Abstract
Background:
In guided bone regeneration (GBR), there are various problems that occur in the bone defect after the wound healing period. This study aimed to investigate the enhancement of the osteogenic ability of the dual scaffold complex and identify the appropriate concentration of growth factors (GF) for new bone formation based on the novel GBR concept that is applying rapid bone forming GFs to the membrane outside of the bone defect.
Methods:
Four bone defects with a diameter of 8 mm were formed in the calvaria of New Zealand white rabbits each to perform GBR. Collagen membrane and biphasic calcium phosphate (BCP) were applied to the bone defects with the four different concetration of BMP-2 or FGF-2. After 2, 4, and 8 weeks of healing, histological, histomorphometric, and immunohistochemical analyses were conducted.
Results:
In the histological analysis, continuous forms of new bones were observed in the upper part of bone defect in the experimental groups, whereas no continuous forms were observed in the control group. In the histomorphometry, The group to which BMP-2 0.5 mg/ml and FGF-2 1.0 mg/ml was applied showed statistically significantly higher new bone formation. Also, the new bone formation according to the healing period was statistically significantly higher at 8 weeks than at 2, 4 weeks.
Conclusion:
The novel GBR method in which BMP-2, newly proposed in this study, is applied to the membrane is effective for bone regeneration. In addition, the dual scaffold complex is quantitatively and qualitatively advantageous for bone regeneration and bone maintenance over time.
Keywords: Growth factors, Wound healing, Bone regeneration
Introduction
Implants have been spotlighted as one of the primary treatment options for edentulous patients whose condition is related to periodontitis, trauma, tumors, or congenital deformity. Guided bone regeneration (GBR) is the most common treatment method for reconstructing the alveolar bone or regenerating bone defects around implants [1–4]. The principle of GBR is to protect the slow-growing internal bone regeneration tissue from the fast-growing epithelium and connective tissue using a barrier membrane, and bone grafts can also be used inside the defect to aid bone regeneration [1, 2, 5, 6].
Several studies have been conducted to induce fast and effective bone regeneration by applying growth factors (GF) with collagen membranes in GBR [7–12]. BMP-2 and FGF-2 are the most widely used in clinical practice among the various GFs [13–15].
Studies have been conducted to enhance osteogenic effects by combining the use of these two GFs in GBR [16–20] in addition to research on bone regeneration using the interaction between BMP-2 and FGF-2 [20–22].
Through our previous study, we have found that applying BMP-2 with bone graft material resulted in poor spatial stability due to the increase of adipose tissue in the later phase of healing, and applying FGF-2 to the collagen membrane showed no difference in new bone formation compared to the control group [23]. Based on these results, a design of applying BMP-2, a significant element to improve bone regeneration, to the membrane, while applying bone graft material and FGF-2 inside the defect was considered to enhance the bone regeneration effect of GBR.
Nosho et al. reported that BMP-2 could be suitable for application in extramedullary bone regeneration, whereas FGF-2 could be suitable for medullary bone regeneration [24]. A study on healing calvarial defects in old mice showed that combining low doses of BMP-2 and FGF-2 increased the total volume and histological bone formation compared to using BMP-2 alone [19]. However, most studies used the same scaffold to apply FGF-2 and BMP-2 [16, 19, 24–27].
As a BMP-2 carrier, the collagen membrane continues to release BMP-2 during the healing period, increases ALP and osteocalcin, which evaluates bone regeneration activity [28], and also exhibits a rapid release effect in the early stage of healing [29], making it a good combination for early healing containment. As a bone graft material, Biphasic Calcium Phosphate (BCP) has macro-sized pores and excellent interconnectivity with collagen membranes for bone formation [30, 31], and applying FGF-2 to BCP led to excellent space maintenance during the healing period [32] and increased ALP activity [33].
The purpose of this study is to validate the novel concept of GBR for enhancing and maintaining new bone formation in the defect area through the external stimuli using the rapidly formed bony wall by collagen membrane containing BMP-2. In addition, the effective concentration of BMP-2 was investigated when combined with FGF-2.
Materials and methods
Animals and materials
Experimental animals
24 New Zealand white rabbits weighing 3–3.5 kg aged 16–20 weeks were prepared. The breeding, management, and surgical procedures followed the animal testing standards of the Animal Care and Use Committee, Yonsei Medical Center, Seoul, Korea (Approval no. 2016-0062).
Materials
BMP-2
rhBMP-2 (E.coli-derived, GENOSS, Suwon, Korea) was used for BMP-2. 0.5 mg/ml and 1.0 mg/ml concentration BMP-2 solutions were prepared through dilution with saline. The same amount of BMP-2 dilutions (50 μl) was applied to each group, and each contained 25 μg and 50 μg of rh-BMP2, respectively. Only 50 μl physiological saline was used for the control group.
FGF-2
rhFGF-2 (E.coli-derived, GENOSS, Suwon, Korea) was used for FGF-2. 0.5 mg/ml and 1.0 mg/ml concentration FGF-2 solutions were prepared through dilution with saline. BCP was soaked in 50 μl FGF-2 dilutions of each concentration, and each contained 25 μg and 50 μg of rh-FGF2, respectively. Only 50 μl of physiological saline was used for the control group.
Biphasic calcium phosphate
Osteon II (Genoss, Suwon, Korea) was used for BCP. The ratio of Hydroxyapatite(HA) and β-Tricalcium phosphate(β-TCP) was 30:70, and had a porous structure. Pore size was 250 μm, porosity 70%, and each particle size was 0.5–1.0 mm. The appropriate dose for 8 mm diameter bone defects in rabbit calvaria was 70 mg, and the same dose was used for each bone defect.
Collagen membrane
In terms of the collagen membrane, highly pure type I collagen (Genoss, Suwon, Korea) derived from the bovine tendon was used. It was 300 μm thick and lasted for more than 6 months in the human body.
Control/experimental groups
BCP (Osteon II) and collagen membrane were used on the control group without applying BMP-2 and FGF-2.
Four groups were set by combining BCP with two concentrations of FGF (0.5 mg/ml, 1.0 mg/ml) and collagen membrane with two concentrations of BMP-2 (0.5 mg/ml, 1.0 mg/ml) (Table 1).
Table 1.
Control/experimental group
| Group | BCP + BMP-2 + Membrane + FGF-2 |
|---|---|
| Control group | BCP + Membrane |
| Experimental Group 1 (G1) | Collagen membrane + BMP-2(1.0 mg/ml) + BCP + FGF-2(1.0 mg/ml) |
| Experimental Group 2 (G2) | Collagen membrane + BMP-2(1.0 mg/ml) + BCP + FGF-2(0.5 mg/ml) |
| Experimental Group 3 (G3) | Collagen membrane + BMP-2(0.5 mg/ml) + BCP + FGF-2(1.0 mg/ml) |
| Experimental Group 4 (G4) | Collagen membrane + BMP-2(0.5 mg/ml) + BCP + FGF-2(0.5 mg/ml) |
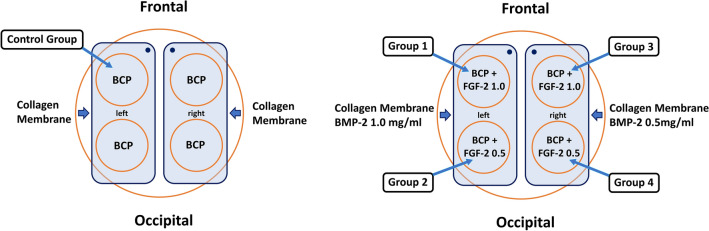
Four bone defects were created in the calvaria (temporal bone) of each of the 24 rabbits. Four concentration groups were arranged in each of the four bone defects of each individual to reduce individual specificity. However, due to the difficulties in fixing 4 collagen membranes of each concentration to prevent interference with each other during the surgical procedure, only two BMP-2 concentration groups were set for each individual in each of the 2 bone defects on the left and right (Fig. 1). The healing period was set at 2 weeks, 4 weeks, and 8 weeks. There were 8 animals in each period.
Fig. 1.
Schematic diagram of surgery of control/experimental groups
Surgical procedures
The New Zealand white rabbits were anesthetized by subcutaneous injection of 1.5 mg/kg Zolazopam (Zoletil, Virbac Korea Co., Seoul, Korea) and intramuscular injection of 5 mg/kg Xylazine HCl (Rompun, Bayer Korea Co., Seoul, Korea). After 10 min of general anesthetic injection, the skull skin was sterilized with Povidone-iodine, and local anesthesia was performed with 2% Lidocaine (lidocaine HCl, Huons, Seongnam, Korea) containing 1:80,000 epinephrine.
Circular bone defects with a depth of 1∼2 mm were formed up to the inferior cortical bone without damaging the underlying dura mater using a Trephine bur (Mr. Curette Tech, Seongnam, Korea) with an outer diameter of 8 mm. The distance between each defect was at least 3 mm.
After implanting the prepared BCP in each bone defect, the collagen membrane was covered and fixed using micropins. The periosteum was first sutured with an absorbable suture (4–0 Vicryl, Ethicon, Somerville, NJ, USA), and then the skin was closed.
Sacrifice
The rabbits were sacrificed after 2, 4, and 8 weeks of healing. After inducing deep anesthesia, the skin was incised to separate the periosteum. Maintaining a sufficient distance from the healed bone defect, a specimen was taken by removing the marginal bone using a handpiece. The front side of the specimens was marked to identify the direction.
Histological and histomorphometric analysis
The specimens were imaged at 12.5 times and 40 times magnification using an optical microscope (Leica DM 2500, Leica Microsystems, Wetzlar, Germany), and histological measurements were performed using H-E stain tissue slides. New bone area and remaining bone graft material area were measured using Image-pro plus (Media Cybernetics, Silver Spring, Maryland, USA). The areas of new bone and remaining bone graft material were calculated as percentages with respect to the total area.
Russell-Movat Pentachrome stain(American MasterTech) tissue slides, a tissue-specific staining method according to tissue development, were used to observe bone development and analyze the differentiation of new bone. (Bone: dark yellow, New osteoid: red, Cartilage: green).
Immunohistochemical analysis
Anti-Osteocalcin antibody (OCG3) immunohistochemical staining was conducted to examine the degree of vascularity and density in osteoblasts. The tissue sections were used by diluting the Anti-Osteocalcin antibody (abcam; ab 13,420; 1/150) and Anti-Mouse HRP (abcam; ab205719; 1/150).
Statistical analysis
Of the 58 specimens, 56 were used for analysis after excluding two outliers. Normality was confirmed using the Shapiro–Wilk test.
The data were presented by mean and standard deviation.
Two-way ANOVA was conducted to check whether there was a difference in bone formation rate according to the BMP-2 and FGF-2 concentration combination and healing period, and a post-hoc test was performed to confirm the difference between groups.
SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used to make the statistical calculations, and the significance level was set at 5%.
Results
Histological findings (H-E stain)
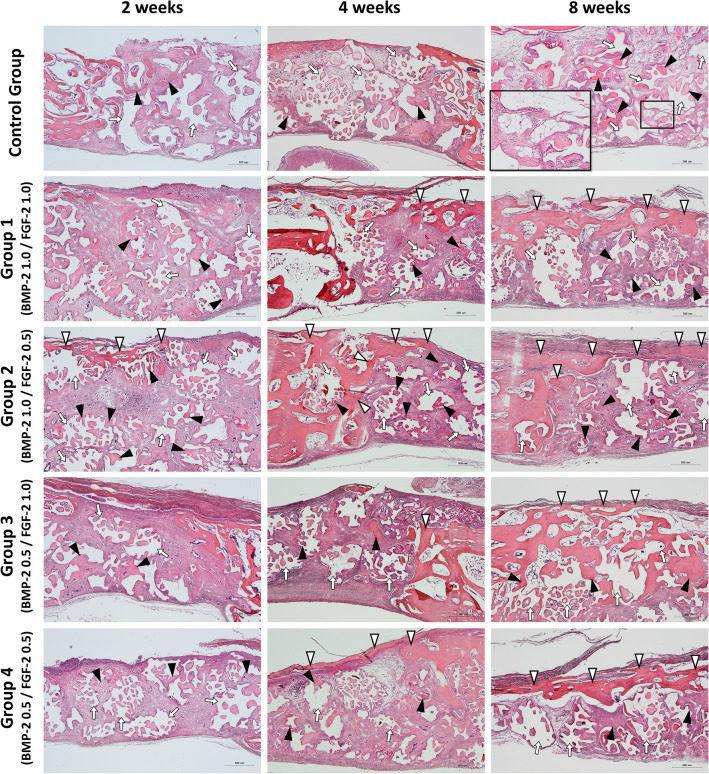
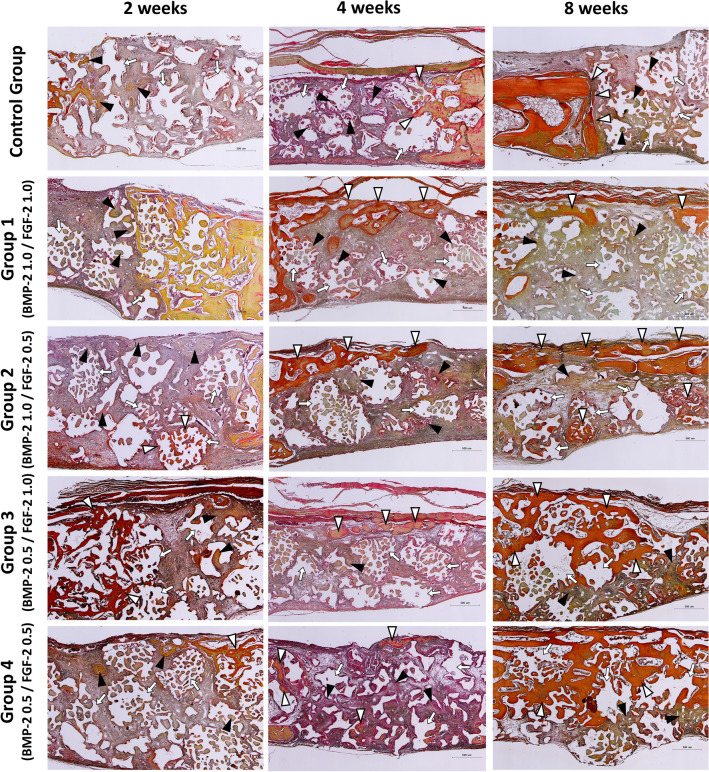
Figure 2 showed images of H-E stained tissue specimens of the control and experimental groups according to the healing process. At 2 weeks after implantation, the formation of new bone (black arrowheads) around the existing bone and bone graft material (white arrows) begins in both the control and experimental groups. Most of the new bone was in the form of woven bone, but in experimental groups 2 and 3, the thin new bone at the top side (white arrowheads) derived from the existing bone.
Fig. 2.
Histological specimens for each group after 2–4–8 weeks healing period. Black arrowhead, new bone; White arrowhead, new bone extending from the existing bone; White arrow, remaining bone graft materials. Scale bar: 500 μm
At 4 weeks after implantation, in the control group, new bone (black arrowheads) was observed around the existing bone and bone graft material (white arrows), similar to the two-week control group. In the experimental groups, new bone extending from the existing bone (white arrowheads) was observed at the top, and the thickness was increased compared to the two- and four-week groups.
At 8 weeks after implantation, all experimental groups had new bone (with arrowheads) covered the defect region at the top continuously, which was not observed in the control group. In the control group, new bone (black arrowheads) was observed around the remaining bone graft material (white arrows) and near the existing bone. Especially, adipose tissue (magnified black square) was observed inside when the new bone covered or wrapped around the bone graft material. The experimental group showed a significant decrease in remaining bone graft material without adipose tissue formation (Fig. 2).
Histomorphometric analysis
New bone area and residual bone graft material area were measured. The areas of new bone and remaining bone graft material with respect to the total area were calculated as percentages.
New bone formation
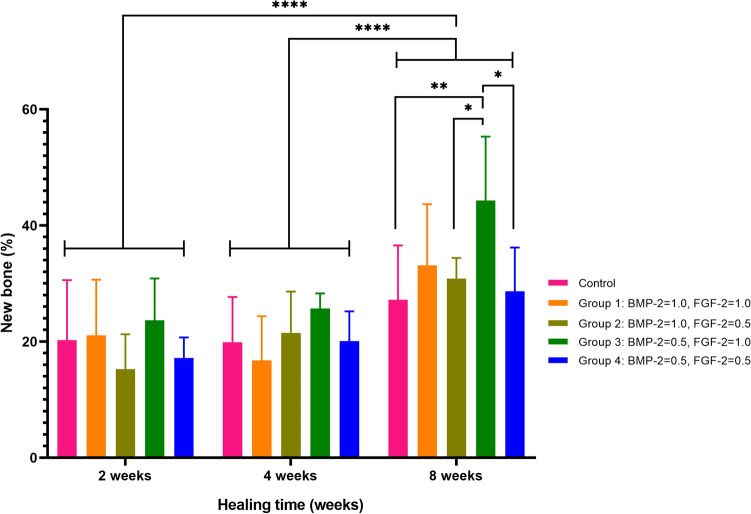
In the two-way ANOVA to determine whether there was a difference in bone formation rates according to BMP-2 and FGF-2 concentration combinations and the healing period, the P-values were 0.04 and < 0.001, respectively, which were less than 0.05, so there were differences in bone formation rates according to BMP-2 and FGF concentration combinations and the healing period, respectively, with a significance level of 5% (Table 2, Fig. 3).
Table 2.
New bone formation analysis
| Variables | N | New bone (%) | P-value | Post-hoc analysis with LSD method |
|---|---|---|---|---|
| Group | 0.040 | Control = G1 = G2 = G4 < G3 | ||
| Control | 12 | 22.42 ± 9.06 | ||
| BMP-2 = 1, FGF-2 = 1 (G1) | 11 | 23.87 ± 11.23 | ||
| BMP-2 = 1, FGF-2 = 0.5 (G2) | 12 | 22.51 ± 8.48 | ||
| BMP-2 = 0.5, FGF-2 = 1 (G3) | 10 | 30.65 ± 11.39 | ||
| BMP-2 = 0.5, FGF-2 = 0.5 (G4) | 11 | 22.14 ± 7.35 | ||
| Healing time | < 0.001 | 2 weeks = 4 weeks < 8 weeks | ||
| 2 weeks | 18 | 19.15 ± 7.31 | ||
| 4 weeks | 19 | 20.80 ± 6.46 | ||
| 8 weeks | 19 | 32.20 ± 9.56 |
Values were presented by mean ± SD and tested by Two-way ANOVA
Fig. 3.
New bone formation between the groups during 2–4–8 weeks healing times
In terms of BMP-2 and FGF-2 concentration combinations, the average bone formation rate was 22.14%, 22.42%, 22.51%, 23.87%, and 30.65%, for Group 4, Control, Group 2, Group 1, and Group 3, respectively. In other words, the BMP-2 0.5 mg/ml and FGF-2 0.5 mg/ml group had the lowest bone formation rate, and the BMP-2 0.5 mg/ml and FGF-2 1.0 mg/ml groups had the highest. In the post-hoc analysis using the LSD method, the relationship between each group was Control = Group 1 = Group 2 = Group 4 < Group 3.
In terms of the healing period, the average bone formation rate was 19.15%, 20.8%, and 32.20%, in the order of 2, 4, and 8 weeks. That is, the longer the healing period, the higher the rate of bone formation. In the post-hoc analysis using the LSD method, the relationship between each healing period was 2 weeks = 4 weeks < 8 weeks.
Histological components
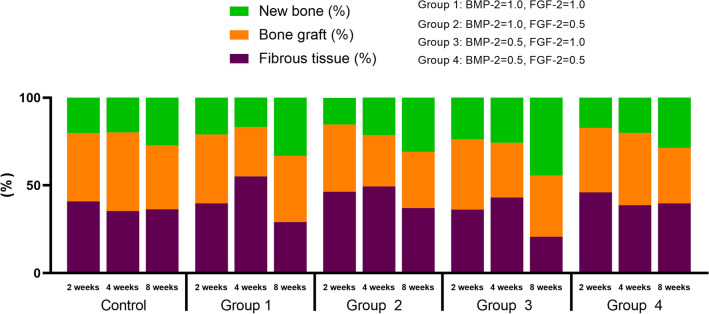
Excluding the new bone and bone graft, the rest of the defect was fibrous tissues. Figure 4 shows a graph of the manner in which the three compositions change in each group according to the healing period. In all groups, the amount of new bone increased and the amount of bone graft decreased as the healing period elapsed (Fig. 4).
Fig. 4.
Cumulative graph of histologic components at groups
Histological findings (Russell-Movat Pentachrome stain)
Figure 5 showed images of pentachrome stained tissue specimens of the control and experimental groups for visualizing the maturity of new bone. Mature bone is stained in yellow to red but immature bone is stained in green. At 2 weeks after implantation, in the control group, new bone (black arrowheads) was observed near the existing bone, and collagen fibers were observed around the bone graft material (white arrows) on pentachrome stained specimens. In the experimental group, mature new bone (white arrowheads) was observed around the existing bone, and new bone (black arrowheads) was observed at the top and inside the bone graft material. These new bones were not yet mature, and many collagen fibers were observed in the upper part and around the bone graft material (white arrows).
Fig. 5.
Histological specimens for each group after 2–4–8 weeks healing period (Pentachrome Stain.). Black arrowhead, immature new bone; White arrowhead, mature new bone; White arrow, remaining bone graft materials. Scale bar: 500 μm
At 4 weeks after implantation, in the control group, mature new bone (white arrowheads) was observed around the existing bone, and immature new bone (black arrowheads) was observed only inside the bone graft (white arrows) in the defect. In the experimental group, mature new bone (white arrowheads) was observed around the existing bone, similar to the control group, but these bones were observed alone at the topor derived from the existing bone. These were not fully connected. New immature bone (black arrowheads) was also observed inside the bone graft material, as in the control group.
At 8 weeks after implantation, in the control group, mature new bone (white arrowheads) was observed around the existing bone, but inside the defect, new bone (black arrowheads) and collagen fibers were observed only around the bone graft material (white arrows). The experimental group had continuous mature new bone (white arrowheads) that completely sealed the upper part of the defect, while the existing bone, it became thinner toward the center of the defect. No immature new bone was observed in the lower part of the defect in the experimental groups.
Between the groups, no mature bone was observed on the control group’s upper part of the defect, but the mature bone was observed on the upper part in all experimental groups. In comparison between the experimental groups, thick mature upper bone was observed in Groups 3 and 4 where 0.5 mg/ml of BMP-2 was applied. In Groups 1 and 2 where BMP-2 was applied at 1.0 mg/ml, thin mature upper bone tended to be observed. Also, in the experimental groups, the thickness of the upper mature bone increased as the healing period elapsed.
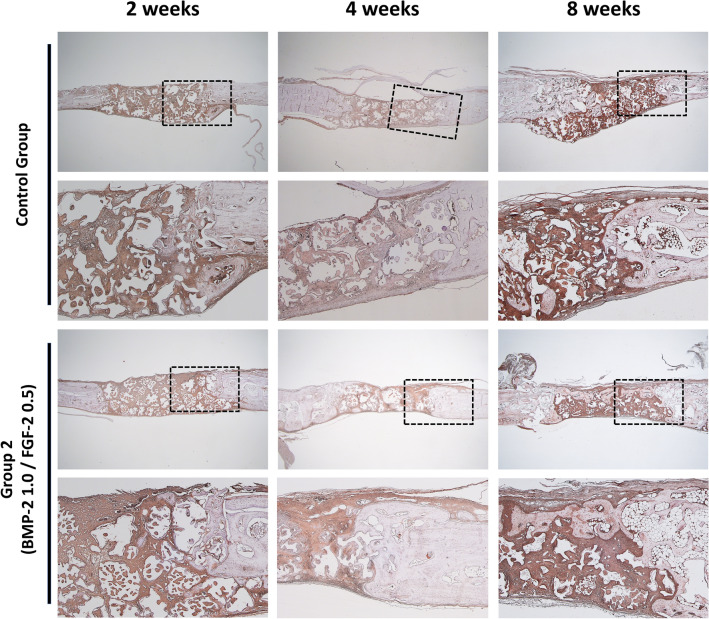
Immunohistochemical findings (Anti-Osteocalcin antibody stain)
At 2 weeks after implantation, osteocalcin was observed around the bone graft material in both the control and experimental group 2. However, the expression of osteocalcin was higher in the bone graft material than in the existing bone.
The 4-week control group showed osteocalcin expression sites similar to those of the two-week group. The 4-week experimental group had different osteocalcin expression sites from the 2-week group. These were mainly observed toward the collagen membrane, and when the upper part was sealed, as shown in Group 2 in Fig. 6, they were near the bone graft material at the center of the defect rather than directly below the new bone.
Fig. 6.
Histological specimens for control group and group 2 after 2–4–8 weeks healing period (Anti-Osteocalcin antibody stain). Scale bar: low magnification, 1000 μm, high magnification, 500 μm
At 8 weeks after implantation, in the control group, similar to the 2 and 4-week groups, osteocalcin was observed near the bone graft material around the existing bone, but there was nothing significant in the defect. In the 8-week experimental group, osteocalcin expression was observed, and when the new bone under the collagen membrane filled the defect, osteocalcin was observed near the internal bone graft material rather than matured covered bone at the top.
Discussion
The aim of this study was to investigate whether GBR using a collagen membrane applying BMP-2 and BCP applying FGF-2 was effective for bone regeneration in critical size defects (CSD) in rabbit calvaria.
BMP-2 is a potent osteogenic agent that accelerates osteoblast differentiation to secrete a new bone matrix, including early collagen and mineral precursors [34], thereby inducing rapid bone regeneration [35]. To confirm the quick bone regeneration ability of BMP-2, Chung et al. used collagen membranes as a BMP-2 carrier and reported that alkaline phosphatase (ALP) activity increased after 2 weeks and OCN increased immediately after surgery (4 days) [28]. The early formed new bone in the upper part can play a role in protecting internal bone regeneration. Several previous studies also reported that rapid upper bone formation can protect internal bone regeneration during the healing period by preventing the penetration of external connective tissue [12, 35, 36]. In the previous study of this laboratory, in the CSD study with the upper BMP-2 and collagen membrane applied, the new bone increased rapidly up to 4 weeks but decreased in the 8-week experimental group. In the experimental group, continuous new bone was observed in the collagen membrane, and the 8-week group showed complete defect occlusion, confirming the role of protecting internal bone regeneration (Fig. 2).
FGF-2 has a high affinity for heparin and prevents heparin degeneration and proteolysis by binding to heparin-like molecules in the basement membrane and extracellular matrix [37]. With these mechanisms, the main action of FGF-2 in wound healing is angiogenesis through revascularization [14]. In addition to angiogenesis, FGF-2 plays a significant role in bone regeneration by inducing marrow-derived mesenchymal cells and promoting osteoblast differentiation [14]. This action or effect can be confirmed by ALP activity and OCN increase [38]. Through an in vitro study, Frank et al. reported that FGF-2 was related to the expression of BMP-2 [39], while Naganawa et al. showed that null mice without the FGF-2 gene do not express BMP-2 [40]. Among the functions of FGF-2, its interaction with BMP-2 is not yet completely determined, but previous studies have confirmed that FGF-2 can affect the bone regeneration function of BMP-2.
However, combined applications of growth factors may result in diverse complications during bone regeneration. Studies have been conducted on approaches to reducing the side effects of BMP-2, including research on using other growth factors, such as using different carrier systems [41], using vascular endothelial growth factor (VEGF) [42], using bFGF conjugates [43] and using Insulin-like growth factor1(IGF1)[44]. It was also reported that using multiple GFs with low concentrations can reduce the risk of side effects compared to using a single GF with a high concentration [19, 44]. Lee et al. reported that the use of BMP-2 inside the defect resulted in excessive adipose tissue after healing, which affected the stability of the graft site or new bone quality [45]. Using low concentrations of BMP-2 and IGF1, new bone formation inside the bone defect and effective bone maintenance was observed over time [44]. When applying BMP-2 and FGF-2, it is necessary to set each concentration to reduce side effects.
This study used two different BMP-2 and FGF-2 concentrations, in which 0.5 mg and 1.0 mg of each were diluted in 1.0 ml of physiological saline. Based on a concentration of 1.0 mg/ml, 0.5–1.0 µg/mm3 of BMP-2 and FGF-2 were used [23]. The amount and size of BCP and collagen membrane that can fill the critical bone defect were determined in a pilot study and two previous experiments conducted in our laboratory. And the amount of saline that can completely wet the BCP and collagen membrane was determined to be 50ul, and 25ug and 50ug of growth factors were applied to prepare scaffolds(23). The experiments involved creating multiple defects in the rabbit calvaria, so the paracrine effect with adjacent defects should have been considered. but the effect on the sites to which a low concentration of growth factors was applied is limited [46, 47], so it was not considered in the results.
In Figs. 2 and 5, compared to the control group, in the experimental group using BMP-2, after a healing period of 4 weeks or more, it was observed that a bridge-shaped bone plate was formed on the upper part of the bone defect. And according to the results of Pentachrome staining, it is considered to be a mature type of bone. And between these experimental groups, Group 3 (BMP-2 0.5 mg/ml, FGF-2 1.0 mg/ml) showed a statistically significant difference in new bone formation compared to other groups (p < 0.05) (Table 2, Fig. 3). These results are consistent with the findings of a previous study (48) on rabbit calvarial defects, which showed that BMP-2 0.5 mg/ml resulted in more bone formation than 1.0 mg/ml. Lee et al. reported no statistically significant difference in new bone formation between FGF-2 0.5 mg/ml and 1.0 mg/ml, but the 1.0 mg/ml group showed 6–7% more bone growth on average than the control group (23).
In terms of comparing new bone formation by healing period, the 8-week a statistically significant difference (p < 0.01) compared to the 2-week and 4-week groups (Table 2, Fig. 4). Considering the histological analysis results in which the 8-week group showed more new bone formation, and the histological analysis results of observing continuous mature bone in the collagen membrane, the novel GBR method adding GFs brings space maintenance and continuous bone regeneration compared to the control group, even after the healing period.
Therefore, the purpose of this study was to designed to isolate bony defect by inducing the new bone formation of upper bone in early osteogenesis using BMP-2 + collagen membrane and FGF-2 + BCP on rabbit CSDs, thereby securing space for bone regeneration and improving bone regeneration ability. During the 8-week healing period, the histological findings showed that forming mature new bone in the collagen membrane increased the total new bone. Also, the concentration combination of BMP-2 (0.5 mg/ml) and FGF-2 (1.0 mg/ml) was statistically significantly higher in new bone formation (p < 0.05). However, some limitations could not be excluded due to the lack of experimental subjects and the specificity of inter-individual healing caused by the limits of animal testing. It will be necessary to find effective internal scaffolds for bone regeneration in defects by deriving experimental designs that show the total volume increase or decrease as an absolute value during the healing period. Also, while we confirmed the possibility of using a low dose of BMP-2, further research should be conducted to reduce the overall concentration. Additional studies on the combined use of GFs other than FGF-2, which is known to interact with BMP-2, for selecting GFs inside defects will lead to the discovery of effective methods for bone regeneration.
In this study, it was confirmed that the new dual scaffold matrix method using a collagen membrane applied with BMP-2 and BCP applied with FGF-2 was effective for bone regeneration in GBR. The concentration combination of BMP-2 0.5 mg/ml and FGF-2 1.0 mg/ml showed a higher osteogenic ability compared to the experimental groups with other concentrations. In addition, the dual scaffold complex is quantitatively and qualitatively advantageous for bone regeneration and bone maintenance over time.
Acknowledgements
We would like to acknowledge the help from Dr. Hyunmin Choi for the help with animal experiments. This research was funded by Yonsei University School of Dentistry Intramural Faculty Research Grant (Y110523) to Y.P.
Declarations
Conflict of interest
The authors have no financial conflict of interest.
Ethical Statement
The breeding, management, and surgical procedures followed the animal testing standards of the Yonsei University Health System Institutional Animal Care and Use Committee, Seoul, Korea. The animal study plan for these experiments (2016–0062) was reviewed and approved by this committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jaehan Park and Narae Jung have contributed equally to this work as co-first authors.
References
- 1.Elgali I, Omar O, Dahlin C, Thomsen P. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017;125:315–37. doi: 10.1111/eos.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Retzepi M, Donos N. Guided bone regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010;21:567–76. doi: 10.1111/j.1600-0501.2010.01922.x. [DOI] [PubMed] [Google Scholar]
- 3.Tolstunov L, Hamrick JFE, Broumand V, Shilo D, Rachmiel A. Bone augmentation techniques for horizontal and vertical alveolar ridge deficiency in oral implantology. Oral Maxillofac Surg Clin North Am. 2019;31:163–91. doi: 10.1016/j.coms.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Khojasteh A, Kheiri L, Motamedian SR, Khoshkam V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann Maxillofac Surg. 2017;7:263–77. doi: 10.4103/ams.ams_76_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buser D, Dula K, Hess D, Hirt HP, Belser UC. Localized ridge augmentation with autografts and barrier membranes. Periodontol 2000. 1999;19:151–163. doi: 10.1111/j.1600-0757.1999.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–6. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Alrasheed A, Al-Ahmari F, Ramalingam S, Nooh N, Wang CY, Al-Hezaimi K. Real-time assessment of guided bone regeneration in standardized calvarial defects using a combination of bone graft and platelet-derived growth factor with and without collagen membrane: an in vivo microcomputed tomographic and histologic experiment in rats. Int J Periodontics Restorative Dent. 2016;36:s173–s186. doi: 10.11607/prd.2645. [DOI] [PubMed] [Google Scholar]
- 8.Kaigler D, Silva EA, Mooney DJ. Guided bone regeneration using injectable vascular endothelial growth factor delivery gel. J Periodontol. 2013;84:230–8. doi: 10.1902/jop.2012.110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalska M, Kozakiewicz M, Bodek KH. Polymer angiogenic factor carrier. Part I. Chitosan-alginate membrane as carrier PDGF-AB and TGF-beta. Polim Med. 2008;38:19–28. [PubMed] [Google Scholar]
- 10.Caridade SG, Monge C, Almodóvar J, Guillot R, Lavaud J, Josserand V, et al. Myoconductive and osteoinductive free-standing polysaccharide membranes. Acta Biomater. 2015;15:139–49. doi: 10.1016/j.actbio.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benic GI, Joo MJ, Yoon SR, Cha JK, Jung UW. Primary ridge augmentation with collagenated xenogenic block bone substitute in combination with collagen membrane and rhBMP-2: a pilot histological investigation. Clin Oral Implants Res. 2017;28:1543–52. doi: 10.1111/clr.13024. [DOI] [PubMed] [Google Scholar]
- 12.Chang YY, Lee JS, Kim MS, Choi SH, Chai JK, Jung UW. Comparison of collagen membrane and bone substitute as a carrier for rhBMP-2 in lateral onlay graft. Clin Oral Implants Res. 2015;26:e13–9. doi: 10.1111/clr.12320. [DOI] [PubMed] [Google Scholar]
- 13.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 14.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000. 2011;56:188–208. [DOI] [PubMed]
- 15.Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. La Chirurgia degli organi di movimento. 2008;92:161–168. doi: 10.1007/s12306-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 16.van der Stok J, Wang H, Amin Yavari S, Siebelt M, Sandker M, Waarsing JH, et al. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue Eng Part A. 2013;19:2605–14. doi: 10.1089/ten.TEA.2013.0181. [DOI] [PubMed] [Google Scholar]
- 17.Su J, Xu H, Sun J, Gong X, Zhao H. Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. Int J Mol Sci. 2013;14:12714–28. doi: 10.3390/ijms140612714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oortgiesen DA, Walboomers XF, Bronckers AL, Meijer GJ, Jansen JA. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med. 2014;8:202–9. doi: 10.1002/term.1514. [DOI] [PubMed] [Google Scholar]
- 19.Charles LF, Woodman JL, Ueno D, Gronowicz G, Hurley MM, Kuhn LT. Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp Gerontol. 2015;64:62–9. doi: 10.1016/j.exger.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorsand B, Nicholson N, Do AV, Femino JE, Martin JA, Petersen E, et al. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J Control Release. 2017;248:53–9. doi: 10.1016/j.jconrel.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marie PJ, Miraoui H, Sévère N. FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors. 2012;30:117–23. doi: 10.3109/08977194.2012.656761. [DOI] [PubMed] [Google Scholar]
- 22.Rice R, Rice DP, Thesleff I. Foxc1 integrates Fgf and Bmp signalling independently of twist or noggin during calvarial bone development. Dev Dyn. 2005;233:847–52. doi: 10.1002/dvdy.20430. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Park YB, Moon HS, Shim JS, Jung HS, Kim HJ, et al. The role of rhFGF-2 soaked polymer membrane for enhancement of guided bone regeneration. J Biomater Sci Polym Ed. 2018;29:825–43. doi: 10.1080/09205063.2017.1354676. [DOI] [PubMed] [Google Scholar]
- 24.Nosho S, Tosa I, Ono M, Hara ES, Ishibashi K, Mikai A, et al. Distinct osteogenic potentials of BMP-2 and FGF-2 in extramedullary and medullary microenvironments. Int J Mol Sci. 2020;21:87. doi: 10.3390/ijms21217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang W, Liang Q, Du L, Shang L, Wang T, Ge S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J Periodontal Res. 2019;54:424–34. doi: 10.1111/jre.12644. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Zou Q, Boerman OC, Nijhuis AW, Jansen JA, Li Y, et al. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J Control Release. 2013;166:172–81. doi: 10.1016/j.jconrel.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn LT, Peng T, Gronowicz G, Hurley MM. Endogenous FGF-2 levels impact FGF-2/BMP-2 growth factor delivery dosing in aged murine calvarial bone defects. J Biomed Mater Res A. 2021;109:2545–55. doi: 10.1002/jbm.a.37249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung EJ, Chien KB, Aguado BA, Shah RN. Osteogenic potential of BMP-2-releasing self-assembled membranes. Tissue Eng Part A. 2013;19:2664–73. doi: 10.1089/ten.tea.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang K, Lu X, Li M, Liu H, Xie C, et al. BMP-2 encapsulated polysaccharide nanoparticle modified biphasic calcium phosphate scaffolds for bone tissue regeneration. J Biomed Mater Res A. 2015;103:1520–32. doi: 10.1002/jbm.a.35282. [DOI] [PubMed] [Google Scholar]
- 31.Hong I, Khalid AW, Pae HC, Cha JK, Lee JS, Paik JW, et al. Distinctive bone regeneration of calvarial defects using biphasic calcium phosphate supplemented ultraviolet-crosslinked collagen membrane. J Periodontal Implant Sci. 2020;50:14–27. doi: 10.5051/jpis.2020.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn B, Hwang M, Kim S, Kim HI, Ku Y. Ridge preservation using basic fibroblast growth factor-2 and collagenated biphasic calcium phosphate in beagle dogs. J Periodontal Implant Sci. 2017;47:381–7. doi: 10.5051/jpis.2017.47.6.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon KS, Choi EJ, Oh S, Kim S. The effect of covalently immobilized FGF-2 on biphasic calcium phosphate bone substitute on enhanced biological compatibility and activity. Biomed Res Int. 2015;5:742192. doi: 10.1155/2015/742192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A. 2012;109:14170–5. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson TB, Siderits B, Nye S, Jeong YH, Han SH, Rhyu IC, et al. Effect of guided bone regeneration on bone quality surrounding dental implants. J Biomech. 2018;80:166–70. doi: 10.1016/j.jbiomech.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz F, Rothamel D, Herten M, Ferrari D, Sager M, Becker J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: an immunohistochemical study in dogs. Clin Oral Implants Res. 2008;19:642–52. doi: 10.1111/j.1600-0501.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 37.Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990;2:857–63. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- 38.Pitaru S, Kotev-Emeth S, Noff D, Kaffuler S, Savion N. Effect of basic fibroblast growth factor on the growth and differentiation of adult stromal bone marrow cells: enhanced development of mineralized bone-like tissue in culture. J Bone Miner Res. 1993;8:919–29. doi: 10.1002/jbmr.5650080804. [DOI] [PubMed] [Google Scholar]
- 39.Frank O, Heim M, Jakob M, Barbero A, Schäfer D, Bendik I, et al. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85:737–46. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- 40.Naganawa T, Xiao L, Coffin JD, Doetschman T, Sabbieti MG, Agas D, et al. Reduced expression and function of bone morphogenetic protein-2 in bones of Fgf2 null mice. J Cell Biochem. 2008;103:1975–88. doi: 10.1002/jcb.21589. [DOI] [PubMed] [Google Scholar]
- 41.Lee JS, Kim TW, Park S, Kim BS, Im GI, Cho KS, et al. Reduction of adipose tissue formation by the controlled release of BMP-2 using a hydroxyapatite-coated collagen carrier system for sinus-augmentation/extraction-socket grafting. Materials (Basel). 2015;8:7634–49. doi: 10.3390/ma8115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aryal R, Chen XP, Fang C, Hu YC. Bone morphogenetic protein-2 and vascular endothelial growth factor in bone tissue regeneration: new insight and perspectives. Orthop Surg. 2014;6:171–8. doi: 10.1111/os.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gittens SA, Bagnall K, Matyas JR, Löbenberg R, Uludag H. Imparting bone mineral affinity to osteogenic proteins through heparin-bisphosphonate conjugates. J Control Release. 2004;98:255–68. doi: 10.1016/j.jconrel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Park Y, Lin S, Bai Y, Moeinzadeh S, Kim S, Huang J, et al. Dual delivery of BMP-2 and IGF-1 through injectable hydrogel promotes cranial bone defect healing. Tissue Eng Part A. 2022;28:760–9. [DOI] [PMC free article] [PubMed]
- 45.Lee JS, Lee SK, Kim BS, Im GI, Cho KS, Kim CS. Controlled release of BMP-2 using a heparin-conjugated carrier system reduces in vivo adipose tissue formation. J Biomed Mater Res A. 2015;103:545–54. doi: 10.1002/jbm.a.35207. [DOI] [PubMed] [Google Scholar]
- 46.Lee JW, Lim HC, Lee EU, Park JY, Lee JS, Lee DW, et al. Paracrine effect of the bone morphogeneticprotein-2 at the experimental site on healing of the adjacent control site: a study in the rabbit calvarial defect model. J Periodontal Implant Sci. 2014;44:178–83. doi: 10.5051/jpis.2014.44.4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JW, Jung IH, Lee KI, Jung UW, Kim CS, Choi SH, et al. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012;100:3304–13. doi: 10.1002/jbm.a.34278. [DOI] [PubMed] [Google Scholar]
- 48.Chung SM, Jung IK, Yoon BH, Choi BR, Kim DM, Jang JS. Evaluation of different combinations of biphasic calcium phosphate and growth factors for bone formation in calvarial defects in a rabbit model. Int J Periodontics Restorative Dent. 2016;36:s49. doi: 10.11607/prd.2633. [DOI] [PubMed] [Google Scholar]