:15:8 | 25:35:12 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Jeehyeon Bae [44] | 2009 | Korea | 82:104:36 | 45:63:14 | 32.6 | 31.2 | More than 2 consecutive abortions | PCR–RFLP | 7 |
| Gonca Imir Yenicesu [45] | 2010 | Turkey | 133:109:30 | 32:24:0 | 27.2 | 29.5 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 7 |
| Agnieszka Seremak-Mrozikiewicz [46] | 2010 | Poland | 44:49:11 | 89:67:13 | 30.15 | 29.4 | 3 or more unexplained consecutive recurrent miscarriages in the first trimester of pregnancy (6–13 week of gestation) | PCR–RFLP | 7 |
| Mohamed A Mohamed [47] | 2010 | Egypt | 6:9:5 | 20:0:0 | 31.4 | 29 | Loss of 3 or more consecutive pregnancies before 20 weeks of pregnancy | PCR-ASO | 7 |
| Ahmad Settin [48] | 2011 | Egypt | 40:26:4 | 67:68:1 | NA | NA | 2 or more events of fetal loss in the form of abortion, miscarriage, or still birth | PCR–RFLP | 6 |
| Mahmood Jeddi-Tehrani [49] | 2011 | Iran | 43:42:15 | 66:25:9 | NA | NA | At least 2 successive pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Chan Woo Park [50] | 2011 | Korea | 14:16:9 | 17:26:7 | 34.9 | 38.5 | 2 or more unexplained pregnancy losses | PCR-DNA sequencing | 7 |
| Oztürk Ozdemir [51] | 2012 | Turkey | 231:239:73 | 76:30:0 | 27.8 | 28.9 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 8 |
| Rohini R Nair [52] | 2012 | India | 75:26:5 | 118:21:1 | NA | NA | 3 or more trimester miscarriages before 12 weeks of gestation | PCR–RFLP | 7 |
| Suat Karata [53] | 2012 | Turkey | 6:54:24 | 40:43:12 | 31.6 | 32.2 | 3 or more consecutive pregnancy losses before 10 weeks of gestation | PCR-ASO | 7 |
| Vajira H W Dissanayake [54] | 2012 | Sri Lanka | 158:39:3 | 169:27:2 | 32.1 | 32.4 | 2 or more consecutive spontaneous abortions with no living children | PCR–RFLP | 8 |
| Farah Idali [55] | 2012 | Iran | 61:36:9 | 66:25:9 | 30.1 | NA | At least 3 pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Ahmad Poursadegh Zonouzi [56] | 2012 | Iran | 53:30:6 | 27:22:1 | 30.17 | 31.54 | First trimester recurrent spontaneous abortions | PCR–RFLP | 6 |
| Raheleh Torabi [57] | 2012 | Iran | 43:42:15 | 66:25:9 | NA | NA | At least 2 recurrent pregnancy losses before the 20th week of gestation | PCR–RFLP | 6 |
| Talieh Kazerooni [58] | 2013 | Iran | 50:6:4 | 54:6:2 | 24.8 | 24.6 | 3 or more consecutive pregnancy losses at less than 20 weeks of gestation | PCR–RFLP | 8 |
| Farah Parveen [59] | 2013 | India | 110:70:20 | 196:90:14 | NA | NA | At least 3 spontaneous miscarriages | PCR–RFLP | 7 |
| Montserrat Creus [60] | 2013 | Spain | 23:26:11 | 13:13:4 | 35 | 35.8 | ≥ 3 consecutive spontaneous miscarriages of unknown etiology ≤ 10 weeks of gestation | PCR-FRET | 9 |
| Lovejeet Kaur [61] | 2013 | India | 86:16:5 | 463:109:21 | 24.89 | 25.32 | 3 or more consecutive unexplained recurrent pregnancy losses before 24 weeks of gestation | PCR–RFLP | 8 |
| Kristin Baumann [62] | 2013 | Germany | 279:287:75 | 66:70:19 | 33.14 | 33.16 | ≥ 2 consecutive miscarriages | PCR–RFLP | 7 |
| Caroline Gross Dutra [63] | 2014 | Brazil | 73:59:13 | 71:53:11 | 31.72 | 29.86 | At least 2 pregnancy losses before 24 weeks of gestation with the same partner and with no report of a full-term pregnancy | TaqMan-qPCR | 8 |
| Yunlei Cao [64] | 2014 | China | 29:43:10 | 53:83:30 | 28.43 | 28.1 | At least 2 consecutive pregnancy losses before 12-week gestational age | PCR-DNA sequencing | 9 |
| Elham Yousefian [65] | 2014 | Iran | 96:90:18 | 63:43:10 | 29.7 | 30.4 | 3 or more consecutive pregnancy losses before the 22nd week of pregnancy, regardless of a previous live birth | PCR-SSOP | 9 |
| A Pietropolli [66] | 2014 | Italy | 55:86:45 | 31:71:27 | 35.2 | 40.4 | 2 or more consecutive spontaneous miscarriages before the 20th week of gestation | PCR-FRET | 8 |
| Fabio L Lino [67] | 2015 | Brazil | 53:43:16 | 46:41:11 | 30.3 | 40.2 | 3 or more idiopathic miscarriages early in pregnancy(≤ 12 weeks) | PCR-ASO | 7 |
| Li Luo [68] | 2015 | China | 40:70:15 | 60:65:10 | 30.89 | 29.4 | 2 or more consecutive spontaneous abortions | PCR–RFLP | 7 |
| Wendell Vilas Boas [69] | 2015 | Brazil | 59:26:4 | 97:47:6 | 29.4 | 23 | At least 2 consecutive miscarriages in the first, second or third trimester of gestation, without any successful pregnancy | PCR–RFLP | 8 |
| Shiny Vanilla [70] | 2015 | India | 13:2:0 | 13:2:0 | NA | NA | 2 or more consecutive miscarriages with or without normal child | PCR–RFLP | 6 |
| L Zhu [71] | 2015 | China | 60:40:18 | 100:72:2 | 29.8 | 28.5 | Spontaneous abortions that occur ≥ 2 times in a row | TaqMan-qPCR | 8 |
| Somayeh-Sadat Tara [72] | 2015 | Iran | 62:114:49 | 70:26:4 | 32.4 | 35.2 | At least 3 successive pregnancy losses below 20th week of gestation | PCR–RFLP | 6 |
| Kamelia Farahmand [73] | 2016 | Iran | 180:114:36 | 230:85:35 | 30.37 | 29.88 | 3 or more consecutive pregnancy losses before 20 weeks of gestation, with no history of full-term pregnancies | PCR-ASO | 8 |
| R O Gonçalves [74] | 2016 | Brazil | 80:51:6 | 59:37:4 | 32.1 | 25.8 | 2 or more consecutive first-trimester abortions (< 12 weeks gestation) | PCR–RFLP | 7 |
| J J López-Jiménez [75] | 2016 | México | 17:26:13 | 12:23:15 | 30 | 30 | 3 consecutive pregnancy losses prior to the 20th week of gestation | PCR–RFLP | 7 |
| Kyu Ri Hwang [76] | 2017 | Korea | 104:153:45 | 94:156:65 | 34.8 | 50.3 | At least 2 unexplained consecutive spontaneous miscarriages before 20 weeks of gestation | TaqMan-qPCR | 6 |
| Walid Al-Achkar [77] | 2017 | Syria | 41:41:18 | 66:39:1 | 30 | 31 | 2 or more miscarriages and diagnosed as RPLs | PCR–RFLP | 8 |
| M Chatzidimitriou [78] | 2017 | Greece | 30:18:0 | 21:3:3 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Hubert Wolski [79] | 2017 | Poland | 165:153:41 | 201:164:35 | 30.99 | 30.05 | ≥ 2 loss of pregnancy before 22 completed weeks of gestation | PCR–RFLP | 7 |
| Alptekin H [80] | 2017 | Turkey | 49:43:14 | 37:20:5 | 27.9 | 29.4 | 2 or more lost pregnancies(early or late miscarriages or stillbirths) for no reason | PCR-DNA sequencing | 7 |
| Amela Jusić [81] | 2018 | Bosnian | 22:29:9 | 47:26:7 | 33.05 | 34.08 | 2 or more consecutive miscarriages before 20 weeks of gestation | PCR–RFLP | 7 |
| Razieh Bigdeli [82] | 2018 | Iran | 91:76:33 | 136:58:6 | 23 | 25.1 | At least 2 pregnancy losses | PCR–RFLP | 8 |
| Domenico Dell'Edera [83] | 2018 | Italy | 220:86:74 | 197:100:90 | NA | NA | At least 2 miscarriages | PCR-FRET | 6 |
| Hanadi El Achi [84] | 2018 | Lebanon | 11:30:29 | 76:26:1 | 32.2 | NA | At least 2 consecutive miscarriages | PCR-ASO | 5 |
| Anil Kumar Sah [85] | 2018 | Nepal | 28:5:2 | 35:0:0 | NA | NA | 2 or more consecutive miscarriages with or without normal child, unexplained cause of losses | PCR–RFLP | 6 |
| Kallur Nava Saraswathy [86] | 2018 | India | 64:16:5 | 66:43:12 | NA | NA | 3 or more consecutive unexplained pregnancy losses before 24 weeks of gestation | PCR–RFLP | 8 |
| Yuanchang Zhu [87] | 2018 | China | 166:157:47 | 66:59:19 | NA | NA | 2 or more clinical pregnancy failures | PCR-DNA sequencing | 8 |
| E A Trifonova [88] | 2019 | Russia | 129:99:25 | 210:112:17 | 29.5 | 27.3 | At least 2 pregnancy losses up to 20 weeks | PCR–RFLP | 8 |
| Yajuan Xu [89] | 2019 | China | 26:87:105 | 40:122:102 | 31.82 | 31.16 | 2 or more spontaneous miscarriages with a diagnosis of recurrent pregnancy loss | TaqMan-qPCR | 8 |
| Najmeh Ahangari [90] | 2019 | Iran | 127:95:23 | 222:22:6 | 32.16 | 31.81 | 2 or more repeated abortions | PCR-DNA sequencing | 8 |
| Yalda Zarfeshan Fard [91] | 2019 | Iran | 15:20:15 | 31:15:4 | 31.26 | 33.76 | 2 or more frequent abortions with normal karyotype and hormone tests | PCR–RFLP | 7 |
| Jyoti Mishra [92] | 2019 | India | 13:9:6 | 25:26:21 | NA | NA | Recurrent miscarriages | PCR–RFLP | 7 |
| Zhong Lin [93] | 2019 | China | 213:153:37 | 253:78:11 | 29.58 | 29.88 | 2 or more consecutive spontaneous abortions | TaqMan-qPCR | 8 |
| Ivana Joksic [94] | 2020 | Serbia | 35:30:5 | 12:18:1 | 33.2 | 33.2 | 3 or more consecutive pregnancy losses | PCR-ASO | 7 |
| Irem Yengel [95] | 2020 | Turkey | 56:68:21 | 50:44:11 | NA | NA | At least 2 recurrent pregnancy losses before the 12th week of gestation | TaqMan-qPCR | 6 |
| Yan Zhang [96] | 2020 | China | 141:85:11 | 313:262:43 | 27.85 | 27.01 | Diagnosed with RPL in compliance with the American Society for Reproductive Medicine definitions of infertility and recurrent pregnancy loss | TaqMan-qPCR | 7 |
| Mai Mahmoud Shaker [97] | 2021 | Egypt | 48:46:6 | 58:38:4 | 26.2 | 25.7 | 2 to three consecutive pregnancy losses earlier to the 20th week of gestation | PCR–RFLP | 8 |
| MTHFR (A1298C) | |||||||||
| Author | Year | Country | Genotype frequency(A/A:A/C:C/C) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| Maria Hohlagschwandtner [24] | 2003 | Austria | 63:67:15 | 35:50:16 | 32 | 56 | 3 or more consecutive spontaneous miscarriages before 20 weeks of gestation | PCR-ASO | 7 |
| Wang XP [28] | 2004 | China | 102:35:10 | 60:20:2 | 27.7 | 32 | 3 or more consecutive pregnancy losses before 20 weeks of gestation | PCR–RFLP | 6 |
| Carolyn B Coulam [34] | 2006 | USA | 50:80:20 | 12:8:0 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| N Mtiraoui [35] | 2006 | Tunisia | 108:65:27 | 130:62:8 | 28.68 | 28.24 | 3 or more consecutive RPLs at 5–30 weeks of gestation | PCR–RFLP | 8 |
| Alexandros Sotiriadis [37] | 2007 | Greece | 44:37:7 | 45:39:6 | 32.2 | 32.2 | 2 or more consecutive miscarriages with the same partner in < 15 weeks gestation | PCR–RFLP | 8 |
| C Ciacci [43] | 2009 | Italy | 18:20:1 | 29:34:9 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Jeehyeon Bae [44] | 2009 | Korea | 144:68:9 | 74:43:4 | 32.6 | 31.2 | More than 2 consecutive abortions | PCR–RFLP | 7 |
| Agnieszka Seremak-Mrozikiewicz [46] | 2010 | Poland | 40:51:13 | 78:74:17 | 30.15 | 29.4 | 3 or more unexplained consecutive recurrent miscarriages in the first trimester of pregnancy (6–13 week of gestation) | PCR–RFLP | 7 |
| Ahmad Settin [48] | 2011 | Egypt | 15:49:6 | 36:97:3 | NA | NA | 2 or more events of fetal loss in the form of abortion, miscarriage, or still birth | PCR–RFLP | 6 |
| Mahmood Jeddi-Tehrani [49] | 2011 | Iran | 69:27:4 | 94:6:0 | NA | NA | At least 2 successive pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Oztürk Ozdemir [51] | 2012 | Turkey | 201:257:85 | 71:35:0 | 27.8 | 28.9 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 8 |
| Farah Idali [55] | 2012 | Iran | 40:46:20 | 94:6:0 | 30.1 | NA | At least 3 pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Ahmad Poursadegh Zonouzi [56] | 2012 | Iran | 35:46:8 | 13:34:3 | 30.17 | 31.54 | First trimester recurrent spontaneous abortions | PCR–RFLP | 6 |
| Vajira H W Dissanayake [54] | 2012 | Sri Lanka | 74:78:43 | 72:79:46 | 32.1 | 32.4 | 2 or more consecutive spontaneous abortions | PCR–RFLP | 8 |
| Farah Parveen [59] | 2013 | India | 88:92:20 | 157:127:16 | NA | NA | At least 3 spontaneous miscarriages | PCR–RFLP | 7 |
| Rohini R Nair [52] | 2013 | India | 48:68:13 | 116:80:6 | 26.89 | 30.76 | 3 or more miscarriages before 12 weeks of gestation | PCR–RFLP | 7 |
| Yunlei Cao [64] | 2014 | China | 49:31:2 | 132:31:3 | 28.43 | 28.1 | At least 2 consecutive pregnancy losses before 12-week gestational age | PCR-DNA sequencing | 9 |
| Elham Yousefian [65] | 2014 | Iran | 98:81:25 | 68:39:9 | 29.7 | 30.4 | 3 or more consecutive pregnancy losses before the 22nd week of pregnancy, regardless of a previous live birth | PCR-SSOP | 9 |
| Fabio L Lino [67] | 2015 | Brazil | 71:32:9 | 52:43:3 | 30.3 | 40.2 | 3 or more idiopathic miscarriages early in pregnancy(≤ 12 weeks) | PCR-ASO | 7 |
| Li Luo [68] | 2015 | China | 82:40:3 | 78:54:3 | 30.89 | 29.4 | 2 or more consecutive spontaneous abortions | PCR–RFLP | 7 |
| Wendell Vilas Boas [69] | 2015 | Brazil | 57:27:5 | 80:62:8 | 29.4 | 23 | At least 2 consecutive miscarriages in the first, second or third trimester of gestation, without any successful pregnancy | PCR–RFLP | 8 |
| L Zhu [71] | 2015 | China | 48:58:12 | 76:88:10 | 29.8 | 28.5 | Spontaneous abortions that occur ≥ 2 times in a row | TaqMan-qPCR | 8 |
| Somayeh-Sadat Tara [72] | 2015 | Iran | 47:116:62 | 59:32:9 | 32.4 | 35.2 | At least 3 successive pregnancy losses below 20th week of gestation | PCR–RFLP | 6 |
| Kamelia Farahmand [73] | 2016 | Iran | 134:152:44 | 329:20:1 | 30.37 | 29.88 | 3 or more consecutive pregnancy losses before 20 weeks of gestation, with no history of full-term pregnancies | PCR–RFLP | 8 |
| J J López-Jiménez [75] | 2016 | México | 42:13:1 | 37:13:0 | 30 | 30 | 3 consecutive pregnancy losses prior to the 20th week of gestation | PCR–RFLP | 7 |
| M Chatzidimitriou [78] | 2017 | Greece | 36:6:6 | 19:8:0 | 35.5 | 35.1 | RPLs | PCR-ASO | 6 |
| Kyu Ri Hwang [76] | 2017 | Korea | 209:86:7 | 210:93:12 | 34.8 | 50.3 | At least 2 unexplained consecutive spontaneous miscarriages before 20 weeks of gestation | TaqMan-qPCR | 6 |
| Hubert Wolski [79] | 2017 | Poland | 152:163:44 | 179:172:49 | 30.99 | 30.05 | ≥ 2 loss of pregnancy before 22 completed weeks of gestation | PCR–RFLP | 7 |
| Alptekin H [80] | 2017 | Turkey | 26:65:15 | 38:17:7 | 27.9 | 29.4 | 2 or more lost pregnancies(early or late miscarriages or stillbirths) for no reason | PCR-DNA sequencing | 7 |
| Walid Al-Achkar [77] | 2017 | Syria | 53:40:8 | 65:40:1 | 30 | 31 | 2 or more miscarriages and diagnosed as RPLs | PCR–RFLP | 8 |
| Razieh Bigdeli [82] | 2018 | Iran | 142:48:10 | 171:28:1 | 23 | 25.1 | At least 2 pregnancy loss | PCR–RFLP | 8 |
| Domenico Dell'Edera [83] | 2018 | Italy | 294:54:32 | 320:35:32 | NA | NA | At least 2 miscarriage | PCR-FRET | 6 |
| Yuanchang Zhu [87] | 2018 | China | 243:114:13 | 83:56:5 | NA | NA | 2 or more clinical pregnancy failures | PCR-DNA sequencing | 8 |
| Hanadi El Achi [84] | 2018 | Lebanon | 9:25:36 | 55:24:24 | 32.2 | NA | At least 2 consecutive miscarriages | PCR-ASO | 5 |
| Yajuan Xu [89] | 2019 | China | 155:58:5 | 214:44:6 | 31.82 | 31.16 | 2 or more spontaneous miscarriages with a diagnosis of recurrent pregnancy loss | TaqMan-qPCR | 8 |
| Najmeh Ahangari [90] | 2019 | Iran | 83:129:33 | 179:57:14 | 32.16 | 31.81 | 2 or more repeated abortions | PCR-DNA sequencing | 8 |
| Zhong Lin [93] | 2019 | China | 231:144:28 | 221:102:19 | 29.58 | 29.88 | 2 or more consecutive spontaneous abortions were diagnosed as RSA | TaqMan-qPCR | 8 |
| Ivana Joksic [94] | 2020 | Serbia | 34:25:11 | 12:19:0 | 33.2 | 33.2 | 3 or more consecutive pregnancy losses | PCR-ASO | 7 |
| Irem Yengel [95] | 2020 | Turkey | 56:64:25 | 43:47:15 | NA | NA | At least 2 recurrent pregnancy losses before the 12th week of gestation | TaqMan-qPCR | 6 |
| Yan Zhang [96] | 2020 | China | 127:89:21 | 381:207:30 | 27.85 | 27.01 | Diagnosed with RPL in compliance with the American Society for Reproductive Medicine definitions of infertility and recurrent pregnancy loss | TaqMan-qPCR | 7 |
| Factor V HR2 | |||||||||
| Author | Year | Country | Genotype frequency(A/A:A/G:G/G) | Mean Age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| A Dilley [20] | 2002 | USA | 53:7:0 | 79:13:0 | 36 | 33 | 3 or more fetal losses, regardless of trimester of loss or previous live birth, or any late loss | PCR–RFLP | 8 |
| Carolyn B Coulam [34] | 2006 | USA | 147:2:1 | 19:1:0 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| Alexandros Sotiriadis [37] | 2007 | Greece | 78:10:0 | 65:23:2 | 32.2 | 32.2 | 2 or more consecutive miscarriages with the same partner in < 15 weeks gestation | PCR–RFLP | 8 |
| C Ciacci [43] | 2009 | Italy | 37:2:0 | 65:7:0 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Oztürk Ozdemir [51] | 2012 | Turkey | 470:73:0 | 103:3:0 | 27.8 | 28.9 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 8 |
| Raheleh Torabi [57] | 2012 | Iran | 86:12:2 | 96:4:0 | NA | NA | At least 2 recurrent pregnancy losses before the 20th week of gestation | PCR–RFLP | 6 |
| Vajira H W Dissanayake [54] | 2012 | Sri Lanka | 186:9:1 | 177:11:1 | 32.1 | 32.4 | 2 or more consecutive spontaneous abortions | PCR–RFLP | 8 |
| Ahmad Poursadegh Zonouzi [56] | 2013 | Iran | 85:4:0 | 48:2:0 | 30.18 | 31.54 | At least 2 consecutive miscarriages | ARMS-PCR | 6 |
| Nadia Arabkhazaeli [98] | 2016 | Iran | 95:5:0 | 91:9:0 | NA | NA | 2 or more spontaneous consecutive abortions at 5–20 weeks of gestation | PCR–RFLP | 6 |
| Mari Izuhara [99] | 2017 | Japan | 74:13:1 | 84:10:1 | 33 | 38.5 | 3 or more consecutive miscarriages of unexplained cause during the first trimester | PCR-DNA sequencing | 6 |
| M Chatzidimitriou [78] | 2017 | Greece | 42:6:0 | 23:4:0 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Razieh Bigdeli [82] | 2018 | Iran | 186:12:2 | 196:4:0 | 23 | 25.1 | At least 2 pregnancy loss | PCR–RFLP | 8 |
| Ivana Joksic [94] | 2020 | Serbia | 47:23:0 | 22:9:0 | 33.2 | 33.2 | 3 or more consecutive pregnancy losses | PCR-ASO | 7 |
| Factor XIII V34L | |||||||||
| Author | Year | Country | Genotype frequency (V/V:V/L:L/L) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| R Anwar [100] | 1999 | UK | 20:15:0 | 29:13:0 | NA | NA | 3 or more recurrent miscarriages | PCR-SSCP | 5 |
| Astrid Dossenbach-Glaninger [101] | 2003 | Austria | 24:21:4 | 31:16:1 | 35.6 | 36.6 | 2 consecutive or 3 to 6 nonconsecutive early pregnancy losses | PCR-ASO | 8 |
| Helena C L Barbosa [102] | 2004 | Brazil | 53:50:3 | 55:27:4 | 30.4 | NA | 3 or more rms accompanied by vaginal elimination of a fetus weighing less than 0.5 kg, with or without vital signs, and/or a gestational age under 20 weeks | PCR–RFLP | 8 |
| Carolyn B Coulam [34] | 2006 | USA | 82:57:11 | 8:11:1 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| Ysabel López Ramírez [103] | 2006 | Venezuela | 25:15:0 | 24:16:0 | 28.3 | 27.3 | ≥ 3 recurrent miscarriages of unknown causes | PCR–RFLP | 7 |
| C Ciacci [43] | 2009 | Italy | 24:14:1 | 49:21:2 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Mahmood Jeddi-Tehrani [49] | 2010 | Iran | 71:25:4 | 83:15:2 | NA | NA | At least 2 recurrent pregnancy losses before the 20th week of gestation | PCR–RFLP | 6 |
| Morteza Bagheri [104] | 2011 | Iran | 35:19:0 | 34:12:0 | NA | NA | 2 or more consecutive fetal losses between the 8th and the 12th week of gestation without a known reason | PCR–RFLP | 8 |
| Ahmad Poursadegh Zonouzi [56] | 2013 | Iran | 59:27:3 | 38:11:1 | 30.18 | 31.54 | At least 2 consecutive miscarriages | ARMS-PCR | 6 |
| Iman Rifaat Elmahgoub [105] | 2014 | Egypt | 81:26:13 | 116:11:3 | 28.5 | 29.1 | Unexplained, recurrent first trimester miscarriage | PCR–RFLP | 8 |
| Fabio L Lino [67] | 2015 | Brazil | 75:34:3 | 60:29:9 | 30.3 | 40.2 | 3 or more idiopathic miscarriages early in pregnancy(≤ 12 weeks) | PCR-ASO | 7 |
| M Chatzidimitriou [78] | 2017 | Greece | 30:16:2 | 11:16:0 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Razieh Bigdeli [82] | 2018 | Iran | 121:72:7 | 146:49:5 | 23 | 25.1 | At least 2 pregnancy loss | PCR–RFLP | 8 |
| Hanadi El Achi [84] | 2018 | Lebanon | 57:13:0 | 77:21:5 | 32.2 | NA | At least 2 consecutive miscarriages | PCR-ASO | 5 |
| Ivana Joksic [94] | 2020 | Serbia | 30:37:3 | 21:10:0 | 33.2 | 33.2 | 3 or more consecutive pregnancy losses | PCR-ASO | 7 |
| β-Fibrinogen-455G > A | |||||||||
| Author | Year | Country | Genotype frequency (G/G:G/A:A/A) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| R Pihusch [19] | 2001 | Germany | 61:33:8 | 69:48:11 | 35 | 32 | 2 or more unexplained consecutive abortions at 25 weeks of gestation | PCR–RFLP | 6 |
| Carolyn B Coulam [34] | 2006 | USA | 121:26:3 | 15:5:0 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| Cai XJ [106] | 2008 | China | 23:7:0 | 21:7:2 | NA | NA | 2 or more consecutive spontaneous abortions | PCR–RFLP | 6 |
| C Ciacci [43] | 2009 | Italy | 28:9:2 | 33:37:2 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Gonca Imir Yenicesu [45] | 2010 | Turkey | 167:88:17 | 28:28:0 | 27.2 | 29.5 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 7 |
| Carlo Ticconi [107] | 2011 | Italy | 58:32:8 | 50:28:0 | 35.5 | 36 | ≥ 2 consecutive miscarriages | PCR-DNA sequencing | 8 |
| Mahmood Jeddi-Tehrani [49] | 2011 | Iran | 64:33:3 | 88:11:1 | NA | NA | At least 2 successive pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Ahmad Poursadegh Zonouzi [56] | 2013 | Iran | 45:38:6 | 24:24:2 | 30.18 | 31.54 | At least 2 consecutive miscarriages | ARMS-PCR | 6 |
| Parisa Maziri [108] | 2017 | Iran | 31:17:2 | 27:20:3 | NA | NA | At least 2 consecutive miscarriages | PCR–RFLP | 6 |
| M Chatzidimitriou [78] | 2017 | Greece | 36:10:2 | 15:9:3 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Razieh Bigdeli [82] | 2018 | Iran | 131:59:10 | 163:36:1 | 23 | 25.1 | At least 2 pregnancy loss | PCR–RFLP | 8 |
| Noha Mahmoud Issa [109] | 2021 | Egypt | 47:28:5 | 43:31:6 | 30 | 31 | At least 3 RPLs at ≤ 24 weeks of gestation | PCR–RFLP | 8 |
| PAI-1 4G/5G | |||||||||
| Author | Year | Country | Genotype frequency (5G/5G:5G/4G:4G/4G) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| Cornelia E Wolf [110] | 2003 | Germany | 7:25:17 | 20:50:32 | 31.9 | 33.1 | At least 2 unexplained early abortions | Allele-specific PCR | 6 |
| T Buchholz [25] | 2003 | Germany | 37:75:72 | 28:58:41 | 35 | 32.8 | At least 2 unexplained consecutive spontaneous miscarriages before 25 weeks of gestation | PCR–RFLP | 7 |
| Astrid Dossenbach-Glaninger [101] | 2003 | Austria | 9:28:12 | 15:25:8 | 35.6 | 36.6 | 2 consecutive or 3 to 6 nonconsecutive early pregnancy losses | PCR-ASO | 8 |
| Li-xue Guan [30] | 2005 | China | 17:52:58 | 28:69:20 | 27 | 26 | At least 3 spontaneous abortions | PCR–RFLP | 7 |
| Carolyn B Coulam [31] | 2006 | USA | 22:117:11 | 5:13:2 | 34.7 | 39.6 | 2 or more consecutive abortions | PCR–RFLP | 6 |
| Chelsi Goodman [111] | 2009 | USA | 25:57:38 | 13:48:23 | 34.7 | NA | 2 or more consecutive spontaneous abortions | PCR-DNA sequencing | 6 |
| C Ciacci [43] | 2009 | Italy | 5:19:15 | 25:30:17 | NA | NA | At least 2 pregnancy losses within the first 3 months of pregnancy | PCR-ASO | 6 |
| Gonca Imir Yenicesu [45] | 2010 | Turkey | 28:185:59 | 12:44:0 | 27.2 | 29.5 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 7 |
| Rami J Al Sallout [112] | 2010 | Palestine | 40:44:16 | 36:48:16 | 28.9 | NA | At least 3 unexplained consecutive spontaneous miscarriages before 25 weeks of gestation | allele-specific PCR | 8 |
| Mahmood Jeddi-Tehrani [113] | 2011 | Iran | 60:31:9 | 72:27:1 | NA | NA | At least 2 successive pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Mahmoud Aarabi [114] | 2011 | Iran | 21:23:10 | 31:66:2 | 32.5 | 32.9 | At least 3 unexplained consecutive spontaneous abortions before 25 weeks of gestation | PCR–RFLP | 7 |
| Farah Idali [55] | 2012 | Iran | 35:54:17 | 72:27:1 | 30.1 | NA | At least 3 pregnancy losses before 20th week of gestation | PCR–RFLP | 6 |
| Oztürk Ozdemir [51] | 2012 | Turkey | 91:331:121 | 34:62:10 | 27.8 | 28.9 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 8 |
| Ivan Subrt [115] | 2013 | Czech | 23:75:59 | 10:54:10 | NA | NA | 2 or more consecutive spontaneous abortions | PCR–RFLP | 6 |
| Kalthoum Magdoud [116] | 2013 | Tunisia | 139:128:37 | 257:104:10 | 32.4 | 31.9 | 3 or more pregnancy losses of unknown etiology with the same partner | PCR-SSCP | 8 |
| Farah Parveen [59] | 2013 | India | 55:100:45 | 95:131:74 | NA | NA | At least 3 spontaneous miscarriages | PCR–RFLP | 7 |
| Young Joo Jeon [117] | 2013 | Korea | 47:132:129 | 39:117:71 | 32.94 | 33.2 | At least 2 consecutive pregnancy losses before 20 weeks of gestation | PCR–RFLP | 8 |
| Ahmad Poursadegh Zonouzi [56] | 2013 | Iran | 26:49:14 | 15:28:7 | 30.18 | 31.54 | At least 2 consecutive miscarriages | ARMS-PCR | 6 |
| Jin Ju Kim [118] | 2014 | Korea | 31:123:73 | 48:154:102 | 36 | 50.3 | At least 2 unexplained consecutive spontaneous miscarriages before 20 weeks of gestation | TaqMan-qPCR | 6 |
| Iman Rifaat Elmahgoub [105] | 2014 | Egypt | 75:37:8 | 99:28:3 | 28.5 | 29.1 | Unexplained, recurrent first trimester miscarriage | PCR–RFLP | 8 |
| Farhad Khosravi [119] | 2014 | Iran | 128:208:85 | 72:27:1 | 29.5 | 33 | At least 2 recurrent miscarriage | PCR–RFLP | 6 |
| A Pietropolli [66] | 2014 | Italy | 57:74:55 | 58:29:42 | 35.2 | 40.4 | 2 or more consecutive spontaneous miscarriages before the 20th week of gestation | PCR-FRET | 8 |
| Fatemeh Shakarami [120] | 2015 | Iran | 33:50:17 | 45:50:5 | NA | NA | At least 2 spontaneous abortions | PCR–RFLP | 6 |
| Fabio L Lino [67] | 2015 | Brazil | 37:57:12 | 42:40:16 | 30.3 | 40.2 | 3 or more idiopathic miscarriages early in pregnancy(≤ 12 weeks) | PCR-DNA sequencing | 7 |
| Magdalena Barlik [121] | 2016 | Poland | 27:75:50 | 32:85:63 | 30.16 | 29.46 | ≥ 2 consecutive loss of pregnancy before 22 completed weeks of gestation | PCR–RFLP | 7 |
| Maria D Salazar Garcia [122] | 2016 | USA | 28:53:32 | 28:50:14 | NA | NA | ≥ 2 spontaneous abortions | allele-specific PCR | cohort study |
| Grażyna Kurzawińska | 2016 | Poland | 27:75:50 | 32:85:63 | 30.16 | 29.46 | At least 2 consecutive pregnancy losses in the first and second trimester | PCR–RFLP | 7 |
| J J López-Jiménez [75] | 2016 | México | 21:25:10 | 25:18:7 | 30 | 30 | 3 consecutive pregnancy losses prior to the 20th week of gestation | PCR–RFLP | 7 |
| Alptekin H [80] | 2017 | Turkey | 21:57:28 | 30:21:11 | 27.9 | 29.4 | 2 or more lost pregnancies(early or late miscarriages or stillbirths) for no reason | allele-specific PCR | 7 |
| M Chatzidimitriou [78] | 2017 | Greece | 2:26:20 | 6:20:1 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Razieh Bigdeli [82] | 2018 | Iran | 70:112:18 | 150:43:7 | 23 | 25.1 | At least 2 pregnancy loss | PCR–RFLP | 8 |
| Amela Jusić [81] | 2018 | Bosnian | 31:22:7 | 50:28:2 | 33.05 | 34.08 | 2 or more consecutive miscarriages before 20 weeks of gestation | PCR–RFLP | 7 |
| E A Trifonova [88] | 2019 | Russia | 38:139:76 | 58:173:108 | 29.5 | 27.3 | At least 2 pregnancy losses up to 20 weeks | PCR-ASO | 8 |
| Dao Anh Thi Le [123] | 2022 | Viet Nam | 16:14:13 | 7:12:11 | 30.1 | 28.6 | At least 2 unexplained RPL before 22 weeks of gestation | ARMS-PCR | 8 |
| ACE (intron 16 I/D) | |||||||||
| Author | Year | Country | Genotype frequency(I/I:I/D:D/D) | Mean age | Definition of RPL | Genotyping method | Quality | ||
| Case | Control | Case | Control | ||||||
| C Fatini [124] | 2000 | Italy | 10:21:28 | 20:30:20 | 31 | 32.5 | 3 or more first-trimester (7 ± 12 weeks of gestation) fetal losses | PCR | 8 |
| T Buchholz [25] | 2003 | Germany | 42:83:59 | 26:71:30 | 35 | 32.8 | At least 2 unexplained consecutive spontaneous miscarriages before 25 weeks of gestation | PCR-AFLP | 7 |
| Venkatesan Vettriselvi [38] | 2008 | India | 42:39:23 | 55:38:27 | NA | NA | 2 or more spontaneous consecutive miscarriages less than 20 weeks of gestation | PCR | 6 |
| Chelsi Goodman [111] | 2009 | USA | 31:55:34 | 22:34:28 | 34.7 | NA | 2 or more consecutive spontaneous abortions | PCR | 6 |
| Morteza Bagheri [125] | 2010 | Iran | 7:26:17 | 12:27:24 | 28.27 | 29.58 | At least 3 pregnancy losses with unknown etiology before 20 weeks gestational age | PCR | 8 |
| Rami J Al Sallout [112] | 2010 | Palestine | 9:42:49 | 12:34:54 | 28.9 | NA | At least 3 unexplained consecutive spontaneous miscarriages before 25 weeks of gestation | PCR | 8 |
| Yi Seul Choi [126] | 2011 | Korea | 77:130:44 | 35:50:41 | 31.97 | 31.22 | At least 3 consecutive spontaneous abortions | PCR | 6 |
| Mahmoud Aarabi [114] | 2011 | Iran | 14:30:19 | 22:47:25 | 32.5 | 32.9 | At least 3 unexplained consecutive spontaneous abortions before 25 weeks of gestation | PCR | 7 |
| Shufang Zhang [127] | 2011 | China | 57:49:21 | 90:34:8 | 30.1 | 28.2 | At least 2 consecutive spontaneous abortions in early pregnancy | PCR | 7 |
| Oztürk Ozdemir [51] | 2012 | Turkey | 71:260:212 | 33:54:19 | 27.8 | 28.9 | 2 or more consecutive early RPL at 5–12 weeks of gestation | PCR-ASO | 8 |
| Ahmad Poursadegh Zonouzi [56] | 2013 | Iran | 23:31:35 | 7:28:15 | 30.18 | 31.54 | At least 2 consecutive miscarriages | ARMS-PCR | 6 |
| Jin Ju Kim [118] | 2014 | Korea | 83:110:34 | 104:148:52 | 36 | 50.3 | At least 2 unexplained consecutive spontaneous miscarriages before 20 weeks of gestation | PCR | 6 |
| Fatemeh Shakarami [120] | 2015 | Iran | 6:60:34 | 0:48:52 | NA | NA | At least 2 spontaneous abortions | PCR–RFLP | 6 |
| Grażyna Kurzawińska [128] | 2016 | Poland | 32:80:40 | 44:84:52 | 30.16 | 29.46 | At least 2 consecutive pregnancy losses in the first and second trimester | PCR–RFLP | 7 |
| Shokoufeh Fazelnia [129] | 2016 | Iran | 23:33:44 | 31:40:29 | NA | NA | 2 or more spontaneous consecutive abortions at 5–20 weeks of gestation | PCR | 6 |
| J J López-Jiménez [75] | 2016 | México | 11:34:10 | 21:19:10 | 30 | 30 | 3 consecutive pregnancy losses prior to the 20th week of gestation | PCR | 7 |
| Fatimah Basil Al-Mukaynizi [130] | 2016 | Saudi Arabia | 3:18:40 | 2:25:32 | 34.1 | 34.6 | 3 or more consecutive pregnancy losses before the 20th week of gestation | PCR | 8 |
| Nina Pereza [131] | 2016 | Croatia | 31:75:43 | 32:62:55 | NA | NA | ≥ 3 consecutive spontaneous abortions of unknown etiology before the 22nd week of gestation | allele-specific PCR | 6 |
| Aisha Mahmood Fageer Hussian [132] | 2016 | Sudan | 3:14:23 | 0:3:37 | 32.3 | 30.9 | 3 or more consequent abortions with no apparent cause | PCR | 6 |
| M Chatzidimitriou [78] | 2017 | Greece | 4:23:21 | 9:10:8 | 35.5 | 35.1 | 2 or more consecutive fetal losses prior to 20 weeks of gestation | PCR-ASO | 6 |
| Parisa Maziri [108] | 2017 | Iran | 1:13:36 | 2:22:26 | NA | NA | At least 2 consecutive miscarriages | PCR | 6 |
| Evren Gumus [133] | 2018 | Turkey | 180:477:350 | 46:75:48 | 25.88 | 26.41 | ≥ 2 consecutive pregnancy losses | PCR | 8 |
| Hanadi El Achi [84] | 2018 | Lebanon | 17:27:26 | 6:30:27 | 32.2 | NA | At least 2 consecutive miscarriages | PCR-ASO | 5 |
| Mohammad Mehdi Heidari [134] | 2019 | Iran | 49:102:51 | 41:99:70 | 27.32 | 29.68 | 3–9 miscarriages with fetal loss | ARMS-PCR | 6 |
| E A Trifonova [88] | 2019 | Russia | 63:129:61 | 85:176:78 | 29.5 | 27.3 | At least 2 pregnancy losses up to 20 weeks | PCR | 8 |
| Noha Mahmoud Issa [109] | 2021 | Egypt | 11:31:38 | 18:37:25 | 30 | 31 | At least 3 RPLs at ≤ 24 weeks of gestation | PCR–RFLP | 8 |
Table 3.
Results of meta-analyses
| Genotype | Genetic model | Pooled ORs (95%CI) | P | I2 | P |
|---|---|---|---|---|---|
| MTHFR C677T | Dominant | 1.43 (1.25, 1.64) | < 0.01 | 79% | < 0.01 |
| Recessive | 1.60 (1.36, 1.87) | < 0.01 | 60% | < 0.01 | |
| MTHFR A1298C | Dominant | 1.66 (1.26, 2.18) | < 0.01 | 90% | < 0.01 |
| Recessive | 1.79 (1.42, 2.26) | < 0.01 | 56% | < 0.01 | |
| ACE I/D | Dominant | 1.23 (1.00, 1.53) | 0.05 | 62% | < 0.01 |
| Recessive | 1.09 (0.87, 1.36) | 0.44 | 71% | < 0.01 | |
| Factor VIII V34L | Dominant | 1.38 (1.02, 1.87) | < 0.05 | 59% | < 0.01 |
| Recessive | 1.28 (0.81, 2.01) | 0.28 | 28% | 0.17 | |
| Factor V R2 | Dominant | 1.12 (0.68, 1.83) | 0.65 | 59% | < 0.01 |
| Recessive | NA | ||||
| PAI-1 4G/5G | Dominant | 1.67 (1.36, 2.06) | < 0.01 | 76% | < 0.01 |
| Recessive | 1.80 (1.39, 2.32) | < 0.01 | 71% | < 0.01 | |
| β-Fibrinogen-455G/A | Dominant | 0.92 (0.62, 1.37) | 0.69 | 74% | < 0.01 |
| Recessive | 1.60 (1.02, 2.51) | < 0.05 | 22% | 0.23 | |
NA not available
Table 4.
Results of subgroup analyses
| MTHFR C677T | |||||
| Subgroup | Genetic model | No. of study | OR(95% CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 0.99 | 0.32 | ||
| Caucasian | 67 | 1.47(1.25, 1.74) | |||
| Non-Caucasian | 18 | 1.28(1.03, 1.59) | |||
| Recessive | 0.33 | 0.57 | |||
| Caucasian | 67 | 1.65(1.37, 1.98) | |||
| Non-Caucasian | 18 | 1.47(1.06, 2.05) | |||
| Number of PL | Dominant | 0.06 | 0.8 | ||
| ≥ 2 | 51 | 1.46(1.24, 1.73) | |||
| ≥ 3 | 28 | 1.52(1.18, 1.96) | |||
| Recessive | 0.82 | 0.37 | |||
| ≥ 2 | 51 | 1.54(1.25, 1.90) | |||
| ≥ 3 | 28 | 1.79(1.39, 2.32) | |||
| Gestational age at PL | Dominant | 0.02 | 0.89 | ||
| Early pregnancy loss | 14 | 1.39(0.99, 1.95) | |||
| Late pregnancy loss | 31 | 1.43(1.16, 1.67) | |||
| Recessive | 0.12 | 0.73 | |||
| Early pregnancy loss | 14 | 1.38(0.98, 1.95) | |||
| Late pregnancy loss | 31 | 1.49(1.17, 1.89) | |||
| MTHFR A1298C | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 5.09 | 0.02 | ||
| Caucasian | 30 | 1.88(1.32, 2.69) | |||
| Non-Caucasian | 10 | 1.15(0.91, 1.46) | |||
| Recessive | 4.13 | 0.04 | |||
| Caucasian | 30 | 2.14(1.55, 2.94) | |||
| Non-Caucasian | 10 | 1.36(1.01, 1.82) | |||
| Number of PL | Dominant | 1.09 | 0.3 | ||
| ≥ 2 | 26 | 1.51(1.15, 1.98) | |||
| ≥ 3 | 12 | 2.23(1.12, 4.42) | |||
| Recessive | 3.96 | 0.05 | |||
| ≥ 2 | 26 | 1.48(1.15, 1.90) | |||
| ≥ 3 | 12 | 2.72(1.58, 4.69) | |||
| Gestational age at PL | Dominant | 0.81 | 0.37 | ||
| Early pregnancy loss | 7 | 1.35(0.78, 2.33) | |||
| Late pregnancy loss | 15 | 1.96(1.07, 3.60) | |||
| Recessive | 0.08 | 0.78 | |||
| Early pregnancy loss | 7 | 1.97(1.29, 3.01) | |||
| Late pregnancy loss | 15 | 2.10(1.20, 3.69) | |||
| ACE I/D | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 0.07 | 0.79 | ||
| Caucasian | 22 | 1.25(0.99, 1.57) | |||
| Non-Caucasian | 4 | 1.13(0.56, 2.29) | |||
| Recessive | 0.87 | 0.35 | |||
| Caucasian | 22 | 1.17(0.95, 1.43) | |||
| Non-Caucasian | 4 | 0.63(0.18, 2.24) | |||
| Number of PL | Dominant | 0.15 | 0.7 | ||
| ≥ 2 | 16 | 1.25(0.93, 1.69) | |||
| ≥ 3 | 10 | 1.15(0.86, 1.55) | |||
| Recessive | 1.76 | 0.19 | |||
| ≥ 2 | 16 | 1.23(0.96, 1.58) | |||
| ≥ 3 | 10 | 0.88(0.57, 1.35) | |||
| Gestational age at PL | Dominant | 4.11 | 0.04 | ||
| Early pregnancy loss | 2 | 2.40(1.24, 4.65) | |||
| Late pregnancy loss | 14 | 1.19(1.00, 1.41) | |||
| Recessive | 0.26 | 0.61 | |||
| Early pregnancy loss | 2 | 1.62(0.48, 5.53) | |||
| Late pregnancy loss | 14 | 1.17(0.95, 1.43) | |||
| PAI-1 4G/5G | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 3.89 | 0.05 | ||
| Caucasian | 30 | 1.76(1.41, 2.19) | |||
| Non-Caucasian | 4 | 1.22(0.91, 1.63) | |||
| Recessive | 0.32 | 0.57 | |||
| Caucasian | 30 | 1.87(1.41, 2.48) | |||
| Non-Caucasian | 4 | 1.50(0.74, 3.05) | |||
| Number of PL | Dominant | 0.01 | 0.94 | ||
| ≥ 2 | 25 | 1.67(1.31, 2.14) | |||
| ≥ 3 | 8 | 1.64(1.06, 2.55) | |||
| Recessive | 0.81 | 0.37 | |||
| ≥ 2 | 25 | 1.60(1.25, 2.04) | |||
| ≥ 3 | 8 | 2.31(1.07, 4.97) | |||
| Gestational age at PL | Dominant | 4.98 | 0.03 | ||
| Early pregnancy loss | 5 | 2.05(1.56, 2.68) | |||
| Late pregnancy loss | 15 | 1.33(1.02, 1.73) | |||
| Recessive | 1.69 | 0.19 | |||
| Early pregnancy loss | 5 | 2.12(0.96, 4.68) | |||
| Late pregnancy loss | 15 | 1.23(0.98, 1.54) | |||
| Factor VIII V34L | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Number of PL | Dominant | 0.12 | 0.73 | ||
| ≥ 2 | 10 | 1,31(0.92, 1.86) | |||
| ≥ 3 | 4 | 1.19(0.75, 1.88) | |||
| Recessive | 5.14 | 0.02 | |||
| ≥ 2 | 10 | 1.55(0.79, 3.05) | |||
| ≥ 3 | 4 | 0.38(0.14, 1.05) | |||
| Gestational age at PL | Dominant | 0.35 | 0.55 | ||
| Early pregnancy loss | 4 | 1.58(0.77, 3.21) | |||
| Late pregnancy loss | 2 | 1.35(0.73, 2.50) | |||
| Recessive | 0.32 | 0.57 | |||
| Early pregnancy loss | 4 | 1.12(0.17, 7.27) | |||
| Late pregnancy loss | 2 | 2.23(0.50, 10.01) | |||
| Factor V R2 | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 0.3 | 0.58 | ||
| Caucasian | 12 | 1.09(0.63, 1.88) | |||
| Non-Caucasian | 1 | 1.44(0.62, 3.38_ | |||
| Number of PL | Dominant | 0 | 1 | ||
| ≥ 2 | 11 | 1.13(0.62, 2.05) | |||
| ≥ 3 | 2 | 1.12(0.59, 2.14) | |||
| Gestational age at PL | Dominant | 0.73 | 0.39 | ||
| Early pregnancy loss | 3 | 1.70(0.50, 5.80) | |||
| Late pregnancy loss | 4 | 0.84(0.28, 2.45) | |||
| β-Fibrinogen-455G/A | |||||
| Subgroup | Genetic model | No. of study | OR(95%CI) | Test for subgroup differences | |
| χ2 | P | ||||
| Ethnicity | Dominant | 0.19 | 0.66 | ||
| Caucasian | 11 | 0.93(0.61, 1.43) | |||
| Non-Caucasian | 1 | 0.71(0.22, 2.25) | |||
| Recessive | 1.56 | 0.21 | |||
| Caucasian | 11 | 1.37(0.79, 2.39) | |||
| Non-Caucasian | 1 | 0.19(0.01,4.06) | |||
| Number of PL | Dominant | 0.26 | 0.61 | ||
| ≥ 2 | 10 | 0.94(0.58, 1.53) | |||
| ≥ 3 | 2 | 0.80(0.53, 1.19) | |||
| Recessive | 1.5 | 0.22 | |||
| ≥ 2 | 10 | 1.75(0.77, 3.96) | |||
| ≥ 3 | 2 | 0.87(0.41, 1.85) | |||
| Gestational age at PL | Dominant | 1.3 | 0.25 | ||
| Early pregnancy loss | 2 | 0.49(0.27, 0.90) | |||
| Late pregnancy loss | 3 | 1.15(0.31, 4.30) | |||
| Recessive | 1.84 | 0.18 | |||
| Early pregnancy loss | 2 | 3.03(0.59, 15.49) | |||
| Late pregnancy loss | 3 | 0.82(0.32, 2.10) | |||
| PL, pregnancy loss | |||||
MTHFR polymorphisms
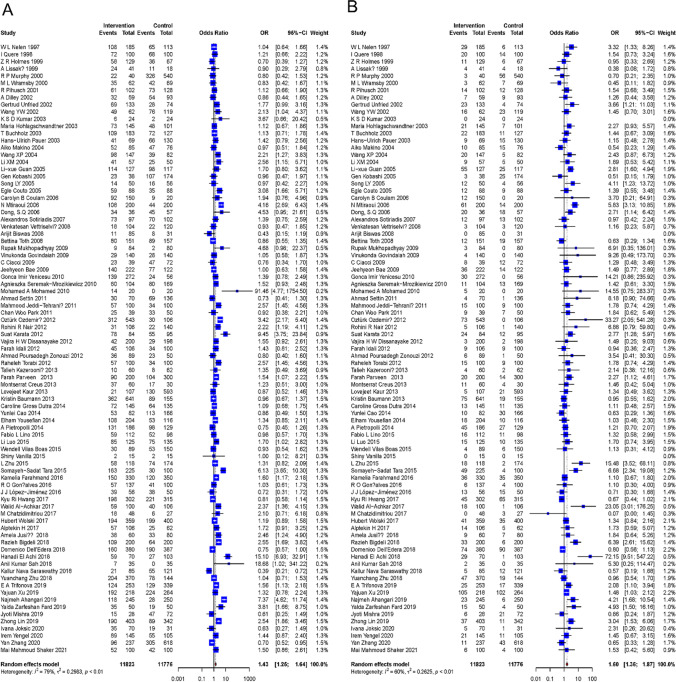
For C677T, 85 studies consisted of 11,823 patients and 11,776 controls for meta-analysis. Under the dominant model (CT + TT vs. CC), a high degree of among-study heterogeneity was observed (I2 = 79%, P < 0.01). The pooled ORs and 95% CI showed great significance for the relationship between this polymorphism and RPL (OR: 1.43; 95% CI: 1.25–1.64; P < 0.01) (Fig. 2A). Under the recessive model (CC + CT vs. TT), a high degree of among-study heterogeneity was observed (I2 = 60%, P < 0. 01). The pooled ORs and 95% CI also showed statistical significance (OR: 1.60; 95% CI: 1.36–1.87; P < 0.01) (Fig. 2B). As for the sensitivity analysis, the risk estimate did not materially change for both the dominant model and the recessive model. The examination for publication bias via the Egger’s line regression test did not show a significant bias for the dominant model (t = 1.46, P = 0.14); however, there was quite a significant publication bias in the recessive model (t = 3.47, P < 0.01). After adding 18 studies and dropping out 3 studies via trim and fill method, the pooled ORs and 95% CI were consistent with the original result (OR: 1.27; 95% CI: 1.05–1.54; P < 0.05), suggesting the publication bias had no significant impact on the result. Subgroup analyses showed that the number of pregnancy loss, gestational age at pregnancy loss, and ethnicity were not associated with among-study heterogeneity under both the two genetic models.
Fig. 2.
Forest plot of the association between risk of RPL and MTHFR C677T polymorphism. A Forest plot under a dominant model; B forest plot under a recessive model. RPL, recurrent pregnancy loss
For A1298C, 40 studies consisted of 7121 patients and 6739 controls for meta-analysis. Under the dominant model (AC + CC vs. AA), an extremely high degree of among-study heterogeneity was observed (I2 = 90%, P < 0. 01). The pooled ORs and 95% CI showed significance for the relationship between MTHFR A1298C and RPL (OR: 1.66; 95% CI: 1.26–2.18; P < 0.01) (Fig. 3A). Under the recessive model (AA + AC vs. CC), a high degree of among-study heterogeneity was observed (I2 = 56%, P < 0. 01). The pooled ORs and 95% CI also showed great statistical significance (OR: 1.79; 95% CI: 1.42–2.26; P < 0. 01) (Fig. 3B). As for the sensitivity analysis, the risk estimate did not materially change for both the dominant model and the recessive model. The examination for publication bias via the Egger’s line regression test showed no significant bias for the dominant model (t = 1.07, P = 0.29), but a significant bias for the recessive model (t = 3.28, P < 0.01). After adding 9 studies via trim and fill method, the pooled ORs and 95% CI were consistent with the original result (OR: 1.51; 95% CI: 1.16–1.97; P < 0.01), suggesting the publication bias had no significant impact on the result. Subgroup analyses showed that ethnicity under both the two models and the number of pregnancy loss under the recessive model were associated with among-study heterogeneity (P < 0.05), while gestational age at pregnancy loss was not.
Fig. 3.
Forest plot of the association between risk of RPL and MTHFR A1298C polymorphism. A Forest plot under a dominant model; B forest plot under a recessive model. RPL, recurrent pregnancy loss
PAI-1 polymorphisms
Thirty-four studies consisted of 5294 patients and 4254 controls for meta-analysis. Under the dominant model (4G5G + 4G4G vs. 5G5G), a high degree of among-study heterogeneity was observed (I2 = 76%, P < 0.01). The pooled ORs and 95% CI showed great significance for the relationship between PAI-1 mutation and RPL (OR: 1.67; 95% CI: 1.36–2.06; P < 0.01) (Supplementary Fig. 1). Under the recessive model (5G5G + 4G5G vs. 4G4G), a high degree of among-study heterogeneity was observed (I2 = 71%, P < 0.01). The pooled ORs and 95% CI also showed great statistical significance (OR: 1.80; 95% CI: 1.39–2.32; P < 0.01) (Supplementary Fig. 2). As for the sensitivity analysis, the risk estimate did not materially change for both the dominant genetic model and the recessive genetic model. The examination for publication bias via the Egger’s line regression test showed no significant bias for the dominant model (t = − 0.98, P = 0.33), but a significant bias for the recessive model (t = 4.54, P < 0.01). After adding 10 studies via trim and fill method, the pooled ORs and 95% CI were inconsistent with the original result (OR: 1.27; 95% CI: 0.90–1.80; P = 0.17), suggesting the publication bias for the recessive model of this polymorphism had a significant impact on the result. Subgroup analyses showed that ethnicity and gestational age at pregnancy loss under the dominant model were associated with among-study heterogeneity (P < 0.05), while the number of abortions was not.
ACE polymorphisms
Twenty-six studies consisted of 4284 patients and 2992 controls for meta-analysis. Under the dominant model (ID + DD vs. II), the among-study heterogeneity was significant (I2 = 62%, P < 0.01). The pooled ORs and 95% CI showed a weak correlation between this polymorphism and RPL (OR: 1.23; 95% CI: 1.00–1.53; P = 0.05) (Supplementary Fig. 3). Under the recessive model (II + ID vs. DD), a high degree of among-study heterogeneity was observed (I2 = 71%, P < 0.01). The pooled ORs and 95% CI showed no statistical significance (OR: 1.09; 95% CI: 0.87–1.36; P = 0.44) (Supplementary Fig. 4). As for the sensitivity analysis, the risk estimate did not materially change for the recessive models, but for the dominant model, the result was unstable (Supplementary Figure X). The examination for publication bias via the Egger’s line regression test did not show a significant bias for both the two models (dominant model: t = − 0.47, P = 0.65; recessive model: t = 0.48, P = 0.63). Subgroup analyses showed that gestational age at pregnancy loss under the dominant model was associated with among-study heterogeneity (P < 0.05), while the number of pregnancy loss and ethnicity was not.
Factor V polymorphisms
Thirteen studies consisted of 1775 patients and 1183 controls for analysis. Under the dominant model (AG + GG vs. AA), a high degree of among-study heterogeneity was observed (I2 = 59%, P < 0.05). The pooled ORs and 95% CI showed no significant relationship between Factor V R2 polymorphisms and RPL (OR: 1.12; 95% CI: 0.68–1.83; P = 0.65) (Supplementary Fig. 5). Owing to very few cases and controls with homozygotic mutation, the recessive model is not suitable for this polymorphism. As for the sensitivity analysis, the risk estimate did not materially change. The examination for bias via the Egger’s line regression test did not show a significant bias (t = 0.36, P = 0.73). Subgroup analyses showed that none of the variables including the gestational age at pregnancy loss, the number of abortions, and ethnicity were associated with among-study heterogeneity for this polymorphism.
Factor XIII polymorphisms
Fifteen studies consisted of 1262 patients and 1093 controls for meta-analysis. Under the dominant model (GT + TT vs. GG), a high degree of among-study heterogeneity was observed (I2 = 59%, P < 0.05). The pooled ORs and 95% CI showed significance for the relationship between this polymorphism and RPL (OR: 1.38; 95% CI: 1.02–1.87; P < 0.05) (Supplementary Fig. 6). Under the recessive model (GG + GT vs. TT), the statistical heterogeneity was not significant (I2 = 28%, P = 0.17), so a fixed-effect model was chosen. The pooled ORs and 95% CI showed no statistical significance (OR: 1.28; 95% CI: 0.81–2.01; P = 0.28) (Supplementary Fig. 7). As for the sensitivity analysis, the risk estimate did not materially change for the recessive models, but for the dominant model, the result was unstable (Supplementary Fig. 11). The examination for publication bias via the Egger’s line regression test did not show a significant bias for both models (dominant model: t = − 0.98, P = 0.34; recessive model: t = 0.06, P = 0.95). Subgroup analyses showed that the number of abortions was associated with among-study heterogeneity under the recessive model (P < 0.05), while the gestational age at pregnancy loss was not. We did not analyze the effect of ethnicity because populations in all included studies were Caucasian.
β-Fibrinogen polymorphisms
Twelve studies consisted of 1258 patients and 891 controls for meta-analysis. Under the dominant model (GA + AA vs. GG), a high degree of among-study heterogeneity was observed (I2 = 74%, P < 0.01). The pooled ORs and 95% CI showed no significance (OR: 0.92; 95% CI: 0.62–1.37; P = 0.69) (Supplementary Fig. 8). Under the recessive model (GG + GA vs. AA), the statistical heterogeneity was not significant (I2 = 22%, P = 0.23), so a fixed-effect model was chosen. The pooled ORs and 95% CI showed statistical significance (OR: 1.60; 95% CI: 1.02–2.51; P < 0.05) (Supplementary Fig. 9). As for the sensitivity analysis, the risk estimate did not materially change for the dominant models, but for the recessive model, the result was unstable (Supplementary Fig. 12). The examination for publication bias via the Egger’s line regression test did not show a significant bias for both two models (dominant model: t = − 1.49, P = 0.17; recessive model: t = 1.29, P = 0.23). Subgroup analyses showed that none of the variables mentioned before were associated with among-study heterogeneity.
Discussion
In this meta-analysis of thrombophilic gene polymorphisms and RPL, 7 polymorphisms in 6 genes related to inherited thrombophilias were involved including MTHFR C677T, MTHFR A1298C, PAI-1 4G/5G, ACE I/D, Factor V R2, Factor XIII V34L, and β-fibrinogen-455G/A. The result of the analysis has shown that MTHFR C677T, MTHFR A1298C, and PAI-1 4G/5G mutations increase the risk of RPL both in the dominant genetic model and in the recessive model. ACE I/D and Factor XIII V34L are positively associated with RPL only in the dominant model, and β-fibrinogen-455G/A is positively associated with RPL only in the recessive model. This may be related to the specific genetic characteristics and their different effects on the disease phenotype. Besides, the evidence in this current study could not support any association between Factor V R2 polymorphisms and RPL significantly.
In light of previous meta-analyses, some studies which analyzed the relation between MTHFR C677T and/or A1298C and RPL were consistent with our findings [135–138]. But no significant association between MTHFR A1298C polymorphism and unexplained RPL was found in several studies [139–141], which was probably due to the relatively small sample size or the selection of literature. There were 3 studies performing meta-analyses about the PAI-1 4G/5G and RPL before, but the findings conflicted possibly due to the limited included studies [142–144]. As for the ACE I/D polymorphism, all had the same findings regardless of the number of included studies, similar to ours [144–146]. Jung et al. explored the association of Factor XIII V34L and RPL, and also found the Val34Leu polymorphism can act as a prognostic factor of RPL [147]. Based on these previous studies, we made a more comprehensive and the most up-to-date analysis. But for the other two polymorphisms, Factor V R2, and β-fibrinogen-455G/A, it is the first time to integrate published data to perform a meta-analysis.
There are two strengths in the present meta-analysis. Our literature search strategy, using MeSH searching in PubMed and Embase, ensured all the related articles were included to the greatest extent under strict inclusion and exclusion criteria, resulting in a relatively large quantity of literature. The integration of sample sizes increased the statistical power, allowing for more accurate risk assessments. On the other hand, the statistical work was done by two reviewers independently, minimizing the omissions and contrived errors to an extreme.
Remarkably, the inclusion criteria of the RPL group were inconsistent in the included studies, including the number of abortions and the gestational age at pregnancy loss. We included all related studies regardless of their definition of RPL. But according to the results of subgroup analyses, we found that the differences in the risk for RPL were associated with both the number of abortions and the gestational age at pregnancy loss, suggesting that the definition of RPL may have a substantial impact on the relationship between thrombophilic gene polymorphisms and RPL, which might be one of the potential sources of among-study heterogeneity. Furthermore, by performing the subgroup analyses according to ethnicity, most of the results showed different risks of RPL for patients with thrombophilic gene mutations in different ethnicities. The inconsistency of results suggests that these thrombophilic gene polymorphisms may have different functional influences on the etiology of RPL, possibly owing to the varied distribution of genotypes among ethnicities. Notably, non-Caucasian people made up a relatively smaller proportion and may not be powered to address ethnic differences, and more studies are necessary to conduct in non-Caucasian regions to compensate for this limitation. Altogether, when clinicians assess the risk of RPL for patients, they should pay attention to these variables.
From the results of the heterogeneity analysis, different degrees of heterogeneity existed in most of the polymorphisms, and many were at medium or relatively high degrees. Given this limitation, subgroup analyses were performed to explore the sources of heterogeneity, and we found that ethnicity and the criteria for the case group, according to the definition of RPL, might be potential sources. Besides, many of the included studies ignored the comparability between case group and control group, namely the control of confounding factors, such as maternal age, BMI, and smoking. All of these factors may contribute to heterogeneity, particularly the maternal age, which has a great impact on fertility. However, the lack of sufficient information hindered further exploration of these factors. In addition, using the random-effect model could reduce the effect of among-study heterogeneity in our results.
Another limitation is publication bias, which is an inevitable issue for all meta-analyses. Studies with positive results are more likely to be published, thereby omitting those unpublished researches with negative results, exacerbating the effect of genetic polymorphisms. In the present meta-analysis, no publication bias was found by Egger’s line regression test in most polymorphisms with P > 0.05. However, for PAI-1 4G/5G under the recessive model, conclusions should be adopted with caution considering the significant publication bias.
Of note, sensitivity analyses of some polymorphisms showed unstable results after excluding each included study and repeating the meta-analysis, including ACE I/D polymorphisms under the dominant genetic model, Factor XIII V34L polymorphisms under the dominant genetic model, and β-fibrinogen-455G/A polymorphisms under the recessive genetic model. The instability of the findings is possibly owing to the limited number of included studies, substantial between-study heterogeneity, or some other important potential bias factors, which need to be verified by further studies.
Our meta-analysis provided evidence that thrombophilic gene polymorphisms were positively associated with RPL and improved risk prediction influencing diagnosis and treatments for clinicians. For those patients with a family history of inherited thrombophilia or suffered unexplained RPL, acquiring more insight into genetic risk factors is important, which may allow for targeted treatment. However, inherited thrombophilia testing is still a double-edged sword [148]. Firstly, the testing has some limitations itself. For instance, it is costly and has risks of both false-positive and false-negative results, increasing the psychological and economic burden on patients [148]. Secondly, there was still no solid evidence currently to support the effectiveness of therapeutic options to prevent pregnancy complications in patients with inherited thrombophilia. Several case–control studies explored the effect of anticoagulant therapies using aspirin, heparins, or low molecular weight heparins; however, results remain discrepant [149]. Some large randomized clinical trials are required to provide higher-level evidence for this question. Overall, whether inherited thrombophilia testing is used for risk prediction of pregnancy complications or not requires clinicians to take all other factors into account.
In conclusion, the present meta-analysis showed significant associations between the increased risk of RPL and thrombophilic gene polymorphisms, especially MTHFR C677T, MTHFR A1298C, PAI-1 4G/5G, ACE I/D, Factor XIII V34L, and β-fibrinogen-455G/A, which may be useful clinical markers to evaluate the risk of RPL or to help unexplained RPL patients identify possible causes, allowing for targeted treatment during pregnancy if necessary.
Supplementary information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to the study conception and design. Literature review, data collection, and analysis were performed by Yuanjia Wen and Haodong He. The first draft of the manuscript was written by Yuanjia Wen. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
All the materials and data are available.
Declarations
Ethics approval
No ethical approval is required.
Consent
No consent is required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanjia Wen and Haodong He contributed equally to the article as the co-first authors.
References
- 1.Practice Committee of the American Society for Reproductive Medicine Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Haberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmark RE, Matthiesen L, Rylance R, Christiansen OB. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet Gynecol Scand. 2017;96:1365–1372. doi: 10.1111/aogs.13210. [DOI] [PubMed] [Google Scholar]
- 4.Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6:98. doi: 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- 5.Alijotas-Reig J, Garrido-Gimenez C. Current concepts and new trends in the diagnosis and management of recurrent miscarriage. Obstet Gynecol Surv. 2013;68:445–466. doi: 10.1097/OGX.0b013e31828aca19. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. 2020;26:356–367. doi: 10.1093/humupd/dmz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertig AT, Rock J, Adams EC. A description of 34 human ova within the first 17 days of development. Am J Anat. 1956;98:435–493. doi: 10.1002/aja.1000980306. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Chen Y, Ye C, Xing D, Wu R, Li F, et al. Hereditary thrombophilia and recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod. 2021;36:1213–1229. doi: 10.1093/humrep/deab010. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2022. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelen WL, Steegers EA, Eskes TK, Blom HJ. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet. 1997;350:861. doi: 10.1016/S0140-6736(97)24038-9. [DOI] [PubMed] [Google Scholar]
- 14.Quere I, Bellet H, Hoffet M, Janbon C, Mares P, Gris JC. A woman with five consecutive fetal deaths: case report and retrospective analysis of hyperhomocysteinemia prevalence in 100 consecutive women with recurrent miscarriages. Fertil Steril. 1998;69:152–154. doi: 10.1016/s0015-0282(97)00451-2. [DOI] [PubMed] [Google Scholar]
- 15.Holmes ZR, Regan L, Chilcott I, Cohen H. The C677T MTHFR gene mutation is not predictive of risk for recurrent fetal loss. Br J Haematol. 1999;105:98–101. [PubMed] [Google Scholar]
- 16.Lissak A, Sharon A, Fruchter O, Kassel A, Sanderovitz J, Abramovici H. Polymorphism for mutation of cytosine to thymine at location 677 in the methylenetetrahydrofolate reductase gene is associated with recurrent early fetal loss. Am J Obstet Gynecol. 1999;181:126–130. doi: 10.1016/s0002-9378(99)70447-3. [DOI] [PubMed] [Google Scholar]
- 17.Murphy RP, Donoghue C, Nallen RJ, D'Mello M, Regan C, Whitehead AS, et al. Prospective evaluation of the risk conferred by factor V Leiden and thermolabile methylenetetrahydrofolate reductase polymorphisms in pregnancy. Arterioscler Thromb Vasc Biol. 2000;20:266–270. doi: 10.1161/01.atv.20.1.266. [DOI] [PubMed] [Google Scholar]
- 18.Wramsby ML, Sten-Linder M, Bremme K. Primary habitual abortions are associated with high frequency of factor V Leiden mutation. Fertil Steril. 2000;74:987–991. doi: 10.1016/s0015-0282(00)01545-4. [DOI] [PubMed] [Google Scholar]
- 19.Pihusch R, Buchholz T, Lohse P, Rubsamen H, Rogenhofer N, Hasbargen U, et al. Thrombophilic gene mutations and recurrent spontaneous abortion: prothrombin mutation increases the risk in the first trimester. Am J Reprod Immunol. 2001;46:124–131. doi: 10.1111/j.8755-8920.2001.460202.x. [DOI] [PubMed] [Google Scholar]
- 20.Dilley A, Benito C, Hooper WC, Austin H, Miller C, El-Jamil M, et al. Mutations in the factor V, prothrombin and MTHFR genes are not risk factors for recurrent fetal loss. J Matern Fetal Neonatal Med. 2002;11:176–182. doi: 10.1080/jmf.11.3.176.182. [DOI] [PubMed] [Google Scholar]
- 21.Unfried G, Griesmacher A, Weismuller W, Nagele F, Huber JC, Tempfer CB. The C677T polymorphism of the methylenetetrahydrofolate reductase gene and idiopathic recurrent miscarriage. Obstet Gynecol. 2002;99:614–619. doi: 10.1016/s0029-7844(01)01789-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang YW, Li F, Li YP, Xue MZ, Yu XW, Li XC, et al. The study on the relationship between the methylenetetrahydofolate reductase 677C→T mutation and unexplained recurrent pregnancy loss. Chinese Journal of Practical Gynecology and Obstetrics. 2002: 37–9.
- 23.Kumar KS, Govindaiah V, Naushad SE, Devi RR, Jyothy A. Plasma homocysteine levels correlated to interactions between folate status and methylene tetrahydrofolate reductase gene mutation in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol. 2003;23:55–58. doi: 10.1080/0144361021000043263. [DOI] [PubMed] [Google Scholar]
- 24.Hohlagschwandtner M, Unfried G, Heinze G, Huber JC, Nagele F, Tempfer C. Combined thrombophilic polymorphisms in women with idiopathic recurrent miscarriage. Fertil Steril. 2003;79:1141–1148. doi: 10.1016/s0015-0282(02)04958-0. [DOI] [PubMed] [Google Scholar]
- 25.Buchholz T, Lohse P, Rogenhofer N, Kosian E, Pihusch R, Thaler CJ. Polymorphisms in the ACE and PAI-1 genes are associated with recurrent spontaneous miscarriages. Hum Reprod. 2003;18:2473–2477. doi: 10.1093/humrep/deg474. [DOI] [PubMed] [Google Scholar]
- 26.Pauer HU, Voigt-Tschirschwitz T, Hinney B, Burfeind P, Wolf C, Emons G, et al. Analyzes of three common thrombophilic gene mutations in German women with recurrent abortions. Acta Obstet Gynecol Scand. 2003;82:942–947. doi: 10.1034/j.1600-0412.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Makino A, Nakanishi T, Sugiura-Ogasawara M, Ozaki Y, Suzumori N, Suzumori K. No association of C677T methylenetetrahydrofolate reductase and an endothelial nitric oxide synthase polymorphism with recurrent pregnancy loss. Am J Reprod Immunol. 2004;52:60–66. doi: 10.1111/j.1600-0897.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang XP, Lin QD, Ma ZW. Zhao AM [C677T and A1298C mutation of the methylenetetrahydrofolate reductase gene in unexplained recurrent spontaneous abortion] Zhonghua Fu Chan Ke Za Zhi. 2004;39:238–241. [PubMed] [Google Scholar]
- 29.Li XM, Zhang YZ, Xu YX. Jiang S [Study on the relationship of MTHFR polymorphisms with unexplained recurrent spontaneous abortion] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:39–42. [PubMed] [Google Scholar]
- 30.Guan LX, Du XY, Wang JX, Gao L, Wang RL, Li HB, et al. Association of genetic polymorphisms in plasminogen activator inhibitor-1 gene and 5,10-methylenetetrahydrofolate reductase gene with recurrent early spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:330–3. [PubMed] [Google Scholar]
- 31.Kobashi G, Kato EH, Morikawa M, Shimada S, Ohta K, Fujimoto S, et al. MTHFR C677T Polymorphism and factor V Leiden mutation are not associated with recurrent spontaneous abortion of unexplained etiology in Japanese women. Semin Thromb Hemost. 2005;31:266–271. doi: 10.1055/s-2005-872430. [DOI] [PubMed] [Google Scholar]
- 32.Song LY, Qi QH, She XD, et al. Relationship between genetic polymorphism of homocysteine metabolism enzyme and unexplained repeated spontaneous abortion. Chin J Perinatal Med. 2005;160–4.
- 33.Couto E. Association of anticardiolipin antibody and C677T in methylenetetrahydrofolate reductase mutation in women with recurrent spontaneous abortions: a new path to thrombophilia. Sao Paulo Med J. 2005;123:15–20. doi: 10.1590/S1516-31802005000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulam CB, Jeyendran RS, Fishel LA, Roussev R. Multiple thrombophilic gene mutations rather than specific gene mutations are risk factors for recurrent miscarriage. Am J Reprod Immunol. 2006;55:360–368. doi: 10.1111/j.1600-0897.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 35.Mtiraoui N, Zammiti W, Ghazouani L, Braham NJ, Saidi S, Finan RR, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy losses. Reproduction. 2006;131:395–401. doi: 10.1530/rep.1.00815. [DOI] [PubMed] [Google Scholar]
- 36.Dong SQ, Du XY, Liu XY, Xinying D, Xueyun L. Correlation analysis of MTHFR gene polymorphism and unexplained habitual abortion. J Pract Obstet Gynecol. 2006: 500–1.
- 37.Sotiriadis A, Vartholomatos G, Pavlou M, Kolaitis N, Dova L, Stefos T, et al. Combined thrombophilic mutations in women with unexplained recurrent miscarriage. Am J Reprod Immunol. 2007;57:133–141. doi: 10.1111/j.1600-0897.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 38.Vettriselvi V, Vijayalakshmi K, Paul SF, Venkatachalam P. ACE and MTHFR gene polymorphisms in unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2008;34:301–306. doi: 10.1111/j.1447-0756.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- 39.Biswas A, Choudhry P, Mittal A, Meena A, Ranjan R, Choudhry VP, et al. Recurrent abortions in Asian Indians: no role of factor V Leiden Hong Kong/Cambridge mutation and MTHFR polymorphism. Clin Appl Thromb Hemost. 2008;14:102–104. doi: 10.1177/1076029607303774. [DOI] [PubMed] [Google Scholar]
- 40.Toth B, Vocke F, Rogenhofer N, Friese K, Thaler CJ, Lohse P. Paternal thrombophilic gene mutations are not associated with recurrent miscarriage. Am J Reprod Immunol. 2008;60:325–332. doi: 10.1111/j.1600-0897.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay R, Saraswathy KN, Ghosh PK. MTHFR C677T and factor V Leiden in recurrent pregnancy loss: a study among an endogamous group in North India. Genet Test Mol Biomarkers. 2009;13:861–865. doi: 10.1089/gtmb.2009.0063. [DOI] [PubMed] [Google Scholar]
- 42.Govindaiah V, Naushad SM, Prabhakara K, Krishna PC, Radha RDA. Association of parental hyperhomocysteinemia and C677T Methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clin Biochem. 2009;42:380–386. doi: 10.1016/j.clinbiochem.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Ciacci C, Tortora R, Scudiero O, Di Fiore R, Salvatore F, Castaldo G. Early pregnancy loss in celiac women: the role of genetic markers of thrombophilia. Dig Liver Dis. 2009;41:717–720. doi: 10.1016/j.dld.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Bae J, Choi DH, Kang MS, Cha SH, Oh D, Kim NK. Effect of methylenetetrahydrofolate reductase and thymidylate synthase enhancer region polymorphisms on the risk of idiopathic recurrent spontaneous abortion in a Korean population. Fertil Steril. 2009;91:1560–1562. doi: 10.1016/j.fertnstert.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 45.Yenicesu GI, Cetin M, Ozdemir O, Cetin A, Ozen F, Yenicesu C, et al. A prospective case-control study analyzes 12 thrombophilic gene mutations in Turkish couples with recurrent pregnancy loss. Am J Reprod Immunol. 2010;63:126–136. doi: 10.1111/j.1600-0897.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- 46.Seremak-Mrozikiewicz A, Drews K, Kurzawinska G, Bogacz A, Grzeskowiak E, Mrozikiewicz PM. The significance of 1793G>A polymorphism in MTHFR gene in women with first trimester recurrent miscarriages. Neuro Endocrinol Lett. 2010;31:717–723. [PubMed] [Google Scholar]
- 47.Mohamed MA, El MMA, El KAF, Mohamed SA, Ali AI. Thrombophilic gene mutations in women with repeated spontaneous miscarriage. Genet Test Mol Biomarkers. 2010;14:593–597. doi: 10.1089/gtmb.2010.0052. [DOI] [PubMed] [Google Scholar]
- 48.Settin A, Elshazli R, Salama A, ElBaz R. Methylenetetrahydrofolate reductase gene polymorphisms in Egyptian women with unexplained recurrent pregnancy loss. Genet Test Mol Biomarkers. 2011;15:887–892. doi: 10.1089/gtmb.2011.0049. [DOI] [PubMed] [Google Scholar]
- 49.Jeddi-Tehrani M, Torabi R, Zarnani AH, Mohammadzadeh A, Arefi S, Zeraati H, et al. Analysis of plasminogen activator inhibitor-1, integrin beta3, beta fibrinogen, and methylenetetrahydrofolate reductase polymorphisms in Iranian women with recurrent pregnancy loss. Am J Reprod Immunol. 2011;66:149–156. doi: 10.1111/j.1600-0897.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 50.Park CW, Han AR, Kwak-Kim J, Park SY, Han JY, Koong MK, et al. The role of methylenetetrahydrofolate reductase C677T polymorphism on the peripheral blood natural killer cell proportion in women with unexplained recurrent miscarriages. Clin Exp Reprod Med. 2011;38:168–173. doi: 10.5653/cerm.2011.38.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozdemir O, Yenicesu GI, Silan F, Koksal B, Atik S, Ozen F, et al. Recurrent pregnancy loss and its relation to combined parental thrombophilic gene mutations. Genet Test Mol Biomarkers. 2012;16:279–286. doi: 10.1089/gtmb.2011.0191. [DOI] [PubMed] [Google Scholar]
- 52.Nair RR, Khanna A, Singh R, Singh K. Association of maternal and fetal MTHFR A1298C polymorphism with the risk of pregnancy loss: a study of an Indian population and a meta-analysis. Fertil Steril. 2013;99:1311–8.e4. doi: 10.1016/j.fertnstert.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Karata S, Aydin Y, Ocer F, Buyru A, Balci H. Hereditary thrombophilia, anti-beta2 glycoprotein 1 IgM, and anti-annexin V antibodies in recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:251–255. doi: 10.1111/j.1600-0897.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- 54.Dissanayake VH, Sirisena ND, Weerasekera LY, Gammulla CG, Seneviratne HR, Jayasekara RW. Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res. 2012;38:1168–1176. doi: 10.1111/j.1447-0756.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- 55.Idali F, Zareii S, Mohammad-Zadeh A, Reihany-Sabet F, Akbarzadeh-Pasha Z, Khorram-Khorshid HR, et al. Plasminogen activator inhibitor 1 and methylenetetrahydrofolate reductase gene mutations in iranian women with polycystic ovary syndrome. Am J Reprod Immunol. 2012;68:400–407. doi: 10.1111/aji.12002. [DOI] [PubMed] [Google Scholar]
- 56.Poursadegh ZA, Chaparzadeh N, Asghari EM, Mehrzad SM, Farzadi L, Ghasemzadeh A, et al. Methylenetetrahydrofolate reductase C677T and A1298C mutations in women with recurrent spontaneous abortions in the Northwest of Iran. ISRN Obstet Gynecol. 2012;2012:945486. doi: 10.5402/2012/945486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torabi R, Zarei S, Zeraati H, Zarnani AH, Akhondi MM, Hadavi R, et al. Combination of thrombophilic gene polymorphisms as a cause of increased the risk of recurrent pregnancy loss. J Reprod Infertil. 2012;13:89–94. [PMC free article] [PubMed] [Google Scholar]
- 58.Kazerooni T, Ghaffarpasand F, Asadi N, Dehkhoda Z, Dehghankhalili M, Kazerooni Y. Correlation between thrombophilia and recurrent pregnancy loss in patients with polycystic ovary syndrome: a comparative study. J Chin Med Assoc. 2013;76:282–288. doi: 10.1016/j.jcma.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTHFR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet. 2013;288:1171–1177. doi: 10.1007/s00404-013-2877-x. [DOI] [PubMed] [Google Scholar]
- 60.Creus M, Deulofeu R, Penarrubia J, Carmona F, Balasch J. Plasma homocysteine and vitamin B12 serum levels, red blood cell folate concentrations, C677T methylenetetrahydrofolate reductase gene mutation and risk of recurrent miscarriage: a case-control study in Spain. Clin Chem Lab Med. 2013;51:693–699. doi: 10.1515/cclm-2012-0452. [DOI] [PubMed] [Google Scholar]
- 61.Kaur L, Puri M, Kaushik S, Sachdeva MP, Trivedi SS, Saraswathy KN. Genetic thromobophilia in pregnancy: a case-control study among North Indian women. J Thromb Thrombolysis. 2013;35:250–256. doi: 10.1007/s11239-012-0797-4. [DOI] [PubMed] [Google Scholar]
- 62.Baumann K, Beuter-Winkler P, Hackethal A, Strowitzki T, Toth B, Bohlmann MK. Maternal factor V Leiden and prothrombin mutations do not seem to contribute to the occurrence of two or more than two consecutive miscarriages in Caucasian patients. Am J Reprod Immunol. 2013;70:518–521. doi: 10.1111/aji.12144. [DOI] [PubMed] [Google Scholar]
- 63.Dutra CG, Fraga LR, Nacul AP, Passos EP, Goncalves RO, Nunes OL, et al. Lack of association between thrombophilic gene variants and recurrent pregnancy loss. Hum Fertil (Camb) 2014;17:99–105. doi: 10.3109/14647273.2014.882022. [DOI] [PubMed] [Google Scholar]
- 64.Cao Y, Zhang Z, Zheng Y, Yuan W, Wang J, Liang H, et al. The association of idiopathic recurrent early pregnancy loss with polymorphisms in folic acid metabolism-related genes. Genes Nutr. 2014;9:402. doi: 10.1007/s12263-014-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yousefian E, Kardi MT, Allahveisi A. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism in Iranian women with idiopathic recurrent pregnancy losses. Iran Red Crescent Med J. 2014;16:e16763. doi: 10.5812/ircmj.16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietropolli A, Giuliani E, Bruno V, Patrizi L, Piccione E, Ticconi C. Plasminogen activator inhibitor-1, factor V, factor II and methylenetetrahydrofolate reductase polymorphisms in women with recurrent miscarriage. J Obstet Gynaecol. 2014;34:229–234. doi: 10.3109/01443615.2013.836476. [DOI] [PubMed] [Google Scholar]
- 67.Lino FL, Traina E, Barreto JA, Moron AF, Mattar R. Thrombophilic mutations and polymorphisms, alone or in combination, and recurrent spontaneous abortion. Clin Appl Thromb Hemost. 2015;21:365–372. doi: 10.1177/1076029613520465. [DOI] [PubMed] [Google Scholar]
- 68.Luo L, Chen Y, Wang L, Zhuo G, Qiu C, Tu Q, et al. Polymorphisms of Genes Involved in the Folate Metabolic Pathway Impact the Occurrence of Unexplained Recurrent Pregnancy Loss. Reprod Sci. 2015;22:845–851. doi: 10.1177/1933719114565033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boas WV, Goncalves RO, Costa OL, Goncalves MS. Metabolism and gene polymorphisms of the folate pathway in Brazilian women with history of recurrent abortion. Rev Bras Ginecol Obstet. 2015;37:71–76. doi: 10.1590/SO100-720320140005223. [DOI] [PubMed] [Google Scholar]
- 70.Vanilla S, Dayanand CD, Kotur PF, Kutty MA, Vegi PK. Evidence of paternal N5, N10 - methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with recurrent spontaneous abortions (RSAs) in Kolar District- a South West of India. J Clin Diagn Res. 2015;9:15–8. doi: 10.7860/JCDR/2015/10856.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu L. Polymorphisms in the methylene tetrahydrofolate reductase and methionine synthase reductase genes and their correlation with unexplained recurrent spontaneous abortion susceptibility. Genet Mol Res. 2015;14:8500–8508. doi: 10.4238/2015.July.28.19. [DOI] [PubMed] [Google Scholar]
- 72.Tara SS, Ghaemimanesh F, Zarei S, Reihani-Sabet F, Pahlevanzadeh Z, Modarresi MH, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in male partners of recurrent miscarriage couples. J Reprod Infertil. 2015;16:193–198. [PMC free article] [PubMed] [Google Scholar]
- 73.Farahmand K, Totonchi M, Hashemi M, Reyhani SF, Kalantari H, Gourabi H, et al. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2016;29:1269–1273. doi: 10.3109/14767058.2015.1044431. [DOI] [PubMed] [Google Scholar]
- 74.Goncalves RO, Fraga LR, Santos WV, Carvalho AF, Veloso CBA, Sarno M, et al. Association between the thrombophilic polymorphisms MTHFR C677T, Factor V Leiden, and prothrombin G20210A and recurrent miscarriage in Brazilian women. Genet Mol Res. 2016; 15. [DOI] [PubMed]
- 75.Lopez-Jimenez JJ, Porras-Dorantes A, Juarez-Vazquez CI, Garcia-Ortiz JE, Fuentes-Chavez CA, Lara-Navarro IJ, et al. Molecular thrombophilic profile in Mexican patients with idiopathic recurrent pregnancy loss. Genet Mol Res. 2016; 15. [DOI] [PubMed]
- 76.Hwang KR, Choi YM, Kim JJ, Lee SK, Yang KM, Paik EC, et al. Methylenetetrahydrofolate reductase polymorphisms and risk of recurrent pregnancy loss: a case-control study. J Korean Med Sci. 2017;32:2029–2034. doi: 10.3346/jkms.2017.32.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Achkar W, Wafa A, Ammar S, Moassass F, Jarjour RA. Association of methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms with recurrent pregnancy loss in Syrian women. Reprod Sci. 2017;24:1275–1279. doi: 10.1177/1933719116682874. [DOI] [PubMed] [Google Scholar]
- 78.Chatzidimitriou M, Chatzidimitriou D, Mavridou M, Anetakis C, Chatzopoulou F, Lialiaris T, et al. Thrombophilic gene polymorphisms and recurrent pregnancy loss in Greek women. Int J Lab Hematol. 2017;39:590–595. doi: 10.1111/ijlh.12703. [DOI] [PubMed] [Google Scholar]
- 79.Wolski H, Barlik M, Drews K, Klejewski A, Kurzawińska G, Ożarowski M, et al. Contribution of inherited thrombophilia to recurrent miscarriage in the Polish population. Ginekol Pol. 2017;88:385–392. doi: 10.5603/GP.a2017.0072. [DOI] [PubMed] [Google Scholar]
- 80.Alptekin H, Alptekin N, Selimoğlu RS, Cengiz T, Barış SB. Inherited thrombophilia and thromboprophylaxis: a retrospective analysis of pregnancy outcomes in 106 patients. Clin Exp Obstet Gynecol. 2017;44:749–754. [Google Scholar]
- 81.Jusic A, Balic D, Avdic A, Podanin M, Balic A. The association of factor V G1961A (factor V Leiden), prothrombin G20210A, MTHFR C677T and PAI-1 4G/5G polymorphisms with recurrent pregnancy loss in Bosnian women. Med Glas (Zenica) 2018;15:158–163. doi: 10.17392/948-18. [DOI] [PubMed] [Google Scholar]
- 82.Bigdeli R, Younesi MR, Panahnejad E, Asgary V, Heidarzadeh S, Mazaheri H, et al. Association between thrombophilia gene polymorphisms and recurrent pregnancy loss risk in the Iranian population. Syst Biol Reprod Med. 2018;64:274–282. doi: 10.1080/19396368.2018.1456576. [DOI] [PubMed] [Google Scholar]
- 83.Dell'Edera D, L'Episcopia A, Simone F, Lupo MG, Epifania AA, Allegretti A. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms and susceptibility to recurrent pregnancy loss. Biomed Rep. 2018;8:172–175. doi: 10.3892/br.2018.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El AH, Awwad J, Abou DS, Halabi S, Damianos S, Mahfouz R. The association between cardiovascular disease gene mutations and recurrent pregnancy loss in the Lebanese population. Mol Biol Rep. 2018;45:911–916. doi: 10.1007/s11033-018-4237-1. [DOI] [PubMed] [Google Scholar]
- 85.Sah AK, Shrestha N, Joshi P, Lakha R, Shrestha S, Sharma L, et al. Association of parental methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with unexplained recurrent pregnancy loss. BMC Res Notes. 2018;11:233. doi: 10.1186/s13104-018-3321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saraswathy KN, Kaur L, Talwar S, Mishra J, Huidrom S, Sachdeva MP, et al. Methylenetetrahydrofolate reductase gene-specific methylation and recurrent miscarriages: a case- control study from North India. J Hum Reprod Sci. 2018;11:142–147. doi: 10.4103/jhrs.JHRS_145_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Wu T, Ye L, Li G, Zeng Y, Zhang Y. Prevalent genotypes of methylenetetrahydrofolate reductase (MTHFR) in recurrent miscarriage and recurrent implantation failure. J Assist Reprod Genet. 2018;35:1437–1442. doi: 10.1007/s10815-018-1205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trifonova EA, Swarovskaya MG, Ganzha OA, Voronkova OV, Gabidulina TV, Stepanov VA. The interaction effect of angiogenesis and endothelial dysfunction-related gene variants increases the susceptibility of recurrent pregnancy loss. J Assist Reprod Genet. 2019;36:717–726. doi: 10.1007/s10815-019-01403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Y, Ban Y, Ran L, Yu Y, Zhai S, Sun Z, et al. Relationship between unexplained recurrent pregnancy loss and 5,10-methylenetetrahydrofolate reductase) polymorphisms. Fertil Steril. 2019;111:597–603. doi: 10.1016/j.fertnstert.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 90.Ahangari N, Doosti M, Mousavifar N, Attaran M, Shahrokhzadeh S, Memarpour S, et al. Hereditary thrombophilia genetic variants in recurrent pregnancy loss. Arch Gynecol Obstet. 2019;300:777–782. doi: 10.1007/s00404-019-05224-7. [DOI] [PubMed] [Google Scholar]
- 91.Zarfeshan FY, Kooshkaki O, Kordi TD, Anani SG. Investigation of the association between C677T polymorphism of the MTHFR gene and plasma homocysteine level in recurrent fetal miscarriage. J Obstet Gynaecol Res. 2019;45:1442–1447. doi: 10.1111/jog.13989. [DOI] [PubMed] [Google Scholar]
- 92.Mishra J, Talwar S, Kaur L, Chandiok K, Yadav S, Puri M, et al. Differential global and MTHFR gene specific methylation patterns in preeclampsia and recurrent miscarriages: a case-control study from North India. Gene. 2019;704:68–73. doi: 10.1016/j.gene.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 93.Lin Z, Li Q, Sun Y, Huang J, Wang W, Fu J, et al. Interactions between genetic variants involved in the folate metabolic pathway and serum lipid, homocysteine levels on the risk of recurrent spontaneous abortion. Lipids Health Dis. 2019;18:143. doi: 10.1186/s12944-019-1083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joksic I, Mikovic Z, Filimonovic D, Munjas J, Karadzov ON, Egic A, et al. Combined presence of coagulation factor XIII V34L and plasminogen activator inhibitor 1 4G/5G gene polymorphisms significantly contribute to recurrent pregnancy loss in Serbian population. J Med Biochem. 2020;39:199–207. doi: 10.2478/jomb-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yengel I, Yorulmaz T, Api M. Association between FVL G1691A, FII G20210A, and MTHFR C677T and A1298C polymorphisms and Turkish women with recurrent pregnancy loss. Med Glas (Zenica) 2020;17:129–135. doi: 10.17392/1062-20. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Zhan W, Du Q, Wu L, Ding H, Liu F, et al. Variants c.677 C>T, c.1298 A>C in MTHFR, and c.66 A>G in MTRR affect the occurrence of recurrent pregnancy loss in chinese women. Genet Test Mol Biomarkers. 2020;24:717–22. doi: 10.1089/gtmb.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shaker MM, Shalabi TA, Amr KS. Correlation of methylation status in MTHFR promoter region with recurrent pregnancy loss. J Genet Eng Biotechnol. 2021;19:44. doi: 10.1186/s43141-021-00147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arabkhazaeli N, Ghanaat K, Hashemi-Soteh MB. H1299R in coagulation Factor V and Glu429Ala in MTHFR genes in recurrent pregnancy loss in Sari. Mazandaran Int J Reprod Biomed. 2016;14:329–334. [PMC free article] [PubMed] [Google Scholar]
- 99.Izuhara M, Shinozawa K, Kitaori T, Katano K, Ozaki Y, Fukutake K, et al. Genotyping analysis of the factor V Nara mutation, Hong Kong mutation, and 16 single-nucleotide polymorphisms, including the R2 haplotype, and the involvement of factor V activity in patients with recurrent miscarriage. Blood Coagul Fibrinolysis. 2017;28:323–328. doi: 10.1097/MBC.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 100.Anwar R, Gallivan L, Edmonds SD, Markham AF. Genotype/phenotype correlations for coagulation factor XIII: specific normal polymorphisms are associated with high or low factor XIII specific activity. Blood. 1999;93:897–905. [PubMed] [Google Scholar]
- 101.Dossenbach-Glaninger A, van Trotsenburg M, Dossenbach M, Oberkanins C, Moritz A, Krugluger W, et al. Plasminogen activator inhibitor 1 4G/5G polymorphism and coagulation factor XIII Val34Leu polymorphism: impaired fibrinolysis and early pregnancy loss. Clin Chem. 2003;49:1081–1086. doi: 10.1373/49.7.1081. [DOI] [PubMed] [Google Scholar]
- 102.Barbosa HC, Carvalho EC, Barini R, Siqueira LH, Costa DS, Annichino-Bizzacchi JM. Tyr204Phe and Val34Leu polymorphisms in two Brazilian ethnic groups and in patients with recurrent miscarriages. Fertil Steril. 2004;82:1455–1457. doi: 10.1016/j.fertnstert.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 103.Lopez RY, Vivenes M, Miller A, Pulido A, Lopez MJ, Arocha-Pinango CL, et al. Prevalence of the coagulation factor XIII polymorphism Val34Leu in women with recurrent miscarriage. Clin Chim Acta. 2006;374:69–74. doi: 10.1016/j.cca.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 104.Bagheri M, Rad IA, Omrani MD, Nanbaksh F. The Val34Leu genetic variation in the A subunit of coagulation factor XIII in recurrent spontaneous abortion. Syst Biol Reprod Med. 2011;57:261–264. doi: 10.3109/19396368.2011.576308. [DOI] [PubMed] [Google Scholar]
- 105.Elmahgoub IR, Afify RA, Abdel AAA, El-Sherbiny WS. Prevalence of coagulation factor XIII and plasminogen activator inhibitor-1 gene polymorphisms among Egyptian women suffering from unexplained primary recurrent miscarriage. J Reprod Immunol. 2014;103:18–22. doi: 10.1016/j.jri.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Cai XJ, Zhen ML, Meiling Z. Study on association of B-fibrinogen gene G/A-455 polymorphisms with recurrent spontaneous abortion. Chin J Birth Health Hered. 2008: 15–6.
- 107.Ticconi C, Mancinelli F, Gravina P, Federici G, Piccione E, Bernardini S. Beta-fibrinogen G-455A polymorphisms and recurrent miscarriage. Gynecol Obstet Invest. 2011;71:198–201. doi: 10.1159/000317522. [DOI] [PubMed] [Google Scholar]
- 108.Maziri P, Tehrani GA. Association between thrombophilic gene polymorphisms and recurrent pregnancy loss in Iranian Women. Iranian Journal of Neonatology. 2017; 8.
- 109.Issa NM, El-Neily DAM, El TSS, El-Attar LM. The prevalence of specific gene polymorphisms related to thrombophilia in Egyptian women with recurrent pregnancy loss. J Hum Reprod Sci. 2021;14:73–80. doi: 10.4103/jhrs.JHRS_24_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolf CE, Haubelt H, Pauer HU, Hinney B, Krome-Cesar C, Legler TJ, et al. Recurrent pregnancy loss and its relation to FV Leiden, FII G20210A and polymorphisms of plasminogen activator and plasminogen activator inhibitor. Pathophysiol Haemost Thromb. 2003;33:134–137. doi: 10.1159/000077821. [DOI] [PubMed] [Google Scholar]
- 111.Goodman C, Hur J, Goodman CS, Jeyendran RS, Coulam C. Are polymorphisms in the ACE and PAI-1 genes associated with recurrent spontaneous miscarriages? Am J Reprod Immunol. 2009;62:365–370. doi: 10.1111/j.1600-0897.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 112.Al SRJ, Sharif FA. Polymorphisms in NOS3, ACE and PAI-1 genes and risk of spontaneous recurrent miscarriage in the Gaza Strip. Med Princ Pract. 2010;19:99–104. doi: 10.1159/000273067. [DOI] [PubMed] [Google Scholar]
- 113.Jeddi-Tehrani M, Torabi R, Mohammadzadeh A, Arefi S, Keramatipour M, Zeraati H, et al. Investigating association of three polymorphisms of coagulation Factor XIII and recurrent pregnancy loss. Am J Reprod Immunol. 2010;64:212–217. doi: 10.1111/j.1600-0897.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- 114.Aarabi M, Memariani T, Arefi S, Aarabi M, Hantoosh ZS, Akhondi MA, et al. Polymorphisms of plasminogen activator inhibitor-1, angiotensin converting enzyme and coagulation factor XIII genes in patients with recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:545–548. doi: 10.3109/14767058.2010.511331. [DOI] [PubMed] [Google Scholar]
- 115.Subrt I, Ulcova-Gallova Z, Cerna M, Hejnalova M, Slovanova J, Bibkova K, et al. Recurrent pregnancy loss, plasminogen activator inhibitor-1 (-675) 4G/5G polymorphism and antiphospholipid antibodies in Czech women. Am J Reprod Immunol. 2013;70:54–58. doi: 10.1111/aji.12099. [DOI] [PubMed] [Google Scholar]
- 116.Magdoud K, Herbepin VG, Touraine R, Almawi WY, Mahjoub T. Plasminogen activator inhibitor 1 4G/5G and -844G/A variants in idiopathic recurrent pregnancy loss. Am J Reprod Immunol. 2013;70:246–252. doi: 10.1111/aji.12116. [DOI] [PubMed] [Google Scholar]
- 117.Jeon YJ, Kim YR, Lee BE, Choi YS, Kim JH, Shin JE, et al. Genetic association of five plasminogen activator inhibitor-1 (PAI-1) polymorphisms and idiopathic recurrent pregnancy loss in Korean women. Thromb Haemost. 2013;110:742–750. doi: 10.1160/TH13-03-0242. [DOI] [PubMed] [Google Scholar]
- 118.Kim JJ, Choi YM, Lee SK, Yang KM, Paik EC, Jeong HJ, et al. The PAI-1 4G/5G and ACE I/D polymorphisms and risk of recurrent pregnancy loss: a case-control study. Am J Reprod Immunol. 2014;72:571–576. doi: 10.1111/aji.12302. [DOI] [PubMed] [Google Scholar]
- 119.Khosravi F, Zarei S, Ahmadvand N, Akbarzadeh-Pasha Z, Savadi E, Zarnani AH, et al. Association between plasminogen activator inhibitor 1 gene mutation and different subgroups of recurrent miscarriage and implantation failure. J Assist Reprod Genet. 2014;31:121–124. doi: 10.1007/s10815-013-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shakarami F, Akbari MT, Zare KS. Association of plasminogen activator inhibitor-1 and angiotensin converting enzyme polymorphisms with recurrent pregnancy loss in Iranian women. Iran J Reprod Med. 2015;13:627–632. [PMC free article] [PubMed] [Google Scholar]
- 121.Barlik M, Seremak-Mrozikiewicz A, Drews K, Klejewski A, Kurzawinska G, Lowicki Z, et al. Correlation between factor VII and PAI-1 genetic variants and recurrent miscarriage. Ginekol Pol. 2016;87:504–509. doi: 10.5603/GP.2016.0034. [DOI] [PubMed] [Google Scholar]
- 122.Salazar GMD, Sung N, Mullenix TM, Dambaeva S, Beaman K, Gilman-Sachs A, et al. Plasminogen activator inhibitor-1 4G/5G polymorphism is associated with reproductive failure: metabolic, hormonal, and immune profiles. Am J Reprod Immunol. 2016;76:70–81. doi: 10.1111/aji.12516. [DOI] [PubMed] [Google Scholar]
- 123.Le DAT, Ta VT, Nguyen AD. Associations of MTHFR and PAI-1 4G/5G Polymorphisms with Unexplained Recurrent Pregnancy Loss. Intern Med J. 2022;29:20–22. [Google Scholar]
- 124.Fatini C, Gensini F, Battaglini B, Prisco D, Cellai AP, Fedi S, et al. Angiotensin-converting enzyme DD genotype, angiotensin type 1 receptor CC genotype, and hyperhomocysteinemia increase first-trimester fetal-loss susceptibility. Blood Coagul Fibrinolysis. 2000;11:657–662. doi: 10.1097/00001721-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Bagheri M, Abdi RI, Omrani MD, Nanbaksh F. Polymorphisms of the angiotensin converting enzyme gene in Iranian Azeri Turkish women with unexplained recurrent pregnancy loss. Hum Fertil (Camb) 2010;13:79–82. doi: 10.3109/14647273.2010.484844. [DOI] [PubMed] [Google Scholar]
- 126.Choi YS, Kwon H, Kim JH, Shin JE, Choi Y, Yoon TK, et al. Haplotype-based association of ACE I/D, AT1R 1166A>C, and AGT M235T polymorphisms in renin-angiotensin-aldosterone system genes in Korean women with idiopathic recurrent spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 2011;158:225–228. doi: 10.1016/j.ejogrb.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 127.Zhang S, Wang J, Wang B, Ping Y, Ma X. Strong association between angiotensin I-converting enzyme I/D polymorphism and unexplained recurrent miscarriage of Chinese women–a case-control study. Reprod Sci. 2011;18:743–746. doi: 10.1177/1933719111415865. [DOI] [PubMed] [Google Scholar]
- 128.Kurzawinska G, Barlik M, Drews K, Rozycka A, Seremak-Mrozikiewicz A, Ozarowski M, et al. Coexistence of ACE (I/D) and PAI-1 (4G/5G) gene variants in recurrent miscarriage in Polish population. Ginekol Pol. 2016;87:271–276. doi: 10.17772/gp/62203. [DOI] [PubMed] [Google Scholar]
- 129.Fazelnia S, Farazmandfar T, Hashemi-Soteh SM. Significant correlation of angiotensin converting enzyme and glycoprotein IIIa genes polymorphisms with unexplained recurrent pregnancy loss in north of Iran. Int J Reprod Biomed. 2016;14:323–328. [PMC free article] [PubMed] [Google Scholar]
- 130.Al-Mukaynizi FB, AlKhuriji A, Babay Z, Addar M, AlDaihan S, Alanazi M, et al. Lack of Association between angiotensin converting enzyme I/D polymorphism and unexplained recurrent miscarriage in Saudi Arabia. J Med Biochem. 2016;35:166–173. doi: 10.1515/jomb-2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pereza N, Ostojic S, Zdravcevic M, Volk M, Kapovic M, Peterlin B. Insertion/deletion polymorphism in intron 16 of ACE gene in idiopathic recurrent spontaneous abortion: case-control study, systematic review and meta-analysis. Reprod Biomed Online. 2016;32:237–246. doi: 10.1016/j.rbmo.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 132.Hussian AMF, Mohammed NEA, Ahmed MAM, et al. Angiotensin converting enzyme insertion/deletion (I/D) polymorphism and risk of recurrent pregnancy loss among sudanese women. Obstet Gynecol. 2016;4:7–10. [Google Scholar]
- 133.Gumus E. The powerful association of angiotensin-converting enzyme insertion/deletion polymorphism and idiopathic recurrent pregnancy loss. Ginekol Pol. 2018;89:573–576. doi: 10.5603/GP.a2018.0098. [DOI] [PubMed] [Google Scholar]
- 134.Heidari MM, Sheikholeslami M, Yavari M, Khatami M, Seyedhassani SM. The association of renin-angiotensinogen system genes polymorphisms and idiopathic recurrent pregnancy loss. Hum Fertil (Camb) 2019;22:164–170. doi: 10.1080/14647273.2017.1388545. [DOI] [PubMed] [Google Scholar]
- 135.Ren A, Wang J. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of unexplained recurrent pregnancy loss: a meta-analysis. Fertil Steril. 2006;86:1716–1722. doi: 10.1016/j.fertnstert.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 136.Wang G, Lin Z, Wang X, Sun Q, Xun Z, Xing B, et al. The association between 5, 10 - methylenetetrahydrofolate reductase and the risk of unexplained recurrent pregnancy loss in China: A Meta-analysis. Medicine (Baltimore) 2021;100:e25487. doi: 10.1097/MD.0000000000025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu X, Zhao L, Zhu H, He D, Tang W, Luo Y. Association between the MTHFR C677T polymorphism and recurrent pregnancy loss: a meta-analysis. Genet Test Mol Biomarkers. 2012;16:806–811. doi: 10.1089/gtmb.2011.0318. [DOI] [PubMed] [Google Scholar]
- 138.Yang Y, Luo Y, Yuan J, Tang Y, Xiong L, Xu M, et al. Association between maternal, fetal and paternal MTHFR gene C677T and A1298C polymorphisms and risk of recurrent pregnancy loss: a comprehensive evaluation. Arch Gynecol Obstet. 2016;293:1197–1211. doi: 10.1007/s00404-015-3944-2. [DOI] [PubMed] [Google Scholar]
- 139.Cao Y, Xu J, Zhang Z, Huang X, Zhang A, Wang J, et al. Association study between methylenetetrahydrofolate reductase polymorphisms and unexplained recurrent pregnancy loss: a meta-analysis. Gene. 2013;514:105–111. doi: 10.1016/j.gene.2012.10.091. [DOI] [PubMed] [Google Scholar]
- 140.Du B, Shi X, Yin C, Feng X. Polymorphisms of methalenetetrahydrofolate reductase in recurrent pregnancy loss: an overview of systematic reviews and meta-analyses. J Assist Reprod Genet. 2019;36:1315–1328. doi: 10.1007/s10815-019-01473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rai V. Methylenetetrahydrofolate reductase gene A1298C polymorphism and susceptibility to recurrent pregnancy loss: a meta-analysis. Cell Mol Biol (Noisy-le-grand) 2014;60:27–34. [PubMed] [Google Scholar]
- 142.Chen H, Nie S, Lu M. Association between plasminogen activator inhibitor-1 gene polymorphisms and recurrent pregnancy loss: a systematic review and meta-analysis. Am J Reprod Immunol. 2015;73:292–300. doi: 10.1111/aji.12321. [DOI] [PubMed] [Google Scholar]
- 143.Li X, Liu Y, Zhang R, Tan J, Chen L, Liu Y. Meta-analysis of the association between plasminogen activator inhibitor-1 4G/5G polymorphism and recurrent pregnancy loss. Med Sci Monit. 2015;21:1051–1056. doi: 10.12659/MSM.892898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Su MT, Lin SH, Chen YC, Kuo PL. Genetic association studies of ACE and PAI-1 genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Thromb Haemost. 2013;109:8–15. doi: 10.1160/TH12-08-0584. [DOI] [PubMed] [Google Scholar]
- 145.Aslbahar F, Neamatzadeh H, Tabatabaiee RS, Karimi-Zarchi M, Javaheri A, Mazaheri M, et al. Association of angiotensin-converting enzyme insertion/deletion polymorphism with recurrent pregnancy loss: a meta-analysis of 26 case-control studies. Rev Bras Ginecol Obstet. 2018;40:631–641. doi: 10.1055/s-0038-1672137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang C, Fangfang W, Jie L, Yanlong Y, Jie W, Xuefei L, et al. Angiotensin-converting enzyme insertion/deletion (I/D) polymorphisms and recurrent pregnancy loss: a meta-analysis. J Assist Reprod Genet. 2012;29:1167–1173. doi: 10.1007/s10815-012-9870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jung JH, Kim JH, Song GG, Choi SJ. Association of the F13A1 Val34Leu polymorphism and recurrent pregnancy loss: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;215:234–240. doi: 10.1016/j.ejogrb.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 148.Middeldorp S. Inherited thrombophilia: a double-edged sword. Hematology Am Soc Hematol Educ Program. 2016;2016:1–9. doi: 10.1182/asheducation-2016.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simcox LE, Ormesher L, Tower C, Greer IA. Thrombophilia and pregnancy complications. Int J Mol Sci. 2015;16:28418–28428. doi: 10.3390/ijms161226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gopel W, Ludwig M, Junge AK, Kohlmann T, Diedrich K, Moller J. Selection pressure for the factor-V-Leiden mutation and embryo implantation. Lancet. 2001;358:1238–1239. doi: 10.1016/S0140-6736(01)06354-1. [DOI] [PubMed] [Google Scholar]
- 151.Nair RR, Khanna A, Singh K. MTHFR C677T polymorphism and recurrent early pregnancy loss risk in north Indian population. Reprod Sci. 2012;19:210–215. doi: 10.1177/1933719111417888. [DOI] [PubMed] [Google Scholar]
- 152.Poursadegh ZA, Chaparzadeh N, Ghorbian S, Sadaghiani MM, Farzadi L, Ghasemzadeh A, et al. The association between thrombophilic gene mutations and recurrent pregnancy loss. J Assist Reprod Genet. 2013;30:1353–1359. doi: 10.1007/s10815-013-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the materials and data are available.