Abstract
Introduction
Helicobacter pylori (H. pylori) is a prevalent stomach bacterium that can cause a range of clinical outcomes, including gastric cancer. In recent years, soluble suppression of tumorigenicity-2 (sST2) has gained attention as a biomarker associated with various diseases, such as gastric cancer. The purpose of this study was to explore the possible connection between H. pylori infection and sST2 levels in patients who do not exhibit symptoms.
Methods
A total of 694 patients from the Salzburg Colon Cancer Prevention Initiative (Sakkopi) were included in the study. The prevalence of H. pylori infection was determined by histology, and sST2 levels were measured in serum samples. Clinical and laboratory parameters, such as age, sex, BMI, smoking status, hypertension, and metabolic syndrome, were also collected.
Results
The median sST2 concentration was similar between patients with (9.62; 7.18–13.44 ng/mL; p = 0.66) and without (9.67; 7.08–13.06 ng/mL) H. pylori. Logistic regression analysis did not show any association (OR 1.00; 95%CI 0.97–1.04; p = 0.93) between sST2 levels and H. pylori infection, which remained so (aOR 0.99; 95%CI 0.95–1.03; p = 0.60) after adjustment for age, sex, educational status, and metabolic syndrome. In addition, sensitivity analyses stratified by age, sex, BMI, smoking status, educational status, and the concomitant diagnosis of metabolic syndrome could not show any association between sST2 levels and H. pylori infection.
Conclusion
The results indicate that sST2 may not serve as a valuable biomarker in the diagnosis and treatment of H. pylori infection. Our findings are of relevance for further research investigating sST2, as we could not find an influence of asymptomatic H. pylori infection on sST2 concentration.
What Is Already Known?
Soluble suppression of tumorigenicity-2 (sST2) has gained attention as a biomarker associated with various diseases, such as gastric cancer.
What Is New in This Study?
The median sST2 concentration was similar between patients with (9.62; 7.18–13.44 ng/mL; p = 0.66) and without (9.67; 7.08–13.06 ng/mL) H. pylori.
What Are the Future Clinical and Research Implications of the Study Findings?
The results indicate that sST2 may not serve as a valuable biomarker in the diagnosis and treatment of H. pylori infection.
Keywords: Biomarker, H. pylori, Cancer, sST2, Soluble suppression of tumorigenicity-2
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that colonizes the human stomach, with an estimated prevalence of 50% worldwide [1–3]. Although H.pylori infection is usually asymptomatic, it can cause a range of clinical manifestations, including gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma [1, 2]. The impact of H.pylori on individual and public health is significant, as it is estimated to be responsible for 90% of duodenal ulcers, 80% of gastric ulcers, and 60% of gastric cancers [4]. Further, H.pylori infection is associated with extraintestinal systemic diseases such as immune thrombocytopenia[5] and iron deficiency anemia of unknown origin [6]. Therefore, H. pylori infection should be treated once diagnosed [4].
Soluble suppression of tumorigenicity-2 (sST2) is a biomarker that has been extensively studied in recent years [7–9]. It is a member of the interleukin-1 receptor family [10] and is secreted in response to various forms of cellular stress, including inflammation, tissue injury, and mechanical strain [11]. sST2 was proposed as a marker for heart failure [12, 13], but emerging evidence suggests that it may also play a role in autoimmune diseases [14], infectious diseases [9], and cancer [15]. Of note, sST2 has been shown to be elevated in patients with gastric cancer [16]. Further, IL-33 and ST2 have been shown to be involved in exacerbated H. pylori infection in vitro [17].
Our study is predicated on the hypothesis that sST2 levels may be altered in asymptomatic patients with H. pylori infection, which holds significant implications for the diagnosis and management of H. pylori infection. While H.pylori infection can be diagnosed through invasive procedures like endoscopy and biopsy, less invasive methods such as stool antigen testing, breath tests, or serology are also available, albeit with potentially lower accuracy [2]. As a non-invasive biomarker, sST2 could offer a promising addition in combination to current diagnostic methods. Moreover, if H. pylori infection does influence sST2 levels, this may have implications for other disease contexts, such as autoimmune diseases and heart failure, where sST2 has been implicated. To the best of our knowledge, this is the first study to investigate the potential association between H. pylori infection and sST2 levels in asymptomatic patients. We aim to compare sST2 levels in patients with and without H. pylori infection, while assessing the relationship between sST2 and other clinical and laboratory parameters. It is worth noting that both sST2 and H. pylori infection have been linked to metabolic risk factors like obesity, insulin resistance, and metabolic syndrome, which may also play a role in our findings [18]. Another objective was to investigate whether there is a correlation between H. pylori infection and changes in sST2 levels, while also examining whether this correlation remains significant regardless of the presence of common metabolic risk factors.
In summary, this paper investigates the potential association between H. pylori infection and sST2 levels in asymptomatic patients. H. pylori infection is a significant public health issue, and sST2 is a biomarker that has been proposed to play a role in various disease settings. Our study aims to shed light on the potential relationship between these two factors and may have implications for the diagnosis and management of H. pylori infection as well as other disease settings.
Methods
Subjects
Our study recruited participants from the Salzburg Colon Cancer Prevention Initiative (Sakkopi), a cohort of asymptomatic patients screened for colorectal cancer at a single center in Austria who were offered a screening esophagogastroduodenoscopy (EGD). We assessed all patients (n = 1047) enrolled between 2018 and 2020. In 718 patients we had serum samples available for sST2 measurement. We further excluded 24 patients who refused EGD. Ultimately, our analysis consisted of 694 patients who had both serum samples available for sST2 measurement as well as undergone EGD with biopsy. All enrolled subjects had clinical and laboratory parameters collected as part of the study [19, 20]. In addition, patients were asked to complete a medical history questionnaire. We assessed and calculated various parameters, including body mass index (BMI), smoking status, arterial hypertension, and metabolic syndrome, based on current guidelines [21]. We assessed the presence of H. pylori through histology, using biopsies collected during EGD. We performed the study and all procedures according to the principles of the Declaration of Helsinki. The local ethics committee for the province Salzburg approved the study protocol (Approval No. 415-E/1262). Written informed consent was obtained from every participant.
Measurement of sST2
Serum levels of sST2 were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Duoset DY206; R&D Systems, USA). The assay was performed according to the manufacturer’s protocol. Serum samples and standard protein were added to the wells on a 96-well plate (Maxisorp plate, Nunc-Immuno, Sigma Aldrich, Austria) and incubated for 2 h. After this incubation period, 96-well plates were washed for three times with washing buffer containing Tween-20. Then, a biotin-labelled secondary antibody was added to the wells and plates were incubated for further 2 h. Later, plates were washed again and Streptavidin-horseradish-peroxidase was added. A colour reaction was achieved using tetramethylbenzidine (TMB; Sigma Aldrich, USA). The reaction was stopped using diluted sulfuric acid. Optical density values were measured at 450 nm on an ELISA plate-reader (Bio-Rad Laboratories, Austria). Serum levels of sST2 were then calculated for each sample using a Excel spreadsheet.
Statistical Analysis
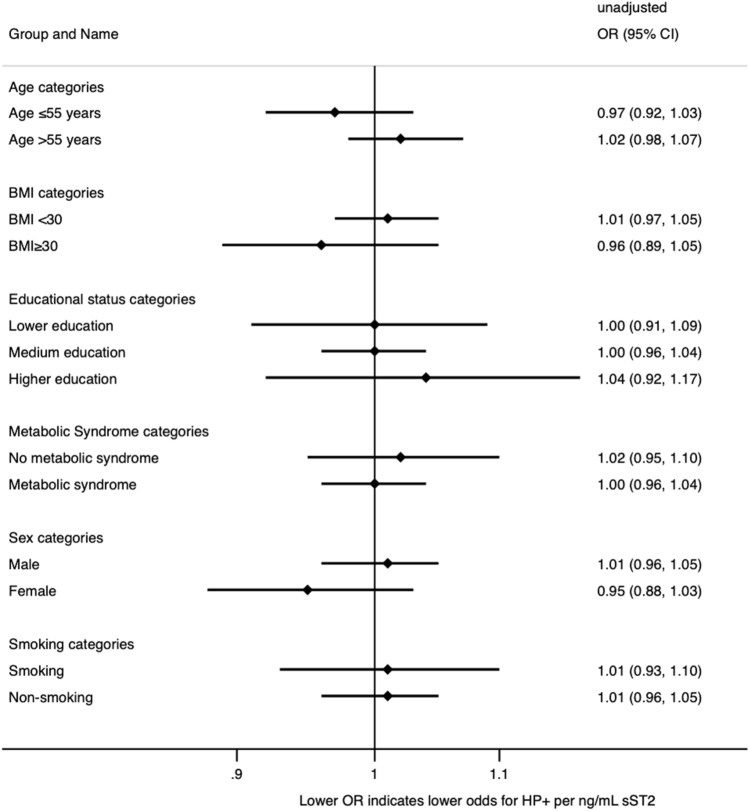
Continuous variables were presented as median with interquartile range (IQR) and compared using Mann–Whitney U Test. Categorical variables were expressed as numbers with percentages and analyzed using the chi-square test. We visualized the distribution of sST2 (Fig. 1) in patients with and without H. pylori infection using the vioplot command [22]. All statistical tests were two-tailed, and a p value less than 0.05 was considered statistically significant. The primary endpoint in our study was the histological diagnosis of H. pylori, and the primary exposure was the concentration of sST2. We fitted logistic regression models to examine the relationship between sST2 concentration and H. pylori status. In Model-1, sST2 concentration was the independent variable, and the occurrence of H. pylori was the dependent variable. In Model-2, sST2 concentration, age, sex, educational status and concomitant metabolic syndrome diagnosis were fixed effect-dependent variables. Sensitivity analyses (Fig. 2) were also performed to explore the relationship between H. pylori status and sST2 concentration, with patients stratified by sex (male versus female), age (patients aged ≤ 55 years and patients aged > 55 years), BMI (BMI < 30 kg/m2 versus BMI ≥ 30 kg/m2), concomitant metabolic syndrome diagnosis, and current smoking status. We obtained adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs). All statistical analyses were conducted using Stata/IC 17.
Fig. 1.
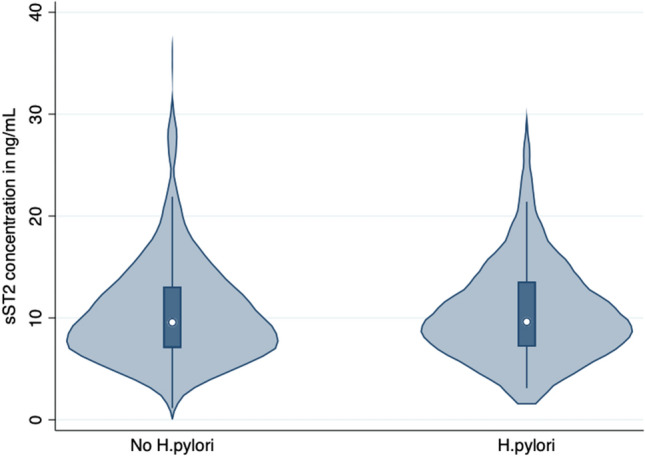
We visualized the distribution of sST2 in ng/mL (this figure) using vioplot command [22] in patients with and without H. pylori
Fig. 2.
To investigate the association between sST2 concentration and H. pylori infection, we performed sensitivity analyses using logistic regression models. The occurrence of H. pylori was set as the dependent variable, and sST2 concentration was the independent variable. The patients were stratified by sex, age, BMI, concomitant metabolic syndrome diagnosis, smoking, and educational status. However, we did not observe any significant relationship between sST2 concentration and H. pylori infection in any of the subgroups analyzed
Results
We compared various clinical and laboratory parameters between patients with (n = 131) and without (n = 563) H. pylori infection. There was no significant difference in age, with both groups having a median age of 57 years (p = 0.75). Similarly, there was no difference in the proportion of patients aged 55 years or younger versus those over 55 years of age (p = 0.95). The proportion of female patients was similar in both groups, with 48% of patients without H. pylori infection and 39% of patients with H. pylori infection being female (p = 0.73). The median BMI was slightly higher in patients with H. pylori infection compared to those without infection (27 kg/m2 vs. 26 kg/m2, p = 0.02). However, there was no significant difference in the proportion of patients with a BMI less than 30 kg/m2 versus those with a BMI of 30 kg/m2 or greater (p = 0.11). The frequency of hypertension, diabetes, and metabolic syndrome did not show any significant difference between the two groups (Table 1). Additionally, smoking status was comparable in both groups. We also found no significant variations between the two groups in the laboratory parameters analyzed, including HbA1c, cholesterol, LDL, HDL, creatinine, hemoglobin, white blood cell count, CRP, and platelet count. There was no significant difference in the median sST2 concentration between patients without (9.67; 7.08–13.06 ng/mL) and those with (9.62; 7.18–13.44 ng/mL; p = 0.66) H. pylori infection (Fig. 1). In the logistic regression, sST2 was not associated with the diagnosis of H. pylori (OR 1.00; 95%CI 0.97–1.04; p = 0.93) and remained so after multivariable adjustment for age, sex, educational status and the diagnosis of metabolic syndrome (aOR 0.99; 95%CI 0.95–1.03; p = 0.60). In the sensitivity analyses stratified by age, sex, BMI, smoking and educational status as well as the concomitant diagnosis of metabolic syndrome, we found no association between sST2 and H. pylori infection in any of the subgroups.
Table 1.
Baseline characteristics of patients with (n = 131) and without (n = 563) H. pylori infection
| No H. pylori | H. pylori | p value | |
|---|---|---|---|
| N = 563 | N = 131 | ||
| Age (years) | 57 (52–63) | 57 (53–64) | 0.75 |
| Age categories | 0.95 | ||
| Age ≤ 55 | 40% (226) | 40% (53) | |
| Age > 55 | 60% (337) | 60% (78) | |
| Female sex | 48% (268) | 39% (51) | 0.73 |
| BMI (kg/m2) | 26 (24–29) | 27 (25–30) | 0.02 |
| BMI categories | 0.11 | ||
| BMI < 30 kg/m2 | 80% (452) | 74% (97) | |
| BMI ≥ 30 kg/m2 | 20% (111) | 26% (34) | |
| Hypertension (yes/no) | 61% (342) | 62% (81) | 0.82 |
| Metabolic syndrome (yes/no) | 83% (469) | 80% (105) | 0.39 |
| Diabetes (yes/no) | 31% (174) | 32% (42) | 0.80 |
| Hba1c (%) | 5.4 (5.2–5.6) | 5.4 (5.2–5.7) | 0.44 |
| Cholesterol (mg/dL) | 224 (194–253) | 220 (195–244) | 0.31 |
| LDL (mg/dL) | 147 (118–173) | 143 (121–165) | 0.49 |
| HDL (mg/dL) | 57 (48–68) | 56 (47–66) | 0.59 |
| Creatinine (mg/dL) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.24 |
| Hemoglobine (mg/dL) | 14.6 (13.8–15.4) | 14.9 (13.9–15.6) | 0.12 |
| White blood count (G/L | 5.6 (4.7–6.7) | 6.0 (5.0–7.2) | 0.35 |
| CRP (mg/dL) | 0.1 (0.1–0.3) | 0.2 (0.1–0.3) | 0.68 |
| Platelets (G/L) | 240 (208–276) | 238 (208–274) | 0.89 |
| Smoking status | 0.14 | ||
| Non-smoker | 83% (433) | 78% (94) | |
| Current smoker | 17% (86) | 22% (27) | |
| ST2 (pg/mL) | 9.57 (7.08–13.06) | 9.62 (7.18–13.55) | 0.66 |
Continuous data are given as median ± inter-quartile range (IQR) and compared using Mann’s Whitney U Test. Categorical data are given as numbers (percentage) and compared using the chi-square test. All tests were two-sided, and a p value of < 0.05 was considered statistically significant
Discussion
The previous literature on sST2 and H. pylori infection is scarce. Bergis et al. investigated the role of Interleukin-33 (IL-33), its membrane bound cellular receptor ST2L, and its soluble receptor sST2 in gastric cancer [16]. Thirty gastric cancer patients, 51 gastritis patients, and 40 healthy volunteers were enrolled, and the levels of IL-33 and sST2 were determined by ELISA. The results showed that sST2 levels in gastric cancer were significantly higher than in gastritis or healthy controls, and higher levels of sST2 were seen in patients with lower degree of tumor differentiation. Soluble ST2 was significantly associated with a more advanced tumor stage, metastatic disease, and significantly correlated with the duration of the disease [16]. Another study investigated the interaction between H. pylori and the proinflammatory cytokine IL-33 and its receptor ST-2 [17]. The results showed that H. pylori infection elevated expression levels of IL-33 and ST-2 and caused ST-2 mobilization into membrane lipid rafts. Depletion of membrane cholesterol reduced H. pylori-induced IL-33 and IL-8 production. In vivo studies revealed that H. pylori infection led to upregulation of IL-33/ST-2 and severe leukocyte infiltration in gastric tissues, indicating that ST-2 recruitment into the lipid rafts serves as a platform for IL-33-dependent H. pylori infection, which aggravates inflammation in the stomach. Two other studies showed that IL-33, for which sST2 is the soluble decoy receptor, is involved in inflammation mediated by H.pylori infection [23, 24]. Based on these preliminary studies, we speculated about a role of sST2 in asymptomatic H. pylori infection. However, our study did not find any significant association between sST2 levels and H. pylori infection in asymptomatic patients. This suggests that sST2 may not serve as a valuable biomarker for the diagnosis and management of H. pylori infection. All of our patients were asymptomatic and underwent gastroscopy because it was offered to them as part of an opportunistic colon cancer screening program using colonoscopy. However, some of our patients may have had more severe gastritis than others, or even subtle symptoms. Unfortunately, we do not have this data.sST2 is a biomarker that has been extensively studied in recent years [7–9]. An association between sST2 and mortality has already been demonstrated in several populations [25, 26]. sST2 was proposed as a marker for heart failure [27], but emerging evidence suggests that it may also play a role in autoimmune diseases [28], infectious diseases [9], and cancer [14]. In autoimmune diseases, sST2 has been shown to be associated with disease activity in conditions such as systemic lupus erythematosus [29] and rheumatoid arthritis [30]. In infectious diseases, sST2 levels have been reported to be elevated in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [31] and sepsis [9, 32]. In cancer, sST2 levels have been linked to tumor progression and metastasis [15, 16]. sST2 has also been investigated in cardiovascular diseases, such as acute myocardial infarction [33], heart failure [12], and atherosclerosis [34]. Furthermore, sST2 has been studied in the context of chronic obstructive pulmonary disease [35], chronic kidney disease [11], and type 2 diabetes mellitus [36].
Therefore, it is actually surprising that there has been no data on the association between sST2 and H. pylori infection so far. Based on our data, we can exclude such an association for asymptomatic patients. Nevertheless, we consider it important to document and publish our unequivocal negative results. First, to avoid reporting bias. Reporting bias refers to the systematic error that can arise in research studies when there is selective reporting or publication of certain results or outcomes over others [37]. It can occur when the researchers choose to report only the results that support their hypothesis or research question, or if journals are more likely to accept and publish positive results rather than negative or inconclusive ones [38, 39]. This bias can lead to an incomplete or misleading understanding of the research question being studied, as important information may be left out or overemphasized. It can also lead to a skewed perception of the efficacy or safety of a particular intervention or treatment. Reporting bias can be minimized by ensuring that all study outcomes are reported, regardless of whether they are statistically significant or not. Second, to state that sST2 is definitely not a suitable biomarker for the detection of H. pylori. And third, to show scientists who are investigating other diseases (diabetes, cancer, cardiovascular diseases) and sST2 that any concomitant asymptomatic H. pylori infection does not bias their results.
Conclusion
In conclusion, this study found no significant association between H. pylori infection and sST2 levels in asymptomatic patients. The median sST2 concentration was similar in both infected and non-infected patients. Logistic regression analysis and sensitivity analyses also did not show any association between sST2 levels and H. pylori infection. These results suggest that sST2 may not serve as a useful biomarker for the diagnosis and management of H. pylori infection. These findings have relevance for researchers investigating sST2 in various diseases and indicate that asymptomatic H. pylori infection does not bias the results of sST2 studies.
Author's contribution
SW, BW, ML and CD conceived the presented idea. All authors contributed to the final version of the manuscript and approved the final version.
Funding
Open access funding provided by Paracelsus Medical University. There is no funding to report for this submission.
Data availability
Data are available upon reasonable request.
Declarations
Competing interests
CD is part of the scientific advisory board of SPAR Austria.
Ethical approval
We performed the study and all procedures according to the principles of the Declaration of Helsinki. The local ethics committee for the province Salzburg approved the study protocol (Approval No. 415-E/1262). Written informed consent was obtained from every participant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 2.Braden B. Diagnosis of Helicobacter pylori infection. BMJ. 2012;344:e828. doi: 10.1136/bmj.e828. [DOI] [PubMed] [Google Scholar]
- 3.Kavitt RT, Cifu AS. Management of Helicobacter pylori infection. JAMA. 2017;317:1572–1573. doi: 10.1001/jama.2017.1949. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Hong J, Chung H, et al. Author Correction: Helicobacter pylori eradication affects platelet count recovery in immune thrombocytopenia. Sci Rep. 2020;10:18198. doi: 10.1038/s41598-020-74316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monzón H, Forné M, Esteve M, et al. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol. 2013;19:4166–4171. doi: 10.3748/wjg.v19.i26.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Chen Z, Ma M, et al. Soluble ST2 in coronary artery disease: clinical biomarkers and treatment guidance. Front Cardiovasc Med. 2022;9:924461. doi: 10.3389/fcvm.2022.924461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boga S, Alkim H, Koksal AR, et al. Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Investig Med. 2016;64:1016–1024. doi: 10.1136/jim-2016-000062. [DOI] [PubMed] [Google Scholar]
- 9.Hur M, Kim H, Kim HJ, et al. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. 2015;35:570–577. doi: 10.3343/alm.2015.35.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwahana H, Yanagisawa K, Ito-Kosaka A, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 11.Homsak E, Gruson D. Soluble ST2: a complex and diverse role in several diseases. Clin Chim Acta. 2020;507:75–87. doi: 10.1016/j.cca.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Savarimuthu S, Goel P, Harky A. Soluble ST2: a valuable prognostic marker in heart failure. Heart Fail Rev. 2022;27:2155–2164. doi: 10.1007/s10741-022-10258-2. [DOI] [PubMed] [Google Scholar]
- 13.Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:3B–7B. doi: 10.1016/j.amjcard.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 14.De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: role in health and disease. Cytokine Growth Factor Rev. 2015;26:615–623. doi: 10.1016/j.cytogfr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Lu D-P, Zhou X-Y, Yao L-T, et al. Serum soluble ST2 is associated with ER-positive breast cancer. BMC Cancer. 2014;14:198. doi: 10.1186/1471-2407-14-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergis D, Kassis V, Radeke HH. High plasma sST2 levels in gastric cancer and their association with metastatic disease. Cancer Biomark. 2016;16:117–125. doi: 10.3233/CBM-150547. [DOI] [PubMed] [Google Scholar]
- 17.Kuo C-J, Chen C-Y, Lo H-R, et al. Helicobacter pylori induces IL-33 production and recruits ST-2 to lipid rafts to exacerbate inflammation. Cells. 2019 doi: 10.3390/cells8101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernly S, Wernly B, Semmler G, et al. Non-alcoholic fatty liver disease is not independently associated with Helicobacter pylori in a Central European screening cohort. Minerva Med. 2022 doi: 10.23736/S0026-4806.22.07928-9. [DOI] [PubMed] [Google Scholar]
- 19.Wernly S, Semmler G, Völkerer A, et al. Cardiovascular risk assessment by SCORE2 predicts risk for colorectal neoplasia and tumor-related mortality. J Pers Med. 2022 doi: 10.3390/jpm12050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semmler G, Wernly S, Wernly B, et al. Machine learning models cannot replace screening colonoscopy for the prediction of advanced colorectal adenoma. J Pers Med. 2021 doi: 10.3390/jpm11100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter N, Nichols A. VIOPLOT: Stata module to produce violin plots with current graphics. Published Online First: 17 June 2012. https://EconPapers.repec.org/RePEc:boc:bocode:s456902 (Accessed 28 Feb 2023).
- 23.Gonciarz W, Krupa A, Moran AP, et al. Interference of LPS H pylori with IL-33-driven regeneration of caviae porcellus primary gastric epithelial cells and fibroblasts. Cells. 2021 doi: 10.3390/cells10061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buzzelli JN, Chalinor HV, Pavlic DI, et al. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell Mol Gastroenterol Hepatol. 2015;1:203–21.e3. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfetsch V, Sanin V, Jaensch A, et al. Increased plasma concentrations of soluble ST2 independently predict mortality but not cardiovascular events in stable coronary heart disease patients: 13-year follow-up of the KAROLA study. Cardiovasc Drugs Ther. 2017;31:167–177. doi: 10.1007/s10557-017-6718-1. [DOI] [PubMed] [Google Scholar]
- 26.Hammer F, Genser B, Dieplinger B, et al. Soluble suppression of tumorigenesis-2 is a strong predictor of all-cause, cardiovascular and infection-related mortality risk in haemodialysis patients with diabetes mellitus. Clinical Kidney J. 2022;15:1915–1923. doi: 10.1093/ckj/sfac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gürgöze MT, van Vark LC, Baart SJ, et al. Multimarker analysis of serially measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, creatinine, and prognosis in acute heart failure. Circ Heart Fail. 2023;16:e009526. doi: 10.1161/CIRCHEARTFAILURE.122.009526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakerian L, Kolahdooz H, Garousi M, et al. IL-33/ST2 axis in autoimmune disease. Cytokine. 2022;158:156015. doi: 10.1016/j.cyto.2022.156015. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Wei F, Liu Y, et al. IL-33/ST2 activation is involved in Ro60-regulated photosensitivity in cutaneous lupus erythematosus. Mediators Inflamm. 2022;2022:4955761. doi: 10.1155/2022/4955761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L-J, Liu C, Li J-H, et al. Elevated levels of soluble ST2 were associated with rheumatoid arthritis disease activity and ameliorated inflammation in synovial fibroblasts. Chin Med J. 2018;131:316–322. doi: 10.4103/0366-6999.223847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park M, Hur M, Kim H, et al. Soluble ST2 as a useful biomarker for predicting clinical outcomes in hospitalized COVID-19 patients. Diagnostics (Basel) 2023 doi: 10.3390/diagnostics13020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Nynatten LR, Slessarev M, Martin CM, et al. Novel plasma protein biomarkers from critically ill sepsis patients. Clin Proteomics. 2022;19:50. doi: 10.1186/s12014-022-09389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Søndergaard FT, Beske RP, Frydland M, et al. Soluble ST2 in plasma is associated with post-procedural no-or-slow reflow after primary percutaneous coronary intervention in ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2023;12:48–52. doi: 10.1093/ehjacc/zuac146. [DOI] [PubMed] [Google Scholar]
- 34.Pfetsch V, Sanin V, Jaensch A, et al. Increased concentrations of soluble ST2 independently predict cardiac and total mortality but not non-fatal cardiovascular events in stable coronary heart disease patients. Atherosclerosis. 2017;263:e46–e47. doi: 10.1016/j.atherosclerosis.2017.06.159. [DOI] [PubMed] [Google Scholar]
- 35.Urban MH, Stojkovic S, Demyanets S, et al. Soluble ST2 and all-cause mortality in patients with chronic obstructive pulmonary disease—a 10-year cohort study. J Clin Med Res. 2021;11:56. doi: 10.3390/jcm11010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeyda M, Wernly B, Demyanets S, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes. 2013;37:658–665. doi: 10.1038/ijo.2012.118. [DOI] [PubMed] [Google Scholar]
- 37.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 38.Chan A-W, Krleza-Jerić K, Schmid I, et al. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ. 2004;171:735–740. doi: 10.1503/cmaj.1041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahni P, Aggarwal R. Reporting and Publishing Research in the Biomedical Sciences. Singapore: Springer; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.