Abstract
Purpose
To study the efficacy of bupivacaine liposome injectable suspension in prolonging sensory blocks of the median and ulnar nerves for subjects with Dupuytren contracture release by collagenase injection. We hypothesized that combining liposome bupivacaine and bupivacaine hydrochloride would extend the duration of blocks without added complications.
Methods
We randomized 32 subjects scheduled for Dupuytren contracture release with collagenase Clostridium histolyticum injections to receive forearm blocks of the median and ulnar nerves with a mixture of 5 mL liposome bupivacaine 1.33% plus 2.5 mL bupivacaine hydrochloride 0.5% per nerve (n = 16) or 7.5 mL bupivacaine hydrochloride 0.5% alone per nerve (n = 16). Sensory block and analgesia were assessed through the first posttreatment week.
Results
Sensory block was nearly 4 times longer in subjects who received the liposome bupivacaine mixture compared with subjects who received bupivacaine hydrochloride alone. Most subjects (13 of 16) who received the liposome bupivacaine mixture had adequate analgesia for finger manipulation to rupture the cords, whereas most subjects (15 of 16) who received bupivacaine hydrochloride alone required additional anesthesia. Subjects in the liposome mixture group reported lower pain scores through the first 3 days after treatment. There were no serious side effects.
Conclusions
Addition of liposome bupivacaine to forearm blocks for Dupuytren contracture release prolonged sensory block and improved pain scores without increasing side effects or impairing hand function. Supplemental lidocaine injections for the painful phases of Dupuytren contracture release with collagenase C histolyticum injections were not required by most subjects who received liposome bupivacaine.
Type of study/level of evidence
Therapeutic I.
Key words: bupivacaine liposome injectable suspension, collagenase Clostridium histolyticum, Dupuytren contracture release, forearm block
Dupuytren contracture is a common disease of the connective tissue of the hand in which the formation of subcutaneous nodules and cords causes disabling flexion contracture of one or more fingers.1,2
The cords of these contractures can be enzymatically weakened after injection of collagenase Clostridium histolyticum (CCH), allowing the fingers to be manipulated to break up the cords.3
Pain is caused by multiple injections of collagenase into the cords (phase 1 of treatment), during manipulation of the fingers to break up the cords (phase 2, 24 to 72 hours after injection), and from the inflammatory response, which can last several days.4 Ideal analgesia would provide adequate pain relief for all phases of treatment (Fig. 1). Although forearm (median and ulnar) nerve blocks can provide adequate analgesia for phase 1 of treatment, they have a relatively short duration.5,6
Figure 1.
Phases of treatment in Dupuytren contracture release with CCH injections.
A recent study4 reported that 43% of patients had severe pain during finger manipulation even when analgesia was provided with a wrist block (10 mL of 2% mepivacaine); 53% of patients had pain after injection of collagenase despite treatment with an oral analgesic combination of acetaminophen 650 mg, ibuprofen 600 mg, or metamizole 575 mg every 8 hours. Bupivacaine liposome injectable suspension (EXPAREL, Pacira Pharmaceuticals, Inc, Parsippany, NJ) is an extended-release formulation of a local anesthetic that has been approved by the Food and Drug Administration for infiltration into the surgical wound, and recently (April, 2018) for use in interscalene brachial plexus blocks. Although the efficacy of liposome bupivacaine for postoperative pain has been studied with respect to soft tissue infiltration,7 it has not been examined in the median and ulnar nerve blocks of the forearm. Thus, although local blocks can be used for CCH treatment of Dupuytren contracture, we chose forearm nerve blocks to study the effects of liposome bupivacaine in the small peripheral nerves.
The objective of this study was to evaluate the analgesic benefit of liposome bupivacaine in prolonging forearm blocks of the median and ulnar nerves and analgesia for subjects with Dupuytren contracture release with injections of CCH into the affected cords. We primarily tested the hypothesis that adding liposome bupivacaine in forearm blocks would sufficiently prolong the sensory block to provide adequate pain relief for Dupuytren contracture release with CCH. We secondarily tested the hypothesis that adding liposome bupivacaine in forearm blocks would improve pain scores in the first posttreatment week.
Materials and Methods
This double-blind, randomized, controlled trial was approved by the Ethics Committee of Ziekenhuis Oost-Limburg, Genk, Belgium (16/011), and by FAGG, the Belgian federal agency for medicines and health products (2016-001656-22). The trial was registered at www.clinicaltrials.gov (NCT03106519) in March, 2017. Patients scheduled for Dupuytren contracture release who were aged 18 to 85 years, had an American Society of Anesthesiologists physical status of I to III, and were able to understand the purpose and risks of the study were eligible to participate. Patients were excluded if they were pregnant, had a history of allergic or adverse reaction to local anesthetics, used pain medications within 28 hours before treatment, had a suspected or known recent history (less than 3 months) of drug or alcohol abuse, had an infection at the planned block site, had a body mass index greater than 44 kg/m2, or had any chronic condition or psychiatric disorder that could compromise neurological or study assessments.
Consenting subjects were randomized in a computer-generated 1:1 ratio to receive a mixture of 5 mL bupivacaine liposome injectable suspension 1.33% plus 2.5 mL bupivacaine HCl 0.5% or 7.5 mL bupivacaine HCl 0.5% alone to the median and ulnar nerves before CCH injection (Xiapex, Swedish Orphan Biovitrum, AB, Stockholm, Sweden). Each subject received a total drug volume of 15 mL (7.5 mL/nerve). The traditional bupivacaine HCl used in common clinical practice was mixed with the extended-release formulation of liposome bupivacaine (EXPAREL). This model has been used to facilitate early interscalene brachial plexus block onset and prolong block duration.
After treatment, all subjects received the standardized multimodal regimen for pain control with acetaminophen 1 g every 6 hours and diclofenac 75 mg 2 times daily. Transmucosal tramadol 50 mg (every 6 hours as needed) was used for breakthrough pain. In addition, subjects received rescue medication upon request for breakthrough pain or as necessary upon discharge home.
Anesthesiology staff members performing the nerve blocks were not blinded to drug treatment but did not participate in subject assessments. Surgeons and research personnel were blinded to drug treatment because blocks were performed in a separate procedure room outside the operating theater. Subjects were not told their allocated treatment. A strict blind was maintained so that the surgeon (who injected CCH and then manipulated the fingers) and the research personnel (who conducted the follow-up assessments of the subjects) would not know the assigned drug arms. The use of supplementary local anesthesia was based on subject request for pain relief and not the surgeon’s perception of need. Subjects left the hospital facility on the same day of the CCH injection.
Nerve blocks
Study medication (bupivacaine liposome injectable suspension mixed with bupivacaine HCl or bupivacaine HCl alone) was administered at least 30 minutes before CCH injection and was deposited in the tissue plane around the median and ulnar nerves at the level of the mid-forearm. All blocks were administered under ultrasound guidance. The injectate was deemed adequately distributed when it encircled the nerve, as documented by ultrasound.
The forearm blocks were the sole anesthetic modality for the CCH injections and were administered without premedication. We chose to use a more proximal approach to median and ulnar nerve blocks at the level of the forearm because a more distal approach (wrist blocks) failed to provide analgesia during finger manipulation in 43% of patients in a study by Sanjuan-Cerveró et al.4
Time to onset and offset of sensory blockade was tested in 5-minute intervals from injection of the study medication up to 30 minutes, and hourly thereafter until discharge. Sensory block onset was defined as time from end of each block procedure to no sensation in the median and ulnar nerve distributions. Sensory block duration was defined as time from block onset to time to return of complete sensation. Block success or failure was defined as absence or presence of full sensation in the areas and muscles supplied by the median and ulnar nerves as assessed by pinprick.8 Inadequate analgesia for phase 2 (finger manipulation to break up the cords) was defined as the need for additional injections of lidocaine 1% into the affected tissues of the hand to allow for effective manipulation and cord breakage.
Vital signs (blood pressure, heart rate, and oxygen saturation) were monitored every 3 minutes for the first 30 minutes after the block, every 5 minutes up to 1 hour after the block, and every 15 minutes until discharge. We obtained 12-lead electrocardiograms before block and approximately 2 hours after nerve blocks.
Posttreatment assessments
Worst pain (modified Brief Pain Inventory) was reported as a numeric rating scale (NRS) score ranging from 0 (no pain) to 10 (most extreme pain).9 The NRS was recorded before and after block.
Before discharge home, subjects were given clear instructions regarding scheduling and questions that would be asked during the posttreatment telephone interviews. Specifically, subjects were trained to assess sensation and distinguish among complete sensation, light touch, and no sensation. They were also trained to assess the presence or absence of weakness in the blocked hand. Subjects were given a daily diary in which to record the NRS score before taking each dose of rescue medication (tramadol). The blinded research staff used standardized scripts to collect pain scores via phone interviews at 24 hours (D1 am), 36 hours (D1 pm), 48 hours (D2 am), 60 hours (D2 pm), 72 hours (D3 am), and 84 hours (D3 pm), and at D4, D5, D6, and D7. The worst pain was asked as question 1 from the modified Brief Pain Inventory: “Please rate your pain at its worst in the last 24 hours from 0 (no pain) to 10 (pain as bad as you can imagine).” This item, based on the NRS, was already familiar to subjects because it was asked during screening and in phase 1 of the study. The phone interview inquired about numbness and weakness or dysesthesias.
Polystyrene foam cups were given to subjects to take home upon discharge; subjects were instructed that use of these cups referred to use with the operated hand. The functionality of the hand was assessed by asking subjects, “Are you able to use a polystyrene foam cup?” Training in motor assessments using the polystyrene foam cup was done before the research intervention and repeated before discharge home. In addition, we assessed the presence or absence of the sensory and motor block by asking the patient to report numbness and/or weakness in the operated hand every 12 hours. At the 48-hour visit, the presence or absence of sensory block and hand weakness of the intrinsic muscles were evaluated, as well as the ability to use the polystyrene foam cup.
Subjects returned for finger manipulation and cord rupture 48 hours after the CCH injections. Lidocaine 1% was injected if the subject reported pain during manipulation of the fingers.
Side effects were recorded through day 7, including nausea, vomiting, fever, constipation, severe itching of the skin, dizziness, sleepless nights, excessive sweating, urinary retention, headache, and heart palpitations.
Statistical analysis
The sample size calculation, estimated on duration of sensory block, assuming a minimum difference important to detect at 48 hours (SD, 32 hours), α = .01, and power of .90, yielded 15 subjects/group. This was increased to 16 subjects/group to accommodate block failures and losses to follow-up. If bupivacaine liposome injectable suspension is capable of prolonging nerve block, its pharmacokinetic data suggest that the formulation should have a conduction block at least 48 hours longer than non-encapsulated bupivacaine HCl, the active component of liposome bupivacaine.10 We selected the SD of 32 hours because there is substantial variability in duration of nerve blocks with the currently available local anesthetics. Because liposome bupivacaine is an encapsulated formulation that releases an active substance, over 72 hours, an even wider variability in block duration might be anticipated.
Continuous variables are presented as means (SD) and categorical (nominal and ordinal) variables as n (%) or as a ratio as in gender and treated limb laterality. Duration of sensory and motor block was compared between groups by Student t test or Mann-Whitney U test, as appropriate.
The proportions of subjects requiring additional anesthesia for phase 2 of treatment were compared between groups by chi-square test.
Efficacy analyses followed intent-to-treat principles. Worst pain was analyzed by generalized estimating equations (GEE) to examine group differences over time. The GEE method is flexible with respect to the type of outcome variable (including possibly skewed continuous distributions and ordinal measures) and to observations that are unequally spaced over time. For instance, worst pain is reported at discharge (D0 pm), at D1 am and pm, D2 am and pm, and D3 am and pm, and then on D4, D5, D6, and D7. In the GEE analyses, a conservative unstructured correlation structure was used so as not to assume the relative magnitude of correlation between any 2 pairs of observations (although GEE is robust against choosing an incorrect correlation structure). Link function was identity for these continuous outcome measures.
Tests of differences between groups for reported side effects were not planned, because the number of each side effect was anticipated to be small, and subjects could report more than one side effect. Instead, the relative risk (with 95% confidence interval) for at least one side effect through day 7 was reported.
P < .05 was deemed statistically different.
Results
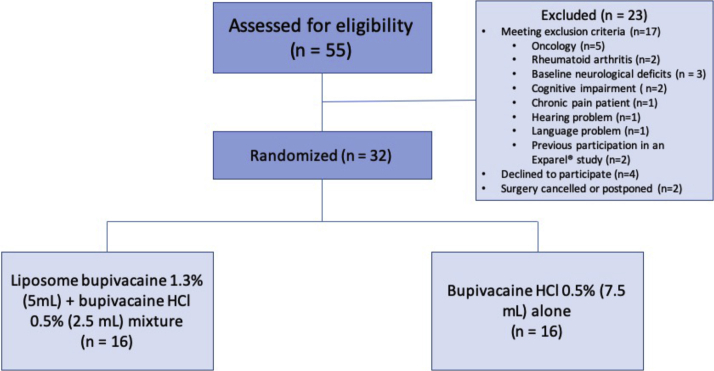
We assessed 55 patients for eligibility; 32 were randomly assigned (16/group) (Fig. 2). The groups did not differ in sociodemographic characteristics or severity of disease (Table 1).
Figure 2.
Consolidated Standards of Reporting Trials diagram for 2-arm study of bupivacaine liposome injectable suspension mixed with bupivacaine HCl or bupivacaine HCl alone.
Table 1.
Sociodemographic Characteristics and Clinical Features of 32 Patients Undergoing Forearm Blocks of Median and Ulnar Nerves for Dupuytren Contracture Release
| Demographics, pain, and arm functionality before surgery | Mixture of 5 mL Liposome Bupivacaine 1.3% Plus 2.5 mL Standard Bupivacaine 0.5% (per Median and Ulnar Nerve) (n =16) | 7.5 mL Standard Bupivacaine 0.5% per Median and Ulnar Nerve) (n = 16) |
|---|---|---|
| Gender (M : F) | 14 : 2 | 14 : 2 |
| Age, y (range) | 66.2 (48–76) | 63.5 (35–82) |
| Body mass index, kg/m2∗ | 25.7 (0.05) | 26.5 (0.04) |
| Race (%) | ||
| American Indian/Alaska native | 0 | 0 |
| Asian | 0 | 0 |
| Black/African American | 0 | 0 |
| Native Hawaiian/Pacific Islander | 0 | 0 |
| White | 16 (100) | 16 (100) |
| Other | 0 | 0 |
| American Society of Anesthesiologists physical status (%) | ||
| I | 4 (25) | 7 (44) |
| II | 10 (63) | 8 (50) |
| III | 2 (12) | 1 (6) |
| Surgical side (R : L)† | 3 : 13 | 8 : 8 |
| Functionality of surgical hand (before surgery) (%) | ||
| Unable to use | 0 | 0 |
| Only light activity | 0 | 0 |
| Able to do some activities | 0 | 0 |
| Able to do most activities | 0 | 1 (6) |
| Slight restrictions only | 8 (50) | 6 (38) |
| Normal | 8 (50) | 9 (56) |
| NRS (0–10) | ||
| At rest | 0.6 (2.0) | 0.2 (1.0) |
| During movement | 1.5 (2.4) | 0.4 (0.9) |
Data are shown as means (SD) or mean (range) for continuous variables and n (%), ratio, or median (range) for discrete (nominal, ordinal) variables.
Body mass index was missing for one subject (active comparator group).
Pearson chi-square (2-sided) P = .063; Fisher exact (2-sided) P = .14.
Duration of sensory block was significantly longer in the liposome bupivacaine mixture group compared with the bupivacaine HCl–alone group (3.8 [1.1] vs 1.0 [0.3] days, respectively; P < .001). Additional anesthetic intervention to complete phase 2 of treatment was required by 15 of 16 subjects (94%) who received bupivacaine HCl alone, compared with 3 of the 16 subjects (20%) who received the liposome bupivacaine mixture (P < .001). Hence, no additional anesthesia was required in 80% of subjects who received the liposome bupivacaine mixture.
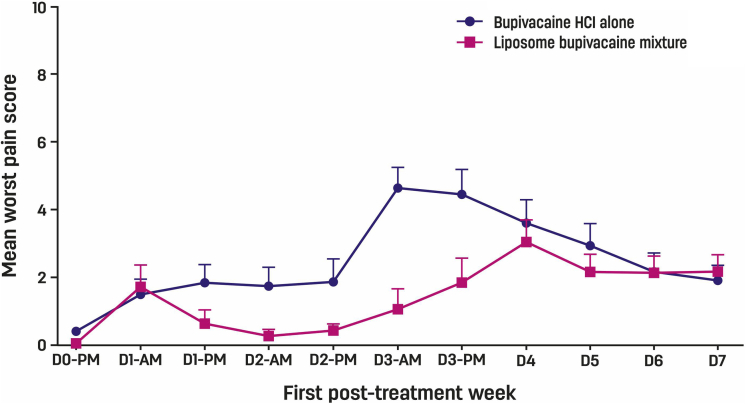
Previous pharmacokinetic analyses in femoral nerve block demonstrated that the peak concentration of liposome bupivacaine occurs during the first 72-h interval.10,11 In the current study, subject-reported worst pain (modified Brief Pain Inventory question 1) over the first 3 posttreatment days was analyzed. Generalized estimating equations (GEE) showed that pain over this interval was significantly lower in subjects who received the liposome bupivacaine mixture compared with those who received bupivacaine HCl alone (GEE P = .010) (Fig. 3).
Figure 3.
Mean worst (NRS) pain scores with 95% confidence interval from discharge after phase 2 through first posttreatment week in 32 subjects undergoing forearm blocks for Dupuytren contracture release (GEE P = .01 over the first 3 days).
All subjects underwent a multimodal oral pain regimen throughout the study period. Three subjects took tramadol through day 7 for breakthrough pain: 2 in the liposome bupivacaine mixture group (total dose of 400 and 150 mg, respectively) and one in the bupivacaine HCl–alone group (100 mg).
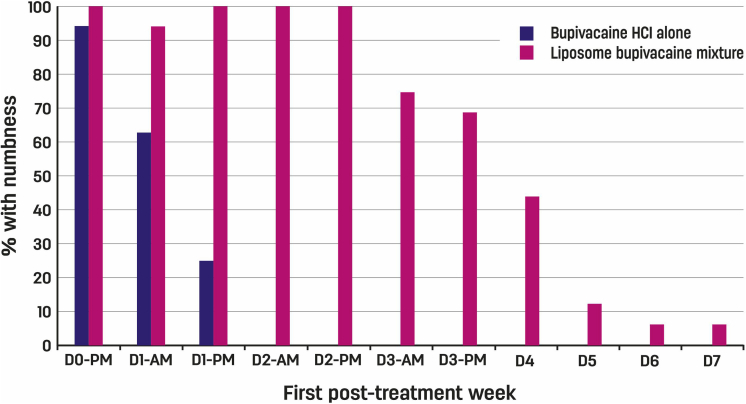
Among subjects who received bupivacaine HCl alone, the proportion who reported numbness decreased rapidly within the first 48 hours. In contrast, numbness appeared to persist through days 3 and 4 in at least 7 subjects (greater than 40%) who received the liposome bupivacaine mixture (GEE P = .008) (Fig. 4). Among subjects who received bupivacaine HCl alone, no weakness was reported within 24 hours after injection. In contrast, subjects who received the liposome bupivacaine mixture experienced weakness, which subsided by the end of the fourth treatment day (GEE P = .03). Assessment of subjects’ sensorimotor block and ability to adduct and abduct the fingers (intrinsic hand muscles) by the research staff just before finger manipulation at 48 hours revealed that no subject who received bupivacaine HCl alone had residual sensory or motor block. In contrast, all subjects who received liposome bupivacaine had some degree of both sensory and motor block and weakness in adduction-abduction of the fingers (P < .001). This indicated that the duration of the blockade with liposome bupivacaine mixture exceeded that of bupivacaine HCl alone. Prolonged numbness and subjectively reported weakness did not appear to affect hand function, as assessed by subjects’ ability to use a polystyrene foam cup throughout the first posttreatment week.
Figure 4.
Sensory block (numbness) from discharge after phase 2 through first posttreatment week in 32 subjects undergoing forearm blocks for Dupuytren contracture release. Among subjects who received bupivacaine HCl alone, the percentage who reported numbness decreased rapidly within the first 48 hours; in contrast, numbness appeared to persist through days 3 and 4 in at least 40% of subjects who received the liposome bupivacaine mixture (GEE P = .008).
Skin tears frequently occur during finger manipulation for Dupuytren contracture release. In this study, almost all subjects had skin tears that were successfully treated by bandaging the hand after the cords were released. No subject reported symptoms consistent with local anesthetic systemic toxicity, including bradycardia, hypotension, arrhythmia, and seizure. There were no differences in occurrence of reported side effects between treatment arms (relative risk for at least one side effect = 1.33; 95% confidence interval, 0.35–5.03) (Table 2).
Table 2.
Frequency of Side Effects Among 32 subjects Undergoing Forearm Blocks of Median and Ulnar Nerves for Dupuytren Contracture Release
| Side Effect | Mixture of 5 mL Liposome Bupivacaine 1.3% Plus 2.5 mL Bupivacaine HCl 0.5% (per Median and Ulnar Nerve) (n = 16) | 7.5 mL Bupivacaine HCl 0.5% per Median and Ulnar Nerve) (n = 16) |
|---|---|---|
| None | 12 (75) | 13 (81) |
| Unique subjects with side effects, n | 4 (25) | 3 (19) |
| Total side effects, n∗ | 6 (38) | 5 (31) |
| Specific side effect∗ | ||
| Bleeding wound | 1 (6) | 0 |
| Dizziness | 0 | 1 (6) |
| Headache | 2 (12) | 1 (6) |
| Itching of skin | 1 (6) | 1 (6) |
| Nausea | 0 | 1 (6) |
| Sleepless night | 2† (12) | 1 (6) |
Data are shown as n (%). Percentages of specific side effects are based on n = 16 in each group. Relative risk for at least one side effect = 1.33 (95% confidence interval, 0.35–5.03).
Percentages for total number of side effects and specific side effects do not sum to 100% because some subjects reported more than one side effect.
Sleepless nights in the same subject on 2 separate dates.
Discussion
The addition of liposome bupivacaine 1.33% to bupivacaine HCl 0.5% in median and ulnar nerve blocks prolonged the duration of sensory block and analgesia compared with bupivacaine HCl alone in subjects with Dupuytren contracture release with injections of CCH. Injections of collagenase resulted in inflammatory response, ecchymosis, and swelling of the treated hand in all research subjects, requiring adequate analgesia. As opposed to subjects treated with the liposome bupivacaine mixture, nearly all in the active comparator group required lidocaine injections for additional analgesia. Overall, pain intensity was relatively low in both groups, probably because all subjects received multimodal analgesia. Regardless, worst pain in the first 72 hours was lower among subjects who received the liposome bupivacaine mixture than in those who received bupivacaine HCl alone. This extended analgesic effect likely resulted from the prolongation of sensory blockade by sustained release of free bupivacaine released from liposomes and was longer by approximately threefold compared with bupivacaine HCl alone (3.8 vs 1.1 days, respectively; P < .001). The prolonged sensory block did not prevent any subject from using a polystyrene foam cup.
Liposome bupivacaine is a novel formulation of bupivacaine HCl, and no dosing recommendations or dose–response studies in the distal peripheral nerves of the upper extremity were available from the literature. Nonetheless, limited dosing information is available from several studies in which the drug was used for the brachial plexus block and the larger nerve blocks. For instance, injection of 133 mg (10 mL) of liposome bupivacaine resulted in successful femoral block.12 Moreover, 5 mL of 0.25% bupivacaine HCl immediately followed by 10 mL of liposome bupivacaine 133 mg in the interscalene brachial plexus block prolonged sensory block and analgesia with the same volume (15 mL) of bupivacaine 0.25%.6 Because the median and ulnar nerve surface areas to be blocked in the current study are 30% to 50% that of the femoral nerve, we empirically chose to mix 5 mL of liposome bupivacaine and 2.5 mL of bupivacaine HCl 0.5%.13,14 We relied on the relative difference in anatomical size between the femoral nerve and the smaller peripheral nerves to approximate the dosing for the current study. Although this may not be an ideal dose or mixture, there was no guidance in the literature suggesting a different dose. Bupivacaine HCl 0.5% was added to liposome bupivacaine to speed the onset of anesthesia for the CCH injection procedure because the liposome bupivacaine suspension contains only 3% of free drug available for immediate blockade, which would not be adequate to result in fast onset of the blockade.
The liposome bupivacaine group received a larger total dose of bupivacaine: 79 mg of bupivacaine HCl (a combination of 66.5 mg bupivacaine HCl in liposome bupivacaine 5 mL plus 12.5 mg bupivacaine HCl) compared with the bupivacaine HCl–alone group (37.5 mg bupivacaine HCl). Nonetheless, the actual dose of free bupivacaine available for nerve blockade after injection was larger in the bupivacaine HCl–alone group than in the liposome bupivacaine group (37.5 versus 14.5 mg, respectively). The liposome bupivacaine suspension contains only 3% of free bupivacaine (66.5 mg × 3% ∼ 2 mg + 12.5 mg = 14.5 mg), and pharmacokinetic studies showed that free bupivacaine is gradually released from the liposomes over 72 hours or more, which is distinctly different from an injection of bupivacaine HCl alone, in which the entire dose (7.5 mL bupivacaine 0.5% = 37.5 mg) is immediately available for nerve blockade. The duration of blockade by free bupivacaine from the liposome bupivacaine suspension is limited because only a small amount of free drug is quickly absorbed. Hence, any duration of analgesia beyond 36 hours is likely caused by the extended release of free bupivacaine from liposome bupivacaine, rather than a function of the larger total mass of bupivacaine HCl in the liposome bupivacaine group.11,15,16
A limitation of this study is that surgical dressings made motor assessments difficult. Nonetheless, analgesic benefit, rather than hand function, was the primary purpose of the study. We therefore opted to ask the subjects whether they could use a polystyrene foam cup, and about their perception of numbness or weakness in the surgical hand. Indirect assessment over the phone of their ability to hold a polystyrene foam cup and their perception of numbness and/or weakness of the surgical hand suggested that any motor block was minor or nonexistent. This could be because liposome bupivacaine releases small amounts of bupivacaine HCl over 72 hours after injection, which may be adequate for autonomic and sensory blockade but not for motor blockade. The study did not include plasma pharmacokinetic determination; however, no subjects had signs or symptoms of systemic toxicity or electrocardiogram abnormality suggestive of local anesthetic systemic toxicity.
Another limitation of the study is that a comparison with local infiltration was not performed, although it is a common practice among hand surgeons. Nerve block and infiltration models require different methods of drug administration. We believe that a more direct comparison of the efficacy of liposome bupivacaine versus bupivacaine HCl was examined by using the nerve block model alone. Nonetheless, because local infiltration is a common practice among hand surgeons, future studies that include an infiltration arm of the trial should be conducted.
The addition of liposome bupivacaine prolonged sensory block for both phases of Dupuytren contracture release and improved pain scores in the first posttreatment week. Future studies of the efficacy of liposome bupivacaine in more extensive and reconstructive hand surgeries are indicated. Studies should also be conducted to determine the dose–response and best anatomical sites of application of liposome bupivacaine that optimize nerve exposure to small amounts of active drug released in a sustained fashion from the liposomes, detailed examination of the hand function after forearm blocks with liposome bupivacaine, and cost-efficacy of the treatments.
Acknowledgments
This study was supported by a research grant from Pacira Pharmaceuticals, Inc (Parsippany, NJ). Pacira Pharmaceuticals was not involved in study design; in data collection, analysis, or interpretation; in writing of this report; or in the decision to submit this article for publication. The authors would like to acknowledge the orthopedic surgical and nursing team at Ziekenhuis Oost-Limburg who facilitated patient care (Joëlle Caretta, RN, Sonja Moonen, RN, Birgit Lohmar, RN, and Ine Vanweert, RN) and the research team at Ziekenhuis Oost-Limburg who collected the subject assessments and followed the subjects by phone after discharge (Ingrid Meex, PhD, Marijke Cipers, RN, and Gülhan Özyürek, MSc).
Footnotes
Declaration of interests: Admir Hadzic serves a consultant for Pacira Pharmaceuticals and has received consulting honoraria and educational and research grants. No benefits in any form have been received or will be received by the other authors related directly or indirectly to the subject of this article.
References
- 1.DiBenedetti D.B., Nguyen D., Zografos L., Ziemiecki R., Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand (N Y) 2011;6(2):149–158. doi: 10.1007/s11552-010-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hindocha S., McGrouther D.A., Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y) 2009;4(3):256–269. doi: 10.1007/s11552-008-9160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai S.S., Hentz V.R. The treatment of Dupuytren disease. J Hand Surg Am. 2011;36(5):936–942. doi: 10.1016/j.jhsa.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Sanjuan-Cerveró R., Carrera-Hueso F.J., Vazquez-Ferreiro P., et al. Pain associated with treatment of Dupuytren contracture with collagenase Clostridium histolyticum. J Hand Surg Am. 2017;42(2):e104–e114. doi: 10.1016/j.jhsa.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Dasta J., Ramamoorthy S., Patou G., Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28(10):1609–1615. doi: 10.1185/03007995.2012.721760. [DOI] [PubMed] [Google Scholar]
- 6.Vandepitte C., Kuroda M., Witvrouw R., et al. Addition of liposome bupivacaine to bupivacaine HCl versus bupivacaine HCl alone for interscalene brachial plexus block in patients having major shoulder surgery. Reg Anesth Pain Med. 2017;42(3):334–341. doi: 10.1097/AAP.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton T.W., Athanassoglou V., Mellon S., et al. Infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;2:CD011419. doi: 10.1002/14651858.CD011419.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigley G.K. In: Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Walker H.K., Hall W.D., Hurst J.W., editors. Butterworths; Boston, MA: 1990. Sensation. [PubMed] [Google Scholar]
- 9.Atkinson T.M., Mendoza T.R., Sit L., et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Med. 2010;11(3):337–346. doi: 10.1111/j.1526-4637.2009.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilfeld B.M., Viscusi E.R., Hadzic A., et al. Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks. Reg Anesth Pain Med. 2015;40(5):572–582. doi: 10.1097/AAP.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth J., IV . Hadzic’s Textbook of Regional Anesthesia and Acute Pain Management. 2nd ed. McGraw-Hill; New York, NY: 2017. Clinical pharmacology of local anesthetics. [Google Scholar]
- 12.Hadzic A., Minkowitz H.S., Melson T.I., et al. Liposome bupivacaine femoral nerve block for postsurgical analgesia after total knee arthroplasty. Anesthesiology. 2016;124(6):1372–1383. doi: 10.1097/ALN.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 13.Eichenberger U., Stöckli S., Marhofer P., et al. Minimal local anesthetic volume for peripheral nerve block. Reg Anesth Pain Med. 2009;34(3):242–246. doi: 10.1097/AAP.0b013e31819a7225. [DOI] [PubMed] [Google Scholar]
- 14.Keplinger M., Marhofer P., Marhofer D., et al. Effective local anaesthetic volumes for sciatic nerve blockade: a clinical evaluation of the ED99. Anaesthesia. 2015;70(5):585–590. doi: 10.1111/anae.13013. [DOI] [PubMed] [Google Scholar]
- 15.Hadzic A., Abikhaled J., Harmon W. Impact of volume expansion on the efficacy and pharmacokinetics of liposome bupivacaine. Local Reg Anesth. 2015;8:105–111. doi: 10.2147/LRA.S88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu D., Onel E., Singla N., Kramer W.G., Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109–115. doi: 10.1007/s40261-012-0043-z. [DOI] [PubMed] [Google Scholar]