Key Points
Anti-TNF–treated patients with IBD have reduced Ab responses over four vaccine doses.
Third and fourth doses increase the breadth and durability of neutralization responses.
T cell IL-4 levels increase with successive vaccine doses.
Visual Abstract
Abstract
Previous studies have reported impaired humoral responses after SARS-CoV-2 mRNA vaccination in patients with immune-mediated inflammatory diseases (IMIDs), particularly those treated with anti-TNF biologics. We previously reported that IMID patients diagnosed with inflammatory bowel disease, psoriasis, psoriatic arthritis, ankylosing spondylitis, or rheumatoid arthritis exhibited greater waning of Ab and T cell responses than healthy control subjects after SARS-CoV-2 vaccine dose 2. Fewer data are available on the effects of third and fourth doses. This observational cohort study collected plasma and PBMCs from healthy control subjects and untreated or treated patients with IMIDs prevaccination and after one to four doses of SARS-CoV-2 mRNA vaccine (BNT162b2 or mRNA-1273). SARS-CoV-2–specific Ab levels, neutralization, and T cell cytokine release were measured against wild-type and Omicron BA.1 and BA.5 variants of concern. Third vaccine doses substantially restored and prolonged Ab and T cell responses in patients with IMIDs and broadened responses against variants of concern. Fourth-dose effects were subtle but also prolonged Ab responses. However, patients with IMIDs treated with anti-TNF, especially patients with inflammatory bowel disease, exhibited lower Ab responses even after the fourth dose. Although T cell IFN-γ responses were maximal after one dose, IL-2 and IL-4 production increased with successive doses, and early production of these cytokines was predictive of neutralization responses at 3–4 mo postvaccination. Our study demonstrates that third and fourth doses of the SARS-CoV-2 mRNA vaccines sustain and broaden immune responses to SARS-CoV-2, supporting the recommendation for three- and four-dose vaccination regimens in patients with IMIDs.
Introduction
With the frequent emergence of new SARS-CoV-2 variants, the COVID-19 pandemic remains a pressing global health concern (1). Patients with immune-mediated inflammatory diseases (IMIDs) have dysregulated immune systems, are often treated with immune-modifying medications, and are predisposed to higher risks of SARS-CoV-2 infection and severe outcomes following infection, including critical care admission and death (2, 3).
The initial clinical trials of the SARS-CoV-2 mRNA vaccines (BNT162b2 [Pfizer/BioNTech] and mRNA-1273 [Moderna]) excluded patients with IMIDs (4, 5), raising concerns about how to optimally protect these vulnerable patients. There is now substantial evidence to suggest that certain immunomodulatory therapies, such as B-cell–depleting therapies, glucocorticoids, TNF inhibitors, mycophenolate mofetil, JAK inhibitors, and methotrexate (MTX), can attenuate humoral and cellular responses to the primary series (two doses) of SARS-CoV-2 mRNA vaccination (6–15). Data are lacking regarding the immunogenicity of the SARS-CoV-2 mRNA vaccines in patients with IMIDs after repeated vaccine doses. Moreover, many studies primarily focused on humoral responses after vaccination. However, adaptive immunity to SARS-CoV-2 depends not only on Ab and neutralization responses but also on concomitant T-cell–mediated responses (16–21). To our knowledge, there is a paucity of studies that longitudinally examine both humoral and cellular immunogenicity of SARS-CoV-2 mRNA vaccines in patients with IMIDs throughout a primary series of vaccination and booster (third and fourth) doses. These data are necessary to inform the optimal vaccination strategy for this vulnerable population.
To further the knowledge of the impact of immunomodulatory drugs on the quality and magnitude of SARS-CoV-2 vaccine–induced immunity, we recently investigated the immune response after COVID-19 vaccination during maintenance therapy in IMIDs in the IMPACT study by prospectively following a cohort of vaccinated patients with IMIDs with inflammatory bowel disease (IBD) or rheumatic or skin disease who were receiving immunosuppressive therapies compared with healthy control subjects (22, 23). We demonstrated that patients with IMIDs exhibit greater waning of Ab and T cell responses to SARS-CoV-2 by 3–4 mo after dose 2 compared with healthy control subjects (22). Notably, anti-TNF–treated patients showed the greatest reductions in Ab responses in our cohort, had reduced efficacy of neutralization of variants of concern (VOCs), and could not neutralize Omicron BA.1 (22). TNF is critical for proper lymphoid organ architecture and organization of germinal centers (GCs) (24, 25). The GCs, in turn, are needed to generate high-affinity Abs, long-lived plasma cells, and memory B cells. In the context of SARS-CoV-2, we and others have reported impaired Ab and neutralization responses to a primary series of vaccination in anti-TNF–treated patients with IMIDs (8, 9, 12, 22, 26). This indicated that patients with IMIDs treated with TNF inhibitors should be closely monitored over time for loss of humoral immunity to SARS-CoV-2.
There are limited data on whether third and fourth doses of SARS-CoV-2 vaccines can correct the deficits in immune responses of anti-TNF–treated patients after two doses of vaccine and on whether successive boosters will augment the magnitude and durability of immunity to SARS-CoV-2 in healthy and IMID populations. To this end, here we report an extension of the IMPACT study to investigate the immunogenicity of third and fourth vaccine doses. We measured spike (S)- and receptor binding domain (RBD)-specific Ab levels, neutralization, and T cell cytokine responses at 2–4 wk and 3–4 mo after each booster dose of vaccine. The primary aim was to evaluate the effect of booster doses on the magnitude and durability of immune responses to wild-type (WT) SARS-CoV-2 and Omicron VOCs as compared with the initial two-dose strategy. As we previously reported, relative to healthy control subjects, patients with IMIDs exhibit accelerated waning of SARS-CoV-2–specific Ab, neutralization, and T cell responses after the second dose. The third dose restored and maximized responses to WT SARS-CoV-2 and broadened Ab responses against VOCs. Furthermore, the third and fourth doses enhanced the durability of immune responses after vaccination compared with the second dose. We additionally demonstrate that SARS-CoV-2–specific T cell cytokine release increases with each successive booster vaccine. Finally, we show that anti-TNF treatment results in greater deficits in Ab responses in patients with IBD compared with other anti-TNF–treated patients with IMIDs. These results highlight the importance of third and fourth doses of mRNA vaccines in patients with IMIDs to prolong and broaden responses to SARS-CoV-2 vaccination.
Materials and Methods
Study design and participants
In the prospective observational cohort IMPACT study, we investigated the immune response after COVID-19 vaccination during maintenance therapy in IMIDs. The methods of the IMPACT study were previously reported (22, 23). In brief, we recruited adult participants, including healthy control subjects and patients diagnosed with one or more of the following IMIDs: IBD, psoriasis, psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, or hidradenitis suppurativa. Patients with IMIDs were untreated or treated with anti–IL-12/IL-23 therapy, anti–IL-17 therapy, anti–IL-23 therapy, anti-TNF therapy, MTX or azathioprine (MTX/AZA) monotherapy, or anti-TNF plus MTX/AZA combination therapy. Participants received one to four homologous or heterologous doses of BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccines. Participants less than 18 y of age, those with prior SARS-CoV-2 infection, patients receiving oral steroids or B cell depletion agents, and those receiving non-mRNA SARS-CoV-2 vaccines were excluded.
Sample collection
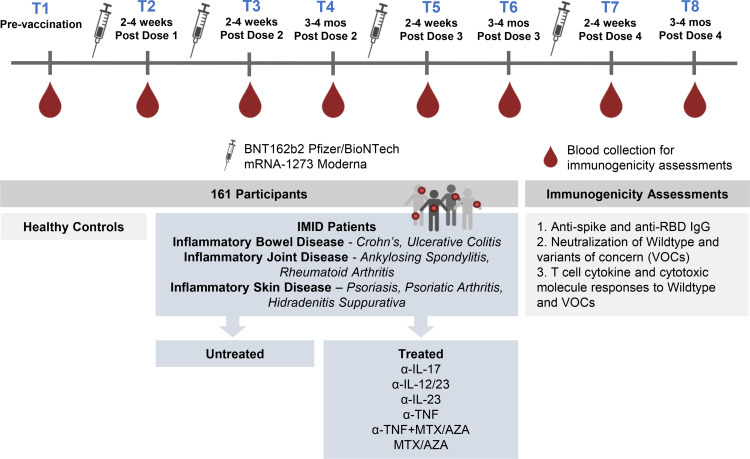
Blood samples were collected from each participant at up to eight time points spanning before and after one to four doses of vaccine, as defined in Table I: T1, prevaccination; T2, 2–4 wk after dose 2; T3, 2–3 wk after dose 2; T4, 3–4 mo after dose 2; T5, 2–4 wk after dose 3; T6, 3–4 mo after dose 3; T7, 2–4 wk after dose 4; T8, 3–4 mo after dose 4. Plasma and PBMCs were isolated from blood (via centrifugation and using SepMate PBMC isolation tubes) for immunogenicity assessment.
Immunogenicity assessment
Ab (IgG) responses against coronavirus S protein, RBD, and nucleocapsid (NP) were measured by automated ELISA (22). Data were compared with median convalescent values (anti-S and anti-RBD IgG) for serum samples from 340 PCR-confirmed COVID-19 cases 21–115 d after symptom onset (22). S-pseudotyped lentivirus neutralization assays were performed to assess neutralization capacity against WT SARS-CoV-2 and VOCs, including Omicron BA.1 and BA.5 (22). To assess SARS-CoV-2–specific T cell responses, PBMCs were stimulated for 48 h with WT or Omicron BA.1 or BA.4/5 S peptide pools (PepMix, JPT Peptide Technologies). The release of nine cytokines and cytotoxic molecules (IL-2, IL-4, IL-17A, IFN-γ, granzyme A, granzyme B, sFasL, perforin, TNF-α) in cell culture supernatants was measured using the LEGENDplex CD8/NK multiplex cytokine bead-based immunoassay (BioLegend) according to the manufacturer’s instructions. Results are reported after subtracting background values from negative control (DMSO stimulation) wells. More complete details are provided in Reference 22.
Statistics
Data from time points T1–T5 were previously reported (22, 23) and reanalyzed for the present article using additional statistical approaches as indicated in the figure legends. For analyses at individual time points, multiple linear regression models controlled for age, body mass index (BMI), sex, and vaccine type. Mixed-effects multivariate linear regression models controlled for age, BMI, sex, and vaccine type, including an interaction term between time point and study group. For the longitudinal analyses, eight time points were available for the patients with IMIDs, but only six time points were available for the healthy control subjects, because healthy control subjects were not eligible for a fourth dose at the time the patient groups were. Mixed-effects models were chosen for this analysis because they are robust in the context of longitudinal studies with missing data on participants over time (27). Comparisons were made only between groups with existing data. Differences in the effect of anti-TNF treatment by disease group were tested using linear regression models as described above, but with an interaction term between treatment with anti-TNF therapy and diagnosis with IBD and an additional control for days after vaccination. Associations between T cell responses (IL-2 and IL-4) in the 2–4 wk following vaccination and neutralizing responses 3–4 mo later were tested using regression models that controlled for age, BMI, sex, and vaccine type, and they included an interaction term between the cytokine level and treatment with anti-TNF therapy. Samples provided by participants who presented evidence of a SARS-CoV-2 infection (seropositive for NP IgG or self-reported a positive PCR or rapid test result) or who were missing key covariates were excluded from analyses. A p value less than 0.05 was considered significant. Regression modeling was conducted in STATA/BE 17.0. Additional software used in creating graphics and/or statistical analyses includes the LEGENDplex Data Analysis Software Suite and R (version 4.2.1) with packages haven, ggpubr, and custom R scripts.
Study approval
This study was approved by the ethics boards of the University of Toronto (research ethics board [REB] protocol 27673), Mount Sinai Hospital/Sinai Health System (MSH REB 21-0022-E), University Health Network–Toronto Western Hospital division (REB 21-5096), and Women’s College Hospital (REB approval 2021-0023-E). Written informed consent was obtained from all participants prior to participation.
Results
Participant characteristics
A total of 161 participants contributed 607 samples over eight time points beginning in January 2021 (Fig. 1, Table I). Inclusion criteria were adult patients diagnosed with one or more IMIDs (IBD, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, or hidradenitis suppurativa), untreated or treated with maintenance immunosuppressive therapy (anti–IL-12/IL-23, anti–IL-17, anti–IL-23, anti-TNF, MTX/AZA, anti-TNF+MTX/AZA), and vaccinated with a SARS-CoV-2 mRNA vaccine (BNT162b2 [Pfizer/BioNTech] or mRNA-1273 [Moderna]). Henceforward, in our analyses, we denote patients treated with anti-TNF monotherapy or anti-TNF+MTX/AZA combination therapy as “TNF IMID” and patients with IMIDs not treated with anti-TNF therapy as “non-TNF IMID.” The most common diagnosis was IBD (n = 71 [63.4%] at T2), and the most common treatments were anti-TNF (n = 37 [33%] at T2), anti-TNF+MTX/AZA (n = 16 [14.3%] at T2), and anti–IL-12/IL-23 therapy (n = 27 [24.1%] at T2). Healthy control subjects (n = 17 at T2) were included only up to 3–4 mo after dose 3. Most study subjects received the Pfizer vaccine. Age did not have significant effects on responses to vaccination among patients with IMIDs (data not shown). There were no significant differences between vaccine intervals for doses 1 and 2 or doses 2 and 3 among treatment groups, with anti-TNF–treated patients having slightly shorter intervals between third and fourth doses than anti–IL-12/IL-23– and anti–IL-23–treated patients (data not shown). Sample sizes used at each time point for the analyses of IgG, neutralization, and T cell cytokine and cytotoxic molecule data are shown in Supplemental Table I.
FIGURE 1.
Schematic diagram of the IMPACT study. Blood was sampled from each patient at up to eight time points spanning prevaccination to after one to four SARS-CoV-2 mRNA vaccine doses for immunogenicity assessment. α-TNF, anti-TNF.
Table I. Participant characteristics.
| T1:PreD1 | T2:2–4 WkPD1 | T3:2–4 WkPD2 | T4:3–4 MoPD2 | T5:2–4 WkPD3 | T6:3–4 MoPD3 | T7:2–4 WkPD4 | T8:3–4 MoPD4 | |
|---|---|---|---|---|---|---|---|---|
| Patients with IMIDs | ||||||||
| Included in analysis, n | 94 | 112 | 103 | 84 | 53 | 41 | 25 | 20 |
| Recruited | 96 | 119 | 112 | 90 | 57 | 49 | 32 | 25 |
| Excludeda | 2 | 7 | 9 | 6 | 4 | 8 | 7 | 5 |
| Female, n (%) | 55 (59) | 63 (56) | 58 (56) | 47 (56) | 28 (53) | 20 (49) | 14 (56) | 11 (55) |
| Age, y, median (IQR) | 39 (29–52) | 42.5 (30–55) | 43 (31–57) | 43 (30–56) | 44 (35–57) | 41 (30–49) | 58 (46–64) | 56 (36–65) |
| BMI, kg/m2, median (IQR) | 24 (22–27) | 24 (22–27) | 24 (22–27) | 24 (22–27) | 26 (23–31) | 25 (23–29) | 24 (22–29) | 24 (21–28) |
| Diagnosisb, n (%) | ||||||||
| Psoriasis | 5 (5.3) | 16 (14.3) | 16 (15.5) | 18 (21.4) | 10 (18.9) | 5 (12.2) | 4 (16.0) | 4 (20.0) |
| Rheumatoid arthritis | 2 (2.1) | 3 (2.7) | 4 (3.9) | 3 (3.6) | 2 (3.8) | 1 (2.4) | 2 (8.0) | 1 (5.0) |
| Ankylosing spondylitis | 11 (11.7) | 11 (9.8) | 11 (10.7) | 3 (3.6) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Psoriatic arthritis | 15 (16.0) | 20 (17.9) | 17 (16.5) | 13 (15.5) | 10 (18.9) | 8 (19.5) | 8 (32.0) | 5 (25.0) |
| Hidradenitis suppurativa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 1 (2.4) | 0 (0.0) | 0 (0.0) |
| IBD | 69 (73.4) | 71 (63.4) | 65 (63.1) | 56 (66.7) | 35 (66.0) | 28 (68.3) | 14 (56.0) | 12 (60.0) |
| Other | 6 (6.4) | 4 (3.6) | 2 (1.9) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Treatment group, n (%) | ||||||||
| Untreated | 9 (9.6) | 9 (8.0) | 9 (8.7) | 9 (10.7) | 5 (9.4) | 6 (14.6) | 0 (0.0) | 0 (0.0) |
| Anti-TNF | 37 (39.4) | 37 (33.0) | 34 (33.0) | 23 (27.4) | 16 (30.2) | 9 (22.0) | 5 (20.0) | 4 (20.0) |
| Anti-IL-17A | 3 (3.2) | 6 (5.4) | 6 (5.8) | 6 (7.1) | 6 (11.3) | 5 (12.2) | 4 (16.0) | 3 (15.0) |
| MTX/AZA | 3 (3.2) | 7 (6.2) | 7 (6.8) | 4 (4.8) | 2 (3.8) | 1 (2.4) | 1 (4.0) | 2 (10.0) |
| Anti-TNF + MTX/AZA | 15 (16.0) | 16 (14.3) | 14 (13.6) | 10 (11.9) | 8 (15.1) | 6 (14.6) | 6 (24.0) | 5 (25.0) |
| Anti-IL-12/IL-23 | 26 (27.7) | 27 (24.1) | 23 (22.3) | 22 (26.2) | 11 (20.8) | 11 (26.8) | 7 (28.0) | 5 (25.0) |
| Anti-IL-23 | 1 (1.1) | 10 (8.9) | 10 (9.7) | 10 (11.9) | 5 (9.4) | 3 (7.3) | 2 (8.0) | 1 (5.0) |
| Days since vaccination, median (IQR) | 26 (23–31) | 16 (14–19) | 104 (97 – 116) | 24 (18–34) | 110 (97 – 124) | 23 (19–28) | 100 (92 – 130) | |
| Vaccine type, n (%) | ||||||||
| Pfizer | 97 (86.6) | 78 (75.7) | 58 (69.0) | 32 (60.4) | 23 (56.1) | 15 (60.0) | 15 (75.0) | |
| Moderna | 11 (9.8) | 7 (6.8) | 8 (9.5) | 3 (5.7) | 3 (7.3) | 0 (0.0) | 0 (0.0) | |
| Mix of Pfizer and Moderna | 4 (3.6) | 18 (17.5) | 18 (21.4) | 18 (34.0) | 15 (36.6) | 10 (40.0) | 5 (25.0) | |
| Healthy control subjects | ||||||||
| Included in analysis, n | 15 | 17 | 21 | 11 | 5 | 6 | ||
| Recruited | 16 | 18 | 22 | 12 | 6 | 6 | ||
| Excludeda | 1 | 1 | 1 | 1 | 1 | 0 | ||
| Female, n (%) | 7 (47) | 7 (41) | 8 (38) | 4 (36) | 3 (60) | 3 (50) | ||
| Age, y, median (IQR) | 33 (25–46) | 34 (26–42) | 34 (26–46) | 27 (25–47) | 27 (25–37) | 31 (26–37) | ||
| BMI, kg/m2, median (IQR) | 24 (23–27) | 25 (23–27) | 25 (22–28) | 23 (22–27) | 23 (23–28) | 24 (22–27) | ||
| Days since vaccination, median (IQR) | 23 (21–27) | 16 (13–19) | 101 (95 – 105) | 21 (15–30) | 94 (92 – 99) | |||
| Vaccine type, n (%) | ||||||||
| Pfizer | 16 (94.1) | 19 (90.5) | 9 (81.8) | 4 (80.0) | 4 (66.7) | |||
| Moderna | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Mix of Pfizer and Moderna | 1 (5.9) | 2 (9.5) | 2 (18.2) | 1 (20.0) | 2 (33.3) |
Excluded from analysis due to evidence of SARS-CoV-2 infection or missing covariates used in analysis (i.e., BMI [n = 13] or vaccine type [n = 1]).
Diagnoses are not mutually exclusive. Participants may have reported multiple diagnoses.
IQR, interquartile range; PD, postdose.
Reduced humoral and cellular responses in patients with IMIDs
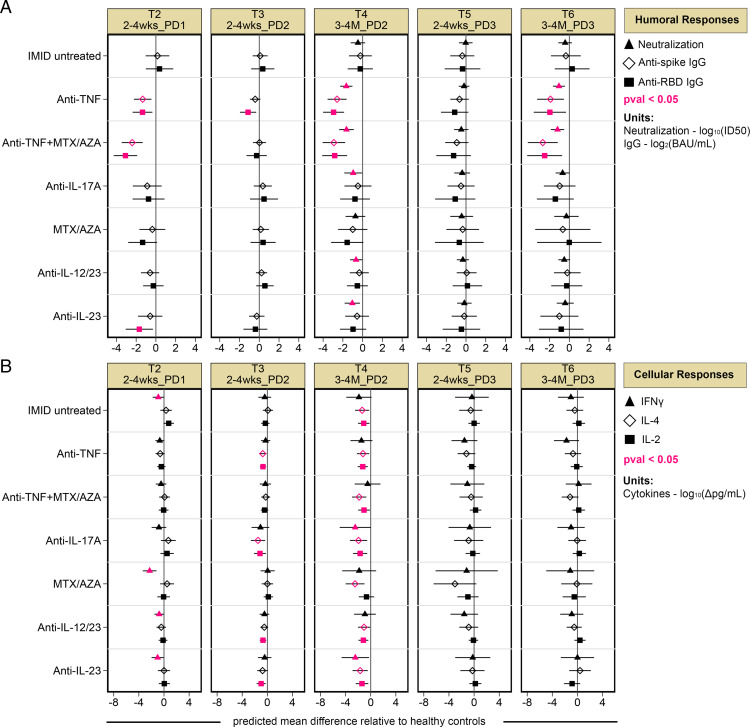
To compare humoral and cellular responses after vaccination in untreated and treated patients with IMIDs with those of healthy control subjects, multiple linear regression models (controlling for age, BMI, sex, and vaccine type) were generated at each time point following the first, second, and third vaccine doses (T2–T6, as defined in Fig. 1 and Table I). The predicted mean difference between each study group relative to healthy control subjects is plotted in Fig. 2. Anti-TNF–, anti-TNF+MTX/AZA–, and anti-IL-23–treated patients displayed reduced S- or RBD-specific Ab levels relative to healthy control subjects 2–4 wk after the first dose of vaccine (T2); however, most deficits were corrected by a second dose (T3) (Fig. 2A). By 3–4 mo after the second dose (T4), anti-TNF– and anti-TNF+MTX/AZA–treated patients displayed significantly reduced SARS-CoV-2–specific Ab responses, whereas all other patients with IMIDs maintained levels comparable to those of healthy control subjects (Fig. 2A). All patients with IMIDs tended to have a reduced capacity to neutralize WT SARS-CoV-2 compared with healthy control subjects by 3–4 mo after dose 2 (T4) in an S-pseudotyped lentiviral assay (Fig. 2A). A third dose of vaccine (T5) corrected deficits in S- and RBD-specific Ab levels and neutralization responses observed in patients with IMIDs (Fig. 2A). At 3–4 mo after dose 3 (T6), only anti-TNF– and anti-TNF+MTX/AZA–treated patients displayed reduced Ag-specific Ab and neutralization responses compared with healthy control subjects (Fig. 2A).
FIGURE 2.
Reduced humoral and cellular responses in patients with IMIDs. Patients are stratified by treatment group. The predicted mean difference in humoral and cellular responses in treated patients with IMIDs relative to healthy control subjects after one to three vaccine doses are shown, with time points defined above each panel, as described in Table I. (A) Mean difference in neutralization against WT SARS-CoV-2 (triangle) and anti-S and anti-RBD IgG (diamond and square, respectively). (B) Mean difference in T cell IFN-γ (triangle), IL-4 (diamond), and IL-2 (square) responses after vaccination in patients with IMIDs. (A and B) Multiple linear regression models controlled for age, BMI, sex, and vaccine type. Significant mean differences (p < 0.05) are colored in pink, and 95% confidence intervals are plotted across each point. (A and B) For neutralization, IgG, and cytokine data, sample sizes are listed in Supplemental Table I. Δ, background subtracted; BAU/ml, binding Ab units per milliliter; log10(ID50), serum dilution that inhibits 50% of lentiviral infection; PD, postdose.
With regard to memory T cell responses to SARS-CoV-2, after one dose of vaccine (T2), IMID untreated, MTX/AZA-, anti-IL-12/IL-23–, and anti-IL-23–treated patients had reduced IFN-γ production relative to healthy control subjects, and these deficits were corrected by a second dose (T3) (Fig. 2B). IL-2 and/or IL-4 production was reduced in several treated IMID groups after dose 2 (T3) (Fig. 2B). Three to 4 mo later (T4), all patients with IMIDs displayed significantly reduced IL-2 and IL-4 production relative to healthy control subjects (Fig. 2B). Reduced IFN-γ production was also observed in anti-IL-17A– and anti-IL-23–treated patients at T4 (Fig. 2B). No deficits in cytokine responses relative to healthy control subjects were observed after the third dose (T5, T6) (Fig. 2B).
In sum, patients with IMIDs had significantly reduced SARS-CoV-2–specific Ab, neutralization, and T cell responses to SARS-CoV-2 at 3–4 mo after dose 2. A third vaccine dose was critical for boosting these responses close to the levels of healthy control subjects. The greatest deficits were observed in the SARS-CoV-2–specific Ab responses of anti-TNF– and anti-TNF+MTX/AZA–treated patients. Therefore, in subsequent analyses, we included comparisons between the TNF IMID and non-TNF IMID groups.
Reduced Ab responses in anti-TNF–treated subjects across four vaccine doses
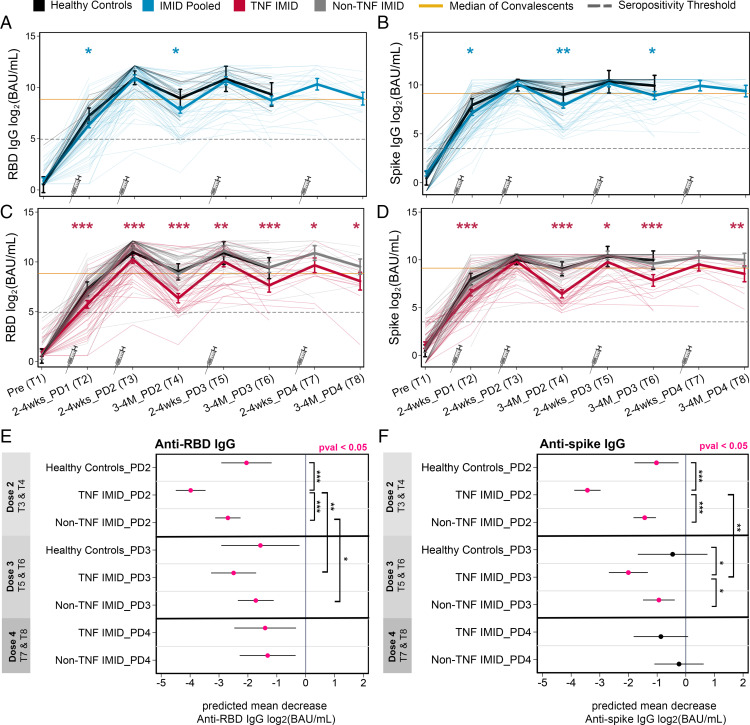
To parse the effects of the third and fourth vaccine doses on the magnitude of Ab responses, mixed-effects linear regression models (controlling for age, BMI, and sex, with an interaction term between time point and study group) were generated to predict the mean anti-RBD and anti-S IgG responses at each time point (Fig. 3). First and second vaccine doses significantly increased anti-RBD and anti-S IgG levels compared with baseline, with maximal levels achieved after two vaccine doses in all participants (Fig. 3A–3D). As noted in the pairwise analysis in Fig. 1, this longitudinal analysis confirms that by 3–4 mo after dose 2 (T4), patients with IMIDs exhibited reduced anti-RBD and anti-S levels compared with healthy control subjects (Fig. 3A, 3B). This was largely driven by the TNF IMID group (Fig. 3C, 3D). The third and fourth vaccine doses (T5–T8) did not result in significant measurable increases in Ag-specific Ab levels over the peak dose 2 response, with no overall differences between healthy control subjects and patients with IMIDs (Fig. 3A–3D). A limitation to this conclusion is that many healthy control subjects and patients in the non-TNF IMID group reached the upper limit of quantification for anti-S IgG levels after the second dose of vaccine (T3), with the percentage of patients reaching saturating Ab levels increasing with subsequent doses (Supplemental Table II). This was less of an issue for RBD-specific IgG responses because levels were generally lower. Although patients with IMIDs as a whole reached the same peak Ab responses as healthy control subjects, the TNF IMID group consistently showed reduced RBD- and S-specific Ab levels compared with healthy control subjects or the non-TNF IMID group across one to four doses of vaccine (Fig. 3C, 3D).
FIGURE 3.
Effect of anti-TNF on levels and durability of Ab responses across four vaccine doses. The figure represents a longitudinal analysis of linked samples before and after one to four doses of SARS-CoV-2 mRNA vaccine, with time points defined in Table I. Predicted mean anti-RBD IgG (A and C) and anti-S IgG (B and D) levels log2(BAU/ml) from prevaccination to after one to four doses of vaccine in healthy control subjects and patients with IMIDs. (A–D) Mixed-effects linear regression models controlled for age, BMI, and sex (thick lines), with an interaction term between time point and study group. Individual patients are plotted in thin lines. Study groups: healthy control subjects (black), patients with IMIDs pooled (blue), TNF IMID (anti-TNF– or anti-TNF+MTX/AZA–treated) (red), and patients with IMIDs not treated with anti-TNF (gray). The dashed gray lines represent the IgG seropositivity threshold. The yellow lines represent the median IgG levels from 340 convalescent cases (PCR-confirmed COVID-19 cases 21–115 d after symptom onset). An additional control for vaccine type was incorporated into mixed-effects regression models to compare groups: IMID pooled versus healthy control subjects (blue asterisks) or TNF IMID versus IMID pooled (red asterisks). (E and F) Predicted mean decrease of anti-RBD IgG (E) and anti-S IgG (F) in healthy control subjects,TNF IMID patients, and patients with IMIDs not treated with anti-TNF (between 2–4 wk and 3–4 mo) after dose 2 (decrease between T3 and T4), after dose 3 (decrease between T5 and T6), and after dose 4 (decrease between T7 and T8). Mixed-effects linear regression models controlled for age, BMI, sex, and vaccine type, with an interaction between time point and study group. Values that are significant (p < 0.05) are colored in pink. Significant pairwise comparisons are indicated by asterisks and brackets. (A–F) Sample sizes are listed in Supplemental Table I. *p < 0.05, **p < 0.01, ***p < 0.001. BAU/ml, binding Ab units per milliliter; PD, postdose.
Third and fourth doses enhance the durability of IgG responses in patients with IMIDs
Although the third and fourth vaccine doses had little or no effect on the overall magnitude of Ab responses, booster doses were critical for limiting the decay of Ab levels after vaccination. Using mixed-effects linear regression models, we predicted the mean decrease in RBD- and S-specific IgG levels between two time points (2–4 wk and 3–4 mo) after doses 2, 3, and 4 (Fig. 3E, 3F). All groups exhibited significant decreases in anti-RBD and anti-S levels by 3–4 mo after dose 2, with the greatest decreases observed in the TNF IMID group (Fig. 3E, 3F). The degree of waning between anti-RBD and anti-S IgG following the third and fourth vaccine doses showed distinct kinetics. For anti-RBD IgG, all study groups showed waning of responses at 3–4 mo after dose 3, albeit of reduced magnitude relative to that observed after the second dose, whereas there were no differences in the magnitude of waning between the third and fourth doses (Fig. 3E). Anti-S IgG levels did not significantly decline in healthy control subjects by 3–4 mo after dose 3, whereas the anti-S IgG levels of patients with IMIDs still waned after dose 3 (Fig. 3F). The fourth dose maintained S-specific IgG levels such that decreases observed in patients with IMIDs in the 3–4 mo following the fourth vaccine dose were no longer significant (Fig. 3F). In sum, the third and fourth doses of vaccine in participants with IMIDs enhanced the duration of Ab responses to SARS-CoV-2.
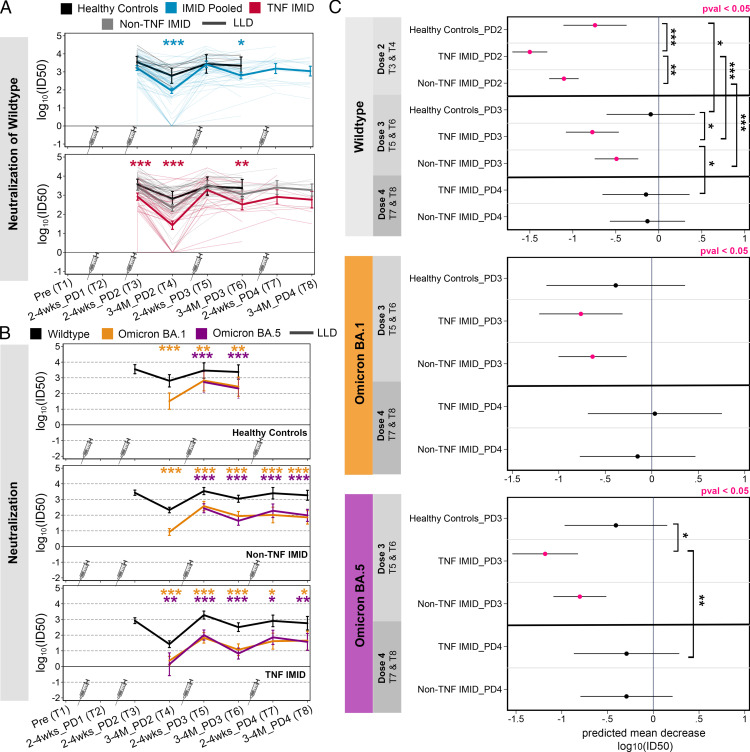
Effect of third and fourth vaccine doses on neutralization activity against wild-type SARS-CoV-2 and VOCs
Our previous work demonstrated that after two vaccine doses, patients with IMIDs had a reduced ability to neutralize WT SARS-CoV-2 compared with healthy control subjects and the TNF IMID group could not neutralize the Omicron BA.1 VOC (22). Here we used mixed-effects linear regression models (controlling for age, BMI, sex, and vaccine type, with an interaction term between time point and study group) to predict neutralization activity following two to four vaccine doses (Fig. 4).
FIGURE 4.
Effect of third and fourth vaccine doses on neutralization activity against WT SARS-CoV-2 and VOCs. Data are reported as a longitudinal analysis of linked samples for the neutralization responses after two to four doses of SARS-CoV-2 mRNA vaccine. Time points are defined in Table I. (A) Predicted neutralization [log10(ID50), NT: neutralization] activity against WT SARS-CoV-2 after two to four doses of vaccine, grouped by healthy control subjects (black), patients with IMIDs pooled (blue), patients with IMID treated with anti-TNF or anti-TNF+MTX/AZA (red), and patients with IMID not treated with anti-TNF (gray). Comparisons between groups are indicated: IMID pooled versus healthy control subjects (blue asterisks) or TNF IMID versus IMID pooled (red asterisks). (B) Predicted neutralization [log10(ID50)] activity against WT, Omicron BA.1, and Omicron BA.5 after two to four doses of vaccine, grouped by healthy control subjects, patients with IMIDs not treated with anti-TNF, and patients with IMID treated with anti-TNF or anti-TNF+MTX/AZA. Neutralization activity against WT is depicted in black, against Omicron BA.1 in orange, and against Omicron BA.5 in purple. Paired t tests were conducted to compare responses to Omicron BA.1 or BA.5 versus WT. (C) Predicted mean decrease of neutralization against WT, Omicron BA.1 and BA.5 in healthy control subjects, TNF IMID, and patients with IMIDs not treated with anti-TNF (between 2–4 wk and 3–4 mo) after dose 2 (decrease between T3 and T4), after dose 3 (decrease between T5 and T6), and after dose 4 (decrease between T7 and T8). Mean decrease values that are significant (p < 0.05) are colored in pink. Significant pairwise comparisons are indicated by asterisks and brackets. (A, C) Sample sizes are listed in Supplemental Table I. (B) Sample sizes are as follows: [WT versus BA.1] n = 9 for HC/T4; n = 5 for HC/T5; n = 6 for HC/T6; n = 48 for non-TNF/T4; n = 24 for non-TNF/T5; n = 25 for non-TNF/T6; n = 8 for non-TNF/T7; n = 11 for non-TNF/T8; n = 32 for TNF/T4; n = 19 for TNF/T5; n = 15 for TNF/T6; n = 9 for TNF/T7; n = 6 for TNF/T8; [WT versus BA.5] n = 5 for HC/T5; n = 6 for HC/T6; n = 24 for non-TNF/T5; n = 25 for non-TNF/T6; n = 8 for non-TNF/T7; n = 11 for non-TNF/T8; n = 4 for TNF/T4; n = 19 for TNF/T5; n = 15 for TNF/T6; n = 9 for TNF/T7; n = 6 for TNF/T8. (A–C) Mixed-effects linear regression models controlled for age, BMI, sex, and vaccine type, with an interaction term between time point and study group. *p < 0.05, **p < 0.01, ***p < 0.001. BAU/ml, binding Ab units per milliliter; HC, healthy control subjects; LLD, lower limit of detection; log10(ID50), serum dilution that inhibits 50% of lentiviral infection; PD, postdose.
At 3–4 mo after dose 2, 10% of patients with IMIDs had no detectable neutralizing activity (lower limit of detection) against WT SARS-CoV-2, and the IMID group overall had significantly reduced neutralization activity compared with healthy control subjects (Fig. 4A). This difference between healthy control subjects and patients with IMIDs was corrected by the third dose. Although peak neutralization levels were reached in healthy donors after two doses of vaccine, for patients with IMIDs, the third dose increased neutralization levels from dose 2 (p = 0.019), driven by an increase in the TNF IMID group (p = 0.005), with no further enhancement by the fourth dose (Fig. 4A). In contrast to the ELISA data, the neutralization results were not impacted by saturation of the responses (see Supplemental Table III).
Neutralization activity against BA.1 and BA.5 VOCs was significantly weaker than neutralization against WT at all time points for all study groups where data were available (Fig. 4B; all p < 0.05). The majority of the TNF IMID group were unable to neutralize either Omicron BA.1 or Omicron BA.5 at 3–4 mo after dose 2 but developed neutralization activity after the third dose (Fig. 4B). At each time point, a significant proportion of the TNF IMID group had neutralization responses to BA.1 and BA.5 that were at the lower limit of detection (Supplemental Table III); hence, the deficits in their responses to VOCs relative to the WT may be underestimated.
Although the third and fourth doses minimally affected the magnitude of neutralization activity against WT and VOCs, compared with the peak response after the second dose, the boosters were critical for prolonging responses. Between 2–4 wk and 3–4 mo after dose 2, all participants displayed significant decreases in neutralization activity against WT SARS-CoV-2, with the TNF IMID group displaying the largest decrease (Fig. 4C). Neutralization levels against WT SARS-CoV-2 and VOCs did not significantly decline in healthy donors by 3–4 mo after the third dose, whereas patients with IMIDs continued to show waning in neutralization responses after dose 3 (Fig. 4C). However, 3–4 mo after dose 4, there was no longer a significant decay in neutralization responses to WT or VOCs among all patients with IMIDs (TNF IMID and non-TNF IMID) (Fig. 4C).
Together, these data suggest that third doses are important in healthy control subjects and patients with IMIDs for reducing the decay of neutralization responses to variants of SARS-CoV-2, thereby broadening neutralization activity, with fourth doses showing additional stabilization effects in patients with IMIDs.
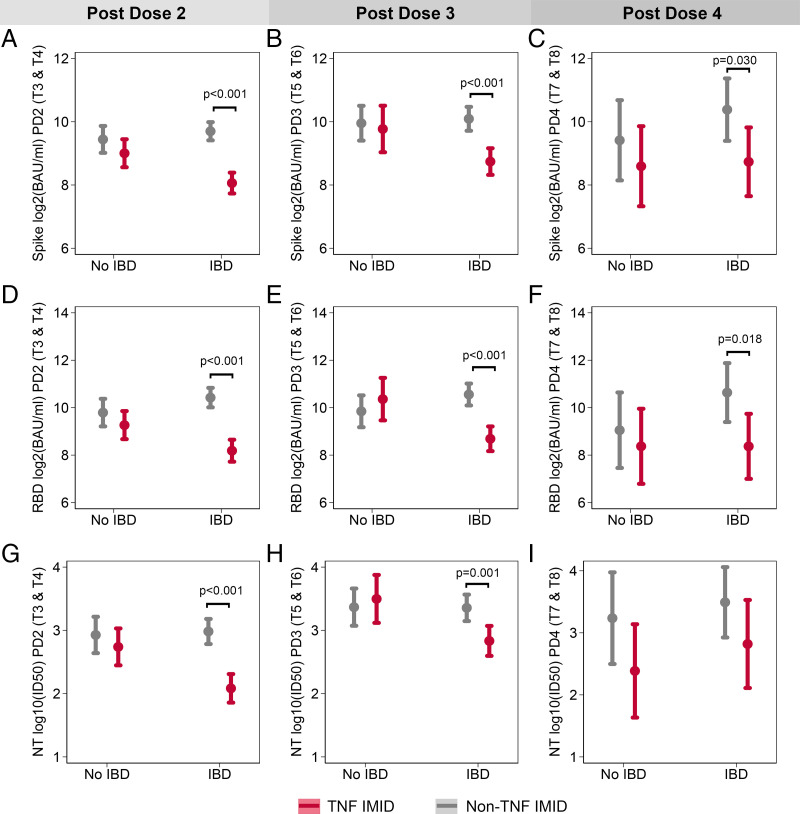
The effect of anti-TNF therapy in reducing Ab responses is significant only in the IBD patient group
Given the observation that anti-TNF therapy had the most profound effect on Ab responses to the SARS-CoV-2 vaccine, we next investigated whether the specific disease type impacted these results. Based on the sample size, this analysis was limited to the stratification of patients with IMIDs by IBD diagnosis. Ab responses after dose 2 (T3 and T4), after dose 3 (T5 and T6), and after dose 4 (T7 and T8) were pooled. Analyses controlled for age, sex, BMI, days after vaccination, and vaccine type. Regardless of vaccine dose, anti-S IgG and anti-RBD IgG levels were significantly lower for patients with IBD treated with anti-TNF therapy than for those who were not treated with anti-TNF therapy (Fig. 5A–5F). At time points after doses 2 and 3, neutralization capacity was lower for patients with IBD receiving anti-TNF therapy than for patients with IBD not treated with anti-TNF (Fig. 5G, 5H). In contrast, treatment status had no significant impact on Ab responses among patients with IMID who did not report an IBD diagnosis (Fig. 5). For each outcome, at time points after doses 2 and 3, there was a significant interaction between IBD status and anti-TNF treatment, such that the effect of anti-TNF therapy was significantly greater for patients with IBD than for other patients with IMIDs (Fig. 5A, 5B, 5D, 5E, 5G, 5H). For this analysis, anti-TNF monotherapy–treated groups, as well as patients treated with anti-TNF+MTX/AZA combination therapy, were pooled into the anti-TNF–treated category (TNF IMID). However, the removal of the combination therapy groups did not affect the significance of these results (data not shown).
FIGURE 5.
The effect of anti-TNF therapy in reducing Ab responses is significant only in the IBD patient group. The effect of anti-TNF therapy on anti-S IgG (A–C), anti-RBD IgG (D–F), and neutralization of WT SARS-CoV-2 (G–I) was tested for participants with and without IBD using mixed-effects models with an interaction term between IBD status and treatment with anti-TNF therapy. Models controlled for days after vaccination, sex, age, BMI, and vaccine type and were repeated for postdose 2 (A, D, G), postdose 3 (B, E, H), and postdose 4 (C, F, I) time points. For IgG data, samples sizes for each subgroup were as follows: n = 38 for non-IBD/non-TNF/PD2, n = 28 for non-IBD/TNF/PD2, n = 68 for IBD/non-TNF/PD2, n = 58 for IBD/TNF/PD2, n = 21 for non-IBD/non-TNF/PD3, n = 9 for non-IBD/TNF/PD3, n = 34 for IBD/non-TNF/PD3, n = 29 for IBD/TNF/PD3, n = 10 for non-IBD/non-TNF/PD4, n = 9 for non-IBD/TNF/PD4, n = 44 IBD/non-TNF/PD4, and n = 11 for IBD/TNF/PD4. For neutralization data, samples sizes for each subgroup were as follows: n = 36 for non-IBD/non-TNF/PD2, n = 28 for non-IBD/TNF/PD2, n = 67 for IBD/non-TNF/PD2, n = 51 for IBD/TNF/PD2, n = 20 for non-IBD/non-TNF/PD3, n = 9 for non-IBD/TNF/PD3, n = 29 for IBD/non-TNF/PD3, n = 25 for IBD/TNF/PD3, n = 8 for non-IBD/non-TNF/PD4, n = 8 for non-IBD/TNF/PD4, n = 11 for IBD/non-TNF/PD4, n = 6 for IBD/TNF/PD4. PD, postdose.
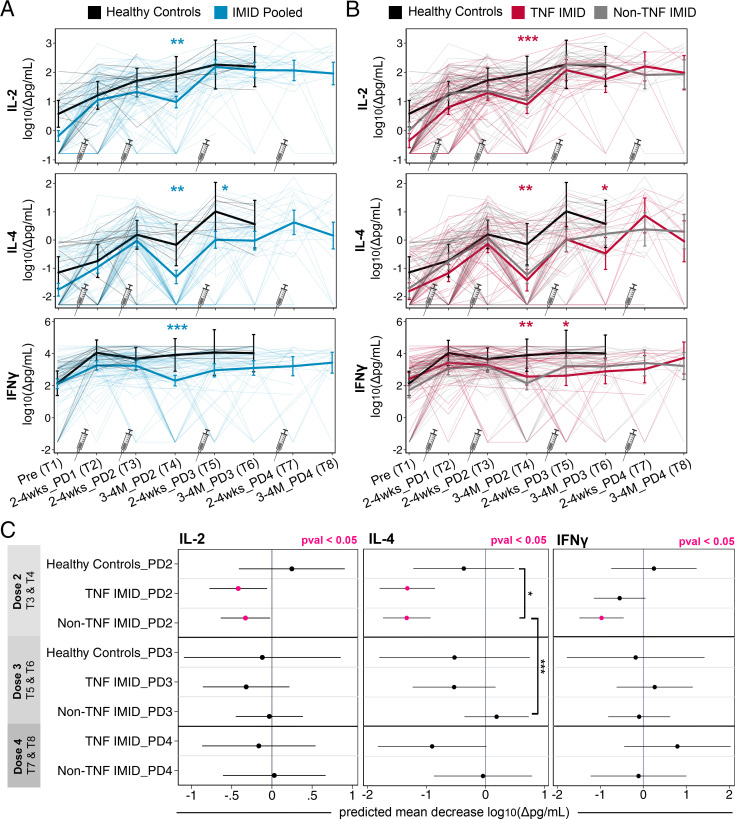
Third and fourth doses increase the magnitude and durability of T cell cytokine responses
To elucidate the effects of the third and fourth doses of vaccine on the magnitude and durability of T cell responses after vaccination, mixed-effects linear regression models (controlling for age, sex, and BMI, with an interaction term between time point and study group) were used to estimate the mean levels of secreted T cell cytokine IL-2, IL-4, and IFN-γ (Fig. 6), as well as IL-17A and cytotoxic molecule responses (Supplemental Fig. 1), across one to four doses of vaccine.
FIGURE 6.
Third and fourth doses increase the magnitude and durability of T cell cytokine responses. Data are reported as a longitudinal analysis of linked samples with time points defined in Table I. (A and B) Predicted mean T cell cytokines IL-2, IL-4, IFN-γ [log10 (Δpg/ml)] from prevaccination to after one to four doses of vaccine. Mixed-effects linear regression models controlled for age, BMI, and sex, with an interaction term between time point and group (thick lines). Individual patients are plotted in thin lines. Groupings: healthy control subjects (black), patients with IMIDs pooled (blue), patients with IMIDs treated with anti-TNF or anti-TNF+MTX/AZA (red), and patients with IMIDs not treated with anti-TNF (gray). An additional control for vaccine type was incorporated into mixed-effects regression models to compare groups: IMID pooled versus healthy control subjects (blue asterisks) or TNF IMID versus healthy control subjects (red asterisks). (C) Predicted mean decrease of T cell cytokines IL-2, IL-4, IFN-γ [log10 (Δpg/ml)] in healthy control subjects, TNF IMID, and non-TNF IMID groups between 2–4 wk and 3–4 mo after dose 2 (decrease between T3 and T4), after dose 3 (decrease between T5 and T6), and after dose 4 (decrease between T7 and T8). Mixed-effects linear regression models controlled for age, BMI, sex, and vaccine type, with an interaction between time point and group. Mean decrease values that are significant (p < 0.05) are colored in pink. Significant pairwise comparisons are indicated by asterisks and brackets. (A–C) Sample sizes are listed in Supplemental Table I. *p < 0.05, **p < 0.01, ***p < 0.001. Δ, background subtracted; PD, postdose.
Relative to baseline (T1), IL-2 and IL-4 production in healthy control subjects and patients with IMID was greater after the first and second doses of vaccine (T2, T3) (Fig. 6A), whereas IFN-γ responses peaked after the first vaccine dose for all participants (Fig. 6A, 6B). Peak IL-2 responses were observed by the third dose in healthy control subjects and patients with IMIDs, with no differences evident between the groups after dose 3 and no further impact of dose 4. T cell IL-4 responses increased with each successive dose, measured up to dose 3 in healthy donors and dose 4 in patients with IMIDs (Fig. 6A). Notably, there was a significant increase in IL-4 between dose 3 and dose 4 for the TNF IMID group (p = 0.026), which was not apparent in the non-TNF IMID group (Fig. 6B). This effect was driven primarily by patients receiving combination therapy with anti-TNF plus MTX/AZA (data not shown). After dose 2, patients with IMID exhibited reductions in IFN-γ production compared with healthy control subjects, and the third dose restored but did not further boost IFN-γ responses (Fig. 6A, 6B). The fourth dose did not increase the magnitude of IFN-γ responses beyond the maximum achieved after the first dose. The TNF IMID and non-TNF IMID groups exhibited similar IL-2, IL-4, and IFN-γ responses, with waning of cytokines after dose 2 of vaccine relative to healthy control subjects, and restoration of responses with third and fourth doses (Fig. 6B).
Other molecules assessed included IL-17A and cytotoxic molecules granzyme A, granzyme B, perforin, and sFasL. In all cases, production peaked after one dose of vaccine. Analogous to the IL-2, IL-4, and IFN-γ responses, patients with IMIDs exhibited reduced levels of cytotoxic molecules compared with healthy control subjects at 3–4 mo after dose 2 (Supplemental Fig. 1A), with these lower levels corrected by the third or fourth doses of vaccine.
To assess the effects of the third and fourth doses on prolonging T cell cytokine and cytotoxic molecule responses after vaccination, we compared the predicted decrease in responses between two time points (2–4 wk and 3–4 mo) after the second, third, and fourth doses of vaccination, respectively (Fig. 6C, Supplemental Fig. 1B). Although healthy donor T cell responses were stable out to 3–4 mo after dose 2, both the TNF IMID and non-TNF IMID groups exhibited significant decreases in IL-2 and IL-4 secretion after the second dose, with IL-4 exhibiting the most substantial decrease (Fig. 6C). The non-TNF IMID group also exhibited decreases in IFN-γ responses after dose 2 (Fig. 6C). However, after the third or fourth dose, there was no longer any significant decay in T cell cytokine responses over the next 3–4 mo (Fig. 6C), highlighting the enhanced durability of cytokine responses in patients with IMIDs after booster doses of vaccine. Parallel phenomena were observed with IL-17A, granzyme A, granzyme B, sFasL, and perforin responses; all patients with IMID, but not healthy control subjects, displayed significant decreases in response after dose 2, with no significant decreases after booster doses (Supplemental Fig. 1B).
We additionally assessed T cell responses to BA.1 and BA.4/5 VOCs after the third dose of vaccine (T5, T6). Healthy control subjects and patients with IMIDs (pooled) did not exhibit significant differences in IL-2, IL-4, IFN-γ, sFasL, and granzyme A responses to Omicron BA.1 or BA.4/5 compared with WT (data not shown). Reduced IL-17A responses to BA.1 and BA.4/5 were observed at T6, and minor reductions in granzyme B and perforin responses to BA.1 or BA.4/5 were observed at T5 and T6 (data not shown). Altogether, these data show that after three vaccine doses, T cell responses of patients with IMIDs to WT and the BA.1 and BA.4/5 VOCs are largely equivalent.
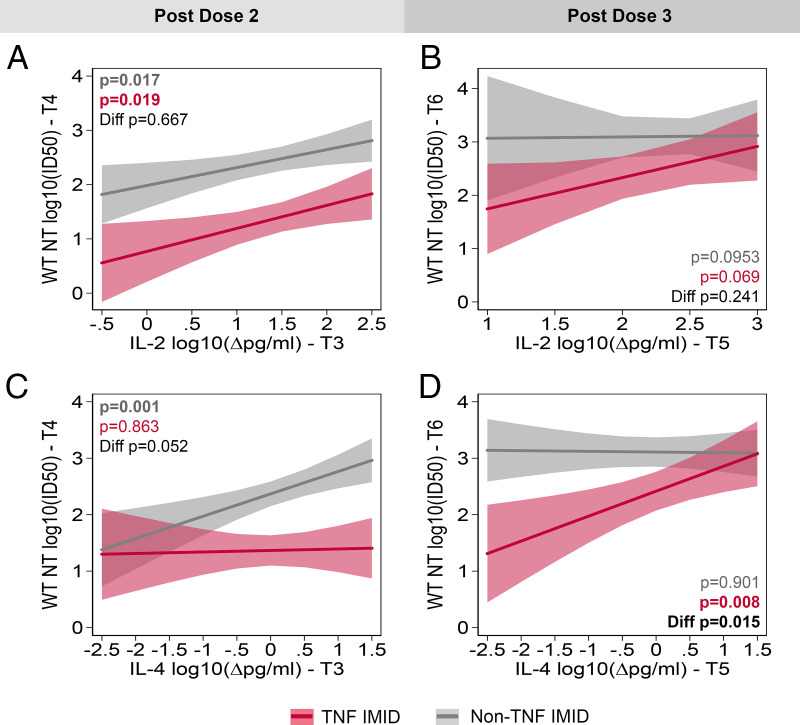
Association between T cell responses 2–4 wk postvaccination and neutralizing Ab titers 3–4 mo later
Multivariate linear regression models were used to estimate the association between T cell and neutralization responses, focusing on IL-2 and IL-4 responses in patients with IMIDs. Of note, IL-2 responses after dose 2 significantly predicted neutralization 3–4 mo later in both the TNF IMID (p = 0.019) and non-TNF IMID groups (p = 0.017) (Fig. 7A). This effect was no longer significant after dose 2, perhaps suggesting saturation of the T cell IL-2 response with respect to help for Ab responses (Fig. 7B). There was also a significant correlation between IL-4 production at 2–4 wk after dose 2 and neutralizing capacity 3–4 mo later, among non-TNF IMID (p = 0.001) but not among TNF IMID (Fig. 7C). Following dose 3, the effect of IL-4 was no longer predictive of neutralizing capacity for the non-TNF IMID group but became predictive in the TNF IMID group (p = 0.008; Fig. 7D). Accordingly, there is a significant difference in the effect of IL-4 on neutralizing capacity between study groups (p = 0.015; Fig. 7D). These data suggest that the early T cell responses may be important in defining the duration of the Ab response, with the timing of the impact of T cells related to when the maximal T cell signal is achieved.
FIGURE 7.
Association between T cell responses 2–4 wk postvaccination and neutralizing Ab titers 3–4 mo later. The association of IL-2 (A and B) or IL-4 (C and D) shortly after vaccination (T3: 2–4 wk_PD2 or T5: 2–4 wk_PD3) with neutralization capacity of the SARS-CoV-2 WT strain 3 mo later (T4: 3–4M_PD3 or T6: 3–4M_PD4) was tested using linear regression models that controlled for age, BMI, sex, and vaccine type. Regression models included an interaction term between cytokine levels and study group (i.e., non-TNF IMID and TNF IMID) and were repeated postdose 2 (A and C) and postdose 3 (B and D). PD, post dose.
Discussion
In this longitudinal study, we followed a cohort of patients diagnosed with one or more IMIDs to assess their responses to SARS-CoV-2 after one through four vaccine doses. We and others have reported that specific immunosuppressants significantly attenuate the magnitude and stability of humoral and cellular responses after two doses of vaccine in patients with IMIDs compared with healthy control subjects, with the most pronounced deficits in IMID patients treated with anti-TNF biologics (7, 9, 10, 12, 22, 26).
Although T cell cytokine responses increased with successive vaccine doses, we observed no difference in the magnitude of the peak Ab response following the third or fourth dose in healthy donors and patients with IMIDs. There is debate in the literature regarding the effect of a third dose on the serological responses in patients with IMIDs. In studies in which patients have absent or weak serological responses after the second dose, a third dose significantly boosted Ab levels (13, 28, 29), whereas some patients with IMIDs do not respond even after a third dose (14). It has also been observed that a third dose does not significantly boost levels compared with the peak after the second dose, which may be attributed to the stronger response seen in these individuals after the second dose as compared with weak responders (10). There are less data on the effects of the fourth vaccine dose; however, one study has reported that patients with IMIDs show higher anti-RBD Ab concentrations following four vaccine doses than after three doses (30). Although in our study the third and fourth doses had little or no measurable effect on the magnitude of Ab responses, the third dose, and to a lesser extent the fourth dose, was critical in enhancing the durability of Ab responses in the ensuing 3–4 mo after vaccination in healthy control subjects and patients with IMIDs. These data are consistent with other studies in patients with IMIDs and healthy patients demonstrating a slower decline in Ab levels after the third dose of vaccine (13, 31, 32). It is important to note that the immunogenicity of a third and fourth dose may differ on the basis of the strength of response after the primary series of vaccination and the degree of immunosuppression, disease indication, type of treatment, and variability in assay dynamic ranges.
Regardless of one to four doses of vaccine, anti-TNF–treated patients consistently showed reduced Ab levels compared with healthy control subjects and patients with IMID not treated with anti-TNF, suggesting a long-term impact of anti-TNF treatment on the humoral response to vaccines. These results are consistent with those of another recent study (33). In contrast, T cell responses in patients receiving anti-TNF therapy and those not on anti-TNF therapy were similar. Strikingly, the negative effect of anti-TNF therapy on humoral responses was observed only in patients with IBD. Removal of the combination-treated anti-TNF patients from this analysis did not affect the differences observed. This finding is in line with literature reporting an effect of anti-TNF therapy on SARS-CoV-2 vaccine response in patients with IBD (12, 26, 34–37), but not in other patients with IMIDs without an IBD diagnosis (38, 39). The mechanisms underlying the differential effect of anti-TNF therapy on vaccine-induced immunity based on disease indication are unclear but may be attributed to higher doses or a higher proportion receiving drugs i.v. in the IBD group compared with patients with IMID who do not have an IBD diagnosis, although this remains to be determined. Regardless of the mechanism, patients with IBD receiving anti-TNF therapy have impaired humoral responses to vaccination and thus may be more susceptible to SARS-CoV-2 without regard to vaccination status. These data support early booster vaccination regimens for these patients.
The third dose of vaccine was necessary for patients with IMIDs to develop robust neutralization activity against BA.1 and BA.5 VOCs, which was lacking in anti-TNF–treated patients with IMIDs 3–4 mo after dose 2 (22), thereby broadening immune responses against SARS-CoV-2. Because recall responses to booster doses are dominated by preexisting memory B cells recognizing WT SARS-CoV-2 (40), the increased breadth of neutralization responses after a booster may be due to the expansion of and additional rounds of affinity maturation in existing clones, such that cross-reactivity for VOCs now becomes detectable.
The kinetics of neutralization responses against WT and VOCs varied between healthy subjects and patients with IMIDs. Although healthy control subjects had stable neutralization responses to WT and VOCs out to 3–4 mo after a third dose of vaccine, patients with IMIDs required a fourth dose to achieve this effect. Regardless of the study group, neutralization activity against BA.1 and BA.5 VOCs was significantly weaker than neutralization of WT across two to four doses of vaccine, in line with studies demonstrating reduced geometric mean titers of neutralization against Omicron sublineages compared with the original WT strain after three and four doses of vaccine (33, 41, 42). Regarding T cell responses to VOCs, we observed that patients with IMIDs retain robust T-cell–mediated immunity against Omicron variants, consistent with studies demonstrating that T cells are largely unaffected by Omicron variants and important for providing potent protection against emerging variants capable of escaping neutralization responses (20, 43–45). Future studies should investigate the humoral immunogenicity of the bivalent vaccines targeting BA.4 and BA.5 VOCs in patients with IMIDs.
Analogously to humoral responses, patients with IMIDs exhibited accelerated waning of T cell cytokine and cytotoxic molecule responses after a second dose of vaccine, which was corrected by a third dose. The decrease in responses by 3–4 mo after vaccination was no longer significant after three doses. IL-4 is of particular interest for vaccine-induced immunity because it is produced by T follicular helper (Tfh) cells and promotes GC B cell proliferation, class switching, and differentiation (46). We noted that IL-4 levels increased with each of the first three doses of vaccine in healthy control subjects and patients with IMIDs, with an additional increase in patients with the TNF IMID group after the fourth dose. Although IL-4 levels correlated with the neutralization responses in non-TNF IMID patients after the second dose, this effect was only apparent in the TNF IMID group after the third dose, hinting at the mechanisms underlying the necessity of booster doses. Because TNF inhibitors are known to disrupt the organization of GCs in patients with rheumatoid arthritis (47), it is possible that the cooperation between Tfh and GC B cells is disrupted in anti-TNF–treated patients relative to other groups, hence providing a potential explanation for the lack of correlation of T cell IL-4 and Ab responses after dose 2. It is possible that by increasing the number of Ag-specific T and B cells and the cumulative magnitude of their functional responses, dose 3 may overcome this organizational deficit, allowing Tfh cells to provide help to B cells, leading to enhanced neutralizing Ab responses, although this remains speculative. A caveat of our study design is that we measured the total cytokine produced after T cell restimulation, but not the number of SARS-CoV-2–specific IL-4–producing T cells. Regardless, the results show that booster doses of vaccination are necessary to increase the magnitude of T cell cytokine production.
This study has several limitations. Each study group (i.e., TNF IMID and non-TNF IMID) was heterogeneous in terms of disease type and specific treatment regimen; therefore, our study was underpowered to evaluate these effects within each group. Sample sizes declined for time points after the third and fourth doses (T5–T8) of the vaccine because participants did not receive booster doses, withdrew from the study, or were excluded because of evidence of natural infection with SARS-CoV-2. Thus, after the fourth dose, it was difficult to draw conclusions about specific treatment groups. The latest time point assessed after each dose of vaccine was at the 3–4-mo mark; we did not track long-term waning beyond that mark. Additionally, we did not assess hybrid immunity, because we were limited by the low numbers of vaccinated and infected patients. In Canada, fourth vaccine doses were offered to immunocompromised populations several months before they became available to healthy adults, resulting in a lack of healthy control subjects to compare with patients with IMIDs for the time points after dose 4.
Another limitation is the narrow dynamic range of the ELISAs, meaning that saturating IgG levels above the upper limit of quantification could contribute to an underestimation of the effects of booster doses on the magnitude of responses and/or an underestimation of the deficits observed in patients with TNF IMID compared with patients with non-TNF IMID and healthy control subjects. However, because neutralization data in our study did not reach saturation, we believe the broad conclusions of the article are not impacted by saturation of some of the readouts. Another limitation is that our study assessed only systemic immunity and did not examine mucosal immunity. Finally, the scope of the IMPACT study lies within investigating the immunogenicity of mRNA vaccines; our results do not make any inferences regarding vaccine efficacy in patients with IMIDs.
The mechanism by which anti-TNF therapy impairs humoral responses to SARS-CoV-2 vaccination is incompletely understood. Given the negative impact of TNF inhibitors on GC reactions (47), it is likely that there is less affinity maturation of the B cell responses of the patients with IBD treated with anti-TNF biologics (37). Follow-up studies to assess the amount of somatic hypermutation and the avidity of anti-S and anti-RBD Abs from healthy control subjects compared with anti-TNF–treated patients will be of interest.
Taken together, our study presents comprehensive data on the longitudinal course of vaccine-induced adaptive immunity in patients with IMIDs treated with systemic or targeted immune-modifying drugs. Our study suggests that repeated doses of vaccine prolong and broaden immune responses to SARS-CoV-2, supporting the recommendation for three- and four-dose vaccine regimens in immunocompromised patients.
Supplementary Material
Acknowledgments
We thank Juan and Stefania Speck for their generous donations to the IMPACT study. We thank Birinder Ghumman for technical support and Natalie Simard for assistance in the flow cytometry facility. We thank Reuben Samson, Queenie Hu, and W. Rod Hardy, and the Coronavirus Variants Rapid Response Network for contributing the lentiviral particles for neutralization assays. We thank Dr. Karen Colwill, Tulunay Tursun, Jenny Wang, Freda Qi, and Adrian Pasculescu for help with generating and analyzing ELISA data.
Footnotes
This work was supported by funding from the Public Health Agency of Canada through the Vaccine Surveillance Reference Group and the COVID-19 Immunity Task Force and by a donation from Juan and Stefania Speck. Additional funding was provided by Canadian Institutes of Health Research/COVID-Immunity Task Force (CITF) Grants VR-1 172 711 and vs1-175545 (to T.H.W. and A.-C.G.); Canadian Institutes of Health Research Grants FDN-143250 (to T.H.W.), FDN-143301 (to A.-C.G.), GA2-177716 (to V.C., A.-C.G., and T.H.W.), and GA1-177703 (to A.-C.G.); and the Canadian Institutes of Health Research Coronavirus Variants Rapid Response Network (to A.-C.G.). The equipment used for ELISAs is housed in the Network Biology Collaborative Centre at the Lunenfeld-Tanenbaum Research Institute, a facility supported by Canada Foundation for Innovation funding (Grant CFI 33474) and by the Government of Ontario, Genome Canada, and Ontario Genomics (OGI-139). V.C. is supported by a Pfizer Chair Research Award, Rheumatology, University of Toronto. D.C. is supported by the Clinical Scientist Training Program at the Department of Medicine, University of Toronto. T.H.W. holds the Canada Research Chair in antiviral immunity. A.-C.G. is the Canada Research Chair in Functional Proteomics and a pillar lead for the Coronavirus Variants Rapid Response Network. J.R.S. holds a Canadian Immunization Research Network fellowship. M.W.C. holds a Canadian Institutes of Health Research Canada Graduate Scholarships–Master’s Program award.
The online version of this article contains supplemental material.
Tania H. Watts is a Distinguished Fellow of AAI.
- AZA
- azathioprine
- BMI
- body mass index
- GC
- germinal center
- IBD
- inflammatory bowel disease
- IMID
- immune-mediated inflammatory disease
- MTX
- methotrexate
- non-TNF IMID
- patients with IMID excluding those treated with anti-TNF agents
- NP
- nucleocapsid
- RBD
- receptor binding domain
- REB
- research ethics board
- S
- spike
- Tfh
- T follicular helper
- TNF-IMID
- patients with IMID treated with anti-TNF biological agents
- VOC
- variant of concern
- WT
- wild type
Disclosures
A.-C.G. has received research funds from a research contract with Providence Therapeutics Holdings, Inc., for other projects, participated in the COVID-19 Immunity Task Force Immune Science and Testing working party, chaired the Canadian Institutes of Health Research Institute of Genetics Advisory Board, and chairs the Science Advisory Board of the National Research Council of Canada Human Health Therapeutics Board. V.C. has received research grants from AbbVie, Amgen, and Eli Lilly and has received honoraria for advisory board member roles from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. His spouse is an employee of AstraZeneca. V.P. has no personal financial ties with any pharmaceutical company. He has received honoraria for speaker and/or advisory board member roles from AbbVie, Celgene, Janssen, Kyowa Kirin Co. Ltd, LEO Pharma, Novartis, Pfizer, Sanofi, UCB, and Union Therapeutics. In his role as Department Division Director of Dermatology at the University of Toronto, V.P. has received departmental support in the form of unrestricted educational grants from AbbVie, Bausch Health, Celgene, Janssen, LEO Pharma, Eli Lilly, L’Oréal, NAOS, Novartis, Pfizer, Sandoz, and Sanofi in the past 36 months. V.P. has received research grants from Sanofi, AbbVie, and Novartis. M.S.S. has received research support, consulting fees, and speaker honoraria from AbbVie, Janssen, Takeda, Pfizer, Gilead, and Amgen. All other authors have no financial conflicts of interest.
References
- 1. World Health Organization . Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed: January 31, 2023.
- 2. MacKenna, B., Kennedy N. A., Mehrkar A., Rowan A., Galloway J., Matthewman J., Mansfield K. E., Bechman K., Yates M., Brown J., et al. 2022. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 4: e490–e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akiyama, S., Hamdeh S., Micic D., Sakuraba A.. 2021. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 80: 384–391. [DOI] [PubMed] [Google Scholar]
- 4. Baden, L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., et al. COVE Study Group . 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack, F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., et al. C4591001 Clinical Trial Group . 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander, J. L., Liu Z., Muñoz Sandoval D., Reynolds C., Ibraheim H., Anandabaskaran S., Saifuddin A., Castro Seoane R., Anand N., Nice R., et al. VIP study investigators . 2022. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 7: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edelman-Klapper, H., Zittan E., Bar-Gil Shitrit A., Rabinowitz K. M., Goren I., Avni-Biron I., Ollech J. E., Lichtenstein L., Banai-Eran H., Yanai H., et al. REsponses to COVid-19 vaccinE IsRaeli IBD group (RECOVER) . 2022. Lower serologic response to COVID-19 mRNA vaccine in patients with inflammatory bowel diseases treated with anti-TNFα. Gastroenterology 162: 454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furer, V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R., et al. 2021. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. [Published erratum appears in 2022 Ann. Rheum. Dis. 81: e133.] Ann. Rheum. Dis. 80: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 9. Geisen, U. M., Sümbül M., Tran F., Berner D. K., Reid H. M., Vullriede L., Ciripoi M., Longardt A. C., Hoff P., Morrison P. J., et al. 2021. Humoral protection to SARS-CoV2 declines faster in patients on TNF alpha blocking therapies. RMD Open 7: e002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wieske, L., van Dam K. P. J., Steenhuis M., Stalman E. W., Kummer L. Y. L., van Kempen Z. L. E., Killestein J., Volkers A. G., Tas S. W., Boekel L., et al. T2B! Immunity against SARS-CoV-2 study group . 2022. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 4: e338–e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haberman, R. H., Herati R., Simon D., Samanovic M., Blank R. B., Tuen M., Koralov S. B., Atreya R., Tascilar K., Allen J. R., et al. 2021. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 80: 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin, S., Kennedy N. A., Saifuddin A., Sandoval D. M., Reynolds C. J., Seoane R. C., Kottoor S. H., Pieper F. P., Lin K.-M., Butler D. K., et al. CLARITY IBD study . 2022. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat. Commun. 13: 1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Syversen, S. W., Jyssum I., Tveter A. T., Tran T. T., Sexton J., Provan S. A., Mjaaland S., Warren D. J., Kvien T. K., Grødeland G., et al. 2022. Immunogenicity and safety of standard and third-dose SARS-CoV-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol. 74: 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jyssum, I., Kared H., Tran T. T., Tveter A. T., Provan S. A., Sexton J., Jørgensen K. K., Jahnsen J., Kro G. B., Warren D. J., et al. 2022. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 4: e177–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boekel, L., Steenhuis M., Hooijberg F., Besten Y. R., van Kempen Z. L. E., Kummer L. Y., van Dam K. P. J., Stalman E. W., Vogelzang E. H., Cristianawati O., et al. 2021. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 3: e778–e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni, L., Ye F., Cheng M.-L., Feng Y., Deng Y.-Q., Zhao H., Wei P., Ge J., Gou M., Li X., et al. 2020. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 52: 971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Notarbartolo, S., Ranzani V., Bandera A., Gruarin P., Bevilacqua V., Putignano A. R., Gobbini A., Galeota E., Manara C., Bombaci M., et al. 2021. Integrated longitudinal immunophenotypic, transcriptional, and repertoire analyses delineate immune responses in patients with COVID-19. Sci. Immunol. 6: eabg5021. [DOI] [PubMed] [Google Scholar]
- 18. Bergamaschi, L., Mescia F., Turner L., Hanson A. L., Kotagiri P., Dunmore B. J., Ruffieux H., De Sa A., Huhn O., Morgan M. D., et al. Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) COVID BioResource Collaboration . 2021. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 54: 1257–1275.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woldemeskel, B. A., Garliss C. C., Blankson J. N.. 2021. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Invest. 131: e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarke, A., Coelho C. H., Zhang Z., Dan J. M., Yu E. D., Methot N., Bloom N. I., Goodwin B., Phillips E., Mallal S., et al. 2022. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185: 847–859.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMahan, K., Yu J., Mercado N. B., Loos C., Tostanoski L. H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. 2021. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590: 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dayam, R. M., Law J. C., Goetgebuer R. L., Chao G. Y. C., Abe K. T., Sutton M., Finkelstein N., Stempak J. M., Pereira D., Croitoru D., et al. 2022. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune-mediated inflammatory diseases. JCI Insight 7: e159721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung, M. W., Dayam R. M., Law J. C., Goetgebuer R. L., Chao G. Y. C., Finkelstein N., Stempak J. M., Pereira D., Croitoru D., Acheampong L., et al. 2022. Third dose corrects waning immunity to SARS-CoV-2 mRNA vaccines in immunocompromised patients with immune-mediated inflammatory diseases. RMD Open 8: e002622. [Google Scholar]
- 24. Pasparakis, M., Alexopoulou L., Episkopou V., Kollias G.. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184: 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumoto, M., Mariathasan S., Nahm M. H., Baranyay F., Peschon J. J., Chaplin D. D.. 1996. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271: 1289–1291. [DOI] [PubMed] [Google Scholar]
- 26. Qui, M., Le Bert N., Chan W. P. W., Tan M., Hang S. K., Hariharaputran S., Sim J. X. Y., Low J. G. H., Ng W., Wan W. Y., et al. 2022. Favorable vaccine-induced SARS-CoV-2-specific T cell response profile in patients undergoing immune-modifying therapies. J. Clin. Invest. 132: e159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibrahim, J. G., Molenberghs G.. 2009. Missing data methods in longitudinal studies: a review. Test (Madr) 18: 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmiedeberg, K., Vuilleumier N., Pagano S., Albrich W. C., Ludewig B., Kempis J. V., Rubbert-Roth A.. 2022. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 4: e11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connolly, C. M., Teles M., Frey S., Boyarsky B. J., Alejo J. L., Werbel W. A., Albayda J., Christopher-Stine L., Garonzik-Wang J., Segev D. L., Paik J. J.. 2022. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann. Rheum. Dis. 81: 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bjørlykke, K. H., Ørbo H. S., Tveter A. T., Jyssum I., Sexton J., Tran T. T., Christensen I. E., Kro G. B., Kvien T. K., Jahnsen J., et al. 2023. Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study. Lancet Rheumatol. 5: e36–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilboa, M., Regev-Yochay G., Mandelboim M., Indenbaum V., Asraf K., Fluss R., Amit S., Mendelson E., Doolman R., Afek A., et al. 2022. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 Omicron infection. JAMA Netw. Open 5: e2231778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ailsworth, S. M., Keshavarz B., Richards N. E., Workman L. J., Murphy D. D., Nelson M. R., Platts-Mills T. A. E., Wilson J. M.. 2023. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann. Allergy Asthma Immunol. 130: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geisen, U. M., Rose R., Neumann F., Ciripoi M., Vullriede L., Reid H. M., Berner D. K., Bertoglio F., Hoff P., Hust M., et al. 2022. The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFα blockade. J. Med. Virol. 94: 5780–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vollenberg, R., Tepasse P.-R., Lorentzen E., Nowacki T. M.. 2022. Impaired humoral immunity with concomitant preserved T cell reactivity in IBD patients on treatment with infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA vaccine BNT162b2: a pilot study. J. Pers. Med. 12: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doherty, J., Morain N. O., Stack R., Girod P., Tosetto M., Inzitiari R., Sheridan J., Cullen G., McDermott E., Buckley M., et al. 2022. Reduced serological response to COVID-19 vaccines in patients with IBD is further diminished by TNF inhibitor therapy; early results of the VARIATION study [VAriability in Response in IBD Against SARS-COV-2 ImmunisatiON]. J. Crohns Colitis 16: 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cerna, K., Duricova D., Lukas M., Machkova N., Hruba V., Mitrova K., Kubickova K., Kostrejova M., Teplan V., Vasatko M., et al. 2022. Anti-SARS-CoV-2 vaccination and antibody response in patients with inflammatory bowel disease on immune-modifying therapy: prospective single-tertiary study. Inflamm. Bowel Dis. 28: 1506–1512. [DOI] [PubMed] [Google Scholar]
- 37. Pape, K. A., Dileepan T., Matchett W. E., Ellwood C., Stresemann S., Kabage A. J., Kozysa D., Evert C., Matson M., Lopez S., et al. 2022. Boosting corrects a memory B cell defect in SARS-CoV-2 mRNA-vaccinated patients with inflammatory bowel disease. JCI Insight 7: e159618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geisen, U. M., Berner D. K., Tran F., Sümbül M., Vullriede L., Ciripoi M., Reid H. M., Schaffarzyk A., Longardt A. C., Franzenburg J., et al. 2021. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 80: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venerito, V., Stefanizzi P., Fornaro M., Cacciapaglia F., Tafuri S., Perniola S., Iannone F., Lopalco G.. 2022. Immunogenicity of BNT162b2 mRNA SARS-CoV-2 vaccine in patients with psoriatic arthritis on TNF inhibitors. RMD Open 8: e001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alsoussi, W. B., Malladi S. K., Zhou J. Q., Liu Z., Ying B., Kim W., Schmitz A. J., Lei T., Horvath S. C., Sturtz A. J., et al. 2023. SARS-CoV-2 Omicron boosting induces de novo B cell response in humans. Nature 617: 592–598. [DOI] [PubMed] [Google Scholar]
- 41. Xie, X., Zou J., Kurhade C., Liu M., Ren P., Pei-Yong Shi. 2022. Neutralization of SARS-CoV-2 Omicron sublineages by 4 doses of the original mRNA vaccine. Cell Rep. 41: 111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurhade, C., Zou J., Xia H., Cai H., Yang Q., Cutler M., Cooper D., Muik A., Jansen K. U., Xie X., et al. 2022. Neutralization of Omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. Nat. Commun. 13: 3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao, Y., Cai C., Grifoni A., Müller T. R., Niessl J., Olofsson A., Humbert M., Hansson L., Österborg A., Bergman P., et al. 2022. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 28: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maringer, Y., Nelde A., Schroeder S. M., Schuhmacher J., Hörber S., Peter A., Karbach J., Jäger E., Walz J. S.. 2022. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 7: eadd3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kartnig, F., Mrak D., Simader E., Tobudic S., Radner H., Mandl P., Göschl L., Hommer N., Mayer M., Hofer P., et al. 2023. Safety and immunogenicity of a third COVID-19 vaccination in patients with immune-mediated inflammatory diseases compared with healthy controls. Ann. Rheum. Dis. 82: 292–300. [DOI] [PubMed] [Google Scholar]
- 46. Crotty, S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29: 621–663. [DOI] [PubMed] [Google Scholar]
- 47. Anolik, J. H., Ravikumar R., Barnard J., Owen T., Almudevar A., Milner E. C. B., Miller C. H., Dutcher P. O., Hadley J. A., Sanz I.. 1950. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J. Immunol. 180: 688–692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.