Abstract
Objectives
This study aimed to assess the prevalence of undiagnosed hypertension and associated factors among people aged 18 years and above in Mizan-Aman town of Bench Sheko Zone in Southwest Ethiopia. Study Design. A community-based cross-sectional study design was carried out among people aged 18 years old and above from April 1 to 30, 2021, in Mizan Aman town.
Methods
A community-based cross-sectional study design was carried out among people aged 18 years old and above from April 1 to 30, 2021, in Mizan Aman town. Seven hundred fifty-nine subjects were selected by the multistage sampling technique. A structured pretested STEPwise questionnaire was used to interview the participants. Data entry and analysis were done using EpiData 3.1 and SPSS version 25 statistical software, respectively. Descriptive analysis was undertaken, and the results were presented using frequency tables, graphs, and statistical summaries. The dependent variable has a dichotomized response of yes and no, and hence binary logistic regression was used to predict a dependent variable based on independent variables, and predictors having p ≤ 0.25 on the bivariable analysis were considered as candidates for the multivariable analysis. Odds ratios with their 95% confidence intervals were calculated to measure the strength of association, and finally a p value <0.05 was considered statistically significant.
Result
The prevalence of undiagnosed hypertension was 14.8% with 95% CI [12.3–15.6]. Older age (AOR = 3.1, 95% CI [1.5–6.5]), male (AOR = 2.2, 95% CI [1.3–3.9]), low physical activity (AOR = 3.9, 95% CI [1.8–8.3]), low consumption of fruit and vegetable (AOR = 4.5, 95% CI [2.4–8.8]), and higher BMI (AOR = 2.7, 95% CI [1.6–4.6]) were significantly associated with undiagnosed hypertension.
Conclusion
The current study outlined that the prevalence of undiagnosed hypertension was high in the study area. In addition, most of the risk factors identified were modifiable, and hence community-based preventive approaches like lifestyle modification, increasing awareness, and strengthening routine screening at primary health service facilities resulted in a substantial change in tackling the burden effectively.
1. Background
Worldwide levels of undiagnosed hypertension (HTN) represent the global public health crisis. It affected around 22% of people aged 18 years and over and is responsible for an estimated 9.4 million deaths per year globally [1, 2]. Approximately 11 million U.S. adults with a usual source of health care have undiagnosed hypertension, placing them at increased risk for cardiovascular events [3]. In South-East Asian countries, more than 50% of people with hypertension remain undiagnosed [4]. The prevalence of undiagnosed hypertension was found to be 30% in sub-Saharan Africa (SSA) [5].
The study conducted in Dabat, Northern Ethiopia, reveals that the largest proportion (83.4%) of patients had not been diagnosed and/or treated for HTN [6]. The prevalence of undiagnosed HTN was 13.3% in Gulele sub-city, Addis Ababa, Ethiopia [7]. Another study conducted in Hawela Tula sub-city, Hawassa, Southern Ethiopia, reveals that the prevalence of undiagnosed hypertension was 12.3% [8].
Signs and symptoms of HTN are often undetectable during the early stages, and hence many people with the disease are left undiagnosed [9]. The preventable and modifiable factors for high blood pressure include behavioral risk factors, such as weight problems, excessive nutritional salt intake, low nutritional consumption of calcium and potassium, alcohol consumption, psychosocial stress, low tiers of physical activity, and non-behavioral risk factors like family history of high blood pressure, age, and sex. These risk factors result in various long-term disease processes, ending in high mortality rates attributable to stroke, heart attack, tobacco- and nutrition-induced cancers, and obstructive lung diseases [10–13]. However, diagnosis and treatment along with lifestyle modification are essential for the management of HTN. In developing countries, the prevention and control measures are grossly inadequate, although the prevalence of the disease is very high [14, 15]. The lifestyle of the Mizan Aman town population is changing due to economic development, urbanization, and demographic transition. These rapid changes have led to the necessity of conducting a study on undiagnosed HTN and its associated factors at the community level. Further, many studies have been done at the facility level and focus on the overall prevalence of HTN. Therefore, this study is aimed to assess the prevalence of undiagnosed HTN and associated factors among adults in Mizan Aman town in the southwestern region of Ethiopia.
2. Methods
2.1. Study Setting, Design, and Period
A community-based cross-sectional study was conducted from April 1 to 30, 2021, in Mizan Aman town, the capital and administrative center of Bench Sheko Zone in the Southwestern Nations Nationalities Peoples Region (SWNNPR). Based on the report from the sub-city administration office, the total population of Mizan Aman town is estimated to be 54,951; of the total, 26,925 of them are male and the remaining 28,026 are female. Among the total population of 54,951 residing in the town, about 60.3% are estimated to be above 18 years old. In the study area, 1 teaching hospital, 1 HC, 9 medium private clinics, 5 drug stores, and 5 health posts (HPs) provided curative and preventive services through 287 healthcare workers (HCWs).
2.2. Sampling Techniques
A multistage sampling method was employed to recruit study participants. First, three kebeles (the smallest administration localities) (Addis Ketema, Edget, and Kometa Keble) were selected using simple random sampling from the five kebeles in the town. Second, households were allocated proportionally to each of the selected Keble and the households were selected within each kebele using the computer-generated random sampling technique (Figure 1). The sampling frame was prepared using the list of households from the family folder available at health posts. The frame is appropriate for this sampling since it contains the updated complete list of households within each of the kebeles considered for the study. Finally, one adult whose age was 18 years and above was selected from each household using a simple random sampling technique if there were two or more eligible adults living in the same household.
Figure 1.
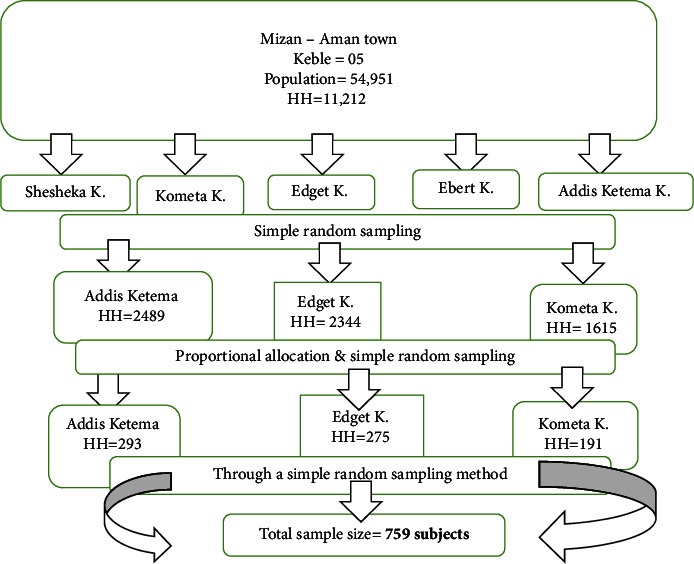
Schematic representation of a sampling procedure to assess the prevalence of undiagnosed HTN and associated factors among adult residents in Mizan Aman town, Bench Sheko Zone, Southwest Ethiopia, 2021.
2.2.1. Inclusion
Inclusion criteria included all adults aged 18 years and above who resided in the study area during the study period.
2.2.2. Exclusion
Exclusion criteria included mentally ill patients, hospitalized patients, and medically confirmed cases of HTN since the objective of the study was on undiagnosed HTN only.
2.3. Sample Size Determination
The sample size was calculated for both specific objectives separately.
For the prevalence, the sample size was calculated using a single population proportion formula based on the following assumptions. The prevalence of undiagnosed HTN n Hawela Tula sub-city, Hawassa, southern Ethiopia (p=12.3%) was taken from a previous study [9]. For this study, 95% level of confidence, 3% margining of error (d), a design effect of 1.5 and 10% for a possible non-response rate was taken and the formula applied as shown below.
| (1) |
Hence, the calculated sample size for the first specific objective becomes 759 subjects.
For the second objective, the sample size was calculated by using EPI INFO stat calc for population proportions to estimate the sufficient sample size. The variables considered in this estimation were being a male gender, family history of having HTN, Khat chewing, being in the age group of above 55 years, having BMI between 25 to 29.9, being in the age group from 35-54. From these variables, being a male gender yielded the maximum sample size of 238 with 16.13% of outcome in unexposed group, with an AOR of 2.5 at (1.2, 5.2) of CI, taking 95% confidence interval, 80% power, 1.5 a design effect and, 10% of non-response [9]. Hence, the calculated sample size for the second specific objective was 238 subjects.
Therefore, the determination for prevalence yielded the maximum number than the determination for factors and hence the final sample size for this study was found to be 759 subjects.
3. Data Collection
A structured and pretested questionnaire adapted from the WHO STEPwise approach for surveillance of NCDs in developing countries was used to interview the participants [16].
Data collection was done sequentially in a two-step process:
Step 1. Interview-based questionnaire on selected major health risk behaviors including smoking, alcohol consumption, poor fruit and vegetable consumption, and physical inactivity.
Step 2. Physical measures of health risks such as height, weight, blood pressure, body mass, and waist and hip circumference.
4. Data Collection Procedure
Using a pretested structured questionnaire, a face-to-face interview was conducted. After completing the interview, the physical measurements were conducted using the tape meter, digital automatic sphygmomanometer, and weight and height scales. We used calibrated instruments and standardized techniques to take the measurements.
Anthropometric measurements were taken based on the WHO guidelines, as specified in the Food and Nutrition Technical Assistance (FANTA) anthropometry manual [17]. Weight was measured to the nearest 0.1 kg with light clothes on a prestandardized digital body weighing scale, and height was measured to the nearest 0.1 cm in the standing position with no shoes, using a portable stadiometer, and BMI was calculated using the formula weight in kilogram divided by height in meters squared. Waist circumference was measured to the nearest 0.1 cm at the end of a normal expiration, at the midpoint between the lower part of the last rib and the top of the hipbone, using an inelastic measuring tape while the participants stood erect with their arms relaxed at the sides, and the hip circumference was a measure to the nearest 0.1 cm at the maximum circumference around the hips/maximum part of the buttocks. The waist circumference (cm) is divided by the hip circumference (cm) to estimate the waist-to-hip ratio (WHR). We take two measurements of weight, height waist, and hip circumference, and if the gap between the first two measurements was >0.1 kg for weight and >0.5 cm for height, hip, and waist circumference, we took the third measurement.
Blood pressure was measured in a sitting position with a supported back, and a digital automatic blood pressure (BP) device was used to measure the BP of the participants. The participants were taking rest for at least 5 min before measurement. Three measurements of BP on a single visit were taken at least one minute apart, and this survey considered the last two measures of BP levels and used their mean to detect HTN. At least two visits were made for those study participants whose BP was elevated at the first contact. According to the WHO guideline, a participant with systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg will be diagnosed as an HTN case [18].
4.1. Data Quality Assurance
Data were collected by two senior nurses under one supervisor for each kebele following the training on interviewing techniques, anthropometric measurements, and handling of data collection instruments for one day. The questionnaire was prepared in English, then translated into Amharic, and then retranslated back to English to check its consistency. A pretest was done on 5% of the sample out of the study area and then an appropriate revision of the tool was done. Double-entry of the data to EpiData software for data verification was also performed.
4.2. Data Analysis
Data analysis was done by SPSS for Windows version 25, and the descriptive analysis was undertaken; the result was presented using frequency tables, graphs, and descriptive statistical summaries. The undiagnosed HTN status has a dichotomized response of yes and no, and hence bivariable analysis was performed using binary logistic regression to identify candidate variables for the multivariable logistic regression model to identify explanatory variables associated with the outcome variable. Then, those variables with a p value <0.25 were included in multivariable logistic regression for adjustment of confounding factors. Odds ratios (ORs) with 95% confidence intervals were calculated to measure the strength of association, and p value <0.05 was considered statistically significant. The Hosmer–Lemeshow test was used to assess the fitness of the model (chi-square: 3.9 with a p value of 0.9).
4.3. Operational Definition
Standard operational definitions were adapted for key variables to maintain consistency and uniformity of the information.
Undiagnosed HTN: adults (aged 18 years and above) will be considered undiagnosed for HTN if, at the time of the survey, he or she was diagnosed as hypertensive (SBP ≥140 mmHg or DBP ≥90 mmHg) but never took any prescribed antihypertensive medicine to lower or control blood pressure and was never been told by a health professional that they have HTN before this study [18].
Harmful use of alcohol: alcohol consumption of more than 14 units/week for men and more than 8 units/week for women in the last 12 months before the survey. Its calculation is then as follows: unit of alcohol = vol (in ml) X % alcohol/1000, and for different local alcoholic beverages, it is as follows: tella (4%), Tej (10%), and arake (40–45%) (glass: 250 ml and bottle: 330 ml) [19].
Low consumption of fruits and vegetables: less than 5 servings (<400 gm) of fruit and or vegetables per day in that 1 serving is defined as one orange/apple/banana or three tablespoons of cooked vegetables [16].
Current smoker: an adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes. Previous smoker: an adult who has smoked at least 100 cigarettes in his or her lifetime but who had quit smoking at the time of the interview. Non-smoker: an adult who has never smoked or who has smoked less than 100 cigarettes in his or her lifetime [20].
Physical activity: the subjects' physical activity was classified as high, moderate, and low.
High physical activity is defined as a vigorous-intensity activity of at least 3 days achieving a minimum total physical activity of at least 1500 min/week or 7 or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities achieving a minimum total physical activity of at least 3000 min/week. Moderate physical activity is defined as 3 or more days of vigorous-intensity activity of at least 20 min per day or 5 or more days of moderate-intensity activity and/or walking of at least 30 min per day or 5 or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities achieving a minimum total physical activity of at least 600 min/week. Low physical activity: not fulfilling the criteria for moderate and high physical activity [21].
A family history of HTN is considered if a person's first-degree relative (parent, grandparent, or sibling) had been diagnosed with HTN and/or received drug therapy for HTN [22].
5. Result
5.1. Sociodemographic Characteristics
A total of 759 participants were randomly selected and included in the study in Mizan Aman town with 97.2% response, and data were collected from 738 study subjects, 482 (65.3%) males and 256 (34.7%) females. The highest percentage (51.9%) of the study participants was in the age categories of 18–34 years, while 13% were in the age of 55 years and above. The mean age of the respondents was 38.85 (SD: 14.57) years (Table 1).
Table 1.
Sociodemographic characteristics of the study participants in Mizan-Aman town, Bench Sheko Zone, Southwest Ethiopia, 2021.
| Variable | Category | Frequency (n = 738) | |
|---|---|---|---|
| Number | Percent (%) | ||
| Age (years) | 18–34 | 383 | 51.9 |
| 35–54 | 259 | 35.1 | |
| >55 | 96 | 13.0 | |
|
| |||
| Sex | Male | 482 | 65.3 |
| Female | 256 | 34.7 | |
|
| |||
| Educational level | Primary education and less | 334 | 45.3 |
| Secondary education | 302 | 40.9 | |
| College and above | 102 | 13.8 | |
|
| |||
| Religion | Orthodox | 364 | 49.3 |
| Protestant | 318 | 43.1 | |
| Muslim | 56 | 7.6 | |
|
| |||
| Ethnicity | Bench | 307 | 41.6 |
| Kaffa | 266 | 36.0 | |
| Amhara | 94 | 12.7 | |
| Others | 71 | 9.6 | |
|
| |||
| Marital status | Single | 220 | 29.8 |
| Married | 434 | 58.8 | |
| Divorced | 61 | 8.3 | |
| Widowed | 23 | 3.1 | |
|
| |||
| Occupation status | Govt. employee | 241 | 32.7 |
| NG employee | 100 | 13.6 | |
| Self-employed | 308 | 41.7 | |
| Others | 89 | 12.1 | |
|
| |||
| Average monthly income (ETB) | <1644 | 473 | 64.2 |
| ≥1644 | 264 | 35.8 | |
|
| |||
| Family history of HTN | Yes | 143 | 19.4 |
| No | 595 | 80.6 | |
|
| |||
| Family history of DM | Yes | 195 | 26.4 |
| No | 543 | 73.6 | |
5.2. Behavioral Characteristics
The number of current smokers was 196 (26.6%) and all smoked manufactured tobacco products. The mean age when first started smoking was 26.4 (SD: 6.3). The majority (63.8%) of respondents declared that someone smoked at the workplace in their presence within the past seven days. The proportion of current and past drinkers was 30.3% and 30.6%, respectively. Among the current drinkers, 20.1% of them were men, but only 5.7% of women drank four or more days in the last week before the survey. About 62.3% of the study participants consumed more than five servings of fruit and vegetables per day for more than three days in a typical week. Among the study participants who responded about their physical activity, 22.1%, 19.1%, and 58.8% had low, moderate, and high levels of physical activity, respectively. Above one-fourth of the study subjects' body mass index (BMI) was greater than 25 kg/m2 (Table 2).
Table 2.
Behavioral characteristics of the study participants in Mizan-Aman town, Bench Sheko Zone, Southwest Ethiopia, 2021.
| Variable | Category | Frequency (n = 738) | ||
|---|---|---|---|---|
| Number | Percent (%) | |||
| Smoking habit | Current smoker | 196 | 26.6 | |
| Previous smoker | 44 | 6.0 | ||
|
| ||||
| Ever consumed alcohol | Yes | 22 | 30.6 | |
|
| ||||
| Currently consumed alcohol | Yes | 200 | 27.1 | |
|
| ||||
| Eat saturated oil | Frequently | 526 | 71.3 | |
|
| ||||
| Eat fatty food | Frequently | 241 | 32.7 | |
|
| ||||
| Add salt to food | Frequently | 622 | 84.3 | |
|
| ||||
| Serving of fruit and vegetable | Less than five servings | 278 | 37.7 | |
| Five and more servings | 460 | 62.3 | ||
|
| ||||
| Physical activity | High | 434 | 58.8 | |
| Moderate | 141 | 19.1 | ||
| Low | 163 | 22.1 | ||
|
| ||||
| Sedentary life | Yes | 221 | 29.9 | |
|
| ||||
| Chat chewing | Ever | Yes | 209 | 28.3 |
| Current | Yes | 178 | 24.1 | |
|
| ||||
| BMI | <25 kg/m2 | 579 | 78.5 | |
| ≥25 kg/m2 | 159 | 21.5 | ||
|
| ||||
| History of raised blood glucose/diabetes | Yes | 180 | 24.4 | |
5.3. Prevalence of Undiagnosed HTN and Associated Risk Factors
5.3.1. Prevalence of Undiagnosed HTN
The mean SBP and mean DBP of the study participants were 119.97 mmHg (SD: 17.4) and 80.48 mmHg (SD: 11.74), respectively. In the current study, the prevalence of undiagnosed HTN in Mizan-Aman was 14.8% [12.3–15.6] 95% CI. The prevalence of undiagnosed HTN among the study participants varied across their age, sex, serving fruit and vegetables, physical activity, and BMI. The prevalence of undiagnosed HTN was higher among older age participants (≥55 years and above age group) than the younger age group, and again the prevalence of undiagnosed HTN was higher among participants with higher BMI (25.2%) than normal BMI (11.9%). It was also higher among participants who had low physical activity (37.4%) compared with high physical activity (7.4%).
5.3.2. Factors Associated with Undiagnosed HTN
Those in the age group of 55 years and above were 3.1 times higher at risk for undiagnosed HTN compared to the 18–34 years of age group (AOR = 3.138, 95% CI [1.511–6.516]). The odds of having undiagnosed HTN was 2.2 times higher in male than in female (AOR = 2.239, 95% CI [1.295–3.870]).
Low consumption of fruit and vegetables was positively associated with undiagnosed HTN. Those eating fruit and vegetables less than five servings per day for less than three days per week had 4.5 times (AOR = 4.549, 95% CI [2.352–8.800]) increased risk of undiagnosed HTN compared to those eating more than five servings per day for more than three days per week. Besides, the odds of having undiagnosed HTN were 3.9 times higher among those who did not take part in high physical activity compared to those who took part in high physical activity (AOR = 3.878, 95% CI [1.803–8.341]).
In addition, the odds of having undiagnosed HTN were 2.7 times higher among those whose BMI was greater than or equal to 25 kg/m2 when compared to those whose BMI was less than 25 kg/m2 (AOR = 2.667, 95% CI [1.551–4.588]) (Table 3).
Table 3.
Bivariable and multivariable logistic regression model showing associated factors with undiagnosed HTN among the study participants in Mizan-Aman town, Bench Sheko Zone, Southwest Ethiopia, 2021.
| Variable | Category | Undiagnosed HTN | COR [95% CI] | AOR [95% CI] |
|---|---|---|---|---|
| Num (%) | ||||
| Age (years) | 18–34 | 60 (15.7) | 1 | |
| 35–54 | 22 (8.5) | 2 (1.19–3.35)∗ | 1.5 (0.65–3.19) | |
| >55 | 27 (28.1) | 0.5 (0.28–0.80)∗ | 3.1 (1.51–6.51) ∗∗ | |
|
| ||||
| Sex | Male | 60 (12.4) | 1.7 (1.10–2.52)∗ | 2.2 (1.29–3.87) ∗∗ |
| Female | 49 (19.1) | 1 | ||
|
| ||||
| Marital status | Single | 23 (10.5) | 1 | |
| Married | 60 (13.8) | 0.7 (0.43–1.21)∗ | 0.4 (0.09–1.55) | |
| Divorced | 20 (32.8) | 0.2 (0.12–0.47)∗ | 0.4 (0.09–1.39) | |
| Widowed | 6 (26.1) | 0.3 (0.12–0.92)∗ | 0.3 (0.07–1.07) | |
|
| ||||
| Occupation status | Govt. employee | 40 (16.6) | 0.9 (0.39–1.96)∗ | 1.1 (0.47–2.58) |
| NG employee | 25 (25.0) | 0.5 (0.16–0.81)∗ | 0.7 (0.26–1.76) | |
| Self-employed | 31 (10.1) | 1.5 (0.32–1.45)∗ | 2.2 (0.94–5.26) | |
| Others | 13 (14.6) | 1 | ||
|
| ||||
| Family history of HTN | Yes | 29 (20.3) | 0.6 (0.38–0.98)∗ | 0.6 (0.30–1.02) |
| No | 80 (13.4) | 1 | ||
|
| ||||
| Smoking habit | Current smoker | 44 (22.6) | 0.4 (0.28–0.67)∗ | 2 (0.78–5.03) |
| Previous smoker | 9 (20.5) | 0.5 (0.22–1.07)∗ | 0.7 (0.26–1.88) | |
| Non-smoker | 56 (11.2) | 1 | ||
|
| ||||
| Ever consumed alcohol | Yes | 41 (19.6) | 0.6 (0.39–0.92)∗ | 0.6 (0.34–1.17) |
| No | 68 (12.9) | 1 | ||
|
| ||||
| Serving of fruit and vegetable | Less than five servings | 16 (5.8) | 4.2 (2.38–7.22)∗ | 4.5 (2.35–8.80) ∗∗ |
| Five or more servings | 93 (20.2) | 1 | ||
|
| ||||
| Eat saturated oil | Frequently | 69 (13.1) | 1.5 (1.00–2.36)∗ | 1.2 (0.69–2.03) |
| Not frequently | 40 (18.9) | 1 | ||
|
| ||||
| Eat fatty food | Frequently | 28 (11.6) | 1.5 (0.93–2.34)∗ | 1.5 (0.87–2.63) |
| Not frequently | 81 (16.3) | 1 | ||
|
| ||||
| Add salt to food | Frequently | 86 (13.8) | 1.5 (0.92–2.56) | 1.5 (0.83–2.86) |
| Not frequently | 23 (19.8) | 1 | ||
|
| ||||
| Physical activity | High | 61 (37.4) | 1 | |
| Moderate | 16 (11.3) | 7.5 (4.65–12.1)∗ | 7.3 (3.85–13.74) ∗∗ | |
| Low | 32 (7.4) | 4.7 (2.54–8.59)∗ | 3.9 (1.80–8.34) ∗∗ | |
|
| ||||
| Sedentary life | Yes | 51 (23.9) | 0.4 (0.26–0.59)∗ | 0.7 (0.36–1.29) |
| No | 58 (11.0) | 1 | ||
|
| ||||
| History of diabetes | Yes | 37 (21.1) | 0.5 (0.35–084)∗ | 0.7 (0.38–1.25) |
| No | 72 (12.8) | 1 | ||
|
| ||||
| Ever chat chewing | Yes | 52 (17.4) | 0.7 (0.46–1.06)∗ | 0.8 (0.42–1.35) |
| No | 57 (13.0) | 1 | ||
|
| ||||
| BMI | Less than 25 kg/m2 | 69 (11.9) | 1 | |
| Greater than 25 kg/m2 | 40 (25.2) | 2.5 (1.60–3.85)∗ | 2.7 (1.55–4.58) ∗∗ | |
∗ Candidate variables in bivariable logistic regression at p value <0.25; ∗∗statistically significant variables in the final model of logistic regression at p value <0.05. Hosmer and Lemeshow's goodness-of-fit test produces a chi-square of 3.853 with a p value of 0.870.
6. Discussion
This community-based study has attempted to determine the prevalence, which is 14.8%, and identified factors associated with undiagnosed HTN. The high prevalence observed might be related to increasing urbanization, lack of awareness, and willingness to participate in regular health check-ups in the absence of health problems and barriers to screening services in Mizan Aman town.
Characteristics such as age, sex, physical activity, servings of fruit and vegetables, and BMI were predicted undiagnosed HTN.
The finding on the prevalence of undiagnosed HTN was in line with the findings of the study in the Gulele sub-city of Addis Ababa (13.3%), Hawassa (12.3%), in Ethiopia [7, 9]. This finding was closer to findings outside of Ethiopia, India (15.2%), and Bangladesh (11.1%) [13, 23]. The current finding was lower than the findings of studies conducted in a rural area of West Bengal (24.1%), Gadarif in eastern Sudan (33.5%), and in River Nile State, Sudan (38.2%) [15, 23, 24]. This difference might be due to the study setting and sample size in that their studies were conducted in rural communities and used large sample sizes attributable to the differences in lifestyle in a different setting. However, it is higher than the findings reported from a study done in the Gilgel Gibe area in Ethiopia (7.5%) [25]. These differences might be related to increasing urbanization, lack of awareness, and willingness to participate in regular health check-ups in the absence of health problems, coupled with accessibility, barriers to screening services, and differences in the study population used in various studies.
This study showed that undiagnosed HTN significantly increased as age increased. Those with age groups of 55 years and above were 3.1 times more likely to develop undiagnosed HTN as compared to the age group of 18–34 years. This finding sharply contrasted with a study conducted in Nepal, which showed that elderly patients (≥65 years of age) had a lower likelihood of being undiagnosed for HTN than patients aged 15–24 years [4]. But this finding is in line with the study findings conducted in Addis Ababa, Southwest Ethiopia, and Gimbi in Ethiopia [3, 26, 27]. The finding was also supported by studies conducted outside of Ethiopia, Bangladesh and South India, which revealed that the magnitude of HTN increased with the increment of age [27–31]. It is mostly related to the biological effect that increases arterial resistance due to age-related changes in the arterial wall and the thickening of the arterial wall or arteriosclerotic structural alterations and calcification in old age.
Regarding the gender difference, males are 2.2 times more likely to be undiagnosed for HTN compared to females. This finding was similar to a study done in Hawela Tula sub-city, Hawassa, that men were at 2.5 times higher risk of undiagnosed HTN than women [9]. Again, this study finding was supported by a study conducted in Southwest Ethiopia, which showed that men were at higher risk for undiagnosed HTN than their counterparts [26]. Other studies which were done in Southern Ethiopia, Nepal, and Southern Tanzania uncovered that HTN was significantly higher in males than in females [4, 32, 33]. However, some studies demonstrated that the odds of having HTN were higher in women [24, 27]. Thus, the significant difference might be due to the presence of coexisting risk factors and having a lower frequency of health facility visits trained in males and hormonal variation. That is, androgens increase blood pressure via the renin-angiotensin system (RAS), which promotes oxidative stress leading to the production of vasoconstrictor substances and a reduction in nitric oxide availability [34]. Other studies suggested that ovarian hormones, especially estrogen, may have the potential to keep blood pressure lower, as well as the cellular, biochemical, and molecular mechanisms by which sex hormones may modify the effects of HTN on the cardiovascular system [35].
The current study revealed that there is an association between undiagnosed HTN and infrequent consumption of fruit and vegetable. Those who consume fruits and/or vegetables less than five servings per day for three or fewer days in a typical week were 4.5 times more likely to develop undiagnosed HTN compared to those who consume fruits and/or vegetables for more than five servings per day for three or more days in a typical week. This finding was supported by a study done in the Gulele sub-city of Addis Ababa, which shows that the prevalence of undiagnosed HTN in people who did not consume fruits and vegetables in a typical week was 3 times more likely than those who consume fruits and vegetables 4–7 times in a typical week [7]. Studies conducted in Southwest Ethiopia also pointed out that eating fruit and vegetables three or fewer days per week was associated with undiagnosed HTN [26]. It is widely accepted that fruit and/or vegetables are considered an important component of a healthy diet and that their consumption could help prevent a wide range of CVDs including HTN CDs, and the World Health Organization (WHO) aims to promote this consumption [36].
The odds of having undiagnosed HTN were increased by 3.9 times among those who were not involved in high physical activity compared to those who did a high physical activity. This finding was concordant with a study conducted in Southwest Ethiopia and Nepal, which conveyed that those involved in vigorous activity were less likely to develop HTN than their counterpart [26, 37]. Hence, these findings suggest that several cardiometabolic problems may arise as a consequence of insufficient physical activity. Rapid urbanization, high population density, increased use of motorized vehicles, and modern technology might be predisposing factors for low physical activity among this study population.
The other important finding was about the association of BMI and undiagnosed HTN that being overweight or obese increases the risk of undiagnosed HTN. Those who had a BMI greater than or equal to 25 kg/m2 were about 2.7 times more likely at risk of undiagnosed HTN compared to those with a BMI of less than 25 kg/m2. This finding was similar to a study conducted in Jigjiga town of Ethiopia that those who had BMI ≥25 were 2 and 2.8 times more likely to be undiagnosed hypertensive when compared to those who had BMI less than 25, respectively [26, 38, 39]. Moreover, the current study agreed with different findings from Gondar city, Durame town, and Hawela Tula sub-city of Hawassa in Ethiopia, India, and Nigeria [9, 25, 40–42]. It could be related to urbanization, changes in dietary habits, and reduced physical activity that leads to obesity, while this study was quite lower than a study conducted in Hawassa that identified BMI was among the factors associated with HTN BMI 25 kg/m2 where 5 times more at risk for HTN than those with BMI less than 25 kg/m2 as well as in a hospital-based study in Bahir Dar Felege-Hiwot referral hospital that peoples of BMI ≥25 kg/m2 were 4.79 folds more likely to develop undiagnosed HTN than those whose BMI is less than 18.5 Kg/m2 (underweight) individuals [38]. Some of the variability between these reports could be due to the study setting and the difference in lifestyle. There are several mechanisms hypothesized to explain the link between obesity and HTN. It is generally thought that the accumulation of visceral and ectopic fat in several tissues and organs alters the metabolic and hemodynamic pathways, and additionally, insulin resistance and inflammation may promote an altered profile of vascular function and consequently lead to the development of HTN in obese people [43]. The reduction of overweight and obesity by improving nutrition and increasing regular physical activity is the best way to avoid or improve HTN [43]. It is important to teach people with high BMI to use interventions that could reduce their BMI and check their BP regularly to reduce the risk of undiagnosed HTN and its consequences.
This article was in preprint in Research Square since October 2022 [44].
7. Strengths and Limitations
To our knowledge, this is the first study to assess the prevalence of undiagnosed hypertension and its associated factors among adults in Mizan-Aman town, and the findings of this study provide a snapshot of the burden of hypertension among the population. Unlike other studies, this study was conducted at community level which can help interventions and future researchers to have more reliable findings. However, the WHO STEPwise protocol employed in this study involved gathering self-reported information on the participants' sociodemographic characteristics and information on participants' behavioral variables and hence might be subjective to respondent bias and readers might consider this limitation. Furthermore, this study lacked some important measurements like biochemicals and hence was unable to determine the level of blood glucose and/or cholesterol in the study.
8. Conclusion
This study revealed that the prevalence of undiagnosed HTN was high in Mizan-Aman town. It indicated that there might be a large number of people who have HTN but are not aware of it. Generally, it was observed that undiagnosed HTN existed in all age groups and both sexes, indicating the vulnerability of the whole population, not just a specific segment.
9. Recommendation
Community-based preventive approaches like lifestyle modification, increasing awareness, and strengthening routine screening for early detection at primary health service facilities bring a considerable change in undertaking the problem effectively.
Acknowledgments
We would like to thank Jimma University for granting ethical approval. We would also like to extend our deep appreciation to the Mizan Aman Health Office staff for data collection of this study through unreserved support and full cooperation in providing the necessary information and logistics during the data collection.
Abbreviations
- AOR:
Adjusted odds ratio
- BMI:
Body mass index
- CI:
Confidence interval
- DBP:
Diastolic blood pressure
- HTN:
Hypertension
- IRB:
Institutional Review Board
- MMHG:
Millimeter of mercury
- NCD:
Non-communicable disease
- OR:
Odds ratio
- SBP:
Systolic blood pressure
- SD:
Standard deviation
- SNNPR:
South Nation Nationalities and Peoples Region
- SPSS:
Statistical Package for Social Sciences
- WC:
Waist circumference
- WHO:
World Health Organization
- WHR:
Waist-to-hip ratio.
Data Availability
All data used during this study are available upon request from the corresponding author.
Ethical Approval
This study was conducted in line with the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethical Review Committee of the Institute of Health of Jimma University, and permission was also obtained from the Mizan Aman town administration office. Brief explanations about the purpose and benefit of the study were described to all study participants. Names and other personal information that can violate the confidentiality of the respondents were not used. Confidentiality and privacy were ensured for collecting information from the study participants, and the right of the respondents to withdraw or not to participate was respected. Participants' feedback form was prepared and provided an overview of results from the physical measurements, and also, counseling service was provided to those with high BP on lifestyle modification and to have regular check-ups. Finally, linking to the nearest health facility was also done.
Consent
For their full cooperation, verbal and written consent was obtained from each study participant.
Disclosure
This manuscript was published as a preprint in Research Square [44].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Sebsibe Elias formulated and designed the research topic, collected and supervised the data collection, analyzed the data, interpreted the results, and drafted the manuscript. Teshome Kabeta Dadi contributed to the design of the study, performed data analysis, and critically reviewed the drafted manuscript. Both authors had full access to all data and took responsibility for the integrity of the data and the accuracy of the analysis, and both authors have read and approved the final manuscript.
References
- 1.Lim S., Vos T., Flaxman A. D., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factorclusters in 21 regions, 1990-2010: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 systematic analysis for the Global Burden of Disease Study 2010. The Lancet . 2012;380(9859):p. 2224. doi: 10.1016/s0140-6736(12)61766-8.226060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar M. H., Liu P., Roth G. A., Ng M., Biryukov S., Marczak L. Global burden of hypertension and systolic blood pressure of at least 110 to 115mmHg, 1990-2015. The Journal of the American Medical Association . 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 3.Ciemins E. L., Ritchey M. D., Vaishali, Joshi V., Loustalot F., Hannan J. Morbidity and mortality weekly report application of a tool to identify undiagnosed hypertension-United States 2016. Centers for Disease Control and Prevention . 2018;67(29):798–802. doi: 10.15585/mmwr.mm6729a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan M., Tasnim F., Tariqujjaman M., et al. Examining the prevalence, correlates and inequalities of undiagnosed hypertension in Nepal: a population-based cross-sectional study. BMJ Open . 2020;10 doi: 10.1136/bmjopen-2020-037592.e037592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ataklte F., Erqou S., Kaptoge S., et al. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension . 2015;65(2):291–2988. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 6.Abebe S. M., Berhane Y., Worku A., Getachew A. Prevalence and associated factors of hypertension: a crossectional community based study in northwest Ethiopia. PLoS One . 2015;10(4) doi: 10.1371/journal.pone.0125210.e0125210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getachew F., Dirar A., Solomon D. Prevalence of undiagnosed hypertension and associated factors among residents in Gulele sub-city, Addis Ababa, Ethiopia. Journal of Community Medicine & Health Education . 2018;8:p. 590. doi: 10.4172/2161-0711.1000590. [DOI] [Google Scholar]
- 8.Wachamo D., Geleta D., Woldesemayat E. M. Undiagnosed hypertension and associated factors among adults in Hawela Tula sub-city, Hawassa, southern Ethiopia: a community-based cross-sectional study. Risk Management and Healthcare Policy . 2020;13:2169–2177. doi: 10.2147/RMHP.S276955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachamo D., Geleta D., Woldesemayat E. M. Undiagnosed hypertension and associated factors among adults in Hawela Tula sub-city, Hawassa, southern Ethiopia: a community-based cross-sectional study. Risk Management and Healthcare Policy . 2020;13:2169–2177. doi: 10.2147/RMHP.S276955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Fernandez R., Ng N., Susilo D., Prawira J., Bangs MJ., Amiya RM. The double burden of disease among mining workers in Papua, Indonesia: at the crossroads between Old and New health paradigms. BMC Public Health . 2016;16(1):p. 951. doi: 10.1186/s12889-016-3630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisay Bissa J., Tomas Benti T., Muktar Beshir A. Prevalence of tobacco consumption, alcohol, khat (catha edulis) use and high blood pressure among adults in Jimma town, South west Ethiopia. Science Journal of Public Health . 2015;3(5):650–654. doi: 10.11648/j.sjph.20150305.19. [DOI] [Google Scholar]
- 12.Maji G. Who & World Health Day. Journal of the Indian Medical Association . 2019;117(5):7–8. [Google Scholar]
- 13.Nansseu J., Noubiap J., Mengnjo M. K., et al. The highly neglected burden of resistant hypertension in Africa: a systematic review and meta-analysis. BMJ Open . 2016;6(9) doi: 10.1136/bmjopen-2016-011452.e011452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudina E. K., Michael Y., Assegid S. Prevalence of hypertension and its risk factors in southwest Ethiopia: a hospital-based cross-sectional survey. Integrated Blood Pressure Control . 2013 Jul 31;6:111–117. doi: 10.2147/IBPC.S47298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty N., Mandal A. K. A study on undiagnosed hypertension and its associated factors among adults residing in a rural area of West Bengal. Natl J Community Med . 2018;9(1):60–63. [Google Scholar]
- 16.Who. The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance . Geneva, Switzerland: WHO STEPS Surveill Man; 2015. [Google Scholar]
- 17.Cogill B. Anthropometric indicators measurement guide. 2013. http://www.developmentgateway.org/download/202582/anthro_2003.pdf .
- 18.Banigbe B. F., Itanyi I. U., Ofili E. O., Ogidi A. G., Patel D., Ezeanolue E. E. High prevalence of undiagnosed hypertension among men in north Central Nigeria: results from the healthy beginning initiative. PLoS One . 2020;15(11):26–37. doi: 10.1371/journal.pone.0242870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minstry of Health Ethiopia. Addis Ababa, Ethiopia: Minstry of Health Ethiopia; 2016. Guidelines on Clinical and Programmatic Management of Major Non Communicable Diseases. [Google Scholar]
- 20.Global Adult Tobacco Survey Collaborative Group. Tobacco Questions for Surveys: A Subset of Key Questions from the Global Adult Tobacco Surveys. 2011. http://www.who.int/tobacco/surveillance/en_tfi_tqs.pdf .
- 21.Samal D., Greisenegger S., Auff E., et al. The relation between knowledge about hypertension and education in hospitalized patients with stroke in Vienna. Stroke . 2007 Apr;38(4):p. 1304. doi: 10.1161/01.STR.0000259733.43470.27.13088 [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Family History and High Blood Pressure. Natinal Hear Lung Blood Institude. 2005. https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-heart-lung-blood-institute-nhlbi .
- 23.Omar S., Musa I R., Osman O. E., Adam I. R., Osman O. E. Prevalence and associated factors of hypertension among adults in Gadarif in eastern Sudan: a community-based study. BMC Public Health . 2020;20(1):p. 291. doi: 10.1186/s12889-020-8386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushara S., Noor S. K., Elmadhoun W M., et al. Undiagnosed hypertension in a rural community in Sudan and association with some features of the metabolic syndrome: how serious is the situation? Renal Failure . 2015;37(6):1022–10266. doi: 10.3109/0886022X.2015.1052951. [DOI] [PubMed] [Google Scholar]
- 25.Helelo T., Gelaw Y. A., Adane A A., Gelaw Y. A., Adane A. A. Prevalence and Prevalence and Associated Factors of Hypertension among Adults in Durame Town, Southern Ethiopiassociated factors of hypertension among adults in Durame town, southern Ethiopia. PLoS One . 2014;9(11) doi: 10.1371/journal.pone.0112790.e112790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulatu K. A Community Based Cross-Sectional Study . Vol. 17. Insight Medical Publishing Group; 2020. Factors associated with hypertension among age groups of 18 Years and above in southwestern Ethiopia; pp. 202–208. [Google Scholar]
- 27.Hanif A., Shamim A. A., Hossain M M., et al. Gender-specific prevalence and associated factors of hypertension among elderly Bangladeshi people: findings from a nationally representative cross-sectional survey. BMJ Open . 2021;11(1) doi: 10.1136/bmjopen-2020-038326.e038326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanam M., Lindeboom W., Razzaque A., et al. Undiagnosed and uncontrolled hypertension among the adults in rural Bangladesh: findings from a community-based study. Journal of Hypertension . 2015;33(12):p. 2399. doi: 10.1097/HJH.0000000000000712.2406406 [DOI] [PubMed] [Google Scholar]
- 29.Bharati D., Nandi P., Yamuna T., et al. Prevalence and covariates of undiagnosed hypertension in the adult population of puducherry, South India. Nepal Journal of Epidemiology . 2012;2(2):191–199. doi: 10.3126/nje.v2i2.6576. [DOI] [Google Scholar]
- 30.Sarki A. M., Nduka C. U., Stranges S., Kandala N. B., Uthman O. A. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore) . 2015;94(50) doi: 10.1097/MD.0000000000001959.e1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotwani P., Kamya M. R., Clark T. D., et al. Epidemiology and awareness of hypertension in a rural Ugandan community: Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study cross-sectional study. BMC Public Health . 2013;13(1):p. 1151. doi: 10.1186/1471-2458-13-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zekewos A., Egeno T., Loha E. The magnitude of hypertension and its risk factors in southern Ethiopia: a community based study. PLoS One . 2019;14(8):1–12. doi: 10.1371/journal.pone.0221726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mwita. The burden of hypertension and its associated factors among adults in Ruvuma, Southern Tanzania. South Sudan Medical Journal . 2020;13(1):12–18. [Google Scholar]
- 34.Reckelhoff J. F. Gender differences in the regulation of blood pressure. Hypertension . 2001 May;37(5):p. 1199. doi: 10.1161/01.hyp.37.5.1199.1208208 [DOI] [PubMed] [Google Scholar]
- 35.Dubey R. Sex hormones and hypertension. Cardiovascular Research . February;53(3):688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 36.Alemseged F., Haileamlak A., Tegegn A., et al. Risk factors for chronic non-communicable diseases at gilgel gibe field research center, southwest Ethiopia: population based study. Ethiop J Health Sci . 2012 Aug;22(S):19–28. [PMC free article] [PubMed] [Google Scholar]
- 37.Dhungana R. R., Pandey A. R., Bista B., Joshi S., Devkota S. Prevalence and associated factors of hypertension: a community-based cross-sectional study in municipalities of kathmandu, Nepal. International Journal of Hypertension . 2016;2016:10. doi: 10.1155/2016/1656938.1656938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belachew A., Tewabe T., Miskir Y., Melese E., Wubet E., Alemu S. Prevalence and associated factors of hypertension among adult patients in Felege-hiwot Comprehensive Referral Hospitals, northwest, Ethiopia:a cross-sectional study. BMC Res Notes . 2018;6 doi: 10.1186/s13104-018-3986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripathy J., Thakur J. S., Jeet G., et al. Alarmingly high prevalence of hypertension and pre-hypertension in North India-results from a large cross-sectional STEPS survey. 2017;12(12) doi: 10.1371/journal.pone.0188619.e0188619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent-Onabajo G. O., Adaji J. O., Umeonwuka C. I. Prevalence of undiagnosed hypertension among traders at A regional market in Nigeria. Annals of Medical and Health Sciences Research . 2017;7:97–101. [Google Scholar]
- 41.Oyekale A. S. Effect of obesity and other risk factors on hypertension among women of reproductive age in Ghana: an instrumental variable probit model. International Journal of Environmental Research and Public Health . 2019;16(23):p. 4699. doi: 10.3390/ijerph16234699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirier P., Giles T. D., Bray G. A., Hong Y., Stern J. S., Pi-sunyer F. X. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss an Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation . 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 43.Goodpaster B. H., Delany J. P., Otto A. D., et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA . 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias S., Dadi T. K. Prevalence of undiagnosed hypertension and associated factors among adults in mizan aman town, Bench Sheko zone, Southwest Ethiopia: a community-based cross-sectional study. Research Square . 2022 doi: 10.21203/rs.3.rs-2142875/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used during this study are available upon request from the corresponding author.


