Abstract
Chronic wounds are characterized by their inability to heal within an expected timeframe and have emerged as an increasingly important clinical problem over the last several decades, due to their higher incidence and greater recognition of associated morbidity and socio-economic burden. Even up to a few years ago, the management of chronic wounds relied on standards of care that were outdated. However, our approach to these chronic conditions has recently enjoyed a renaissance in better prevention, diagnosis, and treatment. Such improvements are due to major advances in cellular and molecular aspects of basic science, in innovative and technological breakthroughs from biomedical engineering, and in our ability to conduct well controlled and reliable clinical research. The evidence-based approaches resulting from these recent advances have become the new standard of care. At the same time, satisfaction with these improvements is tempered by the recognition that persistent gaps exist in our scientific knowledge of impaired healing and our ability to lessen morbidity, loss of limb, and mortality. Therefore, taking stock of what we know and what is needed to improve our understanding of chronic wounds and their associated failure to heal is critical to ensuring better treatments and outcomes.
INTRODUCTION
Chronic wounds are characterized by their inability to heal within an expected timeframe, The definition of chronic wounds is itself challenging. Some authors have proposed guidance based on how long it might take for the wound to heal. This approach is artificial, but unfortunately a better definition that is scientifically acceptable is not available. A major part of the definitional challenge is that chronic wounds are quite heterogeneous in terms of etiology, pathogenesis, size, body location, morbidity, loss of affected limb, host factors, and several other variables. Figure 1 shows examples of the major and typical types of chronic wounds (V.F.). These wounds generally affect the adult population, and are the result of complications from venous insufficiency, diabetes and neuropathies, inability to move and/or spinal cord injury (pressure ulcers), and arterial insufficiency. There are several other clinical settings where the initial injury is the result of genetic factors (for example, the spectrum of epidermolysis bullosa in children) or radiation (accidental or therapeutic). Also, some chronic ulcers have a more predominant immunological basis. Pyoderma gangrenosum and atypical ulcers (such as those due to cryofibrinogenemia or cryoglobulinemia, are examples of that group of chronic wounds.1 It is estimated that in developed countries and worldwide, about 1–2% of the population will experience a chronic wound sometime in their lives. In less developed countries the etiologies may also be quite different; examples include nutritional deficiencies, parasitic and chronic fungal infestation, leprosy. Still, a common denominator does exist, regardless of the underlying cause. That common denominator is “impaired healing” or what some authors and clinicians call “failure to heal.”
Figure 1:
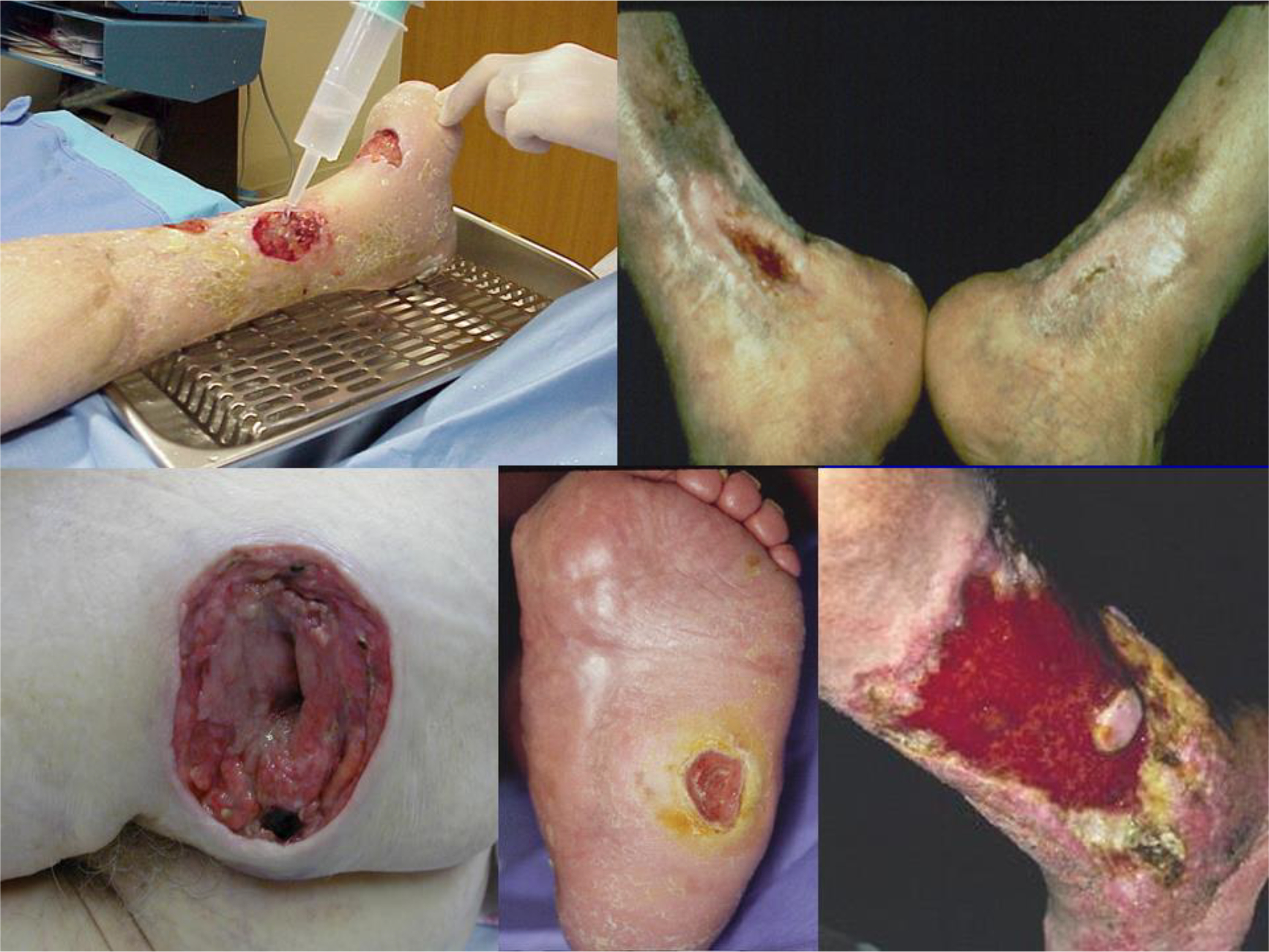
Representative clinical photos of typical chronic wounds. A) Diabetic ulceration due to both neuropathy and arterial insufficiency. The forefoot has been previously amputated; B) Venous ulcer with surrounding lipodermatosclerosis on the medial aspect of the ankles; C) Deep decubitus (pressure) ulcer in the sacral area; D) Neuropathic diabetic ulcer on the sole in a diabetic patient with Charcot foot; E) Extensive ulceration of the lower leg due to combined venous and lymphatic disease. The deep red granulation tissue is not normal and may signify bacterial colonization. The edges of the wound (surrounded by indurated and fibrotic skin) and the island of skin in the wound be are unable to migrate onto the wound bed.
In the last few years, a renaissance has taken place in the understanding and treatment of chronic wounds and impaired healing. Basic science, mostly cellular and molecular, has uncovered many of the processes underlying extracellular matrix deposition, cell migration, and inflammatory responses. A direct result of advances in the basic sciences has been the development of technological breakthroughs for treatment, particularly in the areas of tissue engineering, stem cells, and growth factors. Devices and surgical approaches have been developed or improved for better therapeutic approaches. Therefore, the outlook for better understanding of impaired healing and therapies is brighter than it was even a few years ago. 2,3 Yet, critical gaps in our knowledge of chronic wounds in general and their characteristic feature of impaired healing remain. In this Primer, we will address the epidemiology (D.M.) and pathophysiologic mechanisms (R.R.I., A.M.S.) underlying the difficulties to overcome in the treatment of chronic wounds and how these difficulties also create opportunities for new discoveries. We will explore how these wounds can best be diagnosed (M.R., V.F, M.G.) and characterized to focus on targeted treatment modalities (K.H.), and how they affect patients’ quality of life (S.K). We will then discuss the outlook (V.F.) and initiate a discussion about what the needs are in these various areas of research clinical approaches to render chronic wounds less healing impaired.
EPIDEMIOLOGY
Chronic wounds are a worldwide problem. Epidemiologic discussions of chronic wounds are complicated by variations in terminology, underlying diseases, and regional distribution, as well as healing or effects of management. First, the term chronic wound includes many human aliments for which etiologies, outcomes, treatment, and prognosis vary widely, hampering a unified definition of what is a chronic wound.4 5 In some instances, wounds are described as complex wounds, which are defined as superficial, partial or full thickness skin loss wounds healing by secondary intention. These have a point prevalence of 1.47 per 1000 in the UK. 6 Second, some wounds are defined as a co-morbidity of another illness. For example, in order to have a diabetic foot ulcer, the patient must have diabetes (e.g., population at risk); however, those with diabetes are also more prone to have other chronic wounds like venous leg ulcers. 7 Most chronic wounds are more common in the elderly; therefore epidemiologic discussions of chronic wounds will vary based on population age. Third, the rate of chronic wounds can vary by geographic location and community or facility under study.8–10 Finally, chronic wounds can heal, be treated with limb altering interventions and recur, altering estimates of prevalence and incidence.
PUs are a worldwide problem especially among patients who are infirm. PU rates are highly dependent on residence (hospital, nursing home, etc.), patient characteristics, and age. 11–13 In a worldwide review from 2019, prevalence rates from cross-sectional studies of PU ranged from 3% to 31% in long-term care facilities.13 Prevalence varied by country and many of reports reviewed were more than a decade old.13 A 2019 study in Portugal emphasized the variation by residence and noted a prevalence rate of 5.8% in the hospital, 4.0% in nursing homes, and 0.02% in the community.14 A similar community rate was noted in a study from Spain and a similar nursing home rate was noted in a study from China. 15,16 An assessment of fifty-one nursing homes in Switzerland revealed a prevalence of 0% to 19%. 12 This rate was highly dependent on patient age and Braden score (a risk score based on patient factors such as mobility, activity, nutrition, patent’s sensory/perception, etc.). 12,17,18
VLUs are the most common chronic wound. It is believed that between 1.5 and 3 per one thousand persons have a venous leg ulcer and that these wounds are more common in women. 15,19–21 The prevalence of VLU has been somewhat stable over time and by country. 22 VLU Prevalence also increases by the patient’s age. 19,20 The prevalence also varies by location (full population or primary care setting). 23 The prevalence for those over 65 years of age is about 1.5% and a yearly incidence of 1.2%. 20 However, epidemiologic studies for VLU are also generally more than a decade old.
Probably the best studied chronic wound is the DFU. 9 This is likely because of the public health importance of diabetes as well as the association of this wound with an important complication of diabetes, lower extremity amputation (LEA), as well as death. 24,25 Those with diabetes and a DFU are more than 10 times more likely to have a LEA than those with diabetes and no DFU. 26 Those with a DFU or an LEA are also at a 2- or 3-times increased risk of death, respectively. 24,25 The prevalence of DFU varies from 1.2% to 20% for patients with diabetes in the hospital and from 0.02% to 10% for patients with diabetes in the community. 9,23,27 Among those with diabetes, the worldwide incidence of DFUs has been reported to vary from five per one-hundred-person years to 41 per 100 person years. 9,28 However, DFU prevalence also varies by age, other complications of diabetes, and region. 8,9,26,29 For example, in a study of all US Medicare beneficiaries, the yearly prevalence of DFU was about 8.0% but varied by age from a low of 6.1% for those with diabetes between 65 and 74 years of age to a high of 15.0% for those over 95. 30 Similar variation was noted for the incidence, with an overall rate of 6 per hundred person-years, a lower rate of 4.6 for those between 65 and 74 and a high of 11.5 per hundred person-years for those over 95. 30 All rates varied 3 to 5-fold by US hospital referral region (5,25,26). Regional variation has also been noted in the United Kingdom. 31
It is expected that epidemiological work will continue to play a key role in prevention of chronic wounds and in identifying targeted therapies, both established and novel. For diabetic ulcers, the focus may necessarily shift to a concerted effort to prevent leg and foot amputation. Information on that topic will continue to accumulate within the United States Diabetes Surveillance System (US CDC: https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html
Finally, the likelihood of a wound healing is highly associated with wound-based and patient-based factors or attributes. 9,32 Examples of these attributes include the size, duration, and depth of the wound. 33,34 A published model making use of more detailed attributes (wounds ≤2cm2, ≤2 months old, ≤ 2 in grade) has been used to successfully predict healing as well as to risk stratify wound severity. 35
MECHANISMS/PATHOPHYSIOLOGY
a). Basic mechanisms of wound repair
A recent and extensive review of the physiological phases of the normal wound healing process details the cellular and molecular processes involved in these fundamental events.36 The normal wound healing process is made of overlapping phases of immediate hemostasis, followed by inflammation, and the proliferative and then remodeling phases.37 However, the process of tissue repair in chronic wounds is unlikely to fit easily this “linear” paradigm of sequential, overlapping phases.36 37,38 Figure 2 shows a diagrammatic representation of the non-linear feature of the phases of wound healing in chronic wounds. Another important consideration is that much of what we know about in vivo aspects of wound healing in humans comes from experimental studies in animal models. Such models do not convincingly approximate chronic wounds39, and often suffer (especially in rodent skin) from excessive contraction.40 In fact, there are no recognized valid animal models for chronic wounds. Therefore, in the absence of true animal models, much effort has been made by developing animal models that have some features approximating delayed healing and by minimizing wound contraction, which is particularly a problem with rodent skin. 39 41 42,43Nevertheless, suitable animal models will continue to play a key role in achieving a better understanding of pathophysiological processes (cellular and molecular) and in the testing of new therapeutic agents.44–51 A valuable FDA guide for the use of animal testing is available: https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf.
Figure 2.
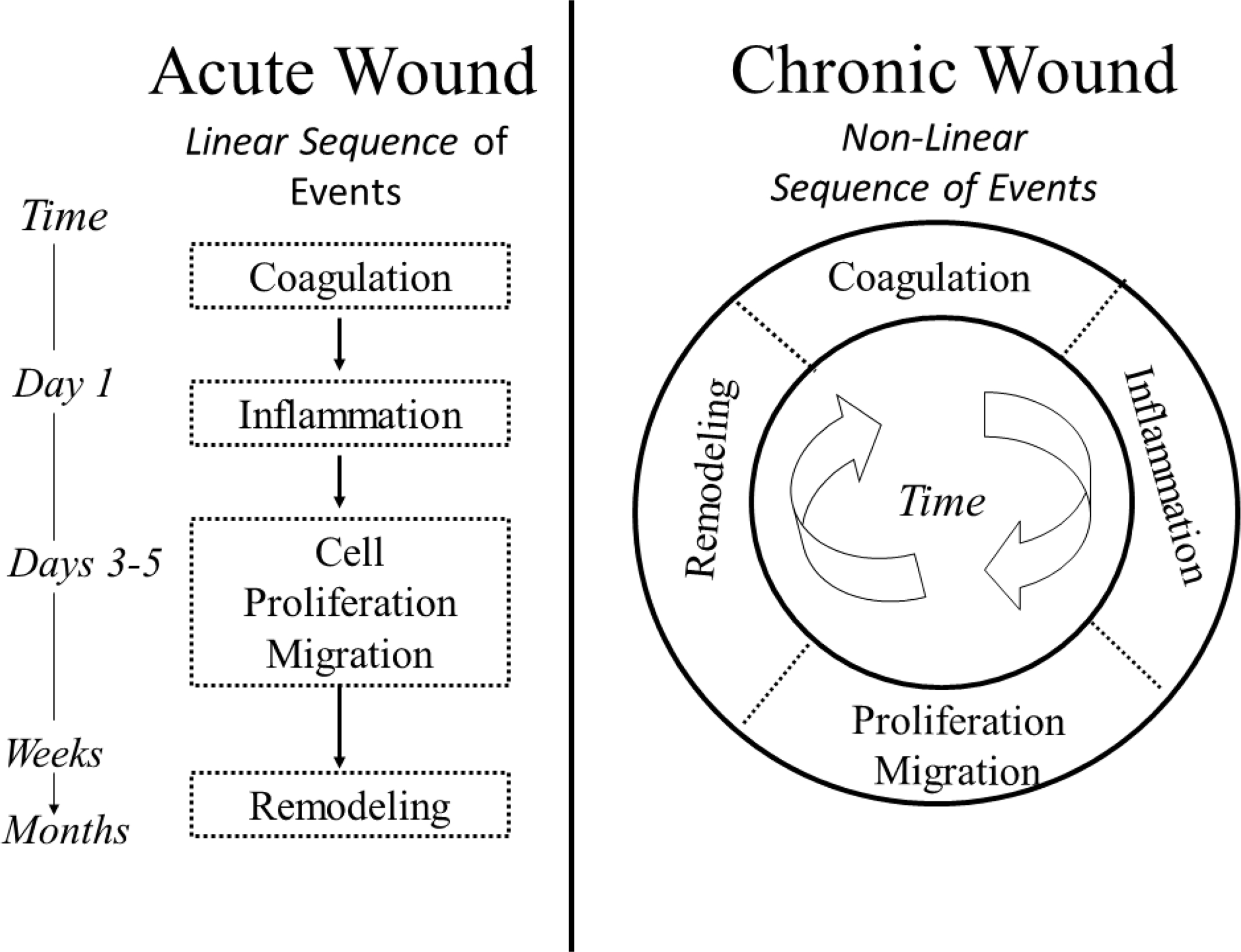
Diagrammatic representation of the wound healing process in acute wound healing and in chronic wounds. Unlike the linear relationship of the recognized phases of normal wound healing (A: left panel), chronic wounds (B: right panel) are characterized by a process whereby the different phases take place at random and with no defined timeframe. Some parts of the wound are in different phases of healing.
As we stated above, reviews of the overall phases of wound healing have been recently published and address that subject very well.36 In light of that, we will instead pay greater attention to one of those phases (inflammation) where we feel a greater focus is needed. Figure 3 illustrates the inflammatory phase of the wound healing process which, as detailed in the next section, is being increasingly recognized as playing a key role in the repair process. In addition, as in Figure 4, the focus is on investigative aspects of impaired healing that do not rely exclusively on animal studies but, instead, are derived from evidence obtained from human chronic wounds.
Figure 3:
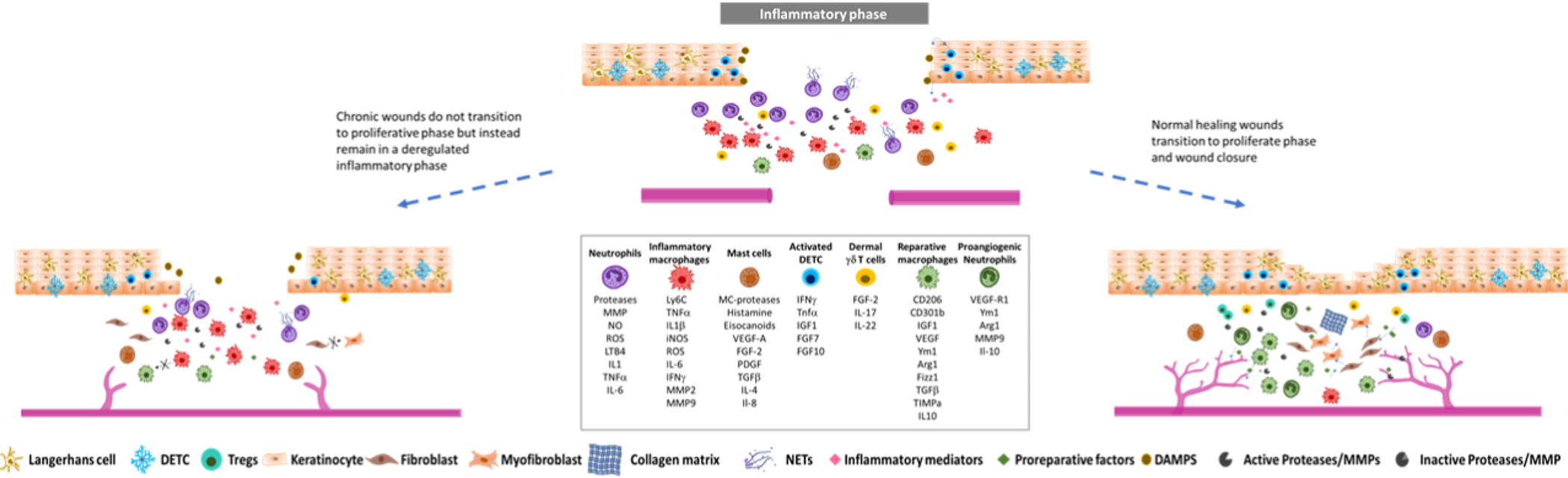
Schematic representation of the inflammatory component of wound healing process in normal healing and chronic wounds. After the inflammatory phase, normal healing wounds can transition to the proliferative phase of wound healing which is hallmarked by the shift of the responses of immune cells to anti-inflammatory and proliferative to allow tissue repair (right panel). Chronic wounds are instead characterized by a stagnant and deregulated phase which fails to quench local inflammatory responses and does not progress to tissue repair (left panel).
Figure 4:
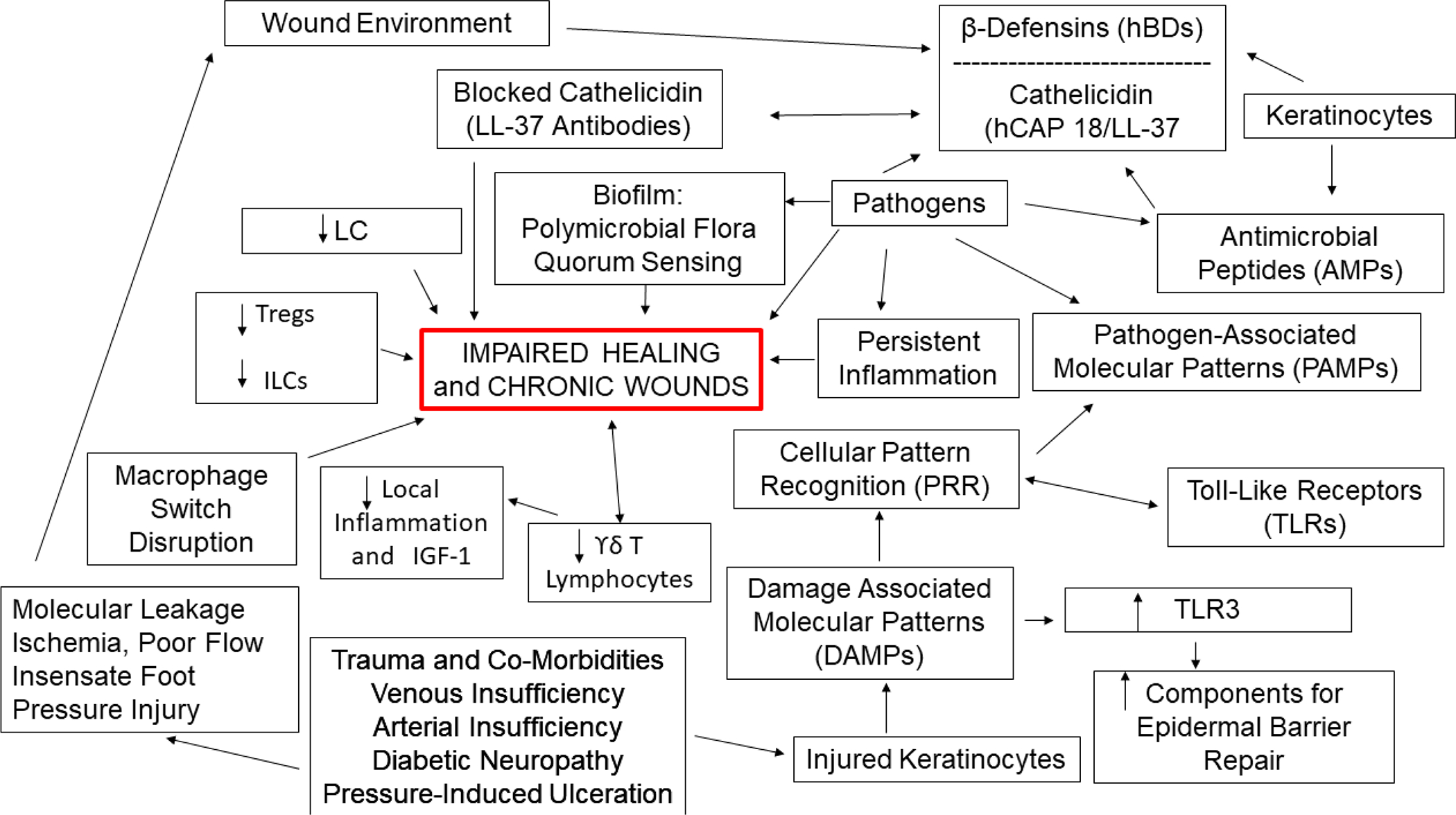
Representation of key component that characterize the path to impaired healing in chronic wounds.
b). Innate Immunity in Wound Healing
Innate immunity is defined as the primary nonspecific first-line defense system protecting against pathogens and tissue damage, rather than to a specific antigen as does adaptive immunity. A continuously updated Nature.com collection on the subject is available: https://nature.com/subjects/innate-immunity. Recent reviews focused on innate immunity and the skin are available.52–55 Most of the current knowledge on the effects of innate immunity in wound healing comes from studies in rodents. However, new studies using advanced sequencing techniques are starting to shed light on the mechanisms that may be associated with chronic wounds in humans. Below the current literature in both rodent and human wound healing is reviewed. We will be sure to distinguish mechanisms that are clearly applicable to and focused on human chronic wounds (Figure 4).
When discussing pathophysiological mechanisms leading to impaired healing and chronic wounds, a major challenge is that much of what we know has been derived from animal research studies. Therefore, a special effort is required to identify those mechanisms that have been uncovered and which are more clearly applicable or proven to take place in human chronic wounds. Figure 4 is meant to show those pathophysiological mechanisms leading to impaired healing in human chronic wounds. When injured, the initial epidermal skin barrier allows invasion by pathogens that activate the innate system and pro-reparative functions. Injured keratinocytes release danger signals, or Damage Associated Molecular Patterns (DAMPs)56 that are recognized by cellular pattern recognition receptors (PRR) such as Toll like receptors (TLRs). PRRs also respond to pathogen-associated molecular patterns (PAMPs) present on or in invading pathogens.57 These pathways, including increasing PRR activity, have been investigated in the context of impaired healing.58–60 Responses are also ligand specific. DAMPs such as host noncoding double-stranded RNA (dsRNA) upregulate the keratinocyte expression of TLR3,61 and activation of TLR3 in human keratinocytes then initiates synthesis of components needed for epidermal barrier repair such as sphingomyelin, and transglutaminase-1.62
As shown in Figure 4, keratinocytes are also important producers of antimicrobial peptides or proteins (AMPs). The best-studied human keratinocyte AMPs are the β-defensin (hBDs) and cathelicidin (hCAP18/LL-37) families. Keratinocytes constitutively express hBD-1, and wounding upregulates the expression of LL-37 and hBD-2, −3.63–65. The cathelicidin family AMP LL-37 is upregulated at edge of acute human surgical wounds, but absent from the wound edge in chronic non-healing ones; blocking its function with anti-LL-37 antibodies impairs wound epithelialization,66 Moreover, treatment with LL-37 accelerates the healing of hard-to-heal venous ulcers.65 Other cells may contribute to innate immunity and tissue repair, such as one subtype of myofibroblasts derived from dermal adipose precursor cells.67 Conversely, in murine wounds, hair follicle cells can signal to myofibroblasts and reprogram them into adipocytes, suggesting new avenues for decreasing scarring.68
A recent study using scRNA-seq analysis showed that keratinocytes in pressure ulcers with poor healing outcomes upregulate MHC-II in response to increased IFNg levels at the wound site 69. Furthermore, keratinocytic MHC-II upregulation impaired activation of T cells at the wound edges (Figure 4).
Langerhans cells are the antigen presenting cell in the epidermis. They express elevated levels of MHC-II and migrate to draining lymph nodes for antigen presentation. In addition, they express C-Lectins, including langerin (CD207) CD205, and CD206, which serve as PRRs and facilitate LCs motility.70 Increased LC numbers are found at the edges of healing wounds, and lower number of LCs were found in diabetic wounds of both mice and humans.71,72 Deletion of langerin+ cells has resulted in improved healing.73
Both murine and human skin contain γδ T lymphocytes,74,75 which can promote repair. In the murine epidermis γδ T cells are also known as dendritic epidermal T cells, or DETC. Studies have shown that murine skin injury results in the activation of DETC.76 Following activation, DETCs express keratinocyte growth factors FGF7 and FGF10, and IGF1 which can promote wound healing.74,75 γδ T cells are also found in the dermis and express IL17 in excisional wounds, which can promote local inflammation and suppresses IGF-1.77 In chronic wounds, γδ T cells are reduced and dysfunctional,78 further supporting a pro-repair role of these cells in normal healing wounds,
Other resident lymphocytes, such as Tregs, have been shown to accelerate wound repair. 79,80 Tregs are usually identified by Foxp3 expression, but also express GATA-3 (a transcription factor associated with Th2 responses) via which they are able to curb fibrosis.79 Non-cytotoxic innate lymphoid cells (ILCs) have also implicated in wound healing, and one of their subgroups (Group 2), if depleted, results in impaired re-epithelialization (Figure 4). 81,82
Cells of myeloid origin populate the skin after injury and exhibit either inflammatory (often detrimental) or pro-reparative functions. Although neutrophils were long regarded as mainly playing a role in fighting microbial infection and in removing damaged tissues, they are actually integral to wound repair.83–85 Persistent neutrophilic presence, however, is associated with degradation of newly formed collagen and impaired tissue repair; slow-healing wounds such as diabetic wounds are populated by large numbers of neutrophils.49,86–88 In excisional wounds, pan-neutrophil depletion using the GR1 antibody resulted in accelerated healing in both diabetic and non-diabetic mice.89 Interestingly, healing is impaired upon deletion of CXCR2 90, one of the main receptors that mediates neutrophil migration.73,91 Neutrophil extracellular traps (NETs) are made of histone- containing nuclear DNA, protease and other proteins that aid in trapping and killing microbes.92 Diabetic patients with non-healing wounds have higher levels of neutrophils with NET markers, and non-infected diabetic mice in which NET formation was impaired healed faster.49,88 Neutrophils exhibit phenotypic heterogeneity and functional plasticity, with a pro-reparative, pro-angiogenic subpopulation that expresses MMP9 for tissue remodeling roles which, in turn, liberates VEGF-A from the extracellular matrix.93 Furthermore, neutrophils have been shown to express VEGF themselves.94
Other granulocytes that have been studied in wound healing are mast cells, whose pro-reparative functions include secretion of growth factors that result in activation of endothelial cell angiogenesis, fibroblast collagen synthesis, and restoration of epithelial barrier.95 High numbers of mast cells are found in chronic wounds, where they impair tissue repair due to increased degranulation and increased protease activity.96 Additionally, mast cells have been associated with fibrosis, although certain studies suggest this may not be the case.97
The best recognized myeloid cells in wound healing are macrophages, possibly due to their plasticity. Macrophage shift from an inflammatory, classically activated phenotype, and although this nomenclature is evolving and macrophages exhibit phenotypes along a spectrum, these are still frequently called M1and characterized by secretion of IL-1, TNFα, IL-6, IL-12, MMPs, and other cytokines, to the M2 (also called alternatively activated), anti-inflammatory, pro-reparative phenotype, characterized by production of arginase, TGFβ, CCL18, PGE2, and IL-10, and up- regulation of scavenger receptors (CD206, CD163). In in vitro settings, these two polarization states are characterized as being distinct and more easily controlled. However, in the wound microenvironment, macrophage polarization stages may present as a continuum, rather than a bi-polar paradigm.98,99 In murine injury models, generation of the cytokine CCL2 leads to recruitment of CCR2+ macrophages, initially as a pro-inflammatory Ly6Chi phenotype; that population subsequently transitions to a CCR2lo/Ly6Clo phenotype that is pro-angiogenic and critical for re-vascularization.100 Furthermore, CD301b+ macrophages have been associated with myofibroblast proliferation and wound repair, although sustained presence of these macrophages at the wound site may be linked to fibrosis and keloid formation.67 Studies using conditional deletion of macrophages during different stages of murine healing have revealed stage-specific functionalities: deletion early in the wound trajectory diminishes granulation tissue and myofibroblast formation while depletion during the mid- stage of healing destabilizes the existing vasculature and impairs epithelialization.101,102 Interestingly, persistence of proinflammatory macrophages at the wound site has been associated with impaired wound healing.103
The switch from M1-rich to the M2-rich wound milieu is crucial for the transition from the inflammatory to proliferative phase of wound healing, and this transitional process may be disrupted in chronic non-healing wounds or even exhibit heterogeneity within the same wound. Various molecules have been identified as cues for the conversion, including Th2 cytokines, and efferocytosis of neutrophils.104 IFNβ-induced epigenetic changes are crucial for the transition of M1 to M2 macrophages. Normal healing wounds upregulate IFNβ, which promotes the expression of the histone methyltransferase Setdb2 in macrophages.105 Setdb2 trimethylates lysine 9 on histone 3 (H3K9me3) and results in abolishment of NFκB DNA binding and transition from M1 to M2. Diabetic wounds exhibited less IFNβ levels and thus decreased levels of Setdb2, leading to increased M1 macrophages at the wound site. The metabolic status of the wound is also critical in these events and responses.106,107
On the flip side, studies also show that supplementation of diabetic and acute wounds with M2 polarized macrophages not only does not promote wound healing but rather delays it 108,109. In support of this, sc-RNAseq analysis showed that healing diabetic foot ulcers (DFUs) have more M1 macrophages compared to non-healing DFUs 110, while others note that DFUs have lower numbers of neutrophils and macrophages compared to non-diabetic acute wounds 111. These presumably contradictory results may be due to timing of the investigation relative to the transition from one subtype to the other and also lack of understanding of the specific characteristic a “healing” macrophage vs a macrophage that impairs healing 112. In fact, a longitudinal study showed that the ratio of M1/M2 macrophages was increased in the early phases of healing DFUs compared to non-healing DFUs but during the later stages, non-healing DFUs had increased M1/M2 ratios compared to the DFUs that healed. This suggests that a sustained inflammatory response and is associated with healing failure 113.
Fibroblast deregulation has also been associated with impaired wound healing. A single cell RNAseq and spatial transcriptomic study using skin from non-diabetic, diabetic without foot ulcer and diabetic with foot ulcer donors identified a novel fibroblast cell type expressing Il6, Tnfaip6, Mmp1,Mmp3 Mmp11, Hif1a and Chi3l1 among other genes 110. In a mouse model of wound healing Engrailed-1 + fibroblasts were associated with increased fibrosis 114. In agreement with this, a study examining single cell transcriptome of diabetic vs normal foot ulcers showed that fibroblasts populations were dysregulated in DFU tissues and were associated with increased expression of fibrotic and inflammatory genes 115.
The ever-expanding network of cytokines and their signaling pathways53,55 presents a major opportunity for further investigation in failure to heal. Some of the most studied cytokines contributing to healing include CCL2 (recruiting the first wave of macrophages to the wound), IL-8 (signaling through CXCR2 in human epidermis to support re- epithelialization),116 and Il-6. The latter is pro-inflammatory in the initial stages of healing and stimulates keratinocyte migration. IL-6 −/− animals have delayed healing, as do animals where IL-17 or IL-22 is deleted.75,117
The pathophysiological mechanism that converts an acute wound destined to heal into one that does not is unclear. Many factors have been associated with wound chronicity including, but not limited to the above noted aberrations in innate immune players in the wound with sustained inflammation, alterations in angiogenesis, dysregulated matrix deposition, neuropathy and impaired neuropeptide signaling, cell senescence, bacterial infection and biofilm formation and wound hypoxia. 118
Biofilms and their formation are now well documented in chronic wounds, and likely play a critical role in impaired healing, chronic wound recurrence and, possibly, chronic wound initial occurrence. The field of biofilm is vast but several recent and representative publications now address the issue of biofilm in chronic wounds. 119–122 Biofilms effectively shield bacterial microorganisms from systemic antibiotics. Moreover, it is becoming clear that the evolution of biofilms has included the strategic evolutionary approach of them becoming polymicrobial and thus less susceptible to antibiotics that could eliminate a more uniform bacterial population. As in other sections dealing with pathophysiologic mechanisms leading to impaired healing, Figure 4 shows these various complex approaches.
Hypoxia in the wound environment results in the release of mediators that regulate angiogenesis and re-epithelialization, via activation of the transcription factor Hypoxia Inducible Factor 1 (HIF-1). 123,124 The in vitro and pre-clinical in vivo evidence demonstrating the downstream pro-reparative consequences of HIF-1 translocation to the nucleus have been extensively reviewed.125,126 These findings have led to the proposed use of deferoxamine, a HIF-1α inducer and stabilizer as a therapeutic for chronic wounds. 123,124 Indeed, a current clinical trial is underway to examine if topically applied deferoxamine can improve healing in diabetic foot ulcers 127, and a patch engineered for its transdermal delivery deferoxamine is being trialed for the treatment of sickle cell ulcers.128,129 The outcome of these trials may provide novel approaches to both preventing and treating chronic ulcers.
d). Advances in Mechanistic Approaches to Impaired Healing
Non-coding RNA
Since their discovery the role of noncoding RNAs (ncRNA) has been expanding. Most of the work has focused on a subset of ncRNAs, the microRNA (miRNA or miR), which are about 22–23 nucleotides long and perform regulatory functions.130 Their expression is tissue and cell-type specific, and early investigations of the cutaneous role of miRNAs focused on their expression patterns and roles in maintaining stemness in epithelial and hair stem cell populations.131,132 The expression pattern of a multitude of miRNAs changes during different phases of wound repair.133–136 One of the most studied is miR-21 that is upregulated in both keratinocytes and fibroblasts at the wound margin in murine wounds and whose inhibition impairs healing in that animal model.137 Its expression promotes an anti-inflammatory phenotype in human macrophages in vitro and modulates conversion of rat mesenchymal stem cells to fibroblasts.138 However, it is over-expressed in venous ulcers,139 possibly inhibiting epithelialization. Dermal remodeling is modulated by miR-29, which targets several transcripts responsible for generating the extracellular matrix and can decrease wound contraction and collagen deposition.140,141 On the other hand, suppression of miR-29 downstream actions by competitive binding to the long non-coding (lnc) RNA H19n is associated with increased extracellular matrix synthesis and fibroblast proliferation; however, over-expression of lncRNA H19 may improve healing in diabetic mice.142 There is evidence that miR-127–3p regulates the transition from the proliferative phase to the remodeling phase of murine wounds, possibly by inhibiting the proliferation of myofibroblasts.143 The role of this miRNA in human wound healing has just recently been explored, and may function as an epigenetic activator regulating the transition from repair to remodeling during skin wound healing.143,144 Another miR under investigation for potential therapeutic modulation of healing is miR-210, which is induced by tissue hypoxia and targets keratinocyte proliferation.145 Wound inflammation and its resolution may be controlled by expression of specific miRNAs such as miR-132, which is diminished in human diabetic wounds.146,147
The subset of long ncRNA (lncRNA), with 200 or more nucleotides, targets genetic networks and has been found to have epithelial functions.148 Notably, two lncRNAs have been found to regulate the balance between proliferation and differentiation in human organotypically cultured epidermis, with the Anti-differentiation Noncoding RNA (ANCR) maintaining progenitor status of basal keratinocytes in human epidermis; its counterpart, Terminal Differentiation-Induced Noncoding RNA (TINCR) promotes differentiation in the upper epidermal compartment.149,150 Recent studies have suggested therapeutic approaches to accelerated healing by targeting specific lncRNAS, either by inhibition of a novel lncRNA, Wound and Keratinocyte Migration Associated lncRNA 2 (WAKMAR2) present in non-healing wounds, or induction of expression of the pro-proliferative lnc-RNA Gas5 by the use of statins.151,152 One recently discovered mechanism by which lncRNAs can regulate cell function is by competitively bonding, or “sponging”, multiple other miRNAs to limit their function.142,153,154
Microbiome
With the advent of new molecular tools, the role of the wound microbiome is being increasingly investigated as a contributor to wound chronicity.155,156 The prolonged inflammatory state of the chronic wound is associated with persistent infection or biofilm formation.120 A causal role has been demonstrated in many studies using animal models. Application of a bacterial inoculum or transfer of preformed biofilm results in delayed healing.157 The transfer of the skin microbiome of a mouse having a mutation that impairs the innate immune response and healing to a wild type mouse results in WT acquisition of the impaired healing phenotype. 158 Staphylococcus aureus and Pseudomonas aeruginosa are among the most commonly encountered by either molecular or culture- based techniques.156,159 However, the classic skin commensal bacterium, Staphylococcus epidermidis, has recently been shown to induce a type17 immune program and accelerated repair through mechanisms linked to antimicrobial peptide production and a subset of skin resident T cells that recognize specific commensals (‘commensal-specific T cells’).160 This commensal organism also upregulates the expression of the antimicrobial molecule, Perforin-2 in keratinocytes.156 Thus, the link between specific microbes within the wound microbiome and wound chronicity remains tenuous.
A better prognosticator of wound chronicity may be the determination of overall bacterial diversity of the wound microbiome.161 A negative linear relationship has been documented between decreasing wound diversity and wound healing rate in two cohorts (each of about 80 patients) with chronic wounds.162 Similar associations have been noted in the wounds of patients with dystrophic epidermolysis bullosa.163 Additional mechanistic progress may be achieved in future studies through deeper sequencing afforded by shotgun metagenomics.164
In addition, host neuroimmune mechanisms that mediate the inflammatory response to pathogens and impact wound repair are being discovered. Nociceptors in the skin that express the transient receptor potential cation channel subfamily V member 1 (TRPV1) are activated by a cutaneous challenge of Candida albicans and respond with expression of the neurotransmitter CGRP. The downstream consequences are activation of dermal dendritic cells to generate IL-23, which then induces the production of the protective, antimicrobial and pro-reparative cytokine IL-17A by dermal γδ T cells.165 On the other hand, skin infection with Streptococcus pyogenes also activates TRPV1+ nociceptors to release CGRP, but the result is inhibition of neutrophil recruitment and function, which could be reversed by administration of a CGRP antagonist.166,167 These divergent nociceptor responses may be due to splice variants, or may be pathogen specific.162 The potential for TRPV1- targeted therapies to improve healing may require personalized medicine approach tailored to the channel splice variant expressed or specific wound pathogen.168
While most studies of the wound microbiome have focused on bacteria, emerging work implicates the wound ‘mycobiome’ and ‘virome’ as potential contributors to wound chronicity. Fungal components are present in the wound microbiome and their abundance is associated with longer healing times.169 Fungal activation of a fungal-specific T cell Th17 response can exacerbate local inflammation and regulate healing.170 Although viral components are recognized as members of the wound microbiome, and viral modulation of inflammatory responses are documented, at this time there are few studies of the impact of the viral component on wound outcomes or chronicity.59
The common presence of antibiotic-resistant bacteria in wounds155 has prompted the search for non-antibiotic approaches. Indeed, a recent study has demonstrated that application of topical antibiotics to a wound significantly impairs healing in mice, and possibly in humans as well.171 Multiple agents are being investigated including therapeutic bacteriophages, antimicrobial peptides, repurposed drugs, cold plasma treatment, photodynamic therapy, probiotics and bioelectric dressings.172,173 Trials of both topically administered probiotics in preclinical wound models, and orally administered probiotics in patients have been reported.174 In one randomized controlled study, supplementing patients with orally ingested probiotics improved healing in the treated cohort of diabetic foot ulcer patients.175 The gut-skin axis and gut dysbiosis are being implicated in the pathogenesis of skin diseases such as atopic dermatitis and psoriasis and ongoing investigation may uncover the impact of this signaling axis on wound healing.176
DIAGNOSIS, SCREENING AND PREVENTION
Chronic wounds are quite heterogeneous in their etiology, pathogenesis, approach to therapy, and prevention. Therefore, clinical evaluation is an essential step to reach a final diagnosis and to establish a specific therapeutic strategy. Table 1 describes the salient features of the main types of chronic wounds: arterial, venous, diabetic and pressure ulcers. Table 1 also lists what are not regarded as atypical wounds, which encompass a group of disorders that are less common but have also proven difficult to heal. These atypical wounds and their diagnosis and management are described in recent publications. 1 Regardless of the type of wound and underlying cause or etiology, a comprehensive assessment of the patient’s condition, including the medical and pharmacologic history, is essential. Table 1 summarizes the diagnostic features to be analyzed from the history, physical exam, and imaging or laboratory testing. In addition to immediate surgical considerations that arise during the diagnostic phase, such as surgical debridement and planning for grafting or vascular and orthopedic procedures, the fundamental clinical criteria involve three major components of the wound: the wound bed, wound edges, and surrounding skin. Figure 5 outlines the diagnostic and interventional approaches.
Table 1.
Chronic Wounds and Common Diagnostic Features
| Type of Chronic Wound | ||||
|---|---|---|---|---|
| Typical Body Location | Cause | Physical Exam Findings | Diagnostic Testing | |
| Pressure Ulcers | Sacral areas and Hips | Inability to move, pressure | Paralysis, aftermath of spinal injury | Rule out osteomyelitis |
| Arterial Ulcers | Lower extremities, especially ankle and toes | Athero-sclerosis | Pale skin and poor or absent peripheral arterial pulses. Hair loss on lower extremities |
Doppler ankle/brachial index measurements. Vascular studies, angiography. |
| Venous Ulceration | Medial aspect of lower legs | Venous hypertension | Lower leg varicosities, hard indurated skin (lipodermatosclerosis) hyperpigmentation, edema | Doppler Duplex Scanning. If suspected, biopsy to rule out malignancy. Doppler ankle/brachial index measurement |
| Diabetic Ulcers | Toes, soles | Neuropathy ± arterial obstruction | Insensate foot, lower extremity, and foot arterial pulses | Neuropathy testing. Vascular studies if pulses are not adequate |
| Atypical chronic wounds | Any location, but more common on lower extremities | Livedo net-like skin pattern, purpura, signs of previous radiation. Undermined wound edges | Serum cryoglobulin and plasma cryofibrinogen. Biopsy to rule out skin cancer, occluded dermal blood vessels or vasculitis |
Figure 5:
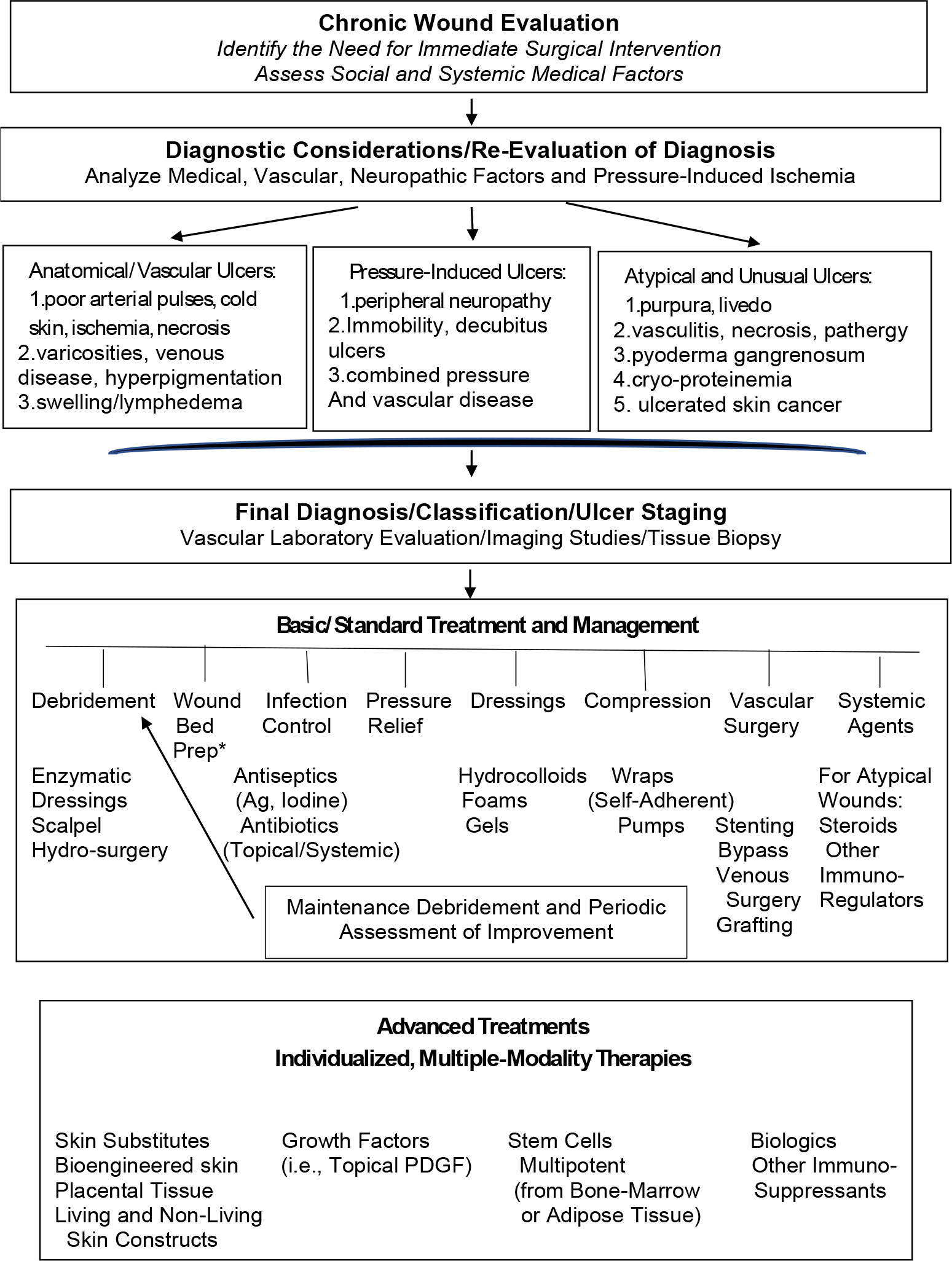
General algorithm for the evaluation, diagnosis, and treatment strategies for chronic wounds.
Clinical features of chronic wounds
The major category of typical chronic wounds are vascular ulcers (including those due to venous and/or arterial insufficiency, diabetic foot ulcers (neuropathic, vascular, or of mixed etiology from neuropathy and vascular obstruction), and pressure or decubitus ulcers. The etiology of these chronic wounds is easily diagnosed by clinical inspection due to such characteristics as body location, adequate arterial pulses, presence of neuropathy, wound bed, unusual features in the surrounding skin, among other typical features (Table 1). The features of venous leg ulcers (VLU or stasis ulcers) reflect the main pathophysiology involving incompetent perforator (or communicating) veins between the deep and venous system and failure of the venous ambulatory pressure to decrease upon walking or exercise (venous hypertension). 177,178 The CEAP classification for VLU has been found useful in different guidelines, and refers to the use of Clinical signs, Etiological cause, Anatomical distribution, and Pathophysiological dysfunction.179,180 With this scoring system each parameter is divided into several stages, which enables the clinician to score the complexity of the patient’s condition. The VLU are typically located above the medial malleolus, more commonly on the medial side of the leg, have irregular edges, and tends to be superficial, i.e., not involving muscle or bone. Surrounding varicosities and edema are common. The wound bed is typically yellow or pale red due to fibrinous material deposition, and there can be considerable exudate even in the absence of infection. The ulcer is not very painful, notably except for when the ulceration is close to or over the malleoli and thus near the nerve-rich periosteum. The surrounding skin is often characterized by indurated and hyperpigmented skin, which is labeled lipodermatosclerosis and represents a deeply fibrotic process.48,178,181–183 Arterial ulcers tend to occur more commonly on the lateral side of the lower leg, are often distally located (such as the dorsum of the foot of toes), and display poor proximal (femoral or popliteal) or distal (dorsalis pedis or posterior tibialis) pulses by palpation or Doppler examination. Arterial ulcers are smaller in size than VLU, are deeper and often down to muscle and even tendons or bone, more painful, and regular in configuration (punched-out appearance). The wound bed often displays black necrotic tissue. Their surrounding skin may show atrophy, with pale skin color and loss of hair. Diabetic foot ulcers a categorized as being neuropathic, ischemic, or neuro-ischemic, according to their preponderant etiological and pathogenic mechanisms. Neuropathic diabetic ulcers usually occur in areas of high pressure on the plantar surface of the foot and, typically, over the metatarsal heads. They may be preceded by an inflammatory process leading to distortion of the underlying bones (Charcot foot). Callus formation is common and contributes to the pressure on the insensate foot. Diabetic ulcers in which ischemia plays a substantial role will show absence of or poor pedal pulses, typical ischemic atrophic changes in the skin, and a necrotic wound bed at an early stage. It is critical to realize that small diabetic ulcers may involve a much larger portion of the foot and may become easily infected, with tunneling and the need to amputation. Thus, diabetic ulcers are always in danger of causing devastating clinical effects. Pressure ulcers are located over bony prominences, such as the heel, hip, and sacral areas. These ulcerations may exhibit skin redness (Stage I) that becomes non-blanchable during digital palpation. They may rapidly advance to deep ulcers involving the dermis (Stage II) and subcutaneous tissues (Stage III), and even tendons and bone (Stage IV). An extensive review of the subject was recently published. 184
Diagnostic work-up
The clinical guidelines for vascular wounds assessment are well established.185 In venous leg ulcers there is no valid test other than clinical evaluation and the need to always exclude arterial insufficiency. However, the detection of incompetent perforators on Doppler Duplex Scanning is helpful in the diagnosis. The vascular diagnostic work up will always start with pedal pulse palpation, which might be difficult in obese patients, those with leg edema, and in patients with concomitant diabetes having extensive vascular calcification.186 184 Diagnostic tests for ulcers due to arterial insufficiency include continuous wave Doppler (CWD), ankle brachial pressure index (ABI), and transcutaneous oximetry (TcPO2). In patients with diabetes the Doppler probe must be used according to systolic toe pressure (TP) and toe brachial index (TBI).187 The results and, particularly, the interpretation of those tests will guide the medical and surgical management and the possible complications. The ABI includes the measurement of the Doppler signal, by dividing the ankle systolic blood pressure over the brachial systolic blood pressure; a value of 1.0 or 1.1 is considered normal, while a ratio of 0.8 or less is abnormal. However, because of calcification in blood vessels, the ABI is unreliable in the assessment of diabetic ulcers. Palpation of the pedal pulses is a subjective test for the detection and intensity of the pulse of the dorsalis pedis and posterior tibialis arteries; the detection depends on the clinician’s skills and experience. The use of a Doppler probe for CWD test helps by recognizing the arterial signal with either tri-phasic or bi-phasic sounds in normal patients, and monophasic or absent sounds in peripheral arterial disease. Measurements of TcPO2 are time consuming, and they are most valid for diabetic foot assessment, where a value of less than 40mm Hg is related to poor oxygen perfusion and may even be used to determine the level of eventual amputation.188 Pressure (decubitus) ulcer assessment is mainly used to understand the true wound size and depth, and has evolved from pure clinical evaluation to imaging techniques in more difficult cases.189 The main problem with pressure ulcers assessment is to completely assess the depth and size, as these wounds typically have undermined edges; there are several 2D and 3D assessment options ranging from high frequency ultrasound to laser scanner imaging or optical coherence tomography.190
The role of the bacterial swab in chronic wound management is now clear; it should be considered only if clinical signs of infection are present.191 Symptoms and signs of wound infection are more critical in diabetic foot and pressure ulcers compared to VLU, and they present with the following clues: increased exudate and foul odor from the wound, increased wound pain, localized swelling, increased heat of the surrounding skin, friable and hyper-granulating wound bed, and increased erythema and cellulitis. Increasing evidence suggests that biofilm formation and persistence plays an important role in chronic wound formation, chronicity, and possibly recurrence.
Wound biopsy
When wounds fail to heal in spite of appropriate therapeutic management, it is important to perform a wound biopsy, which is essential in confirming the clinical wound diagnosis, to understand the failure to heal and respond to treatment, and for ruling out neoplasia. The biopsy should be performed at the wound edge, and should include a portion of the surrounding skin.192 It has been shown that, peculiarly, the biopsy site will heal faster than the actual wound bed tissue.193 The wound biopsy is also helpful in the diagnostic work up in atypical wounds, which include inflammatory wounds, such as vasculitis and those wounds due occlusion of microvascular channels from cryoglobulins and cryofibrinogens.194 The latter ulcers due to cryoproteins are surrounded by a small blood vessels of linear hyperpigmentation often termed “micro livedo”.194 In pyoderma gangrenosum, a diagnosis of exclusion, the biopsy is going to exclude other vasculopathies, including calciphylaxis.195
Biomarkers
TNF-alpha assessment in pyoderma gangrenosum and in non-healing VLU has shown a good correlation with the prognosis of specific groups of patients.196,197 TNF alpha was found to be elevated in non-healing VLU, and, when elevated, its therapeutic use has proved helpful in chronic wounds.196 Osteopontin is another biomarker used to detect the presence of calciphylaxis.198 The level of specific metalloproteinases have shown promising results in non-healing chronic wounds.199 The elevated level of matrix metalloproteinases in wound fluid from non-healing wounds has been correlated with reduced healing rates.200
Prevention
Various guidelines and recommendations have been proposed for the primary and secondary prevention of chronic wounds.201 An increasingly recognized important component of prevention and ulcer recurrence is that, in the absence of proper and continued guidance and teaching, patients with chronic wounds are generally not able to follow effective self-management strategies. Prevention of VLU is mainly using compression leg bandages and stockings. The continued use of compression stockings will help reduce the rate of recurrence. Modern textile materials have helped in increasing compliance (adherence) from patients and practitioners. Control of leg edema is best achieved using stockings and leg elevation. A United Kingdom initiative called Lindsay Leg Club has helped in reinforcing compliance/adherence. Prevention of diabetic foot ulcers is very complex and demanding and is best achieved using a multidisciplinary foot care unit. The risk of developing foot ulcers has been stratified into low, moderate, and high risk according to the extent of neuropathy and limb ischemia.202 Assessment of foot biomechanics and sequential use of orthoses, together with constant follow up, have shown to reduce the risk of foot ulceration in patients with diabetes.203 Prevention of pressure (decubitus) pressure ulcers is challenging but essential, and is initially achieved by using a risk assessment scale The Braden scale is widely used,184,204 but several assessment scales have been implemented according to different categories of patients at risk. The approach in patients with pressure ulcers is to minimize pressure, shear, and moisture by repositioning the patient with scheduled protocols, and by avoiding contact of the chronic wound with urine, feces, and other fluids. In patients simply not able to receive proper repositioning the use of multi-layered silicone dressings in areas at risk has shown to decrease the incidence of pressure ulcers.205 There is a need to more actively screen certain populations at risk for chronic wound development. Finally, it should be noted that the chronic wounds described in this discussion are more applicable to first world “developed” countries, and less so in less developed countries, where lifespan is decreased, tropical and infectious diseases are more common, and medical and laboratory assessment less available. It should be noted here that a more proactive approach is needed in identifying populations at risk for different types of ulcerations. Such an approach would help in determining how we can best prevent ulcerations from occurring in vulnerable groups.
MANAGEMENT
Scale of the clinical problem
The extent and cost of treating wound problems in modern day practice is not completely known but recent publications would suggest that chronic wounds may affect up to 5% of the adult population and consume up to 10% of health care expenditure. It has also been shown that the number of patients with wounds increased by 70% with an increase in cost of care of 45% over a 5-year period.206–208 In the past, clinicians may have been slow to recognize the nature and extent of the challenge of this problem, and there was a general lack of appreciation of the need to develop a system of optimal clinical delivery and care that takes advantage of emerging treatments and technologies. This situation is compounded by the fact that “precision medicine” is still in its infancy when it comes to chronic wounds; better molecular diagnostic and therapeutic tools are needed to focus on individual patients.
Despite the above considerations, the management of chronic wounds has improved dramatically in the last 2–3 decades. The term chronic wound is often used but there are several definitions of chronic wounds that can result in an inability to compare results of an intervention from different studies.4,209,210 Tables 2 illustrates therapeutic options to be considered, including dressings, devices, drugs, and surgical and biological approaches.120,211–214 For the 3 main types of chronics wounds, Table 3 illustrates which therapies might be considered to treat specific wound types if a comprehensive treatment were to be provided. Figure 5 is an algorithm approach for how to address different interventions for treatment. It is important to recognize that basic clinical approaches also rely on proper surgical management. In addition to vascular reconstruction, grafting, and limb salvage, debridement is critical for removing necrotic tissue. Indeed, what had been termed “maintenance debridement” may be required to keep the process of healing going in the right direction.
Table 2.
Standard Treatments of Chronic Wounds
| Types of Chronic Wounds | ||
|---|---|---|
| Venous Leg Ulceration | Diabetic Foot Disease | Pressure Injury |
| Compression | Off-loading device | Pressure relieving mattress |
| Vascular reconstruction | Padding and protection | Incontinence management |
| Endovascular stenting | Vascular/endovascular intervention | Nutritional support |
| Superficial vein ablation | Oral/IV antibiotics | Mobility maximized |
| Steroids topical/systemic | Debridement | Adaptions to home |
| Cytotoxic/immunosuppressive drugs | Amputation | Adapted wheelchairs |
| Skin grafting/flap surgery | Metabolic control | Surgery |
Table 3.
Spectrum of Standard Therapeutic Options for Chronic Wounds
| Therapeutic Approaches | ||||
|---|---|---|---|---|
| Wound Dressings | External Devices | Drugs/Pharmaceuticals | Surgery | Biological Approaches |
| Low adherent | Negative pressure | Antibiotics | Debridement | Protease modulating agents |
| Alginates | Electrical stimulation | Anti -inflammatory agents | Revascularization | Platelet rich plasma |
| Hydrogels | Off-loading foot devices | Vasodilators | Bone resection | Recombinant growth factors |
| Hydrocolloids | Specialized mattresses | Corticosteroids | Skin graft | Tissue engineering constructs (acellular or cellular; living or non-living cells) |
| Foams | Biofilm disruptors | Immunosuppressants | Flap surgery | Extracellular matrix replacement |
| Antimicrobial | Phototherapy | Topical stimulatory agents | Amputation | Stem cells |
Evidence-based approach for treatment selection
The Cochrane Wounds Database details many useful reviews and provides valuable insights: https://wounds.cochrane.org/news/reviews. The available evidence shows the paucity of currently available data on which to draw conclusions, including studies focused on dressings, devices, drugs, surgical and biological approaches. The inability to draw definite conclusions is multifactorial, and it may have to do with poor statistical power analysis when designing clinical research trials, among other clinical and treatment factors. At the present time several recommendations are still based on expert opinion and clinical guidelines.
The stimulus for establishing chronic wounds management guidelines came largely from an emphasis on wound bed preparation (WBP), which has continued up to date.120,211–214 There are basic approaches to the treatment of chronic wounds that are rooted in both experience and evidence: compression for venous ulcers, off-loading for diabetic neuropathic ulcers and pressure ulcers, debridement of necrotic tissue, stenting and revascularization to restore blood flow and oxygen, hyperbaric oxygen for chronic wounds unresponsive to treatment. We now have better wound dressings than we had even 10–20 years ago, and which are based on the recognition that moist wound healing can help chronic wounds as well, or at least the formation of granulation tissue. We like to use film wound dressings for less exudative wounds, foams to absorb excessive exudate, hydrocolloids to initiate debridement, alginates to provide moisture to very dry wound beds. Composite of these dressings and variations have been developed, as for example the delivery of antiseptics, antimicrobials from the dressings.215 For standard and basic therapies, certain considerations are in order.
Diabetic foot ulceration
Diabetic foot ulceration is a complex, common, and challenging problem.210,216–218 It consumes around 25% of total cost of managing all complications of diabetes including diabetic eye, heart, and renal disease. The major underlying reasons for the development of diabetic foot ulcers are peripheral nerve damage, alterations in vascular flow and/or blood vessel physiology may be present and be associated with increased risk of infection.219
Venous Disease
For patients with venous ulceration the cornerstone of standard treatment remains lower leg compression. Much research has centered around the level of sub bandage pressure that needs to be applied and how to achieve it with bandages or stockings. A systemic agents that acts in part through vasodilation or effect on white blood cells was finally shown to enhance the healing of venous ulcers when given at double the dose that was previously tested in controlled randomized trials.220 This finding was later confirmed by a meta-analysis.221
Pressure injury
Pressure ulcers or injuries remain a common and expensive problem.222 Much effort has been spent on developing and evaluating pressure relieving properties in mattress and cushion material. However, many factors in individual patients including incontinence, nutrition, mobility pain level, co-morbidities, environment and skill level of staff caring for a patient the impact of intervention on both the development and resolution of pressure injury. There are also special subgroups of patients at increased risk of developing pressure-induced ulcers including spinal cord injury, congenital or progressive neurological diseases, and individuals who are elderly and mentally infirm. It is likely that varied factors are operating in individual patients who develop these wounds. At this time, it is unknown how biological differences can explain different outcomes in subgroups or individual patients.223
Biologically-based treatments
Despite the difficulties in identifying and studying specific treatments, over the last 2–3 decades enormous progress has been made in advanced therapeutic approaches that could potentially benefit chronic wounds. One of the first such targets was the use of topical growth factors that are important in the healing cascade. 37,224 The first topically applied recombinant growth factor approved by a regulatory agency (FDA) for the treatment of diabetic neuropathic foot ulcers was PDGF.37,225–229 The topical PDGF approval also helped to highlight the importance of wound debridement. 225,230 A review of the present situation with the topical application of growth factors, including PDGF, suggests that there are limitations to this type of therapy. 231
Following and in fact around the same time as interest was focused on growth factors, there was a great deal of activity in tissue engineering to treat chronic wounds. Here the ability to grow epidermal and dermal cells in the laboratory were felt to be another way to enhance healing.232–235 The use of this “artificial skin” or bioengineered skin (cellular or cellular) has led to several publications and, in some cases, regulatory approval for the treatment of venous and diabetic foot ulcers, as well as mechanisms of action.186,232,233,236–239 Important and interesting take-away points from some of these clinical research studies and laboratory research are the following: bioengineered skin constructs (both cellular and acellular) can accelerate the healing/closure of venous and diabetic foot ulcers; the percentage of improved healing over control treatment is no more than about 20%, and that the constructs are short-lived within the wound, thus not acting as a skin replacement but rather as a stimulus for wound repair. A peculiar and interesting consideration is that many of these bioengineered constructs have been approved and marketed with a name ending in “graft”. This decision has actually led to disappointment by clinicians, especially surgeons, who view “grafts” a more permanent solution to wound resurfacing. Moreover, repeated treatments with these advanced therapies may be required, thus diminishing their cost effectiveness. Another important consideration is that such advanced therapies are for the most part not available to less privileged patients and those in third world countries.
Despite these setbacks in advanced approaches progress being made in the more mechanistic aspects of wound healing Is likely to lead to improved and more cost-effective treatment. It could still be stated that expensive ore focus on a technology that might have theoretical benefits in assisting healing rather than identifying the aberrations that are present in an individual patient and which are preventing healing.240
In addition to biological factors influencing healing, it is increasingly recognized that social and psychological factors can influence outcome.241 An important example of the challenge of research is the wounds seen in patients suffering from terminal disease from other comorbidities. It is clearly inappropriate to expect full wound closure in that setting. However, it is both proper and desirable to evaluate interventions that influence pain, smell, discharge, and patients Health Related Quality of Life by measures other than complete wound closure or speed of healing.242
QUALITY OF LIFE
Ways in which quality of life is altered by chronic wounds
Chronic wounds have a negative effect on health-related quality of life on account of the physical injury, the treatment required, the chronicity of the condition and the likelihood of recurrence. Over two decades ago, research conducted by Franks and Moffat243 indicated that leg ulcer patients experienced significantly poorer quality of life than matched controls in the areas of emotional, social and physical health. Today it is well established that chronic wounds are painful and affect physical role and function,244 can cause mental health concerns245 and may limit social and workforce participation.246 Chronic wounds can present a financial burden to those who must self-fund their treatment.247 Wound treatment and prevention have improved in recent decades. However, mitigation of the negative effect of chronic wounds on quality of life remains an unresolved challenge. Box 1 illustrates the many facets of quality of life, and how patients are affected. There are three main domains that are altered in patients with chronic wounds: physical, emotional, and social. For each domain, Box 1 lists the main facets/components of one’s existence that are altered, often in a fundamental way. It is important to recognize that these domains overlap, and the consequences of each are interrelated. In many ways, Box 1 exposes the need for what, ideally, should be a multidisciplinary approach to patients with chronic wounds; it is extremely challenging to address all the listed needs.
Box 1. Quality of life domains affected in patients with chronic wounds.
Physical domain
Physical deformity
Wound pain, odor, exudate
Reduced mobility
Suboptimal sleep
Impaired activities of daily living
Feeling unwell from side effects of treatment modalities
Worsening of comorbidities
Emotional domain
Stress, anxiety, depression
Frustration, worrying, helplessness
Dissatisfaction with health care givers
Fear of trauma to the wound, recurrence
Stigma and worsening self-esteem
Lack of life enjoyment
Social domain
Withdrawal from family and friends
Loss of independence
Altered sexual
Difficulty with domestic duties, hobbies, recreation
Lack of acceptable clothing and footwear
Difficulty with work, studying, shopping
Time required for wound treatment, medical appointments
Measuring quality of life
The introduction of patient reported outcomes and patient centered care has required healthcare providers to identify and respond to what is most important to the patient. It is now common for research to measure quality of life, for example using generic instruments such as the SF 36248 and the EQ-5D.249 Disease specific measures include the Cardiff Wound Impact Schedule250 and the Diabetic Foot Scale251 which are clinically useful and responsive as they include items that are specific to the condition of interest. Quality of life is often a secondary outcome measure and research that investigates quality of life as the primary purpose of the study should be conducted if quality of life improvement is to be realized by people who have chronic wounds.
Management of the wound and care of the patient
Quality of life must be assessed early in the care episode so that needs are identified and interventions can be targeted. Interdisciplinary team work is necessary252 and a genuine partnership with the person who has the wound is essential. Traditional models of treatment and care (provided in the doctor’s clinic and in the home by nurses) have evolved to better meet the holistic need of patients. Advancements include specialist wound clinics that bring together multidisciplinary expertise,252 community based interventions such as Leg Clubs which offer opportunity for social support253 and self-management approaches to optimize the persons involvement in their care.254 Engagement with informal care-givers who support those affected by chronic wounds has also been recognized and their participation in wound care may enable improved quality of life.255
Co-morbidities and chronic wounds
Chronic wounds are a symptom, manifestation or consequence of the health conditions that cause the greatest disease burden in society (for example vascular disease and diabetes) therefore chronic wounds should be considered a priority at the health policy level.256 People who experience chronic wounds often present with multiple co-morbidities257 and therefore many inter-related factors that may affect their quality of life. Our understanding of quality of life is, in the most part, informed by research conducted with the patients who are the easiest to access but not necessarily the most vulnerable. Future research should target people who experience less prevalent wounds, who live in developing countries, who are from diverse backgrounds, who experience cognitive impairment and who are at end of life.
OUTLOOK
Considerable progress has been made in our understanding of the healing process of the skin and the impaired healing that characterizes chronic wounds, and we have discussed the epidemiology and the adverse effects on quality of life. Still, many opportunities exist for improving the outlook in this challenging field.
Stem cells
Although all tissues, including skin, have resident stem cells, bone marrow mesenchymal stem cells (BM MSC) have been the most widely investigated for their potential to accelerate and/or improve aspects of wound healing. In vitro and in vivo studies demonstrate that MSC exhibit multiple pro-reparative functions, including the ability to migrate to sites of injury, stimulation of proliferation of wound resident cells, and wound angiogenesis; these effects take place via secretion of growth factors and cytokines, suppression of inflammation, and generation of anti-microbial peptides.258–260 Additionally, MSC are easily harvested and cultured, and autologous or allogenic MSC can be administered without much risk of immune rejection. Adipose tissue is another easily accessible source for stem cells having some multipotent properties like bone marrow-derived MSC. 261,262 Tissue resident stem cells, such as dermal or epidermal stem cells, have also been proposed as therapeutic mediators of repair.263,264 but require autologous sources to prevent immune rejection, a factor that limits their availability. Other sources of stem cells that have been less fully investigated for use in impaired healing are derived from umbilical cord or Wharton’s jelly.265 An emerging concept had been termed “priming” of advanced therapeutic agents.266 Several lines of investigations support this concept. The pro-reparative efficacy of stem cells may be amplified by conditioning by hypoxia, TGF-β1, or other drugs, or by lentiviral transduction of MSC for VEGF expression.266–269 This priming approach resembles the one proposed for bioengineered skin before its clinical use in chronic wounds.266 Additional strategies to improve therapeutic efficacy include co-administration with other cell types, such as fibroblasts, or modulation of the co-administered extracellular matrix ‘niche’.268,270,271 A promising study was focused on well characterized cultured autologous bone marrow-derived MSC delivered to hard-to-heal wounds in a fibrin spray.272 Subsequent trials in chronic wounds treated with autologous BM MSC have reported improvement in healing.273,274 Although promising, MSC therapy has limitations that include the very few clinical trials from which to draw conclusive evidence, and the lack of uniform protocols for clinical administration. Ongoing work will address these issues, as in a preliminary recently published controlled and prospective randomized clinical trial.275
Exosomes
One approach to standardization of cellular therapy may be through the use of MSC-generated exosomes that have been shown to have similar pro-reparative functions as their cellular parents, primarily in animal models of wound repair and in vitro. Their cargo of miRNAs, Wnt ligands, growth factors, cytokines and signaling lipids provide the mechanism for paracrine stimulation of wound resident cells.276–279 Exosomes cargo has also been demonstrated to orchestrate cell migration, including that of murine dermal fibroblasts.280 Numerous preclinical studies, using many variations of cellular sources of exosomes, have shown improvement in healing.281 The lack of standardization precludes identification of the optimal exosome approach, and will undoubtedly be remedied. The limitations for therapeutic use may also lie with the large numbers of cells required to generate quantities sufficient to effect pro-reparative functions, and the variable cargo contents dependent on parental cell strain.277
Unifying concept of wound bed preparation
This concept of wound bed preparation (WBP) was first proposed to provide diagnostic/prognostic considerations, a framework for unifying mechanisms of tissue repair, and for maximizing available and future treatments. The concept of WBP also recognized the need to address biochemical and molecular factors. 211 This all-encompassing concept was later refined to better address biochemical and therapeutic considerations.212–214 Figure 6 shows a proposed scoring system for WBP (VF, 2021), addressing certain parameters to address and improve.
Figure 6:
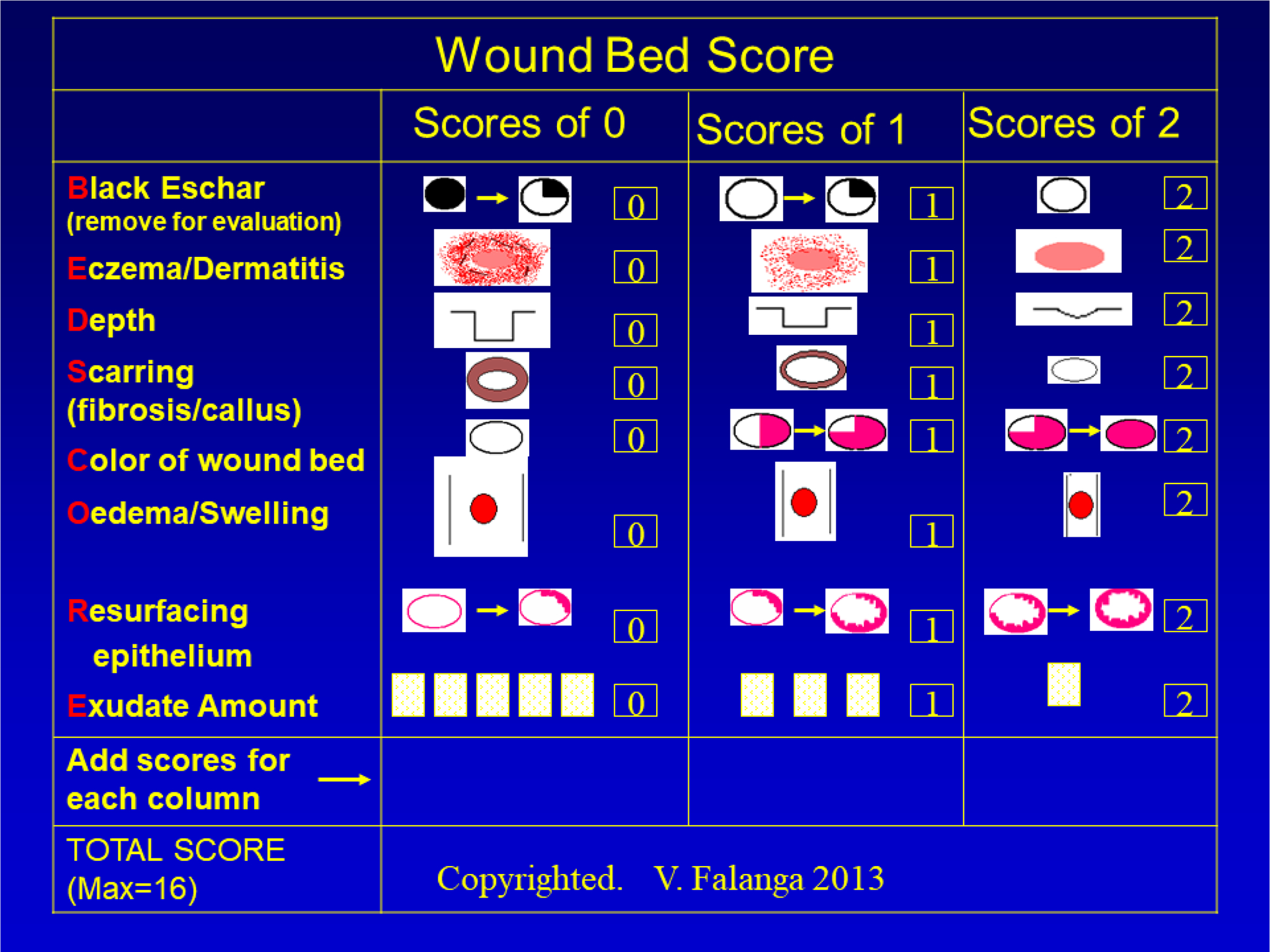
Representation of some critical clinical components of wound bed preparation, with a scoring system (wound bed score or WBS) that may be useful for assessment, follow up, and for decision making in the use of therapeutic agents.
Final comments
Despite established progress and emerging technologies, certain obstacles remain quite challenging; these obstacles are also fertile ground for research. The finite speed at which keratinocytes are capable of migrating from the edge and resurface the wound is a perplexing phenomenon.282,283 These studies suggest the existence of what has been called “keratinocyte speed limit”.37,284 Indeed, a rate of healing (advancement of keratinocytes from the edge towards the wound’s edge) of less than 0.75 mm/week is associated with a poor diagnosis and failure to heal. Conversely, we have not been able to demonstrate a rate greater than 1.4–1.5 mm/week even in healing wounds.284 Another major challenge is that chronic wounds have anatomical and physiological abnormalities that cannot be adequately corrected with present knowledge and approaches. Regrettably, at least until a so-called quantum jump in our capabilities is achieved, we might need to attenuate our ideal focus on tissue regeneration. Certain realities must be recognized. As we evolved into more complex organisms, we lost the ability to heal by a regenerative process. Instead, we developed mechanisms to stop bleeding after injury and achieve closure of acute wounds rapidly, even if that meant reliance on scarring. That evolutionary approach served us well as long as our lifespan was limited. However, with increasing aging, we developed disease processes (diabetes, circulatory problems, pressure injury, etc.) that evolution simply did not prepare us for. Yet another possible reality, supported by early experimental evidence, is that chronic wounds may not be as dormant as we think; additional stimulation may actually increase their energy requirements. 285 Certain approaches deserve additional consideration. We may also have to explore ways to render cellular components and extracellular matrix more resistant to the effects of pressure and ischemia. Therefore, although much of our focus has been to find new ways to stimulate and accelerate the healing process, opposite and unusual paradigm shifts may be required from the laboratory and clinical standpoints.
Footnotes
Competing interests:
The authors declare no competing interests.
REFERENCES
- 1.Janowska A, Dini V, Oranges T, Iannone M, Loggini B, Romanelli M. Atypical Ulcers: Diagnosis and Management. Clin Interv Aging 2019;14:2137–2143. DOI: 10.2147/CIA.S231896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 2015;4(9):560–582. DOI: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen CK. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care (New Rochelle) 2021;10(5):281–292. DOI: 10.1089/wound.2021.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130(4):489–93. (https://www.ncbi.nlm.nih.gov/pubmed/8166487). [PubMed] [Google Scholar]
- 5.Lim HW, Collins SAB, Resneck JS Jr., et al. The burden of skin disease in the United States. J Am Acad Dermatol 2017;76(5):958–972 e2. DOI: 10.1016/j.jaad.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Hall J, Buckley HL, Lamb KA, et al. Point prevalence of complex wounds in a defined United Kingdom population. Wound Repair Regen 2014;22(6):694–700. DOI: 10.1111/wrr.12230. [DOI] [PubMed] [Google Scholar]
- 7.Margolis DJ, Bilker W, Knauss J, Baumgarten M, Strom BL. The incidence and prevalence of pressure ulcers among elderly patients in general medical practice. Ann Epidemiol 2002;12(5):321–5. DOI: 10.1016/s1047-2797(01)00255-1. [DOI] [PubMed] [Google Scholar]
- 8.Margolis DJ, Hoffstad O, Nafash J, et al. Location, location, location: geographic clustering of lower-extremity amputation among Medicare beneficiaries with diabetes. Diabetes Care 2011;34(11):2363–7. DOI: 10.2337/dc11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis DJ, Jeffcoate W. Epidemiology of foot ulceration and amputation: can global variation be explained? Med Clin North Am 2013;97(5):791–805. DOI: 10.1016/j.mcna.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher J Measuring the prevalence and incidence of chronic wounds. Prof Nurse 2003;18(7):384–8. (https://www.ncbi.nlm.nih.gov/pubmed/12674045). [PubMed] [Google Scholar]
- 11.Mervis JS, Phillips TJ. Pressure ulcers: Prevention and management. J Am Acad Dermatol 2019;81(4):893–902. DOI: 10.1016/j.jaad.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 12.Courvoisier DS, Righi L, Bene N, Rae AC, Chopard P. Variation in pressure ulcer prevalence and prevention in nursing homes: A multicenter study. Appl Nurs Res 2018;42:45–50. DOI: 10.1016/j.apnr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Anthony D, Alosoumi D, Safari R. Prevalence of pressure ulcers in long-term care: a global review. J Wound Care 2019;28(11):702–709. DOI: 10.12968/jowc.2019.28.11.702. [DOI] [PubMed] [Google Scholar]
- 14.Lopes TS, Videira L, Saraiva D, Agostinho ES, Bandarra AJF. Multicentre study of pressure ulcer point prevalence in a Portuguese region. J Tissue Viability 2020;29(1):12–18. DOI: 10.1016/j.jtv.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Herrera MA, Martinez-Riera JR, Verdu-Soriano J, et al. Multicentre Study of Chronic Wounds Point Prevalence in Primary Health Care in the Southern Metropolitan Area of Barcelona. J Clin Med 2021;10(4). DOI: 10.3390/jcm10040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei M, Yang D, Chen L, et al. The prevalence and prevention of pressure ulcers: A multicenter study of nine nursing homes in eastern China. J Tissue Viability 2021;30(1):133–136. DOI: 10.1016/j.jtv.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Baumgarten M, Margolis D, Berlin JA, et al. Risk factors for pressure ulcers among elderly hip fracture patients. Wound Repair Regen 2003;11(2):96–103. DOI: 10.1046/j.1524-475x.2003.11204.x. [DOI] [PubMed] [Google Scholar]
- 18.Baumgarten M, Margolis DJ, Localio AR, et al. Extrinsic risk factors for pressure ulcers early in the hospital stay: a nested case-control study. J Gerontol A Biol Sci Med Sci 2008;63(4):408–13. DOI: 10.1093/gerona/63.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid 2016;2016 (https://www.ncbi.nlm.nih.gov/pubmed/26771825). [PMC free article] [PubMed]
- 20.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46(3):381–6. DOI: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 21.Homs-Romero E, Romero-Collado A, Verdu J, Blanch J, Rascon-Hernan C, Marti-Lluch R. Validity of Chronic Venous Disease Diagnoses and Epidemiology Using Validated Electronic Health Records From Primary Care: A Real-World Data Analysis. J Nurs Scholarsh 2021;53(3):296–305. DOI: 10.1111/jnu.12639. [DOI] [PubMed] [Google Scholar]
- 22.Forssgren A, Fransson I, Nelzen O. Leg ulcer point prevalence can be decreased by broad-scale intervention: a follow-up cross-sectional study of a defined geographical population. Acta Derm Venereol 2008;88(3):252–6. DOI: 10.2340/00015555-0433. [DOI] [PubMed] [Google Scholar]
- 23.Berenguer Perez M, Lopez-Casanova P, Sarabia Lavin R, Gonzalez de la Torre H, Verdu-Soriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre-A time series study (2010–2014). Int Wound J 2019;16(1):256–265. DOI: 10.1111/iwj.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33(11):1493–1498. DOI: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 25.Hoffstad O, Mitra N, Walsh J, Margolis DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care 2015;38(10):1852–7. DOI: 10.2337/dc15-0536. [DOI] [PubMed] [Google Scholar]
- 26.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 2008;31(7):1331–6. DOI: 10.2337/dc07-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyer K, Herberger K, Protz K, Glaeske G, Augustin M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen 2016;24(2):434–42. DOI: 10.1111/wrr.12387. [DOI] [PubMed] [Google Scholar]
- 28.Graves N How costs change with infection prevention efforts. Curr Opin Infect Dis 2014;27(4):390–3. DOI: 10.1097/QCO.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 29.Malay DS, Margolis DJ, Hoffstad OJ, Bellamy S. The incidence and risks of failure to heal after lower extremity amputation for the treatment of diabetic neuropathic foot ulcer. J Foot Ankle Surg 2006;45(6):366–74. DOI: 10.1053/j.jfas.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.margolis D, Malay DS, Hoffstad OJ, et al. Prevelance of diabetes, diabetic foot ulcer, and lower extremity amputation among Medicare beneficiaries, 2006–2008, Rockville, MD. Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 31.Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 2012;55(7):1919–25. DOI: 10.1007/s00125-012-2468-6. [DOI] [PubMed] [Google Scholar]
- 32.Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15. DOI: 10.1016/j.annepidem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26(6):1696–700. DOI: 10.2337/diacare.26.6.1696. [DOI] [PubMed] [Google Scholar]
- 34.Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol 2000;136(12):1531–5. DOI: 10.1001/archderm.136.12.1531. [DOI] [PubMed] [Google Scholar]
- 35.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med 2005;22(2):172–6. DOI: 10.1111/j.1464-5491.2004.01375.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev 2019;99(1):665–706. DOI: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falanga V Wound healing and its impairment in the diabetic foot. Lancet 2005;366(9498):1736–43. DOI: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 38.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6(265):265sr6. DOI: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zindle JK, Wolinsky E, Bogie KM. A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health. J Tissue Viability 2021;30(3):291–300. DOI: 10.1016/j.jtv.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Falanga V, Schrayer D, Cha J, et al. Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen 2004;12(3):320–6. DOI: 10.1111/j.1067-1927.2004.012316.x. [DOI] [PubMed] [Google Scholar]
- 41.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Disease models & mechanisms 2014;7(11):1205–13. (In eng). DOI: 10.1242/dmm.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grada A, Mervis J, Falanga V. Research Techniques Made Simple: Animal Models of Wound Healing. J Invest Dermatol 2018;138(10):2095–2105 e1. DOI: 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Elliot S, Wikramanayake TC, Jozic I, Tomic-Canic M. A Modeling Conundrum: Murine Models for Cutaneous Wound Healing. J Invest Dermatol 2018;138(4):736–740. DOI: 10.1016/j.jid.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Ahn ST, Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg 1990;24(1):17–23. DOI: 10.1097/00000637-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Buck DW 2nd, Jin DP, Geringer M, Hong SJ, Galiano RD, Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg 2011;128(5):427e–437e. DOI: 10.1097/PRS.0b013e31822b7333. [DOI] [PubMed] [Google Scholar]
- 46.Davis SC, Mertz PM. Determining the effect of an oak bark formulation on methicillin-resistant staphylococcus aureus and wound healing in porcine wound models. Ostomy Wound Manage 2008;54(10):16–8, 20, 22–5. (https://www.ncbi.nlm.nih.gov/pubmed/18927480). [PubMed] [Google Scholar]
- 47.Dhall S, Do DC, Garcia M, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:562625. DOI: 10.1155/2014/562625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eaglstein WH, Mertz PM. New methods for assessing epidermal wound healing: the effects of triamcinolone acetonide and polyethelene film occlusion. J Invest Dermatol 1978;71(6):382–4. DOI: 10.1111/1523-1747.ep12556814. [DOI] [PubMed] [Google Scholar]
- 49.Fadini GP, Menegazzo L, Rigato M, et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes 2016;65(4):1061–71. DOI: 10.2337/db15-0863. [DOI] [PubMed] [Google Scholar]
- 50.Olson HM, Nechiporuk AV. Using Zebrafish to Study Collective Cell Migration in Development and Disease. Front Cell Dev Biol 2018;6:83. DOI: 10.3389/fcell.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J 2015;56(1):127–38. DOI: 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- 52.Arenas Gomez CM, Sabin KZ, Echeverri K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Dev Dyn 2020;249(7):834–846. DOI: 10.1002/dvdy.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Adv Wound Care (New Rochelle) 2016;5(2):65–78. DOI: 10.1089/wound.2014.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weavers H, Martin P. The cell biology of inflammation: From common traits to remarkable immunological adaptations. J Cell Biol 2020;219(7). DOI: 10.1083/jcb.202004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen AV, Soulika AM. The Dynamics of the Skin’s Immune System. International journal of molecular sciences 2019;20(8). DOI: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009;9(10):679–91. DOI: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140(6):805–20. DOI: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 58.Dasu MR, Isseroff RR. Toll-like receptors in wound healing: location, accessibility, and timing. J Invest Dermatol 2012;132(8):1955–8. DOI: 10.1038/jid.2012.208. [DOI] [PubMed] [Google Scholar]
- 59.Kirchner S, Lei V, MacLeod AS. The Cutaneous Wound Innate Immunological Microenvironment. Int J Mol Sci 2020;21(22). DOI: 10.3390/ijms21228748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilgus TA. Alerting the body to tissue injury: The role of alarmins and DAMPs in cutaneous wound healing. Curr Pathobiol Rep 2018;6(1):55–60. DOI: 10.1007/s40139-018-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson AM, Reddy SK, Ratliff TS, et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 2015;17(2):139–51. DOI: 10.1016/j.stem.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borkowski AW, Park K, Uchida Y, Gallo RL. Activation of TLR3 in keratinocytes increases expression of genes involved in formation of the epidermis, lipid accumulation, and epidermal organelles. J Invest Dermatol 2013;133(8):2031–40. DOI: 10.1038/jid.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol 2016;25(3):167–73. DOI: 10.1111/exd.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strbo N, Yin N, Stojadinovic O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv Wound Care (New Rochelle) 2014;3(7):492–501. DOI: 10.1089/wound.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gronberg A, Mahlapuu M, Stahle M, Whately-Smith C, Rollman O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2014;22(5):613–21. DOI: 10.1111/wrr.12211. [DOI] [PubMed] [Google Scholar]
- 66.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 2003;120(3):379–89. DOI: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 67.Shook BA, Wasko RR, Rivera-Gonzalez GC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018;362(6417). DOI: 10.1126/science.aar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plikus MV, Guerrero-Juarez CF, Ito M, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017;355(6326):748–752. DOI: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li D, Cheng S, Pei Y, et al. Single-Cell Analysis Reveals Major Histocompatibility Complex IIExpressing Keratinocytes in Pressure Ulcers with Worse Healing Outcomes. The Journal of investigative dermatology 2022;142(3 Pt A):705–716. DOI: 10.1016/j.jid.2021.07.176. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol 2017;18(10):1068–1075. DOI: 10.1038/ni.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joshi N, Pohlmeier L, Ben-Yehuda Greenwald M, et al. Comprehensive characterization of myeloid cells during wound healing in healthy and healing-impaired diabetic mice. Eur J Immunol 2020;50(9):1335–1349. DOI: 10.1002/eji.201948438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res 2013;57(1–3):222–8. DOI: 10.1007/s12026-013-8474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajesh A, Stuart G, Real N, et al. Depletion of langerin(+) cells enhances cutaneous wound healing. Immunology 2020;160(4):366–381. DOI: 10.1111/imm.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol 2010;184(10):5423–8. DOI: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacLeod AS, Hemmers S, Garijo O, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest 2013;123(10):4364–74. DOI: 10.1172/JCI70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science 2002;296(5568):747–9. DOI: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Wang Y, Zhou L, et al. Vgamma4 T Cells Inhibit the Pro-healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17A. Front Immunol 2018;9:240. DOI: 10.3389/fimmu.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor KR, Mills RE, Costanzo AE, Jameson JM. Gammadelta T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFalpha in mouse models of obesity and metabolic disease. PLoS One 2010;5(7):e11422. DOI: 10.1371/journal.pone.0011422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathur AN, Zirak B, Boothby IC, et al. Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity 2019;50(3):655–667 e4. DOI: 10.1016/j.immuni.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nosbaum A, Prevel N, Truong HA, et al. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol 2016;196(5):2010–4. DOI: 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015;517(7534):293–301. DOI: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 82.Laurent P, Jolivel V, Manicki P, et al. Immune-Mediated Repair: A Matter of Plasticity. Front Immunol 2017;8:454. DOI: 10.3389/fimmu.2017.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 1989;140(2):396–402. DOI: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- 84.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. The Journal of clinical investigation 2019;129(7):2629–2639. DOI: 10.1172/JCI124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol 2019;40(7):635–647. DOI: 10.1016/j.it.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Wilgus TA, Roy S, McDaniel JC. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv Wound Care (New Rochelle) 2013;2(7):379–388. DOI: 10.1089/wound.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J Neutrophils in tissue injury and repair. Cell Tissue Res 2018;371(3):531–539. DOI: 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21(7):815–9. DOI: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pollenus E, Malengier-Devlies B, Vandermosten L, et al. Limitations of neutrophil depletion by anti-Ly6G antibodies in two heterogenic immunological models. Immunol Lett 2019;212:30–36. DOI: 10.1016/j.imlet.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knockout mice. The Journal of investigative dermatology 2000;115(2):234–44. DOI: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kienle K, Lammermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunological reviews 2016;273(1):76–93. DOI: 10.1111/imr.12458. [DOI] [PubMed] [Google Scholar]
- 92.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009;30(11):513–21. DOI: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Deniset JF, Kubes P. Recent advances in understanding neutrophils. F1000Res 2016;5:2912. DOI: 10.12688/f1000research.9691.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res 2010;339(2):437–48. DOI: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 95.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018;282(1):121–150. DOI: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tellechea A, Leal EC, Kafanas A, et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016;65(7):2006–19. DOI: 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilgus TA, Ud-Din S, Bayat A. A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. International journal of molecular sciences 2020;21(24). DOI: 10.3390/ijms21249673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ Res 2016;119(3):414–7. DOI: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol 2017;61:3–11. DOI: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Kratofil RM, Kubes P, Deniset JF. Monocyte Conversion During Inflammation and Injury. Arterioscler Thromb Vasc Biol 2017;37(1):35–42. DOI: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 101.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184(7):3964–77. (In eng). DOI: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 102.Shook B, Xiao E, Kumamoto Y, Iwasaki A, Horsley V. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. The Journal of investigative dermatology 2016;136(9):1885–1891. DOI: 10.1016/j.jid.2016.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pang J, Maienschein-Cline M, Koh TJ. Enhanced Proliferation of Ly6C(+) Monocytes/Macrophages Contributes to Chronic Inflammation in Skin Wounds of Diabetic Mice. J Immunol 2021;206(3):621–630. DOI: 10.4049/jimmunol.2000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 2019;97(3):258–267. DOI: 10.1111/imcb.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimball AS, Davis FM, denDekker A, et al. The Histone Methyltransferase Setdb2 Modulates Macrophage Phenotype and Uric Acid Production in Diabetic Wound Repair. Immunity 2019;51(2):258–271 e5. DOI: 10.1016/j.immuni.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aloysius A, Saxena S, Seifert AW. Metabolic regulation of innate immune cell phenotypes during wound repair and regeneration. Curr Opin Immunol 2021;68:72–82. DOI: 10.1016/j.coi.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017;356(6342):1026–1030. DOI: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 108.Jetten N, Roumans N, Gijbels MJ, et al. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PloS one 2014;9(7):e102994. DOI: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dreymueller D, Denecke B, Ludwig A, Jahnen-Dechent W. Embryonic stem cell-derived M2-like macrophages delay cutaneous wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2013;21(1):44–54. DOI: 10.1111/j.1524-475X.2012.00858.x. [DOI] [PubMed] [Google Scholar]
- 110.Theocharidis G, Thomas BE, Sarkar D, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nature communications 2022;13(1):181. DOI: 10.1038/s41467-021-27801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nature communications 2020;11(1):4678. DOI: 10.1038/s41467-020-18276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev 2017;122:74–83. DOI: 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative Expression of Proinflammatory and Antiinflammatory Genes Reveals Differences between Healing and Nonhealing Human Chronic Diabetic Foot Ulcers. The Journal of investigative dermatology 2015;135(6):1700–1703. DOI: 10.1038/jid.2015.30. [DOI] [PubMed] [Google Scholar]
- 114.Mascharak S, desJardins-Park HE, Davitt MF, et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021;372(6540). DOI: 10.1126/science.aba2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Januszyk M, Chen K, Henn D, et al. Characterization of Diabetic and Non-Diabetic Foot Ulcers Using Single-Cell RNA-Sequencing. Micromachines (Basel) 2020;11(9). DOI: 10.3390/mi11090815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, Waaijman T, Scheper RJ, Gibbs S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol 2012;132(1):216–25. DOI: 10.1038/jid.2011.245. [DOI] [PubMed] [Google Scholar]
- 117.Johnson BZ, Stevenson AW, Prele CM, Fear MW, Wood FM. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020;8(5). DOI: 10.3390/biomedicines8050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life (Basel) 2021;11(7). DOI: 10.3390/life11070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect 2013;19(2):107–12. DOI: 10.1111/j.1469-0691.2012.04001.x. [DOI] [PubMed] [Google Scholar]
- 120.Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017;25(5):744–757. DOI: 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 121.Granick M, Boykin J, Gamelli R, Schultz G, Tenenhaus M. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen 2006;14 Suppl 1:S1–10. DOI: 10.1111/j.1743-6109.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 122.Versey Z, da Cruz Nizer WS, Russell E, et al. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front Immunol 2021;12:648554. DOI: 10.3389/fimmu.2021.648554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ram M, Singh V, Kumawat S, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. European journal of pharmacology 2015;764:9–21. DOI: 10.1016/j.ejphar.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 124.Tchanque-Fossuo CN, Dahle SE, Buchman SR, Isseroff RR. Deferoxamine: potential novel topical therapeutic for chronic wounds. Br J Dermatol 2017;176(4):1056–1059. DOI: 10.1111/bjd.14956. [DOI] [PubMed] [Google Scholar]
- 125.Bonham CA, Kuehlmann B, Gurtner GC. Impaired Neovascularization in Aging. Advances in wound care 2020;9(3):111–126. DOI: 10.1089/wound.2018.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DeFrates KG, Franco D, Heber-Katz E, Messersmith PB. Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling. Biomaterials 2021;269:120646. DOI: 10.1016/j.biomaterials.2020.120646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Medicine. NUNLo. (https://www.clinicaltrials.gov/ct2/show/NCT03137966?term=NCT03137966&draw=2&rank=1). [Google Scholar]
- 128.Duscher D, Trotsyuk AA, Maan ZN, et al. Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds. J Control Release 2019;308:232–239. DOI: 10.1016/j.jconrel.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 129.NIH US Library of Medicine. (https://www.clinicaltrials.gov/ct2/show/NCT04058197?term=NCT04058197&draw=2&rank=1).
- 130.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136(2):215–33. DOI: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell 2011;8(3):294–308. DOI: 10.1016/j.stem.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hildebrand J, Rutze M, Walz N, et al. A comprehensive analysis of microRNA expression during human keratinocyte differentiation in vitro and in vivo. J Invest Dermatol 2011;131(1):20–9. DOI: 10.1038/jid.2010.268. [DOI] [PubMed] [Google Scholar]
- 133.Banerjee J, Sen CK. microRNA and Wound Healing. Adv Exp Med Biol 2015;888:291–305. DOI: 10.1007/978-3-319-22671-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li D, Wang A, Liu X, et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J Clin Invest 2015;125(8):3008–26. DOI: 10.1172/JCI79052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herter EK, Xu Landen N. Non-Coding RNAs: New Players in Skin Wound Healing. Adv Wound Care (New Rochelle) 2017;6(3):93–107. DOI: 10.1089/wound.2016.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mori R, Tanaka K, Shimokawa I. Identification and functional analysis of inflammation-related miRNAs in skin wound repair. Dev Growth Differ 2018;60(6):306–315. DOI: 10.1111/dgd.12542. [DOI] [PubMed] [Google Scholar]
- 137.Yang X, Wang J, Guo SL, et al. miR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int J Biol Sci 2011;7(5):685–90. DOI: 10.7150/ijbs.7.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li CX, Talele NP, Boo S, et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 2017;16(3):379–389. DOI: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 139.Pastar I, Khan AA, Stojadinovic O, et al. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem 2012;287(35):29324–35. DOI: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vanden Oever M, Muldoon D, Mathews W, McElmurry R, Tolar J. miR-29 Regulates Type VII Collagen in Recessive Dystrophic Epidermolysis Bullosa. J Invest Dermatol 2016;136(10):2013–2021. DOI: 10.1016/j.jid.2016.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suh EJ, Remillard MY, Legesse-Miller A, et al. A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts. Genome Biol 2012;13(12):R121. DOI: 10.1186/gb-2012-13-12-r121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li B, Zhou Y, Chen J, et al. Long noncoding RNA H19 acts as a miR-29b sponge to promote wound healing in diabetic foot ulcer. FASEB J 2021;35(1):e20526. DOI: 10.1096/fj.201900076RRRRR. [DOI] [PubMed] [Google Scholar]
- 143.Auler M, Bergmeier V, Georgieva VS, et al. miR-127–3p Is an Epigenetic Activator of Myofibroblast Senescence Situated within the MicroRNA-Enriched Dlk1-Dio3Imprinted Domain on Mouse Chromosome 12. J Invest Dermatol 2021;141(4S):1076–1086 e3. DOI: 10.1016/j.jid.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 144.Liu P, Zhu Y, Li Q, Cheng B. Comprehensive Analysis of Differentially Expressed miRNAs and mRNAs Reveals That miR-181a-5p Plays a Key Role in Diabetic Dermal Fibroblasts. J Diabetes Res 2020;2020:4581954. DOI: 10.1155/2020/4581954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biswas S, Roy S, Banerjee J, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U S A 2010;107(15):6976–81. DOI: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li X, Li D, Wang A, et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J Invest Dermatol 2017;137(12):2630–2638. DOI: 10.1016/j.jid.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 147.Naqvi RA, Gupta M, George A, Naqvi AR. MicroRNAs in shaping the resolution phase of inflammation. Semin Cell Dev Biol 2021. DOI: 10.1016/j.semcdb.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhai X, Zhao J, Wang Y, et al. Bibliometric Analysis of Global Scientific Research on lncRNA: A Swiftly Expanding Trend. Biomed Res Int 2018;2018:7625078. DOI: 10.1155/2018/7625078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kretz M, Siprashvili Z, Chu C, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 2013;493(7431):231–5. DOI: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kretz M, Webster DE, Flockhart RJ, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev 2012;26(4):338–43. DOI: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herter EK, Li D, Toma MA, et al. WAKMAR2, a Long Noncoding RNA Downregulated in Human Chronic Wounds, Modulates Keratinocyte Motility and Production of Inflammatory Chemokines. J Invest Dermatol 2019;139(6):1373–1384. DOI: 10.1016/j.jid.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 152.Sawaya AP, Pastar I, Stojadinovic O, et al. Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5. J Biol Chem 2018;293(4):1439–1449. DOI: 10.1074/jbc.M117.811240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X, Zhou Y, Chen S, Li W, Chen W, Gu W. LncRNA MACC1-AS1 sponges multiple miRNAs and RNA-binding protein PTBP1. Oncogenesis 2019;8(12):73. DOI: 10.1038/s41389-019-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li X, Xu Landen N. Evaluation of MicroRNA Therapeutic Potential Using the Mouse In Vivo and Human Ex Vivo Wound Models. Methods Mol Biol 2021;2193:67–75. DOI: 10.1007/978-1-0716-0845-6_7. [DOI] [PubMed] [Google Scholar]
- 155.Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020;8(10). DOI: 10.3390/microorganisms8101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tomic-Canic M, Burgess JL, O’Neill KE, Strbo N, Pastar I. Skin Microbiota and its Interplay with Wound Healing. Am J Clin Dermatol 2020;21(Suppl 1):36–43. DOI: 10.1007/s40257-020-00536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnson TR, Gomez BI, McIntyre MK, et al. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int J Mol Sci 2018;19(9). DOI: 10.3390/ijms19092699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Williams H, Crompton RA, Thomason HA, et al. Cutaneous Nod2 Expression Regulates the Skin Microbiome and Wound Healing in a Murine Model. J Invest Dermatol 2017;137(11):2427–2436. DOI: 10.1016/j.jid.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wolcott RD, Hanson JD, Rees EJ, et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2016;24(1):163–74. DOI: 10.1111/wrr.12370. [DOI] [PubMed] [Google Scholar]
- 160.Harrison OJ, Linehan JL, Shih HY, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019;363(6422). DOI: 10.1126/science.aat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Loesche M, Gardner SE, Kalan L, et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J Invest Dermatol 2017;137(1):237–244. DOI: 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tipton CD, Wolcott RD, Sanford NE, et al. Patient genetics is linked to chronic wound microbiome composition and healing. PLoS Pathog 2020;16(6):e1008511. DOI: 10.1371/journal.ppat.1008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bar J, Sarig O, Lotan-Pompan M, et al. Evidence for cutaneous dysbiosis in dystrophic epidermolysis bullosa. Clin Exp Dermatol 2021. DOI: 10.1111/ced.14592. [DOI] [PubMed] [Google Scholar]
- 164.Kalan LR, Meisel JS, Loesche MA, et al. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe 2019;25(5):641–655 e5. DOI: 10.1016/j.chom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015;43(3):515–26. DOI: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pinho-Ribeiro FA, Baddal B, Haarsma R, et al. Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 2018;173(5):1083–1097 e22. DOI: 10.1016/j.cell.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen JA, Edwards TN, Liu AW, et al. Cutaneous TRPV1(+) Neurons Trigger Protective Innate Type 17 Anticipatory Immunity. Cell 2019;178(4):919–932 e14. DOI: 10.1016/j.cell.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bagood MD, Isseroff RR. TRPV1: Role in Skin and Skin Diseases and Potential Target for Improving Wound Healing. Int J Mol Sci 2021;22(11). DOI: 10.3390/ijms22116135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kalan L, Loesche M, Hodkinson BP, et al. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio 2016;7(5). DOI: 10.1128/mBio.01058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hurabielle C, Link VM, Bouladoux N, et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc Natl Acad Sci U S A 2020;117(28):16465–16474. DOI: 10.1073/pnas.2003022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang G, Sweren E, Liu H, et al. Bacteria induce skin regeneration via IL-1beta signaling. Cell Host Microbe 2021;29(5):777–791 e6. DOI: 10.1016/j.chom.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karinja SJ, Spector JA. Treatment of Infected Wounds in the Age of Antimicrobial Resistance: Contemporary Alternative Therapeutic Options. Plast Reconstr Surg 2018;142(4):1082–1092. DOI: 10.1097/PRS.0000000000004799. [DOI] [PubMed] [Google Scholar]
- 173.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010;7(9):503–14. DOI: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fijan S, Frauwallner A, Langerholc T, et al. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed Res Int 2019;2019:7585486. DOI: 10.1155/2019/7585486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohseni S, Bayani M, Bahmani F, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev 2018;34(3). DOI: 10.1002/dmrr.2970. [DOI] [PubMed] [Google Scholar]
- 176.De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021;9(2). DOI: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eaglstein WH, Falanga V. Chronic wounds. Surg Clin North Am 1997;77(3):689–700. DOI: 10.1016/s0039-6109(05)70575-2. [DOI] [PubMed] [Google Scholar]
- 178.Falanga V Wound healing and chronic wounds. J Cutan Med Surg 1998;3 Suppl 1:S1–1–5. (https://www.ncbi.nlm.nih.gov/pubmed/10082600). [PubMed] [Google Scholar]
- 179.O’Donnell TF Jr., Passman MA. Clinical practice guidelines of the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF)--Management of venous leg ulcers. Introduction. J Vasc Surg 2014;60(2 Suppl):1S–2S. DOI: 10.1016/j.jvs.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 180.O’Donnell TF Jr., Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery (R) and the American Venous Forum. J Vasc Surg 2014;60(2 Suppl):3S–59S. DOI: 10.1016/j.jvs.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 181.Browse NL, Burnand KG. The cause of venous ulceration. Lancet 1982;2(8292):243–5. DOI: 10.1016/s0140-6736(82)90325-7. [DOI] [PubMed] [Google Scholar]
- 182.Burnand KG, Clemenson G, Whimster I, Gaunt J, Browse NL. The effect of sustained venous hypertension on the skin capillaries of the canine hind limb. Br J Surg 1982;69(1):41–4. DOI: 10.1002/bjs.1800690114. [DOI] [PubMed] [Google Scholar]
- 183.Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993;28(4):623–7. DOI: 10.1016/0190-9622(93)70085-8. [DOI] [PubMed] [Google Scholar]
- 184.Morton LM, Phillips TJ. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol 2016;74(4):589–605; quiz 605–6. DOI: 10.1016/j.jaad.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 185.Li WW, Carter MJ, Mashiach E, Guthrie SD. Vascular assessment of wound healing: a clinical review. Int Wound J 2017;14(3):460–469. DOI: 10.1111/iwj.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol 2015;173(2):379–90. DOI: 10.1111/bjd.13953. [DOI] [PubMed] [Google Scholar]
- 187.Alvaro-Afonso FJ, Garcia-Morales E, Molines-Barroso RJ, Garcia-Alvarez Y, Sanz-Corbalan I, Lazaro-Martinez JL. Interobserver reliability of the ankle-brachial index, toe-brachial index and distal pulse palpation in patients with diabetes. Diab Vasc Dis Res 2018;15(4):344–347. DOI: 10.1177/1479164118767599. [DOI] [PubMed] [Google Scholar]
- 188.Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3283. DOI: 10.1002/dmrr.3283. [DOI] [PubMed] [Google Scholar]
- 189.Moore ZE, Patton D. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev 2019;1:CD006471. DOI: 10.1002/14651858.CD006471.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oliveira AL, Moore Z, T OC, Patton D. Accuracy of ultrasound, thermography and subepidermal moisture in predicting pressure ulcers: a systematic review. J Wound Care 2017;26(5):199–215. DOI: 10.12968/jowc.2017.26.5.199. [DOI] [PubMed] [Google Scholar]
- 191.Gottrup F, Apelqvist J, Bjarnsholt T, et al. Antimicrobials and Non-Healing Wounds. Evidence, controversies and suggestions-key messages. J Wound Care 2014;23(10):477–8, 480, 482. DOI: 10.12968/jowc.2014.23.10.477. [DOI] [PubMed] [Google Scholar]
- 192.Tang JC, Vivas A, Rey A, Kirsner RS, Romanelli P. Atypical ulcers: wound biopsy results from a university wound pathology service. Ostomy Wound Manage 2012;58(6):20–2, 24, 26–9. (https://www.ncbi.nlm.nih.gov/pubmed/22688856). [PubMed] [Google Scholar]
- 193.Panuncialman J, Hammerman S, Carson P, Falanga V. Wound edge biopsy sites in chronic wounds heal rapidly and do not result in delayed overall healing of the wounds. Wound Repair Regen 2010;18(1):21–5. DOI: 10.1111/j.1524-475X.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Falanga V, Kirsner RS, Eaglstein WH, Katz MH, Kerdel FA. Stanozolol in treatment of leg ulcers due to cryofibrinogenaemia. Lancet 1991;338(8763):347–8. DOI: 10.1016/0140-6736(91)90483-6. [DOI] [PubMed] [Google Scholar]
- 195.Baby D, Upadhyay M, Joseph MD, et al. Calciphylaxis and its diagnosis: A review. J Family Med Prim Care 2019;8(9):2763–2767. DOI: 10.4103/jfmpc.jfmpc_588_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dini V, Romanelli M, Bertone M, Talarico S, Bombardieri S, Barachini P. Improvement of idiopathic pyoderma gangrenosum during treatment with anti-tumor necrosis factor alfa monoclonal antibody. Int J Low Extrem Wounds 2007;6(2):108–13. DOI: 10.1177/1534734607300912. [DOI] [PubMed] [Google Scholar]
- 197.Fox JD, Baquerizo-Nole KL, Keegan BR, et al. Adalimumab treatment leads to reduction of tissue tumor necrosis factor-alpha correlated with venous leg ulcer improvement: a pilot study. Int Wound J 2016;13(5):963–6. DOI: 10.1111/iwj.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis 2001;37(6):1267–76. DOI: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 199.Tardaguila-Garcia A, Garcia-Morales E, Garcia-Alamino JM, Alvaro-Afonso FJ, Molines-Barroso RJ, Lazaro-Martinez JL. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen 2019;27(4):415–420. DOI: 10.1111/wrr.12717. [DOI] [PubMed] [Google Scholar]
- 200.Dini V, Papadia F, Francesco FD, et al. Potential correlation of wound bed score and biomarkers in chronic lower leg wounds: an exploratory study. J Wound Care 2017;26(Sup9):S9–S17. DOI: 10.12968/jowc.2017.26.Sup9.S9. [DOI] [PubMed] [Google Scholar]
- 201.Boyd G, Butcher M, Glover D, Kingsley A. Prevention of non-healing wounds through the prediction of chronicity. J Wound Care 2004;13(7):265–6. DOI: 10.12968/jowc.2004.13.7.26633. [DOI] [PubMed] [Google Scholar]
- 202.Nukada H Ischemia and diabetic neuropathy. Handb Clin Neurol 2014;126:469–87. DOI: 10.1016/B978-0-444-53480-4.00023-0. [DOI] [PubMed] [Google Scholar]
- 203.Crews RT, King AL, Yalla SV, Rosenblatt NJ. Recent Advances and Future Opportunities to Address Challenges in Offloading Diabetic Feet: A Mini-Review. Gerontology 2018;64(4):309–317. DOI: 10.1159/000486392. [DOI] [PubMed] [Google Scholar]
- 204.Lim E, Mordiffi Z, Chew HSJ, Lopez V. Using the Braden subscales to assess risk of pressure injuries in adult patients: A retrospective case-control study. Int Wound J 2019;16(3):665–673. DOI: 10.1111/iwj.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Padula WV, Chen YH, Santamaria N. Five-layer border dressings as part of a quality improvement bundle to prevent pressure injuries in US skilled nursing facilities and Australian nursing homes: A cost-effectiveness analysis. Int Wound J 2019;16(6):1263–1272. DOI: 10.1111/iwj.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J 2016;13(6):1193–1197. DOI: 10.1111/iwj.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17(6):763–71. DOI: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open 2020;10(12):e045253. DOI: 10.1136/bmjopen-2020-045253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141(5):1085–95. (https://www.ncbi.nlm.nih.gov/pubmed/1279979). [PMC free article] [PubMed] [Google Scholar]
- 210.Boulton AJ, Meneses P, Ennis WJ. Diabetic foot ulcers: A framework for prevention and care. Wound Repair Regen 1999;7(1):7–16. DOI: 10.1046/j.1524-475x.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 211.Falanga V Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 2000;8(5):347–52. (https://www.ncbi.nlm.nih.gov/pubmed/11115147). [PubMed] [Google Scholar]
- 212.Ayello EA, Dowsett C, Schultz GS, et al. TIME heals all wounds. Nursing 2004;34(4):36–41; quiz, 41–2. DOI: 10.1097/00152193-200404000-00040. [DOI] [PubMed] [Google Scholar]
- 213.Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11 Suppl 1:S1–28. DOI: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 214.Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound Bed Preparation 2021. Adv Skin Wound Care 2021;34(4):183–195. DOI: 10.1097/01.ASW.0000733724.87630.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barros Almeida I, Garcez Barretto Teixeira L, Oliveira de Carvalho F, et al. Smart Dressings for Wound Healing: A Review. Adv Skin Wound Care 2021;34(2):1–8. DOI: 10.1097/01.ASW.0000725188.95109.68. [DOI] [PubMed] [Google Scholar]
- 216.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217–28. DOI: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 217.Clokie M, Greenway AL, Harding K, et al. New horizons in the understanding of the causes and management of diabetic foot disease: report from the 2017 Diabetes UK Annual Professional Conference Symposium. Diabet Med 2017;34(3):305–315. DOI: 10.1111/dme.13313. [DOI] [PubMed] [Google Scholar]
- 218.Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med 2014;31(12):1498–504. DOI: 10.1111/dme.12545. [DOI] [PubMed] [Google Scholar]
- 219.Lipsky BA, Peters EJ, Berendt AR, et al. Specific guidelines for the treatment of diabetic foot infections 2011. Diabetes Metab Res Rev 2012;28 Suppl 1:234–5. DOI: 10.1002/dmrr.2251. [DOI] [PubMed] [Google Scholar]
- 220.Falanga V, Fujitani RM, Diaz C, et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen 1999;7(4):208–13. DOI: 10.1046/j.1524-475x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 221.Jull A, Waters J, Arroll B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet 2002;359(9317):1550–4. DOI: 10.1016/S0140-6736(02)08513-6. [DOI] [PubMed] [Google Scholar]
- 222.Clark M, Semple MJ, Ivins N, Mahoney K, Harding K. National audit of pressure ulcers and incontinence-associated dermatitis in hospitals across Wales: a cross-sectional study. BMJ Open 2017;7(8):e015616. DOI: 10.1136/bmjopen-2016-015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rappl LM. Physiological changes in tissues denervated by spinal cord injury tissues and possible effects on wound healing. Int Wound J 2008;5(3):435–44. DOI: 10.1111/j.1742-481X.2007.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Falanga V, Carson P, Greenberg A, Hasan A, Nichols E, McPherson J. Topically applied recombinant tissue plasminogen activator for the treatment of venous ulcers. Preliminary report. Dermatol Surg 1996;22(7):643–4. DOI: 10.1111/j.1524-4725.1996.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 225.Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double-blind trial in healing chronic diabetic foot ulcers. CT-102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15(11):1598–604. DOI: 10.2337/diacare.15.11.1598. [DOI] [PubMed] [Google Scholar]
- 226.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21(1):71–8; discussion 79–81. DOI: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 227.Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen 1999;7(3):141–7. DOI: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 228.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999;7(5):335–46. DOI: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 229.Wieman TJ. Clinical efficacy of becaplermin (rhPDGF-BB) gel. Becaplermin Gel Studies Group. Am J Surg 1998;176(2A Suppl):74S–79S. DOI: 10.1016/s0002-9610(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 230.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 1996;183(1):61–4. (https://www.ncbi.nlm.nih.gov/pubmed/8673309). [PubMed] [Google Scholar]
- 231.Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019;7:10. DOI: 10.1186/s41038-019-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol 1998;134(3):293–300. DOI: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- 233.Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 1999;7(4):201–7. DOI: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 234.Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer S. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24(2):290–5. DOI: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 235.Progress MacNeil S. and opportunities for tissue-engineered skin. Nature 2007;445(7130):874–80. DOI: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 236.Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J 2013;10(2):132–7. DOI: 10.1111/iwj.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study G. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26(6):1701–5. DOI: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 238.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D, Group OVUS. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41(5):837–43. DOI: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 239.Omar AA, Mavor AI, Jones AM, Homer-Vanniasinkam S. Treatment of venous leg ulcers with Dermagraft. Eur J Vasc Endovasc Surg 2004;27(6):666–72. DOI: 10.1016/j.ejvs.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 240.Bosanquet DC, Sanders AJ, Ruge F, et al. Development and validation of a gene expression test to identify hard-to-heal chronic venous leg ulcers. Br J Surg 2019;106(8):1035–1042. DOI: 10.1002/bjs.11161. [DOI] [PubMed] [Google Scholar]
- 241.Jeffcoate WJ, Price PE, Phillips CJ, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 2009;13(54):1–86, iii-iv. DOI: 10.3310/hta13540. [DOI] [PubMed] [Google Scholar]
- 242.Grey JE, Leaper D, Harding K. How to measure success in treating chronic leg ulcers. BMJ 2009;338:b1434. DOI: 10.1136/bmj.b1434. [DOI] [PubMed] [Google Scholar]
- 243.Franks PJ, Moffatt CJ. Who suffers most from leg ulceration? J Wound Care 1998;7(8):383–5. DOI: 10.12968/jowc.1998.7.8.383. [DOI] [PubMed] [Google Scholar]
- 244.Olsson M, Jarbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen 2019;27(1):114–125. DOI: 10.1111/wrr.12683. [DOI] [PubMed] [Google Scholar]
- 245.Zhou K, Jia P. Depressive symptoms in patients with wounds: A cross-sectional study. Wound Repair Regen 2016;24(6):1059–1065. DOI: 10.1111/wrr.12484. [DOI] [PubMed] [Google Scholar]
- 246.Kapp S, Miller C, Santamaria N. The quality of life of people who have chronic wounds and who self-treat. J Clin Nurs 2018;27(1–2):182–192. DOI: 10.1111/jocn.13870. [DOI] [PubMed] [Google Scholar]
- 247.Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J 2017;14(6):1108–1119. DOI: 10.1111/iwj.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83. (https://www.ncbi.nlm.nih.gov/pubmed/1593914). [PubMed] [Google Scholar]
- 249.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199–208. DOI: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 250.Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1(1):10–7. DOI: 10.1111/j.1742-481x.2004.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Martinez-Gonzalez D, Doria M, Martinez-Alonso M, et al. Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. J Clin Med 2020;9(8). DOI: 10.3390/jcm9082497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Moore Z, Butcher G, Corbett LQ, McGuiness W, Snyder RJ, van Acker K. Exploring the concept of a team approach to wound care: Managing wounds as a team. J Wound Care 2014;23 Suppl 5b:S1–S38. DOI: 10.12968/jowc.2014.23.Sup5b.S1. [DOI] [PubMed] [Google Scholar]
- 253.Lindsay E The Lindsay Leg Club Model: a model for evidence-based leg ulcer management. Br J Community Nurs 2004;Suppl:S15–20. DOI: 10.12968/bjcn.2004.9.sup2.13128. [DOI] [PubMed] [Google Scholar]
- 254.Kapp S, Santamaria N. How and why patients self-treat chronic wounds. Int Wound J 2017;14(6):1269–1275. DOI: 10.1111/iwj.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Miller C, Kapp S. Informal carers and wound management: an integrative literature review. J Wound Care 2015;24(11):489–90, 492, 494−-7. DOI: 10.12968/jowc.2015.24.11.489. [DOI] [PubMed] [Google Scholar]
- 256.Kapp S, Santamaria N. Chronic wounds should be one of Australia’s National Health Priority Areas. Aust Health Rev 2015;39(5):600–602. DOI: 10.1071/AH14230. [DOI] [PubMed] [Google Scholar]
- 257.Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc 2015;63(3):427–38. DOI: 10.1111/jgs.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int 2018;2018:6901983. DOI: 10.1155/2018/6901983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, Wilk R, Hudecki J, Los MJ. Novel trends in application of stem cells in skin wound healing. Eur J Pharmacol 2019;843:307–315. DOI: 10.1016/j.ejphar.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 260.Otero-Vinas M, Falanga V. Mesenchymal Stem Cells in Chronic Wounds: The Spectrum from Basic to Advanced Therapy. Adv Wound Care (New Rochelle) 2016;5(4):149–163. DOI: 10.1089/wound.2015.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li P, Guo X. A review: therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem Cell Res Ther 2018;9(1):302. DOI: 10.1186/s13287-018-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.van Dongen JA, Harmsen MC, van der Lei B, Stevens HP. Augmentation of Dermal Wound Healing by Adipose Tissue-Derived Stromal Cells (ASC). Bioengineering (Basel) 2018;5(4). DOI: 10.3390/bioengineering5040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Brockmann I, Ehrenpfordt J, Sturmheit T, et al. Skin-Derived Stem Cells for Wound Treatment Using Cultured Epidermal Autografts: Clinical Applications and Challenges. Stem Cells Int 2018;2018:4623615. DOI: 10.1155/2018/4623615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gonzales KAU, Fuchs E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev Cell 2017;43(4):387–401. DOI: 10.1016/j.devcel.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Himal I, Goyal U, Ta M. Evaluating Wharton’s Jelly-Derived Mesenchymal Stem Cell’s Survival, Migration, and Expression of Wound Repair Markers under Conditions of Ischemia-Like Stress. Stem Cells Int 2017;2017:5259849. DOI: 10.1155/2017/5259849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lin X, Kwak T, Fiore D, et al. An in vitro priming step increases the expression of numerous epidermal growth and migration mediators in a tissue-engineering construct. J Tissue Eng Regen Med 2017;11(3):713–723. DOI: 10.1002/term.1967. [DOI] [PubMed] [Google Scholar]
- 267.Magne B, Lataillade JJ, Trouillas M. Mesenchymal Stromal Cell Preconditioning: The Next Step Toward a Customized Treatment For Severe Burn. Stem Cells Dev 2018;27(20):1385–1405. DOI: 10.1089/scd.2018.0094. [DOI] [PubMed] [Google Scholar]
- 268.Xu W, Xu R, Li Z, Wang Y, Hu R. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1alpha signalling. J Cell Mol Med 2019;23(3):1899–1907. DOI: 10.1111/jcmm.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yang HY, Fierro F, So M, et al. Combination product of dermal matrix, human mesenchymal stem cells, and timolol promotes diabetic wound healing in mice. Stem Cells Transl Med 2020;9(11):1353–1364. DOI: 10.1002/sctm.19-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ariyanti AD, Zhang J, Marcelina O, et al. Salidroside-Pretreated Mesenchymal Stem Cells Enhance Diabetic Wound Healing by Promoting Paracrine Function and Survival of Mesenchymal Stem Cells Under Hyperglycemia. Stem Cells Transl Med 2019;8(4):404–414. DOI: 10.1002/sctm.18-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Larsen L, Tchanque-Fossuo CN, Gorouhi F, et al. Combination therapy of autologous adipose mesenchymal stem cell-enriched, high-density lipoaspirate and topical timolol for healing chronic wounds. J Tissue Eng Regen Med 2018;12(1):186–190. DOI: 10.1002/term.2390. [DOI] [PubMed] [Google Scholar]
- 272.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13(6):1299–312. DOI: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 273.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008;121(3):860–877. DOI: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 274.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009;12(5):359–66. DOI: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 275.Falanga V, Grada A, Otero-Vinas M, et al. Autologous Cultured Bone Marrow-Derived Mesenchymal Stem Cells in a Fibrin Spray to Treat Venous Ulcers: A Randomized Controlled Double-Blind Pilot Study. Surg Technol Int 2022;40. DOI: 10.52198/22.STI.40.WH1493. [DOI] [PubMed] [Google Scholar]
- 276.Ferreira ADF, Gomes DA. Stem Cell Extracellular Vesicles in Skin Repair. Bioengineering (Basel) 2018;6(1). DOI: 10.3390/bioengineering6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McBride JD, Rodriguez-Menocal L, Badiavas EV. Extracellular Vesicles as Biomarkers and Therapeutics in Dermatology: A Focus on Exosomes. J Invest Dermatol 2017;137(8):1622–1629. DOI: 10.1016/j.jid.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 278.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017;35(4):851–858. DOI: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 279.Roefs MT, Sluijter JPG, Vader P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol 2020;30(12):990–1013. DOI: 10.1016/j.tcb.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 280.Sung BH, Parent CA, Weaver AM. Extracellular vesicles: Critical players during cell migration. Dev Cell 2021. DOI: 10.1016/j.devcel.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bailey AJM, Li H, Kirkham AM, et al. MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: a Systematic Review and Meta-Analysis of Preclinical Animal Studies. Stem Cell Rev Rep 2021. DOI: 10.1007/s12015-021-10164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Margolis DJ, Gross EA, Wood CR, Lazarus GS. Planimetric rate of healing in venous ulcers of the leg treated with pressure bandage and hydrocolloid dressing. J Am Acad Dermatol 1993;28(3):418–21. DOI: 10.1016/0190-9622(93)70061-w. [DOI] [PubMed] [Google Scholar]
- 283.Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol 1997;133(10):1231–4. (https://www.ncbi.nlm.nih.gov/pubmed/9382561). [PubMed] [Google Scholar]
- 284.Falanga V Measurements in wound healing. Int J Low Extrem Wounds 2008;7(1):9–11. DOI: 10.1177/1534734608314570. [DOI] [PubMed] [Google Scholar]
- 285.Otero M, Lin X, MacLauchlan S, Carson P, Falanga V. Dermal Fibroblasts from Chronic Wounds Exhibit Paradoxically Enhanced Proliferative and Migratory Activities that May be Related to the Non-Canonical Wnt Signaling Pathway. Surg Technol Int 2021;39 (https://www.ncbi.nlm.nih.gov/pubmed/34181242). [PubMed] [Google Scholar]


