Abstract
Desulfomonile tiedjei DCB-1, a sulfate-reducing bacterium, conserves energy for growth from reductive dehalogenation of 3-chlorobenzoate by an uncharacterized chemiosmotic process. Respiratory electron transport components were examined in D. tiedjei cells grown under conditions for reductive dehalogenation, pyruvate fermentation, and sulfate reduction. Reductive dehalogenation was inhibited by the respiratory quinone inhibitor 2-heptyl-4-hydroxyquinoline N-oxide, suggesting that a respiratory quinoid is a component of the electron transport chain coupled to reductive dehalogenation. Moreover, reductive dehalogenation activity was dependent on 1,4-naphthoquinone, a possible precursor for a respiratory quinoid. However, no ubiquinone or menaquinone could be extracted from D. tiedjei. Rather, a UV-absorbing quinoid which is different from common respiratory quinones in chemical structure according to mass spectrometric and UV absorption spectroscopic analyses was extracted. ATP sulfurylase, adenosine phosphosulfate reductase, and desulfoviridin sulfite reductase, enzymes involved in sulfate reduction, were constitutively expressed in the cytoplasm of D. tiedjei cells grown under all three metabolic conditions. A periplasmic hydrogenase was detected in cells grown under reductive-dehalogenating and pyruvate-fermenting conditions. A membrane-bound, periplasm-oriented formate dehydrogenase was detected only in cells grown with formate as electron donor, while a cytoplasmic formate dehydrogenase was detected in cells grown under reductive-dehalogenating and pyruvate-fermenting conditions. Results from dehalogenation assays with D. tiedjei whole-cell suspensions and cell extracts suggest that the membrane-bound reductive dehalogenase is cytoplasm oriented. The data clearly demonstrate an enzyme topology in D. tiedjei which produces protons directly in the periplasm, generating a proton motive force by a scalar mechanism.
Reductive dehalogenation is an important mechanism of pollutant biodegradation. A number of highly chlorinated environmental contaminants, such as polychlorinated biphenyls, pentachlorophenol, tetrachloroethene (PCE), and hexachlorobenzene, are toxic and recalcitrant. These pollutants are resistant to oxidative transformation in natural environments. Reductive dehalogenation is the only known mechanism of biodegradation of certain highly chlorinated compounds. As well, reductive dehalogenation appears to be involved in the anaerobic biodegradation of most chlorinated organic compounds. Dehalogenation generally makes compounds less toxic and more amenable to degradation under aerobic conditions (25, 42).
There is strong evidence suggesting that some bacteria use chloroaromatic compounds or PCE as catabolic terminal electron acceptors, reductively dehalogenating these pollutants and conserving energy for growth. This process is sometimes referred to as dehalorespiration and has been shown for Desulfitobacterium spp. (8, 22, 48, 55), Dehalospirillum multivorans (50), Dehalobacter restrictus (24, 25), Dehalococcoides ethenogenes 195 (36), and Desulfomonile tiedjei DCB-1 (16, 40, 41). D. tiedjei, a gram-negative sulfate-reducing bacterium (14, 39), is a well-characterized pure culture capable of dehalorespiration. This bacterium obtains energy for growth by coupling formate or hydrogen oxidation to reductive dehalogenation of 3-chlorobenzoate (3CB). Use of those electron donors suggests that energy is conserved by a chemiosmotic process, as fermentative growth is unlikely with hydrogen or formate. Chemiosmotic coupling of reductive dehalogenation and ATP synthesis in D. tiedjei occurs via a proton-driven ATPase (41). A proton motive force (PMF) is presumably generated during reductive dehalogenation by an uncharacterized electron transport system. Recently, a membrane-bound, inducible 3CB reductive dehalogenase which is proposed to be the terminal reductase of this electron transport chain was purified from D. tiedjei (45).
Details of chemiosmotic processes in D. tiedjei are unknown. The 3CB reductive dehalogenase has been shown to be an integral membrane protein (45), but it is unclear to which side of the cytoplasmic membrane the active site of the dehalogenase is oriented. Enzymes which catalyze sulfate reduction are normally cytoplasmic, but this has not been confirmed in D. tiedjei. The primary dehydrogenases, hydrogenase and formate dehydrogenase, can be located on either side of the cytoplasmic membrane and have not been localized in D. tiedjei. Electron carriers have generally not been characterized in D. tiedjei; the direct electron donor for the reductive dehalogenase is unidentified. We recently characterized a peripheral membrane cytochrome c of D. tiedjei, which is coinduced with reductive dehalogenation (34) and so is likely a component of the electron transport chain during dehalogenation. DeWeerd et al. (14) discovered that 1,4-naphthoquinone or menadione is required for optimal growth of D. tiedjei in defined media. These quinoids are similar in structure to the aromatic nuclei of menaquinones, suggesting that they are used as precursors for synthesizing a menaquinone which functions as an electron carrier in D. tiedjei. However, the existence of a respiratory quinoid in D. tiedjei has never been proven.
Knowing the topology of the respiratory enzymes and the components of the electron transport chain will improve our understanding of how D. tiedjei generates a PMF during dehalorespiration and sulfate respiration. In the work presented here, hydrogenase, formate dehydrogenase, sulfate-reducing enzymes, and the reductive dehalogenase of D. tiedjei were localized. Also, we performed preliminary studies investigating the dehalorespiratory electron transport chain of D. tiedjei. The results provide evidence that a PMF can be generated by a scalar mechanism, involving oxidation of hydrogen or formate in the periplasm and transfer of electrons across the cytoplasmic membrane to a reductive dehalogenase oriented toward the cytoplasm.
MATERIALS AND METHODS
Organism and growth conditions.
D. tiedjei DCB-1 (ATCC 49306) was grown on reduced anaerobic medium, as previously described (34). Briefly, 20 mM pyruvate was used as the sole energy source for growing D. tiedjei with 2 g of yeast extract per liter and 2 g of tryptone per liter as supplements. When the cells were grown under dehalogenating conditions, 500 μM 3CB was added from a 100 mM anoxic filter-sterilized stock. Desulfovibrio gigas (ATCC 19364) was grown stationarily at 30°C on reduced anaerobic medium containing 31 mM sodium lactate, 20 mM Na2SO4, 30 mM NaHCO3, 17 mM NaCl, 6.8 mM KCl, 2.5 mM MgCl2 · 6H2O, 1.0 mM CaCl2 · 2H2O, 5.8 mM NH4Cl, 1.5 mM KH2PO4, 1 mM Na2S, and 1 g of yeast extract per liter, with a 95% N2–5% CO2 headspace. The pH of the medium was adjusted to 7.5.
Cell fractionation.
Unless indicated otherwise, all fractionation steps were carried out at 4°C without protection against oxygen. Late-exponential-phase cultures were harvested either by centrifugation (10,000 × g, 20 min) or with a bench-scale cross-flow filtration unit equipped with a 0.3-μm-pore-size microfiltration membrane (Filtron Technology Corp., Northborough, Mass.). Cells were suspended in 10 mM Tris-HCl buffer (pH 7.7) and were broken by passing the cell suspensions four times through a French pressure cell at a cell pressure of 103.4 MPa. Cell lysates were then centrifuged (12,000 × g, 20 min). The pelleted, unbroken cells and debris were suspended in 10 mM Tris-HCl buffer (pH 7.7) and passed through the French pressure cell four more times and centrifuged as described above to improve the yield of cell lysate. The combined supernatants were ultracentrifuged (180,000 × g, 2 h). The pellets were washed and suspended in the Tris-HCl buffer and ultracentrifuged again. The resulting pellets were considered to be membrane fractions. The supernatants of the two ultracentrifugation preparations were combined and considered to be soluble fractions.
Cell suspensions.
D. tiedjei cells were harvested by centrifugation (10,000 × g, 20 min, 4°C) in centrifuge tubes which were previously flushed with N2 or stored overnight in an anaerobic glove box. Cell pellets were washed with sterile, anaerobic 10 mM HEPES plus 10 mM potassium phosphate buffer (pH 7.5) reduced with 0.1 mM titanium(III) citrate. The cells were harvested by centrifugation again and suspended in sterile anaerobic buffer at 5 to 10 times their original concentration, and the cells were transferred to sterile 25-ml serum bottles with N2 in the headspace. As an electron acceptor, 3CB was added to the cell suspensions from a 100 mM anoxic, filter-sterilized stock solution. The cell suspensions were incubated stationarily in darkness at 37°C with no electron donor for 48 h to deplete any endogenous reducing power. Different electron donors and metabolic inhibitors were then added from sterile anoxic stock solutions, and the cell suspensions were further incubated. Reductive dehalogenation activity was analyzed as benzoate formation, by high-performance liquid chromatography (HPLC), as previously described (34).
Enzyme assays.
Hydrogenase activity was assayed in a butyl rubber-stoppered glass cuvette by spectrophotometrically recording the hydrogen-dependent reduction of 5 mM methyl viologen (ɛ582 = 9,600 M−1 cm−1) in H2-saturated 100 mM Tris-HCl (pH 8.8) buffer. The reaction was started by injection of cellular fractions or whole cells into the cuvette. One unit of hydrogenase activity is defined as the reduction of 2 μmol of methyl viologen per min. Formate dehydrogenase activity was assayed similarly, except that N2-saturated 50 mM Tris-HCl (pH 8.0) buffer was used. The reaction was started by injection of 0.1 ml of sodium formate from a 100 mM anoxic stock solution into the cuvette, which contained cellular fractions or whole cells. One unit of formate dehydrogenase activity is defined as the reduction of 2 μmol of methyl viologen per min.
ATP sulfurylase was assayed by measuring adenosine phosphosulfate (APS)- and pyrophosphate (PPi)-dependent ATP production in a coupled spectrophotometric test (29). ATP production was monitored with a kit (Sigma Chemical Co., St. Louis, Mo.) which measured ATP production via monitoring NADH oxidation spectrophotometrically (ɛ340 = 6,220 M−1 cm−1) in the reaction mixture. One unit of ATP sulfurylase activity is defined as the oxidation of 1 μmol of NADH per min. APS reductase activity was assayed under aerobic conditions, according to the method of Odom and Peck (47), by measuring sulfite- and AMP-dependent reduction of ferricyanide (ɛ420 = 990 M−1 cm−1). One unit of APS reductase activity is defined as the reduction of 2 μmol of ferricyanide per min, after correction for nonenzymatic reduction of ferricyanide. Desulfoviridin sulfite reductase was identified in D. tiedjei cellular fractions spectrophotometrically by its unique absorption at 630 nm and quantified according to the method of Badziong and Thauer (4).
Reductive dehalogenase activity in D. tiedjei cell extracts was assayed according to the method of Ni et al. (45). Hydrogen was the electron donor for dehalogenation. Hydrogenase present in the cell extracts reduced methyl viologen, which functioned as the artificial electron donor for the reductive dehalogenase. The reaction was stopped by precipitating the proteins with 1% ice-cold trichloroacetic acid. Dehalogenation was measured as conversion of 3CB to benzoate production by HPLC (34).
Analysis of respiratory quinoids.
Respiratory quinoids were extracted overnight with a mixture of chloroform-methanol (2:1 [vol/vol]) from lyophilized D. tiedjei and Desulfovibrio gigas (11). The extract was then filtered to remove cell debris and evaporated to dryness at 37°C by a rotary evaporator. The dried extract was suspended in a small volume of acetone and loaded onto a Kieselgel 60F254 thin-layer chromatography plate (E. Merck AG, Darmstadt, Germany). The thin-layer chromatography plate was developed with a mixture of hexane-diethyl ether (85:15 [vol/vol]). Menaquinones isolated from Desulfovibrio gigas and separated on the thin-layer chromatography plate were revealed by brief irradiation with UV light (254 nm) and eluted from the silica gel with ethanol. The ethanol eluant was further purified by HPLC with an ODS Hypersil column (Hewlett-Packard Co., Palo Alto, Calif.; 5 by 250 by 4 mm). Menaquinones were monitored at 270 nm and eluted with methanol-isopropyl ether (85:15 [vol/vol]) at 1 ml/min. A quinoid isolated from D. tiedjei was extracted and separated from other lipids by thin-layer chromatography, as described above. The ethanol eluant containing the quinoid was further purified by HPLC with the above-described column. The quinoid was monitored at 280 nm and eluted with methanol-water, by using a gradient (70:30 from 0 to 4 min; a linear gradient from 4 to 11 min increasing to 90:10; 90:10 from 11 to 19 min; and 70:30 from 19 to 25 min to reequilibrate). Air-oxidized and sodium-borohydride-reduced UV absorption spectra of the HPLC-purified menaquinone isolated from Desulfovibrio gigas, the quinoid isolated from D. tiedjei, and a mixture of ubiquinones extracted from activated sludge (a gift from H. Satoh, University of Tokyo, Tokyo, Japan) were collected with a Cary 1E spectrophotometer.
An electron impact mass spectrum of the HPLC-purified quinoid was recorded by the Mass Spectrometry Laboratory, Department of Chemistry, University of British Columbia, according to the conditions for analyzing menaquinones reported by Collins and Widdel (11).
Chemicals.
Sodium salts of AMP, APS, and PPi; methyl viologen; potassium ferricyanide; propyl iodide; and 2-heptyl-4-hydroxyquinoline N-oxide (HQNO) were purchased from Sigma. 3CB was purchased from Aldrich Chemical Co., Inc., Milwaukee, Wis. All of the organic solvents were purchased from Fisher Scientific Co., Pittsburgh, Pa.
Replication.
All experiments were repeated at least once with results consistent with those shown.
RESULTS
A putative respiratory quinoid.
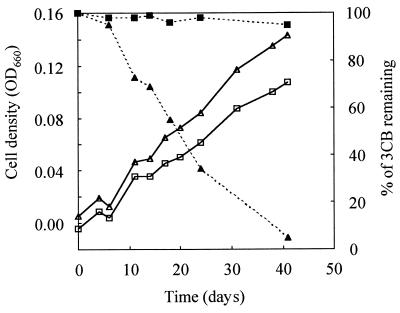
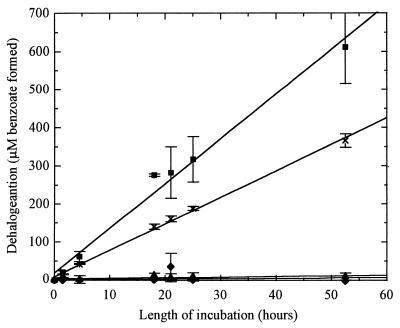
A quinoid appears essential to dehalorespiration by D. tiedjei. Reductive dehalogenation of D. tiedjei was dependent on 1,4-naphthoquinone, a possible respiratory quinoid precursor (Fig. 1). Moreover, 150 nmol of HQNO, a respiratory quinoid inhibitor, per mg of protein inhibited reductive dehalogenation in D. tiedjei cell suspensions (Fig. 2). The same concentration of HQNO had no inhibitory effect on reductive dehalogenation by D. tiedjei cell extracts, indicating that the dehalogenase is not directly affected by HQNO and suggesting that the inhibitory effect of HQNO is through its effect on a respiratory quinoid.
FIG. 1.
Growth curves (open symbols) and reductive dehalogenation (closed symbols) of D. tiedjei after five passages on medium with no vitamin deficiency (triangles) and on medium deficient in 1,4-naphthoquinone (squares). Pyruvate was supplied as the primary energy source to the two cultures. Yeast extract and tryptone were not added to these media to avoid 1,4-naphthoquinone contamination. OD660, optical density at 660 nm.
FIG. 2.
Effect of HQNO on reductive dehalogenation activity of D. tiedjei cell suspensions with formate as the electron donor. ■, no HQNO; ×, 150 nmol of HQNO per mg of protein; ▴, 1.5 μmol of HQNO per mg of protein; ⧫, heat-killed cell suspension. Data are means of triplicates with standard errors.
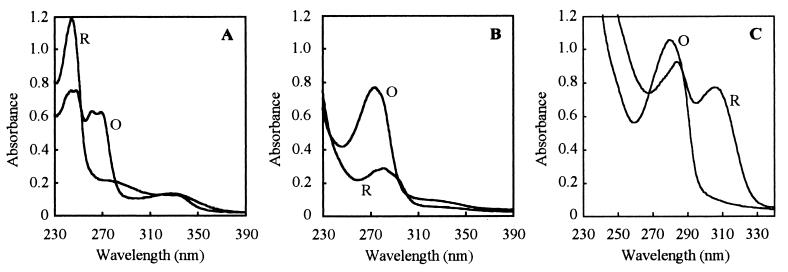
A quinone-like compound was purified from D. tiedjei. Numerous attempts, by different methods, failed to isolate menaquinone or ubiquinone from D. tiedjei cells. However, a different UV-absorbing compound was purified. In the oxidized form, this compound had a peak of maximal absorbance at 280 nm (Fig. 3). The oxidized and reduced UV absorption spectra of the purified compound were very different from those of menaquinone purified from Desulfovibrio gigas. The oxidized absorption spectrum of the purified compound was similar to that of oxidized ubiquinone, but the reduced absorption spectrum was very different from that of ubiquinone.
FIG. 3.
Oxidized (O) and reduced (R) UV absorption spectra of menaquinone extracted from Desulfovibrio gigas (A), ubiquinone extracted from activated sludge (B), and the quinoid extracted from D. tiedjei (C).
The mass spectrum of the quinoid purified from D. tiedjei also indicated that the compound is distinct from menaquinones and ubiquinones. The molecular ion of the purified quinoid was identified as a peak with an m/z value of 340 (Fig. 4). This molecular weight is different from those of menaquinones and ubiquinones. Moreover, the mass spectra of menaquinones and ubiquinones have a characteristic fragmentation pattern, with major peaks differing by 68 mass units, due to successive fragmentation of the isoprenoid side chain. This fragmentation pattern was not observed in the mass spectrum of the purified quinoid, suggesting that the purified quinoid does not have an isoprenoid side chain. In addition, the aromatic nuclear fragments of menaquinones and ubiquinones result in major peaks with m/z values of 235 and 225, respectively. Such peaks were not observed in the mass spectrum of the purified quinoid.
FIG. 4.
Electron impact mass spectrum of the quinoid purified from D. tiedjei.
Reductive dehalogenase activity in cell extracts.
We were unable to identify the electron donor for the dehalogenase in vivo. Reduced methyl viologen functioned as an electron donor in the in vitro dehalogenase assay, supporting a specific activity of 1.1 nmol of benzoate formed per mg of protein per h. Activity was dependent on H2 and cell extract, and the activity was abolished by boiling the cell extract. The quinoid, purified in this study, and a cytochrome c, which is coinduced with reductive dehalogenation activity and which we had previously purified (34), failed to function as the electron donor. The quinoid and cytochrome c together also failed to function. Prereducing these two potential electron donors with 2 mM titanium(III) citrate before adding them to the reaction mixture did not change the results. Reductive dehalogenation in D. tiedjei apparently does not require a corrinoid, as this activity was not inhibited by 250 mM propyl iodide, an agent which interferes in corrinoid-dependent processes by alkylating cobalamins and which inhibits PCE dechlorination by Dehalobacter restrictus (51, 52), Dehalococcoides ethenogenes 195 (35), Desulfitobacterium sp. strain PCE-S (38), and Dehalospirillum multivorans (43, 44).
Topology of the reductive dehalogenase.
The membrane-associated reductive dehalogenase of D. tiedjei is probably oriented toward the cytoplasm. Washed-cell suspensions of D. tiedjei, without an added electron donor (i.e., with only endogenous reducing power), had a specific dehalogenation activity of 14.6 nmol of benzoate formed per mg of protein per h. Addition of reduced methyl viologen failed to increase this dehalogenation rate and even had an inhibitory effect. Although the cause of the inhibition is not clear, this result is not consistent with the presence of a periplasm-oriented reductive dehalogenase. Reduced methyl viologen, which is a monovalent cationic radical, cannot efficiently permeate cytoplasmic cell membranes (27). The ability of reduced methyl viologen to function as an artificial electron donor for the dehalogenase in cell extracts but not in whole cells is most likely due to the location of the active site of the reductive dehalogenase on the cytoplasmic side of the membrane.
Hydrogenase.
The evidence indicates that D. tiedjei has a periplasmic hydrogenase which is active during pyruvate fermentation, whether or not reductive dechlorination is also induced. The hydrogenase activities in soluble fractions of cells grown on pyruvate with or without 3CB were much higher than the hydrogenase activities in the soluble fractions of cells grown on formate plus sulfate (Table 1). Hydrogenase activity measured in whole cells grown on pyruvate with 3CB was relatively high, as in the soluble fractions. Since the redox dye, methyl viologen, which was used as the artificial electron acceptor has been shown to be inefficient in permeating the cytoplasmic membrane in both oxidation states (27), detection of significant levels of hydrogenase activity with whole cells suggests that the soluble hydrogenase is located in the periplasm, rather than in the cytoplasm. Furthermore, the whole-cell hydrogenase activity was completely inhibited by Cu2+ ions, a membrane-impermeable hydrogenase inhibitor (12, 20, 21). This observation further supports the conclusion that the soluble hydrogenase is a periplasmic enzyme.
TABLE 1.
Specific activities of different enzymes of D. tiedjei grown under different metabolic conditions (n = 3)
| Enzyme | Growth substrate(s) | Sp act (U/g of protein) in:
|
||
|---|---|---|---|---|
| Membrane fraction | Soluble fraction | Whole cells | ||
| Hydrogenase | Formate plus sulfate | 1.0 | 3.0 | NDa |
| Pyruvate | 2.8 | 673.0 | ND | |
| Pyruvate plus 3CB | 9.1 | 1,358.6 | 730.0 | |
| Formate dehydrog-enase | Formate plus sulfate | 13.0 | 2.3 | 74.1 |
| Pyruvate | 1.3 | 18.3 | ND | |
| Pyruvate plus 3CB | 3.1 | 37.4 | 0.0 | |
| ATP sulfurylase | Formate plus sulfate | 0.0 | 240.0 | ND |
| Pyruvate | 0.0 | 310.0 | ND | |
| Pyruvate plus 3CB | 0.0 | 340.0 | ND | |
| APS reductase | Formate plus sulfate | 0.0 | 180.0 | ND |
| Pyruvate | 0.0 | 170.0 | ND | |
| Pyruvate plus 3CB | 0.0 | 140.0 | ND | |
| Desulfoviridinb | Formate plus sulfate | 0.0 | 1.8 | ND |
| Pyruvate | 0.0 | 1.5 | ND | |
| Pyruvate plus 3CB | 0.0 | 1.4 | ND | |
ND, not determined.
Specific amount (grams per gram of protein) of desulfoviridin, instead of specific activity, is reported.
Formate dehydrogenase.
The evidence indicates that D. tiedjei has two formate dehydrogenases, one being cytoplasmic and active during pyruvate fermentation and the other being membrane associated, oriented toward the periplasm, and active during growth on formate. Whole cells grown on pyruvate plus 3CB had no measurable formate dehydrogenase activity with membrane-impermeable methyl viologen as the artificial electron acceptor (Table 1), suggesting that there is no periplasm-oriented formate dehydrogenase. When the cells were lysed, a soluble formate dehydrogenase activity was detected with methyl viologen as the electron acceptor. This finding therefore indicated that this soluble formate dehydrogenase is probably a cytoplasmic enzyme. Essentially the same activity was detected in fractions of cells that were grown on only pyruvate. In cells grown on formate plus sulfate, soluble formate dehydrogenase activity was at a reduced level, while a membrane-associated formate dehydrogenase was detected. This membrane-associated formate dehydrogenase is probably periplasm oriented, as whole cells grown on formate plus sulfate had high formate dehydrogenase activity with membrane-impermeable methyl viologen as the artificial electron acceptor. The higher specific activity detected in whole cells, relative to that in the cellular membrane fraction, could be due to underestimation of total protein in whole cells as well as partial loss of enzyme activity during cell fractionation.
Enzymes involved in sulfate reduction.
Three enzymes involved in dissimilatory sulfate reduction, ATP sulfurylase, APS reductase, and desulfoviridin (sulfite reductase), were each active at similar levels in the soluble fractions of D. tiedjei cells grown under conditions for reductive dehalogenation, pyruvate fermentation, and sulfate reduction (Table 1). These enzyme activities were not present in the periplasmic washes, prepared by a standard procedure (4), nor were they present in the membrane fractions. Therefore, these enzymes appear to be constitutively expressed in the cytoplasm. The other three types of sulfite reductases commonly found in sulfate-reducing bacteria (32) were not detected. These findings also indicate that the periplasmic washes and membrane fractions of D. tiedjei cells prepared in this study were free of cytoplasmic contaminants.
DISCUSSION
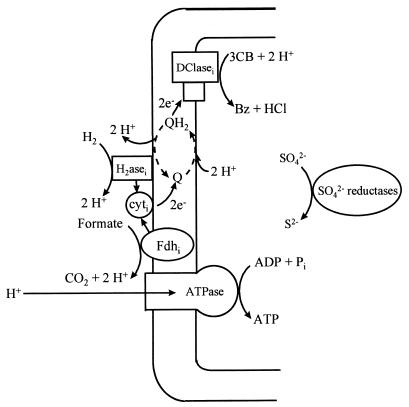
The findings of this study indicate a spatial organization of respiratory enzymes which suggests a tentative model of dehalorespiration in D. tiedjei (Fig. 5). In this model, the membrane-associated, periplasm-oriented formate dehydrogenase and the periplasmic hydrogenase are the primary dehydrogenases. The membrane-bound, cytoplasm-oriented 3CB reductive dehalogenase functions as the terminal reductase. During oxidation of formate or hydrogen, protons are produced in the periplasm. During reduction of 3CB, protons are consumed in the cytoplasm. The net result of this enzyme topology is generation of a PMF via a scalar mechanism (i.e., no protons are translocated). This study provides substantial evidence for this enzyme topology. The electrons produced by the primary dehydrogenases are hypothesized to be transported across the membrane via both the inducible cytochrome c and the quinoid, although we do not currently have any direct evidence to support this electron transport pathway. This electron transport system might further increase the PMF via the indicated redox loop involving the quinoid, a vectorial mechanism (i.e., protons are translocated). It is also possible that the reductive dehalogenase contributes to the PMF by another vectorial mechanism, functioning as a proton pump similar to the mitochondrial terminal oxidase complex. The available evidence neither supports nor excludes vectorial proton translocation during dehalorespiration by D. tiedjei.
FIG. 5.
A tentative chemiosmotic model of dehalorespiration in D. tiedjei. H2asei, inducible hydrogenase; Fdhi, inducible formate dehydrogenase; cyti, 50-kDa inducible cytochrome c; Q, a quinoid; SO42− reductases, the three enzymes, ATP sulfurylase, APS reductase, and desulfoviridin, which are involved in sulfate reduction; Bz, benzoate.
A previous study (41) measuring medium acidification by D. tiedjei cell suspensions suggests an H+/3CB ratio of 2.1. This ratio agrees with the formation of only two to three protons during H2 oxidation and could be interpreted as evidence against vectorial proton translocation. Moreover, the growth yield of D. tiedjei appears to be consistent with this H+/3CB ratio. The growth yield on formate or H2 plus 3CB was 2 to 3 g of protein per mol of 3CB dehalogenated (40), which corresponds to approximately 4 to 6 g (dry weight) per mol of 3CB dehalogenated (or per mol of H2 oxidized). This molar growth yield is slightly lower than that estimated for Desulfovibrio vulgaris Marburg grown on hydrogen plus sulfate (3). If the ATP yield per H2 molecule of D. tiedjei is also similar to that of strain Marburg, 1 ATP molecule per H2 molecule, and if the ATPase of D. tiedjei translocates three protons per molecule of ATP produced, it would be further evidence against vectorial proton translocation in D. tiedjei. This dehalorespiratory model is similar to the one proposed for Dehalobacter restrictus (51). In the latter organism, a menaquinone appears to mediate electron transport between a periplasmic hydrogenase and a membrane-associated, cytoplasm-oriented PCE dehalogenase (51). In the latter organism, the menaquinone is probably not involved in additional vectorial proton translocation.
Despite the fact that D. tiedjei has been shown to be a sulfate-reducing bacterium, the terminal reductases involved in sulfate reduction in D. tiedjei were not previously examined. Our results indicate that D. tiedjei uses a constitutive, cytoplasmic enzyme system for sulfate reduction (Table 1), similar to that of other sulfate-reducing bacteria (28, 30). The constitutive expression of these proteins suggests that D. tiedjei will use sulfate and sulfite as electron acceptors whenever they are available. This may be energetically beneficial to the bacterium, allowing simultaneous use of environmentally scarce electron acceptors. The cytoplasmic location of these proteins suggests that the above chemiosmotic model (Fig. 5) is also valid for sulfate respiration when hydrogen or formate is used as an electron donor.
Activities of the hydrogenase and formate dehydrogenases of D. tiedjei are regulated in response to growth substrates, as these enzymes were detected only in cells grown under particular conditions (Table 1). This regulation may be genetic. Regulation of primary dehydrogenases in anaerobes has not been widely reported but may be common. Hydrogenase activity in Desulfovibrio vulgaris Groningen cell extracts was 10 times higher when cells were grown on H2 plus sulfate than when they were grown on lactate plus sulfate (23). Immunocytolocalization in ultrathin frozen sections of this organism also showed more periplasmic hydrogenase in cells grown on H2 than in cells grown on lactate. The presence of a membrane-bound hydrogenase in Methanosarcina mazei Gö1 depended on growth of the cells on H2 plus carbon dioxide (13). An apparently inducible formate dehydrogenase was detected in Desulfovibrio gigas cells when the electron donor-carbon source was switched from lactate to formate (47).
The use of periplasmic primary dehydrogenases to generate a PMF via a scalar mechanism, as described above, appears to be a common mechanism in sulfate-reducing bacteria. Desulfovibrio gigas and Desulfovibrio vulgaris Groningen both use a periplasmic hydrogenase for H2 oxidation (23, 46). The above-mentioned formate dehydrogenase of Desulfovibrio gigas is membrane bound and periplasm oriented (47). A formate dehydrogenase was also purified from the periplasmic fraction of Desulfovibrio vulgaris Hildenborough cells (53).
The cytoplasmic formate dehydrogenase detected in D. tiedjei cells grown on pyruvate is probably involved in carbon dioxide reduction. Acetate is the sole product of pyruvate fermentation by D. tiedjei in the presence of carbon dioxide (39). Approximately 20% of this acetate appears to be produced from carbon dioxide (54), via the acetyl coenzyme A pathway which is found in homoacetogens and some sulfate-reducing bacteria (26, 31, 49). The key enzyme of this pathway, carbon monoxide dehydrogenase, was detected in D. tiedjei (39). Formate dehydrogenase is the first enzyme of the reductive acetyl coenzyme A pathway.
Although the pathway of electron transport during dehalorespiration by D. tiedjei is not yet certain, the available evidence is consistent with the hypothesis that electrons are transported across the cell membrane during dehalorespiration and that a cytochrome c and a quinoid are involved in this process. The above enzyme topology (Fig. 5) requires that electrons be transported from the periplasmic to the cytoplasmic side of the cellular membrane. A 50-kDa cytochrome c is coinduced with reductive dehalogenation activity (34). In this study, reductive dehalogenation was dependent on 1,4-naphthoquinone (Fig. 1); the respiratory quinoid inhibitor, HQNO, inhibited reductive dehalogenation (Fig. 2); and a quinoid was purified from D. tiedjei. Reduced forms of the inducible cytochrome c and the quinoid failed to replace reduced methyl viologen in a reductive dehalogenase assay with D. tiedjei cell extract. However, this finding does not exclude these two potential electron carriers as components of the electron transport system coupled to the reductive dehalogenase. One possibility is that another electron carrier or a redox center, required to mediate between the reductive dehalogenase and the inducible cytochrome c or the quinoid, is inactivated during cell fractionation under aerobic conditions. A variety of potential physiological electron donors for 3CB reductive dehalogenation by D. tiedjei were previously tested (15), but none of these compounds could replace reduced methyl viologen in a dehalogenation assay. Thus, the electron donor directly coupled in vivo to the reductive dehalogenase of D. tiedjei remains to be determined.
It is possible that 1,4-naphthoquinone functions as a precursor for biosynthesis of the quinoid purified from D. tiedjei. Although 1,4-naphthoquinone was shown not to be an intermediate in menaquinone biosynthesis by E. coli (5, 37), some bacteria, including Mycobacterium phlei, Bacteroides melaninogenicus, and “Aerobacter aerogenes” 170-44, can use 1,4-naphthoquinone for menaquinone biosynthesis (6).
The structure of the quinoid purified from D. tiedjei is uncertain. We, and others (33), could not extract menaquinones or ubiquinones from D. tiedjei. The quinoid we did extract is distinct from menaquinones and ubiquinones in its UV absorption spectra (Fig. 3) and its mass spectrum (Fig. 4). The purified quinoid has some similarities with pyrroloquinoline quinone (PQQ), a respiratory quinoid which can be tightly associated with dehydrogenases (2) and which is found in methylotrophs (18), acetic acid bacteria (1), E. coli (9), and Acinetobacter calcoaceticus (10). Both the purified quinoid and PQQ lack an isoprenoid side chain. The oxidized absorption spectrum of the purified quinoid is very different from that of PQQ. However, spectral changes observed when the purified quinoid is reduced are similar to those observed when PQQ is reduced (17, 19). The mass spectrum of the purified quinoid is different from that reported for PQQ (7). The molecular weight of the quinoid is 340, based on the molecular ion, while the molecular weight of PQQ is 330. Further elucidation of the structure of the quinoid was not done because of difficulties in obtaining sufficient quantities of this compound and because of uncertainty about its role as an electron carrier.
This study demonstrates a mechanism for energy conservation during dehalorespiration. In D. tiedjei, chemical energy available from the coupled dehalogenation half-reactions is transduced to a PMF via a scalar mechanism. It remains to be determined if a vectorial mechanism further contributes to the PMF in this organism. It will be of interest to see if this chemiosmotic mechanism occurs in dehalorespiratory organisms other than D. tiedjei.
ACKNOWLEDGMENTS
This research was supported by the Natural Science and Engineering Research Council of Canada and the Council of Forestry Industries of British Columbia through an Industrial Research Chair in Forest Products Waste Management and by the Natural Science and Engineering Research Council of Canada through a scholarship to T.M.L.
REFERENCES
- 1.Anthony C. The structure of bacterial quinoprotein dehydrogenase. Int J Biochem. 1992;24:29–39. doi: 10.1016/0020-711x(92)90226-q. [DOI] [PubMed] [Google Scholar]
- 2.Anthony C, Ghosh M, Blake C C F. The structure and function of methanol dehydrogenase and related quinoproteins containing pyrrolo-quinoline quinone. Biochem J. 1994;304:665–674. doi: 10.1042/bj3040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badziong W, Thauer R K. Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as the sole energy sources. Arch Microbiol. 1978;117:209–214. doi: 10.1007/BF00402310. [DOI] [PubMed] [Google Scholar]
- 4.Badziong W, Thauer R K. Vectorial electron transport in Desulfovibrio vulgaris (Marburg) growing in hydrogen plus sulfate as sole energy source. Arch Microbiol. 1980;125:167–174. [Google Scholar]
- 5.Baldwin R M, Snyder C D, Rapoport H. Biosynthesis of bacterial menaquinones. Dissymmetry in the naphthalenic intermediates. Biochemistry. 1974;13:1523–1530. doi: 10.1021/bi00704a031. [DOI] [PubMed] [Google Scholar]
- 6.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffoni F, Cambi S, Moneti G. Pyrroloquinoline quinone: a method for its isolation and identification by mass spectrometry. Biochim Biophys Acta. 1992;1116:297–304. doi: 10.1016/0304-4165(92)90043-t. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen N, Ahring B K. Desulfitobacterium hafniense sp. nov., an anaerobic, reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- 9.Cleton-Jansen A M, Goosen N, Fayet O, van de Putte P. Cloning, mapping, and sequencing of the gene encoding Escherichia coli quinoprotein glucose dehydrogenase. J Bacteriol. 1990;172:6308–6315. doi: 10.1128/jb.172.11.6308-6315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleton-Jansen A M, Goosen N, Odle G, van de Putte P. Nucleotide sequence of the gene coding for quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus. Nucleic Acids Res. 1988;16:6228. doi: 10.1093/nar/16.13.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins M D, Widdel F. Respiratory quinone of sulfate-reducing and sulfur-reducing bacteria: a systematic investigation. Syst Appl Microbiol. 1986;8:8–18. [Google Scholar]
- 12.Cypionka H, Dilling W. Intracellular localization of the hydrogenase in Desulfotomaculum orientis. FEMS Microbiol Lett. 1986;36:257–260. [Google Scholar]
- 13.Deppenmeier U, Blaut M, Lentes S, Herzberg C, Gottschalk G. Analysis of the vhoGAC and vhtGAC operons from Methanosarcina mazei strain Gö1, both encoding a membrane bound hydrogenase and cytochrome b. Eur J Biochem. 1995;227:261–269. doi: 10.1111/j.1432-1033.1995.tb20383.x. [DOI] [PubMed] [Google Scholar]
- 14.DeWeerd K A, Mandelco L, Tanner R S, Woese C R, Suflita J M. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic, dehalogenating, sulfate-reducing bacterium. Arch Microbiol. 1990;154:23–30. [Google Scholar]
- 15.DeWeerd K A, Suflita J M. Anaerobic aryl reductive dehalogenation of halobenzoates by cell extracts of “Desulfomonile tiedjei.”. Appl Environ Microbiol. 1990;56:2999–3005. doi: 10.1128/aem.56.10.2999-3005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153:264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- 17.Duine J A, Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980;187:221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duine J A, Frank J, Jr, Verwiel E J. Structure and activity of the prosthetic group of methanol dehydrogenase. Eur J Biochem. 1980;108:187–192. doi: 10.1111/j.1432-1033.1980.tb04711.x. [DOI] [PubMed] [Google Scholar]
- 19.Duine J A, Frank J, Jr, Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium X. Biochim Biophys Acta. 1978;524:277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez V M, Rua M L, Reyes P, Cammack R, Hatchikian E H. Inhibition of Desulfovibrio gigas hydrogenase with copper salts and other metal ions. Eur J Biochem. 1989;185:449–454. doi: 10.1111/j.1432-1033.1989.tb15135.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitz R M, Cypionka H. A study on electron transport-driven proton translocation in Desulfovibrio desulfuricans. Arch Microbiol. 1989;152:369–376. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- 22.Gerritse J, Renard V, Gomes T M P, Lawson P A, Collins M D, Gottschal J C. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol. 1996;165:132–140. doi: 10.1007/s002030050308. [DOI] [PubMed] [Google Scholar]
- 23.Hatchikian E C, Forget N, Bernadae A, Alazard D, Ollivier B. Involvement of a single periplasmic hydrogenase for both hydrogen uptake and production in some Desulfovibrio species. Res Microbiol. 1995;146:129–141. doi: 10.1016/0923-2508(96)80891-6. [DOI] [PubMed] [Google Scholar]
- 24.Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, Vazquez F, Weiss N, Zehnder A J B. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in anaerobic respiration. Arch Microbiol. 1998;169:313–321. doi: 10.1007/s002030050577. [DOI] [PubMed] [Google Scholar]
- 25.Holliger C, Schumacher W. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek J Microbiol Serol. 1994;66:239–246. doi: 10.1007/BF00871642. [DOI] [PubMed] [Google Scholar]
- 26.Jansen K, Thauer R K, Widdel F, Fuchs G. Carbon assimilation pathways in sulfate reducing bacteria. Formate, carbon dioxide carbon monoxide, and acetate assimilation by Desulfovibrio baarsii. Arch Microbiol. 1984;138:257–262. [Google Scholar]
- 27.Jones R W, Garland P B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977;164:199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K, Morisawa Y, Ishituka T, Ishimoto M. Biochemical studies on sulfate reducing bacteria. XIV. Enzyme levels of adenyl sulfate reductase, inorganic pyrophosphatase, sulfite reductase, hydrogenase and adenosine triphosphatase in cells grown on sulfate, sulfite and thiosulfate. J Biochem (Tokyo) 1975;78:1079–1085. doi: 10.1093/oxfordjournals.jbchem.a130985. [DOI] [PubMed] [Google Scholar]
- 29.Krämer M, Cypionka H. Sulfate formation via ATP sulfurylase in thiosulfate- and sulfite-disproportionating bacteria. Arch Microbiol. 1989;151:232–237. [Google Scholar]
- 30.Kremer D R, Veenhuis M, Fauque G, Peck H D, Jr, Le Gall J, Moura J J G, Hansen T A. Immunocytochemical localization of APS reductase and bisulfite reductase in three Desulfovibrio strains. Arch Microbiol. 1988;150:296–301. [Google Scholar]
- 31.Länge S, Scholtz R, Fuchs G. Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 1. Characterization and metabolic function of the cellular tetrahydropterin. Arch Microbiol. 1989;151:77–83. [Google Scholar]
- 32.LeGall J, Fauque G. Dissimilatory reduction of sulfur compounds. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 587–639. [Google Scholar]
- 33.Löffler, F. E., and J. M. Tiedje. 1995. Personal communication.
- 34.Louie T M, Ni S H, Xun L, Mohn W W. Purification, characterization and gene sequence analysis of a novel cytochrome c co-induced with reductive dechlorination in Desulfomonile tiedjei DCB-1. Arch Microbiol. 1997;168:520–527. doi: 10.1007/s002030050530. [DOI] [PubMed] [Google Scholar]
- 35.Magnuson J K, Stern R V, Gossett J M, Zinder S H, Burris D R. Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Appl Environ Microbiol. 1998;64:1270–1275. doi: 10.1128/aem.64.4.1270-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maymó-Gatell X, Chien Y T, Gossett J M, Zinder S H. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 37.Meganathan R. Biosynthesis of the isoprenoid quinones menaquinone (vitamin K2) and ubiquinone (coenzyme Q) In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 642–656. [Google Scholar]
- 38.Miller E, Wohlfarth G, Diekert G. Comparative studies on tetrachloroethene reductive dechlorination mediated by Desulfitobacterium sp. strain PCE-S. Arch Microbiol. 1997;168:513–519. doi: 10.1007/s002030050529. [DOI] [PubMed] [Google Scholar]
- 39.Mohn W W, Tiedje J M. Catabolic thiosulfate disproportionation and carbon dioxide reduction in strain DCB-1, a reductively dechlorinating anaerobe. J Bacteriol. 1990;172:2065–2070. doi: 10.1128/jb.172.4.2065-2070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohn W W, Tiedje J M. Strain DCB-1 conserves energy for growth from reductive dechlorination coupled to formate oxidation. Arch Microbiol. 1990;153:267–271. doi: 10.1007/BF00249080. [DOI] [PubMed] [Google Scholar]
- 41.Mohn W W, Tiedje J M. Evidence for chemiosmotic coupling of reductive dechlorination and ATP synthesis in Desulfomonile tiedjei. Arch Microbiol. 1991;157:1–6. [Google Scholar]
- 42.Mohn W W, Tiedje J M. Microbial reductive dehalogenation. Microbiol Rev. 1992;56:482–507. doi: 10.1128/mr.56.3.482-507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann A, Wohlfarth G, Diekert G. Properties of tetrachloroethene and trichloroethene dehalogenase of Dehalospirillum multivorans. Arch Microbiol. 1995;163:276–281. [Google Scholar]
- 44.Neumann A, Wohlfarth G, Diekert G. Purification and characterization of tetrachloroethene reductive dehalogenase from Dehalospirillum multivorans. J Biol Chem. 1996;271:16515–16519. doi: 10.1074/jbc.271.28.16515. [DOI] [PubMed] [Google Scholar]
- 45.Ni S, Fredrickson J K, Xun L. Purification and characterization of a novel 3-chlorobenzoate-reductive dehalogenase from the cytoplasmic membrane of Desulfomonile tiedjei DCB-1. J Bacteriol. 1995;177:5135–5139. doi: 10.1128/jb.177.17.5135-5139.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niviere V, Bernadac A, Forget N, Fernandez V M, Hatchikian E C. Localization of hydrogenase in Desulfovibrio gigas cells. Arch Microbiol. 1991;155:579–586. [Google Scholar]
- 47.Odom J M, Peck H D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981;147:161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanford R A, Cole J R, Löffler F E, Tiedje J M. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol. 1996;62:3800–3808. doi: 10.1128/aem.62.10.3800-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schauder R, Preuss A, Jetten M, Fuchs G. Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 2. Demonstration of the enzymes of the pathway and comparison of carbon monoxide dehydrogenase. Arch Microbiol. 1989;151:84–89. [Google Scholar]
- 50.Scholz-Muramatsu H, Neumann A, Mebmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 51.Schumacher W, Holliger C. The proton/electron ratio of the menaquinone-dependent electron transport from dihydrogen to tetrachloroethene in “Dehalobacter restrictus.”. J Bacteriol. 1996;178:2328–2333. doi: 10.1128/jb.178.8.2328-2333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumacher W, Holliger C, Zehnder A J B, Hagen W R. Redox chemistry of cobalamin and iron-sulfur cofactors in the tetrachloroethene reductase of Dehalobacter restrictus. FEBS Lett. 1997;409:421–425. doi: 10.1016/s0014-5793(97)00520-6. [DOI] [PubMed] [Google Scholar]
- 53.Sebban C, Blanchard L, Bruschi M, Guerlesquin F. Purification and characterization of the formate dehydrogenase from Desulfovibrio vulgaris Hildenborough. FEMS Microbiol Lett. 1995;133:143–149. doi: 10.1111/j.1574-6968.1995.tb07875.x. [DOI] [PubMed] [Google Scholar]
- 54.Stevens T O, Tiedje J M. Carbon dioxide fixation and mixotrophic metabolism by strain DCB-1, a dehalogenating anaerobic bacterium. Appl Environ Microbiol. 1988;54:2944–2948. doi: 10.1128/aem.54.12.2944-2948.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Utkin I, Woese C, Wiegel J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]