This randomized clinical trial aims to determine whether a direct oral penicillin challenge is noninferior to the standard of care of penicillin skin testing followed by an oral challenge in patients with a low-risk penicillin allergy.
Key Points
Question
Is direct oral penicillin challenge in adults with a low-risk penicillin allergy, defined as a PEN-FAST score less than 3, safe and effective compared with the standard-of-care penicillin skin testing followed by an oral penicillin challenge?
Findings
In this randomized clinical trial of 382 patients across 6 centers in 3 countries, a positive penicillin oral challenge consistent with an immune-mediated reaction occurred in 0.5% of both the direct oral challenge intervention group and the control group, with an upper 1-sided confidence interval below the noninferiority margin of 5 percentage points.
Meaning
In adult patients with a low-risk penicillin allergy, direct oral penicillin challenge is a safe and effective procedure that may facilitate the removal of a larger number of penicillin allergy labels.
Abstract
Importance
Fewer than 5% of patients labeled with a penicillin allergy are truly allergic. The standard of care to remove the penicillin allergy label in adults is specialized testing involving prick and intradermal skin testing followed by an oral challenge with penicillin. Skin testing is resource intensive, limits practice to specialist-trained physicians, and restricts the global population who could undergo penicillin allergy delabeling.
Objective
To determine whether a direct oral penicillin challenge is noninferior to the standard of care of penicillin skin testing followed by an oral challenge in patients with a low-risk penicillin allergy.
Design, Setting, and Participants
This parallel, 2-arm, noninferiority, open-label, multicenter, international randomized clinical trial occurred in 6 specialized centers, 3 in North America (US and Canada) and 3 in Australia, from June 18, 2021, to December 2, 2022. Eligible adults had a PEN-FAST score lower than 3. PEN-FAST is a prospectively derived and internationally validated clinical decision rule that enables point-of-care risk assessment for adults reporting penicillin allergies.
Interventions
Patients were randomly assigned to either direct oral challenge with penicillin (intervention arm) or a standard-of-care arm of penicillin skin testing followed by oral challenge with penicillin (control arm).
Main Outcome and Measure
The primary outcome was a physician-verified positive immune-mediated oral penicillin challenge within 1 hour postintervention in the intention-to-treat population. Noninferiority was achieved if a 1-sided 95% CI of the risk difference (RD) did not exceed 5 percentage points (pp).
Results
A total of 382 adults were randomized, with 377 patients (median [IQR] age, 51 [35-65] years; 247 [65.5%] female) included in the analysis: 187 in the intervention group and 190 in the control group. Most patients had a PEN-FAST score of 0 or 1. The primary outcome occurred in 1 patient (0.5%) in the intervention group and 1 patient (0.5%) in the control group, with an RD of 0.0084 pp (90% CI, −1.22 to 1.24 pp). The 1-sided 95% CI was below the noninferiority margin of 5 pp. In the 5 days following the oral penicillin challenge, 9 immune-mediated adverse events were recorded in the intervention group and 10 in the control group (RD, −0.45 pp; 95% CI, −4.87 to 3.96 pp). No serious adverse events occurred.
Conclusions and Relevance
In this randomized clinical trial, direct oral penicillin challenge in patients with a low-risk penicillin allergy was noninferior compared with standard-of-care skin testing followed by oral challenge. In patients with a low-risk history, direct oral penicillin challenge is a safe procedure to facilitate the removal of a penicillin allergy label.
Trial Registration
ClinicalTrials.gov Identifier: NCT04454229
Introduction
Patient-reported penicillin allergies remain largely unquestioned. These unverified penicillin allergy labels have been associated with poor patient health outcomes, including increased outpatient mortality, longer hospital stay, inappropriate antibiotic prescribing, development of antibiotic-resistant infections, and excess health care costs.1,2,3,4,5 More than 95% of patients labeled as penicillin allergic will have negative penicillin allergy testing and tolerate subsequent exposure.6 Delabeling, or removing this penicillin allergy label, is increasingly recognized as an essential pillar of global antimicrobial stewardship.7,8
In the majority of countries, the current standard of care in adults to verify or disprove a penicillin allergy includes prick and intradermal skin testing, followed, if negative, by the oral challenge. Observational data support direct oral penicillin challenge without prior skin testing in children and lower-risk adults.9 Regardless of whether skin testing occurs first, oral challenge is considered the necessary final step and is the gold standard to remove the penicillin allergy label, as skin testing alone lacks 100% negative predictive value. However, currently, and to our knowledge, there are no adequately powered randomized controlled data to support direct penicillin challenge without preceding skin testing in adults. This is reflected in updated clinical practice guidelines where direct oral challenge only has conditional recommendations supported by low-quality evidence.10 A prospective, single-center randomized clinical trial in the US evaluated the safety of drug challenges in a low-risk penicillin allergy patient group, including children and adults.11 The investigators identified 3 of 79 patients (3.8%) with a positive challenge 2-step direct challenge (immediate skin manifestations treated with antihistamines and no epinephrine use).11 The safety of direct oral challenge was also demonstrated in a large prospective cohort study of hospitalized patients.12 However, safety and efficacy data in an adequately powered multicenter, international randomized clinical trial remain absent from the literature.
PEN-FAST is a prospectively derived and internationally validated clinical decision rule that enables point-of-care risk assessment for adults reporting penicillin allergies (eFigure 1 in Supplement 1).13,14 A PEN-FAST score lower than 3 can identify low-risk penicillin allergy in adults, with a negative predictive value of 96.3% (95% CI, 94.1%-97.8%).13 The aim of the Penicillin Allergy Clinical Decision Rule (PALACE) study was to evaluate whether risk-stratified direct oral challenge with penicillin was noninferior to penicillin prick and intradermal skin testing followed by an oral challenge in patients with low-risk penicillin allergy (PEN-FAST score <3).
Methods
Study Design
The PALACE study, a multicenter, parallel, 2-arm, noninferiority, international, open-label, randomized clinical trial, was conducted in outpatient clinics at 6 centers, 3 in North America (US [Vanderbilt University Medical Centre and Duke University Medical Center] and Canada [McGill University Health Centre]) and 3 in Australia (Austin Health, Peter MacCallum Cancer Centre, and The Royal Melbourne Hospital). The rationale and design of the PALACE study have been published previously.15 The study was designed and overseen by a Trial Management Group (eMethods in Supplement 1). Study administration, data management, and statistical analyses were performed at Austin Health in Melbourne, Victoria, Australia. To ensure the safety and well-being of patients throughout the study, adverse events were reviewed by an independent data and safety monitoring board every 2 months.
The trial protocol (Supplement 2) was approved by an institutional review board at Austin Health and subsequently by the independent institutional review board at each site. All participants provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Outpatient adults (18 years or older) labeled with a penicillin allergy referred to an allergy clinic with a calculated PEN-FAST score of less than 3 were eligible for enrollment (eMethods in Supplement 1). Key exclusion criteria included anaphylaxis associated with any drug and a known history of chronic spontaneous urticaria or mast cell disease. Patients with histories of non–IgE-mediated severe reactions such as severe delayed organ or skin reactions were also excluded. Patients with self-reported penicillin allergy were also not eligible if their reaction was inconsistent with an allergy and compatible with adverse effects such as nausea or headache. The full inclusion and exclusion criteria are provided in the protocol15 (Supplement 2) and the eMethods in Supplement 1. Racial classification was defined by the investigator following verbal confirmation from the participant. Site investigators confirmed eligibility before a participant underwent randomization.
Randomization and Masking
Participants were randomized in a 1:1 ratio to either the intervention group (direct oral penicillin challenge) or the control group (prick followed by intradermal testing followed by 1-step oral challenge with penicillin, if negative). Randomization was performed remotely using a centralized web-based REDCap (Vanderbilt University) by permuted block design (block sizes ranging from 4-14), stratified by hospital site. Group allocation was concealed until randomization, and blinding postrandomization was not possible. The sequence was computer generated by the trial statistician (S.V.). The trial investigators enrolled the patients and performed the randomization.
Procedures
Skin-testing reagents and interpretation of prick and intradermal testing used in the control arm were performed using standardized procedures (eTable 1 in Supplement 1). Open-label administration of the lowest available therapeutic dose at each site of oral penicillin (eg, amoxicillin, penicillin VK) was performed as per protocol (eTable 1 in Supplement 1). Participants were directly observed following the penicillin challenge with measurement of vital signs at baseline, on any associated symptoms as necessary, and at 60 minutes. Site investigators were responsible for safety evaluation. On day 5 following the oral challenge, study research personnel contacted participants to prompt them to report any delayed adverse events occurring after the period of in-clinic observation.
Outcomes
The primary outcome was a physician-verified positive oral penicillin challenge. Positive oral challenge reactions were defined as an immediate reaction occurring within the hour after ingestion of the penicillin that was consistent with an immune-mediated reaction, including diffuse erythema, rash or urticaria, angioedema, respiratory compromise (measured by a decrease in the baseline oxygen saturation level), and anaphylaxis (or unexplained cardiovascular collapse). Two independent investigators (1 infectious disease specialist [A.M.C.] and 1 allergist [J.A.T.]), unaware of study group allocations, judged these reactions retrospectively based on reviewing documentation of the reactions. In current clinical practice, skin testing is considered a safety measure to avoid an adverse reaction to oral challenge in those with an unverified penicillin allergy. Therefore, participants who had positive skin testing results were counted as not achieving the primary outcome. The primary outcome was to demonstrate that in those with low-risk allergy, a direct oral challenge without prior skin testing does not lead to an increased number of immediate allergic events. The secondary outcomes are detailed in the eMethods in Supplement 1 and include trial feasibility, efficacy, and safety.
Statistical Analysis
We calculated that we would need to enroll 380 patients (190 per group) to achieve a statistical power of 80%, assuming a conservative event rate in the control group of 4%,13 the type I error probability of 5% (1-sided), and noninferiority margin of 5 percentage points (pp). If the control group had a lower prevalence of the outcome (2%), the power of the study would be 95%. Had the estimated difference between the intervention and control group been larger than 0%, the power of the study would remain above 80% if the risk difference (RD) remained below 2.5 pp. Noninferiority design and a 1-sided confidence interval were chosen because we did not believe that the control group was superior to the intervention group. While a noninferiority margin of 5 pp allows for more than double the risk of outcome in the control group, this was chosen as a clinically relevant margin by consensus among investigators due to the rarity of the outcome, its relative nonseverity, benefits of removal of the penicillin allergy label, and cost, time, and resources required for skin testing (control arm). Due to the randomization, intervention, and the primary outcome being collected within the same visit, loss to follow-up was expected to be minimal.
The primary analysis was performed in the intention-to-treat population with any postrandomization exclusions due to ineligibility excluded from the analysis. A generalized linear model was used with binomial family and identity links to estimate the RD between intervention and control. Due to the low number of events, the clinical site was not included in the model. A 2-sided 90% CI was used to represent a single-sided 95% CI. The intervention arm was defined as noninferior if the upper limit of the confidence interval did not cross the noninferiority margin of 5 pp. Results were also presented using risk ratios with 2-sided 90% CIs, which were analyzed as previously described but using a log link. Due to the low number of events, each predefined subgroup was analyzed separately. Sensitivity analysis for the primary outcome and subgroup analysis included an exact 90% CI, as described by Chan and Zhang.16
Safety and other binary secondary outcomes were analyzed similarly to the primary outcome and presented with 95% CIs. Due to the highly skewed distribution of the continuous secondary outcome of time from challenge to delabeling, this was analyzed using the rank sum test. The median difference with a 95% CI is presented.
Missing data were present in fewer than 5% of cases. Therefore, no missing data imputation was performed, and the results of the complete case analysis are presented. Analyses were performed with Stata, version 16 (StataCorp). The detailed statistical analysis plan (Supplement 3) was prepared before the analysis and published on ClinicalTrials.gov.
Results
Study Population
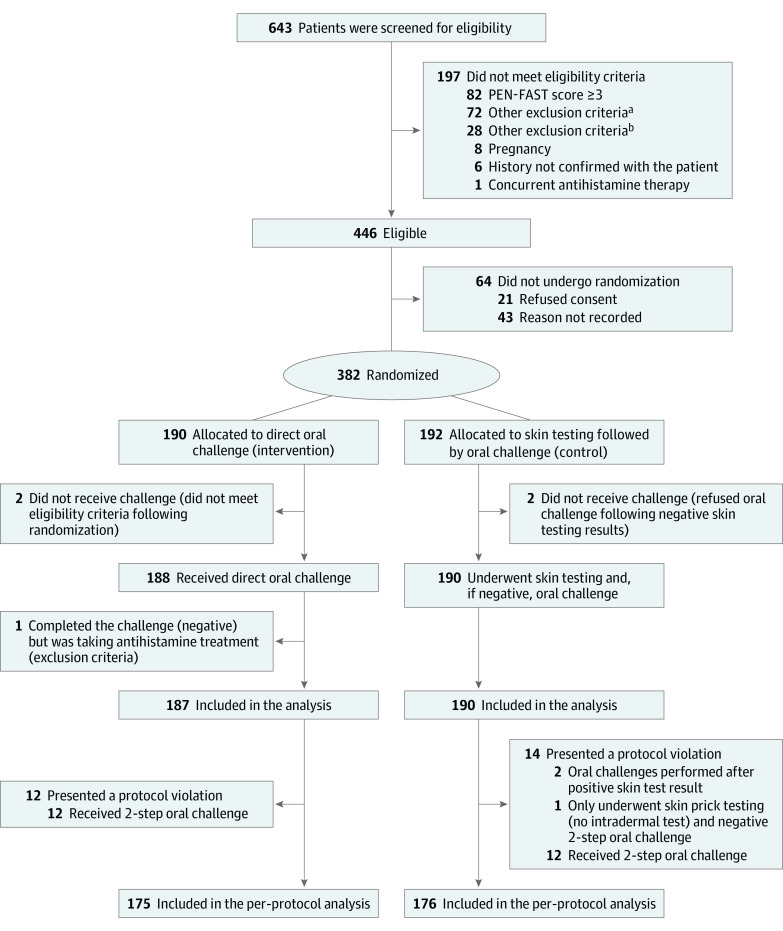
From June 18, 2021, to December 2, 2022, 382 patients were enrolled from the 6 centers; 190 were randomly assigned to the intervention group and 192 to the control group (Figure). The 2 groups were balanced concerning their baseline characteristics (Table 117).
Figure. CONSORT Diagram.
aAny other illness that, in the investigator’s judgment, would substantially increase the risk associated with the patient’s participation in this study, including neurological or psychological conditions.
bPatients with a history of type A adverse drug reaction, drug-associated anaphylaxis, idiopathic urticaria/anaphylaxis, mastocytosis, serum sickness, blistering skin eruption, or acute interstitial nephritis.
Table 1. Baseline Characteristics of the Intention-to-Treat Population.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Intervention group (n = 187) | Control group (n = 190) | |
| Age, median (IQR), y | 52 (36-64) | 50 (35-66) |
| Sex | ||
| Female | 116 (62) | 131 (68) |
| Male | 71 (38) | 59 (31) |
| Race and ethnicity | ||
| White | 174 (93) | 176 (93) |
| Othera | 13 (7) | 14 (7) |
| Recruiting sites | ||
| McGill University Health Centre (Canada) | 67 (36) | 70 (37) |
| Vanderbilt University Medical Center (US) | 14 (8) | 13 (7) |
| Duke University Medical Center (US) | 12 (6) | 12 (6) |
| Austin Health (Australia) | 85 (46) | 87 (46) |
| Peter MacCallum Cancer Centre (Australia) | 7 (4) | 6 (3) |
| The Royal Melbourne Hospital (Australia) | 2 (1) | 2 (1) |
| Atopy | ||
| Asthma | 35 (19) | 49 (26) |
| Allergic rhinitis | 70 (37) | 82 (43) |
| Atopic dermatitis | 24 (13) | 27 (14) |
| Idiopathic urticaria/angioedemab | 8 (4) | 8 (4) |
| History of anaphylaxis (any cause) | 2 (1) | 3 (2) |
| Other antibiotic/antifungal allergy label | 37 (20) | 41 (22) |
| Family history of drug allergy | 13 (7) | 9 (5) |
| Coexisting conditions | ||
| Charlson Comorbidity Index, median (IQR)c | 1 (0-3) | 1 (0-3) |
| Immunocompromisedd | 37 (20) | 45 (24) |
| Medications | ||
| β-Blockers | 15 (8) | 23 (12) |
| Angiotensin-converting enzyme inhibitors | 14 (8) | 15 (8) |
| Immunosuppressive medication | 13 (7) | 15 (8) |
| PEN-FAST score | ||
| 0 | 79 (42) | 73 (38) |
| 1 | 97 (52) | 112 (59) |
| 2 | 11 (6) | 5 (3) |
| Reported allergy labele | ||
| Unspecified penicillin | 146 (78) | 156 (82) |
| Penicillin VK | 3 (2) | 2 (1) |
| Penicillin G | 2 (1) | 6 (3) |
| Amoxicillin | 33 (18) | 20 (11) |
| Ampicillin | 1 (1) | 0 |
| Amoxicillin/clavulanate | 1 (1) | 6 (3) |
| Flucloxacillin | 1 (1) | 0 |
| Concurrent cephalosporin allergy | 4 (2) | 9 (5) |
| Description of penicillin allergy label | ||
| Childhood reaction | 112 (60) | 117 (62) |
| Immediate reaction (<2 h) | 25 (13) | 14 (7) |
| Timing of the index reaction, y | ||
| <1 | 0 | 1 (1) |
| 1-5 | 3 (2) | 1 (1) |
| 6-10 | 8 (4) | 9 (5) |
| 11-15 | 23 (12) | 14 (7) |
| >15 | 147 (79) | 159 (84) |
| Other | 0 | 1 (1) |
| Unknown | 6 (3) | 5 (3) |
| Treatment received for the index reaction | ||
| Any type of treatment | 25 (13) | 26 (14) |
| Oral antihistamines | 19 (10) | 11 (6) |
| Topical corticosteroids | 3 (2) | 7 (4) |
| Systemic corticosteroids | 1 (1) | 2 (1) |
| Intramuscular adrenaline | 0 | 1 (1) |
| Hospitalization | 9 (5) | 13 (7) |
| Antibiotics safely tolerated since the reaction | ||
| Cephalosporins | 47 (25) | 44 (23) |
| Macrolides | 27 (14) | 26 (14) |
| Fluoroquinolones | 12 (6) | 10 (5) |
| Carbapenems | 0 | 2 (1) |
| Glycopeptides | 4 (2) | 1 (1) |
| Aminoglycosides | 3 (2) | 2 (1) |
| Other | 16 (9) | 32 (17) |
| Unknown | 110 (59) | 103 (54) |
The Other category includes African, East Asian, Hispanic or Latino, and Indo-Asian. It is reported together owing to small sample sizes.
Idiopathic urticaria/angioedema is a patient-reported diagnostic, and these patients were not excluded from the trial because the treating clinician considered that the history was inconsistent with idiopathic urticaria.
This score was age adjusted.17
The immunocompromised category includes patients with any of the following conditions: transplant, hematological or oncological cancer in the past 5 years, corticosteroid use of more than 10 mg of prednisolone equivalent per day, connective tissue or autoimmune condition, and AIDS.
A penicillin allergy label was defined as patients reporting an allergy to any of the following drugs: unspecified, penicillin VK, penicillin G, amoxicillin, amoxicillin/clavulanate, ampicillin, flucloxacillin, or dicloxacillin.
Primary Outcome
The primary outcome of a positive oral penicillin challenge consistent with an immune-mediated reaction occurred in 1 of 187 patients (0.5%) in the intervention group and 1 of 190 patients (0.5%) in the control group, with an RD of 0.0084 pp (90% CI, −1.22 to 1.24 pp). Because the upper limit of the 1-sided 95% CI is 1.24 pp, this is below the noninferiority margin of 5 pp. The risk ratio was 1.02 (90% CI, 0.10-10.34). Most of the patients in both groups (>84%) received a single-dose amoxicillin challenge (eTable 1 in Supplement 1). Both patients with positive challenges presented a mild cutaneous skin reaction that resolved following a single dose of antihistamines (eTable 2 in Supplement 1). The intervention had a similar effect when analyzing subgroups of interest; however, the small number of participants and events in each subgroup limited these conclusions (eMethods and eFigure 2 in Supplement 1). Per-protocol analysis of the proportion of positive oral challenges showed similar results (RD, −0.57 pp; 90% CI, −1.50 to 0.36 pp). Sensitivity analysis using exact methods for evaluating confidence intervals showed a similar result (eTable 3 in Supplement 1).
Secondary Outcomes
Feasibility
The study demonstrated feasibility, with 446 of 643 screened patients being eligible to participate (69%; 95% CI, 66%-73%) and 382 of 446 eligible patients being enrolled (86%; 95%, CI 82%-89%). The intervention-to-recruitment ratio is illustrated in the Figure.
Among the 382 randomized patients (190 in the intervention group and 192 in the control group), the intervention as per protocol was completed in 351 participants (92%; 95% CI, 87%-94%). A total of 5 patients were excluded from the analysis due to either missing outcome data or not meeting inclusion criteria.
Adverse Events
In the 5 days following the oral penicillin challenge, 22 cumulative adverse events were recorded in the intervention group (20 participants) and 24 events in the control group (21 participants), with an RD of −0.36 pp (95% CI, −6.64 to 5.93 pp) and a risk ratio of 0.97 (95% CI, 0.54-1.73). There were 9 immune-mediated adverse events recorded in the intervention group and 10 in the control group (RD, −0.45 pp; 95% CI, −4.87 to 3.96 pp). There was no difference between the groups (eTable 4 in Supplement 1).
The overall safety data are summarized in Table 2. The 5-day adverse events occurred after a median (IQR) of 4 (0.67-16.67) hours following the oral penicillin challenge in the intervention group and 6 (2.09-35.10) hours in the control group. Safety events that occurred as part of the primary outcome included immediate diffuse rash or urticaria (<1 hour) in 1 of 187 patients (0.5%) from the intervention group and 1 of 190 patients (0.5%) from the control group. In addition, delayed diffuse rash or urticaria occurring more than 1 hour following the oral penicillin challenge was described in 6 of 187 patients (3.2%) from the intervention group and 3 of 190 patients (1.6%) from the control group (RD, 1.63 pp; 95% CI, −1.46 to 4.72 pp). No serious adverse reactions were reported (Table 2 and eTable 4 in Supplement 1). All reported adverse events were subcategorized into immune or nonimmune mediated and are summarized in eTable 5 in Supplement 1. Treatment for an adverse event was required for 9 events in the intervention group and 4 in the control group, but none led to hospitalization or emergency department presentation (Table 2).
Table 2. Adverse Events Occurring Up to 5 Days After Oral Challenge.
| Variable | Adverse events, No. (%) | |
|---|---|---|
| Intervention group (n = 22) | Control group (n = 24) | |
| Type of adverse event | ||
| An antibiotic-associated adverse event (any nonimmune mediated reaction) | 6 (27) | 2 (8) |
| Nausea/vomiting/diarrhea | 2 (9) | 0 |
| Immediate diffuse rash/urticaria | 2 (9) | 1 (4) |
| Delayed diffuse rash/urticaria (>1 h) | 6 (27) | 3 (12) |
| Other nonsevere adverse events | 6 (27) | 18 (75) |
| Angioedema/laryngeal involvement/respiratory compromise | 0 | 0 |
| Anaphylaxis (or unexplained collapse) | 0 | 0 |
| Death | 0 | 0 |
| Timing | ||
| <1 h | 6 (27) | 5 (21) |
| 1-12 h | 9 (41) | 9 (38) |
| 13-24 h | 3 (14) | 2 (8) |
| 25-48 h | 2 (9) | 4 (17) |
| 2-5 d | 2 (9) | 4 (17) |
| Time since oral challenge, median (IQR), h | 4.1 (0.7-16.7) | 6.9 (2.1-35.1) |
| Severitya | ||
| Grade 1 | 17 (77) | 16 (67) |
| Grade 2 | 5 (23) | 8 (33) |
| Degree of causalityb | ||
| Certain | 2 (9) | 1 (4) |
| Probable/likely | 6 (27) | 4 (17) |
| Possible | 7 (32) | 5 (21) |
| Unlikely | 6 (27) | 14 (58) |
| Unassessable/unclassifiable | 1 (5) | 0 |
| Management | ||
| None | 13 (59) | 16 (67) |
| Rechallenge | 0 | 1 (4) |
| Withdrew from study | 0 | 0 |
| Otherc | 0 | 2 (8) |
| Drug therapy | ||
| Oral antihistamine | 9 (41) | 6 (25) |
| Otherd | 2 (9) | 5 (21) |
| Emergency department referral | 0 | 0 |
| Intensive care unit referral | 0 | 0 |
| Calculated duration of adverse event, median (IQR), h | 11 (1.2-48.3) | 60 (2-76) |
| Recovered/resolved | 22 (100) | 20 (83)e |
Grade 1: asymptomatic or mild symptoms from clinical or diagnostic observations only with no intervention indicated; grade 2: moderate symptoms limiting age-appropriate instrumental activities of daily living with minimal, local, or noninvasive intervention indicated.
Certain: an event with plausible time relationship to drug intake that cannot be explained by disease or other drugs and is definitive pharmacologically or phenomenologically (ie, an objective and specific medical disorder or a recognized pharmacological phenomenon); probable/likely: an event with reasonable time relationship to drug intake that is unlikely to be attributed to disease or other drugs and response to withdrawal is clinically reasonable; possible: an event with reasonable time relationship to drug intake that could also be explained by disease or other drugs and information on drug withdrawal may be lacking or unclear; unlikely: an event with a time to drug intake that makes a relationship improbable (but not impossible) and disease or other drugs provide plausible explanations; and unassessable/unclassifiable: a report suggesting an adverse reaction that cannot be judged because the information is insufficient or contradictory and data cannot be supplemented or verified.
Other management included the use of an intranasal corticosteroid spray (n = 1) and scheduled return to clinic for repeat skin testing and delayed intradermal reads and prolonged oral challenges (n = 1).
Other administered treatments included (1) a combination of acetylsalicylic acid, butalbital, and caffeine for headache, and (2) loperamide in the intervention group, as well as (1) intranasal corticosteroid spray, (2) intravaginal clotrimazole cream, and (3) oral paracetamol/acetaminophen for 3 patients in the control group.
Details regarding the outcomes of the adverse events in the control group: (1) 1 patient presented with a headache and was under treatment with acetaminophen during the assessment; (2) 1 patient presented with Candida vulvovaginitis and received intravaginal clotrimazole for 7 days, though despite resolution of her initial symptoms she had recurrent symptoms 3 weeks later, and it was considered that she recovered with sequelae; (3) 1 patient reported nasal irritation and also noticed recurrent symptoms following the patient’s chemotherapy cycle, and it was considered that the reported symptoms were related to the chemotherapy and not the penicillin challenge; and (4) 1 patient developed a single blister (<1 cm) on the chest that had decreased but was still present at the 5-day assessment. The rest of the patients in control arm (n = 20 [83%]) and all of the patients in the intervention arm (n = 22 [100%]) recovered from their adverse event.
Efficacy
Skin testing was performed in 189 of 190 patients (99.5%) in the control group; 1 patient had an absent response to the histamine-positive control, and intradermal testing was not completed. This patient had a subsequent negative oral challenge (protocol violation) and did not report any adverse reactions. Therefore, this patient was included in the overall but not per-protocol analysis. Four of 190 patients (2.1%) in the control arm had positive intradermal testing results and were excluded from the oral challenge. This included 2 for penicillin G and 1 each for benzylpenicilloyl polylysine and ampicillin. One patient with clinician-diagnosed dermatographism had uninterpretable intradermal skin results due to testing positive to all reagents, including the negative sodium chloride control, and did not proceed to oral challenge.
The allergy label was removed in 186 of 187 participants (99.5%) in the intervention group and 186 of 190 (97.9%) in the control group (RD, 1.57 pp; 95% CI, −0.72 to 3.86 pp). Because 1 patient in both the control and intervention groups reached the primary outcome of a positive oral challenge, the difference in the efficacy of delabeling in the intervention group was explained by 4 patients in the control group who were excluded from the oral challenge due to positive intradermal skin tests. Most patients required a single clinic appointment for their allergy label removal (184 of 186 [98.9%] in both groups). Although the maximum observation and testing time spent in an individual clinic was not controlled by study protocol or procedures, the median (IQR) time from randomization to delabeling was shorter in the intervention group (1.80 [1.33-3.72] hours) compared with the control group (2.28 [1.72-5.48] hours), with a median difference of −0.45 (95% −0.65 to −0.27) hours (eTable 6 in Supplement 1).
Discussion
In this international, multicenter, randomized clinical trial that enrolled participants with low-risk penicillin allergy (PEN-FAST score <3), direct oral penicillin challenge was noninferior to the current standard of prick followed by intradermal skin testing followed, if negative, by 1-step oral challenge based on the primary end point of physician-observed positive immune-mediated penicillin challenge. There was also no difference between immediate or delayed adverse events reported by day 5.
The PEN-FAST tool was previously externally validated in a mixed prospective derivation and a retrospective validation cohort of patients tested for penicillin allergy from Australia and the US and in 2 additional adult populations.13,14,18 This randomized clinical trial now provides evidence that direct oral challenge is an effective and safe method of assessing low-risk penicillin allergies (PEN-FAST score <3) in a predominately White, adult, outpatient population. This supports the work of a previously published explorative single-center randomized clinical trial in North American of 2-step direct oral challenge.11 The present study demonstrated that 2-stage skin testing preceding oral challenge with penicillin provided no additional safety benefit in a well-defined low-risk cohort. The direct oral challenge was associated with a shorter time in the clinic and may have avoided minor skin reactions associated with positive skin testing. Fewer patients were delabeled of their penicillin allergy in the control group (97.9%) vs the intervention group (99.5%), which is related to 4 additional patients in the control group having positive results with intradermal testing (not statistically significant). When the pretest probability of a test is low such as in low-risk penicillin allergy, positive skin tests may represent false positives, and this may lead to unnecessary avoidance of penicillin.19 Of note, 2 patients in this study underwent oral challenge following a positive skin test (protocol violation), with both tolerating the oral challenge dose without complication. Previous data suggest that the positive predictive value of penicillin skin testing is 50% to 75%, which remains a disadvantage of skin testing in a low-risk population. In the US alone, this could lead to more than 100 000 patients labeled as penicillin allergic, based on a false-positive penicillin skin test, who would tolerate penicillin antibiotics and thus highlights that direct oral challenge without preceding skin testing could have an efficacy advantage.
Specialized allergy skin testing is a labor-intensive, expensive, and resource-rich intervention not currently available to large populations of high-income and low- and middle-income countries.20,21 Furthermore, a penicillin allergy label cannot be removed based on skin testing alone, and the oral challenge remains the gold standard. PEN-FAST is a tool to facilitate the identification of low-risk patients labeled as penicillin allergic. A recent survey from South African hospitals demonstrated that PEN-FAST could also be deployed in a low- and middle-income setting; 65% of hospital-admitted patients presented with a low-risk allergy, making them eligible for a direct penicillin oral challenge.21 The PALACE trial is an important strategy to improve global access to care and allow safe, fast, and cheap penicillin delabeling strategies to address this important public health issue. The PALACE trial demonstrates that PEN-FAST is now an internationally validated clinical decision rule that can accurately risk assess penicillin allergy and that 2-stage skin testing followed by oral challenge no longer needs to be the standard of care in low-risk phenotypes.
The World Health Organization has acknowledged the role of antibiotic allergy assessments as a cornerstone intervention of antimicrobial stewardship programs.22 The Infectious Diseases Society of America has identified penicillin allergy testing as a tool to benefit antimicrobial stewardship that requires more evidence for optimal use and scalability.23 The American Academy of Allergy, Asthma, and Immunology Drug Allergy Practice Parameter Update of 2022 recommends proactive penicillin allergy delabeling.10 Although recently published drug allergy practice parameters endorse consideration for a direct oral challenge to penicillin in adults with low-risk penicillin allergy histories, this only carries a conditional recommendation due to low-quality evidence.10 To our knowledge, the present randomized clinical trial provides for the first time a high level of evidence to support the safety and efficacy of direct oral penicillin challenge that will enable globally the implementation and simplification of penicillin delabeling practices in settings that do not require specialized testing.
Limitations
We acknowledge several limitations of this study. The PEN-FAST score was 0 or 1 for more than 94% of participants enrolled, limiting the generalizability of this study to those with a PEN-FAST score of less than 2. Given the PEN-FAST scoring, a similar distribution of lower scores is observed in other outpatient clinics.13 Furthermore, patients with a history of anaphylaxis with any drug were excluded from this study. Indeed, anaphylaxis requiring treatment represents a PEN-FAST score of 3 (main exclusion criteria among 82 of 197 patients). While this can be considered a limitation, patients with anaphylaxis who do not require treatment should be offered a direct challenge, but this is rarely the case in clinical practice. The observed rate of the primary outcome was lower than assumed in the power calculations. However, a lower rate would result in a smaller required sample size (or, alternatively, would increase the power of the study). Similarly, deviations from other assumptions in sample-size calculations (namely, 2-sided confidence intervals) do not affect the conclusions of this trial (the 2-sided 95% CI of −1.46 to 1.48 pp remains well below the noninferiority margin of 5 pp). While an absolute noninferiority margin of 5 pp represents a more than 10 times higher rate of an adverse event when the rate of outcome is 0.5%, we believe that this is still clinically acceptable considering the very low rate of the outcome, clinical nonseverity of the outcome, the effect of allergy label removal, and resources, cost, and time required for skin testing. Because the study was open label and, by design, patients in the intervention group would not have skin testing performed, the only patients presenting adverse reactions related to the skin testing would be in the control group. Although the open-label nature of the trial could have affected the assessment of the primary outcome, the 2 reviewers who classified the adverse events (immune and nonimmune mediated) were blinded to group assignments.
Conclusions
In this randomized clinical trial, in patients with low-risk penicillin allergy and a PEN-FAST score of less than 3, with most patients having a PEN-FAST score of 0 or 1, a direct oral challenge with penicillin was a safe and effective alternative to the current standard, which includes 2-stage skin testing followed by an oral challenge. Compared with skin testing, a direct oral penicillin challenge is less resource and time intensive, is less expensive, and has the potential to be performed outside of the specialist allergy setting, providing a scalable approach to address low-risk, unverified penicillin allergy in diverse treatment settings internationally.
eMethods
eFigure 1. The PEN-FAST clinical decision rule
eFigure 2. Primary outcome, secondary outcomes and subgroup analysis of the primary outcome
eTable 1. Skin testing drug allergy concentrations and type of penicillin and doses used in the trial
eTable 2. Characteristics of patients with positive primary outcomes
eTable 3. Sensitivity analysis using exact methods for confidence intervals
eTable 4. Safety outcomes in the first 2 days and 5 days following oral penicillin challenge
eTable 5. Safety outcome inclusions
eTable 6. Efficacy outcomes
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA; National Antimicrobial Prescribing Survey (NAPS) . Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother. 2016;71(6):1715-1722. doi: 10.1093/jac/dkw008 [DOI] [PubMed] [Google Scholar]
- 2.MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis. 2016;63(7):904-910. doi: 10.1093/cid/ciw462 [DOI] [PubMed] [Google Scholar]
- 3.Trubiano JA, Pai Mangalore R, Baey YW, et al. Old but not forgotten: antibiotic allergies in general medicine (the AGM study). Med J Aust. 2016;204(7):273. doi: 10.5694/mja15.01329 [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ. 2018;361:k2400. doi: 10.1136/bmj.k2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran R, Devchand M, Smibert O, Trubiano JA. Antibiotic allergy labels in hospitalized and critically ill adults: a review of current impacts of inaccurate labelling. Br J Clin Pharmacol. 2019;85(3):492-500. doi: 10.1111/bcp.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi: 10.1016/S0140-6736(18)32218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antimicrobial stewardship interventions: a practice guide. World Health Organization . 2021. Accessed June 10, 2023. https://apps.who.int/iris/bitstream/handle/10665/340709/9789289054980-eng.pdf
- 8.Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? a review of antibiotic allergy and pathways to ‘de-labeling’. Curr Opin Infect Dis. 2013;26(6):526-537. doi: 10.1097/QCO.0000000000000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose MT, Slavin M, Trubiano J. The democratization of de-labeling: a review of direct oral challenge in adults with low-risk penicillin allergy. Expert Rev Anti Infect Ther. 2020;18(11):1143-1153. doi: 10.1080/14787210.2020.1792775 [DOI] [PubMed] [Google Scholar]
- 10.Khan DA, Banerji A, Blumenthal KG, et al. ; Chief Editor(s); Workgroup Contributors; Joint Task Force on Practice Parameters Reviewers . Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333-1393. doi: 10.1016/j.jaci.2022.08.028 [DOI] [PubMed] [Google Scholar]
- 11.Mustafa SS, Conn K, Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract. 2019;7(7):2163-2170. doi: 10.1016/j.jaip.2019.05.037 [DOI] [PubMed] [Google Scholar]
- 12.Chua KYL, Vogrin S, Bury S, et al. The penicillin allergy delabeling program: a multicenter whole-of-hospital health services intervention and comparative effectiveness study. Clin Infect Dis. 2021;73(3):487-496. doi: 10.1093/cid/ciaa653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745-752. doi: 10.1001/jamainternmed.2020.0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Ann Allergy Asthma Immunol. 2022;128(1):27-32. doi: 10.1016/j.anai.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Copaescu AM, James F, Vogrin S, et al. Use of a penicillin allergy clinical decision rule to enable direct oral penicillin provocation: an international multicentre randomised control trial in an adult population (PALACE): study protocol. BMJ Open. 2022;12(8):e063784. doi: 10.1136/bmjopen-2022-063784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics. 1999;55(4):1202-1209. doi: 10.1111/j.0006-341X.1999.01202.x [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Mak R, Yuan Zhang B, Paquette V, et al. Safety of direct oral challenge to amoxicillin in pregnant patients at a Canadian tertiary hospital. J Allergy Clin Immunol Pract. 2022;10(7):1919-1921.e1. doi: 10.1016/j.jaip.2022.03.025 [DOI] [PubMed] [Google Scholar]
- 19.Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381(24):2338-2351. doi: 10.1056/NEJMra1807761 [DOI] [PubMed] [Google Scholar]
- 20.Trubiano JA, Beekmann SE, Worth LJ, et al. Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the Emerging Infections Network. Open Forum Infect Dis. 2016;3(3):ofw153. doi: 10.1093/ofid/ofw153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day C, Mendelson M, Peter J; ADvISE study group . Low self-reported penicillin allergy in South Africa—implications for global public health response. JAC Antimicrob Resist. 2023;5(1):dlad015. doi: 10.1093/jacamr/dlad015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. World Health Organization . 2019. Accessed June 10, 2023. https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf [DOI] [PMC free article] [PubMed]
- 23.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi: 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. The PEN-FAST clinical decision rule
eFigure 2. Primary outcome, secondary outcomes and subgroup analysis of the primary outcome
eTable 1. Skin testing drug allergy concentrations and type of penicillin and doses used in the trial
eTable 2. Characteristics of patients with positive primary outcomes
eTable 3. Sensitivity analysis using exact methods for confidence intervals
eTable 4. Safety outcomes in the first 2 days and 5 days following oral penicillin challenge
eTable 5. Safety outcome inclusions
eTable 6. Efficacy outcomes
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement