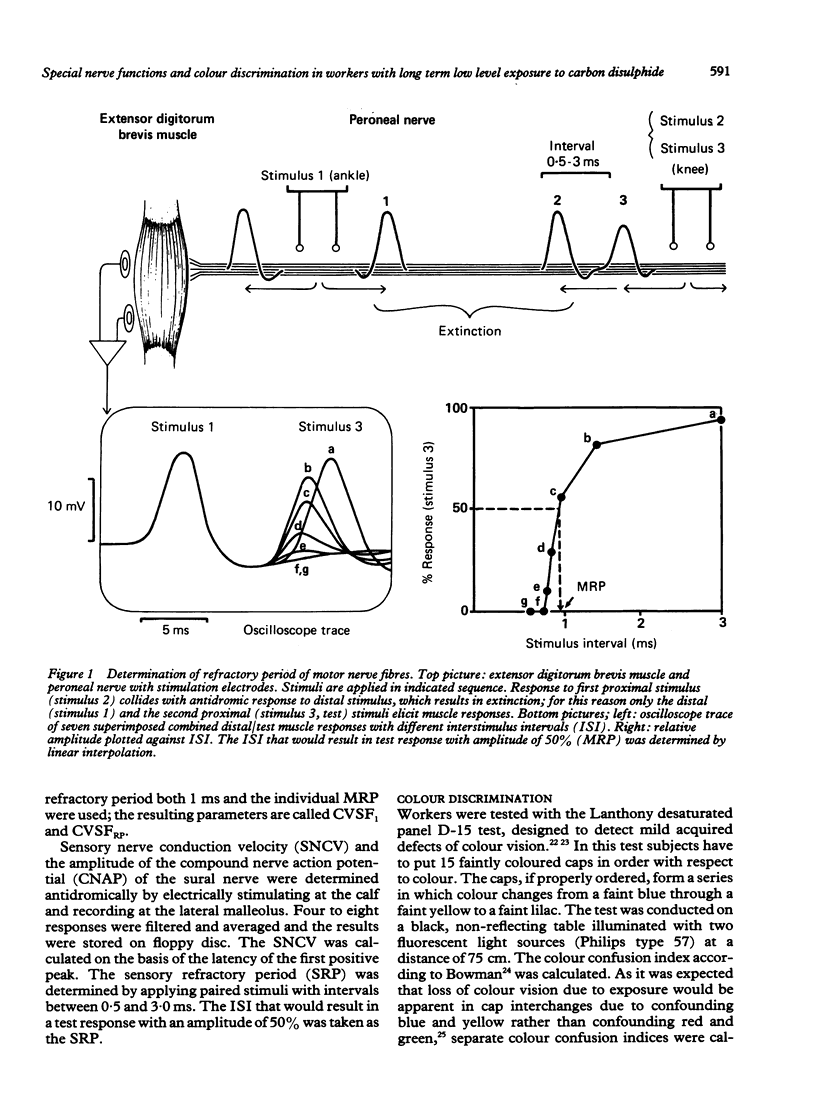
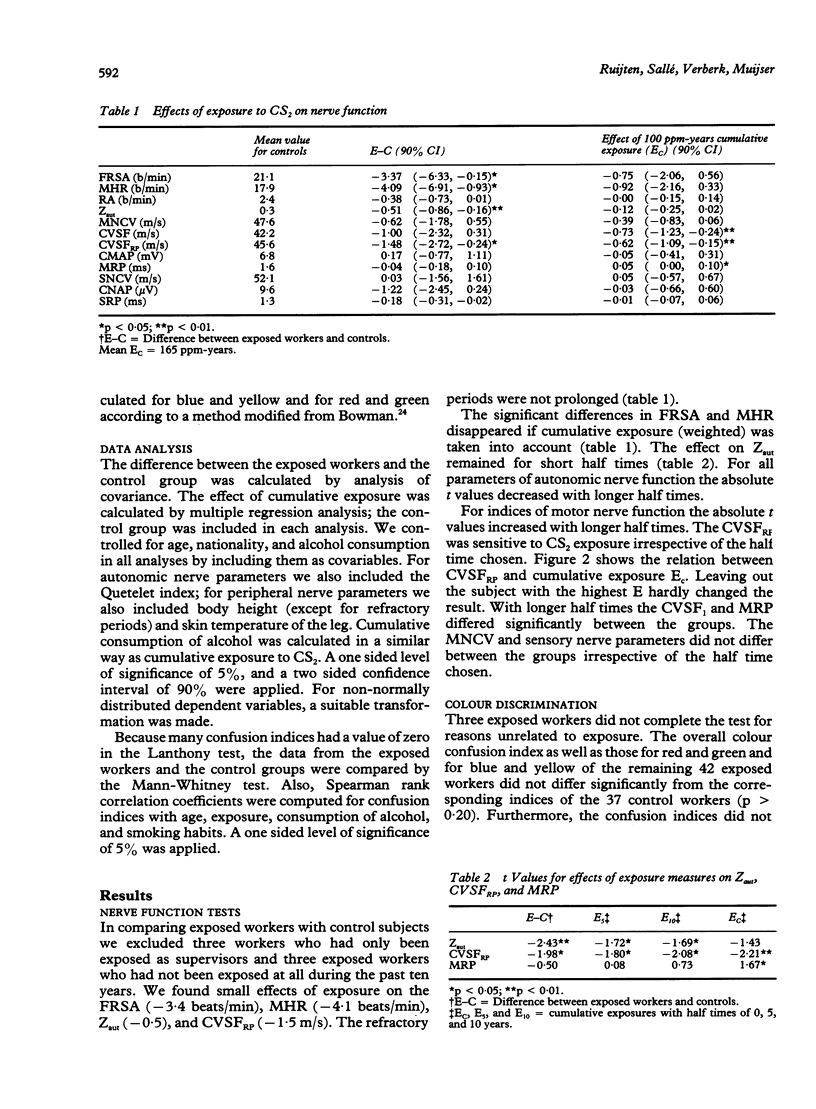
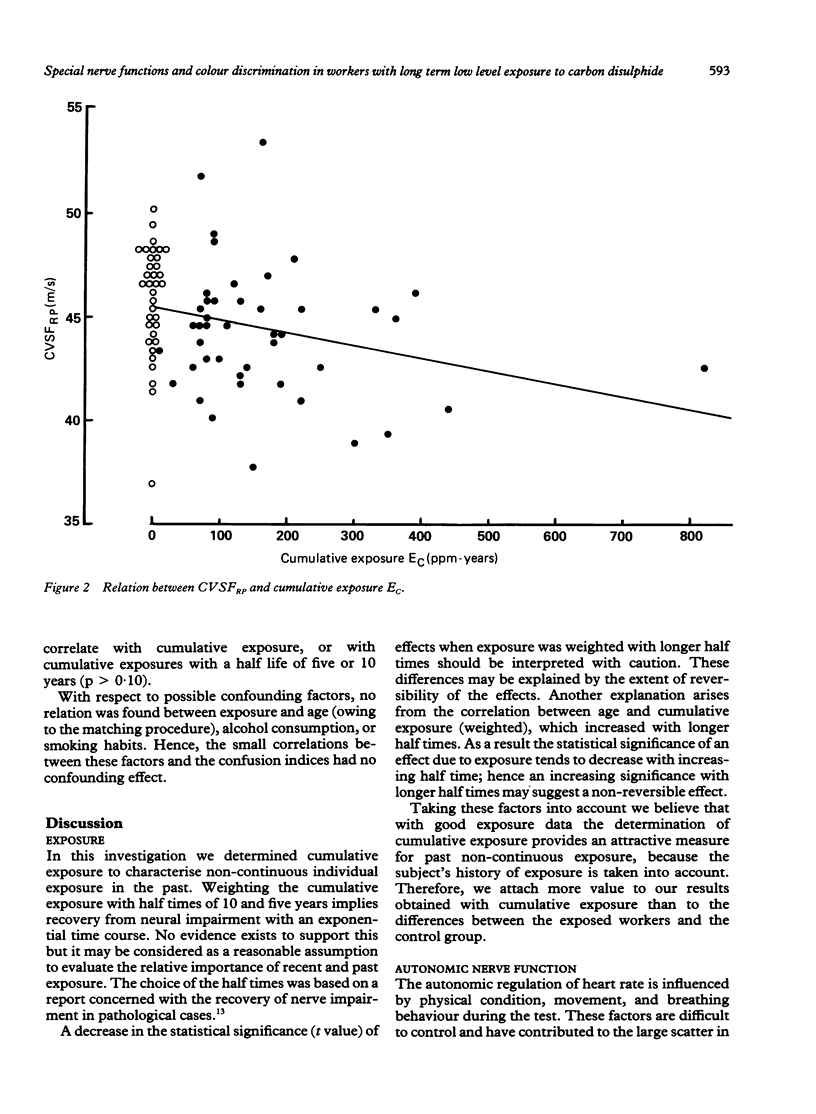
Abstract
Certain functions of the peripheral and autonomic nervous systems, and colour discrimination were examined in 45 workers (mean age 49; mean exposure to carbon disulphide (CS2) 20 years) and 37 controls (mean age 48). Conduction velocity and refractory period of the peroneal and sural nerves were determined. The conduction velocity of the slower fibres of the peroneal nerve was measured by means of an improved method that makes use of the refractory period. Function of autonomic nerves was assessed by measuring the variation in heart rate during rest, during deep breathing, and during isometric muscle contraction. Colour discrimination was evaluated by the Lanthony desaturated test. Individual cumulative exposure to CS2 was calculated on the basis of exposure in the past and individual job history. Mean cumulative exposure was 165 ppm-years. The peroneal nerves of exposed workers showed a decrease (-1.0 m/s) in conduction velocity of the slow fibres and a prolongation (0.1 ms) of the refractory period (mean 1.6 ms) compared with controls. These effects were related to cumulative exposure. No impairment of function of the sural nerve or of colour discrimination was found. The muscle heart reflex was decreased in the exposed group, but this was not related to cumulative exposure. This study has established more firmly that a decrease in conduction velocity of slow motor fibres occurs at low levels of exposure to CS2. Extrapolation of the results suggests that small effects may occur after 40 years of exposure to concentrations below the present threshold limit value (10 ppm).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Honma T., Yanagihara S., Ushio K. Recovery of slowed nerve conduction velocity in lead-exposed workers. Int Arch Occup Environ Health. 1980;46(2):151–157. doi: 10.1007/BF00378193. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. O., Jr, Bus J. S., Popp J. A., Boreiko C. J., Goldberg L. A critical review of the literature on carbon disulfide toxicity. Crit Rev Toxicol. 1983;11(3):169–278. doi: 10.3109/10408448309128255. [DOI] [PubMed] [Google Scholar]
- Bowman K. J. A method for quantitative scoring of the Farnsworth Panel D-15. Acta Ophthalmol (Copenh) 1982 Dec;60(6):907–916. doi: 10.1111/j.1755-3768.1982.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Corn M., Esmen N. A. Workplace exposure zones for classification of employee exposures to physical and chemical agents. Am Ind Hyg Assoc J. 1979 Jan;40(1):47–57. doi: 10.1080/15298667991429318. [DOI] [PubMed] [Google Scholar]
- Corsi G., Bartolucci G. B., Fardin P., Negrin P., Manzoni S. Biochemical and electrophysiological study of subjects with a history of past lead exposure. Am J Ind Med. 1984;6(4):281–290. doi: 10.1002/ajim.4700060406. [DOI] [PubMed] [Google Scholar]
- Corsi G., Maestrelli P., Picotti G., Manzoni S., Negrin P. Chronic peripheral neuropathy in workers with previous exposure to carbon disulphide. Br J Ind Med. 1983 May;40(2):209–211. doi: 10.1136/oem.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer M. A non-invasive computerized measurement of motor neurone refractory period and subnormal conduction in man. Electroencephalogr Clin Neurophysiol. 1981 May;51(5):548–558. doi: 10.1016/0013-4694(81)90231-5. [DOI] [PubMed] [Google Scholar]
- Gilioli R., Bulgheroni C., Bertazzi P. A., Cirla A. M., Tomasini M., Cassitto M. G., Jacovone M. T. Study of neurological and neurophysiological impairment in carbon idsulphide workers. Med Lav. 1978 Mar-Apr;69(2):130–143. [PubMed] [Google Scholar]
- Johnson B. L., Boyd J., Burg J. R., Lee S. T., Xintaras C., Albright B. E. Effects on the peripheral nervous system of workers' exposure to carbon disulfide. Neurotoxicology. 1983 Spring;4(1):53–65. [PubMed] [Google Scholar]
- Kimura J. A method for estimating the refractory period of motor fibers in the human peripheral nerve. J Neurol Sci. 1976 Aug;28(4):485–490. doi: 10.1016/0022-510x(76)90119-2. [DOI] [PubMed] [Google Scholar]
- Lanthony P. The desaturated panel D-15. Doc Ophthalmol. 1978 Oct 16;46(1):185–189. doi: 10.1007/BF00174107. [DOI] [PubMed] [Google Scholar]
- Lowitzsch K., Göhring U., Hecking E., Köhler H. Refractory period, sensory conduction velocity and visual evoked potentials before and after haemodialysis. J Neurol Neurosurg Psychiatry. 1981 Feb;44(2):121–128. doi: 10.1136/jnnp.44.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D., Blain L. Assessing color vision loss among solvent-exposed workers. Am J Ind Med. 1987;12(2):195–203. doi: 10.1002/ajim.4700120208. [DOI] [PubMed] [Google Scholar]
- Mergler D., Blain L., Lemaire J., Lalande F. Colour vision impairment and alcohol consumption. Neurotoxicol Teratol. 1988 May-Jun;10(3):255–260. doi: 10.1016/0892-0362(88)90025-6. [DOI] [PubMed] [Google Scholar]
- Mergler D., Bélanger S., De Grosbois S., Vachon N. Chromal focus of acquired chromatic discrimination loss and solvent exposure among printshop workers. Toxicology. 1988 May;49(2-3):341–348. doi: 10.1016/0300-483x(88)90017-0. [DOI] [PubMed] [Google Scholar]
- Muijser H., Hoogendijk E. M., Hooisma J., Twisk D. A. Lead exposure during demolition of a steel structure coated with lead-based paints. II. Reversible changes in the conduction velocity of the motor nerves in transiently exposed workers. Scand J Work Environ Health. 1987 Feb;13(1):56–61. doi: 10.5271/sjweh.2081. [DOI] [PubMed] [Google Scholar]
- Raitta C., Teir H., Tolonen M., Nurminen M., Helpiö E., Malmström S. Impaired color discrimination among viscose rayon workers exposed to carbon disulfide. J Occup Med. 1981 Mar;23(3):189–192. [PubMed] [Google Scholar]
- Schütt P., Muche H., Lehmann H. J. Refractory period impairment in sural nerves of diabetics. J Neurol. 1983;229(2):113–119. doi: 10.1007/BF00313450. [DOI] [PubMed] [Google Scholar]
- Seppäläinen A. M., Hernberg S. Sensitive technique for detecting subclinical lead neuropathy. Br J Ind Med. 1972 Oct;29(4):443–449. doi: 10.1136/oem.29.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P. S., Schaumburg H. H., Sabri M. I., Veronesi B. The enlarging view of hexacarbon neurotoxicity. Crit Rev Toxicol. 1980 Oct;7(4):279–356. doi: 10.3109/10408448009037489. [DOI] [PubMed] [Google Scholar]
- Wieling W., Borst C., van Dongen Torman M. A., van der Hofstede J. W., van Brederode J. F., Endert E., Dunning A. J. Relationship between impaired parasympathetic and sympathetic cardiovascular control in diabetes mellitus. Diabetologia. 1983 Jun;24(6):422–427. doi: 10.1007/BF00257340. [DOI] [PubMed] [Google Scholar]
- Wieling W., van Brederode J. F., de Rijk L. G., Borst C., Dunning A. J. Reflex control of heart rate in normal subjects in relation to age: a data base for cardiac vagal neuropathy. Diabetologia. 1982 Mar;22(3):163–166. doi: 10.1007/BF00283745. [DOI] [PubMed] [Google Scholar]


