Abstract
Purpose
Maintaining a stable pH at optimal level in human embryo culture media is crucial for embryo development but poses a challenge for all IVF laboratories. We validate analytically reliable conditions for pH measurement that are as close as possible to the embryo microenvironment during IVF.
Methods
This was a multicentric study. A Siemens EPOC portable blood gas analyzer was used. The analytical validation was carried out under the culture medium (Global Total HSA®) conditions of use (microdroplets, under oil overlay, in a IVF incubator with (EmbryoScope®) or without a time lapse system (K system G210+®) and using IVF dishes. The validation included repeatability (“within-run” precision), total precision (between-day precision), trueness based on inter-laboratory comparison, inaccuracy based on external quality assessment and comparison to the reference technique. We also assessed the pre-analytical medium incubation time required to obtain a target value.
Results
A measurement after an incubation period of 24 to 48 h is more representative of the pH to which the embryo will be exposed throughout the culture. The “within-run” and “between-day” precision show very low coefficients of variation (CV%): 0.17 to 0.22% and 0.13 to 0.34%, respectively, with IVF culture media. Trueness (% bias) range from − 0.07 to − 0.03%. We demonstrate good correlation between EPOC and reference pH electrode with an overestimation of 0.03 pH units of EPOC.
Conclusion
Our method demonstrates good analytical performance for IVF laboratories wishing to implement a robust quality assurance system to monitor pH in embryo culture media. Compliance with stringent pre-analytical and analytical conditions is essential.
Keywords: pH, Analytical validation, Embryo, IVF, Culture media
Introduction
There is growing evidence that culture conditions during IVF are critical not only for the development of pre- and post-implantation embryos but also for long-term health effects [1–5]. Under IVF conditions, the extracellular pH (pHe) in the embryo culture medium is the result of a balance between CO2 concentration in the incubator and bicarbonate concentration in the culture medium (determined by the manufacturer). Numerous animal data suggest the importance of pH control in the IVF culture medium. Maintaining the intracellular pH (pHi) is one of the vital roles of embryo homeostasis since pHi regulates many cellular processes. Indeed, variations in pHi can affect embryo development [6]. In the cytoskeleton, for example, alkalinization and acidification of pHi alter the actin network (microfilaments) and can affect hamster embryo development [7]. Furthermore, at metabolic level, the pHi value can impact enzymatic activities [8] or blastocoel formation [9, 10]. The pHi of the human embryo is maintained by regulatory mechanisms (mainly Na+/H+ and HCO3-/Cl- exchangers) that can be exceeded, particularly in oocytes and early stage embryos [11–15]. These regulatory mechanisms do not activate above or below a specific pHi threshold, so there may be a lag time during which pHi follows pHe [16]. Moreover, if pHe variations are too abrupt or too marked, the regulation mechanisms may be exceeded. This explains why monitoring the pH of IVF culture media is of paramount importance.
Beyond the clinical interest of controlling the pH of IVF culture medium, it is important to consider the analytical difficulty and relative reliably of measuring pH in such media. Indeed, pH probes are not always very reliable. The frequency of calibration is important for certain types of probes or devices (before each use). There is a need to use a sample of medium specifically for the measurement given the fact that it is impossible to measure pH in the real medium in which the embryo evolves. Exposure of the IVF culture medium to air must be avoided. The variability between batches of commercial IVF culture media should also be noted [17] as well as the fact that research on the precise analytical reliability of pH measurements in microdroplets of IVF culture media specifically has not yet been published, even if many labs commonly used blood gas analyzers for pH measurement of IVF culture media [18, 19]. All of these factors adversely impact the recording of pH levels. Some authors have demonstrated a quite good analytical reliability of conventional blood gas analyzers for pH measurement in IVF culture media in tubes in terms of precision, but not in terms of accuracy [19]. There is a need to evaluate the analytical performances of small measurement devices, adapted to the IVF lab and in real embryo culture conditions (microdrops under oil in IVF-specific dishes). Embryologists face numerous questions when it comes to the analytical conditions to be respected in order to record pH levels with good analytical reliability on the one hand, and under conditions that best reflect the pH in which the embryo evolves before implantation, on the other hand.
The purpose of this work is to initially set out the pre-analytical conditions (i.e., time of incubation of the culture medium before pH measurement), and mainly the analytical performance (i.e., accuracy, precision including repeatability and total precision and comparison with a reference apparatus) that can be reached when measuring pH under real embryo culture conditions. The embryo culture medium sample was analyzed in microdroplets under an oil overlay. Assessments were performed in two types of IVF culture dishes widely used for time-lapse (EmbryoSlide+ and 16-well culture Dish, Vitrolife, Sweden) and conventional incubators, after incubation in two types of IVF incubators. The measurement was recorded using a Siemens EPOC portable blood gas analyser in order to measure the pH in small volumes of medium with well-defined pre-examination and examination processes.
Material and methods
Measurement with the Siemens EPOC device
All pH measurements in the study were recorded using the EPOC portable blood gas analyzer (Siemens, Munich, Germany) [20, 21]. EPOC was initially designed and marketed for blood gas analysis during emergency rescue. The device dimensions are 60 × 180 × 30 mm. It weighs 500 g and has a touch-screen display, integrated barcode scanner, and optional external printer. A minimum volume of 150 μL is required to measure the pH in IVF culture media. The latter is collected using a 1-mL syringe (BD, New Jersey, US), a thin 25-gauge needle (BD, New Jersey, US) and by aspirating several microdroplets under an oil overlay in IVF dishes using binoculars. The culture media is then inserted into a small cavity of a BGEM (blood gases, electrolytes, and metabolites) test card containing sensors for the analysis. The single-use EPOC BGEM test card contains three types of analytical sensors. Potentiometric ISEs (ion selective electrodes) are used to measure the pH (among other parameters). The quality assurance (QA) of the EPOC system includes automated internal quality control, liquid quality control, and calibration verification procedures. Calibration and analysis take approximately 7 minutes. The results are shown on the display and can be transmitted by wireless (Bluetooth) connection to a printer and computer, as needed. The EPOC test cards can be stored at room temperature (15–30 °C). The gas analyzer was set for measurement at 37 °C. The syringe and needle were placed at 37 °C before sampling, as was the culture medium. The culture medium was sampled on a heating stage. For pH measurement in IVF culture media, in lab 1, 25-μL drops of Global Total HSA (CooperSurgical, Trumbull, US) were placed in 12-well dishes (Vitrolife, Sweden) covered by 4.5 mL of oil FertiCult™ Mineral Oil, FertiPro (Beernem, Belgium). In lab 2, two 180-μL drops of Global Total HSA (Cooper Surgical Trumbull, US) were placed in EmbryoSlide+ (Vitrolife, Sweden) covered by 1.6 mL of oil Ovoil™ (Vitrolife, Sweden). The pH readings obtained with the different pH analyzer were adjusted for temperature at 37 °C.
CO2 monitoring in the two types of incubators
In lab 1, CO2 concentration inside K-system G210+™ incubators was measured by an internal CO2 probe (K-system) and an external CO2 probe (Vaisala™). Both were annually checked according to the standard ISO/IEC 17025:2017. CO2 levels were adjusted according to internal C02 probe values.
In lab 2, CO2 concentration inside Embryoscope™ was measured by an internal CO2 probe (Embryoscope) biannually checked with an external probe according to ISO/IEC 17025:2017.
Pre-examination processes: length of the culture media incubation period prior to pH measurement
In order to assess the incubation period required to reach the target pH value and to evaluate whether pH variation could occur after a prolonged incubation period, the pH of an IVF culture medium (Global Total HSA, Cooper Surgical) was measured after incubation periods of 0, 2, 4, 7, 12, 24, 48, 72, 96, 120, 144, and 168 hours in two dry incubators (K System G210+). One incubator was set at 5.2% and the other at 7.9% CO2 in order to cover the entire pH range (7.2 to 7.4) provided by the manufacturer for this medium. The maximum incubation period (168 hours) was chosen to mimic an initial incubation period of 24 hours to equilibrate the medium before embryo culture and an embryo culture period up to day 6. pH measurements were performed as described above by aspirating 12 × 25 μL culture medium droplets in a 12-well IVF dish (Vitrolife, Sweden).
Validation of the examination procedure
Analytical validation included an assessment of the standard criteria described in ISO 15189 for a quantitative parameter (repeatability = within-run precision, total precision = between-day precision, trueness based on inter-laboratory comparison (ILC), inaccuracy based on external quality assessment (EQA) and comparison to reference technique) [22]. Inter-sample contamination tests were not performed as the EPOC BGEM test cards are for single use only.
- Measurement precision
-
1.1.Measurement repeatability (=“within-run” precision)
-
1.1.
Repeatability was assessed under 6 different conditions (Table 1). The first 4 conditions (conditions 1 to 4, Table 1) used the Global medium in 2 different incubators K System G210+ (CooperSurgical, Trumbull, US) for lab 1; EmbryoScope (Vitrolife, Sweden) for lab 2 at two different CO2 concentrations. The last two conditions (conditions 5 and 6, Table 1) used a buffered medium with a stable pH in order to limit the matrix effect: EPOC Eurotrol BGEM level 1 for condition 5 and Eurotrol BGEM level 3 for condition 6. For conditions 1 to 4, the medium was in 25-μL microdroplets under an oil overlay and incubated for 24 hours. Ten dishes were prepared for each condition.
-
1.2.
Measurement of total precision = between-day precision
Table 1.
Repeatability tests (= “within-run” precision). Repeatability was assessed under 6 different conditions. The last two conditions (conditions 5 and 6) used a buffered medium with stable pH in order to limit the matrix effect. For conditions 1 to 4, the medium was used in microdroplets under an oil overlay and incubated for 24 hours. Ten dishes were prepared for each condition. The same conditions were measured in succession in the same series
| Condition | Matrix | Incubation | Number of measurements | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|
| 1 | Global Total HSA® |
K system G210 + incubator CO2 level 1 (8%) (lab 1) |
10 | 7.200 | 0.015 | 0.21 |
| 2 | Global Total HSA® |
K system G210 + incubator CO2 level 2 (5%) (lab 1) |
10 | 7.373 | 0.012 | 0.17 |
| 3 | Global Total HSA® | EmbryoScope incubator CO2 level 1 (8.5%) (lab 2) | 10 | 7.240 | 0.016 | 0.22 |
| 4 | Global Total HSA® | EmbryoScope incubator CO2 level 2 (5.5%) (lab 2) | 10 | 7.394 | 0.009 | 0.12 |
| 5 | Medium with stable pH level 1 (Eurotrol BGEM level 1) | No incubation (lab 1) | 9 | 7.013 | 0.005 | 0.07 |
| 6 | Medium with stable pH level 1 (Eurotrol BGEM level 3) | No incubation (lab 1) | 10 | 7.724 | 0.008 | 0.11 |
In the two labs, using 2 incubators, namely, G210+ in lab 1 and time lapse incubators EmbryoScope in lab 2 set at two different CO2 levels, the pH was recorded daily in each incubator. An IVF dish used specifically to record the pH was prepared on a daily basis and incubated for 48 h before measurement. The coefficient of variation (CV%) for the “between day” precision was thus calculated for different batches (for which at least 10 values per batch and per condition are available), using IVF culture medium Global Total HSA and for 3 different batches of internal quality control (IQC) provided by Eurotrol (Table 2). For some batches, CO2 was adjusted when the batch was first used (3 batches out of 7)—hence, pH values for reproduction are only taken after adjustment.
-
2.Measurement inaccuracy and trueness
-
2.1.Measurement of inaccuracy: using the external quality assessment (EQA)
-
2.1.
Table 2.
Assessment of total precision (“between-day” precision) under different conditions (an IVF culture medium Global Total HSA and a stable buffered matrix ICQ Eurotrol of different batches, in two different labs with two levels of CO2. The same conditions were measured on different consecutive days
| Matrix | Incubation | Batch | No. of measurements | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|
| Global Total HSA® | Lab 1–K system G210+incubator–pH target 7.22 | 210114 | 13 | 7.22 | 0.02 | 0.32 |
| Global Total HSA® | Lab 1–K system G210+incubator–pH target 7.22 | 210311 | 13 | 7.21 | 0.01 | 0.17 |
| Global Total HSA® | Lab 1–K system G210+incubator–pH target 7.22 | 21345077 | 14 | 7.22 | 0.02 | 0.25 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.22 | 210506 | 10 | 7.22 | 0.01 | 0.18 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.22 | 21345077 | 9 | 7.22 | 0.02 | 0.23 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.22 | 220506006235 | 9 | 7.20 | 0.02 | 0.24 |
| Global Total HSA® | Lab 1–K system G210+incubator–pH target 7.38 | 210114 | 15 | 7.38 | 0.03 | 0.34 |
| Global Total HSA® | Lab 1–K system G210+ incubator–pH target 7.38 | 210311 | 11 | 7.37 | 0.01 | 0.18 |
| Global Total HSA® | Lab 1–K system G210+incubator–pH target 7.38 | 21345077 | 15 | 7.38 | 0.01 | 0.17 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.38 | 211022001863 | 9 | 7.37 | 0.01 | 0.13 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.38 | 220114003796 | 9 | 7.39 | 0.01 | 0.13 |
| Global Total HSA® | Lab 2–EmbryoScope–pH target 7.38 | 220311005070 | 9 | 7.39 | 0.01 | 0.14 |
| ICQ Eurotrol | Lab 1–no incubation | B002 | 16 | 7.452 | 0.009 | 0.125 |
| ICQ Eurotrol | Lab 2–no incubation | B002 | 9 | 7.456 | 0.009 | 0.125 |
| ICQ Eurotrol | Lab 1–no incubation | B015 | 8 | 7.453 | 0.009 | 0.125 |
| ICQ Eurotrol | Lab 2–no incubation | B015 | 7 | 7.457 | 0.009 | 0.121 |
| ICQ Eurotrol | Lab 1–no incubation | B039 | 19 | 7.463 | 0.006 | 0.077 |
| ICQ Eurotrol | Lab 2–no incubation | B039 | 27 | 7.461 | 0.007 | 0.089 |
Four EQAs were performed by the two IVF labs in the same year. The samples were prepared and provided by Biologie Prospective (accredited body NF EN ISO/CEI 17043). Inaccuracy was calculated as follows: inaccuracy (%) = ((x − v)/v) × 100; x is the value measured by the lab and v is the target value, i.e., mean of peer group.
-
2.2.
Measurement of trueness using inter-laboratory comparison (ILC)
Internal quality control (IQC) provided by Eurotrol (level 2: ref 179.002.010) was performed by both labs and outsourced. Bias (%) = ((m − v)/v) × 100; m is the mean of values measured by the lab for the same batch of ILC and v is the target value provided by the quality control body.
-
3.Comparison of examination procedures
-
3.1.Comparison of two EPOC devices mirrored for pH measurement
-
3.1.
The two different portable gas analyzers used in labs 1 and 2 were compared in lab 1 before being used in each lab. We recorded the pH of 15 samples of different media and controls ranging from 6.92 to 7.77 measured at the same time with the two devices (EPOC lab1 and EPOC lab 2) in the same location (in lab 1). The different media used were Eurotrol BGEM level 1, Eurotrol BGEM level 2, Eurotrol BGEM level 3, G-Gamete (Vitrolife), Leibovitz’s L-15 medium (Gibco), Universal medium (Origio), G-MOPS (Vitrolife), Global Total HSA (CooperSurgical), and G-IVF (Vitrolife). The pH readings obtained with the EPOC devices were adjusted for temperature at 37 °C.
-
3.2.
Comparison of EPOC and conventional blood gas apparatus for measuring the pH in IVF culture media
The Siemens EPOC device was compared to the conventional blood gas analyzer (BGA) RapidLab 1260 Siemens in lab 2 on 19 samples. The pH was measured in IVF culture media incubated in different CO2 concentrations in both devices. The IVF culture media used was Global Total HSA incubated using the EmbryoSlide (Vitrolife, Sweden) and EmbryoScope system (Vitrolife, Sweden). The pH readings obtained with the two devices were adjusted for temperature at 37 °C. Methods were compared using linear regression and Bland–Altman plot. Comparison of means was performed using the Wilcoxon test for paired series.
-
3.3.
Comparison of EPOC and conventional pH analyzer (reference method) with potentiometric probe (CPA) for measuring the pH in IVF culture media
The Siemens EPOC device was compared to the conventional pH analyzer Sension+ pH 3™ with potentiometric probe (Hach, Dusseldorf, Germany) on 16 samples. The pH was measured in 5 mL of IVF culture media incubated in different CO2 concentrations during 24 h. Because of the bigger size of conventional potentiometric probe, 5 mL of IVF culture media was placed in 12-mL Falcon tubes. The IVF culture media used was Global Total HSA. Before each use of the conventional pH analyzer, a 3-point calibration was made at pH 4, 7 and 10 using standard calibration solution HACH™ serial number C02983, C02980, and C02889, respectively. The pH readings obtained with the two devices were adjusted for temperature at 37 °C. Methods were compared using linear regression and Bland–Altman plot. Comparison of means was performed using the Wilcoxon test for paired series.
Statement about institutional review board (IRB) approval
This is not applicable. Our study is an analytical study performed on blank culture media samples. No biological samples or patient data were used for this work.
Results
Pre-examination processes: how long should the culture media be incubated before measuring the pH?
Starting from a preincubation pH of 7,35, we attained the pH target values of 7.21 and 7.38 with 7.9% and 5.2% CO2 in 8 to 12 hours, respectively (Fig. 1). A slight increase in pH during the incubation period was observed between 24 h and 168 h: an increase of 0.014 and 0.029 pH units between 24-h and 168-h incubation (6 days) with 5.9 and 7.2% CO2, respectively (Fig. 1).
Fig. 1.
pH value as a function of incubation time of IVF culture media (Global Total HSA, CooperSurgical) in two dry incubators set with CO2 at 5.2% (orange) and 7.9% (blue) in order to cover the entire pH range (7.2 to 7.4) provided by the manufacturer for this medium. pH value of culture medium before incubation was 7.35. Linear correlations curve were calculated from 24 h incubation to the end of incubation (day 7)
Validation of the examination procedure
- Measurement precision
-
1.1.Measurement repeatability = within-run precision.
-
1.1.
The repeatability tests highlighted very low coefficients of variation under all of the conditions: 0.17 to 0.22% for conditions 1 to 4 using the Global medium and 0.07 to 0.11% for conditions using the buffered medium (Table 1). For condition 5, incorrect medium injection prevented us from obtaining 10 values.
-
1.2.
Measurement of total precision = between-day precision.
Total precision assessed under different conditions (2 different matrices of different batches, in two different labs with two levels of CO2) shows coefficients of variation below 0.4% under all conditions (Table 2) (0.13 to 0.34 with IVF culture media).
-
2.Measurement inaccuracy and trueness
-
2.1.Measurement of inaccuracy using the external quality assessment (EQA)
-
2.1.
Inaccuracy calculated using EQA values range from − 0.15 to 0.22% for lab 1 and from − 0.22 to 0.24% for lab 2 (Table 3).
-
2.2.
Measurement of trueness using Inter-Laboratory Comparison (ILC)
Table 3.
Measurement of inaccuracy in the two labs. Inaccuracy was calculated using the external quality assessment according to the following formula: inaccuracy (%) = ((x − v)/v) × 100; x is the value measured by the lab and v is the target value, i.e., mean of peer group
| EQA 1a | EQA 1b | EQA 2a | EQA 2b | EQA 3a | EQA 3b | EQA 4a | EQA 4b | |
|---|---|---|---|---|---|---|---|---|
| Inaccuracy (%) lab 1 | −0.15 | 0.00 | −0.11 | 0.00 | − 0.07 | − 0.05 | 0.22 | 0.20 |
| Inaccuracy (%) lab 2 | 0.13 | −0.07 | −0.22 | −0.15 | − 0.01 | 0.01 | 0.24 | 0.23 |
Trueness (% bias) calculated using ILC values range from − 0.07 to − 0.02% for lab 1 and from − 0.03 to 0.03% for lab 2 (Table 4).
-
3.Comparison of examination procedures
-
3.1.Comparison of two EPOC devices mirrored for pH measurement in IVF culture media
-
3.1.
Table 4.
Measurement of trueness in the two labs. Bias was calculated using inter-laboratory comparison (ILC) according to the following formula: Bias (%) = ((m − v)/v) × 100; m is the mean of values measured by the lab for the same batch of ILC and v is the target value provided by the quality control body
| ILC 1 | ILC 2 | ILC 3 | |
|---|---|---|---|
| Bias (%) lab 1 | −0.07 | −0.02 | −0.03 |
| Bias (%) lab 2 | −0.02 | −0.03 | 0.03 |
Linear regression and the Bland–Altman plot show that EPOC 1 values and EPOC 2 values are very close (Fig. 2). The linear regression equation is y = 0.98x + 0.14 with coefficient R2 = 0.99. On the Bland–Altman plot, the dispersion of the points is homogeneous around the abscissa axis and the mean of the differences between EPOC 1 values and EPOC 2 values is − 0.004 pH units.
-
3.2.
Comparison between EPOC and conventional blood gas apparatus for pH measurements in IVF culture media
Fig. 2.
Comparison of EPOC 1 (lab 1) and EPOC 2 (lab 2) for pH measurement of 15 samples from 6.92 to 7.77 pH units. At the left, linear regression, and at the right, Bland–Altman plot. For Bland–Altman plot, blue line represents the mean of differences between EPOC 1 values and EPOC 2 values
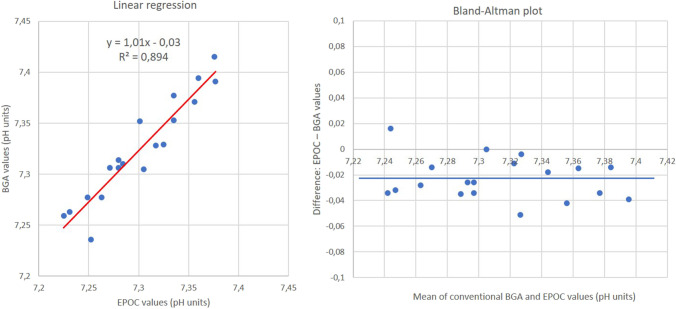
Linear regression and the Bland–Altman plot show good correlation between EPOC and BGA values (Fig. 3). The linear regression equation is y = 1.01x − 0.03 with coefficient R2 = 0.89. On the Bland–Altman plot, the points are dispersed under the abscissa axis indicating an underestimated EPOC measurement compared to the BGA measurements. The mean of the differences between EPOC and BGA values is − 0.023 pH units (p < 0.001).
-
3.3.
Comparison of EPOC and conventional pH analyzer with potentiometric probe (CPA) for measuring the pH in IVF culture media
Fig. 3.
Comparison of EPOC 2 and conventional blood gas analyzer (BGA) for pH measurement of 19 samples from 7.22 to 7.42 pH units. At the left, linear regression, and at the right, Bland–Altman plot. For Bland–Altman plot, blue line represents the mean of differences between EPOC 2 values and BGA values
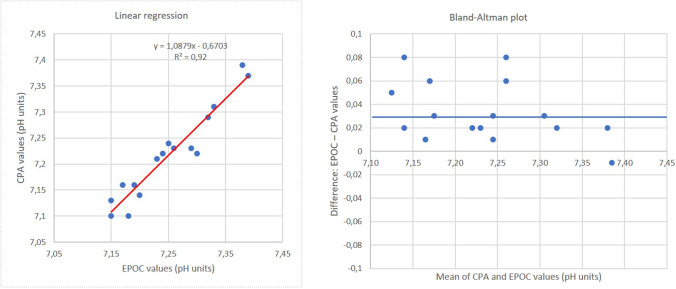
Linear regression and the Bland–Altman plot show good correlation between EPOC and CPA values (Fig. 4). The linear regression equation is y = 1.09x − 0.07 with coefficient R2 = 0.92 (Fig. 4). On the Bland–Altman plot, the points are dispersed over the abscissa axis indicating an overestimated EPOC measurement compared to CPA. The mean of the differences between EPOC and CPA values is + 0.03 pH units (p < 0.001) (Fig. 4).
Fig. 4.
Comparison of EPOC and conventional pH analyzer (CPA) with potentiometric probe for pH measurement of 16 samples from 7.10 to 7.39 pH units. At the left, linear regression, and at the right, Bland–Altman plot. For Bland–Altman plot, blue line represents the mean of differences between EPOC values and CPA values
Discussion
It is crucial to control embryo culture conditions during IVF to ensure pre- and post-implantation embryo development as well as a successful IVF outcome. In order to verify the embryo culture medium pH, different systems can be used but we show here that a portable BGA (Epoc), easy to use, shows excellent performances.
Maintaining a stable culture medium pH at an optimum level poses a challenge for all IVF laboratories worldwide. Indeed, it is very difficult to measure the pH of the culture medium quickly and reliably under conditions of use (in microdroplets under oil overlay in IVF-specific dishes placed in specific incubators with or without a time lapse system). These measurement difficulties can be linked to several causes: small measurement volumes, the rapid variation in embryo culture media pH on exposure to the air, the fact that the analytical performance of pH measuring systems is not always demonstrated and is never highlighted on IVF culture media, and the difficulty sometimes surrounding the use of pH measurement systems in an IVF laboratory (cumbersome blood gas apparatus or device requiring time-consuming calibration before each measurement or transportation of the sample).
Thus, for the first time, we wanted to evaluate the analytical performance of a rapid, compact pH measuring system involving the most widely used embryo culture medium in France (Global Total HSA). The EPOC portable BGA was assessed and proved to be highly accurate using the blood matrix. In this scenario, the EPOC system demonstrated excellent analytical precision and was comparable to other conventional blood gas analyzers including direct ISEs such as Rapidlab RL1265 and Rapidpoint RP500 (indirect ISE such as Dimension Vista and blood count analyzer such as Sysmex XE-2100 [20, 21, 23] in cardiology and oncology units. No performance data for a pH measurement system using IVF culture media have been published to date. In our study, the analytical validation of this pH measuring system was carried out under routine embryo culture conditions of use (in microdroplets, under an oil overlay, in a specific IVF incubator with or without a time lapse system). Analytical validation included validation of the standard criteria described in ISO 15189 for quantitative parameters (repeatability, total precision, trueness based on ILC, inaccuracy based on EQA, and comparison to the reference technique) [22]. In addition, we assessed the medium incubation period required to reach a target value in order to also validate our pre-analytical conditions.
In terms of pre-analytical conditions, despite the medium reaching the pH target value in approximately 8 to 12 hours, a slight increase in pH was noted throughout the incubation period (Fig. 1). Because of slight variations over time, we recommend recording the pH over an incubation period of 24 to 48 h, which is more representative of the pH to which the embryo will be exposed throughout the culture. Tarahomi et al. documented a more significant pH increase of 0.04 to 0.08 over 4 days of sham culture of three commonly used IVF culture media (SC, HTF and G-1) [17]. The slight increase over time was likely due to medium evaporation, which can occur even under oil and results in an increased concentration of bicarbonate ions, and has even been reported in dry incubators [24]. Our results are reassuring since the observed increase is very slight and occurs under very stringent conditions: measurement in microdroplets of 25 μL, dry incubators, and no medium renewal over a period of 7 days.
Regarding pH repeatability in our commercial IVF culture media, we recorded a CV ranging from 0.12 to 0.21%. These values are close to the mean CV (%) values reported in the literature using the EPOC system for blood (CV of 0.07% for Lukonen et al. and Nichols et al.) [21, 23] This CV for repeatability (“within-run” precision) is good and quite as reliable as the one observed using our control with stable pH (Table 1). The minor variability is probably partly related to medium equilibrium and partly to the analytical performance of the EPOC. We have also demonstrated good total precision (=between-day precision) with EPOC under our IVF conditions with a CV (%) ranging from 0.13 to 0.32%. Comparatively, using blood as the matrix, the total precision CV (%) published with this device was close to ours: 0.13 to 0.15% for Lukonen et al. and Nichols et al. [21, 23]. Our repeatability on IVF culture media was assessed on 3 different batches (Table 2) in order to avoid a “batch-effect.” It should be noted that our study is not designed to compare inter-batch variability on the target pH value, unlike others [17]. Indeed, in our study, CO2 levels were sometimes adjusted (on 1 out of 3 occasions) when a new batch was used in order to approach the target value.
Our inter-laboratory comparison confirms the good accuracy of our measurement system. Furthermore, our mirror comparison of both EPOC systems confirms that there is no tendency of over- or under-estimation by one lab compared to the other (Fig. 2).
We show that our technique is very accurate. Percentage bias calculated with ILC and inaccuracy calculated with EQA are both very weak. Inaccuracy calculated using EQA values ranges from − 0.22 to 0.24%, representing less than 0.02 pH units. Moreover, we can show that EPOC pH values are weaker than conventional BGA pH values of 0.02 pH units on average (Fig. 3). The slight difference between EPOC and conventional BGA could be attributed to the longer period between sampling and analysis since the syringe had to be transported for 1 min to reach the BGA outside of the lab. Such minor differences in the blood were even apparent when Luukkonen et al. compared the EPOC device to RP500 and RL1265 BGAs. The r2 was 0.97 for both comparisons with a mean difference of + 0.13 and − 0.05 pH units, respectively, compared to EPOC [21].
We found a slight overestimation of our EPOC analyzer compared to the reference CPA of 0.03 pH units (fixed bias) (Fig. 4). We must keep in mind that the accuracy of the reference CPA announced by the manufacturer is 0.02 pH units. Others authors have reported differences (at higher level) between BGA analyzers and pH electrode (CPA) and have concluded that BGA is not enough accurate for pH measurement in IVF culture media [19]. However, differences between these results [19] and our study should be noted. These authors shown some differences between the 2 techniques (BGA and pH electrode) going up to 0.30 pH unit whereas our maximum difference is 0.08. Moreover, they shown a low correlation between BGA and reference electrode (r = 0.54) whereas we show a good correlation (r = 0.96). These differences could be explained by some evaporation of CO2 during handling. Indeed, these authors used a conventional BGA located in another laboratory (time to arrival: 6 min), whereas we use a portable GDS device that allows instantaneous measurement in the IVF lab. Because of the very sensitivity of our IVF culture media, we raise again two methodological points of primary importance: a sufficient incubation time of culture media before pH measurement and a very short handling time outside the incubator.
Portable BGA devices are invaluable monitoring tools for use in IVF labs because of their portability, ease of use and rapid, and automated calibration process. Indeed, one constraint of some other pH measurement systems is the calibration requirement prior to use because conventional electrodes (CPA) do not give reproducible potentials over prolonged periods of time. However, it is important for EPOC analyzer users to be initially trained in aspirating at least 150 μL of IVF culture media from several microdroplets in IVF dishes. Syringe aspiration of the medium must be carried out as swiftly as possible, under oil overlay, with binoculars and without bubble formation in the syringe. Once the medium has been aspirated, the needle should be removed, any air purged from the syringe, and the medium gradually and safely injected into the EPOC assay card. Human error is obviously one of the main reasons for a lack of results. If the medium is injected incorrectly into the card (insufficient volume and injection speed too slow or too fast), the analysis may be incorrect and will have to be repeated. Among the incubators used for this work, the K-system G210+ series has been recently implemented (G210 InviCell Plus, K-System, CooperSurgical) and now offer an optional pH monitoring system based on fluorescent dyes embedded in disposable sensors. According to the manufacturer, the inaccuracy of this system is 0.05 pH units in a range of pH 7.0 to 7.6, which is good but below the one we evidenced here (0.02) using the EPOC device. Since we have not tested this solution, we cannot compare its characteristics in term of quality performance, ease to use, cost, etc…, with the EPOC solution. However, one advantage of the solution validated here is that it is usable for almost all kinds of incubators in IVF labs worldwide. Our work studies the analytical reliability of a device for measuring pH in embryo culture media in real IVF culture conditions. From an economic point of view, apart from the price of the measuring apparatus, each measurement with an EPOC has an additional cost of about 6 euros per measurement, related to the cost of consumables (single-use card). However, there are still no clinical studies that identify whether it is necessary to have a high analytical reliability in the measurement of the pH for the improvement of IVF outcomes.
We think the main causes of pH variation within a laboratory that does not change its environmental and embryo culture conditions can be a drift of the CO2 probe or variations depending on the media batches. Discrepancies in measurements by CO2 probes have been described [25]. Thus, we recommend a pH control at least each time the medium batch is changed and each time maintenance is carried out on or there is some form of intervention in the incubator. These recommendations should be supported by clinical studies on the impact of small pH variations on human IVF outcomes. The difficulty of giving recommendations is linked to the lack of these clinical studies.
To conclude, despite the difficulties and specific factors surrounding the pH measurement of embryo culture media in an IVF laboratory, we were able to demonstrate good analytical performance levels (repeatability, total precision, trueness, inaccuracy, and comparison to the reference method) for a rapid measuring system that can be adapted to IVF labs, using IVF culture media under embryo culture conditions. We precisely set out analytically reliable conditions for measuring the pH in IVF culture media that are as close/representative as possible to the embryo microenvironment during IVF culture. The following pre-analytical and analytical conditions applied in this study during this analytical validation were: warm syringe, rapid, on-site measurement, dish removed at the last minute, microdroplets under oil overlay and in dishes and incubators of interest and an adequate medium incubation period. It is important to comply with stringent pre-analytical and analytical conditions to obtain reliable outcomes. This work can provide a basis for IVF laboratories wishing to implement a robust quality assurance system to monitor pH levels in embryo culture media.
Acknowledgments
This paper and the research behind it would not have been possible without their exceptional work and effort of the embryology staff of the University Hospital of Toulouse and Bordeaux.
Author contribution
NG designed the study, analyzed the data, performed statistical analysis, and wrote the manuscript. LCD participated in the design of the study, analyzed the data, and critically revised the manuscript. CD, SG, and EG performed pH analysis. MC, JM, and RL analyzed the data and critically revised the manuscript. All authors contributed to the critical discussion and reviewed and approved the final version of the manuscript for submission and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Funding
This study is not a clinical trial but is an ancillary study of a French multicentric Randomized Control Trial ACIDOFIV (trial on impact of pH values of the embryo culture medium on live birth rate after In Vitro Fertilization). This latest was supported by a grant from the French Ministry of Health (PHRCI2019 RC31/19/0503).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25(3):605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 2.Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27(7):1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 3.El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013;99(3):632–641. doi: 10.1016/j.fertnstert.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 4.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62(6):1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 5.Market-Velker BA, Fernandes AD, Mann MR. Side-by-side comparison of five commercial media systems in a mouse model: suboptimal in vitro culture interferes with imprint maintenance. Biol Reprod. 2010;83(6):938–950. doi: 10.1095/biolreprod.110.085480. [DOI] [PubMed] [Google Scholar]
- 6.Gatimel N, Moreau J, Parinaud J, Leandri RD. Need for choosing the ideal pH value for IVF culture media. J Assist Reprod Genet. 2020;37(5):1019–1028. doi: 10.1007/s10815-020-01726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod. 2001;64(6):1845–1854. doi: 10.1095/biolreprod64.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998;50(4):434–442. doi: 10.1002/(SICI)1098-2795(199808)50:4<434::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Barr KJ, Garrill A, Jones DH, Orlowski J, Kidder GM. Contributions of Na+/H+ exchanger isoforms to preimplantation development of the mouse. Mol Reprod Dev. 1998;50(2):146–153. doi: 10.1002/(SICI)1098-2795(199806)50:2<146::AID-MRD4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front Biosci. 2001;6:D708–D730. doi: 10.2741/watson. [DOI] [PubMed] [Google Scholar]
- 11.Phillips KP, Leveille MC, Claman P, Baltz JM. Intracellular pH regulation in human preimplantation embryos. Hum Reprod. 2000;15(4):896–904. doi: 10.1093/humrep/15.4.896. [DOI] [PubMed] [Google Scholar]
- 12.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998;13(12):3441–3448. doi: 10.1093/humrep/13.12.3441. [DOI] [PubMed] [Google Scholar]
- 13.Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005;17(3):371–378. doi: 10.1071/RD04102. [DOI] [PubMed] [Google Scholar]
- 14.Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod Biomed Online. 2010;21(2):219–229. doi: 10.1016/j.rbmo.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Steeves CL, Lane M, Bavister BD, Phillips KP, Baltz JM. Differences in intracellular pH regulation by Na(+)/H(+) antiporter among two-cell mouse embryos derived from females of different strains. Biol Reprod. 2001;65(1):14–22. doi: 10.1095/biolreprod65.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Swain JE. Is there an optimal pH for culture media used in clinical IVF? Hum Reprod Update. 2012;18(3):333–339. doi: 10.1093/humupd/dmr053. [DOI] [PubMed] [Google Scholar]
- 17.Tarahomi M, de Melker AA, van Wely M, Hamer G, Repping S, Mastenbroek S. pH stability of human preimplantation embryo culture media: effects of culture and batches. Reprod Biomed Online. 2018;37(4):409–414. doi: 10.1016/j.rbmo.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Swain JE. Comparison of three pH measuring devices within the IVF laboratory. Fertil Steril. 2013;100:S251. doi: 10.1016/j.fertnstert.2013.07.1186. [DOI] [Google Scholar]
- 19.Diaz de Pool JDN, Van Den Berg SAA, Pilgram GSK, Ballieux B, Van Der Westerlaken LAJ. Validation of the blood gas analyzer for pH measurements in IVF culture medium: Prevent suboptimal culture conditions. PloS One. 2018;13(11):e0206707. doi: 10.1371/journal.pone.0206707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawrocki J, Furian M, Buergin A, Mayer L, Schneider S, Mademilov M, et al. Validation of a portable blood gas analyzer for use in challenging field conditions at high altitude. Front Physiol. 2020;11:600551. doi: 10.3389/fphys.2020.600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luukkonen AA, Lehto TM, Hedberg PS, Vaskivuo TE. Evaluation of a hand-held blood gas analyzer for rapid determination of blood gases, electrolytes and metabolites in intensive care setting. Clin Chem Lab Med. 2016;54(4):585–594. doi: 10.1515/cclm-2015-0592. [DOI] [PubMed] [Google Scholar]
- 22.EN/ISO15189. Medical laboratories — requirements for quality and competence (ISO 15189:2022). The British Standard Institution; 2022. https://www.iso.org/obp/ui/#iso:std:iso:15189:ed-4:v1:en
- 23.Nichols JH, Rajadhyaksha A, Rodriguez M. Evaluation of the enterprise point-of-care (EPOC) system for blood gas and electrolyte analysis. Point of Care. 2008;7:7–11. doi: 10.1097/poc.0b013e3181634d02. [DOI] [Google Scholar]
- 24.Swain JE. Controversies in ART: considerations and risks for uninterrupted embryo culture. Reprod Biomed Online. 2019;39(1):19–26. doi: 10.1016/j.rbmo.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Swain JE. Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality. Reprod Biomed Online. 2010;21(1):6–16. doi: 10.1016/j.rbmo.2010.03.012. [DOI] [PubMed] [Google Scholar]