Abstract
The response of free-swimming Rhodobacter sphaeroides to increases and decreases in the intensity of light of different wavelengths was analyzed. There was a transient (1 to 2 s) increase in swimming speed in response to an increase in light intensity, and there was a similar transient stop when the light intensity decreased. Measurement of changes in membrane potential and the use of electron transport inhibitors showed that the transient increase in swimming speed, following an increase in light intensity, and the stop following its decrease were the result of changes in photosynthetic electron transport. R. sphaeroides has two operons coding for multiple homologs of the enteric chemosensory genes. Mutants in the first chemosensory operon showed wild-type photoresponses. Mutants with the cheA gene of the second operon (cheAII) deleted, either with or without the first operon present, showed inverted photoresponses, with free-swimming cells stopping on an increase in light intensity and increasing swimming speed on a decrease. These mutants also lacked adaptation. Transposon mutants with mutations in cheAII, which also reduced expression of downstream genes, however, showed no photoresponses. These results show that (i) free-swimming cells respond to both an increase and a decrease in light intensity (tethered cells only show the stopping on a step down in light intensity), (ii) the signal comes from photosynthetic electron transfer, and (iii) the signal is primarily channelled through the second chemosensory pathway. The different responses shown by the cheAII deletion and insertion mutants suggest that CheWII is required for photoresponses, and a third sensory pathway can substitute for CheAII as long as CheWII is present. The inverted response suggests that transducers are involved in photoresponses as well as chemotactic responses.
Rhodobacter sphaeroides is a facultative, photoheterotrophic, purple nonsulfur bacterium, a member of the α-subgroup of gram-negative bacteria. It swims by using a single, unidirectionally rotating, subpolar flagellum (1). It responds to a wide range of metabolites, terminal electron acceptors, and light. The most obvious response to a change in stimulus is a transient stop following the removal of an attractant (17, 18).
In Escherichia coli, chemoeffectors bind to transmembrane chemoreceptors and signal via a single phosphorelay pathway to the flagellar motor (6, 16). The phosphorelay pathway comprises CheW, CheA (a histidine protein kinase), and CheY (a response regulator which binds to the flagellar switch). A second set of proteins are involved in signal termination and adaptation: CheZ, which increases the rate of CheY-P dephosphorylation, and CheB and CheR, methyl esterase and transferase enzymes involved in resetting the signalling state of the receptor. In addition to the chemoreceptors, there is an additional receptor protein, Aer, a flavin adenine dinucleotide binding membrane protein, which appears to respond to alterations in the rate of respiratory electron transport and signal via the phosphorelay system to the motor (2, 5, 20).
R. sphaeroides has a more complex sensory system, with two operons containing multiple copies of the chemosensory genes. Together, the operons contain two cheA, three cheY, three cheW, and two cheR homologs and one cheB homolog (10). No copies of cheZ have been identified. Deletion of the first operon results in only minor changes in the response to sugars, while mutations in the second operon result in changes in response to all chemoattractants, particularly to organic acids, the principal attractants of R. sphaeroides. When the behavior of tethered cells was examined, it was found that mutants in the second operon still responded to organic acids, but the response was inverted and showed no short-term adaptation (10).
Previous experiments have shown that the step-down responses of tethered cells to changes in the concentration of terminal electron acceptors and to light shown by R. sphaeroides are not the result of a change in Δp, the electrochemical proton gradient, but are probably signalled by a change in the rate of electron transfer (8), possibly via an Aer homolog (20). There is a great deal of evidence that the response to changes in light intensity correlates with a change in the electron transport rate (9).
Some photosynthetic species have been shown to show an additional repellent response to increases in the intensity of blue light, with the increase in blue light resulting in the cells stopping or reversing. The wavelengths avoided by Ectothiorhodospira halophila are the same as those absorbed by the photoactive yellow protein, PYP, a coumaric acid-containing photoactive protein (18). This protein has now been found in several nonhalophilic species, and it has been suggested that this may play a role in the avoidance of damaging light by these species (21).
In this study, we examined the responses of free-swimming R. sphaeroides cells to short and long flashes of light of different intensities and examined the role of electron transfer and the different chemosensory genes in those responses.
MATERIALS AND METHODS
Strains and growth conditions.
R. sphaeroides WS8N, a spontaneous nalidixic acid-resistant mutant of WS8, and chemotaxis and phototaxis mutants were grown photoheterotrophically to early log phase in succinate medium as previously described, with antibiotics added as required in the following concentrations: nalidixic acid, 20 μg ml−1; and kanamycin, 25 μg ml−1 (10). The mutants chosen for study were JPA 203, which has operon 1 deleted and a Tn5 insertion into the cheA gene of the second operon (cheAII); JPA 211, which has an in-frame deletion of cheAII; and JPA 215, which has both operon 1 and cheAII deleted in frame (10). Cells were grown at three different intensities to the same optical density. High-light grown cells were grown at 200 μM m−2 s−1, normal-light-grown cells were grown at 50 μM m−2 s−1, and low-light-grown cells were grown at 8 μM m−2 s−1. Cells were harvested and washed once before being resuspended in 100 ml of 10 mM N2-sparged Na-HEPES (pH 7.2) containing 50 mg of chloramphenicol ml−1. The bacteriochlorophyll content of the cells was determined spectrophotometrically after solvent extraction (7:2 acetone-ethanol) (4).
The effects of different concentrations of the inhibitors myxathiazol, antimycin A, and carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP) on the photoresponses and the membrane potential were measured after a minimum incubation of 5 min.
Motility measurement.
Cells in anaerobic HEPES buffer were drawn into optically flat microslides (0.05-mm diameter; Camlab., Cambridge, United Kingdom) and sealed with Vaseline. The slides were placed on the stage of a Nikon Optiphot microscope and incubated at a light intensity of 4 μM m−2 s−1 for at least 5 min before measurements were started. The photostimulus was given via a shuttered light source with transmission filters (432 ± 20 nm for blue light and 880 ± 20 nm for far-red light, with 70% peak transmission). Cells were monitored via a CCD camera (Sony Hyperhad SPT-M108CE) with ×100 and ×5 magnification lens. A yellow (transmission, 500 to 900 nm) filter was inserted in front of the camera to stop interference by the stimulating light. The low light intensity made computerized motion analysis difficult; therefore, the speed of individual cells was analyzed manually. At least 10 cells were analyzed manually for 7 s per cell by recording the images on videotape and tracing the tracks onto acetate sheets, and the results presented are the average of a minimum of three experiments. The swimming speed of R. sphaeroides is more variable than those of the majority of bacterial species studied, possibly as a result of the single flagellum, making the standard error fairly large. The results are, however, statistically significant. To analyze the mean speed of a population of cells during 30 s of exposure to light, the Seescan motion analysis system (Seescan, Cambridge, United Kingdom) was used as described previously (19). At least 100 cells were analyzed during each experiment.
Membrane potential measurements.
Membrane potential was determined by measuring the electrochromic bandshift of the membrane-bound carotenoid pigments by using a DW2000 dual-wavelength spectrophotometer (SLM-Aminco) (3). Photosynthetic stimulation was achieved by illumination at 90° with light at either 432 ± 20 nm or with a near-infrared transmission filter (Kodak Wratten 88A).
Genetic techniques.
The mutants used in this study were all isolated by transposon mutagenesis in the phototaxis screen described previously (10). The transposon insertion sites of the different mutants were identified by shotgun cloning of EcoRI fragments into pUC18 and selection of ampicillin- and kanamycin-resistant colonies. The DNA flanking Tn5 was sequenced by using Tn5SEQ primers with an ABI377 automated sequencing facility with dye terminators and universal primers.
RESULTS
Photoresponse of normal-light cells.
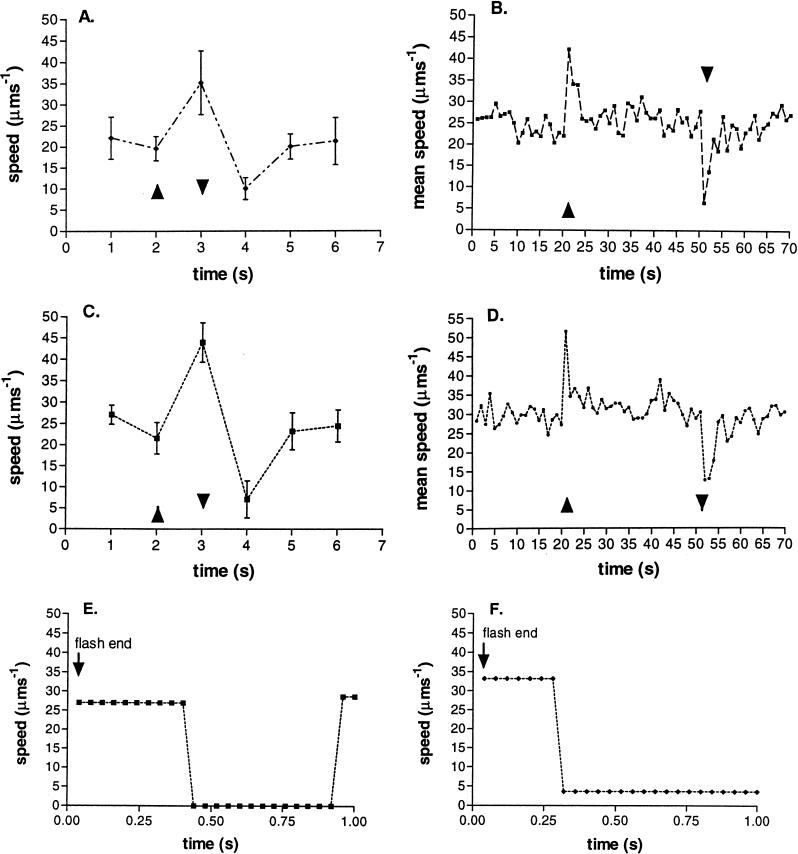
Cells grown under normal light intensity (50 μM m−2 s−1) and then incubated in very low light (4 μM m−2 s−1) showed a marked increase in swimming speed during a step up in light intensity of 1 μM m−2 s−1. This increase occurred at all wavelengths tested. Figures 1A and C show the response of free-swimming cells to a 1-s flash of white light and 432-nm blue light, respectively. There was an increase in speed from an average of 25 μm s−1 to an average of 44 μm s−1 during the flash. At the end of the flash, when the light intensity returned to the low incubation level, all of the cells stopped transiently before a gradual return to the prestimulus swimming speed. Figures 1B and D show the behavior of the population of free-swimming cells in response to a sustained, 30-s increase in white or blue light. The increase in intensity was 25% over the incubation intensity, and all of the cells responded with an increase in speed, the response taking 1 to 2 s. The increase in swimming speed was transient, the cells returning to their prestimulus speed within a few seconds, suggesting the increase in speed was not the result of a long-term change in Δp, but a response followed by adaptation. When the light was switched off, there was a transient stop followed by a return to prestimulus swimming. The results are the average of three experiments with about 100 cells per experiment. As shown in Fig. 1E and F, the length of time taken for an individual cell to stop after the light was removed varied between about 300 and 400 ms and the duration of the stop also varied. The results are the average of the swimming speed measured by motion analysis with time, and the apparent slow swimming is the result of the spread in response times within the population. Direct observation suggested that all of the cells in the population did stop. Previous data suggest that incubation at these light intensities maintains the Δp above that required to saturate a motor of free-swimming R. sphaeroides (11).
FIG. 1.
Change in swimming speed of normal-light-grown R. sphaeroides cells in response to a flash of light of a different wavelength and duration. (A and C) Average behavior of 10 cells in response to a 1-s flash of white (A) or blue (C) light. (B and D) Change in mean speed of the population of at least 100 free-swimming cells in response to a 30-s pulse of white (B) or blue (D) light. (E and F) Behavior of two individual cells at the end of a flash of blue light. ▴, light on; ▾, light off. The apparent slow swimming speed on the step down in light is the result of the distribution of response times shown by cells in the population (E and F).
Most of these experiments were carried out by using, for stimulation, a low-intensity flash of 432-nm blue light, but in all cases, identical results were seen with pulses of broad-spectrum white light (Fig. 1A and B) or far-red light (data not shown). At these intensities, this strain of R. sphaeroides did not show a repellent response to blue light, but the light was shown to increase photosynthetic electron transport (see below).
Effects of electrochemical uncoupler and photosynthetic electron transport inhibitors.
It has already been reported that R. sphaeroides responds to electron transport effectors (light, oxygen, and dimethyl sulfoxide) and that this taxis is influenced by the relative activities of the different electron transport pathways (8, 9). Moreover, recent data show how the response to a step down in light intensity is greatly reduced by inhibitors affecting the photosynthetic electron flow (9). Previous studies concentrated on tethered cells. This study focused on the connection between electron transport and motility in free-swimming cells.
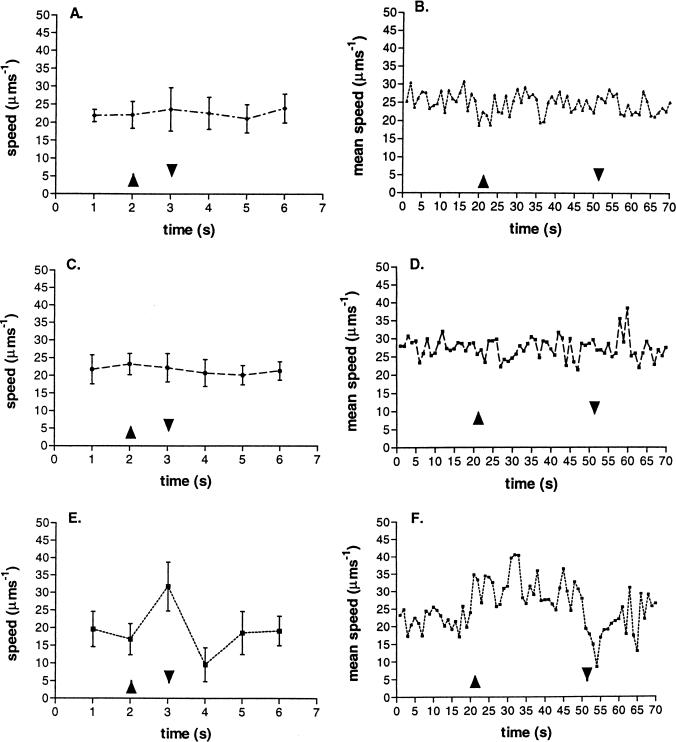
Because light directly affects the magnitude of Δp, experimental conditions allowing the Δp to be maintained, but the rate of electron transport to change, were set up, and the responses of free-swimming cells were measured. Figure 2 shows the responses of free-swimming cells treated with antimycin A, myxothiazol, and FCCP to long and short pulses of light. The low concentrations used (antimycin A, 3 μM; myxothiazol, 1 μM; and FCCP, 10 nM), allowed a membrane potential of at least 70% of the untreated potential to be maintained, as measured by the carotenoid bandshift (data not shown) and previously reported (9). Under these conditions, swimming continued normally. Higher concentrations did alter swimming behavior, but concentrations up to 60 μM antimycin A, 10 μM myxothiazol, and 200 nM FCCP were required to completely abolish the membrane potential. Antimycin A and myxothiazol are competitive inhibitors, acting on the cytochrome bc1 complex and inhibiting electron flow. Both inhibited the response of free-swimming cells to a pulse of light (Fig. 2). The free-swimming cells showed a normal response to a pulse of light, however, in the presence of the proton ionophore FCCP, although the unstimulated swimming behavior was “jerkier” than that of untreated cells (Fig. 2E and F). Swimming speed increased in response to light increase, and the cells stopped when the light was removed, although the kinetics and strength of the population response were reduced.
FIG. 2.
Effect of uncouplers (10 nM FCCP [E and F]) and inhibitors (3 μM antimycin A [A and B] and 1 μM myxothiazol [C and D]) of electron transport on the response of 10 free-swimming cells to a 1-s pulse of blue light (A, C, and E) or a population of 100 cells to a 30-s pulse of blue light (B, D, and F). ▴, light on; ▾, light off.
The pulse of blue light did induce a carotenoid bandshift, although it was only about 60 to 75% of the size of that produced by an equivalent pulse of 850- to 880-nm-wavelength light. This showed that blue light at this wavelength and intensity was photosynthetically active.
Photoresponses of low- and high-light-grown cells.
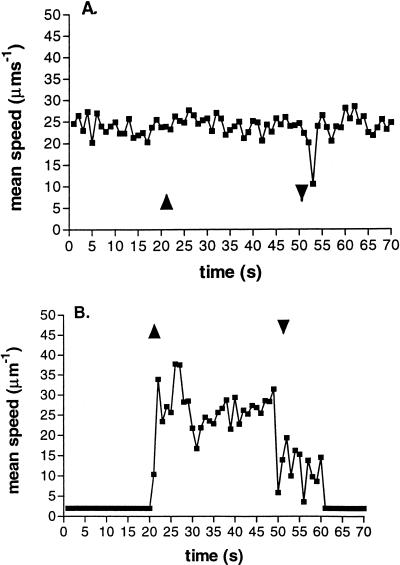
The photoresponses of cells grown at low (8 μM m−2 s−1) and high (200 μM m−2 s−1) light intensities were measured, because differences in their light-harvesting pigments and electron transfer kinetics make their photosynthetic capabilities different (7). Low-light-grown cells showed no response to the increase in light intensity, but responded to the decrease in intensity. High-light-grown cells, however, showed very reduced motility when incubated at the background intensity of 4 μM m−2 s−1, but increased their speed initially to about 35 μm s−1, before settling to about 27 μm s−1, when the light intensity increased (Fig. 3). The high-light-grown cells showed a stop response on the reduction in light similar to that seen with low-light-grown cells (Fig. 3A). Interestingly, the cells must maintain a functional Δp for about 10 s after the step down, because they continued to swim at about 15 μm s−1 before motility was lost. The photosynthetic electron transport rate of the low-light-grown cells was almost certainly saturated under the incubation conditions, hence, the lack of response to a step up in light (7). The step-down response on the return to the prestimulus intensity does, however, suggest that the increase in light intensity changed some aspect of photosynthetic electron transport, which caused a response when the light intensity was reduced back to the prestimulus level.
FIG. 3.
Difference in the responses of populations of low-light-grown (A) and high-light-grown (B) R. sphaeroides cells to a 30-s pulse of blue light. ▴, light on; ▾, light off.
Behavior of chemosensory mutants.
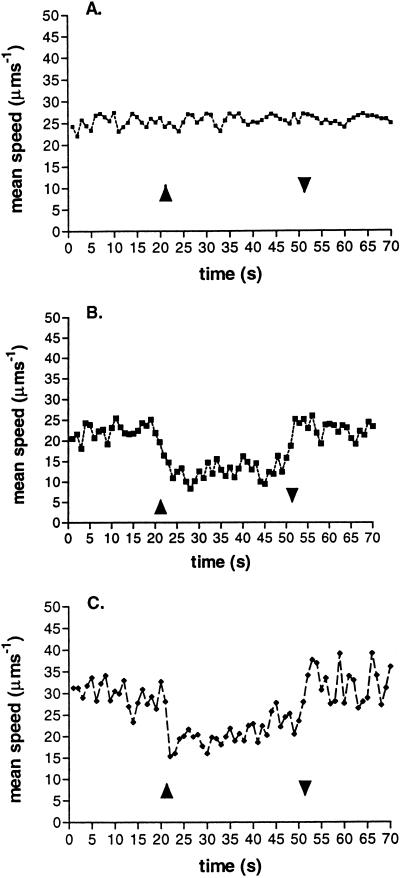
The involvement the Che proteins in photoresponses was investigated in mutants with deletions of genes in the first and second chemotactic operons of R. sphaeroides WS8N (10). While cells with deletions of the first operon showed a wild-type photoresponse, cells with a Tn5 insertion in the cheAII gene (JPA 203) showed no photoresponse, whether or not the first operon was present (Fig. 4). Mutants in which cheAII was deleted in frame in the presence (JPA 211) or absence of the first operon (JPA 215), however, showed an inverted response (Fig. 4B and C), with the cells slowing down when the light intensity was increased and continuing to swim slowly during the period of the pulse, without any clear adaptation, but recovering their prestimulus behavior at the end of the pulse. The size of the response by both JPA 211 and JPA 215 to a pulse of light was not as large as that of the wild type and showed significantly slower kinetics on both the step up and step down in light.
FIG. 4.
Response of a population of three R. sphaeroides mutants to a 30-s pulse of blue light. ▴, light on; ▾, light off. JPA 203 (A) contains a Tn5 insertion in cheAII (Δoperon 1, cheWII), JPA 211 (B) has cheAII deleted in frame, and JPA 215 (C) has both operon 1 and cheAII deleted in frame.
These data suggest that while CheAII must be the primary route for photosensory signalling, but as with so many aspects of R. sphaeroides behavior, an alternative signalling pathway must be available. Five other phototactic mutants isolated in the mutant screen were analyzed. All of the mutants showed no response, and sequencing revealed that all had Tn5 inserted into the cheAII gene (data not shown). No mutations were found in any transducer homolog, perhaps suggesting that more than one transducer may be involved in redox sensing. The first operon could not substitute for the deletion of cheAII, because an inverted response was seen in deletion mutants with and without the first operon (Fig. 4B and C). The responses to photostimuli are the same as the inverted chemosensory responses seen in the cheAII deletion mutants (10). As with wild-type cells, the addition of antimycin A and myxothiazol prevented any response to a pulse of light, while FCCP allowed an almost normal response (data not shown).
DISCUSSION
These data are consistent with the hypothesis that R. sphaeroides responds to a change in the rate of electron transport, the primary signal being transmitted via CheAII to the flagellar motor. Unlike previous studies using tethered cells, in addition to a transient stop when light was switched off, there was a transient increase in the apparent speed of free-swimming cells when the light intensity was increased, in all but cells grown under very low light. It is, however, possible that the change in speed is caused by suppression of very short stops, below the resolution of video rate, on a step up in light resulting in an apparent increase in speed.
Although the low-light-grown cells showed no response to an increase in light, they did respond to its reduction. This suggests that the rate of electron transport, although saturated in low-light-grown cells, probably did change when cells were moved into high light, and this new rate was reduced transiently on the step down, inducing a behavioral response. The loss of the response in cells treated with inhibitors of electron transport, but not in those treated with the proton uncoupler FCCP, supports the hypothesis that a change in electron transport rate generates the primary signal for the photoresponse (9).
Although some photosynthetic bacteria show a repellent response to blue light (12), this strain of R. sphaeroides did not. The carotenoid bandshift showed that the low intensities used here were photosynthetically active and caused a positive response. The repellent response requires much higher light intensities and may therefore use a different pathway (12).
The complete loss of a response in mutants with a Tn5 insertion into cheAII compared to the inverted response of cells in which cheAII was deleted in frame is intriguing, but is similar to the effect on the chemoresponses of the same mutants, supporting the idea that there is a common signaling pathway (10). The Tn5 insertion would be expected to have a polar effect on downstream expression, while the in-frame deletion would not. A polar effect on downstream expression is supported by the observation that eight independent cheAII Tn5 mutants were isolated, and all showed the identical response and the obvious phenotypic difference between the transposon and deletion mutants. The in-frame deletion would therefore produce normal levels of CheWII, CheWIII, CheRII, and CheB. The Tn5 insertion mutant would, on the other hand, have no or little CheWII and reduced levels of CheWIII, CheRII, and CheB. The latter three proteins would be expressed from an internal promoter between cheWII and cheWIII (16a). The loss of the response in the insertion mutants suggests that CheWII may be required to signal a redox change from a transducer to CheA. The inverted response in the deletion mutants indicates that there must be yet another signalling pathway to the motor, because the response occurs in mutants lacking both CheAII and CheAI. Our inability to find any photoresponse mutants with Tn5 insertions in transducer homologs also suggests that there is more than one transducer signalling changes in electron transport to the chemosensory pathways. Recent hybridization studies suggest that R. sphaeroides may have as many as 12 mcp homologs, expressed under different growth conditions (11a). Recent work with a related photosynthetic species, Rhodospirillum centenum, has shown that, in that species, there is only one common pathway signaling from chemoreceptors and photoreceptors to the motor (13, 14, 15).
The inverted photoresponse may be the result of an imbalance in the adaptation pathway, again suggesting the involvement of transducers. There are several transducer and adaptation mutants in E. coli that show inverted responses, and it has been suggested that the inversion may be the result of overmethylation of the transducers sending an aberrant signal through CheA (22). The role of methylation in R. sphaeroides behavior has not been established, but the deletion mutants lack both of the identified CheA phosphodonors required to phosphorylate, and thus activate, the esterase CheB. The methyl transferase, CheRII, would be expressed and be constitutively active, resulting in the transducers being overmethylated. The signal must, however, be sent through a third sensory pathway to the flagella, because both CheAs are absent.
It is clear from the studies of R. sphaeroides that environmental sensing in this species is extremely complex and involves the regulation of several pathways, not all of which have yet been identified. This study shows for the first time that, in this species, the photoresponse signals must be transmitted through one of the identified chemosensory pathways.
ACKNOWLEDGMENTS
We thank the BBSCR and the University of Bologna, Bologna, Italy, for funding the advanced student project.
We thank Ruslan Grishanin for providing the mutants and Helen Packer for help with the motion analysis.
REFERENCES
- 1.Armitage J P, Macnab R M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987;169:514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibikov S I, Biran R, Rudd K E, Parkinson J S. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A J, Cotton N P J, Jackson J B. The relation between membrane ionic current and ATP synthesis in chromatophores from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1983;723:440–453. [Google Scholar]
- 4.Clayton R K. Towards the isolation of a photochemical reaction center in Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1963;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- 5.Dang C V, Niwano M, Ryu J-I, Taylor B L. Inversion of aerotactic response in Escherichia coli deficient in cheB protein methylesterase. J Bacteriol. 1986;166:275–280. doi: 10.1128/jb.166.1.275-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia A F, Venturoli G, Gad’on N, Fernandez-Velasco J G, Melandri B A, Drews G. The adaptation of the electron transfer chain of Rhodopseudomonas capsulata to different light intensities. Biochim Biophys Acta. 1987;890:335–345. [Google Scholar]
- 8.Gauden D E, Armitage J P. Electron transport-dependent taxis in Rhodobacter sphaeroides. J Bacteriol. 1995;177:5853–5859. doi: 10.1128/jb.177.20.5853-5859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grishanin R N, Gauden D E, Armitage J P. Photoresponses in Rhodobacter sphaeroides: role of photosynthetic electron transport. J Bacteriol. 1997;179:24–30. doi: 10.1128/jb.179.1.24-30.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin P A, Maguire B A, Grishanin R N, Armitage J P. Evidence for two chemosensory pathways in Rhodobacter sphaeroides. Mol Microbiol. 1997;26:1083–1096. doi: 10.1046/j.1365-2958.1997.6502022.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrison D M, Packer H L, Armitage J P. Swimming speed and chemokinetic response of Rhodobacter sphaeroides investigated by natural manipulation of the membrane potential. FEBS Lett. 1994;348:37–40. doi: 10.1016/0014-5793(94)00572-9. [DOI] [PubMed] [Google Scholar]
- 11a.Harrison, D. M., J. Skidmore, J. P. Armitage, and J. R. Maddock. Localisation and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol. Microbiol., in press. [DOI] [PubMed]
- 12.Hellingwerf K J, Kort R, Crielaard W. Negative phototaxis in photosynthetic bacteria. In: Caddick M X, Baumberg S, Hodgson D A, Phillips-Jones M K, editors. Microbial responses to light and time. Cambridge, United Kingdom: Cambridge University Press; 1998. pp. 107–123. [Google Scholar]
- 13.Jiang Z-Y, Bauer C E. Analysis of a chemotaxis operon from Rhodospirillum centenum. J Bacteriol. 1997;179:5712–5719. doi: 10.1128/jb.179.18.5712-5719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Z-Y, Gest H, Bauer C E. Chemosensory and photosensory perception in purple photosynthetic bacteria utilize common signal transduction components. J Bacteriol. 1997;179:5720–5727. doi: 10.1128/jb.179.18.5720-5727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z-Y, Rushing B G, Bai Y, Gest H, Bauer C E. Isolation of Rhodospirillum centenum mutants defective in phototactic colony motility by transposon mutagenesis. J Bacteriol. 1998;180:1248–1255. doi: 10.1128/jb.180.5.1248-1255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukat G S, Stock J B. Response regulation in bacterial chemotaxis. J Cell Biochem. 1993;51:41–46. doi: 10.1002/jcb.240510109. [DOI] [PubMed] [Google Scholar]
- 16a.Martin, A. C., and J. P. Armitage. Unpublished data.
- 17.Packer H L, Gauden D E, Armitage J P. The behavioural response of anaerobic Rhodobacter sphaeroides to temporal stimuli. Microbiology. 1996;142:593–599. doi: 10.1099/13500872-142-3-593. [DOI] [PubMed] [Google Scholar]
- 18.Poole P S, Brown S, Armitage J P. Swimming changes and chemotactic responses in Rhodobacter sphaeroides do not involve changes in the steady state membrane potential or respiratory electron transport. Arch Microbiol. 1990;153:614–618. [Google Scholar]
- 19.Poole P S, Sinclair D R, Armitage J P. Real time computer tracking of free-swimming and tethered rotating cells. Anal Biochem. 1988;175:52–58. doi: 10.1016/0003-2697(88)90359-4. [DOI] [PubMed] [Google Scholar]
- 20.Rebbapragada A, Johnson M S, Harding G P, Zuccarelli A J, Fletcher H M, Zhulin I B, Taylor B L. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprenger W W, Hoff W D, Armitage J P, Hellingwerf K J. The eubacterium Ectothiorhodospira halophila is negatively phototactic, with a wavelength dependence that fits the absorption spectrum of the photoactive yellow protein. J Bacteriol. 1993;175:3096–3104. doi: 10.1128/jb.175.10.3096-3104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor B L, Johnson M S. Rewiring a receptor: negative output from positive input. FEBS Lett. 1998;425:377–381. doi: 10.1016/s0014-5793(98)00253-1. [DOI] [PubMed] [Google Scholar]