Abstract
Purpose
To analyze and summarize the efficacy of immune checkpoint inhibitor (ICI) alone or in combination therapy for renal cell carcinoma (RCC) and urothelial carcinoma (UC) stratified by sex.
Methods
Three databases were queried in October 2022 for randomized controlled trials (RCTs) analyzing RCC and UC patients treated with ICIs. We analyzed the association between sex and the efficacy of ICIs in RCC and UC patients across several clinical settings. The outcomes of interest were overall survival (OS) and progression-free survival for the metastatic setting and disease-free survival (DFS) for the adjuvant setting.
Results
Overall, 16 RCTs were included for meta-analyses and network meta-analyses. In the first-line treatment of metastatic RCC (mRCC) and UC (mUC) patients, ICI-based combination therapies significantly improved OS compared to the current standard of care, regardless of sex. Adjuvant ICI monotherapy reduced the risk of disease recurrence in female patients with locally advanced RCC (pooled hazard ratio [HR]: 0.71, 95% confidence interval [CI] 0.55–0.93) but not in male patients, and, conversely, in male patients with muscle-invasive UC (pooled HR: 0.80, 95%CI 0.68–0.94) but not in female patients. Treatment ranking analyses in the first-line treatment of mRCC and mUC showed different results between sexes. Of note, regarding adjuvant treatment for RCC, pembrolizumab (99%) had the highest likelihood of improved DFS in males, whereas atezolizumab (84%) in females.
Conclusions
OS benefit of first-line ICI-based combination therapy was seen in mRCC and mUC patients regardless of sex. Sex-based recommendations for ICI-based regimens according to the clinical setting may help guide clinical decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00345-023-04412-0.
Keywords: Sex, Gender, Immune checkpoint inhibitors, Renal cell carcinoma, Urothelial carcinoma, Metastatic, Advanced, Adjuvant
Introduction
The inclusion of immune checkpoint inhibitors (ICIs) has changed the treatment landscape of metastatic renal cell (mRCC) and urothelial (mUC) carcinoma [1, 2]. Earlier use of these therapies such as in the adjuvant therapy for locally advanced RCC and UC has shown promise in randomized controlled trials (RCTs) [3, 4].
Considering the patients’ sex is one of the first steps towards personalized medicine [5–7]. For example, female sex is established as a prognosticator of worse survival in patients with muscle-invasive bladder UC [7]. In mRCC, similarly, sex-related discrepancies in the distribution of metastases exist [8]. These differences between men and woman suggest that biological, genetic, and social differences between sexes play an important role in the biology and natural history (i.e., response to therapy) of the underlying disease.
Indeed, immunity and immune response varies among sexes [9, 10], as demonstrated in several cancers, such as glioblastoma or non-small cell lung cancer [11, 12]. A pan-cancer meta-analysis including melanoma and non-small cell lung cancer showed that the overall survival (OS) benefit from ICI was significantly worse in female patients than in male patients [13]. However, these data suffer from disease heterogeneity. Specifically, pooled data on sex-specific differences in the efficacy of ICI focusing on urologic cancers is scarce [14]. Therefore, we conducted this systematic review, meta-analysis, and network meta-analysis (NMA) to comprehensively assess the sex-specific differential efficacy of the ICI monotherapy or ICI-based combination therapies on survival outcomes of urologic cancers in both the metastatic and adjuvant settings. Based on different biology, we separately analyzed RCC and UC patients.
Methods
The protocol has been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42022368243).
Search strategy
This systematic review, meta-analysis, and NMA was conducted based on the guidelines of the Preferred Reporting Items for Meta-Analyses of Observational Studies in Epidemiology Statement (Supplementary Table 1)[15]. A literature search on PubMed®, Web of Science™, and Scopus® databases was performed in October 2022 to identify studies investigating the oncologic outcomes of ICI monotherapy or ICI-based combination therapies for RCC or UC. The detailed search strategy is described in Supplementary Appendix. Abstracts presented at recent major conferences were reviewed to include unpublished RCTs and trials’ updates. The outcome measurements of interest were OS and progression-free survival (PFS) for the metastatic setting and disease-free survival (DFS) for the adjuvant setting. Two investigators independently performed the initial screening based on the titles and abstracts to identify eligible studies. Disagreements were resolved by consensus with co-authors.
Inclusion and exclusion criteria
Studies were included if they investigated RCC and UC patients (Patients) and compared the efficacy of the ICI monotherapy or ICI-based combination therapy (Interventions) with the efficacy of standard of care (SOC) at the time of study enrollment (Comparisons) to assess their differential effects on OS and/or PFS between sexes (Outcome) in an RCT (Study design). Studies lacking original patient data, reviews, letters, editorial comments, replies from authors, case reports, and articles not written in English were excluded. References of all papers included were scanned for additional studies of interest.
Data extraction
The following data were independently extracted by two authors; studies and the first author’s name, publication year, inclusion criteria, agents, number of patients stratified by sex, follow-up periods, International Metastatic RCC Database Consortium (IMDC) classification and objective response rates (ORRs) for mRCC patients. Subsequently, the hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and/or PFS, or DFS were retrieved. The IMmotion151 trial, which compared the efficacy of atezolizumab + bevacizumab versus sunitinib in previously untreated mRCC, did not provide data on differential oncologic outcomes stratified by sex [16]. In addition, the IMvigor211 trial, which compared the efficacy of atezolizumab versus chemotherapy in mUC, also did not provide data on relevant oncologic outcomes; therefore, these two RCTs were excluded [17].
Risk of bias assessment
Assessment of study quality and risk of bias was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions risk-of-bias tool (RoB version 2) (Supplementary Fig. 1)[18]. The risk-of-bias assessment of each study was performed independently by two authors.
Statistical analyses
For meta-analysis, forest plots with HRs were used to analyze the association between ICI therapy and oncologic outcomes. PFS was defined as the time from treatment initiation to radiological progression evaluated by investigator-assessed Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1), clinical progression, or death. DFS was defined as the time from randomization to the first documented local or distant recurrence or death, whichever occurred first. A fixed-effect model was used for calculations of HRs [19]. Heterogeneity among the outcomes of included studies in this meta-analysis was assessed using Cochrane’s Q test. When significant heterogeneity (p-value of < 0.05 in Cochrane’s Q test) was observed, we attempted to investigate the cause of heterogeneity [20]. Funnel plots were created to evaluate the publication bias using Review Manager 5.3 Software (RevMan; The Cochrane Collaboration, Oxford, UK, Supplementary Fig. 2).
For network meta-analysis, we performed a network meta-analysis using random-effect models with a frequentist approach for direct and indirect treatment comparisons with regard to OS, PFS, and DFS [21, 22]. In the assessment of oncologic outcomes, contrast-based analyses were applied with estimated differences in the log HR and the standard error calculated from the published HR and CI [23]. The relative effects were presented as HRs and 95% CI [21]. We also estimated the relative ranking of the different regimens for OS, PFS, and DFS using the surface under the cumulative ranking (SUCRA) [21].
All analyses were conducted using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria), and the statistical significance level was set at P < 0.05.
Results
Study selection and characteristics
Our initial search identified 4,654 records. After removing duplicates, 2,533 records remained for screening titles and abstracts (Supplementary Fig. 3). After screening, a full-text review of 48 articles was performed. According to the inclusion criteria, we finally identified 16 RCTs (19 publications) eligible for meta-analyses or NMAs [3, 4, 24–40]. Of the 16 RCTs, nine included RCC patients [3, 24–34] and seven included UC patients [4, 35–40]. The demographics of each included study are shown in Supplementary Table 2 and 3. There were 2,038 female patients out of 7,615 patients (27%) in the RCC studies and 1,181 out of 5,000 patients (24%) in the UC studies.
RCC
Study selection and characteristics
The study demographics and oncologic outcomes of included studies are shown in Supplementary Table 2. Among nine studies included, five studies comprising 4,206 patients, assessed ICI-based combination therapy in mRCC as 1st-line treatment [24, 25, 27–29, 31, 33], one study investigated ICI monotherapy in mRCC in the 2nd-or 3rd line of therapy for disease progression after tyrosine kinase inhibitors (TKIs) [26], and three studies comprising 2,588 patients evaluated ICI monotherapy or combination therapy in locally advanced RCC as adjuvant therapy [3, 30, 32, 34]. Control arms of all studies of 1st line ICI-based combination therapy were sunitinib, however, the control arm of the CheckMate025 trial for 2nd- or 3rd-line therapy of mRCC was everolimus.
Meta-analysis
The results of our meta-analyses and NMAs are summarized in Supplementary Table 4.
Efficacy of ICI-based combination therapy for mRCC
OS: Systemic therapy with ICI significantly reduced the risk of overall mortality in male (pooled HR: 0.75, 95%CI: 0.67–0.84) and female patients (pooled HR: 0.67, 95%CI: 0.56–0.80) compared to TKI or mTOR inhibitors (Supplementary Fig. 1). There was no significant difference between male and female patients in terms of the OS benefit with ICI (p = 0.3).
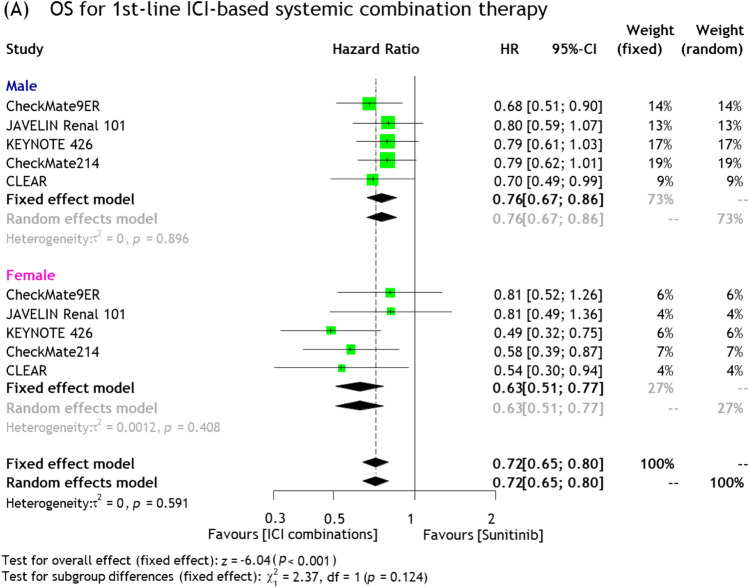
When limited to the 1st-line setting, ICI-based combination therapy significantly improved OS in both male (pooled HR: 0.76, 95%CI 0.67–0.86) and female (pooled HR: 0.63, 95%CI 0.51–0.77) patients compared to sunitinib alone; there was no statistically significant difference between the sexes (p = 0.12, Fig. 1A). There was no significant heterogeneity in these analyses.
Fig. 1.
Forest plots showing association of survival outcomes and ICI therapy for RCC stratified by sex; OS A and PFS B for 1st-line ICI-based systemic combination therapy, and C DFS for adjuvant ICI therapy for locally advanced RCC
PFS: In the 1st-line setting, ICI-based combination therapy also significantly reduced the risk of disease progression in both male (pooled HR: 0.63, 95%CI 0.45–0.88) and female (pooled HR: 0.69, 95%CI 0.58–0.81) patients compared to sunitinib alone, with no statistically significant difference in PFS between the sexes (p = 0.8, Fig. 1B). Cochrane’s Q tests revealed significant heterogeneity in the analysis of male patients (p < 0.001).
ORR: Four RCTs provided data on ORRs stratified by sex. The forest plot showed no statistical differences in ORR between male and female patients (pooled OR: 1.03, 95%CI 0.83–1.28, Supplementary Fig. 5).
Efficacy of adjuvant ICI therapies for locally advanced RCC
As shown in Fig. 1C, adjuvant ICI therapy significantly reduced the risk of disease recurrence in female patients (pooled HR: 0.71, 95%CI 0.55–0.93), whereas there was no statistically significant improvement in the recurrence rate in male patients (pooled HR: 0.86, 95%CI 0.60–1.23). No statistically significant differences were seen in OS between the sexes (p = 0.4). Cochrane’s Q tests revealed significant heterogeneity in the analysis of male patients (p = 0.008).
Network meta-analysis
1st-line ICI-based combination therapies for mRCC
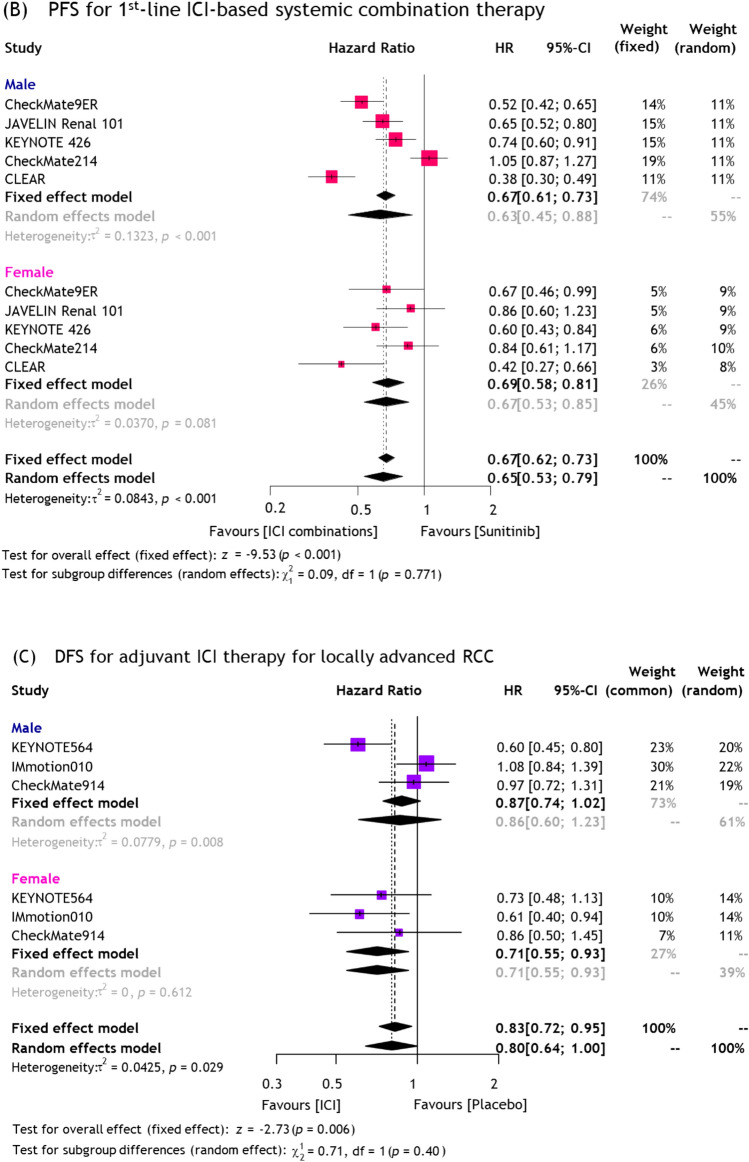
Five different ICI-based regimens, such as nivolumab + cabozantinib, avelumab + axitinib, nivolumab + ipilimumab, pembrolizumab + axitinib, and pembrolizumab + lenvatinib were included in this NMA. Network plots of all NMAs are depicted in Supplementary Fig. 6. As shown in Fig. 2, compared to sunitinib alone, nivolumab + cabozantinib (HR: 0.68, 95%CI 0.51–0.90) and pembrolizumab + lenvatinib (HR: 0.70, 95%CI: 0.49–0.99) reduced the risk of overall mortality in male patients, while pembrolizumab + axitinib (HR: 0.49, 95%CI 0.32–0.75), pembrolizumab + lenvatinib (HR: 0.54, 95%CI 0.30–0.94), and nivolumab + ipilimumab (HR: 0.58, 95%CI 0.39–0.87) reduced the risk of overall mortality in female patients. The SUCRA analysis of treatment rankings revealed that nivolumab + cabozantinib had the highest likelihood of providing the maximal OS benefit in males (78%) and pembrolizumab + axitinib had the highest likelihood of providing the maximal OS benefit in females (84%) (Fig. 2). Analysis for PFS is described in Supplementary Fig. 7.
Fig. 2.
Forest plots and SUCRA graph from NMAs for A OS in mRCC patients treated with 1st-line systemic treatment and B DFS in locally advanced RCC patients treated with adjuvant ICI therapy
Adjuvant ICI therapies for locally advanced RCC
Three different ICI-based regimens, including pembrolizumab, atezolizumab, and nivolumab + ipilimumab, were eligible for this NMA. As shown in Fig. 2, compared to placebo, only pembrolizumab (HR: 0.60, 95%CI 0.45–0.80) reduced the risk of disease recurrence in male patients, while only atezolizumab (HR: 0.61, 95%CI 0.40–0.94) reduced the risk of disease recurrence in female patients. The SUCRA analysis of treatment rankings revealed that pembrolizumab had the highest likelihood of providing the maximal DFS benefit in males (99%) and atezolizumab had the highest likelihood of providing the maximal DFS benefit in females (84%) (Fig. 2).
UC
Study selection and characteristics
The study demographics and oncologic outcomes of included studies are shown in Supplementary Table 3. Of seven studies included, three studies comprising 2,240 patients assessed ICI-based combination therapy for mUC as 1st-line treatment [36, 37, 39], one study investigated pembrolizumab as 2nd-line treatment for progression after 1st line chemotherapy for mUC [35], one study for maintenance treatment after first-line chemotherapy for locally advanced or mUC [38], and two studies comprising 1518 patients evaluated ICI monotherapy as adjuvant therapy in muscle-invasive UC patients after radical surgery [4, 40].
Meta-analysis
Efficacy of ICI-based systemic therapy for mUC
Systemic therapy with ICI significantly reduced the risk of overall mortality in male patients (pooled HR: 0.80, 95%CI: 0.73–0.88) as well as female patients (pooled HR: 0.84, 95%CI: 0.70–1.00) compared to SOC (Supplementary Fig. 8). There was no significant difference between male and female patients in terms of the OS benefit with regards to systemic therapy with ICI (p = 0.7).
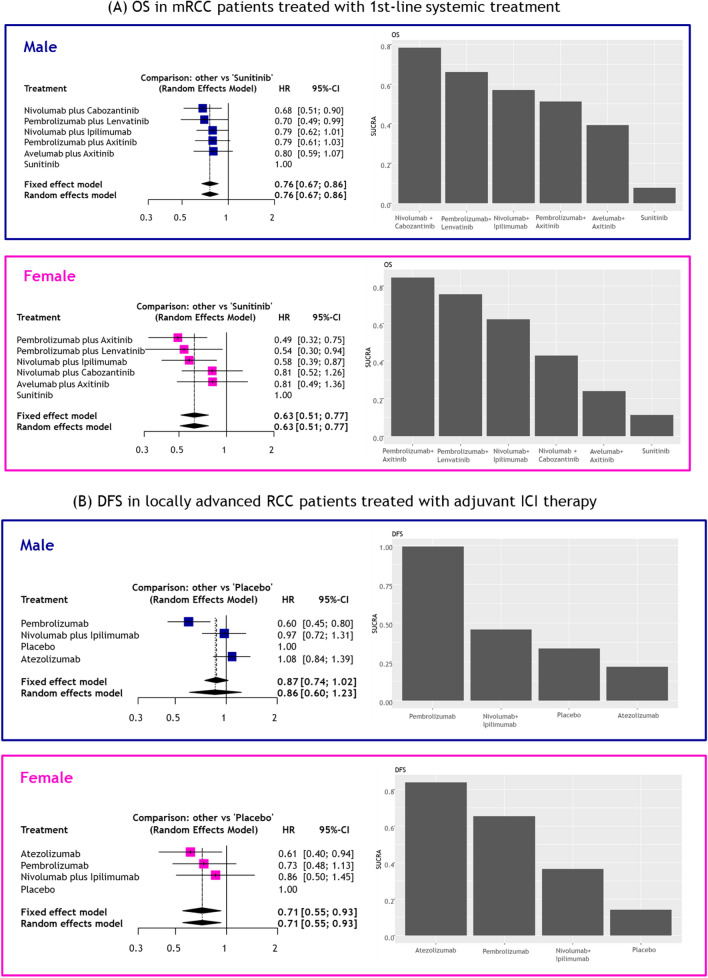
When limited to the 1st-line setting, ICI-based combination therapy significantly reduced the risk of overall mortality in male (pooled HR: 0.86, 95%CI: 0.76–0.96), but not in female (pooled HR: 0.84, 95%CI: 0.68–1.04) patients, compared to chemotherapy (Fig. 3). There were no statistically significant differences in OS between sexes (p = 0.9, Fig. 3A). We did not find any significant heterogeneity in these analyses.
Fig. 3.
Forest plots showing association of survival outcomes and ICI therapy for UC stratified by sex; A OS for 1st-line ICI-based systemic combination therapy and B DFS for adjuvant ICI therapy for locally advanced UC
Efficacy of adjuvant ICI therapies for locally advanced UC
Adjuvant ICI therapy significantly reduced the risk of disease recurrence in male (pooled HR: 0.80, 95%CI: 0.68–0.94), but not in female (pooled HR: 0.87, 95%CI: 0.64–1.17) patients, compared to placebo or observation (Fig. 3B). No statistically significant differences were observed in DFS between sexes (p = 0.6). We did not find significant heterogeneity in the analyses.
Network meta-analysis of 1st-line ICI-based combination therapies for mUC
Three different ICI-based regimens, including atezolizumab + chemotherapy, durvalumab + tremelimumab, and pembrolizumab + chemotherapy, were eligible for this NMA. The SUCRA analysis of treatment rankings revealed that atezolizumab + chemotherapy (77%) in males and pembrolizumab + chemotherapy (81%) in females to provide the highest likelihood of maximal OS benefit (Supplementary Fig. 9).
Discussion
In this meta-analysis and NMA, we comprehensively evaluated the differential impact of sex on oncologic outcomes in both RCC and UC patients treated with ICI-based treatment. We found several key findings. First, in the first-line treatment for mRCC and mUC patients, ICI-based combination therapies significantly improved OS compared to the current standard of care regardless of sex. Second, adjuvant ICI monotherapy significantly reduced the risk of disease recurrence in female patients with locally advanced RCC and in male patients with muscle-invasive UC, whereas the reverse did not reach statistical significance. Third, treatment ranking analyses in each clinical setting of RCC and UC showed different results between sexes.
Sex-dependent immune responses are an emerging area of research [9, 10]. For example, sex hormones and X chromosome number seem to be associated with type-1 interferon (IFN-1) response [10]. The pathway relating to host defense, which is orchestrated by IFN-1, displays different activity between sexes and potentially contributes to differences in immune responses to immunotherapy between the sexes [9]. In the context of pan-cancer analyses of ICI therapy, sex has been reported as an important variable in determining response to treatment, with a trend to inferior response in female patients [13, 41]. Explanation for the observed disparity between sexes in the response to ICI therapy includes differences in the expression of programmed death-ligand 1 (PD-L1) which is partly regulated by estrogen [42]. However, despite these hypotheses and previous findings, our analyses revealed no significant differences between sexes in the efficacy of ICI-based systemic therapy for mRCC and mUC.
In the 1st-line mRCC setting, our meta-analysis revealed that ICI-based combination therapies reduced the risk of death by 24% in male and 37% in female patients, compared to sunitinib alone. The difference between sexes did not reach statistical significance (p = 0.12). Despite this lack of statistical significance, females seem to have a larger benefit of ICI therapy in mRCC as well as the adjuvant RCC setting. This disparity in survival outcomes in RCC patients between sexes could be related to genetic, hormonal, and/or social (i.e., behavioral) differences. Tulchiner et al. found an increase in estradiol and luteinizing hormone (LH)/ follicle-stimulating hormone (FSH) ratio in male patients during nivolumab monotherapy for mRCC; they also reported that the increased LH/FSH ratio was associated with worse PFS and ORR [43]. Sex disparities in oncologic outcomes in mRCC patients remain controversial.
In mRCC patients, our NMAs showed sex-specific differential treatment rankings in which nivolumab + cabozantinib had the highest likelihood of reduced risk of overall mortality in males, while pembrolizumab + axitinib had the highest likelihood in females. Interestingly, nivolumab + cabozantinib ranked fourth in female patients, and pembrolizumab + axitinib also ranked fourth in male patients. Moreover, in a recently published NMA, pembrolizumab + lenvatinib had the highest probability of being the best treatment in terms of OS among all mRCC patients [44]. In addition, in the adjuvant setting, despite atezolizumab not showing a DFS benefit in the entire cohort [32], atezolizumab significantly reduced the risk of disease progression in females compared to placebo. Even though a rationale for these different efficacies was not evaluated, our results might help improve clinical decision-making and personalizing treatment allocation according to the sex. Further investigations of different cancer states with combination regimens are warranted to obtain a definitive supporting rationale for the sex disparity regarding the efficacy of ICI-based therapy for RCC.
In urothelial carcinoma of the bladder (UCB), sex-related differences in the incidence, etiology, and response to immunotherapy are well documented [45]. In muscle-invasive UCB patients, the sex disparity in survival outcomes was demonstrated in recent meta-analyses, while the differential outcomes were not seen in non-muscle invasive bladder cancer (NMIBC) or upper tract UC [6, 7]. The studies concluded that female sex is associated with worse survival outcomes, including cancer-specific and OS [6, 7]. In addition, in the context of immunotherapy for UCB, sex differences are known with regards to response to intravesical Bacillus Calmette-Guérin (BCG) which is used for the treatment of NMIBC [46]. Regarding systemic immunotherapy for UC, our analysis revealed that adjuvant ICI monotherapy significantly reduced the risk of disease recurrence in male patients with locally advanced UC following radical surgery, whereas risk reduction did not reach statistical significance in female patients. This is in line with recent evidence suggesting that estrogens contribute to increased PD-L1 expression [42]. However, in mUC patients, despite worse prognosis and immune response to ICI in females based on previous evidence, our analysis revealed that first-line ICI-based combination therapies reduced the risk of death by the same margin in male and female patients (14% and 16%, respectively) compared to chemotherapy alone. Several biological sex disparities, such as the protective role of estrogen against carcinogenesis or enrichment of basal subtype in females have been reported [45]. Further investigations, specifically in UCB patients treated with ICI-based systemic therapy, are warranted.
In mUC patients, a recent meta-analysis revealed that ICI-based combination therapy significantly reduced the risk of death in the entire cohort and atezolizumab + chemotherapy had the highest likelihood of providing the maximal OS [47]. Interestingly, our differential treatment ranking depending on patients’ sex indicated that atezolizumab + chemotherapy had the highest likelihood of reducing the risk of overall mortality in males, while pembrolizumab + chemotherapy had the highest in females. Despite the limitation of fewer female patients as well as restrictions related to subgroup analysis, our findings might help guide clinical decision-making. Again, more investigations are needed to obtain the rationale for the differences.
The present study has several limitations that need to be considered. First, included RCTs differed in patient populations, such as the proportion of disease, burden, as well as the type of sequential therapies. Second, our analyses were performed based on subgroup analyses of each RCT, therefore sometimes suffering from a limited number of patients. Indeed, fewer female patients were included in all studies with approximately 25% of included patients being female in both RCC and UC studies. Third, for the metastatic setting, most trials assessed ICI-based combination regimen. Therefore, sex differences in efficacy cannot be attributed to ICI alone. Fourth, NMAs have a limited role in facilitating proper patient selection for current treatment options; this approach cannot substitute a direct comparison of each treatment. Finally, other than immune response, anatomical, genetic, and/or hormonal differences can influence outcomes and tumor behaviors. Further investigation of the multifactorial origin of sex-related disparities in the incidence and outcomes of UC and RCC is needed to facilitate a step forward towards personalized medicine in the era of immune therapy.
Conclusions
In mRCC and mUC patients, OS benefit from 1st-line ICI-based combination therapy was comparable, regardless of sex. Our treatment ranking analyses showed different ICI-based regimens to be the preferred according to patient sex and clinical setting, suggesting that recommendations of ICI-based regimens considering the sex might help guide clinical decision-making. Further investigation into potential sex disparities in the immune response to ICI is needed to select the patients most likely to benefit from a specific ICI-based combination therapy. There is no doubt that sex remains an important determinant in the choice and outcome of urologic oncologic therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BCG
Bacillus Calmette-Guérin
- CIs
Confidence intervals
- DFS
Disease-free survival
- FSH
Follicle-stimulating hormone
- ICI
Immune checkpoint inhibitor
- IFN-1
Type 1 interferon
- IMDC
International Metastatic RCC Database Consortium
- LH
Luteinizing hormone
- mRCC
Metastatic renal cell carcinoma
- mTOR
Mechanistic target of rapamycin
- mUC
Metastatic urothelial carcinoma
- NMA
Network meta-analysis
- NMIBC
Non-muscle invasive bladder cancer
- ORR
Objective response rate
- OS
Overall survival
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- RCT
Randomized controlled trial
- RCC
Renal cell carcinoma
- SOC
Standard of care
- SUCRA
Surface under the cumulative ranking
- TKI
Tyrosine kinase inhibitor
- UC
Urothelial carcinoma
- UCB
Urothelial carcinoma of bladder
Author contributions
TY contributed to protocol/project development, data collection and management, data analysis, and manuscript writing/editing.TK (Tatsushi Kawada) and FQ contributed to data analysis and manuscript writing/editing. KB, EL, PR, MvD, MM, MC, BP, KM contributed to manuscript writing/editing. TK (Takahiro Kimura),MS, and PIK contributed to supervision and manuscript editing. SFS contributed to supervision, protocol/project development/management and manuscript editing.
Funding
Open access funding provided by Medical University of Vienna. NA (no external funding provided), EUSP Scholarship of the European Association of Urology (PR).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen and Sanofi. Shahrokh F. Shariat received follows: Honoraria: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, Takeda Consulting or Advisory Role: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, Takeda Speakers Bureau: Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, Takeda, The other authors declare no conflicts of interest associated with this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takafumi Yanagisawa, Email: t.yanagisawa.jikei@gmail.com.
Tatsushi Kawada, Email: tktk5524@yahoo.co.jp.
Fahad Quhal, Email: F.Quhal@hotmail.com.
Kensuke Bekku, Email: gmd421030@s.okayama-u.ac.jp.
Ekaterina Laukhtina, Email: katyalaukhtina@gmail.com.
Pawel Rajwa, Email: pawelgrajwa@gmail.com.
Markus von Deimling, Email: mvondeimling@gmail.com.
Muhammad Majdoub, Email: majdoxm@gmail.com.
Marcin Chlosta, Email: marcin.p.chlosta@gmail.com.
Benjamin Pradere, Email: benjaminpradere@gmail.com.
Keiichiro Mori, Email: morikeiichiro29@gmail.com.
Takahiro Kimura, Email: tkimura0809@gmail.com.
Manuela Schmidinger, Email: manuela.schmidinger@meduniwien.ac.at.
Pierre I. Karakiewicz, Email: pierrekarakiewicz@gmail.com
Shahrokh F. Shariat, Email: shahrokh.shariat@meduniwien.ac.at
References
- 1.Cathomas R, Lorch A, Bruins HM, Compérat EM, Cowan NC, Efstathiou JA, et al. The 2021 updated European association of urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95–103. doi: 10.1016/j.eururo.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. doi: 10.1016/j.eururo.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385(8):683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 4.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384(22):2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucca I, Klatte T, Fajkovic H, de Martino M, Shariat SF. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat Rev Urol. 2015;12(10):585–592. doi: 10.1038/nrurol.2015.232. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Mostafaei H, Enikeev DV, Lysenko I, Quhal F, Kimura S, et al. Differential Effect of Sex on Outcomes after Radical Surgery for Upper Tract and Bladder Urothelial Carcinoma: A Systematic Review and Meta-Analysis. J Urol. 2020;204(1):58–62. doi: 10.1097/ju.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Yanagisawa T, Katayama S, Laukhtina E, Pradere B, Mostafaei H, et al. Impact of sex on outcomes after surgery for non-muscle-invasive and muscle-invasive bladder urothelial carcinoma: a systematic review and meta-analysis. World J Urol. 2022 doi: 10.1007/s00345-022-04116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosiello G, Pecoraro A, Deuker M, Stolzenbach LF, Martin T, Tian Z, et al. The impact of sex and age on distribution of metastases in patients with renal cell carcinoma. Int J Clin Oncol. 2021;26(5):962–970. doi: 10.1007/s10147-021-01874-3. [DOI] [PubMed] [Google Scholar]
- 9.Capone I, Marchetti P, Ascierto PA, Malorni W, Gabriele L. Sexual dimorphism of immune responses: a new perspective in cancer immunotherapy. Front Immunol. 2018;9:552. doi: 10.3389/fimmu.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb K, Peckham H, Radziszewska A, Menon M, Oliveri P, Simpson F, et al. Sex and pubertal differences in the type 1 interferon pathway associate with both x chromosome number and serum sex hormone concentration. Front Immunol. 2018;9:3167. doi: 10.3389/fimmu.2018.03167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang J, Hong J, Tang X, Qiu X, Zhu K, Zhou L, et al. Sex difference in response to non-small cell lung cancer immunotherapy: an updated meta-analysis. Ann Med. 2022;54(1):2606–2616. doi: 10.1080/07853890.2022.2124449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shireman JM, Ammanuel S, Eickhoff JC, Dey M. Sexual dimorphism of the immune system predicts clinical outcomes in glioblastoma immunotherapy: A systematic review and meta-analysis. Neurooncol Adv. 2022;4(1):082. doi: 10.1093/noajnl/vdac082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746. doi: 10.1016/s1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 14.Hassler MR, Abufaraj M, Kimura S, Stangl-Kremser J, Gust K, Glybochko PV, et al. Impact of patients' gender on efficacy of immunotherapy in patients with metastatic kidney cancer: a systematic review and meta-analysis. Clin Genitourin Cancer. 2020;18(2):88–94.e2. doi: 10.1016/j.clgc.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in immotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8(2):275–280. doi: 10.1001/jamaoncol.2021.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/s0140-6736(17)33297-x. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assel M, Sjoberg D, Elders A, Wang X, Huo D, Botchway A, et al. Guidelines for reporting of statistics for clinical research in urology. Eur Urol. 2019;75(3):358–367. doi: 10.1016/j.eururo.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor MJ, Shah TT, Smigielska K, Day E, Sukumar J, Fiorentino F, et al. Additional treatments to the local tumour for metastatic prostate cancer-assessment of novel treatment algorithms (IP2-ATLANTA): protocol for a multicentre, phase II randomised controlled trial. BMJ Open. 2021;11(2):e042953. doi: 10.1136/bmjopen-2020-042953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 23.Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54. doi: 10.1186/1471-2288-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–1039. doi: 10.1016/j.annonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(7):888–898. doi: 10.1016/s1470-2045(22)00290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. doi: 10.1016/s1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Russo P, Gruenwald V, Tomita Y, Zurawski B, Parikh OA, et al. LBA4 Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial. Ann Oncol. 2022;33:S1430. doi: 10.1016/j.annonc.2022.08.069. [DOI] [Google Scholar]
- 31.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2022;400(10358):1103–1116. doi: 10.1016/s0140-6736(22)01658-0. [DOI] [PubMed] [Google Scholar]
- 33.Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/s1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 34.Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133–1144. doi: 10.1016/s1470-2045(22)00487-9. [DOI] [PubMed] [Google Scholar]
- 35.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/s0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 37.Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/s1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 38.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 39.Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/s1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 40.Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525–537. doi: 10.1016/s1470-2045(21)00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang PF, Song HF, Zhang Q, Yan CX. Pan-cancer immunogenomic analyses reveal sex disparity in the efficacy of cancer immunotherapy. Eur J Cancer. 2020;126:136–138. doi: 10.1016/j.ejca.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Carè A, Bellenghi M, Matarrese P, Gabriele L, Salvioli S, Malorni W. Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death Differ. 2018;25(3):477–485. doi: 10.1038/s41418-017-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tulchiner G, Pichler R, Ulmer H, Staudacher N, Lindner AK, Brunner A, et al. Sex-specific hormone changes during immunotherapy and its influence on survival in metastatic renal cell carcinoma. Cancer Immunol Immunother. 2021;70(10):2805–2817. doi: 10.1007/s00262-021-02882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombardi P, Filetti M, Falcone R, Di Bidino R, Iacovelli R, Ciccarese C, et al. New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: A systematic review and network meta-analysis. Cancer Treat Rev. 2022;106:102377. doi: 10.1016/j.ctrv.2022.102377. [DOI] [PubMed] [Google Scholar]
- 45.Koti M, Ingersoll MA, Gupta S, Lam CM, Li X, Kamat AM, et al. Sex differences in bladder cancer immunobiology and outcomes: a collaborative review with implications for treatment. Eur Urol Oncol. 2020;3(5):622–630. doi: 10.1016/j.euo.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Uhlig A, Strauss A, Seif Amir Hosseini A, Lotz J, Trojan L, Schmid M, et al. Gender-specific differences in recurrence of non-muscle-invasive bladder cancer a systematic review and meta-analysis. Eur Urol Focus. 2018;4(6):924–936. doi: 10.1016/j.euf.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Mori K, Pradere B, Moschini M, Mostafaei H, Laukhtina E, Schuettfort VM, et al. First-line immune-checkpoint inhibitor combination therapy for chemotherapy-eligible patients with metastatic urothelial carcinoma: A systematic review and meta-analysis. Eur J Cancer. 2021;151:35–48. doi: 10.1016/j.ejca.2021.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.