Abstract
Background:
Angiotensinogen is the proximal precursor of the angiotensin peptide hormones of the Renin-Angiotensin-Aldosterone System. Clinical trials are ongoing targeting angiotensinogen for the treatment of hypertension and heart failure. The epidemiology of angiotensinogen is not well defined, particularly its relationship to ethnicity, sex, and blood pressure (BP) / hypertension.
Objectives:
We sought to determine the relationship of circulating angiotensinogen levels to ethnicity, sex, BP, incident hypertension, and prevalent hypertension in a modern sex-balanced ethnically diverse cohort.
Methods:
Plasma angiotensinogen levels were measured in 5,786 participants from the Multiethnic Study of Atherosclerosis (MESA) study. Linear, logistic, and Cox Proportional Hazards models were utilized to examine the associations of angiotensinogen with BP, prevalent hypertension, and incident hypertension, respectively.
Results:
Angiotensinogen levels were significantly higher in females than males and differed across self-reported ethnicities with the ordering (from highest to lowest): White, Black, Hispanic, and Chinese adults. Higher levels were associated with higher BP and odds of prevalent hypertension, after adjusting for other risk factors. Equivalent relative differences in angiotensinogen were associated with greater differences in BP in males versus females. In males not taking RAAS-blocking medications, a standard deviation increment in log-angiotensinogen was associated with 2.61 mmHg higher systolic BP (95% CI: [1.49, 3.80]), while in females the same increment in angiotensinogen was associated with 0.97 mmHg higher systolic BP (95% CI: [0.30, 1.65]).
Conclusions:
Significant differences in angiotensinogen levels are present between sexes and ethnicities. A positive association is present between levels and prevalent hypertension and BP, which differs between sexes.
Keywords: Angiotensinogen, Blood Pressure, Hypertension, Sex Differences, Renin-Angiotensin-Aldosterone System
CONDENSED ABSTRACT:
Angiotensinogen is a critical component of the renin-angiotensin-aldosterone system. We measured levels of angiotensinogen in 5,786 participants from a modern sex-balanced ethnically diverse cohort, the Multiethnic Study of Atherosclerosis (MESA). We observed significant differences in angiotensinogen levels between the sexes and across self-reported ethnicities. Levels were observed to be significantly higher in females relative to males. Positive associations between angiotensinogen and blood pressure / prevalent hypertension were observed after controlling for other risk factors for hypertension. Equivalent relative differences in angiotensinogen levels were associated with greater differences in blood pressure among males versus females.
INTRODUCTION
Chronic activation of the renin-angiotensin-aldosterone system (RAAS) results in vasoconstriction, cardiac remodeling and fibrosis, and is a major contributor to hypertension and heart failure1–4. Despite proven benefits of downstream RAAS inhibition with angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin II type 1 receptor blockers (ARBs), chronic monotherapy with these agents have sub-optimal therapeutic efficacy due to the generation of counterbalancing compensatory pathways that may restore Angiotensin II levels to pre-treatment levels5. In particular, chymase-dependent production and other escape mechanisms are present that generate angiotensin II6. On the other hand, efforts to provide a more extensive RAAS blockade using a combination therapy of kidney-active agents such as ACE-I, ARBs, and renin inhibitors increases the risk of side effects, such as hyperkalemia and renal dysfunction7,8. This evidences the need for new pharmacologic approaches to RAAS inhibition to better treat hypertension and heart failure.
Angiotensinogen, the liver-derived protein precursor of all angiotensin (Ang) peptides represents a new target for RAAS pathway inhibition and RNA silencer-based therapeutic strategies targeting this protein have been reported on recently9. Linkage and genetic studies have suggested an association between the angiotensinogen gene (AGT) and hypertension and heart failure10–13. In addition, preclinical and early clinical studies with small numbers of participants have shown that AGT gene silencing therapies reduce blood pressure without associated effects on liver or renal function9,14. The novel approach of targeting angiotensinogen specifically in the liver and minimizing effects in the kidney is therefore hypothesized to provide the benefits of RAAS suppression without the renal complications of dual therapy.
Despite the importance of angiotensinogen, a characterization of angiotensinogen levels in a multi-ethnic human population is lacking, and the magnitude of its contribution to hypertensive cardiovascular disease in humans is unclear. The current study aimed to provide a comprehensive evaluation of angiotensinogen levels, blood pressure and prevalent hypertension in humans (Central Illustration). Given the differences in the prevalence of hypertension between ethnicities and the sexes, our first aim was to characterize the relationship between sex, ethnicity, and circulating levels of angiotensinogen. Second, we sought to determine the relationship between angiotensinogen levels and blood pressure / hypertension and whether these relationships were modified by sex and / or race / ethnicity. This evaluation was conducted in the Multi-Ethnic Study of Atherosclerosis (MESA), an NIH-funded, multi-ethnic, sex-balanced, contemporary prospective cohort study15.
Central Illustration. Determination of the relationship between angiotensinogen, biological sex, ethnicity, and blood pressure / hypertension.
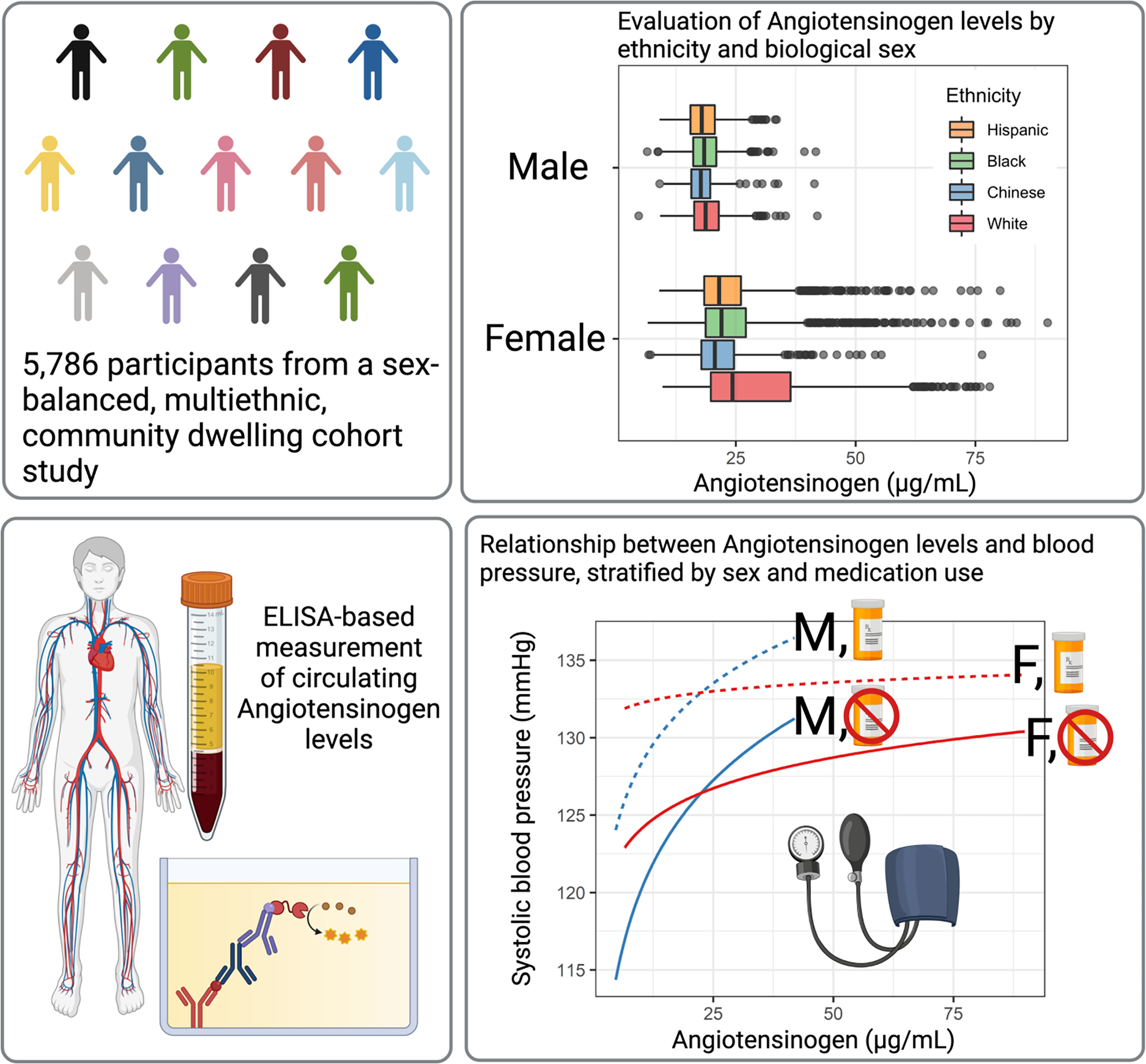
Levels of angiotensinogen were measured from blood plasma in 5,786 participants of the Multi-Ethnic Study of Atherosclerosis. Significant differences in angiotensinogen levels were observed between the biological sexes and race / ethnicity groups. Mean angiotensinogen was significantly higher in female participants than males. Angiotensinogen levels were positively associated with blood pressure and hypertension. In males, the magnitude of the positive association was greater than in females. In participants on medications targeting angiotensinogen metabolism, the positive association between angiotensinogen and blood pressure was diminished.
METHODS
Study participants and design
The Multiethnic Study of Atherosclerosis enrolled 6,814 participants between 2000 and 2002 according to pre-specified race / ethnicity and age strata. Participants of four race / ethnicity groups (White, Chinese, Black, and Hispanic), with age between 45–84 years were enrolled from six communities in the United States (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA). The cohort recruitment was sex-balanced, with approximately equal numbers of men and women participants. In order to study the development of atherosclerosis, participants were all free of clinical atherosclerotic cardiovascular disease at enrollment. Participants provided informed consent and the study was approved by Institutional Review Boards at each field center. The design of the study has been described in detail in earlier publications15. The current ancillary study utilized data from participants that were not included in the MESA-1000. The MESA-1000 is a random sample of approximately 1,000 participants that has undergone additional characterization using baseline blood samples. To minimize depletion of these specimens, ancillary studies exclude the MESA-1000 participants, unless otherwise justified. Consequently, 5,786 participants were included in the current study. Medical history, laboratory data, and anthropometric measurements were ascertained as described previously15,16.
Study outcomes
In addition to measured levels of angiotensinogen, outcome measures of the current study included systolic blood pressure (SBP), diastolic blood pressure (DBP) and prevalent hypertension at baseline (Exam 1) in the MESA study. Prevalent hypertension was defined as SBP over 130 mmHg, DBP over 80 mmHg, or hypertension medication use at Exam 1. Incident hypertension was a secondary outcome measure and was defined as the time to first measurement of: elevated blood pressure, initiation of hypertension medication, or self-report of hypertension in the subset of (N = 2,196) normotensive participants. As these data were only ascertained during Exams 2–6, time-to-incident hypertension was thus an interval censored variable.
Laboratory measurements
Angiotensinogen levels were measured in blood plasma from the baseline (Exam 1) visit of the included MESA participants. The measurements were made using an enzyme-linked immunoassay that has been described previously17 and was executed by Medpace Reference Labs (Cincinnati, Ohio). Briefly, the coating antibody was rabbit anti-human immunoglobulin monoclonal antibody (IBL-America, Catalog #27412, Minneapolis, Minnesota). Total angiotensinogen (including intact angiotensinogen and des(Ang I) angiotensinogen) was then detected from 1:10,000 diluted EDTA plasma with horse radish peroxidase mouse anti-human angiotensinogen monoclonal Fab’ fragment (IBL-America). A standard curve was generated for quantitation using purified human angiotensinogen. The coefficient of variation was observed to be 9% with an analytical range of 13.9 μg/mL to 75.9 μg/mL. Angiotensinogen measurements were log-transformed and standardized (to have mean 0 and standard-deviation 1) prior to modeling efforts, which improved the departure from approximate normality and aids in model interpretability as other continuous variables were standardized prior to modeling efforts.
Statistical analysis
The distribution of cohort characteristics was determined within each sex and within angiotensinogen levels (above versus below the sex-specific median). Median, first quartile, and third quartile levels of angiotensinogen were determined by sex and race / ethnicity. A linear model was estimated regressing circulating angiotensinogen levels on sex, race / ethnicity, age, and the interaction between sex and race / ethnicity. From this model, the total proportion of the variance in angiotensinogen levels accounted for by these variables was determined (model coefficient of determination, R2) as well as the partial coefficient of determination (partial R2) values for each variable. This model also facilitated identifying the relative differences in angiotensinogen levels by sex and race / ethnicity.
Angiotensinogen, blood pressure, and hypertension:
To determine the relationship between circulating angiotensinogen levels and blood pressure, linear models relating systolic and diastolic blood pressure to angiotensinogen were fit. These models were then refitted to incorporate other covariates known to affect blood pressure and the RAAS: age, sex, the interaction between sex and angiotensinogen, race / ethnicity, body mass index (BMI), cigarette use, angiotensin-converting enzyme inhibitors (ACE-I) / angiotensin II receptor blocker (ARB) medication use, and the interaction between these medications and angiotensinogen levels. The partial coefficient of determination was calculated to determine the proportion of variation in blood pressure that was explained by angiotensinogen levels (after accounting for other covariates). To determine the relationship between angiotensinogen levels and hypertension, a cross-sectional analysis was conducted. As a sensitivity analysis, we evaluated including a variable representing all medications for hypertension as opposed to ACE-I / ARB use alone. The cross-sectional analysis identified participants with hypertension at baseline (SBP > 130 or DBP > 80 mmHg or taking blood pressure medication). Unadjusted and adjusted models were fit to evaluate the odds of hypertension given varying angiotensinogen levels at baseline. The adjusted model included age, sex, race / ethnicity, BMI, cigarette use, and the interaction between sex and angiotensinogen.
Adjustment for the multiple comparisons made in the current study:
While the current study involved multiple aims, the relationship between sex, race / ethnicity and angiotensinogen levels was considered primary. Consequently, the confidence intervals reported for comparisons within these primary aims were adjusted to maintain a simultaneous confidence coefficients of 0.95. The remaining analyses were considered exploratory in nature and were not adjusted.
The analyses reported in the current work were conducted using the R statistical language18 (version 4.0.2) and the following packages: survival19, emmeans20, dplyr21, and ggplot222.
RESULTS
Baseline demographics of the 5,786 participants with available angiotensinogen measurements are reported in Table 1. While angiotensinogen measurements were made in 5,787 participants, one extreme outlier in angiotensinogen levels was observed and was removed from the data. Average levels of angiotensinogen differed significantly between males and females, and between race / ethnic groups. Median and interquartile ranges are presented by sex and race / ethnicity along with a boxplot illustrating the distribution of levels in Figure 1. As presented in Table 2, females had significantly higher mean levels of angiotensinogen than males within each race / ethnicity. This difference between the sexes was most pronounced in White participants, where the ratio of female to male angiotensinogen levels was 1.44 with a 95% simultaneous confidence interval of (1.40, 1.48). The difference was least pronounced in Chinese participants, where the ratio of female to male levels was 1.19 with a 95% simultaneous confidence interval of (1.14, 1.24). Within women, 83.2% of the participants had already gone through menopause by Exam 1 and the proportion of women with above and below median angiotensinogen levels was similar (Table 1). In contrasting race / ethnicity, angiotensinogen levels were highest in White participants compared to Chinese, Black, or Hispanic participants (Table 3). These differences between race / ethnic groups were more pronounced in female participants than male participants. Specifically, the ratio of levels in White female participants to Chinese female participants was 1.28 with a 95% simultaneous confidence interval of (1.22, 1.34); in male participants this ratio was 1.06 with a 95% simultaneous confidence interval of (1.01, 1.11). In both females and males, the ordering from highest to lowest of median angiotensinogen levels was White, Black, Hispanic, and Chinese. The model underlying these sex and race / ethnicity contrasts is presented in Supplemental Table 1 and was adjusted for the age of participants. Overall, this model explained 21.0% of the variance in angiotensinogen levels (model adjusted R2). In this model, sex had the greatest explanatory power with a partial R2 of 0.178 (17.8%), followed by race / ethnicity with a R2 of 0.032 (3.2%), and the interaction between sex and race / ethnicity with a partial R2 of 0.013 (1.3%).
Table 1: Cohort Characteristics.
Characteristics of the 5,786 MESA participants stratified by sex and angiotensinogen (below median versus above sex-specific median). Median angiotensinogen was 18.3 μg/mL in males and 22.4 μg/mL in females.
| Male (N = 2776) | Female (N = 3010) | |||
|---|---|---|---|---|
| Characteristic | Below Median | Above Median | Below Median | Above Median |
|
| ||||
| Mean age ± SD, years | 63.4 ± 10.0 | 61.7 ± 10.3 | 63.2 ± 10.7 | 62.2 ± 9.8 |
| Race/Ethnicity, n (%) | ||||
| White | 484 (46.1) | 565 (53.9) | 439 (39.7) | 666 (60.3) |
| Chinese | 216 (61.5) | 135 (38.5) | 226 (63.8) | 128 (36.2) |
| Black | 378 (49.6) | 384 (50.4) | 480 (53.1) | 424 (46.9) |
| Hispanic | 325 (52.9) | 289 (47.1) | 363 (56.1) | 284 (43.9) |
| Post-Menopause | 1234 (49.3) | 1268 (50.7) | ||
| Diabetes, n (%) | 201 (50.6) | 196 (49.4) | 189 (55.1) | 154 (44.9) |
| Mean BMI ± SD, kg/m2 | 27.7 ± 4.5 | 28.0 ± 4.4 | 28.9 ± 6.3 | 28.7 ± 6.1 |
| Mean total cholesterol ± SD, mg/dL | 182.3 ± 32.7 | 193.7 ± 36.5 | 196.3 ± 34.7 | 202.9 ± 36.1 |
| Mean HDL cholesterol ± SD, mg/dL | 44.6 ± 11.5 | 45.6 ± 12.0 | 54.0 ± 14.4 | 58.6 ± 16.1 |
| Mean LDL cholesterol ± SD, mg/dL | 113.5 ± 29.9 | 119.4 ± 32.0 | 119.2 ± 31.3 | 116.4 ± 32.5 |
| Mean systolic BP ± SD, mmHg | 125.6 ± 19.8 | 126.7 ± 19.0 | 128.0 ± 23.8 | 127.6 ± 22.6 |
| Mean diastolic BP ± SD, mmHg | 74.5 ± 9.3 | 75.7 ± 9.5 | 69.2 ± 10.4 | 69.1 ± 9.9 |
| Median hs-CRP (Q1, Q3), mg/L | 1.230 [0.640, 2.780] | 1.590 [0.780, 3.360] | 1.940 [0.860, 4.200] | 3.230 [1.420, 6.907] |
| Creatinine, mg/dL | 1.02 [0.92, 1.12] | 1.02 [0.92, 1.22] | 0.82 [0.72, 0.92] | 0.82 [0.72, 0.92] |
| eGFR | 76.5 ± 15.7 | 75.1 ± 16.6 | 74.3 ± 15.2 | 71.7 ± 16.0 |
| Hypertension, n (%) | 853 (49.9) | 857 (50.1) | 864 (48.3) | 924 (51.7) |
| Any hypertension medication, n (%) | 514 (50.3) | 507 (49.7) | 546 (46.9) | 618 (53.1) |
| ACE Inhibitor, n (%) | 195 (47.6) | 215 (52.4) | 183 (50.8) | 177 (49.2) |
| Angiotensin II receptor blockers, n (%) | 66 (54.1) | 56 (45.9) | 91 (48.7) | 96 (51.3) |
| Alpha blocker use, n (%) | 133 (60.2) | 88 (39.8) | 9 (50.0) | 9 (50.0) |
| Beta blocker use, n (%) | 126 (51.2) | 120 (48.8) | 136 (45.0) | 166 (55.0) |
| Any lipid lowering medication, n (%) | 239 (51.7) | 223 (48.3) | 241 (47.2) | 270 (52.8) |
| Current aspirin use, n (%) | 393 (49.5) | 401 (50.5) | 305 (45.9) | 359 (54.1) |
| Smoking history, n (%) | ||||
| Never | 558 (49.6) | 567 (50.4) | 938 (52.8) | 838 (47.2) |
| Former | 654 (52.4) | 594 (47.6) | 390 (43.8) | 500 (56.2) |
| Current | 186 (47.2) | 208 (52.8) | 176 (52.9) | 157 (47.1) |
| Alcohol history, n (%) | ||||
| Never | 156 (55.5) | 125 (44.5) | 516 (57.1) | 387 (42.9) |
| Former | 459 (60.3) | 302 (39.7) | 349 (54.5) | 291 (45.5) |
| Current | 775 (45.2) | 940 (54.8) | 631 (43.8) | 811 (56.2) |
| Poor diet | 883 (49.7) | 892 (50.3) | 746 (48.7) | 786 (51.3) |
| Physical activity, MET-minutes/week | 4215.0 [2055.0, 8287.5] | 4537.5 [2160.0, 8460.0] | 3420.0 [1616.2, 6465.0] | 3888.8 [1920.0, 6810.0] |
Figure 1: Angiotensinogen levels.
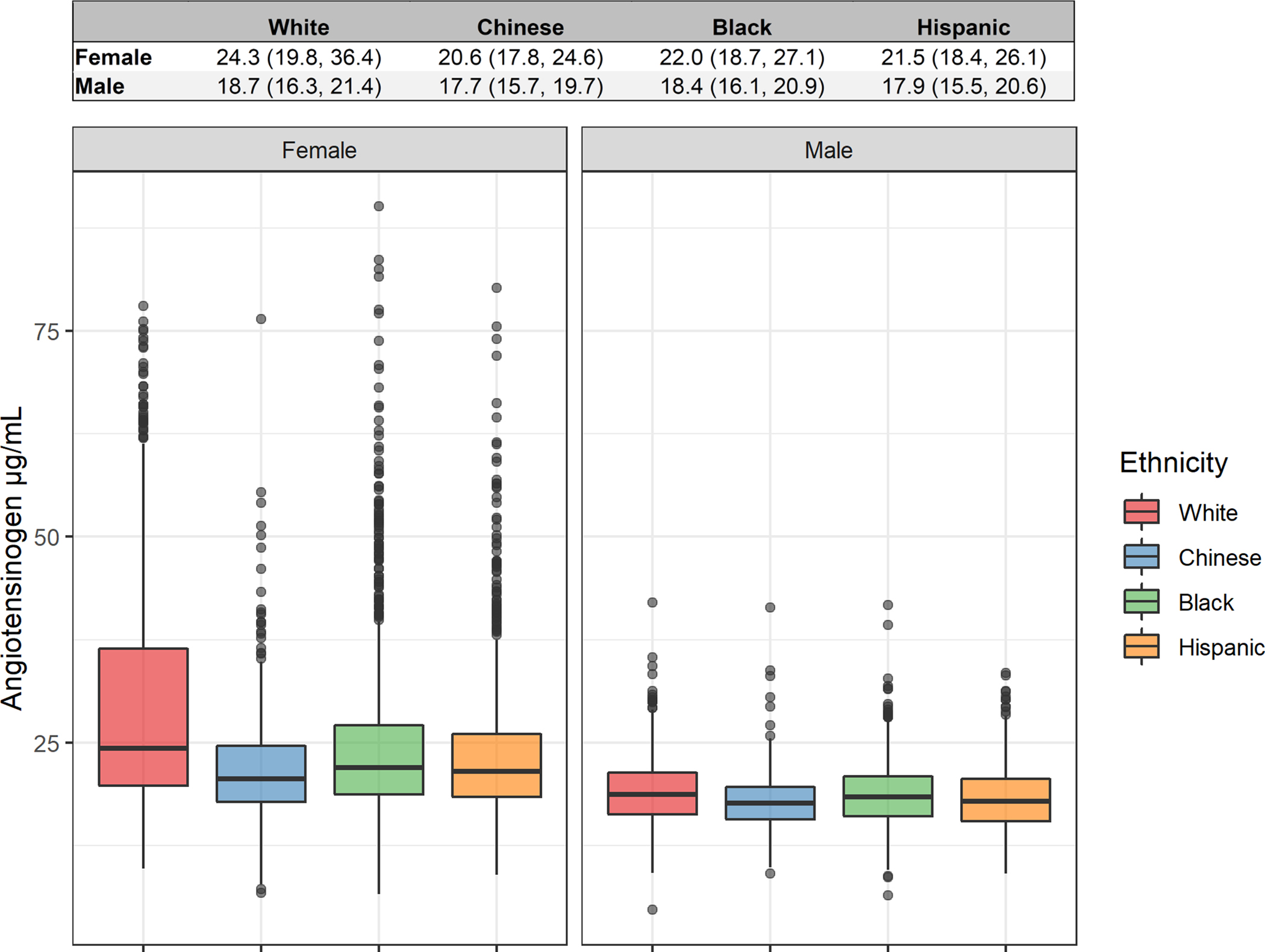
Distribution of angiotensinogen levels by sex and ethnicity in the MESA participants. Values shown above boxplot are median (Q1, Q3), where Q1 is the 25th percentile and Q3 is the 75th percentile.
Table 2: Comparison of angiotensinogen levels between the sexes.
Ratio of model estimated angiotensinogen levels comparing the sexes within ethnicities. The model was adjusted for age. Confidence intervals were determined using Tukey’s method to account for the multiple comparisons and are thus 95% simultaneous confidence intervals.
| Ethnicity | Female / Male Ratio (95% CI) |
|---|---|
|
| |
| White | 1.44 (1.40, 1.48) |
| Chinese | 1.19 (1.14, 1.24) |
| Black | 1.28 (1.24, 1.32) |
| Hispanic | 1.27 (1.23, 1.31) |
Table 3: Comparison of angiotensinogen levels between ethnicities.
Ratio of model estimated angiotensinogen levels comparing ethnicities within each sex. The model was adjusted for age. Confidence intervals were determined using Tukey’s method to account for the multiple comparisons and are thus 95% simultaneous confidence intervals.
| Ratio | Female (95% CI) | Male (95% CI) |
|---|---|---|
|
| ||
| White / Chinese | 1.28 (1.22, 1.34) | 1.06 (1.01, 1.11) |
| White / Black | 1.15 (1.11, 1.19) | 1.02 (0.98, 1.06) |
| White / Hispanic | 1.18 (1.14, 1.23) | 1.04 (1.004, 1.09) |
| Chinese / Black | 0.90 (0.85, 0.94) | 0.96 (0.92, 1.01) |
| Chinese / Hispanic | 0.93 (0.88, 0.97) | 0.99 (0.94, 1.04) |
| Black / Hispanic | 1.03 (0.99, 1.07) | 1.02 (0.98, 1.07) |
Angiotensinogen and blood pressure:
Unadjusted models describing the relationship between SBP and DBP are shown in Supplemental Table 2(a–b). Multivariable models adjusted for race / ethnicity, age, BMI, cigarette use, ACE-I / ARB medication use, and sex are presented in Supplemental Table 3(a–b). The coefficients for angiotensinogen changed substantially between the unadjusted and adjusted models indicating that the unadjusted models might suffer from residual confounding, where relevant variables (e.g., biological sex) are not included. In both adjusted models (for SBP and DBP), the relationship between angiotensinogen and blood pressure differed significantly between the sexes (interaction p-value = 0.02 for SBP, p-value = 0.0001 for DBP), and was moderated by ACE-I or ARB treatment status. Specifically, in male participants not on ACE-I/ARBs, 1-SD higher angiotensinogen levels (log scale) were associated with 2.61 mmHg higher SBP (95% CI: [1.42, 3.80]) and 1.52 mmHg higher DBP (95% CI: [0.93, 2.11]). In female participants, 1-SD higher angiotensinogen levels were associated with 0.97 mmHg higher SBP (95% CI: [0.30, 1.65]), and 0.24 mmHg higher DBP (95% CI: [−0.095, 0.57]). In males on ACE-I/ARBs, this association was less positive at 1.92 mmHg for SBP (95% CI: [0.34, 3.50]) and 0.86 mmHg for DBP (95% CI: [0.08, 1.64]). In females on ACE-I/ARBs, the association was less positive at 0.28 mmHg for SBP (95% CI: [−0.95, 1.52]) and −0.42 mmHg for DBP (95% CI: [−1.03, 0.20]). Figure 2 presents model estimated mean blood pressure from angiotensinogen levels, averaged over race / ethnicity, age, BMI, and cigarette use (using weighted averaging to match actual cohort responses for these variables). Results of the sensitivity analysis utilizing all medications for hypertension as opposed to ACE-I / ARB medication use alone are presented in Supplemental Table 4(a–b). The estimates for each variable were similar in the sensitivity analysis.
Figure 2: Relationship between angiotensinogen levels and blood pressure.
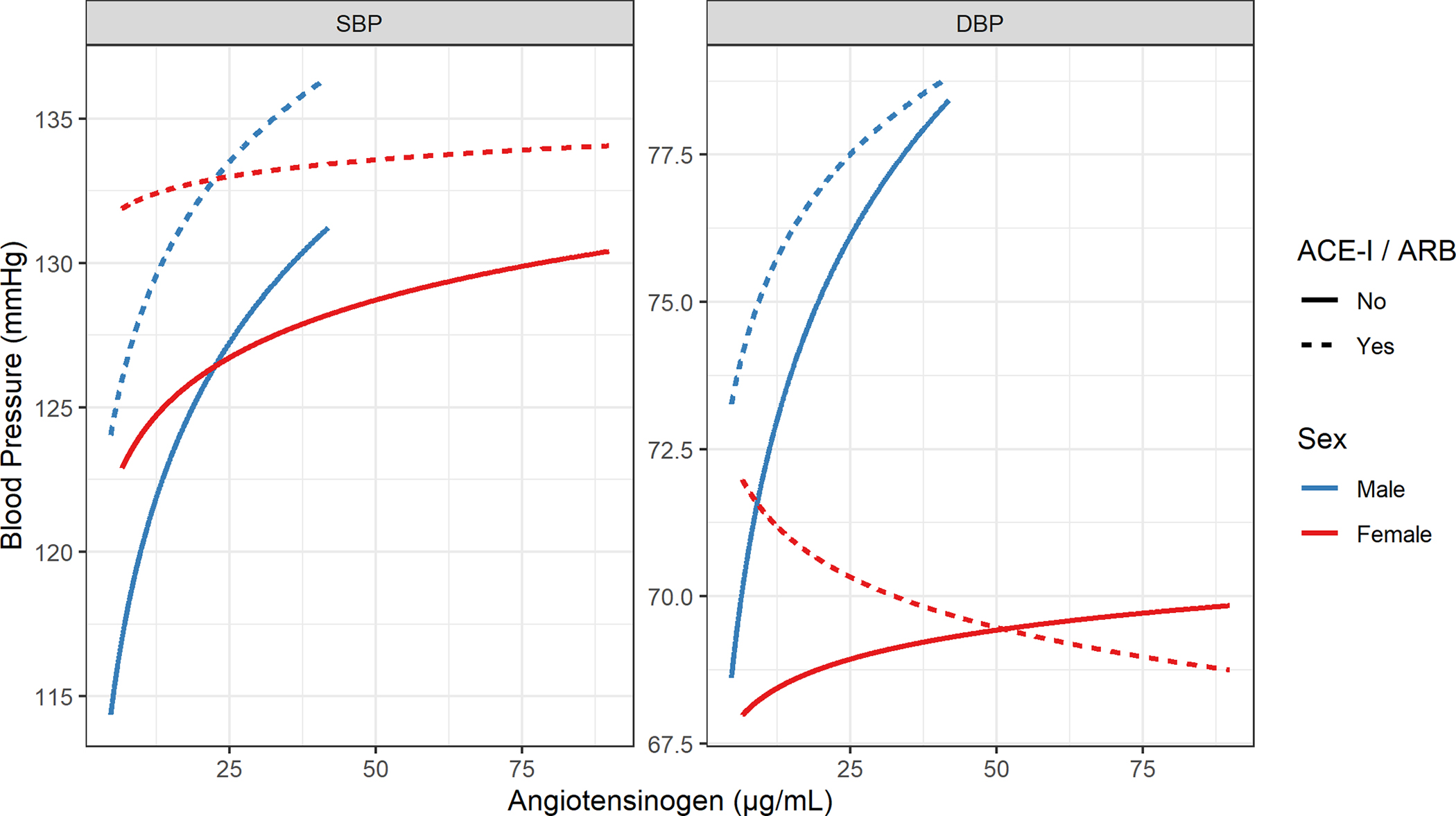
Model estimated mean blood pressure by Sex and ACE-I / ARB treatment status as a function of angiotensinogen levels. Estimates are averaged over age, BMI, and cigarette use.
Angiotensinogen and hypertension:
The multivariable model for hypertension at baseline (cross-sectional analysis) adjusted for the same variables as the blood pressure analysis except for ACE-I / ARB since this was part of the criteria for determining hypertension. This model (Supplemental Table 5) showed 1-SD higher angiotensinogen (log-scale) was associated with 1.22 times greater odds of prevalent hypertension [95% CI: (1.15, 1.31)] at baseline after adjusting for race / ethnicity, age, BMI, cigarette use, and self-reported biological sex. This model was utilized to compare the odds of hypertension by quartiles of angiotensinogen (Figure 3), where angiotensinogen quartiles were determined separately within each sex. Scant evidence of an effect of the interaction between sex and angiotensinogen was observed (Likelihood ratio test p-value = 0.82), and the model without the angiotensinogen by sex interaction term was superior based on comparison of Bayesian Information Criteria (BIC). While evidence was not observed of sex modifying the relationship between angiotensinogen and hypertension, the addition of angiotensinogen substantially modified the relationship between sex and hypertension (Supplemental Table 6). Specifically, while male sex was associated with 1.24 (95% CI: [1.10, 1.39]) higher odds of hypertension in a model without angiotensinogen, the addition of angiotensinogen to the model increased the magnitude of the higher odds of hypertension in males to 1.47 (95% CI: [1.29, 1.68]). Figure 3 presents the estimated odds ratio comparing participants in the second (25th – 50th percentile), third (50th – 75th percentile), and fourth quartiles (75th – 100th) to the first quartile (0th – 25th percentile) of angiotensinogen levels within each sex. Although a significant interaction between sex and angiotensinogen was not observed in predicting the odds of prevalent hypertension, the substantially increased range of angiotensinogen values in females results in larger predicted odds ratios for prevalent hypertension comparing quartiles of angiotensinogen in females than in males.
Figure 3: Relationship between angiotensinogen level and prevalent hypertension.
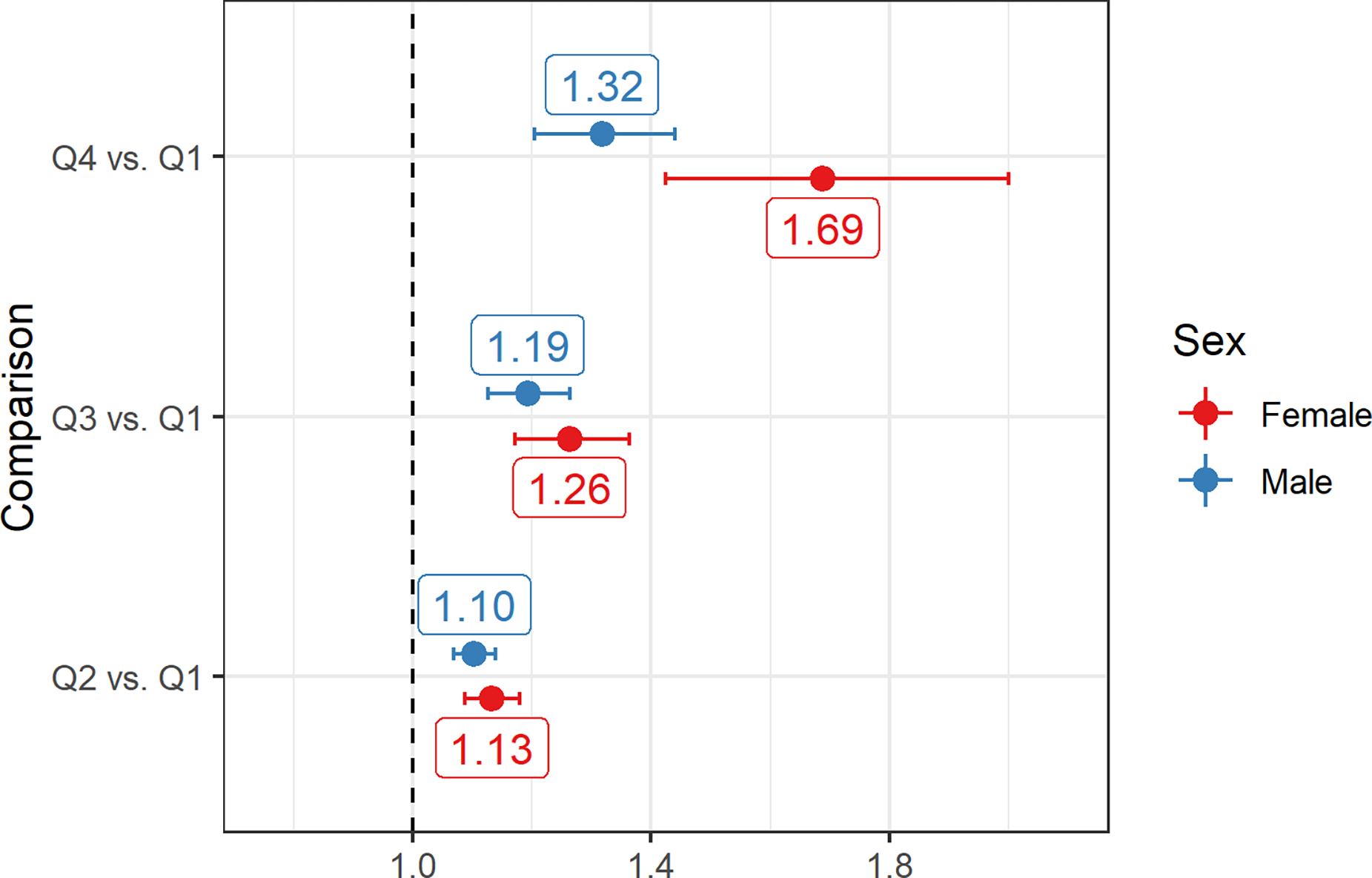
Model adjusted odds ratios for prevalent hypertension comparing subjects in the second (25th – 50th percentile), third (50th – 75th percentile), and fourth quartiles (75th – 100th) to the first quartile (0th – 25th percentile). The midpoint of each interval was utilized for making the comparison. Percentiles were calculated within each sex. Horizontal axis represents the odds ratio (OR) for prevalent hypertension.
To determine the added explanatory power of angiotensinogen for predicting prevalent hypertension after controlling for other risk factors, we compared the multivariable model with angiotensinogen to a model without, which included race / ethnicity, age, BMI, cigarette use, and biological sex. The addition of angiotensinogen did lead to significantly better model fit (Likelihood Ratio Test p-value < 0.0001) and resulted in a superior model by BIC. However, the improvement in the cross-validation estimated AUC was observed to be minimal (increased from 0.737 to 0.741). Given the relationship between angiotensinogen levels and biological sex, we compared the addition of angiotensinogen versus the addition of biological sex to a model with race / ethnicity, age, BMI, and cigarette use. The addition of angiotensinogen resulted in a superior model compared to the addition of biological sex with a residual deviance (error) of 6716.8 versus 6720.4.
The adjusted model for predicting incident hypertension from angiotensinogen levels is shown in Supplemental Table 7. There were 2,192 participants included in this analysis, of which 1,198 (54.7%) were noted to develop hypertension over the course of the follow-up. A likelihood ratio test showed that the inclusion of angiotensinogen did not improve model fit significantly relative to a model with race / ethnicity, age, sex, BMI, and smoking status (p-value = 0.26). The addition of an interaction term between sex and angiotensinogen did not improve model fit significantly relative to a model without this interaction (p-value = 0.50).
DISCUSSION
This study demonstrates several key observations regarding angiotensinogen: (1) females of all race / ethnic groups had ~15–30% higher median angiotensinogen levels than males; (2) across race / ethnicity, median levels were highest in White then Black then Hispanic then Chinese participants; (3) higher levels of angiotensinogen were associated with higher blood pressure and higher odds of prevalent hypertension; (4) despite males having lower levels on average than females, the association between angiotensinogen and blood pressure was more positive in males (steeper slope for a fixed relative difference in levels); (5) the increased range of angiotensinogen levels in females portends a greater difference in the estimated odds of prevalent hypertension in females comparing high versus low levels.
The current work represents the largest human study of the relationship between angiotensinogen levels, blood pressure, and hypertension. The importance of evaluating factors associated with angiotensinogen levels as well as the relationship between levels and hypertension is highlighted by the recent development of novel therapeutics directly targeting angiotensinogen levels 9,14,23,24. These data are pertinent and timely for the further development and testing in humans of multiple new therapeutics targeting the RAAS.
In the current work, we observed that circulating levels of angiotensinogen varied significantly by sex and race / ethnicity. In female participants especially, the variability (interquartile range) in angiotensinogen levels was large within each race / ethnicity. The substantial differences between the sexes in angiotensinogen levels may be due to differences in sex hormones or other effects relating to sex chromosome complement. Research on the relationship between estrogen and angiotensinogen levels was conducted in animal models in the 1990s and showed that treatment with estradiol increased plasma angiotensinogen levels25–28. In humans, causal evidence of a relationship between estrogen and circulating levels of angiotensinogen has also been observed in studies of post-menopausal women administered oral estradiol treatment28. We note, however, that the majority of the women included in the current study (83%) were of post-menopausal age.
We hypothesize that the differences in angiotensinogen levels between ethnicities may have a genetic component. Multiple variants in the promoter and enhancer regions of the AGT gene, as well as other variants in linkage disequilibrium with promoter variants, have been shown to be associated with angiotensinogen levels or mRNA expression in humans 29–31. These variants include rs5050, rs5051, and rs5049 in the AGT promoter. An analysis of haplotype frequencies for these three variants between African, Admixed American (Hispanic), East Asian, and European populations from the 1,000 Genomes Project Phase 3 data32 sheds light on variation in the AGT promoter between ethnicities. The two most prevalent haplotypes are unique between the ethnicities, while within each of these populations the two most prevalent haplotypes account for greater than 65% of the population. Further, there may be a relationship between these ethnicity-associated variants and sex-specific regulation of angiotensinogen levels. Specifically, the rs5050 variant has been shown to modify the binding of estrogen receptor to the AGT promoter region and that transcriptional activity following estrogen treatment is higher when the T allele is present (in a hepatoma cell culture model)31.
We characterized the relationship between circulating levels of angiotensinogen and blood pressure. Given that angiotensinogen is the precursor of Ang II and other Ang peptides, we expected to find a positive relationship between angiotensinogen and blood pressure as others have found33. Following adjustment for race / ethnicity, BMI, cigarette use, ACE-I / ARB use, and sex, we did observe that greater levels of angiotensinogen were associated with higher systolic and diastolic blood pressure. However, the observation that a fixed relative difference in angiotensinogen among males is associated with a greater difference in blood pressure and prevalent hypertension as compared to females is a novel finding. Both female sex and ACE-I / ARB use moderated the association between angiotensinogen on blood pressure. Given the mechanism of action of ACE-I and ARB drugs the finding of a reduced positive association between angiotensinogen and blood pressure supports a direct effect of angiotensinogen levels on blood pressure. However, for both females and males, a significant positive relationship between angiotensinogen and SBP was still observed in those taking ACE-I / ARB medications; for DBP such a relationship was observed in males. To synthesize, our models for blood pressure show a greater magnitude of positive increase in blood pressure associated with a standardized unit change in angiotensinogen in males than females, yet the range of angiotensinogen levels is substantially larger in females than males. It is important to note that in absolute terms, the largest magnitude association observed across a 1-SD difference in angiotensinogen levels was less than 4 mmHg, which is important for interpreting clinical relevance.
To determine the relationship between angiotensinogen and hypertension, we conducted a cross-sectional analysis using exam 1 (baseline) data. A model adjusted for race / ethnicity, age, BMI, cigarette use, and male sex showed a significant positive association between circulating levels of angiotensinogen and the odds of hypertension. Interestingly, the inclusion of angiotensinogen in a model with race / ethnicity, age, BMI, cigarette use explained more of the variability in the prevalence of hypertension than the inclusion of sex, while the inclusion of both sex and angiotensinogen was observed to be the best model among those compared. We observed that the estimated effect of being male on the odds of hypertension was greater when angiotensinogen was included then when it was not.
Our finding that the associated increase in blood pressure per unit increase in angiotensinogen is higher in males may be attributable sex differences in the metabolism of angiotensinogen and in the expression of receptors for Ang peptides34. In a recent study by Toering et al., healthy male and female human subjects were administered Ang II, and males exhibited greater acute phase increases in DBP and mean arterial pressure (MAP) than women35. This complemented previous animal model research into the sex differences in the RAAS system and hypertension that showed that males have greater activation of “classical” RAAS (driven by AT1R mediated effects of Ang II), while females have greater activation of “nonclassical RAAS” (driven by AT2R and Mas receptor mediated effects of Ang 1–7)36,37. The effects mediated by AT2R and Mas receptor including vasodilation and natriuresis are thought to be counter to those mediated by AT1R38. Leveraging an animal model designed to partition sex effects into the effects of sex hormones versus sex chromosome genes, Pessôa et al. reported that both female sex hormones and sex chromosome complement were required for conferring the AT2R-mediated relaxation response to Ang II39.
Our results are timely given that new therapies targeting the reduction of angiotensinogen are being evaluated in human trials. The effect of antisense oligonucleotide IONIS-AGT-LRx targeting angiotensinogen determined from three phase I and II trials was recently reported9. Double-blind, placebo-controlled clinical trials were performed in persons with hypertension using IONIS-AGT-LRx as monotherapy or as an add-on to ACE-I/ARB. IONIS-AGT-LRx was well tolerated with no significant changes in platelet count, potassium levels, or liver and renal function. IONIS-AGT-LRx significantly reduced angiotensinogen levels compared with placebo in all 3 studies. Although these studies were not powered to evaluate blood pressure reduction, trends in blood pressure reduction were observed. Two additional Phase II/IIb trials are ongoing. One trial is in resistant hypertension with a minimum of 3 medications (NCT04714320), and the another is in heart failure with reduced ejection fraction (NCT04836182).
Limitations:
The current study has some important limitations. First, as with all observational studies, the associations presented here do not establish causal evidence for the role of angiotensinogen levels in determining blood pressure and hypertension. Second, in the current work we have used definitions of race / ethnicity that were self-reported by MESA participants. Whether self-reported race / ethnicity is sufficient or optimal for evaluating the association between disease outcomes and race / ethnicity has been debated, and race and ethnicity are largely a social constructs40,41. It has been observed in the MESA study that the self-reported Hispanic group is more heterogenous across genetic-based measures of ancestry than the other groups, and many self-reported Hispanic participants cluster with White participants in the analysis of genetic-based measures of ancestry42. The data on blood pressure should be interpreted with the caution as a measurement of blood pressure at one exam visit may not be sufficient for determining prevalent hypertension. Lack of adherence to reported blood pressure medication use may present a complication when controlling for such medications. Additionally, while the MESA protocol for blood pressure measurement is standardized and consistent across the exams and field centers, the Dinamap device utilized is not listed in the US Blood Pressure Validated Device Listing. Finally, in the current work the ELISA method measures the product of intact angiotensinogen and des (Ang I) angiotensinogen. While beyond the scope of the current study, concurrent measurement of intact angiotensinogen and des (Ang I) angiotensinogen would allow for studying individual associations.
CONCLUSIONS
Circulating levels of angiotensinogen vary substantially between the sexes, with higher levels observed in females. Ethnicity-associated variation in angiotensinogen levels is present with White participants having highest levels compared to Black, Hispanic, and Chinese participants. In those not taking ACE-I or ARB medications, the association between angiotensinogen levels is positive, and greater in magnitude in males than females. ACE-I and ARB medications moderate the positive association between angiotensinogen and blood pressure. Higher angiotensinogen levels are associated with higher odds of prevalent hypertension. This association was not significantly modified by biological sex. This work supports the continued evaluation of therapeutics targeting angiotensinogen. Whether these drugs can be used as monotherapy or as combinations, and whether targeting angiotensinogen primarily in the liver minimizes renal-associated side effects awaits to be determined in future clinical trials.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge:
Circulating levels of angiotensinogen differ between the sexes and by ethnicity, as does the strength of the relationship between angiotensinogen and blood pressure.
Translational Outlook:
Further investigations of the genetic determinants of angiotensinogen concentration and the effect of sex hormones on the relationship of angiotensin to blood pressure are warranted.
Funding:
This research was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under grant number P20GM103451 and a Research Enhancement Award to PT from NIGMS under grant number SC1GM139730. Additional support for this research, including sample processing and angiotensinogen measurements was provided by Ionis Pharmaceuticals, Inc.
This MESA study used in this analysis was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
ABBREVIATIONS
- ACE-I
Angiotensin-Converting Enzyme inhibitor
- AGT
Angiotensinogen gene
- ARB
Angiotensin II receptor blocker
- Ang
Angiotensin
- AUC
area under the curve
- BMI
body mass index
- BP
blood pressure
- MESA
Multiethnic Study of Atherosclerosis
- RAAS
Renin-Angiotensin-Aldosterone System
Footnotes
Disclosures: ST is a co-inventor and receives royalties from patents owned by University of California San Diego (UCSD) and is a co-founder and has an equity interest in Oxitope, LLC and its affiliates, Kleanthi Diagnostics, LLC and Covicept Therapeutics, Inc and has a dual appointment at UCSD and Ionis Pharmaceuticals. Although these relationships have been identified for conflict-of-interest management based on the overall scope of the project, the research findings included in this particular publication may not necessarily relate to the interests of the above companies. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. MB, AEM and ESM are employees of Ionis Pharmaceuticals. The other authors have reported that they have no relationships relevant to this work to disclose.
REFERENCES
- 1.Kahlon T, Carlisle S, Otero Mostacero D, Williams N, Trainor P, DeFilippis AP. Angiotensinogen. JACC: Heart Failure 2022;10:699–713. [DOI] [PubMed] [Google Scholar]
- 2.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 2012;318:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and Pathophysiological Features of Angiotensinogen: A Mini Review. N Am J Med Sci (Boston) 2011;4:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeunemaitre X, Soubrier F, Kotelevtsev YV et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell 1992;71:169–180. [DOI] [PubMed] [Google Scholar]
- 5.Bomback AS, Toto R. Dual Blockade of the Renin-Angiotensin-Aldosterone System: Beyond the ACE Inhibitor and Angiotensin-II Receptor Blocker Combination. American Journal of Hypertension 2009;22:1032–1040. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario CM, Groban L, Wang H et al. The Angiotensin-(1–12)/Chymase axis as an alternate component of the tissue renin angiotensin system. Molecular and Cellular Endocrinology 2021;529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma TKW, Kam KKH, Yan BP, Lam Y-Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. British Journal of Pharmacology 2010;160:1273–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harel Z, Gilbert C, Wald R et al. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. Bmj 2012;344:e42–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan ES, Tami Y, Hu K et al. Antisense Inhibition of Angiotensinogen With IONIS-AGT-LRx. JACC: Basic to Translational Science 2021;6:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi AA, Nordestgaard BG, Tybjærg-Hansen A. Angiotensinogen Gene Polymorphism, Plasma Angiotensinogen, and Risk of Hypertension and Ischemic Heart Disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2003;23:1269–1275. [DOI] [PubMed] [Google Scholar]
- 11.Sethi AA, Nordestgaard BG, Grønholdt M-LM, Steffensen R, Jensen G, Tybjærg-Hansen A. Angiotensinogen Single Nucleotide Polymorphisms, Elevated Blood Pressure, and Risk of Cardiovascular Disease. Hypertension 2003;41:1202–1211. [DOI] [PubMed] [Google Scholar]
- 12.Jeunemaitre X, Gimenez-Roqueplo A-P, Célérier J, Corvol P. Angiotensinogen variants and human hypertension. Current Hypertension Reports 1999;1:31–41. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhang L, Wang H-W, Wang X-Y, Li X-Q, Zhang L-L. The M235T polymorphism in the angiotensinogen gene and heart failure: a meta-analysis. Journal of the Renin-Angiotensin-Aldosterone System 2012;15:190–195. [DOI] [PubMed] [Google Scholar]
- 14.Wu C-H, Wang Y, Ma M et al. Antisense oligonucleotides targeting angiotensinogen: insights from animal studies. Bioscience Reports 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. American Journal of Epidemiology 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 16.Burke G, Lima J, Wong ND, Narula J. The Multiethnic Study of Atherosclerosis. Global Heart 2016;11. [DOI] [PubMed] [Google Scholar]
- 17.Katsurada A, Hagiwara Y, Miyashita K et al. Novel sandwich ELISA for human angiotensinogen. American Journal of Physiology-Renal Physiology 2007;293:F956–F960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 19.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer, 2000. [Google Scholar]
- 20.Lenth R emmeans: Estimated Marginal Means, aka Least-Squares Means (R package). 1.4.8 ed, 2020. [Google Scholar]
- 21.Wickham H, Francois R, Henry L, Muller K. dplyr: A Grammar of Data Manipulation (R package). 1.0.0 ed, 2020. [Google Scholar]
- 22.Wickham H ggplot2 : Elegant Graphics for Data Analysis. Use R!,. 2nd ed. Cham: Springer International Publishing : Imprint: Springer,, 2016:1 online resource (XVI, 260 pages 232 illustrations, 140 illustrations in color. [Google Scholar]
- 23.Uijl E, Mirabito Colafella KM, Sun Y et al. Strong and Sustained Antihypertensive Effect of Small Interfering RNA Targeting Liver Angiotensinogen. Hypertension 2019;73:1249–1257. [DOI] [PubMed] [Google Scholar]
- 24.Ren L, Colafella KMM, Bovée DM, Uijl E, Danser AHJ. Targeting angiotensinogen with RNA-based therapeutics. Current Opinion in Nephrology and Hypertension 2020;29:180–189. [DOI] [PubMed] [Google Scholar]
- 25.Klett C, Ganten D, Hellmann W et al. Regulation of hepatic angiotensinogen synthesis and secretion by steroid hormones. Endocrinology 1992;130:3660–3668. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MS, Chin WW, Shupnik MA. Regulation of angiotensinogen gene expression by estrogen. Journal of Hypertension 1992;10:361–366. [DOI] [PubMed] [Google Scholar]
- 27.Stavréus-Evers A, Parini P, Freyschuss B et al. Estrogenic influence on the regulation of hepatic estrogen receptor-α and serum level of angiotensinogen in female rats. The Journal of Steroid Biochemistry and Molecular Biology 2001;78:83–88. [DOI] [PubMed] [Google Scholar]
- 28.Harvey PJ, Morris BL, Miller JA, Floras JS. Estradiol Induces Discordant Angiotensin and Blood Pressure Responses to Orthostasis in Healthy Postmenopausal Women. Hypertension 2005;45:399–405. [DOI] [PubMed] [Google Scholar]
- 29.Sarzani R, Bordicchia M, Marcucci P et al. Angiotensinogen promoter variants influence gene expression in human kidney and visceral adipose tissue. Journal of Human Hypertension 2009;24:213–219. [DOI] [PubMed] [Google Scholar]
- 30.Inoue I, Nakajima T, Williams CS et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. Journal of Clinical Investigation 1997;99:1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao YY, Zhou J, Narayanan CS, Cui Y, Kumar A. Role of C/A Polymorphism at −20 on the Expression of Human Angiotensinogen Gene. Hypertension 1999;33:108–115. [DOI] [PubMed] [Google Scholar]
- 32.Clarke L, Fairley S, Zheng-Bradley X et al. The international Genome sample resource (IGSR): A worldwide collection of genome variation incorporating the 1000 Genomes Project data. Nucleic acids research 2017;45:D854–D859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umemura S Plasma Angiotensinogen Concentrations in Obese Patients. American Journal of Hypertension 1997;10:629–633. [DOI] [PubMed] [Google Scholar]
- 34.Hilliard LM, Sampson AK, Brown RD, Denton KM. The “His and Hers” of the Renin-Angiotensin System. Current Hypertension Reports 2012;15:71–79. [DOI] [PubMed] [Google Scholar]
- 35.Toering TJ, van der Graaf AM, Visser FW et al. Gender differences in response to acute and chronic angiotensin II infusion: a translational approach. Physiological Reports 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez LA, Sullivan JC. Sex Differences in Hypertension: Where We Have Been and Where We Are Going. American Journal of Hypertension 2018;31:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. American Journal of Physiology-Heart and Circulatory Physiology 2005;288:H2177–H2184. [DOI] [PubMed] [Google Scholar]
- 38.Arendse LB, Danser AHJ, Poglitsch M et al. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacological Reviews 2019;71:539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pessôa BS, Slump DE, Ibrahimi K et al. Angiotensin II Type 2 Receptor– and Acetylcholine-Mediated Relaxation. Hypertension 2015;66:396–402. [DOI] [PubMed] [Google Scholar]
- 40.Flanagin A, Frey T, Christiansen SL. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. Jama 2021;326. [DOI] [PubMed] [Google Scholar]
- 41.Flanagin A, Frey T, Christiansen SL, Bauchner H. The Reporting of Race and Ethnicity in Medical and Science Journals. Jama 2021;325. [DOI] [PubMed] [Google Scholar]
- 42.Divers J, Redden DT, Rice KM et al. Comparing self-reported ethnicity to genetic background measures in the context of the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Genetics 2011;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


