Abstract
Increased energy consumption as a result of population growth and industrialization necessitates the use of renewable energy sources in the field of chemistry. Nonrenewable energy sources release not only greenhouse gases but also other hazardous pollutants that are damaging to all living things. This plainly mandates the researchers' use of a renewable energy source that is both environmentally friendly and cost-effective. This study shows that a renewable energy source (sunlight) can be used to synthesize tetrahydrobenzo[b]pyran scaffolds using the Knoevenagel–Michael cyclocondensation of aldehyde derivatives, malononitrile, and dimedone via a three-condensation domino reaction. This research establishes a new role for solar energy as a renewable energy source for the synthesis of tetrahydrobenzo[b]pyran scaffolds under catalyst-solvent-free conditions, with outstanding yields, shorter reaction time, and great atom economy. This cyclization may also be done on a gram scale with free, safe, and clean energy from concentrated solar radiation (CSR), indicating the reaction's potential for industrial applications.
Subject terms: Chemistry, Green chemistry, Renewable energy, Solar energy
Introduction
Nature has been directing humans on how to accomplish chemistry for a long time. We were unable to comprehend how nature, with its vast capacity, conducts intricate chemical processes in a biological system. Chemistry has relied on non-renewable resources in the past, as well as in the present, such as petroleum-based chemicals, which are commonly used as solvents. Apart from being non-renewable, these solvents are extremely harmful to both the environment and humans. Nonetheless, we have continued to use non-renewable materials in an irresponsible manner, despite the fact that the moment has come for the so-called "6th wave of innovation," in which sustainability is a top priority. As a result, many academic and industrial researchers are attempting to focus more on greener or more sustainable approaches to process development. In reality, most chemical and pharmaceutical companies now have a dedicated unit for environmentally friendly chemistry. Solvent, a medium required to accomplish chemical reactions, is a major difficulty, as is waste from chemical plants. Even a simple swap from organic to water as a solvent could drastically reduce the amount of organic solvent used and hence waste. When compared to organic solvents, one may argue that the expense of water over its life cycle or the treatment of wastewater is substantially higher. The amount of solvent required (water) is significantly smaller than that required for reactions carried out in organic solvents. This chemistry in water is swiftly progressing, however, it will be fascinating to see if the reaction is carried out neatly or without the need for a solvent, as "the best solvent is no solvent"1.
Because of the indiscriminate use of nonrenewable resources and the pressing need for sustainability, the time has come for the 6th wave of innovation, which entails the application of greener and more sustainable principles. In the development of processes, industries require us to consider a variety of factors such as the use of harmful chemicals, waste management, multistep processes, longer duration, reuse and recovery of materials, and so on. Given these considerations, a solvent- and catalyst-free process utilizing renewable energy sources is one of the most attractive approaches2.
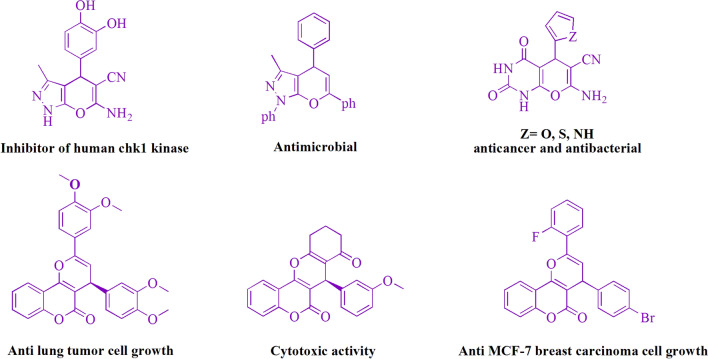
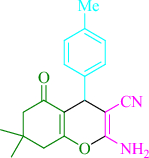
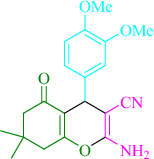
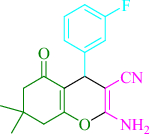
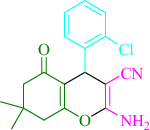
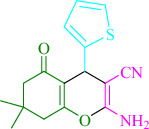
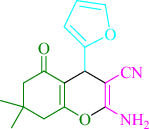
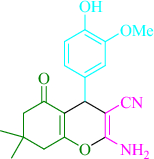
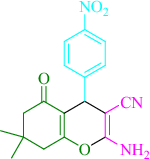
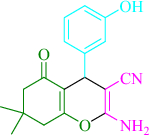
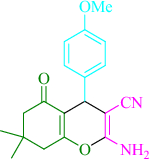
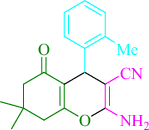
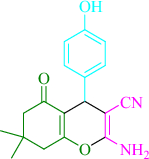
Pyran derivatives with a variety of pharmacological properties (Fig. 1), including Chk1 kinase inhibitory activity3, analgesic properties4, anticancer5, vasodilatory activities6, spamolytic7, antihypertensive, hepatoprotective, cardiotonic8, vasodilator9, anti-leukemic10,11, emetic12, anti-anaphylactic activities13, diuretic14, and anti-alzheimer15.
Figure 1.
Pyran motifs are found in a number of medicinally significant substances.
These compounds can be synthesized in a variety of ways, employing a variety of catalysts like as.
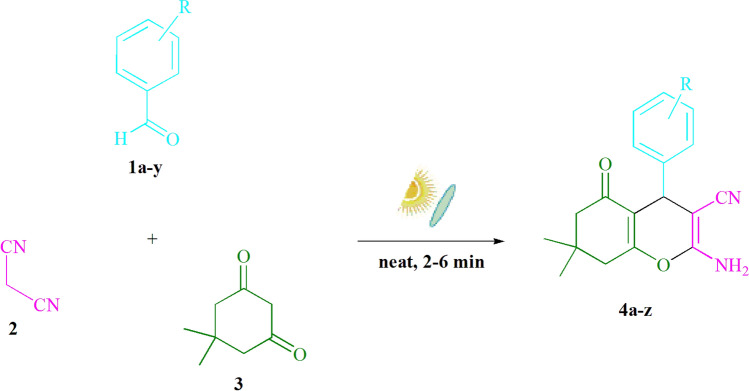
CaHPO416, SiO2NPs17, ethylenediamine diacetate18, SBPPSP19, I220, NH4Al(SO4)2·12H2O21, NH4H2PO4/Al2O322, ACoPc–MNPs23, ZnONPs24, Fe3O4@SiO2–imid–PMA25, NiFe2O4@SiO2–H3PW12O4026, theophylline27, triethanolamine28, NaN329, Fe3O4@SiO2@TiO230, MgFe2O4 nanoparticles31, trichloroisocyanuric acid32, Na2 eosin Y33, DABCO34, Pd nanoparticles35, WELPSA36, Core/Shell CaO@SiO2–SO3H37, Co3O4 nano-flakes38, HMS/Pr–Rh–Zr39, nano-SiO2/DBN40, M–Fe3O4@HAL–SO3H41, Bead-PIL42. It's worth noting that the Knoevenagel–Michael cyclocondensation reaction is a well-known method for producing tetrahydrobenzo[b]pyran scaffolds with either transition metal catalysts or organic solvents and/or conventional heating sources. Given the relevance of this reaction and our ongoing interest in green chemistry43–46, we predicted that this reaction could be performed without the use of a catalyst or solvent, as well as using just solar energy as a clean energy source. We are excited to show that this reaction might be carried out on a wider scale utilizing concentrated solar radiation (CSR), which is a free, safe, and clean energy source.
Experimental
General
The melting points of all compounds were determined using Electrothermal 9100 equipment. Furthermore, nuclear magnetic resonance (1HNMR, and 13CNMR) spectra were recorded using a Bruker DRX-400, Bruker DRX-300 and Bruker DRX-100 Avance instrument using CDCl3 as the solvent. The mass spectra were procured employing a spectrometer from Agilent Innovation (HP) working at a 70 eV ionization potential. The components (Carbon, Hydrogen, and Nitrogen) were examined employing a Heraeus CHN-O-Rapid analyzer. All reagents were purchased from Acros, Merck, and Fluka Chemicals and used without further purification.
The entire procedure for preparing (4a–z)
The synthesis of tetrahydrobenzo[b]pyran derivatives was achieved using a three-condensation domino reaction involving aldehyde derivatives (1, 1.0 mmol), malononitrile (2, 1.0 mmol), and dimedone (3, 1.0 mmol) catalyzed by concentrated sun radiation (CSR) under catalyst-solvent free conditions (Fig. 6). For 2–6 min, the mixture was stirred. TLC was used to monitor the reaction process, with n-hexane/EtOAc (3:1) as the eluent. The achieved solid was filtered, and rinsed with water, and the crude solid was recrystallized from ethanol to yield pure material without the need for further purification. Although we were able to synthesize the above chemicals utilizing gram-scale processes, we wanted to test if it could be scaled up to the level that pharmaceutical process R&D prefers. Under CSR, 50 mmol each of m-tolualdehyde, malononitrile, and dimedone were agitated. As expected, the large-scale reaction occurred well and finished in 2 min, with the product collected by easy filtration as normal. This material's 1HNMR spectrum indicates that it is spectroscopically pure.
Figure 6.
Tetrahydrobenzo[b]pyran scaffold synthesis.
After comparing spectroscopic information, the products were categorized (1HNMR). The following sources and Figs. 2, 3, 4, and 5 provide support for this manuscript:
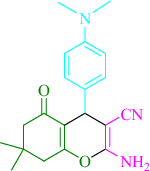
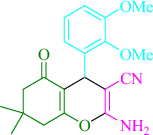
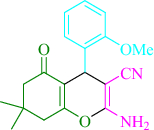
Figure 2.
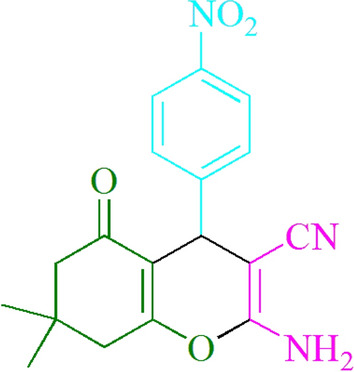
Structure for compound 4j.
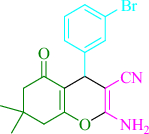
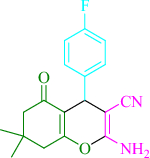
Figure 3.
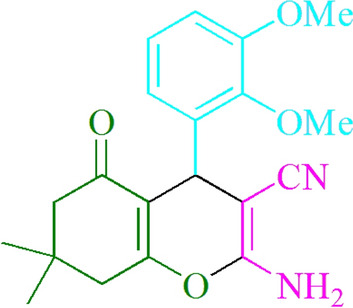
Structure for compound 4 s.
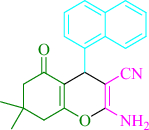
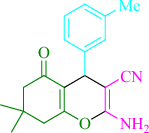
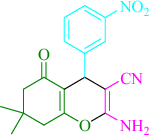
Figure 4.
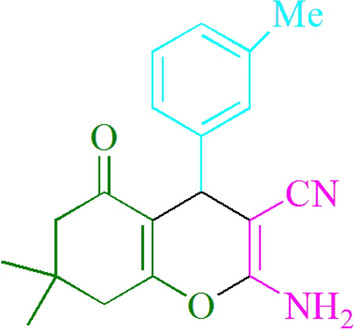
Structure for compound 4u.
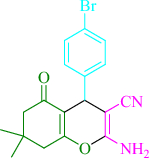
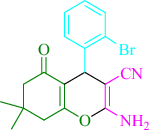
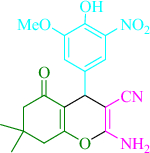
Figure 5.
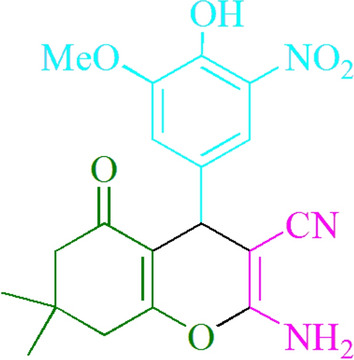
Structure for compound 4z.
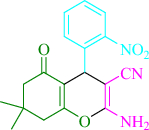
2-Amino-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4Hchromene-3-carbonitrile (4j)
Yield: 95%; M.p. 177–179 °C; 1HNMR (300 MHz, CDCl3) 1.07 (3H, s, CH3), 1.16 (3H, s, CH3), 2.30 (2H, d, J = 14.0 Hz, CH2), 2.52 (2H, s, CH2), 4.55 (1H, s, CHAr), 4.68 (2H, s, NH2), 7.45 (2H, d, J = 11.6 Hz, ArH), 8.20 (2H, d, J = 11.6 Hz, ArH).
2-Amino-4-(2,3-dimethoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4Hchromene-3-carbonitrile (4 s)
Yield: 92%; M.p. 218–220 °C; 1HNMR (300 MHz, CDCl3) 1.10 (3H, s, CH3), 1.14 (3H, s, CH3), 2.25 (2H, s, CH2), 2.47 (2H, s, CH2), 3.77 (3H, s, OCH3), 3.83 (3H, s, OCH3), 4.47 (2H, s, NH2), 4.73 (1H, s, CHAr), 6.68–6.84 (3H, m, ArH).
2-Amino-4-(3-methylphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4Hchromene-3-carbonitrile (4u)
Yield: 96%; M.p. 199–201 °C; 1HNMR (400 MHz, CDCl3) 1.06 (3H, s, CH3), 1.13 (3H, s, CH3), 2.23 (2H, d, J = 5.6 Hz, CH2), 2.31 (3H, s, CH3), 2.46 (2H, s, CH2), 4.38 (1H, s, CHAr), 4.52 (2H, s, NH2), 7.09–7.15 (3H, m, ArH), 7.28 (1H, s, ArH).
2-Amino-4-(3-nitro-4-hydroxy-5-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4Hchromene-3-carbonitrile (4z)
Yield: 89%; M.p. 222–224 °C; 1HNMR (400 MHz, CDCl3) 1.08 (3H, s, CH3), 1.15 (3H, s, CH3), 2.27 (2H, d, J = 5.6 Hz, CH2), 2.50 (2H, s, CH2), 3.99 (3H, s, OCH3), 4.41 (1H, s, CHAr), 4.68 (2H, s, NH2), 7.19–7.47 (2H, m, ArH), 10.68 (1H, s, OH); 13CNMR (100 MHz, CDCl3): 27.5, 28.9, 32.2, 35.2, 40.6, 50.6, 56.8, 62.2, 114.0, 118.3, 134.9, 137.2, 145.5, 149.0, 149.8, 154.9, 157.6, 162.1, 195.9; Anal. Calcd for C19H19N3O6: C, 59.22; H, 4.97; N, 10.90%. Found: C, 59.29; H, 4.86; N, 10.98%; MS (m/z): 386 (M +) (Supplementary Fig. S1).
Results and discussion
CSR was used to stir benzaldehyde (1 mmol), malononitrile (1 mmol), and dimedone (1 mmol) in EtOH for 5 min, yielding 85% of the desired result (Table 1, entry 1). Furthermore, conducting the reaction in H2O, MeOH, DMSO, DMF, THF, CH3CN, or EtOAc did not result in a higher chemical yield (Table 1). We eventually tried heating these with CSR by simply combining them. Surprisingly, the reaction was completed in under 3 min, yielding the quantitatively desired result (Table 1, entry 3). It's only natural to point out that recrystallization from ethanol yielded spectroscopically pure product 4a. Because no column chromatography was necessary, this clearly indicates that the new approach is far superior in terms of product isolation. CSR set-up was used to synthesize tetrahydrobenzo[b]pyran scaffolds according to the published procedures1,2. With the right circumstances in place, a wide range of substrates were investigated (Table 2 and Fig. 6). It's worth mentioning that the benzaldehyde substituent had no effect on the reaction's outcome (Table 2). The reaction conditions were tolerant of polar and halide substitutions. At the current reaction state, reactions with both electron-donating and electron-withdrawing functional groups went smoothly. The yield of all ortho, meta, and para-substituted aromatic aldehydes is exceptionally high. Different aldehydes, such as the bulkier naphthaldehyde, provide a finished product with minimal yield loss. In terms of reactivity, heterocyclic aldehydes followed a similar pattern (Table 2).
Table 1.
The optimization table for the 4a synthesisa.
| Entry | Solvent | Time (min) | Isolated yields (%) |
|---|---|---|---|
| 1 | EtOH | 5 | 85 |
| 2 | H2O | 10 | 47 |
| 3 | – | 3 | 96 |
| 4 | MeOH | 10 | 61 |
| 5 | DMSO | 25 | 23 |
| 6 | DMF | 25 | 18 |
| 7 | THF | 25 | 27 |
| 8 | CH3CN | 15 | 21 |
| 9 | EtOAc | 20 | 16 |
aReaction conditions: In the solvents (3 mL) indicated, benzaldehyde (1 mmol), malononitrile (1 mmol), and dimedone (1 mmol) were agitated under CSR.
Significant values are in bold.
Table 2.
Substrate scope for tetrahydrobenzo[b]pyran scaffolds.
 | |||
|
4a (3 min, 96%) Mp. 224-226 °C Lit. 226-228 °C16 |
4b (2 min, 97%) Mp. 222-224 °C Lit. 223-226 °C16 |
4c (2 min, 94%) Mp. 220-222 °C Lit. 221-223 °C19 |
4d (4 min, 91%) Mp. 228-230 °C Lit. 227-229 °C24 |
|
4e (2 min, 98%) Mp. 210-212 °C Lit. 210-212 °C30 |
4f (4 min, 90%) Mp. 213-215 °C Lit. 214-216 °C18 |
4g (2 min, 93%) Mp. 210-212 °C Lit. 208-210 °C26 |
4h (2 min, 96%) Mp. 214-216 °C Lit. 216-217 °C26 |
|
4i (5 min, 88%) Mp. 229-231 °C Lit. 227-229 °C24 |
4j (3 min, 95%) Mp. 177-179 °C Lit. 180-181 °C18 |
4k (4 min, 91%) Mp. 209-211 °C Lit. 208-210 °C18 |
4l (6 min, 88%) Mp. 227-229 °C Lit. 228-230 °C17 |
|
4m (5 min, 91%) Mp. 216-218 °C Lit. 215-218 °C30 |
4n (6 min, 86%) Mp. 206-208 °C Lit. 204-206 °C16 |
4o (5 min, 85%) Mp. 224-226 °C Lit. 226-228 °C23 |
4p (3 min, 93%) Mp. 200-202 °C Lit. 202-205 °C16 |
|
4q (2 min, 97%) Mp. 212-214 °C Lit. 211-212 °C26 |
4r (6 min, 87%) Mp. 208-210 °C Lit. 210-212 °C23 |
4s (4 min, 92%) Mp. 218-220 °C Lit. 217-219 °C17 |
4t (2 min, 97%) Mp. 200-202 °C Lit. 198-200 °C19 |
|
4u (2 min, 96%) Mp. 199-201 °C Lit. 198-200 °C19 |
4v (4 min, 83%) Mp. 148-150 °C Lit. 150-152 °C30 |
4w (3 min, 96%) Mp. 211-213 °C Lit. 211-212 °C26 |
4x (5 min, 87%) Mp. 229-231 °C Lit. 228-230 °C16 |
|
4y (3 min, 93%) Mp. 208-210 °C Lit. 210-212 °C32 |
4z (5 min, 89%) Mp. 222-224 °Ca |
||
aThe new compound is synthesized in this work.
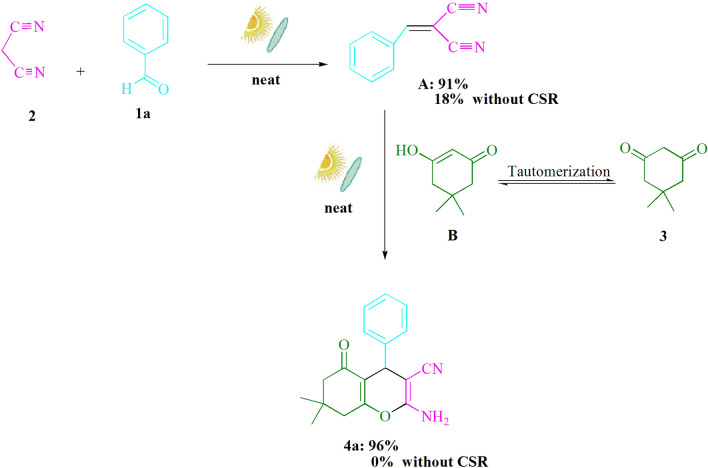
Experiments on intermediates have also been studied as a control. The Knoevenagel-Michael cyclocondensation reaction has two steps: the first is the production of arylidenemalononitrile A, and the second is the condensation of A with B. Separate processes were done to extract intermediates in order to determine which step actually needed a solar heat source (Fig. 7). Under sun radiation, benzaldehyde (1a) and malononitrile (2) were first agitated. In 91% of cases, the intermediate product (A) was separated. Only 18% yield of the intended product A was produced when the reaction was performed without CSR but in refluxing ethanol. In addition, no desirable product 4a was obtained when A was refluxed in ethanol with dimedone. When A was mixed with dimedone in the presence of CSR, however, the desired product 4a was formed 96% of the time. According to the reported methods1,2, we conducted several control experiments (Fig. 7) to determine which step actually requires solar radiation. We discovered that both steps in Fig. 7 required CSR because they did not give any significant conversion at room temperature or conventional heating at 90–100 °C. UV–visible and infrared light are both present in solar radiation. UV radiation's photocatalytic characteristic, along with highly rapid vibrational movements of bonds (IR irradiation), causes a rapid collision of reactants, resulting in a chemical transformation in a short period of time. The enhancement of the chemical reaction rate could be due to the synergistic effect of UV and IR light.
Figure 7.
Control experiments on intermediates.
Table 3 shows a comparison of the catalytic capability of a number of catalysts mentioned in this study for the manufacture of tetrahydrobenzo[b]pyran scaffolds. It could be used in a variety of ways, including the utilization of a renewable energy source (sunlight), catalyst-solvent-free conditions with high yields, and time-saving elements of the reaction. On a multigram scale, the atom-economic protocol is effective and has substantial industrial applications. These goods excel in terms of both performance and purity.
Table 3.
Catalytic ability of some of the catalysts in the manuscript for the production of tetrahydrobenzo[b]pyran scaffoldsa.
| Entry | Catalyst | Conditions | Time/yield (%) references |
|---|---|---|---|
| 1 | WELPSA | H2O, Microwave, 80 °C | 10 h/8836 |
| 2 | Core/Shell CaO@SiO2-SO3H | H2O, 50 °C | 20 min/9337 |
| 3 | Co3O4 nano-flakes | EtOH/H2O, rt | 5 min/9538 |
| 4 | HMS/Pr-Rh-Zr | PEG, 80 °C | 30 min/8739 |
| 5 | nano-SiO2/DBN | H2O/EtOH, 60 °C | 20 min/8540 |
| 6 | M-Fe3O4@HAL-SO3H | H2O/EtOH, 80 °C | 1.5 h/9541 |
| 7 | Bead-PIL | H2O, rt | 40 min/9742 |
| 8 | Catalyst-solvent free conditions | Concentrated solar radiation (CSR) | 3 min/96 (this work) |
Significant values are in bold
aBased on the three-component reaction of benzaldehyde, malononitrile, and dimedone.
Conclusion
To conclude, a very efficient and long-lasting synthesis of tetrahydrobenzo[b]pyran scaffolds has been discovered via a Knoevenagel–Michael cyclocondensation reaction mediated by concentrated sun radiation (CSR). This reaction has been carried out on a variety of substrates using a renewable energy source (sunlight) and a relatively simple experimental apparatus. The reaction was highly quick and did not necessitate the use of a solvent or chromatographic purification. This reaction might be scaled up without sacrificing the outcome, according to a multigram scale reaction of model substrates. Furthermore, the use of this approach to synthesize real-world medicinal compounds demonstrated its broad applicability.
Supplementary Information
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the Apadana Institute of Higher Education.
Author contributions
F.M. wrote the main manuscript text and F.M. prepared Figures 1, 2, 3, 4, 5, 6, 7. F.M. reviewed the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38662-0.
References
- 1.Gadkari YU, Hatvate NT, Takale BS, Telvekar VN. Concentrated solar radiation as a renewable heat source for a preparative-scale and solvent-free Biginelli reaction. New J. Chem. 2020;44:8167–8170. doi: 10.1039/D0NJ01351J. [DOI] [Google Scholar]
- 2.Gadkari YU, Hatvate NT, Telvekar VN. Solar energy as a renewable energy source for preparative-scale as well as solvent and catalyst-free Hantzsch reaction. Sustain. Chem. Pharm. 2021;21:100444. doi: 10.1016/j.scp.2021.100444. [DOI] [Google Scholar]
- 3.Foloppe N, Fisher LM, Howes R, Potter A, Robertson AG, Surgenor AE. Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg. Med. Chem. 2006;14:4792–4802. doi: 10.1016/j.bmc.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Kuo SC, Huang LJ, Nakamura H. Studies on heterocyclic compounds. 6. Synthesis and analgesic and antiinflammatory activities of 3, 4-dimethylpyrano [2, 3-c] pyrazol-6-one derivatives. J. Med. Chem. 1984;27:539–544. doi: 10.1021/jm00370a020. [DOI] [PubMed] [Google Scholar]
- 5.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahluwalia VK, Dahiya A, Garg V. Reaction of 5-amino-4-formyl-3-methyl (or phenyl)-1-phenyl-1H-pyrazoles with active methylene compounds: Synthesis of fused heterocyclic rings. Indian J. Biochem. Biophys. 1997;36:88–90. [Google Scholar]
- 7.Ellis GP, Taylor A. The chemistry of heterocyclic compounds. In: Ellis GP, editor. Chromenes, chromenes, and chromenes. John Wiley; 1977. [Google Scholar]
- 8.Heber D, Heers C, Ravens U. Positive inotropic activity of 5-amino-6-cyano-1, 3-dimethyl-1, 2, 3, 4-tetrahydropyrido [2, 3-d] pyrim idine-2, 4-dione in cardiac muscle from guinea-pig and man. Part 6: Compounds with positive inotropic activity. Pharmazie. 1993;48:537–541. [PubMed] [Google Scholar]
- 9.Coates WJ. Pyrimidopyrimidine derivatives, Eur. Pat. 351058. Chem. Abstr. 1990;113:40711. [Google Scholar]
- 10.Fokialakis N, Magiatis P, Chinou I, Mitaku S, Tillequin F. Megistoquinones I and II, two quinoline alkaloids with antibacterial activity from the bark of Sarcomelicope megistophylla. Chem. Pharm. Bull. 2002;50:413–414. doi: 10.1248/cpb.50.413. [DOI] [PubMed] [Google Scholar]
- 11.Beagley P, Blackie MA, Chibale K, Clarkson C, Meijboom R, Moss JR, Smith PJ, Su H. Synthesis and antiplasmodial activity in vitro of new ferrocene–chloroquine analogues. Dalton Trans. 2003;15:3046–3051. doi: 10.1039/B303335J. [DOI] [Google Scholar]
- 12.Cannon JG, Khonje PR, Long JP. Centrally acting emetics. 9. Hofmann and Emde degradation products of nuciferine. J. Med. Chem. 1975;18:110–112. doi: 10.1021/jm00235a026. [DOI] [PubMed] [Google Scholar]
- 13.Biot C, Glorian G, Maciejewski LA, Brocard JS, Domarle O, Blampain G, Millet P, Georges AJ, Abessolo H, Dive D, Lebibi J. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene− chloroquine analogue. J. Med. Chem. 1997;40:3715–3718. doi: 10.1021/jm970401y. [DOI] [PubMed] [Google Scholar]
- 14.Hafez EAA, Elnagdi MH, Elagamey AGA, El-Taweel FMAA. Nitriles in heterocyclic synthesis: novel synsthesis of benzo [c]-coumarin and of benzo [c] pyrano [3, 2-c] quinoline derivatives. Heterocycles. 1987;26:903–907. doi: 10.3987/R-1987-04-0903. [DOI] [Google Scholar]
- 15.Bayer TA, Schäfer S, Breyhan H, Wirths O, Treiber C, Multhaup G. A vicious circle: role of oxidative stress, intraneuronal Aβ and Cu in Alzheimer's disease. Clin. Neuropathol. 2006;25:163–171. [PubMed] [Google Scholar]
- 16.Bodaghifard MA, Solimannejad M, Asadbegi S, Dolatabadifarahani S. Mild and green synthesis of tetrahydrobenzopyran, pyranopyrimidinone and polyhydroquinoline derivatives and DFT study on product structures. Res. Chem. Intermed. 2016;42:1165–1179. doi: 10.1007/s11164-015-2079-1. [DOI] [Google Scholar]
- 17.Banerjee S, Horn A, Khatri H, Sereda G. A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett. 2011;52:1878–1881. doi: 10.1016/j.tetlet.2011.02.031. [DOI] [Google Scholar]
- 18.Zhou Z, Zhang Y, Hu X. Efficient one-pot synthesis of tetrahydrobenzo [b] pyrans by ethylenediamine diacetate-catalyzed multicomponent reaction under solvent-free conditions. Polycycl. Aromat. Compd. 2017;37:39–45. doi: 10.1080/10406638.2015.1088042. [DOI] [Google Scholar]
- 19.Niknam K, Borazjani N, Rashidian R, Jamali A. Silica-bonded N-propylpiperazine sodium n-propionate as recyclable catalyst for synthesis of 4H-pyran derivatives. Chinese J. Catal. 2013;34:2245–2254. doi: 10.1016/S1872-2067(12)60693-7. [DOI] [Google Scholar]
- 20.Bhosale RS, Magar CV, Solanke KS, Mane SB, Choudhary SS, Pawar RP. Molecular iodine: An efficient catalyst for the synthesis of tetrahydrobenzo [b] pyrans. Synth. Commun. 2007;37:4353–4357. doi: 10.1080/00397910701578578. [DOI] [Google Scholar]
- 21.Mohammadi AA, Asghariganjeh MR, Hadadzahmatkesh A. Synthesis of tetrahydrobenzo [b] pyran under catalysis of NH4Al(SO4)2·12H2O (Alum) Arab. J. Chem. 2017;10:S2213–S2216. doi: 10.1016/j.arabjc.2013.07.055. [DOI] [Google Scholar]
- 22.Maleki B, Sedigh Ashrafi S. Nano α-Al2O3 supported ammonium dihydrogen phosphate (NH4H2PO4/Al2O3): preparation, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. RSC Adv. 2014;4:42873–42891. doi: 10.1039/C4RA07813F. [DOI] [Google Scholar]
- 23.Zolfigol MA, Safaiee M, Bahrami-Nejad N. Dendrimeric magnetic nanoparticle cores with Co-phthalocyanine tags and their application in the synthesis of tetrahydrobenzo [b] pyran derivatives. New J. Chem. 2016;40:5071–5079. doi: 10.1039/C6NJ00243A. [DOI] [Google Scholar]
- 24.Banerjee S, Saha A. Free-ZnO nanoparticles: a mild, efficient and reusable catalyst for the one-pot multicomponent synthesis of tetrahydrobenzo [b] pyran and dihydropyrimidone derivatives. New J. Chem. 2013;37:4170–4175. doi: 10.1039/c3nj00723e. [DOI] [Google Scholar]
- 25.Esmaeilpour M, Javidi J, Dehghani F, Dodeji FN. A green one-pot three-component synthesis of tetrahydrobenzo[b]pyran and 3,4-dihydropyrano[c]chromene derivatives using a Fe3O4@SiO2–imid–PMAn magnetic nanocatalyst under ultrasonic irradiation or reflux conditions. RSC Adv. 2015;5:26625–26633. doi: 10.1039/C5RA01021G. [DOI] [Google Scholar]
- 26.Maleki B, Eshghi H, Barghamadi M, Nasiri N, Khojastehnezhad A, Sedigh Ashrafi S, Pourshiani O. Silica-coated magnetic NiFe2O4 nanoparticlessupported H3PW12O40; synthesis, preparation, and application as an efficient, magnetic, green catalyst for one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed. 2016;42:3071–3093. doi: 10.1007/s11164-015-2198-8. [DOI] [Google Scholar]
- 27.Mohamadpour F. Synthesis of pyran-annulated heterocyclic systems catalyzed by theophylline as a green and bio-based catalyst. Polycycl. Aromat. Compd. 2021;41:160–172. doi: 10.1080/10406638.2019.1575246. [DOI] [Google Scholar]
- 28.Rahnamafa R, Moradi L, Khoobi M. Rapid and green synthesis of 4H-benzo [b] pyrans using triethanolamine as an efficient homogeneous catalyst under ambient conditions. Res. Chem. Intermed. 2020;46:2109–2116. doi: 10.1007/s11164-020-04081-3. [DOI] [Google Scholar]
- 29.Ramesh R, Maheswari S, Malecki JG, Lalitha A. NaN3 Catalyzed highly convenient access to functionalized 4h-chromenes: a green one-pot approach for diversity amplification. Polycycl. Aromat. Compd. 2020;40:1581–1594. doi: 10.1080/10406638.2018.1564678. [DOI] [Google Scholar]
- 30.Khazaei A, Gholami F, Khakyzadeh V, Moosavi-Zare AR, Afsar J. Magnetic core–shell titanium dioxide nanoparticles as an efficient catalyst for domino Knoevenagel–Michael-cyclocondensation reaction of malononitrile, various aldehydes and dimedone. RSC Adv. 2015;5:14305–14310. doi: 10.1039/C4RA16300A. [DOI] [Google Scholar]
- 31.Eshtehardian B, Rouhani M, Mirjafary Z. Green protocol for synthesis of MgFe2O4 nanoparticles and study of their activity as an efficient catalyst for the synthesis of chromene and pyran derivatives under ultrasound irradiation. J. Iran. Chem. Soc. 2020;17:469–481. doi: 10.1007/s13738-019-01783-3. [DOI] [Google Scholar]
- 32.Hojati SF, MoeiniEghbali N, Mohamadi S, Ghorbani T. Trichloroisocyanuric acid as a highly efficient catalyst for the synthesis of tetrahydrobenzo [b] pyran derivatives. Org. Prep. Proced. Int. 2018;50:408–415. doi: 10.1080/00304948.2018.1468982. [DOI] [Google Scholar]
- 33.Mohamadpour F. Photoexcited Na2 eosin Y as direct hydrogen atom transfer (HAT) photocatalyst promoted photochemical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds via visible light-mediated under air atmosphere. J. Taiwan Inst. Chem. 2021;129:52–63. doi: 10.1016/j.jtice.2021.09.017. [DOI] [Google Scholar]
- 34.Tahmassebi D, Bryson JA, Binz SI. 1, 4-Diazabicyclo [2.2.2] octane as an efficient catalyst for a clean, one-pot synthesis of tetrahydrobenzo [b] pyran derivatives via multicomponent reaction in aqueous media. Synth. Commun. 2011;41:2701–2711. doi: 10.1080/00397911.2010.515345. [DOI] [Google Scholar]
- 35.Saha M, Pal AK. Palladium (0) nanoparticles: a novel and reusable catalyst for the synthesis of various pyran derivatives. Adv. Nanoparticles. 2012;1:61–70. doi: 10.4236/anp.2012.13009. [DOI] [Google Scholar]
- 36.Nesaragi AR, Kamble RR, Hoolageri SR, Mavazzan A, Madar SF, Anand A, Joshi SD. WELPSA: A natural catalyst of alkali and alkaline earth metals for the facile synthesis of tetrahydrobenzo [b] pyrans and pyrano [2, 3-d] pyrimidinones as inhibitors of SARS-CoV-2. Appl. Organomet. Chem. 2022;36:e6469. doi: 10.1002/aoc.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sameri F, Mobinikhaledi A, Bodaghifard MA. Preparation of Core/Shell CaO@SiO2-SO3H as a Novel and Recyclable Nanocatalyst for One-Pot Synthesize of Dihydropyrano[2, 3-c] Pyrazoles and Tetrahydrobenzo [b] Pyrans. SILICON. 2022;14:1395–1406. doi: 10.1007/s12633-021-00942-7. [DOI] [Google Scholar]
- 38.Patel G, Patel AR, Maity G, Das S, Patel SP, Banerjee S. Fabrication of self-assembled Co3O4 nano-flake for one-pot synthesis of tetrahydrobenzo [b] pyran and 1, 3-benzothazole derivatives. Curr. Res. Green Sustain. Chem. 2022;5:100258. doi: 10.1016/j.crgsc.2021.100258. [DOI] [Google Scholar]
- 39.Abdolahi S, Hajjami M, Gholamian F. An approach to the synthesis and characterization of HMS/Pr-Rh-Zr as efficient catalyst for synthesis of tetrahydrobenzo [b] pyran and 1, 4-dihydropyrano [2, 3-c] pyrazole derivatives. Res. Chem. Intermed. 2021;47:1883–1904. doi: 10.1007/s11164-021-04411-z. [DOI] [Google Scholar]
- 40.Mehravar M, Mirjalili BBF, Babaei E, Bamoniri A. Nano-SiO2/DBN: an efficacious and reusable catalyst for one-pot synthesis of tetrahydrobenzo [b] pyran derivatives. BMC Chem. 2021;15:1–10. doi: 10.1186/s13065-021-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar P, Gupta P, Sharma C. Surface modified novel magnetically tuned halloysite functionalized sulfonic acid: Synthesis, characterization and catalytic activity. Catal. Sci. Technol. 2021;11:3775–3786. doi: 10.1039/D1CY00285F. [DOI] [Google Scholar]
- 42.Koohestani F, Sadjadi S. Polyionic liquid decorated chitosan beads as versatile metal-free catalysts for catalyzing chemical reactions in aqueous media. J. Mol. Liq. 2021;334:115754. doi: 10.1016/j.molliq.2021.115754. [DOI] [Google Scholar]
- 43.Mohamadpour F. The development of Friedländer heteroannulation through a single electron transfer and energy transfer pathway using methylene blue (MB+) Sci. Rep. 2022;12:7253. doi: 10.1038/s41598-022-11349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamadpour F. Imin-based synthesis of polyfunctionalized dihydro-2-oxypyrroles catalyzed by glycine amino acid via tandem Michael-Mannich cyclocondensation reaction under ambient temperature. Res. Chem. Intermed. 2020;46:1931–1940. doi: 10.1007/s11164-019-04072-z. [DOI] [Google Scholar]
- 45.Mohamadpour F. A new role for photoexcited Na2 eosin Y as direct hydrogen atom transfer (HAT) photocatalyst in photochemical synthesis of dihydropyrano[2,3-c]pyrazole scaffolds promoted by visible light irradiation under air atmosphere. J. Photochem. Photobiol. A: Chem. 2021;418:113428. doi: 10.1016/j.jphotochem.2021.113428. [DOI] [Google Scholar]
- 46.Mohamadpour F. Carbazole-based photocatalyst (4CzIPN) for novel donor–acceptor (D–A) fluorophore-catalyzed visible-light-induced photosynthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones via a proton-coupled electron transfer (PCET) process. RSC Adv. 2023;13:2514–2522. doi: 10.1039/D2RA07064B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].