Abstract
Purpose
Despite the success of ICSI in treating severe male factor infertile patients, total fertilization failure (FF) still occurs in around 1–3% of ICSI cycles. To overcome FF, the use of calcium ionophores has been proposed to induce oocyte activation and restore fertilization rates. However, assisted oocyte activation (AOA) protocols and ionophores vary between laboratories, and the morphokinetic development underlying AOA remains understudied.
Methods
A prospective single-center cohort study involving 81 in vitro matured metaphase-II oocytes from 66 oocyte donation cycles artificially activated by A23187 (GM508 CultActive, Gynemed) (n=42) or ionomycin (n=39). Parthenogenesis was induced, and morphokinetic parameters (tPNa, tPNf, t2-t8, tSB, and tB) were compared between the 2 study groups and a control group comprising 39 2PN-zygotes from standard ICSI cycles.
Results
Ionomycin treatment resulted in higher activation rates compared to A23187 (38.5% vs 23.8%, p=0.15). Importantly, none of the A23187-activated parthenotes formed blastocysts. When evaluating the morphokinetic dynamics between the two ionophores, we found that tPNa and tPNf were significantly delayed in the group treated by A23187 (11.84 vs 5.31, p=0.002 and 50.15 vs 29.69, p=0.005, respectively). t2 was significantly delayed in A23187-activated parthenotes when compared to the double heterologous control embryo group. In contrast, the morphokinetic development of ionomycin-activated parthenotes was comparable to control embryos (p>0.05).
Conclusion
Our results suggest that A23187 leads to lower oocyte activation rates and profoundly affects morphokinetic timings and preimplantation development in parthenotes. Despite our limited sample size and low parthenote competence, standardization and further optimization of AOA protocols may allow wider use and improved outcomes for FF cycles.
Keywords: AOA, Calcium ionophores, Morphokinetics, Time-lapse, Ionomycin, ICSI
Introduction
Intracytoplasmic sperm injection (ICSI) has undoubtedly revolutionized the treatment of severe male factor infertility. Since its introduction in 1992, many couples have been treated successfully, resulting in millions of pregnancies worldwide [1, 2]. ICSI generally provides high fertilization rates, yet despite its success, even in the presence of normal sperm parameters [3], total fertilization failure (FF) occurs in up to 1–3% of cycles [4–10]. Patients confronted with FF after ICSI ultimately face limited options for achieving a pregnancy and obtaining genetically related offspring.
Oocyte activation deficiency (OAD) associated with either male or female factors has been established as the most frequent contributor to FF after ICSI [11–13]. Oocyte activation is driven by phospholipase C zeta (PLCZ) [14], which is present in the perinuclear theca of sperm [15]. In vivo, PLCZ is released into the oocyte cytoplasm by the male gamete during its fusion with the oolemma [16]. This process triggers a pathway by which PLCZ hydrolyzes phosphatidylinositol 4,5-biphospate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3) that binds to the IP3 receptor on the endoplasmic reticulum (ER), ultimately resulting in Ca2+ release [13]. This first transient rise in calcium is followed by a series of shorter calcium transient rises of high amplitude, known as calcium oscillations, which decrease in amplitude and frequency as fertilization progresses until absolute cessation [17]. The induced calcium oscillations lead to a resumption of oocyte meiosis, de-condensation of the sperm nucleus, formation of male and female pronuclei, maternal RNA recruitment, initiation of DNA synthesis, embryonic genome activation, and embryo cleavage [18–20]. An aberrant or suppressed pattern of Ca2+ release due to deficiencies in PLCZ function is thus commonly associated with male infertility and abnormal embryo development [21, 22]. Accordingly, several potentially deleterious PLCZ mutations have been linked to male-factor-related OAD and FF after ICSI [23].
To overcome FF, Tesarik and colleagues demonstrated that ICSI combined with assisted oocyte activation (AOA) using Ca2+ ionophores could artificially induce Ca2+ oscillations, resulting in oocyte activation and normal fertilization rates [24]. Since then, AOA has emerged as a promising treatment option for an increasing number of patients facing FF [4, 25–27]. Ca2+ ionophores act by conferring high permeability to cell membranes, allowing the penetration of Ca2+ ions. Oocytes exposed to Ca2+ ionophores experience an increase in free intracytoplasmic Ca+ resulting from a Ca2+ influx and a Ca2+ release from the intracellular stores [28]. This process induces an increase in the cytoplasmic levels of Ca2+, capable of triggering oocyte activation [29]. The most preferred Ca2+ ionophores for AOA currently include ionomycin and A23187 (calcimycin) [30]. Both are carboxylic antibiotics synthesized by Streptomyces Conglobatus and Streptomyces Chartreusensis, respectively. A23187 has recently become an interesting option for AOA in ART laboratories, as it is commercially available, as a ready-to-use solution (GM508 CultActive, Gynemed). Both ionomycin and A23187 have been shown to restore fertilization in patients with FF after ICSI, with accumulating evidence supporting their safety and efficacy for achieving normal embryonic development and live births [4, 12, 26, 31–35]. However, protocol heterogeneity remains an important concern. AOA protocols vary in the choice of laboratory-generated and commercially available agents, ionophore concentration (undisclosed by manufacturers) [30], and the timing and duration of exposure [12]. There are currently no clinical guidelines or industry standards regarding the use of ionophores for AOA.
When evaluating ionophores, ionomycin appears more potent and specific compared to A23187 [36], with higher activation and calcium-releasing potential [30]. Yet higher activation rates do not necessarily translate into more live births [12]. Accordingly, animal studies have suggested that alterations in Ca2+ levels and oscillatory patterns may profoundly affect embryonic development, blastocyst quality, and implantation potential [37–40]. The effects of different Ca2+ ionophores on human embryo morphokinetic patterns remain limited. Martinez et al. compared parameters of embryos derived from ICSI-AOA and ICSI cycles and suggest that ionomycin-mediated AOA does not affect early development [41]. Similarly, the use of A23187 did not significantly alter major embryo morphokinetic patterns [42]. However, given the complexity of mechanisms regulating Ca2+ levels and oscillations during oocyte activation, further investigations are warranted. As Ca+ oscillation patterns elicited by ionophores do not reflect the physiological oocyte activation process [12, 43], evaluating associations between AOA and embryo morphokinetic parameters may provide greater insights into the safety of this procedure.
In this study, we aimed to compare activation rates, developmental potential, and morphokinetic patterns following the application of ionomycin and A23187 for AOA in human oocytes. Due to the ethical and legal issues surrounding the use of human oocytes and sperm, we performed this comparison using a parthenogenetic model (activating oocytes without injecting sperm). Parthenogenesis can be induced by chemical agents to recapitulate the Ca2+ oscillations induced by sperm during fertilization. Parthenogenetic activation of mammalian oocytes by AOA results in early development and remains a valuable model for understanding factors underlying embryogenesis [44]. Morphokinetic parameters of the parthenotes activated with ionomycin and A23187 were compared to an ICSI control group performed with frozen donor oocytes and donor sperm.
Materials and methods
Study population
This prospective, single-center cohort study included 81 frozen in vitro matured (IVM) metaphase-I oocytes obtained for research from 66 oocyte donation cycles. Two AOA study groups were defined depending on the calcium ionophore used: A23187 (GM508 CultActive, Gynemed) (n=42) and ionomycin (n=39). The control group included 39 2PN zygotes randomly selected from standard ICSI cycles using frozen-warmed donor eggs and donor sperm without AOA. All embryos and parthenotes were cultured until the blastocyst stage in a time-lapse (TL) incubator (EmbryoScope®, Vitrolife, Sweden).
Ovarian stimulation and standard laboratory procedures
All oocyte donors underwent controlled ovarian stimulation induced with either recombinant FSH (Gonal®, Merck-Serono, Spain; Bemfola®, Gedeon Richter Iberica, Spain) or highly purified hCG (Menopur®, Ferring, Spain). Pituitary inhibition was performed with a GnRH antagonist (Orgalutran®, Organon, Netherlands). Ovulation was triggered when ≥3 follicles of ≥18 mm diameter were observed, using 0.3 mg of Triptorelin (Decapeptyl®, Ipsen Pharma, France). Retrieval of cumulus-oocyte complexes (COCs) was performed by ultrasound-guided transvaginal follicular aspiration after 36 h. Oocyte denudation was performed mechanically by exposing oocytes to 80 IU/ml of hyaluronidase (Hyase-10X®, Vitrolife, Goteborg, Sweden), followed by gentle pipetting.
MI oocytes were in vitro matured (IVM) by incubation in G2™ plus medium (Vitrolife, Sweden). Maturation was assessed by evaluating the presence of one polar body 2–6 h after oocyte pick up (OPU). IVM oocytes were vitrified using Kuwayama’s method [45], Kitazato vitrification media, and the cryotop vitrification open system (Kitazato BIOPharma Co., Ltd, Fuji, Japan). The vitrified IVM oocytes were warmed using the Kitazato warming protocol and media (Kitazato BIOPharma Co., Ltd, Fuji, Japan).
For the control group, timelapse data from existing embryos was used. These embryos were generated using frozen-warmed donor MII oocytes, which were first incubated in culture medium (SAGE 1-step™) 2–4 h post OPU and then were vitrified and warmed as above [45]. Standard ICSI was performed using donor sperm. After ICSI, oocytes were incubated at 37°C in 6% CO2 and 95% relative humidity (RH), in a culture medium (SAGE 1-step™) overlayed with mineral oil (OVOIL™, Vitrolife, Sweden), in a TL incubator (Embryoscope®, Vitrolife, Sweden).
Injection with latex microspheres
A latex microsphere of the size of a sperm head (LM; accu-beads+™, Hamilton Thorne INC. USA) was intracytoplasmatically injected into the IVM oocytes to generate parthenotes, as previously described [46]. The injection was performed to mimic ICSI manipulations of the control group. Microspheres were resuspended in SpermRinse™ (Vitrolife, Sweden), and the injections were performed following the standard ICSI technique. ICSI plates included a drop of polyvinylpyrrolidone (PVP) (ICSI™ Vitrolife, Sweden), a drop of microsphere solution, and a drop of buffered medium (G-MOPS™ PLUS, Vitrolife, Sweden) for each oocyte. To perform the microinjection protocol, the microinjection pipettes (MIC 35-30 Origio, Denmark) were first equilibrated in PVP. A single latex microsphere was then aspirated from the microsphere solution, washed in the PVP drop, and finally injected into the IVM oocyte.
Oocyte activation using calcium ionophores
Oocytes were incubated for 15 min in 30 μL drops of A23187 (GM508 CultActive, Gynemed, Germany) immediately after the microsphere injection. Oocytes were then washed twice in 30 μL drops of MOPS- and HEPES-free culture medium (GM501 Cult, Gynemed, Germany). Finally, the oocytes were placed in an Embryoslide® and cultured in an Embryoscope® TL system in standard conditions (37°C, 6% CO2, and 95% RH).
Ca2+ ionophore ionomycin (Strepromyces conglobatus, Ionomycin, I9657, Sigma Aldrich) was diluted in DMSO (Sigma-Aldrich) to a concentration of 0.7 mg/mL. This solution was further diluted in G1™ culture medium (Vitrolife, Sweden) at a final concentration of 10 μM and then equilibrated at 37°C, 6% CO2, and 95% RH overnight. AOA was performed according to Heindryckx et al. (2008) with some modifications. Oocytes were allowed to recover for 30 min in G1™ PLUS (Vitrolife, Göteborg, Sweden) after ICSI and were incubated in an ionomycin solution (10 μmol/L in G1™ PLUS) for 7 min. Next, oocytes were washed 10 times in G1™ PLUS and then incubated for 30 min in G1™ PLUS. The oocytes were subsequently exposed to the ionomycin solution for two more rounds, first for 7 min followed by a 30-min wash in G1™ PLUS. The oocytes were then placed in the Embryoslide® for TL culture to monitor embryo and parthenote development.
Morphokinetic data collection
The morphokinetic timing nomenclature was based on the guidelines proposed by Ciray et al.: time of pronuclei appearance (tPNa), time of pronuclei fading (tPNf), two to eight discrete cells (t2 to t8), initiation of blastulation (tSB), and time of full blastocyst (tB) [47]. All parameters were annotated considering t0 as the end of ICSI.
Statistical analysis
Activation rates and morphokinetic patterns between ionomycin and A23187 AOA groups were compared to controls. Univariate analysis was carried out to evaluate differences in activation rates using Chi’s squared test and all morphokinetic milestones using a non-parametric ANOVA test (Kruskal-Wallis). A p-value < 0.05 was considered statistically significant.
Results
Demographic characteristics
Demographic and cycle characteristics are presented in Table 1. The age of the oocyte donors included in the study ranged from 18 to 35 years, with a mean of 25.9±4.4 years (Table 1). The mean number of CCOs collected was 24.2±12.2, from which an average of 18.1±10.3 were MII, 1.7±1.5 were MI, 2.6 ±3.3 were GV, and 1.8±2.8 included diploid, degenerated, or abnormal oocytes and were classified as “other.” Overall, we obtained 1.7±1.1 IVM per oocyte donation cycle. The number of IVM oocytes is not reported for the control group, since they are routinely discarded in donation treatments (Table 1).
Table 1.
Demographic characteristics overall and by study group
| Overall N=120 |
A23187 N=42 |
Ionomycin N=39 |
Control N=39 |
|
|---|---|---|---|---|
| Female age, mean (SD) | 25.90 (4.40) | 25.40 (4.82) | 26.21 (4.38) | 25.56 (3.95) |
| BMI, mean (SD) | 22.18 (2.91) | 21.85 (3.32) | 21.94 (2.70) | 22.61 (2.85) |
| OPU results, mean (SD) | ||||
| CCOs | 24.22 (12.22) | 24.08 (10.77) | 24.63 (13.25) | 24.51 (13.04) |
| MII | 18.11 (10.28) | 17.00 (7.74) | 17.00 (9.27) | 19.54 (12.49) |
| MI | 1.69 (1.52) | 2.12 (1.33) | 2.08 (1.32) | 1.21 (1.73) |
| GV | 2.58 (3.33) | 2.88 (3.26) | 3.71 (3.64) | 1.90 (2.74) |
| Other | 1.82 (2.78) | 1.96 (3.38) | 1.92 (3.44) | 1.85 (2.39) |
| MIV, mean (SD) | 1.74 (1.07) | 1.72 (1.13) | 1.83 (1.19) | - |
Artificial oocyte activation and morphokinetics analysis
Ionomycin treatment resulted in higher activation rates of IVM MI oocytes (15/39, 38.5%) compared to A23187 (10/42, 23.8%); however, this difference was not statistically significant (p=0.153). In the ionomycin group, 86.7% (13/15) of parthenotes reached the 2-cell stage, 80% (12/15) the 3-cell stage, 60% (9/15) the 4-cell stage, 46.7% (7/15) the 5-cell stage, 20% (3/15) the 8-cell stage, and 13.3% (2/15) reached the blastocyst stage (Table 2). Following A23187 treatment, significantly less oocytes reached the 2-cell stage (50% (5/10, p-value=0.046) and the 3-cell stage (40% (4/10), p-value=0,04), 30% (3/10) reached the 4-cell stage, and 20% (2/10) reached the 5-cell stage (Table 2). Strikingly, none of the parthenotes that underwent A23187-mediated AOA reached the 8-cell stage or formed a blastocyst. As expected, the developmental potential of the parthenotes was significantly lower than in the embryo control group, where the overall blastocyst rate was 71.8% (p-value 1×10−04, Table 2).
Table 2.
Assisted oocyte activation and embryo developmental rates
| Overall parthenotes (n=81) | A23187 (n=42) | Ionomycin (n=39) | Control (n=39) | p-value* | |
|---|---|---|---|---|---|
| Treatment yield | |||||
| Activated zygotes, n (%) | 25 (30.87) | 10 (23.81) | 15 (38.46) | NA | 0.153 |
| 1PN zygotes, n (%) | 21 (84.0) | 9 (90.0) | 12 (80.0) | NA | 0.504 |
| 2-cell stage parthenotes, n (%) | 18 (72.0) | 5 (50.0) | 13 (86.66) | 39 (100) | 0.046 |
| 3-cell stage parthenotes, n (%) | 16 (64.0) | 4 (40.0) | 12 (80.0) | 38 (97.4) | 0.041 |
| 4-cell stage parthenotes, n (%) | 12 (48.0) | 3 (30) | 9 (60) | 36 (92.3) | 0.141 |
| 5-cell stage parthenotes, n (%) | 9 (36) | 2 (20) | 7 (46.66) | 34 (87.2) | 0.216 |
| 8-cell stage parthenotes, n (%) | 3 (12) | 0 | 3 (20) | 31 (79.5) | 0.132 |
| Blastocyst parthenotes, n (%) | 2 (8) | 0 | 2 (13.33) | 28 (71.8) | 0.223 |
*Chi’s squared test (A23187 vs ionomycin)
NA, not applicable
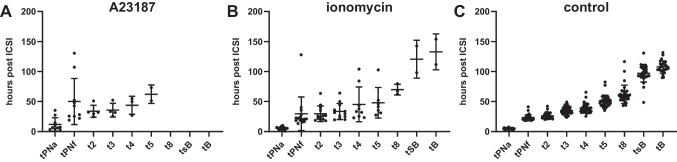
We observed a wide variation of timings to reach each cleavage stage among individual parthenotes (Fig. 1). When comparing the two ionophores (ionomycin and A23187), we detected no differences in morphokinetic patterns after t2 (Table 3). Interestingly however, tPNa and tPNf were significantly delayed in the A23187 group (11.8±11.0 hpi and 50.2±38.4 hpi, respectively) compared to parthenotes that underwent ionomycin activation (5.3±2.3 hpi and 29.7±28.1 hpi; p=0.002 and 0.005, respectively) (Table 3). Notably, these parameters were also significantly delayed in parthenotes following A23187 activation compared to controls (4.9±1.0 hpi and 23.3±4.6 hpi; p=1.8×10−04 and p=0.006 respectively) (Table 3). Yet, parthenotes activated with ionomycin presented with similar mean tPNa and tPNf compared to control zygotes generated using standard ICSI (p=0.961 and p=0.595, respectively) (Table 3). Notably, t2 was significantly delayed following A23187 treatment (p=0.023) when compared to controls (Table 3). Conversely, none of the morphokinetic parameters differed between the ionomycin and control group (Table 3).
Fig. 1.
Developmental timings of parthenotes treated with A23187 (A) or ionomycin (B) and control embryos (C). Dots represent division timings (hpi), and bars represent the mean and standard deviation
Table 3.
Mean morphokinetic timings from parthenotes and control embryos analyzed by time-lapse
| Morphokinetic development | A23187 (A) | Ionomycin (B) | Control (C) | p-value (a–b)* | p-value (a–c)* | p-value (b–c)* |
|---|---|---|---|---|---|---|
| tPNa (h), mean (SD) | 11.84 (11.03) | 5.31 (2.33) | 4.95 (1.02) | 0.002 | 1.8×10−4 | 0.961 |
| tPNf (h), mean (SD) | 50.15 (38.35) | 29.69 (28.11) | 23.33 (4.62) | 0.005 | 0.006 | 0.595 |
| t2 (h), mean (SD) | 33.96 (9.99) | 29.68 (13.03) | 26.04 (4.69) | 0.052 | 0.023 | 0.860 |
| t3 (h), mean (SD) | 35.70 (11.57) | 33.58 (13.42) | 34.64 (7.86) | 0.646 | 0.823 | 0.248 |
| t4 (h), mean (SD) | 43.83 (14.86) | 45.22 (28.99) | 38.68 (7.79) | 0.314 | 0.485 | 0.501 |
| t5 (h), mean (SD) | 62.10 (15.41) | 48.09 (25.54) | 50.88 (9.65) | 0.063 | 0.242 | 0.124 |
| t8 (h), mean (SD) | - | 69.93 (8.86) | 61.99 (15.20) | - | - | 0.078 |
| tsB (h), mean (SD) | - | 120.55 (31.47) | 97.08 (14.51) | - | - | 0.228 |
| tB (h), mean (SD) | - | 132.80 (29.84) | 108.57 (11.04) | - | - | 0.088** |
*Kruskal-Wallis ANOVA test
**Unpaired t-test
Discussion
AOA using Ca2+ ionophores such as ionomycin and A23187 has been applied in clinical practice as a promising treatment for FF or to improve poor fertilization rates after ICSI [4, 20, 25, 29, 31, 43, 48–51]. However, as AOA is exclusively recommended for select patients, comprehensive efficacy and safety assessments remain limited and are occasionally conflicting. While a recent meta-analysis demonstrated significantly improved oocyte activation and clinical outcomes following Ca2+ ionophore treatment [27] an earlier study contradicted these findings [52]. Ultimately, differences in patient baseline characteristics, study methodology, and restricted sample size warrant careful outcome interpretation. Critically, comparisons among studies remain difficult due to the lack of standardization among AOA protocols, particularly the use of different oocyte-activating agents. While Ca2+ ionophores have proven beneficial for oocyte activation, they ultimately fail to elicit physiological patterns of Ca2+ release [12, 22]. Differences in Ca2+ levels and oscillation patterns may ultimately influence both the efficiency and quality of embryo development. Evaluating potential downstream effects of differing oocyte activating agents thus remains paramount for improving AOA procedures.
To provide greater insights into the efficiency and safety of AOA, we compared two Ca2+ ionophores commonly used in clinical practice: ionomycin and commercially available A23187 (GM508 CultActive, Gynemed). Our study employed a parthenogenic model using human in vitro matured oocytes to evaluate activation rates, parthenogenetic developmental competence, and morphokinetic dynamics of the activated oocytes using a TL incubation system. Applying this approach, our study also contributes to a greater understanding of morphokinetic parameters associated with early in vitro development of human parthenotes generated by different AOA methods.
Similar to previous reports surrounding human parthenogenesis, our results revealed an overall reduced developmental potential of haploid parthenotes. Nevertheless, to date, several studies have reported successful parthenogenetic blastocyst formation in human [36, 53–55]. In accordance with earlier studies using similar protocols [54, 56], 10–20% of the activated oocytes progressed to the 8-cell stage of development, while 13.3% of the ionomycin-activated oocytes generated blastocysts.
As previously demonstrated, our results suggest that A23187 elicits a lower oocyte activation rate compared to ionomycin [30, 33, 34, 51]. A recent study in patients at risk of failed or impaired fertilization showed significantly higher fertilization rates when using ionomycin compared to A23187 for AOA, in cases of previous FF or low fertilization, as well as severe OAT [51]. These results are in line with our findings and suggest that ionomycin offers a higher capacity to initiate Ca2+ oscillations compared to A23187. As suggested by Jia et al., this may be particularly relevant in cases of sperm-related defects [51]. Moreover, in contrast to ionomycin oocyte activation, treatment with A23187 appeared to have a more profound impact on preimplantation development, ultimately impeding reproductive potential. Accordingly, the number of parthenotes reaching the 2-cell stage was significantly lower compared to the ionomycin group, while all parthenotes activated with A23187 were arrested before reaching the blastocyst stage. Esbert et al. demonstrated similar results, reporting complete cleavage arrest of unfertilized oocytes activated with A23187 the day after ICSI [57]. Other studies have also corroborated these findings demonstrating poor embryonic development following AOA using A23187 [51], with significantly reduced blastocyst rates ranging from ~3 to 5% [58–60]. Additionally, Economou et al. further showed that blastocysts obtained from AOA using solely A23187 were all chromosomally abnormal [59]. Collectively, these results indirectly suggest that the Ca2+ levels or oscillation patterns elicited by A23187 may not be sufficient to support normal embryo development.
Notably, we observed a significant delay in the timing of PN appearance and fading in parthenotes activated with A23187 compared to both ionomycin-AOA, as well as the control group. Interestingly, in their study, Escribá and colleagues observed significant delays in cleavage divisions in parthenotes activated with A23187 compared to correctly fertilized embryos [44]. While they primarily attribute impaired development to the ploidy of the parthenotes, our findings suggest that the choice of ionophore itself may also impact embryogenesis. Importantly, in our study, early morphokinetic parameters did not differ between the ionomycin-AOA and control groups. These findings corroborate previous results from our group demonstrating similar timings in the appearance and fading of pronuclei between ionomycin-mediated AOA and non-AOA ICSI groups [41]. Accordingly, A23187 treatment may contribute to defects in the first cell cycle or improper regulation of the phases leading to the first mitosis accounting for the observed early arrest of a vast proportion of parthenotes in the A23187-AOA group. While further validation remains necessary, modifications to the AOA protocol and/or culture conditions of A23187-activated oocytes [44, 59] may enhance their cleavage and blastulation potential. Nevertheless, our findings highlight the importance of protocol standardization for obtaining optimal AOA outcomes.
Contrary to our findings, Shebl et al. observed an accelerated 2PN appearance following A23187-mediated AOA and ICSI compared to no-AOA ICSI performed on sibling oocyte controls. However, the patient cohort in their study included couples with suspected fertilization problems rather than previous complete fertilization failure. In such cases, the presence of sperm may lead to a longer latency of the Ca2+ signal compared to oocyte activation in the absence of sperm (as in our study), or in couples with substantial male factor infertility, such as PLCZ mutations [42]. Nevertheless, the abrupt non-physiological increase of free intracytoplasmic Ca2+ associated with the use of Ca2+ ionophores may induce alterations in early morphokinetic events. Further monitoring of the safety of AOA procedures will be paramount for enabling widespread clinical use of this treatment. This is particularly relevant given the increasing application of AOA treatment in patients not suffering from FF after ICSI. Several studies have reported the use of AOA treatment for embryo developmental arrest, PCOS, POI, and unexplained infertility among others [32, 61–63]. For instance, Mingrong and colleagues used A23187-mediated AOA for patients with a variety of male and female idiopathic and non-idopathic infertility indications, including patients with severe teratozoospermia, advanced maternal age, and patients who failed to achieve pregnancy in more than two fresh cycles or with a combination of the above factors. The authors observed significant improvements in clinical outcomes following AOA treatment. However, as the previous cycle of the same patients is used as the control group, their findings warrant careful interpretation. Moreover, strong evidence for AOA overcoming such indications is still lacking [64], while differences in AOA protocols and oocyte-activating agents make comparisons difficult. Establishing guidelines or industry standards regarding the use of ionophores for AOA will be of paramount importance for future clinical use. While the conclusions of our study are limited by the small sample size resulting from low activation rates and overall low parthenote competence in both groups, an important strength is the use of TL technology, allowing comprehensive evaluations of all developmental events. Due to the different exposure duration of the ionophores, caution is also warranted when comparing developmental timings between ionophore and the control embryo groups.
To improve parthenote yield in the future, Ca2+ ionophore treatment can be followed by another chemical, for instance, 6-DMAP (broad protein synthesis inhibitor) or cytochalasin B or D (inhibitors of actin filaments polymerization), which blocks second polar body extrusion. Thus, the resulting parthenote is “pseudodiploid” heterozygous, containing the two sister chromatids of each maternal chromosome present in the MII oocyte [65]. Nonetheless, our parthenogenic model allowed valuable comparisons between differing AOA protocols, without the need of performing a clinical trial on patients with FF. We show that despite successful oocyte activation, the choice of Ca2+ ionophores for AOA may have an effect on early morphokinetic parameters, potentially impacting cleavage and blastocyst utilization rates. Ultimately, care should be applied when selecting ionophores for clinical treatment.
Acknowledgements
We thank all the donors at Clinica Eugin who participated in this study.
Author contribution
AQ-V: involved in study design, experimental methods, data analysis, statistical analysis and manuscript preparation. MM involved in study design, experimental methods and manuscript preparation. IM-E: involved in statistical analysis and manuscript preparation. MJZ, AR, RV, and MP involved in study implementation and supervision, expert knowledge, and manuscript preparation. All authors read and approved the final manuscript.
Data availability
Anonymized data will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval
Permission to conduct the study was granted by the Ethics Committee for Clinical Research of Clinica Eugin (CEIm Eugin, protocol code: IONOTIME). All patients obtained and signed a written informed consent form. All procedures were performed in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration, as revised in 2013.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palermo G, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 2.Palermo GD, et al. Intracytoplasmic sperm injection: state of the art in humans. Reproduction. 2017;154(6):F93–F110. doi: 10.1530/REP-17-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moomjy M, et al. Implications of complete fertilization failure after intracytoplasmic sperm injection for subsequent fertilization and reproductive outcome. Hum Reprod. 1998;13(8):2212–2216. doi: 10.1093/humrep/13.8.2212. [DOI] [PubMed] [Google Scholar]
- 4.Vanden Meerschaut F, et al. Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28(5):560–571. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCzeta): oocyte activation and clinical links to male factor infertility. Adv Biol Regul. 2013;53(3):292–308. doi: 10.1016/j.jbior.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty SP, Payne D, Matthews CD. Fertilization failures and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13(Suppl 1):155–164. doi: 10.1093/humrep/13.suppl_1.155. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357(9274):2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya S, Maheshwari A, Mollison J. Factors associated with failed treatment: an analysis of 121,744 women embarking on their first IVF cycles. PLoS One. 2013;8(12):e82249. doi: 10.1371/journal.pone.0082249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahutte NG, Arici A. Failed fertilization: is it predictable? Curr Opin Obstet Gynecol. 2003;15(3):211–218. doi: 10.1097/00001703-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Esfandiari N, et al. Complete failed fertilization after intracytoplasmic sperm injection--analysis of 10 years’ data. Int J Fertil Womens Med. 2005;50(4):187–192. [PubMed] [Google Scholar]
- 11.Tesarik J, et al. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002;78(3):619–624. doi: 10.1016/s0015-0282(02)03291-0. [DOI] [PubMed] [Google Scholar]
- 12.Kashir J, et al. Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application. Hum Reprod Open. 2022;2022(2):hoac003. doi: 10.1093/hropen/hoac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv Biol Regul. 2018;67:148–162. doi: 10.1016/j.jbior.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Knott JG, et al. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72(4):992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 15.Young C, et al. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril. 2009;91(5 Suppl):2230–2242. doi: 10.1016/j.fertnstert.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe LA, Cross NL, Picheral B. Studies of the voltage-dependent polyspermy block using cross-species fertilization of amphibians. Dev Biol. 1983;98(2):319–326. doi: 10.1016/0012-1606(83)90362-7. [DOI] [PubMed] [Google Scholar]
- 17.McGuinness OM, et al. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development. 1996;122(7):2199–2206. doi: 10.1242/dev.122.7.2199. [DOI] [PubMed] [Google Scholar]
- 18.Cox LJ, et al. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124(5):611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 19.Jones KT. Mammalian egg activation: from Ca2+ spiking to cell cycle progression. Reproduction. 2005;130(6):813–823. doi: 10.1530/rep.1.00710. [DOI] [PubMed] [Google Scholar]
- 20.Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94(2):520–526. doi: 10.1016/j.fertnstert.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Saunders CM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 22.Kashir J, et al. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16(6):690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- 23.Torra-Massana M, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019;34(8):1494–1504. doi: 10.1093/humrep/dez094. [DOI] [PubMed] [Google Scholar]
- 24.Tesarik J, Sousa M. More than 90% fertilization rates after intracytoplasmic sperm injection and artificial induction of oocyte activation with calcium ionophore. Fertil Steril. 1995;63(2):343–349. doi: 10.1016/s0015-0282(16)57366-x. [DOI] [PubMed] [Google Scholar]
- 25.Heindryckx B, et al. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20(8):2237–2241. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 26.Ebner T, Montag M. Artificial oocyte activation: evidence for clinical readiness. Reprod BioMed Online. 2016;32(3):271–3. doi: 10.1016/j.rbmo.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Murugesu S, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017;108(3):468–482 e3. doi: 10.1016/j.fertnstert.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Vasilev F, et al. Effects of ionomycin on egg activation and early development in starfish. PLoS One. 2012;7(6):e39231. doi: 10.1371/journal.pone.0039231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges E, Jr, et al. Artificial oocyte activation using calcium ionophore in ICSI cycles with spermatozoa from different sources. Reprod BioMed Online. 2009;18(1):45–52. doi: 10.1016/s1472-6483(10)60423-3. [DOI] [PubMed] [Google Scholar]
- 30.Nikiforaki D, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798–806 e2. doi: 10.1016/j.fertnstert.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Vanden Meerschaut F, et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012;27(7):1977–1984. doi: 10.1093/humrep/des097. [DOI] [PubMed] [Google Scholar]
- 32.Ebner T, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod BioMed Online. 2015;30(4):359–365. doi: 10.1016/j.rbmo.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer-Buitrago M, et al. Single Ca(2+) transients vs oscillatory Ca(2+) signaling for assisted oocyte activation: limitations and benefits. Reproduction. 2018;155(2):R105–R119. doi: 10.1530/REP-17-0098. [DOI] [PubMed] [Google Scholar]
- 34.Bonte D, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019;112(2):266–274. doi: 10.1016/j.fertnstert.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Shan Y, et al. Assisted oocyte activation with calcium ionophore improves pregnancy outcomes and offspring safety in infertile patients: a systematic review and meta-analysis. Front Physiol. 2021;12:751905. doi: 10.3389/fphys.2021.751905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Versieren K, et al. Developmental competence of parthenogenetic mouse and human embryos after chemical or electrical activation. Reprod BioMed Online. 2010;21(6):769–775. doi: 10.1016/j.rbmo.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Bos-Mikich A, Whittingham DG, Jones KT. Meiotic and mitotic Ca2+ oscillations affect cell composition in resulting blastocysts. Dev Biol. 1997;182(1):172–179. doi: 10.1006/dbio.1996.8468. [DOI] [PubMed] [Google Scholar]
- 38.Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128(6):917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- 39.Ozil JP, et al. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300(2):534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 40.Kim BY, et al. Alterations in calcium oscillatory activity in vitrified mouse eggs impact on egg quality and subsequent embryonic development. Pflugers Arch. 2011;461(5):515–526. doi: 10.1007/s00424-011-0955-0. [DOI] [PubMed] [Google Scholar]
- 41.Martinez M, et al. Assisted oocyte activation effects on the morphokinetic pattern of derived embryos. J Assist Reprod Genet. 2021;38(2):531–537. doi: 10.1007/s10815-020-02025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shebl O, et al. Ionophore application for artificial oocyte activation and its potential effect on morphokinetics: a sibling oocyte study. J Assist Reprod Genet. 2021;38(12):3125–3133. doi: 10.1007/s10815-021-02338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebner T, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012;98(6):1432–1437. doi: 10.1016/j.fertnstert.2012.07.1134. [DOI] [PubMed] [Google Scholar]
- 44.Escriba MJ, et al. Kinetics of the early development of uniparental human haploid embryos. Fertil Steril. 2016;105(5):1360–1368 e1. doi: 10.1016/j.fertnstert.2015.12.139. [DOI] [PubMed] [Google Scholar]
- 45.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Durban M, et al. PLCzeta disruption with complete fertilization failure in normozoospermia. J Assist Reprod Genet. 2015;32(6):879–886. doi: 10.1007/s10815-015-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciray HN, et al. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29(12):2650–2660. doi: 10.1093/humrep/deu278. [DOI] [PubMed] [Google Scholar]
- 48.Heindryckx B, et al. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod BioMed Online. 2008;17(5):662–668. doi: 10.1016/s1472-6483(10)60313-6. [DOI] [PubMed] [Google Scholar]
- 49.Montag M, et al. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod BioMed Online. 2012;24(5):521–526. doi: 10.1016/j.rbmo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Vanden Meerschaut F, et al. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod. 2013;28(1):87–98. doi: 10.1093/humrep/des368. [DOI] [PubMed] [Google Scholar]
- 51.Jia L, et al. Artificial oocyte activation with ionomycin compared with A23187 among patients at risk of failed or impaired fertilization. Reprod BioMed Online. 2023;46(1):35–45. doi: 10.1016/j.rbmo.2022.08.105. [DOI] [PubMed] [Google Scholar]
- 52.Sfontouris IA, et al. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Hum Reprod. 2015;30(8):1831–1841. doi: 10.1093/humrep/dev136. [DOI] [PubMed] [Google Scholar]
- 53.Mai Q, et al. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17(12):1008–1019. doi: 10.1038/cr.2007.102. [DOI] [PubMed] [Google Scholar]
- 54.Paffoni A, et al. In vitro development of human oocytes after parthenogenetic activation or intracytoplasmic sperm injection. Fertil Steril. 2007;87(1):77–82. doi: 10.1016/j.fertnstert.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 55.de Fried EP, et al. Human parthenogenetic blastocysts derived from noninseminated cryopreserved human oocytes. Fertil Steril. 2008;89(4):943–947. doi: 10.1016/j.fertnstert.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 56.Winston N, et al. Parthenogenetic activation and development of fresh and aged human oocytes. Fertil Steril. 1991;56(5):904–912. [PubMed] [Google Scholar]
- 57.Esbert M, et al. Calcium Ionophore A23187 treatment to rescue unfertilized oocytes: a prospective randomized analysis of sibling oocytes. Reprod BioMed Online. 2022;45(5):878–883. doi: 10.1016/j.rbmo.2022.06.021. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, et al. Three-day-old human unfertilized oocytes after in vitro fertilization/intracytoplasmic sperm injection can be activated by calcium ionophore a23187 or strontium chloride and develop to blastocysts. Cell Rep. 2014;16(4):276–280. doi: 10.1089/cell.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Economou KA, et al. The combination of calcium ionophore A23187 and GM-CSF can safely salvage aged human unfertilized oocytes after ICSI. J Assist Reprod Genet. 2017;34(1):33–41. doi: 10.1007/s10815-016-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Z, et al. Calcium Ionophore (A23187) Rescues the activation of unfertilized oocytes after intracytoplasmic sperm injection and chromosome analysis of blastocyst after activation. Front Endocrinol (Lausanne) 2021;12:692082. doi: 10.3389/fendo.2021.692082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv M, et al. Artificial oocyte activation to improve reproductive outcomes in couples with various causes of infertility: a retrospective cohort study. Reprod BioMed Online. 2020;40(4):501–509. doi: 10.1016/j.rbmo.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Mateizel I, et al. Effect of A23187 ionophore treatment on human blastocyst development-a sibling oocyte study. J Assist Reprod Genet. 2022;39(6):1225–1232. doi: 10.1007/s10815-022-02467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai TE, et al. Artificial oocyte activation may improve embryo quality in older patients with diminished ovarian reserve undergoing IVF-ICSI cycles. J Ovarian Res. 2022;15(1):102. doi: 10.1186/s13048-022-01036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cardona Barberan A, et al. Assisted oocyte activation does not overcome recurrent embryo developmental problems. Hum Reprod. 2023; [DOI] [PubMed]
- 65.Bos-Mikich A, et al. Parthenogenesis and human assisted reproduction. Stem Cells Int. 2016;2016:1970843. doi: 10.1155/2016/1970843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on reasonable request to the corresponding author.