Abstract
Colorectal cancer (CRC) is the third most common malignancy in terms of global tumor incidence, and the rates of morbidity and mortality due to CRC are rising. Experimental models of CRC play a vital role in CRC research. Clinical studies aimed at investigating the evolution and mechanism underlying the formation of CRC are based on cellular and animal models with broad applications. The present review classifies the different experimental models used in CRC research, and describes the characteristics and limitations of these models by comparing the research models with the clinical symptoms. The review also discusses the future prospects of developing new experimental models of CRC.
Keywords: colorectal cancer, cellular models, animal models, preclinical studies, drug development
1 Introduction
Colorectal cancer (CRC) is the most common malignancy worldwide, in terms of both morbidity and mortality (Sung et al., 2021). The understanding of the origin of CRC has increased dramatically over the past few decades. However, despite breakthroughs in diagnosis and treatment, CRC continues to be a major health concern worldwide. The morbidity and mortality due to CRC are on the rise owing to the overall low screening rates and changes in lifestyle, including poor diets, irregular lifestyles, smoking, and other factors (Minami et al., 2022). Strategies for the early screening and intervention of precancerous CRC lesions in developed countries have reduced the rates of incidence and mortality due to CRC (Zorzi and Urso, 2022). Similar to studies on other illnesses, research studies on CRC critically depend on experimental models with reliable and distinct characteristics. Although CRC tumors have heterogeneous characteristics, experimental models of CRC are established in such a manner that they represent the characteristics of CRC tumors. Selection of the appropriate model that reflects the tumor system is a crucial challenge in cancer screening. Therefore, experimental models of CRC have been extensively studied for determining the optimum model for studying the invasion, progression, and early detection of CRC. This review discusses the significance of CRC models as a platform for screening drugs and developing novel therapeutic approaches for CRC. The application of cellular and animal models of CRC were also summarized and discussed to aid further preclinical studies on CRC.
2 Cellular models based on intestinal cells and CRC cells
In vitro models of CRC established using intestinal cells and CRC cells are frequently employed for obtaining rapidly growing cellular models of CRC and for facilitating experimental control. In vitro models of CRC can simultaneously generate several populations of homogeneous cells. Specific cellular targets of macroscopic systems can be conveniently studied using these models by analyzing the experimental results (Saeidnia et al., 2015).
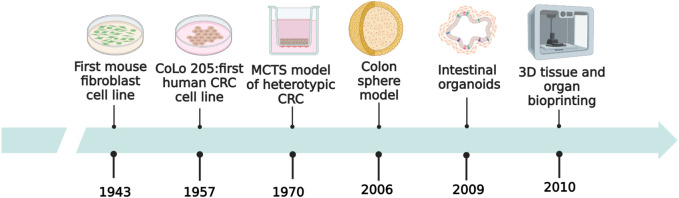
The first mammalian cell line was established in 1943, which served as a prelude to in vitro cell culture. The CoLo 205 CRC cell line was established in 1957, which promoted in vitro studies on CRC. Figure 1 depicts the history of development of in vitro models of CRC (Sanford et al., 1948; Ricci et al., 2007; Sharma et al., 2010; Jedrzejczak, 2017).
FIGURE 1.
History of development of in vitro models of CRC.
2.1 Two-dimensional (2D) cellular models of CRC
CRC cell lines are in vitro tumor models with different origins and types, and serve as fundamental tools for investigating the biomarkers of drug sensitivity, resistance, and toxicity. CRC cell lines are established by isolating CRC cells from patients or animals with CRC followed by culture on artificial media. The appropriate cell lines are selected based on the type of cancer or gene expression levels, according to the aims of the study. SW620, Caco-2, RKO, SW480, HT8, HT29, HT116, LoVo, and LS174 T cell lines are currently widely used in basic research studies on CRC (Akashi et al., 2000; Vécsey et al., 2002; Lind et al., 2004; Barretina et al., 2012; Ahmed et al., 2013; Gemei et al., 2013; Mouradov et al., 2014; Maletzki et al., 2015; Boot et al., 2016; Berg et al., 2017; Mooi et al., 2018; Kim et al., 2020; Bian et al., 2021).
Although the characteristics of CRC cell lines are highly consistent with those of human cancer models, they have certain limitations. CRC cell lines facilitate the investigation of the molecular and phenotypic characteristics of CRC. However, as only one side of the cells is in contact with the medium during culture, the majority of cells gradually flatten, undergo abnormal division, and lose their differentiation phenotype following isolation from tissues and plate culture. Additionally, CRC cells continue to proliferate in vitro, which may cause the cell lines to lose the characteristics of the original tumor. Another limitation of CRC cell lines is the scarcity of matrix ingredients in the tumor microenvironment (TME), including the cells and acellular components constituting the structural complexity of the in vivo environment. Altogether, these indicate that CRC cell lines fail to accurately mimic the in vivo growth characteristics of tumor cells.
2.2 Three-dimensional (3D) cellular models of CRC
Owing to the limitations of 2D cellular models of CRC, researchers are committed towards exploiting novel and physiologically representative models of CRC. In vitro 3D culture models, including spheroids and organoids, are therefore used for overcoming the limitations of 2D cellular models. Spheroids comprise a mixture of single-cell or multicellular systems, while organoids are generally formed of specific stem cells or ancestral cells from organs (Kimlin et al., 2013; Boucherit et al., 2020). Spheroids and organoids are superior at mimicking tumor cell heterogeneity and the complex interactions among different cells (Thoma et al., 2014).
2.2.1 Spheroids
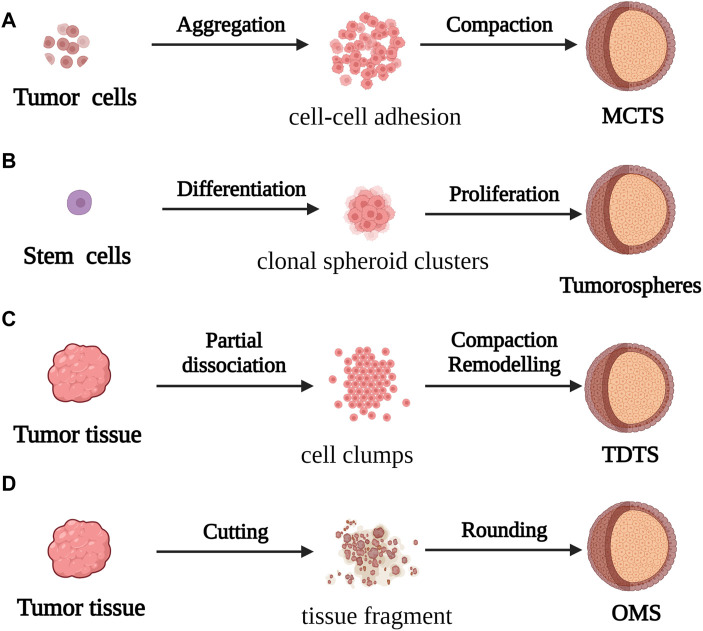
Spheroids are one of the most commonly used models in CRC research. They are constructed by suspending cancer cell lines or isolated tumor tissues from patients in CRC. They have a convenient mode of production and application, and are particularly effective for studying micrometastases or avascular tumors. Spheroid models can be categorized into four types according to the origin and morphology of the cancer cells from which they are derived. These categories include multicellular tumor spheroids (MCTS), tumorospheres, tissue-derived tumor spheres (TDTS), and organotypic multicellular spheroids (OMS; Figure 2) (Weiswald et al., 2015).
FIGURE 2.
For the formation process of spherical cancer models (A) MCTS: Cell suspensions cultured under non-adherent conditions were aggregated and compacted to obtain MCTS; (B) Tumorospheres: Stem cells cultured under low-adherent conditions formed Tumorospheres by clonal proliferation (C) TDTS: Partial dissociation of tumor tissue and compaction/remodeling produced TDTS; (D) OMS: Cut tumor tissue aggregates formed OMS during culture under non-adherent conditions.
MCTS models, first constructed by Bauleth-Ramos, consist of colonic epithelia, human intestinal fiber cells, and human mononuclear cells, and are inoculated into hydrogel microwells to form the spheroid model (Inch et al., 1970; Bauleth-Ramos, T et al., 2020). MCTS models are similar to solid tumors in terms of the growth kinetics, metabolic rate, and resistance to chemotherapy and radiotherapy in vivo (Ivascu and Kubbies, 2006), and have been employed for screening and evaluating the efficacy of drugs. However, the variability of MCTS models makes it difficult to obtain repeatable and stable experimental data, which affects the use of these models in tumor research.
The tumorosphere model of CRC stem cells (CSCs) was used in the early 2000s for evaluating the differentiation capacity of tumors. However, because there are no morphological phenotypes associated with the phenotypic instability of CSCs, the tumorosphere model is unable to faithfully simulate the in vivo 3D framework and physiological condition of tumors (Valent et al., 2012).
The TDTS models consist of cancer and stromal cells, and are commonly used in studies on CRC. TDTS models of CRC tumors have a unique histological feature similar to the poorly differentiated globules produced by permanent cancer cell lines, and can fully simulate the characteristics of in vitro 3D cell culture models of CRC (Santini and Rainaldi, 1999; Weiswald et al., 2009).
OMS models are enriched in stem cells which can represent the complexity of parental tumor cells similar to in vivo tissues by forming an extracellular layer of epithelioid cells and an intracellular layer of mesenchymal cells, and thus maintaining the multicellular nature of CRC (Rajcevic et al., 2014). However, the difficulty of producing homogeneous spheres in a reproducible manner combined with the insufficiency of stable experimental data can prove to be a challenge during the application of the OMS model in CRC research and drug development.
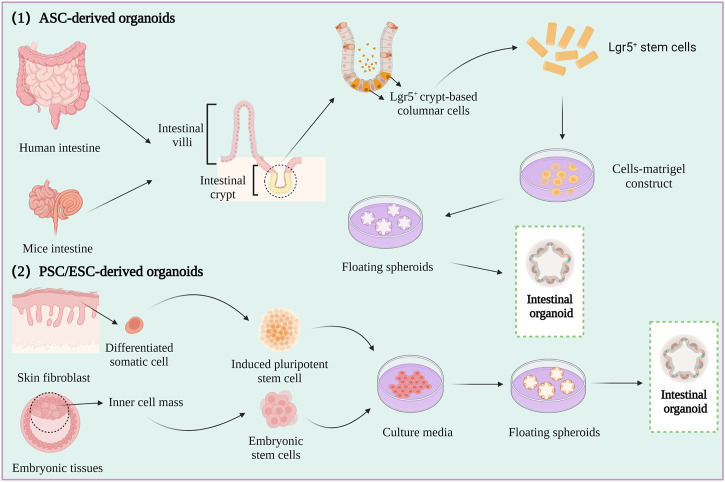
2.2.2 Organoids
Spheroids are a simple experimental model that only partly represent the in vivo characteristics of tumor tissues. However, organoids are relatively complex three-dimensional (3D) culture models that are frequently used in CRC research. Organoids are self-organizing organotypic cultures that are produced from various stem cells, including tissue specific adult stem cells (ASCs), embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs) (Fujii et al., 2018; Fujii and Sato, 2021). The stem cells are grown in matrigel 3D culture conditions to mimic the in vivo growth environment, and to produce stable, near-physiological epithelial structures (Figure 3) (Lancaster and knoblich, 2014; Huch and Koo, 2015).
FIGURE 3.
Intestine organoid cultures.
The first intestinal epithelial 3D organoids were constructed by growing leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5+) intestinal stem cells in a medium containing stem cell niche restatement factors and tissue-specific growth factors (Sato et al., 2011). An increasing number of studies have described the formation of patient-derived organoids (PDOs) by culturing minced human CRC tumors in human intestinal stem cell medium (HISC), and the phenotype and genotype of the PDOs have been reported to be highly similar to those of the original tumor (Van et al., 2015; Vlachogiannis et al., 2018).
Organoids are typically used for investigating the mechanism underlying the development of CRC, screening anti-CRC drugs, and determining the efficacy and mechanism of action of drugs. However, there are various limitations to the application of organoids in studies on CRC, which are described hereafter. First, the current methods for organoid culture lack the technological means for maintaining the blood vessels, immune system, and peripheral nervous system of tumor cells, and organoids lacking these characteristics cannot be used in CRC research (Bredenoord et al., 2017). Second, as PDO models lack the cellular and acellular components of the TME of the original tumor, they cannot equivalently represent the in vivo environment of the tumor (Li X. et al., 2020). Third, there are no specific media for culturing organoids to date. Furthermore, it is unclear whether organoids can represent the overall heterogeneity of the tumor and all cell types in the tumor. Organoids can be applied to relevant studies by optimizing the culture conditions for maintaining the expression of genes related to microsatellite instability, B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutations, poor differentiation, or mucinous phenotypes related to CRC. The application of organoids to CRC research can be improved by employing the co-culture model of organoids in which immune cells and mesenchymal cells are co-cultured for simulating the in vivo TME.
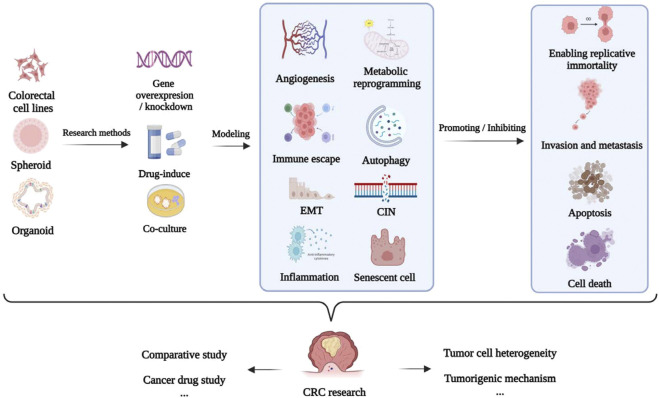
2.3 Application of cellular models of CRC
The establishment of models using the corresponding tumor cells is crucial for investigating the mechanism underlying the development of CRC and discovery of anti-CRC drugs (Senga and Grose, 2021). The applications of different cellular models of CRC according to the different molecular mechanisms underlying tumor formation, including epithelial–mesenchymal transition (EMT), apoptosis, invasion, metastasis, chromosome instability (CIN), and immune escape, are summarized in Table 1 and Figure 4.
TABLE 1.
Applications of cellular models of CRC.
| Mechanism being investigated | Research model | Cell lines | References |
|---|---|---|---|
| Apoptosis | Induction of apoptosis via the overexpression of neurofibromin (NF2), heterogeneous nuclear ribonucleoprotein L (HNRNPL), and other genes | HCT116 and SW620 | Wu et al. (2020) |
| HIEC, Caco2, HCT116, LoVo, and SW480 | Zhao et al. (2021) | ||
| Induction of apoptosis via the knockdown of ribosomal protein lateral stalk subunit P0 pseudogene 2 (RPLP0P2), Cadherin 17 (CDH17), and other genes | HCT116, HT29, SW480, and RKO | Yuan et al. (2021) | |
| KM12SM, KM12C, Colo320, HT29, RKO, and SW480 | Tian et al. (2018) | ||
| Inhibition of apoptosis via the knockdown of receptor interacting protein kinase 3 (RIP3) | SW480, HCT-116, RIP3+/+−MEF, and RIP3−/−MEF | Han et al. (2018) | |
| Inhibition of glycolysis and promotion of apoptosis via the knockdown of hypoxia-inducible factor-1α (HIF-1α) | FHC, CCD841 CoN, HT29, SW480, LoVo, HCT116, and SW620 | Liu et al. (2019) | |
| Cu nanoparticles (CuNPs)-induced apoptosis of CRC cells | SW480 | Ghasemi et al. (2020) | |
| Autophagy | Inhibition of autophagy with chloroquine | HCT116 and SW480 | Ma et al. (2020) |
| Rapamycin-induced model of autophagy | KM12SM, KM12C, Colo320, HT29, RKO, and SW480 | Tian et al. (2018) | |
| Angiogenesis | Inhibition of angiogenesis via the knockdown of cellular-myelocytomatosis viral oncogene (c-Myc), vascular endothelial growth factor (VEGF), and other genes | HCT116 | Yin et al. (2010) |
| Co-culture of patient-derived cancer-associated fibroblasts (CAFs) and HUVECs | Patient-derived CAFs | Unterleuthner et al. (2020) | |
| Invasion and metastasis | Promotion of invasion and metastasis via the overexpression of zinc-finger protein 326 (ZNF326), metastasis associated 1 family member 3 (MTA3), and other genes | SW480, SW620, CL187, and RKO | Yang et al. (2021) |
| LoVo and HCT15 | Jiao et al. (2017) | ||
| Inhibition of invasion and metastasis via the overexpression of t-box transcription factor 5 (TBX5) | HT29, SW620, SW480, LoVo, and HCT116 | Dong et al. (2020) | |
| Inhibition of invasive metastasis via the knockdown of sphingosine phosphate lyase 1 (SGPL1), forkhead Box O6 (FOXO6), and other genes | DLD-1, Caco-2, and CCD 841 CoN | Faqar et al. (2021) | |
| HCT116-CSC | Zou et al. (2022) | ||
| NCM460, Caco2, HT29, HCT116, and SW480 | Li et al. (2019) | ||
| Co-culture of EMT-CRC cells and HUVECs | NCM460, LoVo, HCT-116, DLD-1, SW620, and SW480 | Dou et al. (2021) | |
| Metabolic reprogramming | Reprogramming of energy metabolism via the overexpression of mitochondrial citrate carrier solute carrier family 25 member 1 (SLC25A1), human kallikrein 2 (HK2), and other genes | NCM460, SW480, HCT116, SW620, LoVo, LS174T, and HT29 | Yang et al. (2021a) |
| Inhibition of metabolic reprogramming via HIF-1α knockout | HCT8, HCT15, HCT116, LoVo, SW480, SW1116, HT29, Caco-2, DLD-1, and T84 | Dong et al. (2022) | |
| Immune escape | Promotion of immune escape via lipopolysaccharide (LPS)-induced macrophage infiltration | HCT-8, HCT-116, SW620, SW480, DLD-1, CaCo-2, CT26, and HT-29 | Liu et al. (2020a) |
| Induction of immune escape via the overexpression of antigen-presenting-cell, B7 homolog x (B7x), and other genes | HCA-7, HT-29, 293T, and TALL-104 | Cen et al. (2021) | |
| LoVo, Colo-205, SW480, SW620, HCT-116, CT-26, and MC-38 | Li et al. (2020c) | ||
| Inflammation | LPS-induced model of inflammation | HCT116 and SW480 | Zhu et al. (2019) |
| — | Schafer and Werner (2008) | ||
| Colon 26 | Choo et al. (2005) | ||
| — | Schottelius and Baldwin (1999) | ||
| Induction of tumor necrosis factor-α (TNF-α), nuclear factor-kappa B (NF-kB), and other pro-inflammatory factors | Caco-2, HT29, SW480, SW48, and DLD1 | Li et al. (2012) | |
| Volo | Tai et al. (2012) | ||
| EMT | Suppression of EMT via the knockdown of Pleckstrin homology-like domain family A member 2 (PHLDA2), SRY-Box transcription Factor 2 (SOX2), and other genes | HCT116 and SW480 | Ma et al. (2020) |
| SW480 and SW620 | Zhu et al. (2021) | ||
| HCT116 and LoVo | Qi et al. (2021) | ||
| HCT116 and DLD-1 | Ju et al. (2020) | ||
| HCT116, SW480, HT29, and SW620 | Hua et al. (2020) | ||
| Induction of EMT via interleukin-6 (IL-6), TNF-α, and other inflammatory factors | SW480, SW620, and Caco-2 | Rokavec et al. (2014) | |
| HCT116 and Caco-2 | Wang et al. (2013) | ||
| Induction of EMT via the overexpression of cryopyrin-associated periodic syndromes 1 (CAPS1), nuclear factor of activated T-cells (NFATc1), and other genes | FHC, HT29, SW480, SW620, and DLD1 | Zhao et al. (2019) | |
| SW620, LoVo, Caco-2, SW480, HT29, HCT116, and DLD-1 | Shen et al. (2021) | ||
| HCT116 | Li et al. (2021) | ||
| Induction of EMT by X-ray irradiation | SW480 | Lin et al. (2017) | |
| Genomic instability/mutation (CIN) | — | CRC PDOs | Bolhaqueiro et al. (2019) |
| Induction of CIN by DNA damage caused by the overexpression of iroquois homeobox gene 5 (IRX5), integrin-linked kinase (ILK), and other genes | SW480 and DLD-1 | Sun et al. (2020) | |
| HCT116 | Chadla et al. (2021) | ||
| Senescent cells | Induction of cellular senescence via the overexpression of lamin B1 (LMNB1), tribbles homolog 2 (TRIB2), and other genes | SW480, HT29, and IEC-6 | Liu et al. (2013) |
| HEK 293 T, SW48, and LoVo | Hou et al. (2018) | ||
| Drug-induced senescence of CRC cells using oxaliplatin, adriamycin, aspirin, and other drugs | SW620 and HCT116 | Jung et al. (2015) | |
| SW837, HCT116, and SW48 | Tato-Costa et al. (2016) | ||
| PROb and CT26 | Seignez et al. (2014) | ||
| HCT116 | Vétillard et al. (2015) | ||
| HCT116 and SW480 | Zhang et al. (2011) | ||
| C85 | Dabrowska et al. (2011) | ||
| Dabrowska et al. (2019) |
FIGURE 4.
Application of CRC cellular models.
3 CRC animal models based on experimental animals
The occurrence of diseases such as cancer that occur spontaneously in animals is largely attributed to genetic diversity and immune functions. Therefore, studying the methods for generating animal models of CRC can aid in elucidating the mechanisms underlying the development of cancer (Marian, 2004). Animal models can compensate for the limitations of cellular models that are incapable of simulating the mechanism underlying the development of CRC. Rat and murine models are the most frequently used animal models of CRC, and other animal models of CRC, including fruit fly, zebrafish, and pigs, are also commonly used as sentinels and preclinical models in CRC research.
3.1 Rodent models
Rodent models are conducive tools for conducting cancer research, and are extensively used for elucidating the etiopathogenesis and molecular mechanisms underlying the development of CRC. Previous studies have demonstrated that the protein-coding genes of mice and humans share high homogeneity (Mouse Genome Sequencing Consortium, 2002). Additionally, the use of murine models is advantageous owing to the fact that mice have a short intergenerational interval, high reproducibility, and similar genetic background and formula as humans, compared to other animal models. Murine models of CRC can therefore be used as effective tools for studying the mechanism underlying the pathogenesis of CRC and determining novel strategies for the prevention and treatment of CRC (Doyle et al., 2012).
Transgenic mice models can serve as effective tools for preclinical evaluation and screening during the optimization and development of anticancer drugs. Mutations in APC (adenomatous polyposis coli) are commonly inherited in adenoma-carcinoma transitions observed during the development of CRC (Van et al., 2000). Additionally, the absence of mutations in DNA mismatch repair (MMR) genes increases deletion mutations in APC, which accelerates the formation of adenomas (Huang et al., 2004). It has been reported that mutations in tumor protein 53 (p53), Kirsten rats arcomaviral oncogene homolog (KRAS), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), F-box and WD repeat domain containing 7 (FBXW7), SMAD family member 4 (SMAD4), transcription factor 7-like 2 (TCF7L2), NRAS proto-oncogene (NRAS), AT-rich interaction domain 1 A (ARID1A), SRY-box transcription factor 9 (SOX9), and APC membrane recruitment protein 1 (FAM123B) can also increase the risk of CRC (Cancer Genome Atlas Network, 2012). Transgenic murine models are extensively used for studying the occurrence and elimination of tumors, underlying molecular pathways, and genomic regulation via gain-of-function or loss-of-function mutations in oncogenes and cancer suppressor genes.
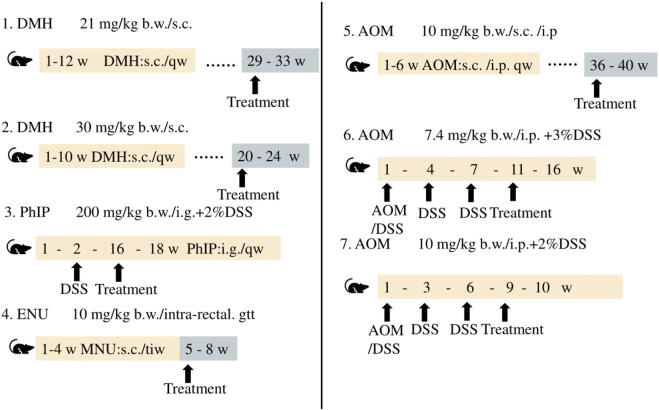
CRC is caused by various risk factors, including poor dietary habits, environment, exposure to carcinogenic chemicals, and other factors (Hecht, 2003; Mehta et al., 2017). Animal models of CRC generated by treatment with chemicals serve as effective models in studies aimed at determining novel therapeutic approaches and investigating the diagnosis, prognosis, and identification of predictive markers. The differences among the methods and duration of treatment for inducing CRC with different chemical agents are depicted in Figure 5.
FIGURE 5.
Chemically induced CRC animal models.
The use of chemical agents for generating models of CRC requires a long duration and these models have longer experimental cycles. Mofikawa et al. established the first orthotopic transplantation model of CRC in 1986 by transplanting human CRC cells under the cecal wall of nude mice. This shortened the period of study using animal models of CRC, and initiated the establishment of tumor transplantation models. Table 2 summarizes the different murine models of CRC, and describes their scope of application and limitations in tumor research.
TABLE 2.
Murine models of CRC.
| Model | Strategy for model generation | Pathological mechanism | Detailed methodology | Range of application | Limitations | References |
|---|---|---|---|---|---|---|
| Spontaneous animal model of CRC | Mutant animal models of CRC | Proliferation | Mutation in APC | FAP model for studying hereditary CRC | Survival time < 4 months, tumor formation in small intestine, difficulty in metastasis | Moser et al. (1990) |
| Shoemaker et al. (1997) | ||||||
| Shoemaker et al. (1998) | ||||||
| Barker et al. (2007) | ||||||
| Mutation in APC/Cre | Induction of colorectal adenoma | Difficulty in metastasis | Robanus-Maandag et al. (2010) | |||
| Chen et al. (2020) | ||||||
| Mutations in Mlh1, Msh2, Msh3, Msh6, and Pms2 | Hereditary nonpolyposis CRC (HNPCC) | Multi-tissue tumors, difficulty in metastasis | Lynch et al. (1997) | |||
| Papadopoulos and Lindblom (1997) | ||||||
| Manceau et al. (2011) | ||||||
| Mutation in SMAD4 | Familial juvenile polyposis model, acceleration of tumor development | Difficulty in metastasis | Takaku et al. (1998) | |||
| Lu et al. (1998) | ||||||
| Mutation in KRAS | Induction of colonic hyperplasia and generation of aberrant crypt foci (ACF) carcinogenesis model | CRC cannot be induced by mutations in single genes, but is induced in combination with other gene mutations that induce carcinogenesis and enhance the incidence of CRC. | Bos et al. (1987) | |||
| Campbell et al. (1998) | ||||||
| Jen et al. (1994) | ||||||
| Janssen et al. (2002) | ||||||
| Janssen et al. (2006) | ||||||
| Calcagno et al. (2008) | ||||||
| Mutation in PIK3CA | Induction of colon adenoma | Single mutations generally do not induce CRC. | Juric et al. (2018) | |||
| Invasion and metastasis | Mutation in FBXW7 | Model of highly invasive colorectal cancer | Single mutations generally do not induce CRC. | Mao et al. (2004) | ||
| Mutation in p53 | Induction of distal intestinal tumor | Single mutations generally do not induce CRC. | Nakayama et al. (2017) | |||
| Kadosh et al. (2020) | ||||||
| Diet- and chemical-induced models of CRC | Diet-induced models of CRC | Inflammation | High-fat diet (HFD)/western diet (NMD) | Colorectal barrier dysfunction and inflammation, invasive adenocarcinoma | Requires a long duration and has a low carcinogenic efficiency | Itano et al. (2012) |
| Yu et al. (2022) | ||||||
| Chemical-induced models of CRC | 2,4,6-Trinitro-benzenesulfonic acid (TNBS) | Induction of colitis-driven CRC | Cannot be used alone, necessary to break the intestinal mucosal screen before use, mortality rate of modeling is high | Scheiffele and Fuss (2002) | ||
| Anaerobic oxidation of methane (AOM) + dextran sodium sulfate (DSS) | Tumors driven by colitis, induced distal CRC | The modeling rate is low and molding time is uncertain | Neufert et al. (2007) | |||
| De-Robertis et al. (2011) | ||||||
| Liang et al. (2017) | ||||||
| Sun et al. (2022) | ||||||
| Proliferation | AOM | ACF and CRC epithelial tumor model | The period of modeling is long and time-consuming, cannot be used for studying CRC metastases | Femia and Caderni (2008) | ||
| Izzo et al. (2008) | ||||||
| Orlando et al. (2008) | ||||||
| 1,2 Dimethyl hydrazine (DMH) | Human sporadic CRC research model, tumorigenicity specificity | Requires a long time and has a low carcinogenic efficiency | Ma et al. (1996) | |||
| Kissow et al. (2012) | ||||||
| Aranganathan and Nalini (2013) | ||||||
| Parahydrogen-induced polarization (PhIP) | ACF-induced rat model | Low incidence, long study cycle | Ito et al. (1991) | |||
| Tanaka et al. (2005) | ||||||
| 3,2′-Dimethyl-4-Aminobiphenyl (DMAB) | Induced colon and small intestinal carcinogenesis | Requires multiple administration, low specificity | Reddy and Mori (1981) | |||
| Reddy (1998) | ||||||
| CIN | N-ethyl-N-nitrosourea (ENU)/N- methyl -N-nitrosourea (MNU)/N-methyl-N-nitrosoguanidine (MNNG) | Induced distal CRC model | Induced mutations are random and drug volume quantification is difficult | Huang et al. (2020) | ||
| Animal model of transplanted CRC | Animal model of orthotopic tumor transplantation | Invasion and metastasis | Cecal transplantation | Induction of primary CRC that can metastasize to local lymphatic vessels, lungs, and liver | Risk of laparotomy is high in this model. CRC originates from the mucosa, and whether tumor metastasis results from the overflow of intraperitoneal cells cannot be excluded | Talmadge et al. (2007) |
| Martin et al. (2013) | ||||||
| Lee et al. (2014) | ||||||
| O'Rourke et al. (2017) | ||||||
| Animal model of ectopic tumor transplantation | Spleen planting | Study of advanced CRC | The operation is complex and requires highly advanced technical skills | Kasuya et al. (2005) | ||
| Bai et al. (2015) | ||||||
| Yang et al. (2021c) | ||||||
| Tail vein injection | Lung metastasis model of CRC | Differs from human CRC metastasis, multiple metastases are prone to occur | Wang et al. (2020) | |||
| Liver implantation | Liver metastasis model of CRC | Only the late metastatic process of CRC is simulated; tumor forms only at the site of implantation | Panis and Nordlinger (1991) | |||
| Kopetz et al. (2009) | ||||||
| Roque et al. (2019) | ||||||
| Intraperitoneal injection of CRC cell for inducing metastasis | Peritoneal metastasis model of CRC | Unsuitable for studying early metastasis of lymph nodes in CRC. | Li et al. (2016) | |||
| Proliferation | Hypodermic implantation | Real-time monitoring of CRC growth | Cannot simulate the in situ growth of CRC, not easy to study tumor invasion and metastasis | Rygaard and poulsen (1969) | ||
| Lehmann et al. (2017) |
3.2 Other animal models of CRC
In addition to rodents, invertebrates such as fruit fly can be used for personalized diagnosis and developing potential therapeutic strategies for CRC. Vertebrates such as zebrafish, dogs, cats, pigs, and non-human primates are also used in studies on CRC. The advantages and disadvantages of the different animal models used in CRC research are summarized in Table 3.
TABLE 3.
Other animal models of CRC.
| Classification | Animal | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Invertebrate | Drosophila melanogaster (fruit fly) | The model can represent the composition of mammalian intestinal cells, aids in avoiding cancer heterogeneity | The model has no acquired immune function and has a short life cycle. It is impossible to simulate the complexity of tumor development | Bhandari and Shashidhara, 2001 Martorell et al., 2014 |
| Vertebrate | Danio rerio (zebrafish) | Histopathological features of intestinal tumors are similar to those of human tumors. High transparency of seedlings, small size, short developmental cycle, in vitro fertilization, and large number of eggs. Requires small experimental dosage and is less time-consuming | The culture temperature is inconsistent with the growth temperature of tumor cells. Long-term tumor transplantation experiments cannot be performed | Amatruda et al. (2002) |
| Trede et al. (2004) | ||||
| Haldi et al. (2006) | ||||
| Brugman et al. (2009) | ||||
| Paquette et al. (2013) | ||||
| Canis lupus familiaris (Dog) | The model has a similar physiological structure to humans, and the mechanism of pathogenesis is similar to sporadic CRC in humans. Gentle character, good experimental coordination, and repeatability | Long duration of modeling, observational inconveniences, not suitable for acute experiments | Kamano et al. (1981) | |
| Kamano et al. (1983) | ||||
| Youmans et al. (2012) | ||||
| Felis catus (Domestic cat) | The histological subtype of the model is similar to that of advanced CRC in humans. Model can be used for studying the germination of intestinal tumor in CRC. | Low incidence, tumors mostly occur in the small intestine | Uneyama et al. (2021) | |
| Groll et al. (2021) | ||||
| Sus scrofa (Pig) | The anatomical structure of the small intestine is similar to that of humans. Model has a moderate size and long life. The progression and accumulation of mutations in CRC can be monitored by colonoscopy screening | The model cannot be used to study acute CRC as the process of cancer formation is slow | Llanos et al. (2006) | |
| Sangild et al. (2006) | ||||
| Flisikowska et al. (2012) | ||||
| Dean (2013) | ||||
| Flisikowska et al. (2017) | ||||
| Gonzalez et al. (2019) | ||||
| Ovis aries (Sheep) | Cellular differentiation in the model is similar to that of colon adenocarcinoma in humans. Model can be used to study advanced CRC. | Adenocarcinoma develops in the small intestine | Munday et al. (2006) | |
| Macaca mulatta (Rhesus monkey) | Shares high genomic homology with humans; anatomical and physiological similarities. Shares same clinicopathological features as human Lynch syndrome | Research cycle or modeling time-consuming | Bakken et al. (2016) | |
| Dray et al. (2018) | ||||
| Ozirmak et al. (2022) |
3.3 Application of animal models of CRC
The carcinogenesis of CRC is affected by several contributing factors. The selection of the animal model of CRC depends on the purpose of the study, as summarized in Table 4.
TABLE 4.
Applications of animal models of CRC.
| Purpose of study | Research methods/models | References |
|---|---|---|
| Studying apoptosis in CRC | Investigation of apoptosis in CRC with CRC xenograft models | Han et al. (2018) |
| Li et al. (2020a) | ||
| Investigation of angiogenesis in CRC | Studying the effect of AOM/DSS-induced expression of severe acute respiratory infection (SARI) gene on angiogenesis in CRC | Dai et al. (2016) |
| DMH/DSS-induced expression of CRC angiogenesis factor in rat model | Liu et al. (2015a) | |
| Induction of tumor angiogenesis in vivo via the expression of VEGF and interleukin-8 (IL-8) | Liu et al. (2015b) | |
| Studying angiogenesis in CRC xenografts following induction with drugs, C-X-C motif chemokine ligand 12 (CXCL12), and CXCL11 | Rupertus et al. (2014) | |
| Yu et al. (2005) | ||
| Jakopovic et al. (2020) | ||
| Drug-induced in vivo inhibition of angiogenesis | Petrović et al. (2020) | |
| Dickkopf associated protein 2 (DKK2)-induced angiogenesis in CRC xenografts | Ding et al. (2016) | |
| Deng et al. (2019) | ||
| Inhibition of angiogenesis by potentially inappropriate medication (PIM) kinase in orthotopically transplanted CRC tumors | Casillas et al. (2018) | |
| Induction of angiogenesis by hepatectomy in CRC xenografts | Lo et al. (2018) | |
| EG-VEGF induced angiogenesis in orthotopically transplanted CRC tumors | Goi et al. (2004) | |
| Investigation of metabolic reprogramming in CRC | Induction of metabolic reprogramming in CRC xenograft model using hexokinase, free fatty acid (FFA), acetyl coenzyme A, citrate, and other agents | Bu et al. (2018) |
| Wang et al. (2018) | ||
| Dong et al. (2022) | ||
| Zhang et al. (2022) | ||
| AOM/DSS-induced CRC model of metabolic reprogramming | Wu et al. (2020) | |
| Yin et al. (2021) | ||
| Initiation of metabolic reprogramming by DSS-induced inflammation | Qu et al. (2017) | |
| Study of invasion and metastasis in CRC | CRC xenograft model for studying invasion and metastasis in CRC | Rokavec et al. (2014) |
| Erreni et al. (2016) | ||
| Li et al. (2019b) | ||
| Study of immune escape in CRC | Gene mutation-induced model of immune escape | Xing et al. (2021) |
| Wei et al. (2022) | ||
| Generation of immune escape model by ablation of zebrafish macrophages using chlorophosphonate liposomes | Póvoa et al. (2021) | |
| Study of inflammation in CRC | TNBS/oxazolone/DSS-induced inflammatory CRC | Wirtz et al. (2007) |
| LPS/DSS-induced inflammation | Garlanda et al. (2004) | |
| DSS-induced inflammation of intestinal epithelium and mucosa | Mashimo et al. (1996) | |
| Van et al. (2006) | ||
| DSS/AOM-induced inflammation in sporadic CRC | De et al. (2019) | |
| Liang et al. (2017) | ||
| TNBS-induced inflammation | Scheiffele and Fuss (2002) | |
| DMH-induced inflammation | Kumar et al. (2019) | |
| Radiofrequency ablation (RFA)-induced inflammation | Shi et al. (2019) | |
| HFD-induced inflammation | Hu et al. (2021) | |
| Gene mutation-induced inflammatory CRC | Puppa et al. (2011) | |
| De et al. (2020) | ||
| High-iron diet-induced inflammatory CRC | Seril et al. (2006) | |
| Investigation of the mechanism of EMT in CRC | Induction of EMT models via mutations/overexpression/knockdown p rostate transmembrane protein androgen induced 1 (PMEPA1), SOX2, histone deacetylase 1 (HDAC1), and other genes | Wang et al. (2014) |
| Matsuda et al. (2016) | ||
| Li et al. (2017a) | ||
| Zhuang et al. (2018) | ||
| Yang et al. (2019) | ||
| Zhang et al. (2019) | ||
| Liu et al. (2020b) | ||
| Shen et al. (2021) | ||
| Qi et al. (2021) | ||
| Zhu et al. (2021) | ||
| Liu et al. (2020) | ||
| Transforming growth factor-β (TGF-β)-induced model of EMT | Li et al. (2021) | |
| Tumor EMT-induced metastatic model of CRC | Adams et al., 2021 | |
| Epigenetic reprogramming | CRC xenograft model for studying epigenetic reprogramming in CRC | Kodach et al. (2021) |
| Induction of gene mutation for studying epigenetic reprogramming in CRC | Hashimoto et al. (2017) | |
| Study of cell aging in CRC | Xenotransplantation model for studying cellular aging in CRC | Gao et al. (2010) |
| Liu et al. (2013) | ||
| Mikuła et al. (2015) | ||
| Hou et al. (2018) | ||
| DMH/DSS-induced model of cellular aging | Liu et al. (2013) | |
| AOM/DSS-induced model of cellular aging | Foersch et al. (2015) | |
| Polymorphic microbiota | AOM/DSS-induced model for studying composition of intestinal microbiota | Wu et al. (2016) |
Traditional Chinese medicine (TCM) and western medicine are two different medical theoretical systems. The research model based on the etiological mechanism theory of TCM is applied to animal studies with TCM syndrome, as shown in Table 5.
TABLE 5.
Applications of animal models of CRC.
| TCM syndrome | Research methods/models | References |
|---|---|---|
| CRC with spleen qi deficiency syndromeHou et al., 2018 (SDS) | Restricted feeding/fatigue/purging + hypodermic implantation of C26 tumor cells to establish a spleen deficiency with cachexia model | Zhang et al. (2020) |
| CRC with damp-heat syndrome (DHS) | HFD/AOM/DSS-induced malignant tumor (stasis-toxin) model | Cao and Zhou (2020) |
| Huang et al. (2022) | ||
| CRC with internal retention of toxin stagnation syndrome (IRTSS) | LPS tail vein and peritoneal injection + hypodermic implantation of C26 tumor cells to establish colorectal tumor-bearing with syndrome of heat-toxicity and blood stasis model | Li et al. (2017b) |
4 Conclusions and future directions
Understanding the inherent advantages and limitations of the different models of CRC, and the appropriate application of these models in drug development and studies on the mechanism of tumor occurrence and development are important in CRC research.
Human cell lines and xenograft models have been extensively employed over the past few decades owing to their low cost and ease of application. However, these models are incapable of reproducing the heterogeneity of CRC tumors (Harma et al., 2010). The cell co-culture technique can overcome the limitations of monolayer cell culture, and enables the construction of in vitro physiological or pathological models that closely represent the in vivo condition, and can be used for studying the interactions between cells, and between cells and the culture environment. It has been reported that 3D models can mimic the physiological characteristics of parental tumors, including tumor heterogeneity (Li et al., 2019). However, the shape, size, and activity of organoids are different under the same culture conditions, and the matrix limits the penetration of drugs and hinders drug screening (Zhao et al., 2020). It is therefore imperative to construct a model that closely represents the characteristics of CRC in vivo.
The intestinal microarray platforms used in CRC research, which consist of intestinal organoids and organic chips, can summarize the important structural features and functions of the natural duodenum. This platform can be applied for studying drug conveyance, metabolism, and drug-drug interactions (Kasendra et al., 2018). Multi-locus transfer chips consist of multiple 3D organoids that connect the CRC-like organs, liver, lungs, and endothelial flow via recirculating fluid systems, and enables cell tracking by fluorescence imaging technology. The transfer sites of CRC cells are also included in multi-locus transfer chips (Aleman and Skardal, 2019).
Animal models of CRC have been widely used for studying the complexity of CRC. There are primarily two types of animal models, namely, in situ models and the cell and tissue transplantation models of CRC. Owing to the relatively simple modeling approach of human tumor xenotransplantation, this model is presently widely used for studying the efficacy of anti-CRC drugs. The effects of CRC xenotransplantation can be closely related to clinical activity via the rational application of these models. For instance, genetically engineered murine models have been used for studying the progression of tissue-specific molecular changes in CRC by determining the effect of specific molecular targets. Chemical induced-CRC animal model is one of the most commonly CRC models, in which CAC model is usually induced by AOM/DSS to study the mechanism of inflammation related-tumorigenesis and development (Zeng et al., 2022). The CRC model with TCM syndrome is an artificial disease and syndrome experimental animal model created by simulating and replicating characteristics of human disease prototype according to TCM theory. An animal model combining with CRC and TCM syndromes might be useful to mimic the clinical characteristics of CRC patients with TCM syndrome (Zhang et al., 2020). Mouse is the commonly used to the models mentioned above, however, it is increasingly accepted that the use of larger animal models, especially dogs and pigs, can provide deeper insights in cancer research (Croker et al., 2009).
The application of molecular tools and genetic strategies has aided the advancement of cancer research, and the cellular and animal models of CRC are being continually improved. Further understanding of the genetic and epigenetic events in CRC, including the alterations in molecular networks associated with the initial stages of development, are facilitated by high-resolution approaches.
Although CRC research has advanced immensely in recent years, several clinical issues remain to be resolved to date, which is partly attributed to the absence of suitable preclinical research models. The application of in vivo and in vitro models in CRC research, combined with advanced scientific techniques for simulating a more realistic tumor environment in vivo and in vitro, can help replicate the complex scenarios of tumor occurrence and development, identify novel therapeutic approaches for inhibiting tumor growth, and elucidate the molecular mechanisms underlying tumor formation.
Acknowledgments
The authors must be grateful to the BioRender (www.biorender.com), as the figures in this review were drawn by using the BioRender platform.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82074318, 81930117 and 82004310), Natural Science Foundation Youth Project of Jiangsu Province (BK 20200846), Natural Science Research of Jiangsu Higher Education Institutions of China (19KJA310007), Qinglan Project of Jiangsu Province, College Students’ Innovative Entrepreneurial Training Plan Program (202010315023Z and 202010315025), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| ACF | Aberrant crypt foci |
| AOM | Anaerobic oxidation of methane |
| APC | Adenomatous polyposis coli |
| ARID1A | AT-rich interaction domain 1A |
| ASCs | Adult stem cells |
| B7x | B7 homolog x |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| CAFs | Cancer-associated fibroblasts |
| CAPS1 | Cryopyrin-associated periodic syndromes 1 |
| CDH17 | Cadherin 17 |
| CIN | Chromosome instability |
| C-Myc | Cellular-myelocytomatosis viral oncogene |
| CRC | Colorectal cancer |
| CSCs | Colorectal cancer stem cells |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| DHS | Damp-heat syndrome |
| DKK2 | Dickkopf associated protein 2 |
| DMH | 1,2 Dimethyl hydrazine |
| DMAB | 3,2′-Dimethyl-4-Aminobiphenyl |
| DSS | Dextran sodium sulfate |
| EMT | Epithelial-mesenchymal transition |
| ENU | N-ethyl-N-nitrosourea |
| ESCs | Embryonic stem cells |
| FAM123B | APC membrane recruitment protein 1 |
| FBXW7 | F-box and WD repeat domain containing 7 |
| FFA | Free fatty acids |
| FOXO6 | Forkhead Box O6 |
| HDAC1 | Histone deacetylase 1 |
| HFD | High-fat diet |
| HIF-1α | Hypoxia-inducible factor-1α |
| HISC | Human intestinal stem cell |
| HK2 | Human kallikrein 2 |
| HNRNPL | Heterogeneous nuclear ribonucleoprotein L |
| HNPCC | Hereditary nonpolyposis colorectal cancer |
| ILK | Integrin-linked kinase |
| iPSCs | Induced pluripotent stem cells |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IRTSS | Internal retention of toxin stagnation syndrome |
| IRX5 | Iroquois homeobox gene 5 |
| KRAS | Kirsten rats arcomaviral oncogene homolog |
| LGR5+ | Leucine-rich repeat-containing G-protein-coupled receptor 5 |
| LMNB1 | Lamin B1 |
| LPS | Lipopolysaccharide |
| MCTS | Multicellular tumor spheroids |
| MNU | N-methyl-N-nitrosourea |
| MNNG | N-methyl-N-nitrosoguanidine |
| MTA3 | Metastasis associated 1 family member 3 |
| NFATc1 | Nuclear factor of activated T-cells |
| NF2 | Neurofibromin 2 |
| NF-kB | Nuclear factor-kappa B |
| NRAS | NRAS proto-oncogene, GTPase |
| OMS | Organotypic multicellular spheroids |
| p53 | Tumor protein 53 |
| PDOs | Patient-derived organoids |
| PhIP | Parahydrogen-induced polarization |
| PHLDA2 | Pleckstrin homology-like domain family A member 2 |
| PIM | Potentially inappropriate medication |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PMEPA1 | Prostate transmembrane protein androgen induced 1 |
| RIP3 | Receptor interacting protein kinase 3 |
| RPLP0P2 | Ribosomal protein lateral stalk subunit P0 pseudogene 2 |
| SARI | Severe acute respiratory infection |
| SDS | Spleen qi deficiency syndrome |
| SGPL1 | Sphingosine phosphate lyase 1 |
| SLC25A1 | Solute carrier family 25 member 1 |
| SMAD4 | SMAD family member 4 |
| SOX2 | SRY-box transcription Factor 2 |
| SOX9 | SRY-box transcription factor 9 |
| TBX5 | T-box transcription factor 5 |
| TCF7L2 | Transcription factor 7-like 2 |
| TCM | Traditional Chinese medicine |
| TDTS | Tissue-derived tumor spheres |
| TGF-β | Transforming growth factor β |
| TME | Tumor microenvironment |
| TNBS | 2,4,6-Trinitro-benzenesulfonic acid |
| TNF-α | Tumor necrosis factor-α |
| TRIB2 | Tribbles homolog 2 |
| VEGF | Vascular endothelial growth factor |
| ZNF326 | Zinc-finger protein 326 |
References
- Ahmed D., Eide P. W., Eilertsen I. A., Danielsen S. A., Eknæs M., Hektoen M., et al. (2013). Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2 (9), e71. 10.1038/oncsis.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi H., Han H. J., Iizaka M., Nakamura Y. (2000). Growth-suppressive effect of non-steroidal anti-inflammatory drugs on 11 colon-cancer cell lines and fluorescence differential display of genes whose expression is influenced by sulindac. Int. J. Cancer 88 (6), 873–880. [DOI] [PubMed] [Google Scholar]
- Aleman J., Skardal A. (2019). A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells. Biotechnol. Bioeng. 116 (4), 936–944. 10.1002/bit.26871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda J. F., Shepard J. L., Stern H. M., Zon L. I. (2002). Zebrafish as a cancer model system. Cancer Cell. 1 (3), 229–231. 10.1016/s1535-6108(02)00052-1 [DOI] [PubMed] [Google Scholar]
- Aranganathan S., Nalini N. (2013). Antiproliferative efficacy of hesperetin (citrus flavanoid) in 1,2-dimethylhydrazine-induced colon cancer. Phytotherapy Res. PTR 27 (7), 999–1005. 10.1002/ptr.4826 [DOI] [PubMed] [Google Scholar]
- Bai J. S., Wang J., Zhao X. F. (2015). Nude mice hemispleen method in hepatic metastases of colon cancer model. 37, 447–450. 10.11724/jdmu.2015.05.08 [DOI] [Google Scholar]
- Bakken T. E., Miller J. A., Ding S. L., Sunkin S. M., Smith K. A., Ng L., et al. (2016). A comprehensive transcriptional map of primate brain development. Nature 535 (7612), 367–375. 10.1038/nature18637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Van Es J. H., Kuipers J., Kujala P., Van den Born M., Cozijnsen M., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 (7165), 1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A. A., Kim S., et al. (2012). The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483 (7391), 603–607. 10.1038/nature11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K. C. G., Eide P. W., Eilertsen I. A., Johannessen B., Bruun J., Danielsen S. A., et al. (2017). Multi-omics of 34 colorectal cancer cell lines - a resource for biomedical studies. Mol. Cancer 16 (1), 116. 10.1186/s12943-017-0691-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P., Shashidhara L. S. (2001). Studies on human colon cancer gene APC by targeted expression in Drosophila. Oncogene 20 (47), 6871–6880. 10.1038/sj.onc.1204849 [DOI] [PubMed] [Google Scholar]
- Bian X., Cao F., Wang X., Hou Y., Zhao H., Liu Y. (2021). Establishment and characterization of a new human colon cancer cell line, PUMC-CRC1. Sci. Rep. 11 (1), 13122. 10.1038/s41598-021-92491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaqueiro A. C. F., Ponsioen B., Bakker B., Klaasen S. J., Kucukkose E., Van Jaarsveld R. H., et al. (2019). Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet. 51 (5), 824–834. 10.1038/s41588-019-0399-6 [DOI] [PubMed] [Google Scholar]
- Boot A., Van Eendenburg J., Crobach S., Ruano D., Speetjens F., Calame J., et al. (2016). Characterization of novel low passage primary and metastatic colorectal cancer cell lines. Oncotarget 7 (12), 14499–14509. 10.18632/oncotarget.7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., et al. (1987). Prevalence of Ras gene mutations in human colorectal cancers. Nature 327 (6120), 293–297. 10.1038/327293a0 [DOI] [PubMed] [Google Scholar]
- Boucherit N., Gorvel L., Olive D. (2020). 3D tumor models and their use for the testing of immunotherapies. Front. Immunol. 11, 603640–640. 10.3389/fimmu.2020.603640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenoord A. L., Clevers H., Knoblich J. A. (2017). Human tissues in a dish: The research and ethical implications of organoid technology. Science 355 (6322), eaaf9414. 10.1126/science.aaf9414 [DOI] [PubMed] [Google Scholar]
- Brugman S., Liu K. Y., Lindenbergh-Kortleve D., Samsom J. N., Furuta G. T., Renshaw S. A., et al. (2009). Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 137 (5), 1757–1767. 10.1053/j.gastro.2009.07.069 [DOI] [PubMed] [Google Scholar]
- Bu P., Chen K. Y., Xiang K., Johnson C., Crown S. B., Rakhilin N., et al. (2018). Aldolase B mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell. Metab. 27 (6), 1249–1262. 10.1016/j.cmet.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno S. R., Li S., Colon M., Kreinest P. A., Thompson E. A., Fields A. P., et al. (2008). Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon. Int. J. Cancer 122, 2462–2470. 10.1002/ijc.23383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. L., Khosravi-Far R., Rossman K. L., Clark G. J., Der C. J. (1998). Increasing complexity of Ras signaling. Oncogene 17 (11), 1395–1413. 10.1038/sj.onc.1202174 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 (7407), 330–337. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Zhou X. (2020). Establishment of intestinal cancer model in mice with damp-heat, phlegm-stagnation and stasis-toxin. J. Hunan Univ. Chin. Med. 40 (01), 38–41. 10.3969/j.issn.1674-070X.2020.01.009 [DOI] [Google Scholar]
- Casali A., Batlle E. (2009). Intestinal stem cells in mammals and drosophila. Cell. Stem Cell. 4 (2), 124–127. 10.1016/j.stem.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Casillas A. L., Toth R. K., Sainz A. G., Singh N., Desai A. A., Kraft A. S., et al. (2018). Hypoxia-inducible PIM kinase expression promotes resistance to antiangiogenic agents. Clin. Cancer Res. 24 (1), 169–180. 10.1158/1078-0432.CCR-17-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B., Wei J., Wang D., Xiong Y., Shay J. W., DuBois R. N. (2021). Mutant APC promotes tumor immune evasion via PD-L1 in colorectal cancer. Oncogene 40 (41), 5984–5992. 10.1038/s41388-021-01972-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadla P., Arbi M., Nikou S., Kalliakoudas T., Papadaki H., Taraviras S., et al. (2021). Integrin-linked-kinase overexpression is implicated in mechanisms of genomic instability in human colorectal cancer. Dig. Dis. Sci. 66 (5), 1510–1523. 10.1007/s10620-020-06364-6 [DOI] [PubMed] [Google Scholar]
- Chen L., Vasoya R. P., Toke N. H., Parthasarathy A., Luo S., Chiles E., et al. (2020). HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology 158 (4), 985–999. 10.1053/j.gastro.2019.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo M. K., Sakurai H., Koizumi K., Saiki I. (2005). Stimulation of cultured colon 26 cells with TNF-alpha promotes lung metastasis through the extracellular signal-regulated kinase pathway. Cancer Lett. 230 (1), 47–56. 10.1016/j.canlet.2004.12.027 [DOI] [PubMed] [Google Scholar]
- Croker A. K., Goodale D., Chu J., Postenka C., Hedley B. D., Hess D. A., et al. (2009). High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 13 (8B), 2236–2252. 10.1111/j.1582-4934.2008.00455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska M., Skoneczny M., Rode W. (2011). Functional gene expression profile underlying methotrexate-induced senescence in human colon cancer cells. Tumour Biol. 32 (5), 965–976. 10.1007/s13277-011-0198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska M., Skoneczny M., Uram L., Rode W. (2019). Methotrexate-induced senescence of human colon cancer cells depends on p53 acetylation, but not genomic aberrations. Anticancer Drugs 30 (4), 374–382. 10.1097/CAD.0000000000000731 [DOI] [PubMed] [Google Scholar]
- Dai L., Cui X., Zhang X., Cheng L., Liu Y., Yang Y., et al. (2016). SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat. Commun. 7, 11996. 10.1038/ncomms11996 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Oliveira T., Ramakrishnan M., Diamanti M. A., Ziegler P. K., Brombacher F., Greten F. R. (2019). Loss of Stat6 affects chromatin condensation in intestinal epithelial cells causing diverse outcome in murine models of inflammation-associated and sporadic colon carcinogenesis. Oncogene 38 (11), 1787–1801. 10.1038/s41388-018-0551-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis M., Massi E., Poeta M. L., Carotti S., Morini S., Cecchetelli L., et al. (2011). The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 10, 9. 10.4103/1477-3163.78279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis S., Verna G., Serino G., Armentano R., Cavalcanti E., Liso M., et al. (2020). Winnie-APCMin/+ mice: A spontaneous model of colitis-associated colorectal cancer combining genetics and inflammation. Int. J. Mol. Sci. 21 (8), 2972. 10.3390/ijms21082972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. G. (2013). Commentary on "The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J. Surg. Res. 185 (2), 541–542. 10.1016/j.jss.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Deng F., Zhou R., Lin C., Yang S., Wang H., Li W., et al. (2019). Tumor-secreted dickkopf-2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer. Theranostics 9 (4), 1001–1014. 10.7150/thno.30056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Li L., Yang T., Fan X., Wu G. (2016). Combined application of anti-VEGF and anti-EGFR attenuates the growth and angiogenesis of colorectal cancer mainly through suppressing AKT and ERK signaling in mice model. BMC Cancer 16 (1), 791. 10.1186/s12885-016-2834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolara P., Luceri C., De Filippo C., Femia A. P., Giovannelli L., Caderni G., et al. (2005). Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. 591 (1-2), 237–246. 10.1016/j.mrfmmm.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Dong M. J., Zhou Y., Duan M., Gao Q. M., Zhao J. H. (2020). Clinical significance and mechanism of TBX5 gene in colorectal cancer. Zhonghua Zhong Liu Za Zhi 42 (5), 383–390. 10.3760/cma.j.cn112152-112152-20190829-00560 [DOI] [PubMed] [Google Scholar]
- Dong S., Liang S., Cheng Z., Zhang X., Luo L., Li L., et al. (2022). ROS/PI3K/AKT and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 41 (1), 15. 10.1186/s13046-021-02229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou R., Liu K., Yang C., Zheng J., Shi D., Lin X., et al. (2021). EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clin. Transl. Med. 11 (12), 595. 10.1002/ctm2.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., McGarry M. P., Lee N. A., Lee J. J. (2012). The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 21 (2), 327–349. 10.1007/s11248-011-9537-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray B. K., Raveendran M., Harris R. A., Benavides F., Gray S. B., Perez C. J., et al. (2018). Mismatch repair gene mutations lead to lynch syndrome colorectal cancer in rhesus macaques. Genes. Cancer 9 (3-4), 142–152. 10.18632/genesandcancer.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreni M., Siddiqui I., Marelli G., Grizzi F., Bianchi P., Morone D., et al. (2016). The fractalkine-receptor Axis improves human colorectal cancer prognosis by limiting tumor metastatic dissemination. J. Immunol. 196 (2), 902–914. 10.4049/jimmunol.1501335 [DOI] [PubMed] [Google Scholar]
- Faqar-Uz-Zaman W. F., Schmidt K. G., Thomas D., Pfeilschifter J. M., Radeke H. H., Schwiebs A. (2021). S1P lyase siRNA dampens malignancy of DLD-1 colorectal cancer Cells. Lipids 56 (2), 155–166. 10.1002/lipd.12282 [DOI] [PubMed] [Google Scholar]
- Femia A. P., Caderni G. (2008). Rodent models of colon carcinogenesis for the study of chemopreventive activity of natural products. Planta Med. 74 (13), 1602–1607. 10.1055/s-2008-1074577 [DOI] [PubMed] [Google Scholar]
- Flisikowska T., Merkl C., Landmann M., Eser S., Rezaei N., Cui X., et al. (2012). A porcine model of familial adenomatous polyposis. Gastroenterology 143 (5), 1173–1175. 10.1053/j.gastro.2012.07.110 [DOI] [PubMed] [Google Scholar]
- Flisikowska T., Stachowiak M., Xu H., Wagner A., Hernandez-Caceres A., Wurmser C., et al. (2017). Porcine familial adenomatous polyposis model enables systematic analysis of early events in adenoma progression. Sci. Rep. 7 (1), 6613. 10.1038/s41598-017-06741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foersch S., Sperka T., Lindner C., Taut A., Rudolph K. L., Breier G., et al. (2015). VEGFR2 signaling prevents colorectal cancer cell senescence to promote tumorigenesis in mice with colitis. Gastroenterology 149 (1), 177–189. 10.1053/j.gastro.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Fujii M., Matano M., Toshimitsu K., Takano A., Mikami Y., Nishikori S., et al. (2018). Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell. Stem Cell. 23 (6), 787–793. 10.1016/j.stem.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Fujii M., Sato T. (2021). Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mat. 20, 20156–20169. 10.1038/s41563-020-0754-0 [DOI] [PubMed] [Google Scholar]
- Gao F. H., Hu X. H., Li W., Liu H., Zhang Y. J., Guo Z. Y., et al. (2010). Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-Myc. BMC Cancer 10, 610. 10.1186/1471-2407-10-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C., Riva F., Polentarutti N., Buracchi C., Sironi M., De Bortoli M., et al. (2004). Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl. Acad. Sci. U. S. A. 101 (10), 3522–3526. 10.1073/pnas.0308680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi P., Shafiee G., Ziamajidi N., Abbasalipourkabir R. (2020). Copper nanoparticles induce apoptosis and oxidative stress in SW480 human colon cancer cell line. Biol. Trace Elem. Res. 10.1007/s12011-022-03458-2 [DOI] [PubMed] [Google Scholar]
- Goi T., Fujioka M., Satoh Y., Tabata S., Koneri K., Nagano H., et al. (2004). Angiogenesis and tumor proliferation/metastasis of human colorectal cancer cell line SW620 transfected with endocrine glands-derived-vascular endothelial growth factor, as a new angiogenic factor. Cancer Res. 64 (6), 1906–1910. 10.1158/0008-5472.can-3696-2 [DOI] [PubMed] [Google Scholar]
- Gonzalez L. M., Stewart A. S., Freund J., Kucera C. R., Dekaney C. M., Magness S. T., et al. (2019). Preservation of reserve intestinal epithelial stem cells following severe ischemic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 316 (4), G482-G494–G494. 10.1152/ajpgi.00262.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M. R., Khomchuk Y., Wrana J. L. (2015). Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526 (7575), 715–718. 10.1038/nature15382 [DOI] [PubMed] [Google Scholar]
- Groll T., Schopf F., Denk D., Mogler C., Schwittlick U., Aupperle-Lellbach H., et al. (2021). Bridging the species gap: Morphological and molecular comparison of feline and human intestinal carcinomas. Cancers (Basel). 13 (23), 5941. 10.3390/cancers13235941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldi M., Ton C., Seng W. L., McGrath P. (2006). Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9, 9139–9151. 10.1007/s10456-006-9040-2 [DOI] [PubMed] [Google Scholar]
- Han Q., Ma Y., Wang H., Dai Y., Chen C., Liu Y., et al. (2018). Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J. Transl. Med. 16 (1), 201. 10.1186/s12967-018-1580-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harma S., Haber D., Settleman J. (2010). Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 10 (4), 241–253. 10.1038/nrc2820 [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Yamada Y., Semi K., Yagi M., Tanaka A., Itakura F., et al. (2017). Cellular context-dependent consequences of APC mutations on gene regulation and cellular behavior. Proc. Natl. Acad. Sci. U. S. A. 114 (4), 758–763. 10.1073/pnas.1614197114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hason M., Bartůněk P. (2019). Zebrafish models of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes. (Basel). 10 (11), 935. 10.3390/genes10110935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S. (2003). Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 3 (10), 733–744. 10.1038/nrc1190 [DOI] [PubMed] [Google Scholar]
- Hou Z., Guo K., Sun X., Hu F., Chen Q., Luo X., et al. (2018). TRIB2 functions as novel oncogene in colorectal cancer by blocking cellular senescence through AP4/p21 signaling. Mol. Cancer 17 (1), 172. 10.1186/s12943-018-0922-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Fatima S., Chen M., Xu K., Huang C., Gong R. H., et al. (2021). Toll-like receptor 4 is a master regulator for colorectal cancer growth under high-fat diet by programming cancer metabolism. Cell. Death Dis. 12 (8), 791. 10.1038/s41419-021-04076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua R., Yu J., Yan X., Ni Q., Zhi X., Li X., et al. (2020). Syndecan-2 in colorectal cancer plays oncogenic role via epithelial-mesenchymal transition and MAPK pathway. Biomed. Pharmacother. 121, 109630. 10.1016/j.biopha.2019.109630 [DOI] [PubMed] [Google Scholar]
- Huang J., Jiang T., Kang J., Xu J., Dengzhang Y., Zhao Z., et al. (2022). Synergistic effect of Huangqin decoction combined treatment with Radix Actinidiae chinensis on DSS and AOM-induced colorectal cancer. Front. Pharmacol. 13, 933070. 10.3389/fphar.2022.933070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zheng S., Jin S. H., Zhang S. Z. (2004). Somatic mutations of APC gene in carcinomas from hereditary non-polyposis colorectal cancer patients. World J. Gastroenterol. 10 (6), 834–836. 10.3748/wjg.v10.i6.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Liu C. A., Cai P. Z., Xu F. P., Zhu W. J., Wang W. W., et al. (2020). Omega-3PUFA Attenuates MNU-induced colorectal cancer in rats by blocking PI3K/AKT/Bcl-2 signaling. Onco Targets Ther. 13, 1953–1965. 10.2147/OTT.S241298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Koo B. K. (2015). Modeling mouse and human development using organoid cultures. Development 142 (18), 3113–3125. 10.1242/dev.118570 [DOI] [PubMed] [Google Scholar]
- Inch W. R., McCredie J. A., Sutherland R. M. (1970). Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth 34 (3), 271–282. [PubMed] [Google Scholar]
- Itano O., Fan K., Yang K., Suzuki K., Quimby F., Dong Z., et al. (2012). Effect of caloric intake on Western-style diet-induced intestinal tumors in a mouse model for hereditary colon cancer. Nutr. Cancer 64 (3), 401–408. 10.1080/01635581.2012.660672 [DOI] [PubMed] [Google Scholar]
- Ito N., Hasegawa R., Sano M., Tamano S., Esumi H., Takayama S., et al. (1991). A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP). Carcinogenesis 12 (8), 1503–1506. 10.1093/carcin/12.8.1503 [DOI] [PubMed] [Google Scholar]
- Ivascu A., Kubbies M. (2006). Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen 11 (8), 922–932. 10.1177/1087057106292763 [DOI] [PubMed] [Google Scholar]
- Izzo A. A., Aviello G., Petrosino S., Orlando P., Marsicano G., Lutz B., et al. (2008). Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J. Mol. Med. Berl. 86 (1), 89–98. 10.1007/s00109-007-0248-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakopovic B., Oršolić N., Kraljević P. S. (2020). Antitumor, immunomodulatory and antiangiogenic efficacy of medicinal mushroom extract mixtures in advanced colorectal cancer animal model. Molecules 25 (21), 5005. 10.3390/molecules25215005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen K. P., Alberici P., Fsihi H., Gaspar C., Breukel C., Franken P., et al. (2006). APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131 (4), 1096–1109. 10.1053/j.gastro.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Janssen K. P., El-Marjou F., Pinto D., Sastre X., Rouillard D., Fouquet C., et al. (2002). Targeted expression of oncogenic KRAS in intestinal epithelium causes spontaneous tumorigenesis in mice. Gastroenterology 123 (2), 492–504. 10.1053/gast.2002.34786 [DOI] [PubMed] [Google Scholar]
- Jedrzejczak S. M. (2017). “History of cell culture,” in In new insights into cell culture technology (Rijeka, Croatia: InTech; ). 10.5772/66905 [DOI] [Google Scholar]
- Jen J., Powell S. M., Papadopoulos N., Smith K. J., Hamilton S. R., Vogelstein B., et al. (1994). Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 54 (21), 5523–5526. [PubMed] [Google Scholar]
- Jiao T., Li Y., Gao T., Zhang Y., Feng M., Liu M., et al. (2017). MTA3 regulates malignant progression of colorectal cancer through Wnt signaling pathway. Tumour Biol. 39 (3), 1010428317695027. 10.1177/1010428317695027 [DOI] [PubMed] [Google Scholar]
- Ju S., Wang F., Wang Y., Ju S. (2020). CSN8 is a key regulator in hypoxia-induced epithelial-mesenchymal transition and dormancy of colorectal cancer cells. Mol. Cancer 19 (1), 168. 10.1186/s12943-020-01285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. R., Kim E. J., Choi H. J., Park J. J., Kim H. S., Lee Y. J., et al. (2015). Aspirin targets SIRT1 and AMPK to induce senescence of colorectal carcinoma cells. Mol. Pharmacol. 88 (4), 708–719. 10.1124/mol.115.098616 [DOI] [PubMed] [Google Scholar]
- Juric D., Rodon J., Tabernero J., Janku F., Burris H. A., Schellens J. H. M., et al. (2018). Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-human study. J. Clin. Oncol. 36 (13), 1291–1299. 10.1200/JCO.2017.72.7107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh E., Snir-Alkalay I., Venkatachalam A., May S., Lasry A., Elyada E., et al. (2020). The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586 (7827), 133–138. 10.1038/s41586-020-2541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamano T., Kishino H., Mizukami K., Azuma N., Tamura J., Katami A., et al. (1983). Histopathological study on N-ethyl-N'-nitro-N-nitrosoguanidine-induced colon cancer in dogs. Int. J. Cancer 32 (2), 255–258. 10.1002/ijc.2910320219 [DOI] [PubMed] [Google Scholar]
- Kamano T., Kurihara M., Kishino H., Mizukami K., Kidokoro T., Wakabayashi K., et al. (1981). Experimental colonic cancer in a dog. Jpn. J. Surg. 11 (3), 214–218. 10.1007/BF02468841 [DOI] [PubMed] [Google Scholar]
- Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A., et al. (2018). Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 8 (1), 2871. 10.1038/s41598-018-21201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya H., Kuruppu D. K., Donahue J. M., Choi E. W., Kawasaki H., Tanabe K. K., et al. (2020). Establishment and characterization of 18 human colorectal cancer cell lines. Sci. Rep. 10 (1), 6801. 10.1038/s41598-020-63812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya H., Kuruppu D. K., Donahue J. M., Choi E. W., Kawasaki H., Tanabe K. K. (2005). Mouse models of subcutaneous spleen reservoir for multiple portal venous injections to treat liver malignancies. Cancer Res. 65 (9), 3823–3827. 10.1158/0008-5472.CAN-04-2631 [DOI] [PubMed] [Google Scholar]
- Kim S. C., Kim H. S., Kim J. H., Jeong N., Shin Y. K., Kim M. J., et al. (2020). Establishment and characterization of 18 human colorectal cancer cell lines. Sci. Rep. 10 (1), 6801. 10.1038/s41598-020-63812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimlin L. C., Casagrande G., Virador V. M. (2013). In vitro three-dimensional (3D) models in cancer research: An update. Mol. Carcinog. 52 (3), 167–182. 10.1002/mc.21844 [DOI] [PubMed] [Google Scholar]
- Kissow H., Hartmann B., Holst J. J., Viby N. E., Hansen L. S., Rosenkilde M. M., et al. (2012). Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul. Pept. 179 (1-3), 91–100. 10.1016/j.regpep.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Kodach L. L., Jacobs R. J., Voorneveld P. W., Wildenberg M. E., Verspaget H. W., Van Wezel T., et al. (2011). Statins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell 'stemness' via the bone morphogenetic protein pathway. Gut 60 (11), 1544–1553. 10.1136/gut.2011.237495 [DOI] [PubMed] [Google Scholar]
- Kopetz S., Lesslie D. P., Dallas N. A., Park S. I., Johnson M., Parikh N. U., et al. (2009). Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 69 (9), 3842–3849. 10.1158/0008-5472.CAN-08-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. L., Verma S., Das P. (2019). Artesunate suppresses inflammation and oxidative stress in a rat model of colorectal cancer. Drug Dev. Res. 80 (8), 1089–1097. 10.1002/ddr.21590 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Knoblich J. A. (2014). Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345 (6194), 1247125. 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lee W. Y., Hong H. K., Ham S. K., Kim C. I., Cho Y. B. (2014). Comparison of colorectal cancer in differentially established liver metastasis models. Anticancer Res. 34 (7), 3321–3328. [PubMed] [Google Scholar]
- Lehmann B., Biburger M., Brückner C., Ipsen-Escobedo A., Gordan S., Lehmann C., et al. (2017). Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci. Immunol. 2 (7), 6413. 10.1126/sciimmunol.aah6413 [DOI] [PubMed] [Google Scholar]
- Li M., Izpisua Belmonte J. C. (2019a). Organoids-preclinical models of human disease. N. Engl. J. Med. 380 (6), 569–579. 10.1056/NEJMra1806175 [DOI] [PubMed] [Google Scholar]
- Li Q., Tang H., Hu F., Qin C. (2019b). Silencing of FOXO6 inhibits the proliferation, invasion, and glycolysis in colorectal cancer cells. J. Cell. Biochem. 120 (3), 3853–3860. 10.1002/jcb.27667 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang S., Hu M., Xu M., Jiang X. (2020a). Silencing of synaptotagmin 13 inhibits tumor growth through suppressing proliferation and promoting apoptosis of colorectal cancer cells. Int. J. Mol. Med. 45 (1), 234–244. 10.3892/ijmm.2019.4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Shi X., Chen M., Xu N., Sun D., Bai R., et al. (2019c). Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int. J. Cancer 145 (5), 1395–1407. 10.1002/ijc.32245 [DOI] [PubMed] [Google Scholar]
- Li S., Zhang J., Qian S., Wu X., Sun L., Ling T., et al. (2021). S100A8 promotes epithelial-mesenchymal transition and metastasis under TGF-β/USF2 axis in colorectal cancer. Cancer Commun. 41 (2), 154–170. 10.1002/cac2.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Larsson P., Ljuslinder I., Öhlund D., Myte R., Löfgren-Burström A., et al. (2020b). Ex vivo organoid cultures reveal the importance of the tumor microenvironment for maintenance of colorectal cancer stem cells. Cancers (Basel) 12 (4), 923. 10.3390/cancers12040923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Deuring J., Peppelenbosch M. P., Kuipers E. J., De Haar C., Van der Woude C. J. (2012). IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis 33 (10), 1889–1896. 10.1093/carcin/bgs214 [DOI] [PubMed] [Google Scholar]
- Li Y., Liu Y., Zhao N., Yang X., Li Y., Zhai F., et al. (2020c). Checkpoint regulator B7x is epigenetically regulated by HDAC3 and mediates resistance to HDAC inhibitors by reprogramming the tumor immune environment in colorectal cancer. Cell. Death Dis. 11 (9), 753. 10.1038/s41419-020-02968-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Qian L. Y., Tang P. L., Guo Y., Men C., Cui Y., et al. (2017b). Cathelicidin LL37 promotes epithelial and smooth-muscle-like differentiation of adipose-derived stem cells through the wnt/β-catenin and NF-κB pathways. Chin. J. Traditional Chin. Med. 82 (03), 1336–1345. 10.1134/S0006297917110116 [DOI] [PubMed] [Google Scholar]
- Li Y., Yang Y., Li J., Liu H., Chen F., Li B., et al. (2017a). USP22 drives colorectal cancer invasion and metastasis via epithelial-mesenchymal transition by activating AP4. Oncotarget 8 (20), 32683–32695. 10.18632/oncotarget.15950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang J., Zhou T., Ye X. (2016). Establishment of a colorectal cancer nude mouse visualization model of HIF-1α overexpression. Oncol. Lett. 11 (4), 2725–2732. 10.3892/ol.2016.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Xie R., Su J., Ye B., Wei S., Liang Z., et al. (2017). Inhibition of RNA polymerase III transcription by triptolide attenuates colorectal tumorigenesis. J. Exp. Clin. Cancer Res. 38 (1), 217. 10.1186/s13046-019-1232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Chen S., Chen Z., Dai Q., Ke C. (2017). X-ray-induced epithelial-mesenchymal transition in SW480 colorectal cancer cells and its potential mechanisms. J. BUON 22 (6), 1457–1462. [PubMed] [Google Scholar]
- Lind G. E., Thorstensen L., Løvig T., Meling G. I., Hamelin R., Rognum T. O., et al. (2004). A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol. Cancer 3, 28. 10.1186/1476-4598-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yao Z., Wang J., Zhang W., Yang Y., Zhang Y., et al. (2020a). Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell. Death Differ. 27 (6), 1765–1781. 10.1038/s41418-019-0460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Deng G. H., Zhang J., Wang Y., Xia X. Y., Luo X. M., et al. (2015a). The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52, 130–142. 10.1016/j.psyneuen.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang J., Shi L., Zhang W., Du X., Wang Z., et al. (2013). β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 20 (6), 512–520. 10.1016/j.phymed.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Liu M., Xiao Y., Tang W., Li J., Hong L., Dai W., et al. (2020b). HOXD9 promote epithelial-mesenchymal transition and metastasis in colorectal carcinoma. Cancer Med. 9 (11), 3932–3943. 10.1002/cam4.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li W., Liu H., Yu X. (2019). Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 15 (11), 2497–2508. 10.7150/ijbs.37481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. X., Gu S. Z., Zhang S., Ren Y., Sang L. X., Dai C. (2015b). Angiopoietin and vascular endothelial growth factor expression in colorectal disease models. World J. Gastroenterol. 21 (9), 2645–2650. 10.3748/wjgv21i9.2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos J. C., Bakonyi Neto A., Lerco M. M., Clark R. M., Polachini do Valle A., Sousa M. M. (2006). Induction of short gut syndrome and transplantation in a porcine model. Transpl. Proc. 38 (6), 1855–1856. 10.1016/j.transproceed.2006.06.085 [DOI] [PubMed] [Google Scholar]
- Lo Dico R., Tijeras-Raballand A., Bonnin P., Launay J. M., Kaci R., Pimpie C., et al. (2018). Hepatectomy increases metastatic graft and growth in an immunocompetent murine model of peritoneal metastases. Eur. J. Surg. Oncol. 44 (6), 784–791. 10.1016/j.ejso.2018.01.096 [DOI] [PubMed] [Google Scholar]
- Lu S. L., Kawabata M., Imamura T., Akiyama Y., Nomizu T., Miyazono K., et al. (1998). HNPCC associated with germline mutation in the TGF-beta type II receptor gene. Nat. Genet. 19 (1), 17–18. 10.1038/ng0598-17 [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. (1997). An update of HNPCC (Lynch syndrome). Cancer Genet. Cytogenet 93 (1), 84–99. 10.1016/s0165-4608(96)00290-7 [DOI] [PubMed] [Google Scholar]
- Ma Q., Hoper M., Anderson N., Rowlands B. J. (1996). Effect of supplemental L-arginine in a chemical-induced model of colorectal cancer. World J. Surg. 20 (8), 1087–1091. 10.1007/s002689900165 [DOI] [PubMed] [Google Scholar]
- Ma Z., Lou S., Jiang Z. (2020). PHLDA2 regulates EMT and autophagy in colorectal cancer via the PI3K/AKT signaling pathway. Aging (Albany NY) 12 (9), 7985–8000. 10.18632/aging.103117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletzki C., Gock M., Randow M., Klar E., Huehns M., Prall F., et al. (2015). Establishment and characterization of cell lines from chromosomal instable colorectal cancer. World J. Gastroenterol. 21 (1), 164–176. 10.3748/wjgv21.i1.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau G., Karoui M., Charachon A., Delchier J. C., Sobhani I. (2011). HNPCC (hereditary non-polyposis colorectal cancer) or lynch syndrome: A syndrome related to a failure of DNA repair system. Bull. Cancer 98 (3), 323–336. 10.1684/bdc.2011.1328 [DOI] [PubMed] [Google Scholar]
- Mao J. H., Perez-Losada J., Wu D., Delrosario R., Tsunematsu R., Nakayama K. I., et al. (2004). Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432, 775–779. 10.1038/nature03155 [DOI] [PubMed] [Google Scholar]
- Marian B. (2004). Colorectal cancer: Modeling causes, prevention and therapy. Drug Discov. Today Dis. Models 1 (1), 11–17. 10.1016/jddmod.2004.07.006 [DOI] [Google Scholar]
- Martin E. S., Belmont P. J., Sinnamon M. J., Richard L. G., Yuan J., Coffee E. M., et al. (2013). Development of a colon cancer GEMM-derived orthotopic transplant model for drug discovery and validation. Clin. Cancer Res. 19 (11), 2929–2940. 10.1158/1078-0432.CCR-12-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell Ò., Merlos-Suárez A., Campbell K., Barriga F. M., Christov C. P., Miguel-Aliaga I., et al. (2014). Conserved mechanisms of tumorigenesis in the Drosophila adult midgut. PLoS ONE 9, 88413. 10.1371/journal.pone.0088413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo H., Wu D. C., Podolsky D. K., Fishman M. C. (1996). Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274 (5285), 262–265. 10.1126/science.274.5285.262 [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Miura K., Yamane J., Shima H., Fujibuchi W., Ishida K., et al. (2016). SERPINI1 regulates epithelial-mesenchymal transition in an orthotopic implantation model of colorectal cancer. Cancer Sci. 07 (5), 619–628. 10.1111/cas.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G., Hsiao A. Y., Ingram M., Luker G. D., Takayama S. (2012). Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 164 (2), 192–204. 10.1016/j.jconrel.2012.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. S., Song M., Nishihara R., Drew D. A., Wu K., Qian Z. R., et al. (2017). Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 152 (8), 1944–1953.e1. 10.1053/j.gastro.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuła-Pietrasik J., Sosińska P., Maksin K., Kucińska M. G., Piotrowska H., Murias M., et al. (2015). Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget 6 (30), 29178–29195. 10.18632/oncotarget.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Kanemura S., Kusaka J., Kinouchi M., Suzuki S., Nishino Y., et al. (2022). Associations of cigarette smoking, alcohol drinking and body mass index with survival after colorectal cancer diagnosis by anatomic subsite: A prospective patient cohort study in Japan. Jpn. J. Clin. Oncol. 52 (12), 1375–1388. 10.1093/jjco/hyac140 [DOI] [PubMed] [Google Scholar]
- Mooi J. K., Luk I. Y., Mariadason J. M. (2018). Cell line models of molecular subtypes of colorectal cancer. Methods Mol. Biol. 1765, 3–26. 10.1007/978-1-4939-7765-9_1 [DOI] [PubMed] [Google Scholar]
- Moser A. R., Pitot H. C., Dove W. F. (1990). A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247 (4940), 322–324. 10.1126/science.2296722 [DOI] [PubMed] [Google Scholar]
- Mouradov D., Sloggett C., Jorissen R. N., Love C. G., Li S., Burgess A. W., et al. (2014). Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 74 (12), 3238–3247. 10.1158/0008-5472.CAN-14-0013 [DOI] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium Waterston R. H., Lindblad-Toh K., Birney E., Rogers J., Abril J. F., Agarwal P., et al. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature 420 (6915), 520–562. 10.1038/nature01262 [DOI] [PubMed] [Google Scholar]
- Munday J. S., Brennan M. M., Jaber A. M., Kiupel M. (2006). Ovine intestinal adenocarcinomas: Histologic and phenotypic comparison with human colon cancer. Comp. Med. 56 (2), 136–141. [PubMed] [Google Scholar]
- Nakayama M., Sakai E., Echizen K., Yamada Y., Oshima H., Han T. S., et al. (2017). Intestinal cancer progression by mutant p53 through the acquisition of invasiveness associated with complex glandular formation. Oncogene 36 (42), 5885–5896. 10.1038/onc.2017.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufert C., Becker C., Neurath M. F. (2007). An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998–2004. 10.1038/nprot.2007.279 [DOI] [PubMed] [Google Scholar]
- O'Rourke K. P., Loizou E., Livshits G., Schatoff E. M., Baslan T., Manchado E., et al. (2017). Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35 (6), 577–582. 10.1038/nbt.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando F. A., Tan D., Baltodano J. D., Khoury T., Gibbs J. F., Hassid V. J., et al. (2008). Aberrant crypt foci as precursors in colorectal cancer progression. J. Surg. Oncol. 98 (3), 207–213. 10.1002/jso.21106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozirmak Lermi N., Gray S. B., Bowen C. M., Reyes-Uribe L., Dray B. K., Deng N., et al. (2022). Comparative molecular genomic analyses of a spontaneous rhesus macaque model of mismatch repair-deficient colorectal cancer. PLoS Genet. 18 (4), 1010163. 10.1371/journal.pgen.1010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panis Y., Nordlinger B. (1991). Experimental models for hepatic metastases from colorectal tumors. Ann. Chir. 45 (3), 222–228. [PubMed] [Google Scholar]
- Papadopoulos N., Lindblom A. (1997). Molecular basis of HNPCC: Mutations of MMR genes. Hum. Mutat. 10 (2), 89–99. [DOI] [PubMed] [Google Scholar]
- Paquette C. E., Kent M. L., Buchner C., Tanguay R. L., Guillemin K., Mason T. J., et al. (2013). A retrospective study of the prevalence and classification of intestinal neoplasia in zebrafish (Danio rerio). Zebrafish 10 (2), 228–236. 10.1089/zeb.2012.0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović J., Glamočlija J., Ilić-Tomić T., Soković M., Robajac D., Nedić O., et al. (2020). Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int. J. Biol. Macromol. 148, 129–139. 10.1016/j.ijbiomac.2020.01.033 [DOI] [PubMed] [Google Scholar]
- Póvoa V., Rebelo de Almeida C., Maia-Gil M., Sobral D., Domingues M., Martinez-Lopez M., et al. (2021). Innate immune evasion revealed in a colorectal zebrafish xenograft model. Nat. Commun. 12 (1), 1156. 10.1038/s41467-021-21421-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppa M. J., White J. P., Sato S., Cairns M., Baynes J. W., Carson J. A. (2011). Gut barrier dysfunction in the APC(Min/+) mouse model of colon cancer cachexia. Biochim. Biophys. Acta 1812 (12), 1601–1606. 10.1016/j.bbadis.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z. P., Yalikong A., Zhang J. W., Cai S. L., Li B., Di S., et al. (2021). HDAC2 promotes the EMT of colorectal cancer cells and via the modular scaffold function of ENSG00000274093.1. J. Cell. Mol. Med. 25 (2), 1190–1197. 10.1111/jcmm.16186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., Shen L., Liu S., Li H., Ma Y., Zhang R., et al. (2017). Chronic inflammation confers to the metabolic reprogramming associated with tumorigenesis of colorectal cancer. Cancer Biol. Ther. 18 (4), 237–244. 10.1080/15384047.2017.1294292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcevic U., Knol J. C., Piersma S., Bougnaud S., Fack F., Sundlisaeter E., et al. (2014). Colorectal cancer derived organotypic spheroids maintain essential tissue characteristics but adapt their metabolism in culture. Proteome Sci. 12, 39. 10.1186/1477-5956-12-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. S. (1998). Colon carcinogenesis models for chemoprevention studies. Hematol. Oncol. Clin. North Am. 12 (5), 963–973. 10.1016/s0889-8588(05)70036-8 [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Mori H. (1981). Effect of dietary wheat bran and dehydrated citrus fiber on 3,2'-dimethyl-4-aminobiphenyl-induced intestinal carcinogenesis in F344 rats. Carcinogenesis 2 (1), 21–25. 10.1093/carcin/2.1.21 [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L., Lombardi D. G., Pilozzi E., Biffoni M., Todaro M., Peschle C., et al. (2007). Identification and expansion of human colon-cancer-initiating cells. Nature 445 (7123), 111–115. 10.1038/nature05384 [DOI] [PubMed] [Google Scholar]
- Robanus-Maandag E. C., Koelink P. J., Breukel C., Salvatori D. C., Jagmohan-Changur S. C., Bosch C. A., et al. (2010). A new conditional APC-mutant mouse model for colorectal cancer. Carcinogenesis 31 (5), 946–952. 10.1093/carcin/bgq046 [DOI] [PubMed] [Google Scholar]
- Rokavec M., Öner M. G., Li H., Jackstadt R., Jiang L., Lodygin D., et al. (2014). IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Investig. 124 (4), 1853–1867. 10.1172/JCI73531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque-Lima B., Roque C. C. T. A., Begnami M. D., Peresi P., Lima E. N. P., Mello C. A. L., et al. (2019). Development of patient-derived orthotopic xenografts from metastatic colorectal cancer in nude mice. J. Drug Target 27 (9), 943–949. 10.1080/1061186X.2018.1509983 [DOI] [PubMed] [Google Scholar]
- Rupertus K., Sinistra J., Scheuer C., Nickels R. M., Schilling M. K., Menger M. D., et al. (2014). Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer. Clin. Exp. Metastasis 31 (4), 447–459. 10.1007/s10585-014-9639-4 [DOI] [PubMed] [Google Scholar]
- Rygaard J., Poulsen C. O. (1969). Heterotransplantation of a human malignant tumour to “nude”mice. Acta Pathol. Microbiol. Scand. 77, 758–760. 10.1111/j.1699-0463.1969.tb04520.x [DOI] [PubMed] [Google Scholar]
- Saeidnia S., Manayi A., Abdollahi M. (2015). From in vitro experiments to in vivo and clinical studies; pros and cons. Curr. Drug Discov. Technol. 12 (4), 218–224. 10.2174/1570163813666160114093140 [DOI] [PubMed] [Google Scholar]
- Sanford K. K., Earle W. R., Likely G. D. (1948). The growth in vitro of single isolated tissue cells. J. Natl. Cancer Inst. 9 (3), 229–246. [PubMed] [Google Scholar]
- Sangild P. T., Siggers R. H., Schmidt M., Elnif J., Bjornvad C. R., Thymann T., et al. (2006). Diet and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130 (6), 1776–1792. 10.1053/j.gastro.2006.02.026 [DOI] [PubMed] [Google Scholar]
- Santini M. T., Rainaldi G. (1999). Three-dimensional spheroid model in tumor biology. Pathobiology 67 (3), 148–157. 10.1159/000028065 [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D. E., Ferrante M., Vries R. G., Van Es J. H., Van den Brink S., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141 (5), 1762–1772. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Schafer M., Werner S. (2008). Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell. Biol. 9 (8), 628–638. 10.1038/nrm2455 [DOI] [PubMed] [Google Scholar]
- Scheiffele F., Fuss I. J. (2002). Induction of TNBS colitis in mice. Curr. Protoc. Immunol. 15, 15.19. 10.1002/0471142735.im1519s49 [DOI] [PubMed] [Google Scholar]
- Schottelius A., Baldwin A., Jr (1999). A role for transcription factor NF-κB in intestinal inflammation. Int. J. Color. Dis. 14, 18–28. 10.1007/s003840050178 [DOI] [PubMed] [Google Scholar]
- Seignez C., Martin A., Rollet C. E., Racoeur C., Scagliarini A., Jeannin J. F., et al. (2014). Senescence of tumor cells induced by oxaliplatin increases the efficiency of a lipid A immunotherapy via the recruitment of neutrophils. Oncotarget 5 (22), 11442–11451. 10.18632/oncotarget.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senga S. S., Grose R. P. (2021). Hallmarks of cancer-the new testament. Open Biol. 11 (1), 200358. 10.1098/rsob.200358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seril D. N., Liao J., West A. B., Yang G. Y. (2006). High-iron diet: Foe or feat in ulcerative colitis and ulcerative colitis-associated carcinogenesis. J. Clin. Gastroenterol. 40 (5), 391–397. 10.1097/00004836-200605000-00006 [DOI] [PubMed] [Google Scholar]
- Sharma S. V., Haber D. A., Settleman J. (2010). Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 10 (4), 241–253. 10.1038/nrc2820 [DOI] [PubMed] [Google Scholar]
- Shen T., Yue C., Wang X., Wang Z., Wu Y., Zhao C., et al. (2021). NFATc1 promotes epithelial-mesenchymal transition and facilitates colorectal cancer metastasis by targeting SNAI1. Exp. Cell. Res. 408 (1), 112854. 10.1016/j.yexcr.2021.112854 [DOI] [PubMed] [Google Scholar]
- Shi L., Wang J., Ding N., Zhang Y., Zhu Y., Dong S., et al. (2019). Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat. Commun. 10 (1), 5421. 10.1038/s41467-019-13204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker A. R., Gould K. A., Luongo C., Moser A. R., Dove W. F. (1997). Studies of neoplasia in the Min mouse. Biochim. Biophys. Acta 1332 (2), 25–48. 10.1016/s0304-419x(96)00041-8 [DOI] [PubMed] [Google Scholar]
- Shoemaker A. R., Moser A. R., Midgley C. A., Clipson L., Newton M. A., Dove W. F. (1998). A resistant genetic background leading to incomplete penetrance of intestinal neoplasia and reduced loss of heterozygosity in APCMin/+ mice. Proc. Natl. Acad. Sci. U. S. A. 95 (18), 10826–10831. 10.1073/pnas.95.18.10826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R., Kartheuser A., Jagmohan-Changur S., Leblanc V., Breukel C., De Vries A., et al. (1997). Loss of APC and the entire chromosome 18 but absence of mutations at the Ras and Tp53 genes in intestinal tumors from APC1638N, a mouse model for APC-driven carcinogenesis. Carcinogenesis 18, 321–327. 10.1093/carcin/18.2.321 [DOI] [PubMed] [Google Scholar]
- Sun Q., Yang H., Liu M., Ren S., Zhao H., Ming T., et al. (2022). Berberine suppresses colorectal cancer by regulation of Hedgehog signaling pathway activity and gut microbiota. Phytomedicine 103, 154227. 10.1016/j.phymed.2022.154227 [DOI] [PubMed] [Google Scholar]
- Sun X., Jiang X., Wu J., Ma R., Wu Y., Cao H., et al. (2020). IRX5 prompts genomic instability in colorectal cancer cells. J. Cell. Biochem. 121 (11), 4680–4689. 10.1002/jcb.29693 [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tai J., Wang G., Liu T., Wang L., Lin C., Li F. (2012). Effects of siRNA targeting c-Myc and VEGF on human colorectal cancer Volo cells. J. Biochem. Mol. Toxicol. 26 (12), 499–505. 10.1002/jbt.21455 [DOI] [PubMed] [Google Scholar]
- Takaku K., Oshima M., Miyoshi H., Matsui M., Seldin M. F., Taketo M. M. (1998). Intestinal tumorigenesis in compound mutant mice of both Dpc4 (SMAD4) and APC genes. Cell. 92, 645–656. 10.1016/s0092-8674(00)81132-0 [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Singh R. K., Fidler I. J., Raz A. (2007). Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 170 (3), 793–804. 10.2353/ajpath.2007.060929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Suzuki R., Kohno H., Sugie S., Takahashi M., Wakabayashi K. (2005). Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine and dextran sodium sulfate in male ICR mice possess β-catenin gene mutations and increases immunoreactivity for β-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis 26, 229–238. 10.1093/carcin/bgh292 [DOI] [PubMed] [Google Scholar]
- Tato-Costa J., Casimiro S., Pacheco T., Pires R., Fernandes A., Alho I., et al. (2016). Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin. Colorectal Cancer 15 (2), 170–178. 10.1016/j.clcc.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Thoma C. R., Zimmermann M., Agarkova I., Kelm J. M., Krek W. (2014). 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 69-70, 29–41. 10.1016/j.addr.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Tian X., Han Z., Zhu Q., Tan J., Liu W., Wang Y., et al. (2018). Silencing of cadherin-17 enhances apoptosis and inhibits autophagy in colorectal cancer cells. Biomed. Pharmacother. 108, 331–337. 10.1016/j.biopha.2018.09.020 [DOI] [PubMed] [Google Scholar]
- Trede N. S., Langenau D. M., Traver D., Look A. T., Zon L. I. (2004). The use of zebrafish to understand immunity. Immunity 20 (4), 367–379. 10.1016/s1074-7613(04)00084-6 [DOI] [PubMed] [Google Scholar]
- Uneyama M., Chambers J. K., Nakashima K., Uchida K., Nakayama H. (2021). Histological classification and immunohistochemical study of feline colorectal epithelial tumors. Vet. Pathol. 58 (2), 305–314. 10.1177/0300985820974279 [DOI] [PubMed] [Google Scholar]
- Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H., et al. (2020). Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 23 (2), 159–177. 10.1007/s10456-019-09688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P., Bonnet D., De Maria R., Lapidot T., Copland M., Melo J. V., et al. (2012). Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 12 (11), 767–775. 10.1038/nrc3368 [DOI] [PubMed] [Google Scholar]
- Van de Wetering M., Francies H. E., Francis J. M., Bounova G., Iorio F., Pronk A., et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 161 (4), 933–945. 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B. A., De Bruijn A. C., Velcich A., Meijerink J. P., Van Goudoever J. B., et al. (2006). MUC2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131 (1), 117–129. 10.1053/j.gastro.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Van R. B., Tops C. M., Vasen H. F. (2000). From gene to disease; the APC gene and familial adenomatous polyposis coli. Ned. Tijdschr. Geneeskd. 144 (42), 2007–2009. [PubMed] [Google Scholar]
- Vécsey-Semjén B., Becker K. F., Sinski A., Blennow E., Vietor I., Zatloukal K., et al. (2002). Novel colon cancer cell lines leading to better understanding of the diversity of respective primary cancers. Oncogene 21 (30), 4646–4662. 10.1038/sj.onc.1205577 [DOI] [PubMed] [Google Scholar]
- Vétillard A., Jonchère B., Moreau M., Toutain B., Henry C., Fontanel S., et al. (2015). Akt inhibition improves irinotecan treatment and prevents cell emergence by switching the senescence response to apoptosis. Oncotarget 6 (41), 43342–43362. 10.18632/oncotarget.6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachogiannis G., Hedayat S., Vatsiou A., Jamin Y., Fernández-Mateos J., Khan K., et al. (2018). Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359 (6378), 920–926. 10.1126/scienceaao2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Yang X., Li C., Cao X., Luo X., Hu J. (2014). PIK3R3 induces epithelial-to-mesenchymal transition and promotes metastasis in colorectal cancer. Mol. Cancer Ther. 13 (7), 1837–1847. 10.1158/1535-7163.MCT-14-0049 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang H. S., Zhou B. H., Li C. L., Zhang F., Wang X. F., et al. (2013). Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One 8 (2), e56664. 10.1371/journal.pone.0056664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen D., Song W., Liu Z., Ma W., Li X., et al. (2020). ATP6L promotes metastasis of colorectal cancer by inducing epithelial-mesenchymal transition. Cancer Sci. 111 (2), 477–488. 10.1111/cas.14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zuo X., Xie K., Wei D. (2018). The role of CD44 and cancer stem cells. Methods Mol. Biol. 1692, 31–42. 10.1007/978-1-4939-7401-6_3 [DOI] [PubMed] [Google Scholar]
- Wei J., Zhang J., Wang D., Cen B., Lang J. D., DuBois R. N. (2022). The COX-2-PGE2 pathway promotes tumor evasion in colorectal adenomas. Cancer Prev. Res. (Phila). 15 (5), 285–296. 10.1158/1940-6207.CAPR-21-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiswald L. B., Bellet D., Dangles M. V. (2015). Spherical cancer models in tumor biology. Neoplasia 17 (1), 1–15. 10.1016/j.neo.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiswald L. B., Richon S., Validire P., Briffod M., Lai-Kuen R., Cordelières F. P., et al. (2009). Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br. J. Cancer 101 (3), 473–482. 10.1038/sj.bjc.6605173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S., Neufert C., Weigmann B., Neurath M. F. (2007). Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2 (3), 541–546. 10.1038/nprot.2007.41 [DOI] [PubMed] [Google Scholar]
- Wu M., Wu Y., Deng B., Li J., Cao H., Qu Y., et al. (2016). Isoliquiritigenin decreases the incidence of colitis-associated colorectal cancer by modulating the intestinal microbiota. Oncotarget 7 (51), 85318–85331. 10.18632/oncotarget.13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Mao F., Li N., Li W., Luo Y., Shi W., et al. (2020). NF2/Merlin suppresses proliferation and induces apoptosis in colorectal cancer cells. Front. Biosci. (Landmark Ed. 25 (3), 513–525. 10.2741/4817 [DOI] [PubMed] [Google Scholar]
- Wu Z., Zuo M., Zeng L., Cui K., Liu B., Yan C., et al. (2021). OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 22 (1), 50827. 10.15252/embr.202050827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C., Wang M., Ajibade A. A., Tan P., Fu C., Chen L., et al. (2021). Microbiota regulate innate immune signaling and protective immunity against cancer. Cell. Host Microbe 29 (6), 959–974.e7. 10.1016/j.chom.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., He J., Zhang B., Zhang Z., Jia G., Liu S., et al. (2021a). SLC25A1 promotes tumor growth and survival by reprogramming energy metabolism in colorectal cancer. Cell. Death Dis. 12 (12), 1108. 10.1038/s41419-021-04411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. S., Wen D., Zhao X. F. (2021c). Sophocarpine can enhance the inhibiting effect of oxaliplatin on colon cancer liver metastasis-in vitro and in vivo . Naunyn Schmiedeb. Arch. Pharmacol. 394 (6), 1263–1274. 10.1007/s00210-020-02032-8 [DOI] [PubMed] [Google Scholar]
- Yang Y., Yan T., Han Q., Zhang M., Zhang Y., Luo Y., et al. (2021b). ZNF326 promotes colorectal cancer epithelial-mesenchymal transition. Pathol. Res. Pract. 225, 153554. 10.1016/j.prp.2021.153554 [DOI] [PubMed] [Google Scholar]
- Yang Z., Wu D., Chen Y., Min Z., Quan Y. (2019). GRHL2 inhibits colorectal cancer progression and metastasis via oppressing epithelial-mesenchymal transition. Cancer Biol. Ther. 20 (9), 1195–1205. 10.1080/15384047.2019.1599664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K., Lee J., Liu Z., Kim H., Martin D. R., Wu D., et al. (2021). Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell. Death Differ. 28 (8), 2421–2435. 10.1038/s41418-021-00760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Cao L. Y., Wu W. Q., Li H., Jiang Y., Zhang H. F ( (2010). Blocking effects of siRNA on VEGF expression in human colorectal cancer cells. World J. Gastroenterol. 16 (9), 1086–1092. 10.3748/wjg.v16.i9.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans L., Taylor C., Shin E., Harrell A., Ellis A. E., Séguin B., et al. (2012). Frequent alteration of the tumor suppressor gene APC in sporadic canine colorectal tumors. PLoS One 7 (12), 50813. 10.1371/journal.pone.0050813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. K., Ahn J. H., Lee H. J., Lee S. K., Hong S. W., Yoon Y., et al. (2005). Expression of human apolipoprotein(a) kringles in colon cancer cells suppresses angiogenesis-dependent tumor growth and peritoneal dissemination. J. Gene Med. 7 (1), 39–49. 10.1002/jgm.638 [DOI] [PubMed] [Google Scholar]
- Yu Y., Cai Y., Yang B., Xie S., Shen W., Wu Y., et al. (2022). High-fat diet enhances the liver metastasis potential of colorectal cancer through microbiota dysbiosis. Cancers (Basel). 14 (11), 2573. 10.3390/cancers14112573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Tu S., Ma Y., Sun Y. (2021). Downregulation of lncRNA RPLP0P2 inhibits cell proliferation, invasion and migration, and promotes apoptosis in colorectal cancer. Mol. Med. Rep. 23 (5), 309. 10.3892/mmr.2021.11948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S., Tan L., Sun Q., Chen L., Zhao H., Liu M., et al. (2022). Suppression of colitis-associated colorectal cancer by scutellarin through inhibiting Hedgehog signaling pathway activity. Phytomedicine 98, 153972. 10.1016/j.phymed.2022.153972 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang X. Y., Zhang P., He T. C., Han J. H., Zhang R., et al. (2022). Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell. Death Dis. 13 (1), 57. 10.1038/s41419-022-04506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang X., Lai C., Zhang H., Lai M. (2019). PMEPA1 induces EMT via a non-canonical TGF-β signalling in colorectal cancer. J. Cell. Mol. Med. 23 (5), 3603–3615. 10.1111/jcmm.14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. L., Li N., Shen Q., Fan M., Guo X. D., Zhang X. W., et al. (2020). Establishment of a mouse model of cancer cachexia with spleen deficiency syndrome and the effects of atractylenolide I. Acta Pharmacol. Sin. 41 (2), 237–248. 10.1038/s41401-019-0275-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao Y., Zhang G., Huang S., Dong Z., Kong C., et al. (2011). DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells. Int. J. Cancer 128 (3), 551–561. 10.1002/ijc.25365 [DOI] [PubMed] [Google Scholar]
- Zhao G. X., Xu Y. Y., Weng S. Q., Zhang S., Chen Y., Shen X. Z., et al. (2019). CAPS1 promotes colorectal cancer metastasis via Snail mediated epithelial mesenchymal transformation. Oncogene 38 (23), 4574–4589. 10.1038/s41388-019-0740-7 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Guan J., Wang X. (2020). Intestinal stem cells and intestinal organoids. J. Genet. Genomics 47 (6), 289–299. 10.1016/j.jgg.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Y., Wang Q. (2021). HNRNPL affects the proliferation and apoptosis of colorectal cancer cells by regulating PD-L1. Pathol. Res. Pract. 218, 153320. 10.1016/j.prp.2020.153320 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang B., Ma Y., Zhao F., Chen J., Wang B., et al. (2022). Colorectal cancer patient-derived 2d and 3d models efficiently recapitulate inter- and intratumoral heterogeneity. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger. 9 (22), e2201539. 10.1002/advs.202201539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Cheng Z., Lin C., Hoffman R. M., Huang Y., Singh S. R., et al. (2019). MyD88 regulates LPS-induced NF-ĸB/MAPK cytokines and promotes inflammation and malignancy in colorectal cancer cells. Cancer Genomics Proteomics 16 (6), 409–419. 10.21873/cgp.20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Huang S., Chen S., Chen J., Wang Z., Wang Y., et al. (2021). SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell. Death Dis. 12 (5), 449. 10.1038/s41419-021-03733-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y. W., Wu C. E., Zhou J. Y., Zhao Z. M., Liu C. L., Shen J. Y., et al. (2018). Solasodine reverses stemness and epithelial-mesenchymal transition in human colorectal cancer. Biochem. Biophys. Res. Commun. 505 (2), 485–491. 10.1016/j.bbrc.2018.09.094 [DOI] [PubMed] [Google Scholar]
- Zorzi M., Urso E. D. L. (2022). Impact of colorectal cancer screening on incidence, mortality and surgery rates: Evidences from programs based on the fecal immunochemical test in Italy. Dig. Liver Dis. S1590-8658 (22), 336–341. 10.1016/j.dld.2022.08.013 [DOI] [PubMed] [Google Scholar]
- Zou W., Zhang Y., Bai G., Zhuang J., Wei L., Wang Z., et al. (2022). siRNA-induced CD44 knockdown suppresses the proliferation and invasion of colorectal cancer stem cells through inhibiting epithelial-mesenchymal transition. J. Cell. Mol. Med. 26 (7), 1969–1978. 10.1111/jcmm.17221 [DOI] [PMC free article] [PubMed] [Google Scholar]