Abstract
Background:
There is a cohort of patients in whom hip preservation surgery is not indicated, because they have developed signs of early osteoarthritis (OA), and nor can they have a hip replacement, as they are too early in the disease process. Management of this cohort of patients is not standardised and both pharmacological and nonpharmacological measures are utilised to reduce pain. Interventions available for early OA include intra-articular injections of steroids, viscosupplementation and more recently platelet-rich plasma (PRP). However, the use of PRP in hip OA has not yet been studied systematically.
Purpose:
To assess intra-articular PRP as a therapeutic intervention for hip OA, including the duration of efficacy, influence of dose and composition of PRP, and the incidence of adverse effects.
Study Design:
A systematic review and meta-analysis; Level of evidence, 4.
Methods:
We performed literature searches on the MEDLINE, EMBASE, CINAHL, WEB OF SCIENCE, COCHRANE, and SCOPUS databases, and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. Data were pooled using random-effects meta-analysis. We assessed the quality of the included studies using the methodological index for non-randomized studies instrument, with an additional assessment for randomized controlled trials with the revised Cochrane risk of bias tool for randomized trials. This is the first study to concisely collate the available data on the use of PRP in hip OA.
Results:
Eight studies were included in the analysis, with data from a total of 331 patients. PRP significantly reduced pain compared with the baseline at multiple time points, with the greatest effect at the 1- to 2-month follow-up, but PRP significantly improved function only at the 1- to 2-month follow-up. A significantly larger reduction in pain was achieved with a single injection of PRP compared with multiple injections, a total injected dose of PRP <15 mL compared with ≥15 mL, and use of a leukocyte-poor PRP preparation compared with leukocyte-rich PRP. There were no lasting adverse effects.
Conclusion:
Low- and moderate-quality evidence suggests that PRP reduces pain and improves function at the end-point follow-up of studies compared with the baseline. Moderate-quality evidence suggests that a larger reduction in pain is achieved with a single injection of PRP compared with multiple injections, and low-quality evidence attributes a larger reduction of pain with a total injected dose of PRP <15 mL compared with ≥15 mL and using leukocyte-poor PRP compared with leukocyte-rich PRP.
Keywords: hip joint, injection, intra-articular, osteoarthritis, platelet-rich plasma
Hip osteoarthritis (OA) is a progressive degenerative disease involving the articular cartilage and surrounding structures of the hip, 17 resulting in pain and dysfunction, often beyond the confines of the hip. 13 It is one of the most prevalent disabling musculoskeletal problems, affecting 11% of adults in England. 23 While end-stage hip OA is successfully treated by total hip arthroplasty, 8 the National Institute for Health and Care Excellence (NICE) guidelines include nonpharmacological and pharmacological management for earlier states of OA. 21 One such option is an intra-articular injection of corticosteroid, considered an adjunct to the core treatments and providing immediate, albeit short-term, pain relief. 39 This has led to research into other injectable therapies for hip OA, 12 although these have not yet appeared in routine use and have not been recommended by the NICE guidelines in the United Kingdom.
One of the interventions that has gained significant popularity over the past decade is platelet-rich plasma (PRP). PRP is an autologous product derived from whole blood that contains elevated platelet levels, 16 as well as higher concentrations of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor β, and vascular endothelial growth factor. 22 There are several PRP collection protocols and preparation characteristics available from many commercial systems. 9 The main steps involved in the production of PRP are shown in Figure 1. Generally, the production of PRP requires the collection of whole venous blood, which is then mixed with an anticoagulant prior to centrifugation. 18 A single or double centrifugation process is then performed to separate the erythrocytes and concentrate the platelets. The concentrated platelets are found with leukocytes in the “buffy coat,” 5 from which various methods can be used to isolate the platelets with or without leukocytes. The platelets can then be activated with calcium chloride or applied directly without activation.
Figure 1.
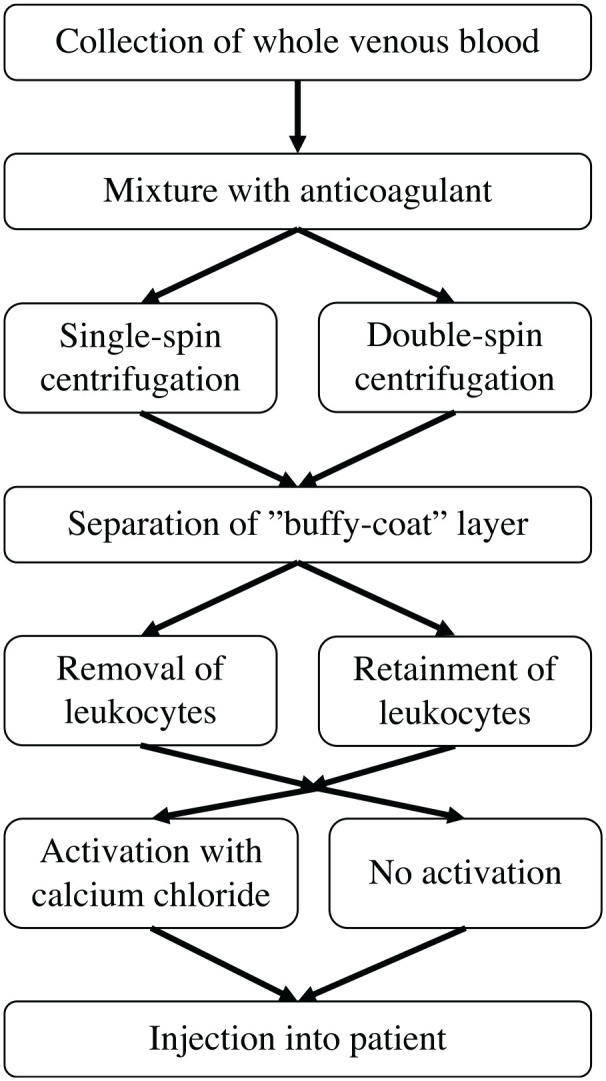
Flowchart of the main steps involved in the production of platelet-rich plasma.
PRP can be categorized into several different types based on differences in platelet isolation and activation method, centrifugation speed, and collection systems —and multiple classification systems exist. 25 One important categorization of PRP is into leukocyte-rich or leukocyte-poor PRP, defined as having a leukocyte concentration above or below the baseline, respectively. 15 The presence of leukocytes has been associated with elevated catabolic cytokines, which may partially antagonize the anabolic cytokines contained within platelets. 32 Regardless of the preparation system, PRP universally contains supraphysiological amounts of platelets and growth factors and has been shown to have an overall anti-inflammatory effect and a positive effect on chondrogenesis, 33 indicating its use as a therapeutic intervention for OA.
While multiple studies have been performed to look into the use of PRP to treat knee OA, 34 there have been fewer looking specifically at hip OA, recommended to be kept separate from knee OA by the European League Against Rheumatism because of differences in anatomy, development, and treatment applicability. 38 Studies that do focus on hip OA have varying conclusions, with controlled studies typically using a comparator of hyaluronic acid (HA)—itself an emerging injectable. Therefore, the aims of this systematic review and meta-analysis were as follows: (1) Assess the efficacy of intra-articular PRP on patient-reported outcomes for hip OA; (2) determine the duration of efficacy after PRP injections; (3) assess the influence of composition and dosage of PRP on efficacy; and (4) review the incidence of adverse effects from PRP therapy.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols checklist 27 (see Appendix 1, available in the online version of this article), following advice from the Cochrane Handbook. 11 The study was registered on the PROSPERO database (ID: CRD42021245553).
Literature Search
We conducted a literature search using MEDLINE (via PUBMED), EMBASE (via OVID), CINAHL (via EBSCOhost), WEB OF SCIENCE, COCHRANE, and SCOPUS databases from database inception to December 2, 2020. No language restrictions were applied, and our search terms were a combination of Medical Subject Headings terms and keywords relating to PRP, OA, and the hip joint (see Appendix 2, available online). The reference lists of included studies were also searched to find additional studies that were not identified by our initial search. We attempted to contact authors of conference abstracts and clinical trials found in the search to include further studies.
Study Selection
After deduplication, 2 authors (A.L., J.B.Z.) independently screened all potentially eligible studies. We included studies that assessed the use of intra-articular injections of PRP as a stand-alone treatment in the treatment of hip OA in adults that reported a patient-reported outcome at any time point as well as the dosage of PRP used and any treatment complications. We excluded studies that used PRP in augmentation to arthroscopy or as an intraosseous injection, as well as any animal studies or those not published in English. Disagreements were resolved through discussion, and a third reviewer (V.K.) made the final decision in the event no consensus was reached.
Data Extraction
The following data were extracted from eligible studies: study details—title, authors, publication year, study design, study setting, and inclusion/exclusion criteria; participant information—sample size, mean age, sex ratio, and hip OA severity; intervention—dose, frequency of administration of PRP injections, and information regarding the preparation methods of PRP; the effect size and P values of both primary and secondary outcome measures for patients treated with PRP from baseline to follow-ups, and adverse effects. Where data were not reported, authors were contacted to obtain the relevant information.
Quality Assessment
The quality of the included studies was assessed independently by the 2 authors using the methodological index for non-randomized studies (MINORS) instrument. 30 Studies were assessed through 12 items (8 items for noncomparative studies), each of which was given a score out of 2 based on whether the item was reported and adequate. In addition, randomized controlled trials (RCTs) were assessed with the revised Cochrane risk of bias tool for randomized trials. 31 This tool included 5 domains, each of which was assessed to determine an item as low (+), high (−), or some concerns (?) over the risk of bias. The overall risk of bias was judged to be low if each domain was assessed as (+), some concerns if at least 1 domain was assessed as (?) but no domains were assessed as (−), and high risk if 2 or more domains were marked as (?), or if 1 or more domains were marked as (−). Risk of bias plots were made using the robvis web app. 19 Any disagreement was discussed and a third reviewer (V.K.) resolved any outstanding conflicts.
Data Analysis
The results of the studies were analyzed in RStudio Version 1.3.1093 (RStudioTeam, 2020). For continuous outcomes, data were preprocessed to obtain mean ± SD using the estimation by Wan et al, 36 if not already recorded within the original papers. Outcomes were expressed as the mean difference (MD) or the standardized mean difference (SMD) depending on the similarity of the used scales. We used the I2 statistic to measure heterogeneity between studies, with a value <50% representing low heterogeneity and a value >75% indicating high heterogeneity. The outcomes were pooled using a random-effects model. A subgroup analysis was performed to identify any sources of heterogeneity.
Results
Literature Search
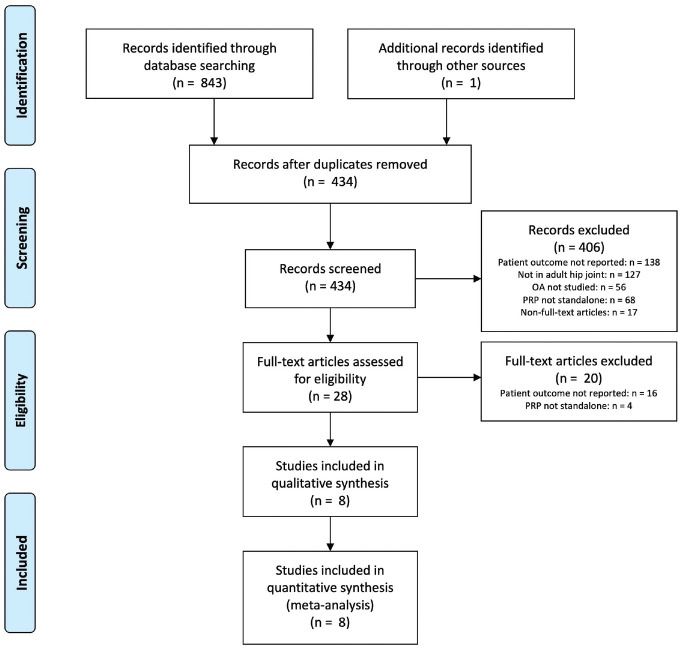
A flow diagram of studies found is presented in Figure 2. After reading the full text, 8 studies1,3,4,6,7,26,29,35 were included for the final analysis. Five studies were reported to be RCTs.3,4,6,7,35 All 5 studies compared PRP with HA, and 1 study additionally compared a combined PRP + HA treatment. 4 Three studies were nonrandomized clinical trials. Two studies evaluated PRP in a single patient cohort26,29 and 1 study compared PRP with combination treatments of PRP + cortisone and PRP + cortisone + recombinant IL1R-antagonist 1 as a retrospective nonrandomized intervention study. Authors of 3 studies were contacted to request further information regarding the composition of PRP used, 1 of which responded.
Figure 2.
Flowchart of the selection process according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. OA, osteoarthritis; PRP, platelet-rich plasma.
Participants
Of participants who received PRP treatment alone, a total of 331 patients were analyzed. There were 50.5% men and 49.5% women, with a mean age of 59.8 ± 11.5 years. Patients were included based on both the clinical criteria and a radiological grading system, with 7 of the 8 studies using the Kellgren and Lawrence (K-L) classification system. Of the patients, 19% were grade I, 35% grade II, 28% grade III, and 18% grade IV. Sánchez et al 26 classified hips with the Tönnis scale, with 30% grade 2 and 70% grade 3.
Intervention
PRP was prepared using a single spin technique in 3 of the 8 studies,1,26,35 with the rest employing a double spin procedure. Most studies involved a total of 3 intra-articular injections at 1-week intervals, with 2 studies29,35 using a single injection, and Baltzer et al 1 reporting 6 injections. A range of 2 to 8 mL of PRP was injected per injection, giving a total dosage ranging from 6 to 24 mL of PRP. Five studies1,6,26,29,35 prepared leukocyte-poor PRP, with the other 33,4,7 using leukocyte-rich PRP.
Outcomes
All studies assessed pain reduction using a 10-cm visual analog scale (VAS). In addition, 5 studies3,4,7,26,35 recorded the Harris Hip Score (HHS), 54,6,7,26,35 recorded the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and 1 29 recorded patient outcomes using the Hip disability and Osteoarthritis Outcome Score (HOOS). Five studies1,4,26,29,35 reported proportions of responders to treatment, defining responders as those experiencing a reduction in pain intensity >20% to 50%, varying between studies. The total length of follow-ups ranged from 4 to 14 months after the last PRP injection.
Quality Assessment
The MINORS scores of the included studies are shown in Appendix 3 (available online). Seven of the 8 studies3,4,6,7,26,29,35 included clear aims and outcomes, and the design was prospective in 6 studies.3,4,6,7,26,35 A summary of the risk of bias of the included RCTs is shown in Appendix 4 (available online). Two RCTs7,35 were judged to have some concerns overall, with the other 3 RCTs3,4,6 judged to be at high risk of bias overall.
Pain Assessment (VAS)
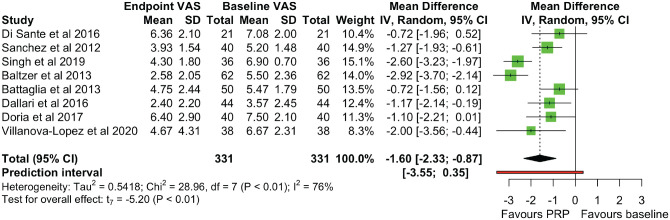
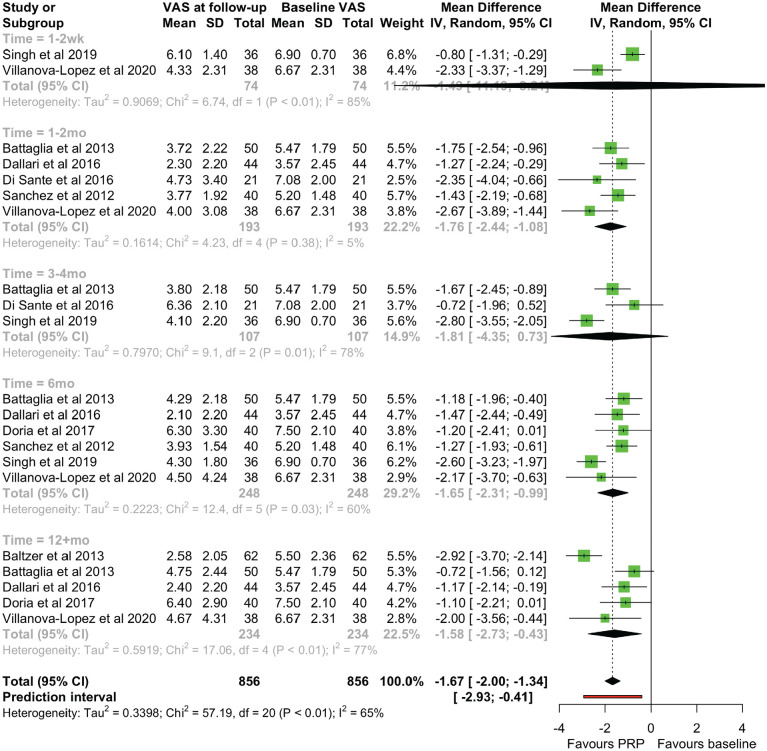
All studies reported VAS scores, with a significant reduction of VAS scores at the endpoint follow-up compared with baseline scores (MD, −1.599 [95% CI, −2.326 to −0.872]; P =.001) (Figure 3). There was significant heterogeneity (χ2 = 28.96 [df = 7]; I2 = 75.8%). Reported VAS scores were grouped into 5 time points after treatment: immediate (1-2 weeks), early (1-2 months), midterm (3-4 months), late (6 months), and long term (12+ months). A statistically significant VAS reduction was seen at early, late, and long-term follow-ups, with significant heterogeneity at all time points except for the early follow-up (χ2 = 4.23 [df = 4]; I2 = 5.4%) (Figure 4). There was a varying effect of OA severity on VAS scores between studies. Three studies4,29,35 found greater improvement with early OA (K-L grades I-II) over severe OA (K-L grades III-IV), with 2 further studies6,7 demonstrating that pain effects were maintained long term in a cohort mainly with early OA, 7 but they disappeared at the 12-month follow-up in a cohort with mainly severe OA. 6 Sánchez et al 26 used the Tönnis scale and found that 10 of the 11 patients who did not respond to treatment (27.5% of patients overall) had Tönnis grade 3 hips. However, 2 studies1,3 found no correlation between the VAS and the K-L grade, with Battaglia et al 3 showing a more profound short-term reduction in the VAS with K-L grade IV OA compared with K-L grade III OA, although these differences were not present by the 12-month follow-up. A short-term effect with severe OA was also noticed by Singh et al, 29 where there was no difference between patients with early or severe OA in terms of the proportion of responders at 12 weeks.
Figure 3.
A forest plot for the visual analog scale (VAS) scores at the endpoint. IV, inverse variance; PRP, platelet-rich-plasma.
Figure 4.
A forest plot for the visual analog scale (VAS) scores at immediate (1-2 wk), early (1-2 mo), midterm (3-4 mo), late (6 mo), and long-term (12+mo) follow-up. IV, inverse variance; PRP, platelet-rich-plasma.
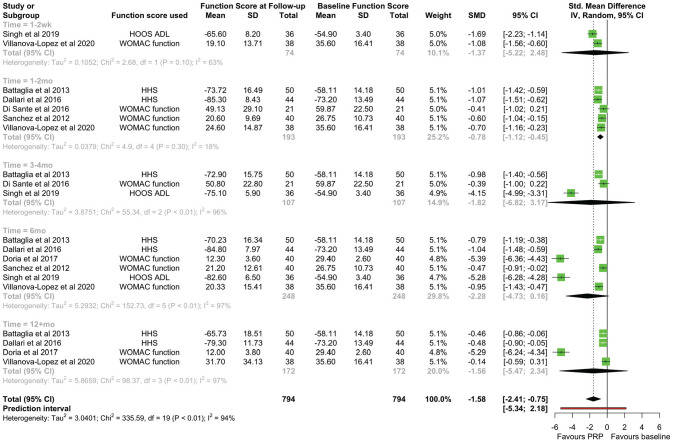
Function Assessment
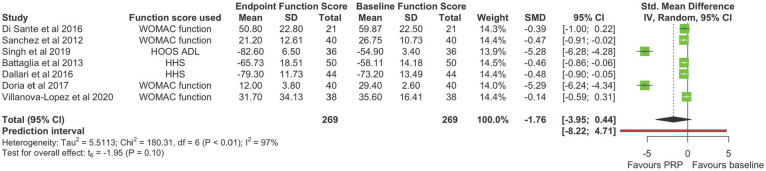
Seven of the 8 studies3,4,6,7,26,29,35 reported an outcome measure that wholly or partially covered function. SMDs were calculated for WOMAC–function, HOOS–Activities of Daily Living, and HHS scores. There was no significant difference in function scores at the endpoint compared with the baseline (SMD, −1.755 [95% CI, −3.953 to 0.442]; P = .098) (Figure 5). When pooling effects at different time points, a statistically significant improvement in function was only seen at the early follow-up (SMD, −0.784 [95% CI, −1.118 to −0.450]; P = .003), with no significant heterogeneity at this timepoint (χ2 = 4.90 [df = 4]; I2 = 18.4%) (Figure 6). There was limited reporting on function outcomes stratified by initial OA severity, with conflicting findings from studies. Similar to VAS scores, Singh et al 29 found an improvement in function only with K-L grades I-II, with no improvement at any time interval in patients with K-L grades III-IV. However, Battaglia et al 3 found no difference in the temporal variation of the HHS between any K-L grade OA.
Figure 5.
A forest plot for the function scores at the endpoint. HHS, Harris Hip Score; HOOS–ADL, Hip disability and Osteoarthritis Outcome Score–Activities of Daily Living; IV, inverse variance; PRP, platelet-rich-plasma; SMD, standardized mean difference; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 6.
A forest plot for the function scores at immediate (1-2 wk), early (1-2 mo), mid (3-4 mo), late (6 mo), and long-term (12+mo) follow-ups. HHS, Harris Hip Score; HOOS–ADL, Hip disability and Osteoarthritis Outcome Score–Activities of Daily Living; IV, inverse variance; PRP, platelet-rich-plasma; SMD, standardized mean difference; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
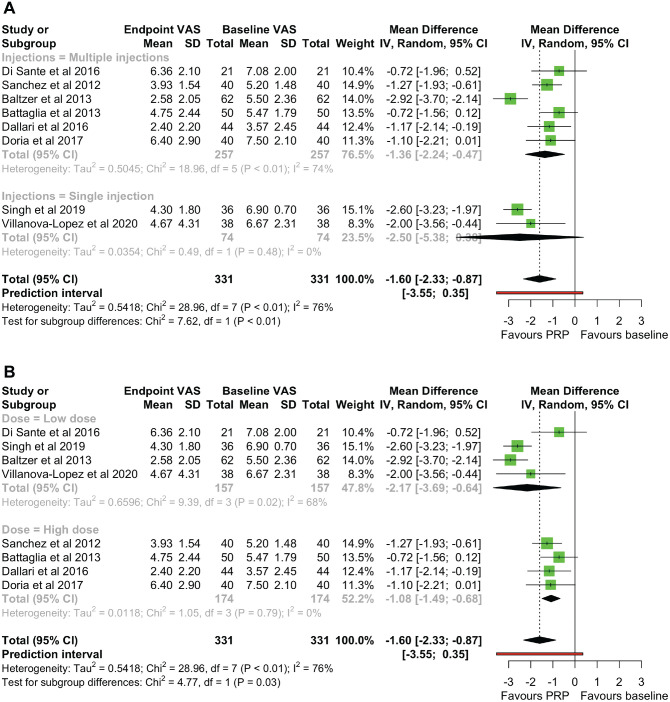
Effect of PRP Dose on Efficacy
Subgroup analyses were performed to investigate the effect of injection number and total dose PRP injected on endpoint VAS scores compared with the baseline. Subgroups were formed based on whether single or multiple PRP injections were administered and whether <15 mL or ≥15 mL of PRP was injected in total, designated as low and high doses, respectively. The test for injection number differences showed a statistically significant quantitative subgroup effect (P = .006), with a greater reduction in the VAS score seen after a single injection (MD, –2.496 [95% CI, –5.377 to 0.384) compared with multiple injections (MD, –1.359 [95% CI, –2.243 to 0.475]) (Figure 7A). In addition, studies that gave a single injection were homogeneous (χ2 = 0.49 [df = 1]; I 2 = 0%), while significant heterogeneity remained among the studies giving multiple injections (χ2 = 18.96 [df = 5]; I2 = 74%).
Figure 7.
(A) A subgroup analysis for endpoint VAS scores with a single versus multiple injections of PRP. (B) A subgroup analysis for endpoint VAS scores with a high versus low total dose of PRP. IV, inverse variance; PRP, platelet-rich-plasma; VAS, visual analog scale.
The results of the subgroup analysis for total dose differences also showed a significant quantitative subgroup effect (P = .029), with a greater reduction in the VAS score after a low total dose (MD, −2.166 [95% CI, −3.693 to −0.639]) compared with a high total dose (MD, −1.081 [95% CI, −1.487 to −0.676]) (Figure 7B). Heterogeneity remained among low dose studies (χ2 = 9.39 [df = 3]; I2 = 68%) but disappeared among high dose studies (χ2 = 1.05 [df = 3]; I2 = 0%).
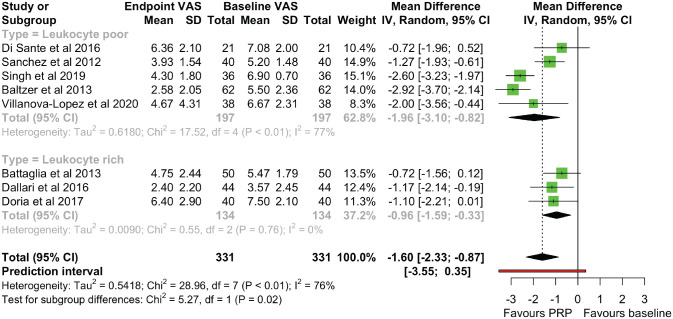
Effect of PRP Composition on Efficacy
A subgroup analysis was performed to investigate the effect of PRP composition on endpoint VAS scores compared with the baseline. Subgroup analyses were formed based on whether leukocyte-poor or leukocyte-rich PRP was injected. The test for subgroup differences showed a significant subgroup effect (P = .022), with a greater reduction in VAS scores after leukocyte-poor PRP was administered (MD = −1.959 [95% CI, −3.098 to −0.819]) compared with leukocyte-rich PRP (MD = −0.958 [95% CI, −1.591 to −0.325]) (Figure 8). Heterogeneity disappeared among the 3 studies3,4,7 that used leukocyte-rich PRP (χ2 = 0.55 [df = 2]; I2 = 0%) but remained among studies that used leukocyte-poor PRP (χ2 = 17.52 [df = 4]; I2 = 77%).
Figure 8.
A subgroup analysis for endpoint visual analog scale (VAS) scores with a leukocyte-rich versus leukocyte-poor platelet-rich-plasma (PRP) preparation. IV, inverse variance.
Adverse Effects
Seven of the 8 studies1,3,4,6,7,26,35 reported the presence or absence of adverse effects, 4 of which1,4,6,35 did not observe any adverse effects among patients treated with PRP. In the other 3 studies,3,7,26 postinjection pain was reported in patients, which universally was transient and self-limiting. In addition, 2 individual events—1 mild rash and 1 superficial hematoma—were reported, both of which spontaneously resolved without long-term complications.
Discussion
This systematic review and meta-analysis aimed to assess the efficacy of PRP as a treatment for hip OA, including any effects of posttreatment duration, dosage, and composition on efficacy. The use of stand-alone PRP injections in the hip OA-specific context is an emerging concept, with the present study suggesting that PRP is beneficial and safe for patients, with demonstratable reductions in pain over a few months.20,37 The present study demonstrates the beneficial effect of PRP in a larger group (N = 331 patients), including an RCT conducted after the previous reviews had been published. 35 It utilized a comprehensive, systematic search strategy to identify all the available evidence in the main electronic databases. There was a significant improvement in pain outcomes as measured by the VAS scale at the endpoint compared with the baseline, with a peak improvement at the midterm (3-4 mo) follow-up, although this was not significant. While there was also a trend for functional scores to improve at the endpoint compared with the baseline, this was not significant, with a significant improvement only seen at the early (1-2 mo) follow-up. This is in contrast to the literature on the use of PRP for knee OA, 28 where the function may be significantly improved even at the 12-month follow-up. However, PRP metrics may be crucial in providing this sustained improvement in function, with a potential critical dose needed with knee OA 2 yet to be achieved with hip OA. In addition, patients with hip OA tend to be younger than those with knee OA, 10 and while joint stability matters more in the knee, range of motion is more crucial in the hip. 24 Therefore, there is a need to focus on hip OA as a stand-alone disease when considering the use of PRP, rather than extrapolating based on results with knee OA.
Although data reporting was too limited to perform a subgroup analysis stratified by OA severity, findings between studies provided conflicting indications about the effect of OA severity on outcomes. While 2 studies1,3 found no correlation between posttreatment outcomes and initial OA severity, most studies found that patients with early OA (K-L grades I-II or Tönnis grade 2) had a greater beneficial effect on outcomes, which was maintained long term at the 12-month follow-up. Nonetheless, there may still be a short-term benefit of PRP even in patients with severe OA, with 2 studies3,29 finding a relatively greater reduction in pain at short-term follow-up in hips with severe OA compared with those with early OA. Future trials should report outcomes stratified by OA grade to establish whether the efficacy of PRP does differ between OA severity and whether there is a short-term benefit when severe OA is treated with PRP.
This study is novel in that it used outcome measures at baseline as the comparator with posttreatment outcomes, unlike previous reviews that use a control group with injected HA. Intra-articular HA injections are a separate emerging treatment for hip OA and do not provide a true gold standard against which PRP can be compared. Few clinical trials have compared PRP injections with control treatments—such as acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroid injections—combined with physical therapy, which form the mainstay of nonoperative hip OA management according to current recommendations. 21
In addition, this is the first study to assess the effect of PRP dosage, injection number, and leukocyte concentration in the management of hip OA. When PRP was given as a single injection, a low total dose (<15 mL), or a leukocyte-poor preparation of PRP, there was a trend for a greater improvement of pain outcomes. Adverse effects in all studies were temporary and self-limiting and did not affect reported outcomes.
There are several limitations to this study. This review included 8 articles analyzing 331 patients treated with PRP, reflecting the very small number of trials that exist. Only 2 studies adopted a double-blind approach, which when combined with the use of baseline scores in the meta-analysis, could present potential bias in outcome measurement. In addition, there may be a bias arising from selective reporting of data, as we cannot exclude the possibility of studies failing to find an effect of PRP treatment and thus remaining unpublished.
Hip OA characteristically involves inflammation and subsequent degeneration of articular cartilage, 17 with the aim of therapeutic intra-articular injections being to directly suppress the inflammation in the joint space. Trials assessing the efficacy of injected PRP therapy should therefore include an objective outcome measure assessing the direct effect of PRP on the articular cartilage using biomarkers or radiographic OA scoring. None of the included studies reported such a measure during the follow-up period, although 1 study 4 did measure growth factor concentration in the PRP from a subsample of patients. In addition, studies differed on therapeutic management during the follow-up period, varying between prohibiting all anti-inflammatory drug consumption throughout 6 to allowing NSAID consumption from 48 hours after treatment. 3 Reported outcomes could therefore have been influenced by concurrent therapy rather than have been attributed to the PRP injection alone.
There was also a large variation in the preparation methods, and reporting thereof, used in producing PRP. This may have contributed to the considerable heterogeneity found, as shown by the removal of heterogeneity in the subgroup analysis of studies where a high total dose of PRP (≥15 mL) was injected, studies that gave a single injection of PRP, or studies that prepared PRP to be leukocyte rich. Future trials should adopt a standard coding system and follow “minimum reporting requirements” 14 when describing PRP preparation to limit this issue.
The scarcity of data available on the use of PRP to treat hip OA may have also contributed to the heterogeneity existing between studies. There was significant heterogeneity between studies at the endpoint and all follow-up points apart from the early 1- to 2-month follow-up in both pain and function scores. Therefore, we can only suggest with any confidence a short-term effect of PRP on improvement of outcomes in hip OA, with only weak evidence for a sustained improvement in pain scores. In each subgroup analysis, heterogeneity remained in 1 of the 2 subgroups, suggesting larger numbers of patients are needed to provide stronger evidence of any differential effect of PRP injection number, dosage, or leukocyte presence.
The future trial design needs to incorporate the aforementioned limitations into a randomized double-blind controlled trial. A large patient cohort with clinically and radiographically diagnosed unilateral hip OA of differing severity treated with a single injection of a low dose of PRP prepared to a leukocyte-poor protocol should be studied. The control group should use the current gold standard therapy of analgesia and physical therapy alongside a single corticosteroid injection. Total follow-up should be extended beyond 12 months, with assessments at 1 to 2 months, 6 months, 12 months, and 24 months to provide a clearer picture of how long any PRP effects may last. At the baseline and each follow-up appointment, radiographic appearance and inflammatory markers should be assessed alongside patient-reported pain and function scores to ascertain whether efficacy from PRP injections is clinically significant.
Conclusion
Low- and moderate-quality evidence suggests that PRP reduces pain and improves function in patients with hip OA compared with baseline, with the strongest evidence for an effect at the 1- to 2-month follow-up. Moderate quality evidence suggests that a larger reduction in pain is achieved with a single injection of PRP compared with multiple injections, and low-quality evidence attributes a larger reduction of pain with a total injected dose of PRP <15 mL compared with ≥15 mL, or using a leukocyte-poor PRP preparation compared with leukocyte-rich PRP. Finally, there were no lasting adverse effects associated with PRP injections. Large, methodologically rigorous trials using gold standard control groups should be conducted to test whether PRP injections have a benefit for patients with hip OA over other currently used forms of nonsurgical management.
Supplemental Material
Supplemental material, sj-pdf-1-ajs-10.1177_03635465221095563 for The Use of Intra-articular Platelet-Rich Plasma as a Therapeutic Intervention for Hip Osteoarthritis: A Systematic Review and Meta-analysis by Anthony Lim, John B. Zhu and Vikas Khanduja in The American Journal of Sports Medicine
Acknowledgments
The authors thank Matthew Pettit for his advice regarding the meta-analysis.
Footnotes
Submitted November 7, 2021; accepted March 10, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: V.K. is an educational consultant for Smith & Nephew and Arthrex. V.K. is also on the Executive Board for BHS as President and ESSKA as Chair of EHPA. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
An online CME course associated with this article is available for 1 AMA PRA Category 1 Credit™ at http://www.sportsmed.org/aossmimis/Members/Education/AJSM_Current_Concepts_Store.aspx. In accordance with the standards of the Accreditation Council for Continuing Medical Education (ACCME), it is the policy of The American Orthopaedic Society for Sports Medicine that authors, editors, and planners disclose to the learners all financial relationships during the past 12 months with any commercial interest (A ‘commercial interest’ is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients). Any and all disclosures are provided in the online journal CME area which is provided to all participants before they actually take the CME activity. In accordance with AOSSM policy, authors, editors, and planners’ participation in this educational activity will be predicated upon timely submission and review of AOSSM disclosure. Noncompliance will result in an author/editor or planner to be stricken from participating in this CME activity.
ORCID iD: Vikas Khanduja  https://orcid.org/0000-0001-9454-3978
https://orcid.org/0000-0001-9454-3978
References
- 1.Baltzer AWA, Ostapczuk MS, Stosch D, Seidel F, Granrath M. A new treatment for hip osteoarthritis: clinical evidence for the efficacy of autologous conditioned serum. Orthop Rev (Pavia). 2013;5(2):59-64. doi: 10.4081/or.2013.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal H, Leon J, Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11(1):3971. doi: 10.1038/s41598-021-83025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36(12):e1501-e1508. doi: 10.3928/01477447-20131120-13 [DOI] [PubMed] [Google Scholar]
- 4.Dallari D, Stagni C, Rani N, et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med. 2016;44(3):664-671. doi: 10.1177/0363546515620383 [DOI] [PubMed] [Google Scholar]
- 5.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7(4):189-197. doi: 10.4103/0974-2077.150734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Sante L, Villani C, Santilli V, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrasonography. 2016;18(4):463-468. doi: 10.11152/mu-874 [DOI] [PubMed] [Google Scholar]
- 7.Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E. Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;5(3):152-155. doi: 10.1055/s-0037-1605584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi R, Perruccio AV, Mahomed NN. Surgical management of hip osteoarthritis. CMAJ. 2014;186(5):347-355. doi: 10.1503/cmaj.121584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gato-Calvo L, Magalhaes J, Ruiz-Romero C, Blanco FJ, Burguera EF. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther Adv Chronic Dis. 2019;10:2040622319825567. doi: 10.1177/2040622319825567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Günther KP, Stürmer T, Sauerland S, et al. Prevalence of generalised osteoarthritis in patients with advanced hip and knee osteoarthritis: the Ulm Osteoarthritis Study. Ann Rheum Dis. 1998;57(12):717-723. doi: 10.1136/ard.57.12.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Cochrane. Published 2020. Accessed January 29, 2021. www.training.cochrane.org/handbook
- 12.Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77-90. doi: 10.1038/s41584-018-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AM, McLoughlin E, Giannakas K, Hutchinson C, Andrew JG. Hip osteoarthritis: where is the pain? Ann R Coll Surg Engl. 2004;86(2):119-121. doi: 10.1308/003588404322827518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kon E, Di Matteo B, Delgado D, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther. 2020;20(12):1447-1460. doi: 10.1080/14712598.2020.1798925 [DOI] [PubMed] [Google Scholar]
- 15.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624-634. doi: 10.1007/s12178-018-9527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med. 2019;38(1):17-44. doi: 10.1016/j.csm.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 17.Lespasio MJ, Sultan AA, Piuzzi NS, et al. Hip osteoarthritis: a primer. Perm J. 2018;22:17-84. doi: 10.7812/TPP/17-084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228. doi: 10.1097/00008505-200110000-00002 [DOI] [PubMed] [Google Scholar]
- 19.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55-61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 20.Medina-Porqueres I, Ortega-Castillo M, Muriel-Garcia A. Effectiveness of platelet-rich plasma in the management of hip osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol. 2020;39:3903-3904. doi: 10.1007/s10067-020-05241-x [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence. Osteoarthritis: Care and Management. Published 2020. Accessed January 29, 2021. https://www.nice.org.uk/guidance/cg177
- 22.Pavlovic V, Ciric M, Jovanovic V, Stojanovic P. Platelet rich plasma: a short overview of certain bioactive components. Open Med (Wars). 2016;11(1):242-247. doi: 10.1515/med-2016-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Public Health England PHP. Musculoskeletal Conditions - PHE. Published 2021. Accessed January 29, 2021. https://fingertips.phe.org.uk/profile/msk/data
- 24.Roaas A, Andersson GBJ. Normal range of motion of the hip, knee and ankle joints in male subjects, 30–40 years of age. Acta Orthop Scand. 1982;53(2):205-208. doi: 10.3109/17453678208992202 [DOI] [PubMed] [Google Scholar]
- 25.Rossi LA, Murray IR, Chu CR, Muschler GF, Rodeo SA, Piuzzi NS. Classification systems for platelet-rich plasma. Bone Joint J. 2019;101(8):891-896. doi: 10.1302/0301-620X.101B8.BJJ-2019-0037.R1 [DOI] [PubMed] [Google Scholar]
- 26.Sánchez M, Guadilla J, Fiz N, Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (UK). 2012;51(1):144-150. doi: 10.1093/rheumatology/ker303 [DOI] [PubMed] [Google Scholar]
- 27.Shamseer L, Moher D, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:G7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12(1):16. doi: 10.1186/s13018-017-0521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh JR, Haffey P, Valimahomed A, Gellhorn AC. The effectiveness of autologous platelet-rich plasma for osteoarthritis of the hip: a retrospective analysis. Pain Med. 2019;20(8):1611-1618. doi: 10.1093/pm/pnz041 [DOI] [PubMed] [Google Scholar]
- 30.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 31.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 32.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi: 10.1177/0363546511417792 [DOI] [PubMed] [Google Scholar]
- 33.Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35-41. doi:10.1177/0363546 513507766 [DOI] [PubMed] [Google Scholar]
- 34.Tang JZ, Nie MJ, Zhao JZ, Zhang GC, Zhang Q, Wang B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020;15(1):403. doi: 10.1186/s13018-020-01919-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villanova-López MM, Núñez-Núñez M, Fernández-Prieto D, et al. Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Rev Esp Cir Ortop Traumatol. 2020;64(2):134-142. doi: 10.1016/j.recot.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 36.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078 [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Doherty M. EULAR recommendations for knee and hip osteoarthritis: a critique of the methodology. Br J Sports Med. 2006;40(8):664. doi: 10.1136/bjsm.2004.016840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong HM, Zhao GF, Lin T, et al. Intra-articular steroid injection for patients with hip osteoarthritis: a systematic review and meta-analysis. Biomed Res Int. 2020;2020:6320154. doi: 10.1155/2020/6320154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajs-10.1177_03635465221095563 for The Use of Intra-articular Platelet-Rich Plasma as a Therapeutic Intervention for Hip Osteoarthritis: A Systematic Review and Meta-analysis by Anthony Lim, John B. Zhu and Vikas Khanduja in The American Journal of Sports Medicine