Abstract
Background: Percutaneous balloon pulmonary valvuloplasty (PBPV) is the treatment of choice for hemodynamically significant pulmonary stenosis (PS). Currently, the Tyshak balloon is preferred but requires multiple dilatations because of its instability across the valve leading to a watermelon seeding effect. Accura balloon (Vascular Concept, UK) offers an advantage in its self-positioning configuration, variable diameter, and rapid inflation-deflation sequence which shortens the procedural time and valve injury. Method: 43 patients with severe pulmonary valve stenosis underwent PBPV using an Accura balloon at LPS Institute of Cardiology, GSVM Medical College, Kanpur, UP, India from March 2018 to February 2022. The procedure was carried out using the standard technique but the metallic straightener was removed when the catheter reached the right atrium to facilitate its delivery across the pulmonary valve. Patients were followed up by 2D echo at 24 hours and 6 months. Result: Successful BPV was done in all 43 patients [with mean age 21.9 (range 18-41); 31 males and 12 females] among which 5 patients had dysplastic valves. The mean diameter of the annulus was 18.5 (range 15-21) mm. Immediate hemodynamic improvement was observed in 38 patients (88%) as peak systolic gradient reduced from 84±13 to 22±12 mmHg (P<0.005) while 5 patients (12%) had <50% reduction of resting gradient, though it came down significantly at 6 months. Fluoroscopy and procedural time were 5.2±1.9 min and 22.6±3.4 min respectively. Major complications (death, cardiac perforation, tamponade, tricuspid regurgitation, requirement of blood transfusion) were none. Minor complications (transient hypotension, ventricular premature contraction, transient bradycardia) were reported in all patients. Accura balloon being bulky were delivered over left atrial and super stiff Amplatz wire in 36 and 7 patients respectively. Conclusion: PBPV using Accura balloon is safe and effective for both stenosed and dysplastic valves. In a few patients, maximal effect will be observed over a period of 6 months.
Keywords: Pulmonary stenosis, Accura balloon, tyshak balloon, percutaneous balloon pulmonary valvuloplasty, cardiac perforation, tamponade
Introduction
Pulmonary valve stenosis (PS) in isolation accounts for 7-9% of all congenital defects but it commonly coexists with other complex defects [1]. Most children and adults with mild-to-moderate PS remain asymptomatic and don’t progress, whereas those with severe PS may experience exertional dyspnea and fatigue. Some develop symptoms of right heart failure (peripheral edema, fatigue, and dyspnea), and rarely patients can present with exertional angina, syncope, or sudden death. Surgical valvotomy was the treatment of choice in the pre-percutaneous era, which now has been replaced by balloon valvuloplasty.
Since the first report of successful balloon pulmonary valvuloplasty by Kan et al. nearly four decades ago, it has become the gold standard treatment modality for hemodynamically significant PS. It offers excellent long-term result thus making it safe and effective [2].
The age at presentation is guided by the amount of degree of stenosis and therefore patients may be symptomatic in early childhood [3]. It is not uncommon to encounter this among adult patients where predominant symptoms are dyspnoea, effort intolerance, and rarely syncope. Data regarding the outcome of percutaneous balloon pulmonary valvuloplasty (PBPV) are limited among adult patients but results are very useful as it saves them from the surgeon’s knife and therefore avoids all potential complication related to surgery [4]. Various balloon catheters have been used for PBPV and its size (diameter and length) is determined by the size of the annulus. Balloon slippage is a commonly encountered problem with the frequently used Tyshak Balloon. A number of studies have been conducted using the Inoue balloon but, the Accura ballon has not been studied for PBPV. Accura balloon (Vascular Concept, Essex, UK), which was originally developed for Mitral valvoplasty in rheumatic Mitral stenosis, offers various advantages over other balloon dilation catheters in BPV as: 1. Because of its peculiar expansible anatomy during inflation its position becomes stable when inflated as compared to the Tyshak which is known to slip. 2. Variable diameter to which it can be expanded, which helps in achieving stepwise dilatation.
We conducted this study to assess the efficacy and safety of the Accura balloon (Vascular Concept, Essex, UK) for balloon pulmonary valvuloplasty in the adult population.
Material and method
Study design and participants
This prospective study was conducted at the LPS Institute of Cardiology, GSVM Medical College, Kanpur, UP, India from March 2018 to February 2022 among patients with pulmonary valve stenosis. The procedure was performed in asymptomatic patients with echocardiographic evidence of suitable morphology with a maximum instantaneous and mean gradient across the pulmonary valve of ≥64 mmHg and ≥40 mmHg respectively and in symptomatic patients with a maximum instantaneous gradient of ≥50 mmHg and mean gradient ≥30 mmHg. Patients with associated infundibular stenosis, supravalvular stenosis, associated complex cyanotic disease requiring palliation, ≥ grade III pulmonary leak, and pulmonary annulus ≤15 mm were excluded from our study. The presence and intensity of pulmonary regurgitation were assessed through colour flow mapping [5]. All patients underwent detailed clinical evaluation, including routine biochemistry, electrocardiogram, chest x-ray, and 2-D transthoracic echocardiogram (TTE) including Doppler interrogation. The Pulmonary valve was evaluated in a parasternal short axis (PSAX) view for its morphology, pressure gradient, and annular diameter. The Primary safety endpoint was assessed by the combination of death, cardiac perforation, tamponade, and major bleeding needing transfusion or a haemoglobin drop of more than 3 g/dl. The procedure was defined as successful if adequate dilatation was achieved with a residual gradient ≤35 mmHg in the absence of any major complication. Restenosis was labelled as a new elevation of pressure gradient ≥35 mmHg across the pulmonary valve on follow-up TTE. Procedures were performed after obtaining signed informed consent from all patients and the study protocol was approved by the institutional ethical committee. The trial was registered in UMIN Clinical Trials Registry (UMIN-CTR) with registration number of UMIN000051063.
Device description
The Accura balloon is a double-lumen balloon catheter made of polyvinyl chloride having a balloon attached at its distal end. There is a nylon mesh layer between the two latex layers. The latex layer imparts it compliance, whereas the mesh layer regulates its diameter, shape, inner pressure, and resistance against rupture. During inflation, the distal part expands followed by the proximal and waist expands at the last. The length and diameter of the shaft are 80 cm and 11 Fr respectively. It has got a separate steel stretching tube to straighten it. It can be delivered over either dedicated left atrial wire or extra stiff 0.035” Amplatz wire. The central lumen allows the wire to pass through while the inflation port helps in the inflation of the balloon. The size of the balloon represents the maximum diameter of the waist of the fully inflated balloon and is available from 19 mm to 30 mm with 2 mm increments.
Procedural details
Transfemoral arterial and venous access were obtained using 5 Fr sheath through modified Seldinger’s technique. Intravenous heparin was given according to the weight-based regimen (80-100 U/Kg). Arterial access was used for pressure monitoring. Right ventricular (RV) pressures were measured using 5F Glide catheter (Terumo Inc.; Japan) and ventriculogram were performed in the anteroposterior (AP) and lateral view to estimate the size of the annulus from hinge to hinge during systole (Figure 1A). The Glide catheter was crossed through the stenosed valve using 0.035” stright tip terumo wire (Terumo Inc., Japan), PA pressure was recorded and was parked deep into the left lower pulmonary artery branch. Terumo wire was exchanged with 0.025” left atrial stainless-steel guidewire while putting its loop in the left PA branch for better support. The venous sheath was withdrawn, and the local site was dilated using 12F dilator to facilitate the entry of the Accura balloon. The balloon was sized according to the size of the annulus and the balloon-annulus ratio (BAR) was kept at 1.1-1.2. In those patients with too tight valves and a hugely dilated right atrium, the Accura balloon could not be negotiated from RA to RV, and rather looped wire tended to prolapse into RV due to its poor support (Figure 2A). The balloon was withdrawn, and the Glide catheter was tracked over the LA wire beyond the pulmonary valve. LA wire was exchanged with 0.035” Amplatz super stiff wire and the glide catheter was withdrawn (Figure 1B) [6]. When the Accura balloon reached RA, the metal stretching tube inside the balloon was removed (Figure 2B), thereby de-slenderizing the balloon. It helped the balloon to resume its more flexible natural shape. It was gradually negotiated across the pulmonary valve with a gentle clockwise turn and push. Once reached beyond the valve, it was positioned in the main pulmonary artery with the guidewire firmly held in place. The distal half of the balloon was inflated with a diluted contrast agent (dilution-1:6). Initial inflation was performed with a balloon size 1-2 mm less than its maximal capacity. It was partially inflated and withdrawn to put the waist across the valve and inflated according to size within 3-5 s and then quickly deflated thereby achieving successful dilatation (Figure 3). Abolition of the “waist” after inflation of the balloon was the endpoint. In case of inadequate dilatation, second inflation was attempted until the waist was abolished (Figures 4, 5). The deflated balloon was then pulled back into RA, re-slenderized using metal stretching tube, and withdrawn while keeping the guidewire in the PA. The glide catheter was repositioned over the same wire to get PA and RV pressures using the pullback technique. PA pressure increased following PBPV (Figure 6). RV angiogram was also performed using the same catheter. Following PBPV, venous and arterial hemostasis were secured by a figure of Z suture and manual compression. TTE was performed the next day and on follow-up at one, three, and six months.
Figure 1.

RV angiogram in left lateral view showing characteristic doming of pulmonary valve (A); Extra-stiff Amplatz guidewire parked in left lower pulmonary artery (B).
Figure 2.

Accura balloon along with metal stretching tube being tracked over LA wire but it could not be negotiated from RA to RV (A); Accura balloon was slenderized by withdrawing the metal stretching tube (B).
Figure 3.

Successful PBPV with Accura balloon over Amplatz wire.
Figure 4.
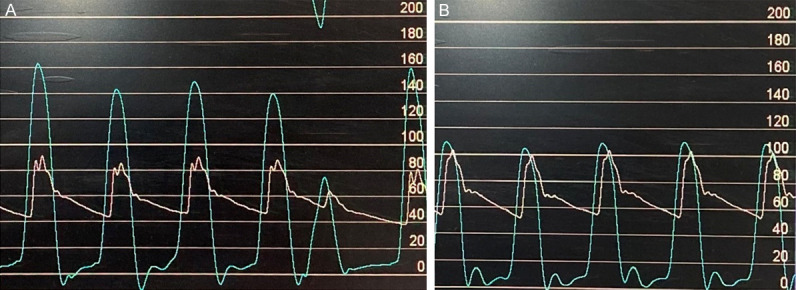
Supra-systemic RV pressure (A); RV pressure decreased but still more than systemic (B).
Figure 5.

After repeated balloon dilatation, RV pressure became sub-systemic (A, B).
Figure 6.

Right ventricular pressure (A: Preprocedure; C: Post procedure); Pulmonary Artery pressure (B: Preprocedure; D: Post preprocedure). Peak-to-peak systolic pressure gradient before the procedure was 159 mmHg (RVSP-178 mmHg, PASP-19 mmHg; red arrow) which came down to 16 mmHg after the procedure (RVSP-64 mmHg, PASP-48 mmHg; green arrow).
Statistical evaluation
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation, and categorical data were recorded as percentages. Continuous variables were analysed using the student t-test. All P values were two sided and P<0.05 was considered statistically significant.
Results
Baseline characteristics
Mean age of patients was 21.9 (range: 18-29) years among which 72% (n=31) were male. The baseline characteristics of patients are summarised in Table 1. The most predominant symptoms were dyspnoea and fatigue which were noted in 28 (65%) and 18 (41%) respectively while cyanosis was observed in 4.5% (n=2) as a result of right to left shunting through foramen ovale. Classical doming was noted in 88% (n=38) patients while 12% (n=5) had mild dysplastic valves because of associated Noonan’s syndrome.
Table 1.
Baseline characteristics and procedural outcome of patients (N=43)
| Variable | Mean |
|---|---|
| Age (years) | 21.9 (18-41) |
| Sex (Male/Female) | 31 (72%)/12 (28%) |
| Weight (kg) | 32.7 (22.6-381.) |
| Height (cm) | 139.8 (123.5-157.2) |
| BSA | 1.48 (1.32-1.72) |
| Clinical presentation | |
| A. Asymtomatic | 8 (18.6%) |
| B. Symtomatic | 35 (81.4%) |
| a. Dyspnoea | 28 (65%) |
| b. Fatigue | 18 (41%) |
| c. Palpitation | 13 (30%) |
| d. Syncope | 3 (7%) |
| e. Evaluation of murmur | 5 (11.6%) |
| f. Cyanosis | 2 (4.5%) |
| PV morphology | |
| a. Doming | 38 (88.4%) |
| b. Dysplastic | 5 (11.6%) |
| Peak echocardiographic gradient (mmHg) | 76 (52-166) |
| RV to PA peak catheter pullback gradient (mmHg) | 68 (52-159) |
| RV/Systemic pressure ratio | 0.85 (0.60-1.3) |
| Pulmonary annulus diameter (mm) | 18.5 (15-21) |
| Balloon/annulus ratio | 1.23 (1.20-1.28) |
| Fluoroscopy time (min) | 5.2±1.9 |
| Procedural time (min) | 22.6±3.4 |
| Procedural Success | 43 (100%) |
| Major complications (death, cardiac perforation, tamponade, Bleeding) | 0 (0%) |
| Minor complications | |
| a. Transient hypotension | 35 (81%) |
| b. Ventricular premature contraction | 39 (91%) |
| c. Transient bradycardia | 36 (84%) |
Efficacy
The Accura balloon dilatation catheter was negotiated across pulmonary valve in all cases and adequate dilatation of the pulmonary valve was achieved, although 8 (18%) patients required repeat dilatation. The mean annular size was 18.5 (16-23) mm, and final balloon-to-annulus ratio was 1.23 (1.20-1.35). Peak gradient by echocardiography and catheter pullback was 76 (52-166) and 68 (52-159) mmHg respectively (Table 1). The RV systolic pressure decreased significantly from 79.4 (58-178) mmHg to 44.6 (34.6-47) following PBPV (Table 2). Similarly, a significant fall in RV-PA systolic pressure gradient was observed (68±13 mmHg vs. 24±10 mmHg; P<0.005) (Table 3). PA systolic pressure also increased significantly (21.2 mmHg vs. 25.2 mmHg; P<0.01). Patients having dysplastic valves and 3 patients with doming valves required repeat dilatation as the gradient did not decrease significantly following first dilatation.
Table 2.
Changes in haemodynamic parameters following percutaneous balloon pulmonary valvuloplasty (N=43)
| Parameters | Before Procedure | After Procedure | P value |
|---|---|---|---|
| RV Systolic Pressure (mmHg) | 79.4 (58-178) | 44.6 (34.6-47) | <0.01 |
| PA Systolic Pressure (mmHg) | 21.2 (15-29) | 25.2 (22-28) | <0.01 |
| RV-PA systolic gradient (mmHg) | 68±13 | 24±10 | <0.005 |
Table 3.
Change of peak pulmonary systolic gradient following PBPV

|
Complications
Minor complications were observed in all patients which included transient hypotension (n=35; 81%), ventricular premature contraction (n=39; 91%), and transient bradycardia (n=36; 84%). All of these were self-limiting. The pulmonary leak was observed in 44.2% (n=19) which was only trivial to mild in nature, and no one had significant pulmonary regurgitation. Similarly, mild and moderate tricuspid leak were noted among 76.8% (n=33) and 23.2% (n=10) patients respectively. None of the patients developed significant tricuspid regurgitation. Procedural success was reported in all patients.
The acute hemodynamic gains of PBPV in our patient population were maintained on follow-up at six months (Table 4).
Table 4.
Follow-up data by Doppler echocardiographic study
| Variable | Number (Percentage) |
|---|---|
| Pulmonary leak | |
| a. None | 24 (65.8%) |
| b. Trivial to mild | 19 (44.2%) |
| c. Moderate to severe | 0 (0%) |
| Tricuspid leak | |
| a. Mild | 33 (76.8%) |
| b. Moderate | 10 (23.2%) |
| c. Severe | 0 (0%) |
| Pulmonary transvalvular peak gradient (mmHg) | |
| a. 1 month | 24±10 |
| b. 3 months | 22±11 |
| c. 6 months | 19±8 |
Discussion
Valvular PS results from the fusion of valve leaflets, which imparts it dome-like shape and orifice that may be either central or eccentric [7]. Morphology is most commonly tricuspid but at times may be bicuspid or rarely unicommissural. Although the most commonly encountered pattern is the doming of the valve, there may be valve ring hypoplasia or dysplastic pulmonary valves as in the case of Noonan’s syndrome [8,9]. With the availability of the current generation low-profile balloon, transcatheter treatment has replaced surgery as the gold standard treatment of valvular PS. Morphologic effects of the balloon forces on the stenotic valves have been attributed to commissure splitting, cusp tear, or a combination of two [10]. Several balloons are used for BPV [1]. However, slipping of the balloon is the main issue faced during valvuloplasty with these catheters. Accura balloon has at least 2 advantages over these balloons. Firstly because of its peculiar expansile shape, it achieves a stable position across the stenosed valve and there are very less chances of slipping during inflation, secondly it can be expanded to a variable diameter that helps in achieving stepwise dilatation. Our study demonstrated PBPV using an Accura balloon catheter through stepwise dilation to be a safe, efficient, and convenient method whose effect was maintained over six months.
A wide array of balloons has been utilized for valvulotomy whose triumph depends on the propensity to attain steady and veritable inflation [1]. Negotiating the balloon across the stenosed valve across hypertrophied RV and dilated RA is the key to success. The valve may be calcified in adult patients so balloons with higher rated burst pressure (RBP) are preferred among adult patients.
The journey which started with the successful use of a ureteral dilatation catheter encountered lower profile Tyshak series of balloons (NuMed, NY), sturdy balloons with higher RBP like Z-med series (NuMed), Diamond (Boston Scientific, MA), Powerflex (Cordis Endovascular, NJ), Marshal (Meditech, MA) and barbell shaped Nucleus balloon (NuMed) [1].
Indications for PBPV among asymptomatic patients are not well-defined and somewhat debatable. In developing countries, loss of follow-up and lack of resources is an important issue. Hence, patients having significant gradient across the pulmonary valve even in the absence of symptoms may undergo the procedure in lieu of disease progression, prevention of secondary changes in the right ventricle, and impending right ventricular failure and systolic dysfunction of RV.
In the case of adult patients having large annulus, an appropriate size single balloon may not be always available. Another disadvantage of a single large balloon is difficulty in maintaining its stability, tracking, and longer deflation time which creates hemodynamic instability. Many operators advocate the use of the double balloon technique which requires two vascular access and occasionally rapid pacing for stabilization but is associated with less risk of hypotension due to continuous flow during balloon inflation but lengthens the procedure. Studies have demonstrated equal efficacy of both strategies [11,12].
Our findings were concordant with findings demonstrated by Lanjewar et al. where 21% of patients were asymptomatic compared to 18% in our study [13]. In our study, patients having a very high baseline gradient (>100 mmHg) had persistent gradient following PBPV compared to those with a less basal gradient but both groups achieved similar degrees of regression over follow-up.
The safety of the Inoue balloon for PBPV was first demonstrated by Chen et al. among adult patients [14]. They concluded that patients with congenital pulmonic stenosis who present in late adolescence or adult life can be treated with percutaneous balloon valvuloplasty with excellent short-term and long-term results that were similar to those in young children. In their study, 13% of patients developed pulmonary regurgitation after the procedure but it resolved in all of them on follow-up. Liu et al. did a retrospective analysis of outcomes following BPV in children using the single balloon and adults using the Inoue balloon, long term results were similar to single balloon among the pediatric population. Gradients were not significantly different from that obtained at one-month follow-up in children, over a follow-up of 15 years [15]. Similarly, Lanjewar et al. showed excellent mid-term results of Inoue balloon in adolescents and adults with isolated pulmonary stenosis. They encountered an increase in pulmonary regurgitation by one grade in 53.2%. In our study, 44.2% of patients had trivial to mild pulmonary regurgitation [13].
In our study, the Accura balloon was used for valve dilatation because of its availability and ease to use. Its advantage over the Inoue catheter is its better trackability. The biggest disadvantage of the Tyshak balloon is the melon seeding effect and maintaining its position while inflation especially if the size is small. The potential risk with the longer balloon is an injury to the tensor apparatus of the tricuspid valve and occasional complete heart block due to atrioventricular node injury. Accura balloon has a short cycle of positioning-inflation-deflation which completes within 5 seconds and therefore minimal hemodynamic instability and faster hemodynamic recovery if at all occurs. During the course of inflation, it becomes a balloon floatation catheter because the distal inflated portion assumes the shape of dog bone when engaged across the valve and subsequently proximal part gets inflated. This provides perfect anchorage for the valve dilation and prevents any slippage either proximal or distal. During terminal inflation, the waist expands achieving perfect commissurotomy. At this time, the procedure can be repeated depending on the need without disengaging the catheter. De-slenderization and modification of standard technique i.e., using super stiff Amplatz guidewire instead of stainless-steel LA wire have been reported by other authors as well [3,6]. Although Inoue and Accura balloons are fundamentally similar, their pressure and volume relationship are unique. The Accura balloon has an advantage over the Inoue balloon in that it can deliver more stable and higher pressure when inflated within the standard diameter range, therefore, better splitting of commissures at the same pressure compared to the Inoue balloon. Furthermore, Balloon sizes attainable with Inoue and Accura balloons are +4 and +3 mm respectively from their lowest given diameter. Also, recommended contrast dilution with Inoue and Accura balloons are 1:4 and 1:6-8 respectively which means that deflation time is lesser with the Accura balloon. Hemodynamic instability was encountered in our case in lieu of critical stenosis and therefore, the Accura balloon scored over the Inoue balloon. One should be careful while negotiating Accura across the RVOT, it should be de-slenderized as stretching stylet never allow it to pass through the tricuspid valve. Therefore, removal of the stretching tube is necessary and very important step once the balloon reaches the right atrium. Another advantage is that this balloon can be tracked with ease over either a dedicated left atrial wire or an extra stiff Amplatz wire.
The balloon annulus ratio was 1.23 (1.20-1.28) in our study which was concordant with the current recommendation of 1.2-1.25 [1]. The slightly oversized diameter was chosen for patients with dysplastic valves where many operators prefer BAR of 1.4 [8,16]. Appropriate size balloon prevents pulmonary leak and at the same time relieves obstruction. Abolition of the waist should be the endpoint while inflation and disappearance of the waist at higher pressure offer no added advantage and in fact may be associated with its rupture and pulmonary regurgitation. The incidence of residual pulmonary leak was 44% in our study and was concordant with 40-90% in the published series. It is the result of commissural tear, separation, and occasionally leaflet injury as seen in balloon mitral valvulotomy. The oversized balloon is one of the underlying factors as well, therefore BAR should be 1.2-1.3.
Conclusion
PBPV using Accura balloon is safe and effective for both stenosed and dysplastic valves. In a few patients, maximal effect will be observed over a period of 6 months.
Limitation
There is scant Indian data on Accura balloon catheter technique for PBPV. The present report describes our experience in BPV using the Accura balloon catheter and its immediate and mid-term results among adult patients. To our knowledge this is the largest data from India with mid-term follow-up of patients undergoing BPV with Accura balloon dilatation catheter. Our study was conducted over small population.
Disclosure of conflict of interest
None.
References
- 1.Rao PS. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc Interv. 2007;69:747–763. doi: 10.1002/ccd.20982. [DOI] [PubMed] [Google Scholar]
- 2.Fawzy ME, Hassan W, Fadel BM, Sergani H, El Shaer F, El Widaa H, Al Sanei A. Long-term results (up to 17 years) of pulmonary balloon valvuloplasty in adults and its effects on concomitant severe infundibular stenosis and tricuspid regurgitation. Am Heart J. 2007;153:433–8. doi: 10.1016/j.ahj.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Patel TM, Dani SI, Shah SC, Shah UG, Patel TK. Inoue-Balloon pulmonary valvuloplasty using a ÒFree-FloatÓ technique. J Invasive Cardiol. 1996;8:374–377. [PubMed] [Google Scholar]
- 4.Bahl VK, Chandra S, Goel A, Goswami KC, Wasir HS. Versatility of Inoue balloon catheter. Int J Cardiol. 1997;59:75–83. doi: 10.1016/s0167-5273(96)02901-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JW, Nanda NC, Philipot EF, Fan P. Evaluation of valvular regurgitation by color Doppler. J Am Soc Echocardiogr. 1989;2:56–66. doi: 10.1016/s0894-7317(89)80030-6. [DOI] [PubMed] [Google Scholar]
- 6.Deora S, Vyas C, Shah S, Patel T. Percutaneous balloon pulmonary valvuloplasty: a modified over-the-wire Inoue balloon technique for difficult right ventricular anatomy. Indian Heart J. 2014;66:211–213. doi: 10.1016/j.ihj.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gikonyo BM, Lucas RV, Edwards JE. Anatomic features of congenital pulmonary valvar stenosis. Pediatr Cardiol. 1987;8:109–16. doi: 10.1007/BF02079465. [DOI] [PubMed] [Google Scholar]
- 8.Rao PS. Balloon dilatation in infants and children with dysplastic pulmonary valves: short-term and intermediate-term results. Am Heart J. 1988;116:1168–1173. doi: 10.1016/0002-8703(88)90435-8. [DOI] [PubMed] [Google Scholar]
- 9.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–e833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 10.Walls JT, Lababidi Z, Curtis JJ. Morphologic effects of percutaneous balloon pulmonary valvuloplasty. South Med J. 1987;80:475–478. doi: 10.1097/00007611-198704000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Al Kasab S, Ribeiro PA, Al Zaibag M, Halim M, Habbab MA, Shahid M. Percutaneous double balloon pulmonary valvotomy in adults: one- to two-year follow-up. Am J Cardiol. 1988;62:822–825. doi: 10.1016/0002-9149(88)91234-9. [DOI] [PubMed] [Google Scholar]
- 12.Rao PS, Fawzy ME. Double balloon technique for percutaneous balloon pulmonary valvuloplasty: comparison with single balloon technique. J Interv Cardiol. 1988;1:257–262. [Google Scholar]
- 13.Lanjewar C, Phadke M, Singh A, Sabnis G, Jare M, Kerkar P. Percutaneous balloon valvuloplasty with Inoue balloon catheter technique for pulmonary valve stenosis in adolescents and adults. Indian Heart J. 2017;69:176–181. doi: 10.1016/j.ihj.2016.11.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CR, Cheng TO, Huang T, Zhou YL, Chen JY, Huang YG, Li HJ. Percutaneous balloon valvuloplasty for pulmonic stenosis in adolescents and adults. N Engl J Med. 1996;335:21–25. doi: 10.1056/NEJM199607043350104. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Xu X, Liu G, Ding X, Zhao X, Qin Y. Comparison of immediate and long-term results between the single balloon and Inoue balloon techniques for percutaneous pulmonary valvuloplasty. Heart Lung Circ. 2015;24:40–5. doi: 10.1016/j.hlc.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Rao PS, Galal O, Patnana M, Buck SH, Wilson AD. Results of three to 10 year follow up of balloon dilatation of the pulmonary valve. Heart. 1998;80:591–595. doi: 10.1136/hrt.80.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]


