Summary
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex debilitating disorder manifesting as severe fatigue and post-exertional malaise. The etiology of ME/CFS remains elusive. Here we present a deep metagenomic analysis of stool combined with plasma metabolomics and clinical phenotyping of two ME/CFS cohorts with short (<4y, n=75) or long-term disease (>10y, n=79) compared to healthy controls (n=79). First, we describe microbial and metabolomic dysbiosis in ME/CFS patients. Short-term patients showed significant microbial dysbiosis, while long-term patients had largely resolved microbial dysbiosis but had metabolic and clinical aberrations. Second, we identified phenotypic, microbial, and metabolic biomarkers specific to patient cohorts. These revealed potential functional mechanisms underlying disease onset and duration, including reduced microbial butyrate biosynthesis together with a reduction in plasma butyrate, bile acids, and benzoate. In addition to the insights derived, our data represent an important resource to facilitate mechanistic hypotheses of host-microbiome interactions in ME/CFS.
eTOC Blurb
ME/CFS is an understudied multisystemic disease. Xiong et al. present an integrated multiomics analysis comparing ME/CFS patients with matched controls. Short-term disease was characterized by greater gut microbial dysbiosis, particularly a depletion of butyrate-producing microbes; long-term disease was associated with more severe phenotypic and metabolic abnormalities.
Graphical Abstract
Introduction
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex, multi-system debilitating illness. The syndrome includes severe fatigue that is not alleviated by rest, post-exertional malaise (PEM), muscle and joint pain, headaches, sleep problems, hypersensitivity to sensory stimuli, and gastrointestinal symptoms.1,2,3 In the US alone, ME/CFS affects up to 2.5 million people.4 Our limited understanding of both the physiological changes associated with the syndrome and the underlying biological mechanisms are major impediments to identifying and developing both specific therapies and reliable biomarker-based diagnostics.5,6
The human microbiome – the body’s trillions of bacteria, fungi, and viruses – has recently emerged as an important potential contributor to, or biomarker of ME/CFS.7 Patients have frequent gastrointestinal (GI) disturbances, and in lower-resolution studies based on 16S rRNA gene sequencing, altered gut microbiota.7,8,9,10,11,12,13 Compared to healthy controls, the microbial dysbiosis observed in ME/CFS patients was characterized by decreased bacterial diversity, overrepresentation of putative proinflammatory species, and reductions in putative anti-inflammatory species.11,13 However, sample sizes for these studies were relatively small with limited taxonomic resolution. Much remains underexplored vis a vis the potential functional consequences of ME/CFS-associated microbial changes.
An important function of the intestinal microbiota is metabolism,14,15 feeding both microbial and host processes in its dynamic, symbiotic, and mutualistic relationship with the host. For example, the metabolic products of gut microbiota can feed into host pathways as energy sources16 or function as immune regulators,17,18,19 including short-chain fatty acids,20 metabolites of bile acids,21 or amino acid metabolites like tryptophan,22 respectively. Thus, the microbiome can modulate host physiology via direct stimulatory effects 23, 24 or through secondary pathways coupled to metabolic processes 25. Such host-microbe interactions can be identified both through mechanistic studies 26. but also inferred by high resolution profiling 27, 28 and integrated analyses of the gut microbiome 29 – the microbial fingerprint, and the host metabolome – the collective chemical fingerprint.
Here, we performed a high-resolution characterization of the gut microbiome and the plasma metabolome in two ME/CFS cohorts compared to healthy controls. In an important departure from current studies, we profiled a ‘short-term’ cohort (diagnosed within the previous four years), vs. a ‘long-term’ cohort (patients who have been suffering from ME/CFS for more than ten years). Our goal was to gain an understanding of the baseline molecular mechanisms by which changes in the ME/CFS microbiome may be reflected in circulating metabolic markers, which could then potentiate further alterations in host physiology. In addition, we sought to identify potential molecular and biological markers of ME/CFS progression between the short- and long-term cohorts. Finally, we collected detailed clinical and lifestyle survey metadata for association analysis. Shotgun sequencing of the fecal microbiome of 149 ME/CFS patients (74 short- and 75 long-term) vs. 79 age- and sex-matched healthy controls, we found that short-term ME/CFS patients had more significant microbial and gastrointestinal abnormalities, and that long-term patients tended to establish a stable, but individualized gut microbiome. However, long-term patients had significantly more irreversible health problems and progressive metabolic aberrations. Finally, integrating detailed clinical and lifestyle survey metadata with these high resolution ‘omics data allowed us to develop a highly accurate ME/CFS disease classifier. Taken together, our study presents a high resolution, multi-cohort and multi-‘omics analysis, and provides a mechanistic hypotheses of host-microbiome interactions in ME/CFS.
Results
Data characteristics.
We enrolled 228 participants; 149 with ME/CFS (74 ‘short-term’ and 75 ‘long-term’) and 79 approximately age- and sex-matched healthy controls (Figure 1, Table S1–2). The short-term and long-term cohorts were designed to obtain a better understanding of the biological processes during the progression of ME/CFS. The cohort was 96.5% Caucasian, with an average age of 43 years and 67% female, characteristics consistent with epidemiological reports that women are 3–4X more susceptible to ME/CFS than men 30. We collected detailed clinical metadata (Table S1–2), stool samples for shotgun metagenomics, and blood for targeted metabolomic analysis. Clinical metadata (n = 228) and blood samples (n = 184) were collected at time of enrollment, followed by self-collection of fecal samples (n = 224) within the following two weeks (n=180 complete datasets of metadata, blood, and stool). We established a workflow to integrate the 906 clinical features into major disease markers to optimize data dimensionality (Figure 1). Whole-genome shotgun metagenomic sequencing of the stool samples generated an average of 10,801,733 high-quality and classifiable reads per sample, which were then reconstructed to examine gut microbiome composition (species N=384, Table S3) and gene function (gene N=9652, Table S4). Plasma was fractionated from blood and sent for targeted LC-MS analysis, where 1278 metabolites were identified for host molecular ‘omics profiling (Table S5). Finally, we analyzed each data type individually, then altogether to build multi-‘omics models to describe and predict onset, stage of disorder, and associated microbial and metabolic features. This allowed us to target microbial pathways likely to affect host-microbiome interactions and alter the disease pathophysiology. For all datatypes, we performed two primary comparisons: 1) ME/CFS vs. healthy controls, to understand the broad differences inherent to the disease, and 2) short- vs. long-term ME/CFS vs. healthy controls, to understand disease progression.
Figure 1. Summary of study design and analytical pipeline.
We collected detailed clinical metadata, fecal samples, and blood samples for 228 individuals in three cohorts: healthy controls, patients with short-term (<4y) or long-term (>10y) ME/CFS. A comprehensive ‘omics workflow was constructed with the multi-data types (phenotypic, metagenomics, and metabolomics, respectively) and multi-computational models to understand potential host-microbe interactions. LC-MS, Liquid chromatography-mass spectrometry.
The host phenotype in ME/CFS
To understand and interpret the host phenotype of ME/CFS compared to healthy controls, we collected comprehensive clinical metadata including detailed demographics and lifestyle information, an itemized dietary intake survey, medical history records, three general questionnaires regarding the physical and mental health of all participants, and five patient-specific surveys encompassing ME/CFS clinical symptoms and measurements (Table S1–2). We first excluded possible dietary biases and then established that the dietary habits were comparable among all groups (Figure S1, Table S1–2). We also found that the frequency of previous acute infections (Figure S1, Chi-square test, p < 0.001), which supports the potential association of infections with the onset of ME/CFS 31. Our naïve Bayesian classification model (Figure S2A, area under the curve (a measure of classification accuracy), AUC = 0.85), which we used to identify clinical features that discriminate healthy controls vs. patients, showed that, besides some announced dysfunctions like orthostatic intolerance and fibromyalgia, most ME/CFS patients also suffered from additional complications, such as depression, headaches, constipation, and anxiety 32, 33 (Figure S2B). Altogether, these high-resolution data echo the known pathophysiologies of ME/CFS and established the clinical characteristics of our cohorts 34.
ME/CFS patients have decreased gut microbial diversity and greater heterogeneity
To begin to identify potential microbial mechanism(s) related to these symptoms, we first decoded the microbiota of ME/CFS patients. After classification of our shotgun metagenomic dataset to the species level (384 species passing quality cutoffs, see Methods, Table S3), we examined community-wide metrics to understand if broad dysbiosis was observed in patients compared to controls. Clustering using principal coordinate analysis (Bray-Curtis dissimilarity distance, which reflects the similarity of microbiome composition between each pairwise set of samples) showed 1) most of sample variation was explained by the onset of ME/CFS (Figure 2F, permutational analysis of variance (PERMANOVA), p = 0.002) and was not influenced by the age difference between the control and patient groups (Figure S3, Table S7), 2) patient samples had higher heterogeneity, as a population compared to controls (Figure 2G, p < 0.01, control N=79, ME/CFS N=149, heterogeneity was calculated over N=384 species, Wilcoxon rank-sum test with Bonferroni correction). Interestingly, high heterogeneity was also observed in our recent study of frail older adults35, 36, suggesting a non-uniform adjustment to the host’s changing physiological conditions.
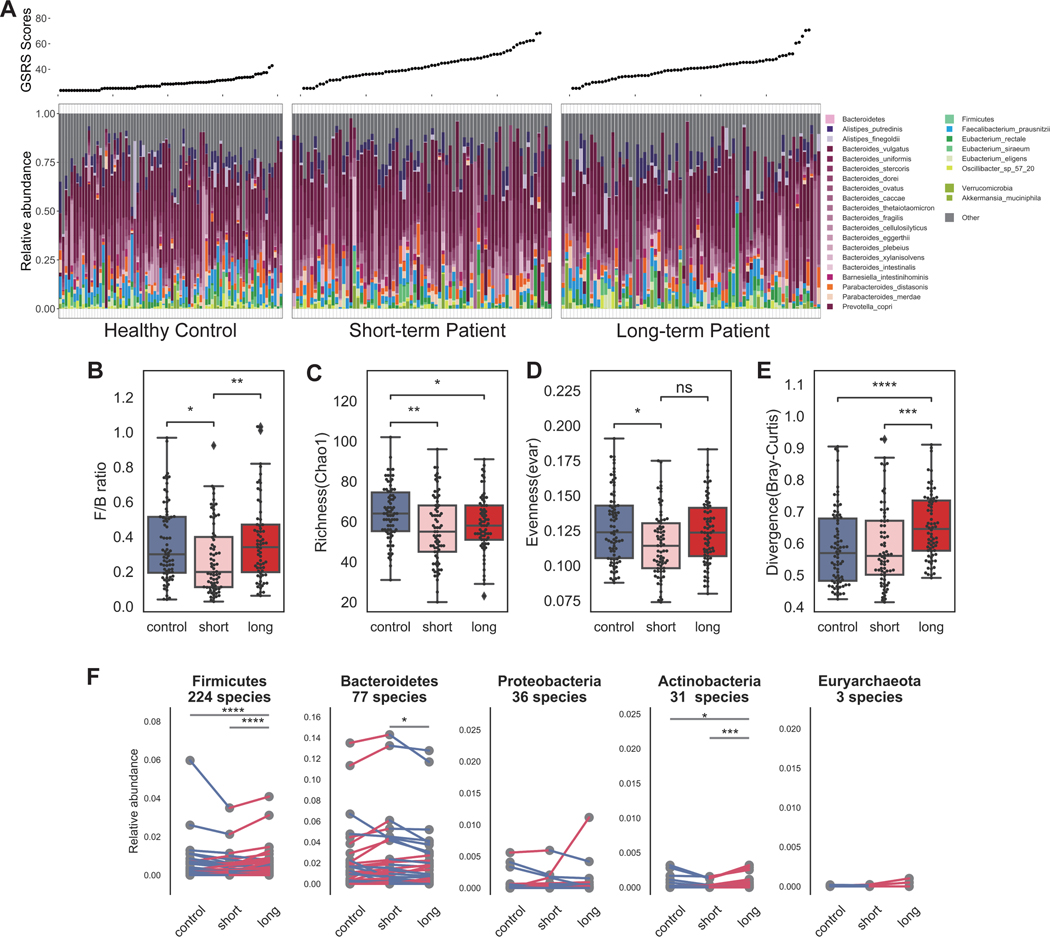
Figure 2. Microbial dysbiosis in ME/CFS is characterized by decreased diversity and greater heterogeneity.
Comparing controls vs. ME/CFS patients (irrespective of disease stage), community structure differed in ME/CFS with A) decreased richness (Chao 1 index, which measures the number of observed species); B) decreased evenness (lower values of Smith and Wilson’s Evar index); C) decreased rarity (smaller proportion of the least abundant species (<0.2% relative abundance); D) decreased inequality (smaller Gini index of the dominant species (>0.2% relative abundance); E) decreased Firmicutes/Bacteroidetes ratio. p-values were computed by Wilcoxon rank-sum test. F) First and second principal coordinates of dimensionality reduction for Bray-Curtis dissimilarity distances, which measures pairwise similarity of two given samples). Values in brackets indicate the amount of total variability explained by each principal coordinates. p-value and R2 were calculated by permutational multivariate analysis of variance (PERMANOVA) test with patient/control as a variable. G) Increased heterogeneity observed in ME/CFS as measured by divergence, or Bray-Curtis dissimilarity. p-value was computed by Wilcoxon rank-sum test. H) Volcano plot showing differences in predicted growth rate in select species in ME/CFS. Each dot indicates a microbe, sized by the value of its inferred growth rate. The x-axis shows the absolute difference (mean growth rate in patient – mean growth rate in control) and the y-axis is the log10(p-value, Wilcoxon rank-sum test). Species that were predicted to grow faster in patients were colored red and slower in blue. p-value > 0.05 was considered not significant (gray). See also Table S3.
High microbial biodiversity has increasingly been associated with ecosystem health 37, with a less diverse (fewer members) and less even structure (i.e., more heavily weighted with fewer members) associated with decreased resilience and susceptibility to pathogenic colonization 38. ME/CFS patients had fewer community members (Figure 2A, Chao 1 index; p <0.001, Control N=79, MECFS N=149, Chao 1 index was calculated over N=384 species, Wilcoxon rank-sum test with Bonferroni correction), and lower Evar evenness (Figure 2B, p <0.05, Wilcoxon rank-sum test with Bonferroni correction). To understand if there were specific species lacking in patients, we calculated a rarity and dominance index. A decrease of Chao 1 (Figure 2A) and rarity (Figure 2C, p <0.05, Wilcoxon rank-sum test with Bonferroni correction) indicated that ME/CFS patients had fewer low abundance members. The lower Gini index from those dominant species implied greater equality (Figure 2D, p <0.01, Wilcoxon rank-sum test with Bonferroni correction), with highly abundant commensal species. We also noted a modest change in the ratio of the overall relative abundance of Firmicutes to Bacteroidetes phyla (Figure 2E), a common metric for comparing broad compositional differences between microbiota, including IBD and other inflammatory diseases 39–41.
Finally, we predicted in-situ growth rate of individual microbes with our Growth Rate InDex (GRiD) algorithm, which leverages coverage differences over a microbe’s genome to infer microbial growth rate, a proxy for metabolic activity in a community 42. Interestingly, the species that grew slower in ME/CFS were primarily Firmicutes (Figure 2H), suggesting a further nuance to the already disproportionate Firmicutes:Bacteroides ratio. Taken together, the gut microbiome of ME/CFS, like in aging and other chronic inflammatory disorders, was characterized by modest but broad dysbiosis, including a less diverse and more uneven gut microbiome community with higher heterogeneity and altered Firmicutes:Bacteroidetes ratio, supported by a group of slower-replicating Firmicutes species.
Microbial dysbiosis occurs in short-term ME/CFS and stabilizes in long-term disease
Our analyses thus far investigated major differentiating features between ME/CFS patients and healthy controls. We wondered if microbial and metabolomic markers changed significantly during the progression of ME/CFS, or if early features could predict later severity. We analyzed our dataset comparing the short-term to the long-term group. First, we evaluated the effect of age as a confounder but found no significant differences (PERMANOVA with age as a variate, p > 0.05, Wilcoxon rank-sum test with Bonferroni correction, p > 0.05 (≤50 years old (yo) vs. >50 yo subgroups, Figure S3, Table S7).
We then examined overall microbial composition differences between the patient cohorts as previously performed (Figure 3A). Interestingly, differences in the gut microbiome were more pronounced and variable during early stages of the disease compared to long-term and controls (Figure 3C–3F, p < 0.05, pairwise Wilcoxon rank-sum test with Bonferroni correction). As noted, heterogeneity was a feature we observed associated with aging, particularly frail aging . As we found no confounding effect of age, we conjectured that these differences were driven by ME/CFS disease duration. A significant shift in the Bacteroidetes (77.5%) to Firmicutes (19.4%) ratio was observed only in short-term but not long-term patients (Figure 3B, pairwise Wilcoxon rank-sum test with Bonferroni correction, p < 0.05 short-term; p > 0.1 long-term vs. controls), explaining the modest difference previously observed (Figure 2E).
Figure 3. Significant microbial dysbiosis in observed in short-term ME/CFS.
A) Taxonomic classification at species-level resolution for all individuals in the three cohorts: healthy controls, short-term patients, and long-term patients. Relative abundances of the most abundant gut species (top 25) are presented with gray representing the aggregate relative abundance of the remaining species. Gastrointestinal Symptom Rating Scale (GSRS) score, indicating the scale of gastrointestinal abnormality, is shown above for each individual. Similarly to Figure 2, we showed differences in the microbial community structures in short-term and long-term patients in: B) composition at phylum-level – decreased Firmicutes/Bacteroidetes ratio in short-term patients; C) reduced richness in short- and long-term (Chao 1 index, which measures the number of observed species); D) decreased evenness in short-term (lower values of Smith and Wilson’s Evar index). p-values were computed by Wilcoxon rank-sum test. E) Increased heterogeneity observed in long-term cohort as measured by divergence, or Bray-Curtis dissimilarity. p-value was computed by Wilcoxon rank-sum test. F) Each species in the five most abundant phyla were compared among three groups to observe the dynamics of gut community with respect to progression of disease. Here, each point represents the average relative abundance for a given species, connected with a line that is colored by increase (red) or decrease (blue). p-values were computed by Wilcoxon signed-rank test. p-value annotation legend: ns: p > 0.05, *: 0.01 < p <= 0.05, **: 0.001 < p <= 0.01, ***: 1e-04 < p <= 0.001, ****: p <= 1e-04. See also Table S3.
To further resolve species-level differences between short-term and long-term patients, we performed a pairwise comparison for the mean relative abundance of every species in the top five most abundant phyla (Figure 3F). We discovered that species that had atypical relative abundance in short-term patients with respect to healthy controls (e.g., Bacteroidetes sp.) tended to stabilize in late-stage patients, i.e., become relatively closer to the relative abundance observed in controls.
Taken together, we found that microbial dysbiosis is most marked early in ME/CFS disease, characterized by a loss of diversity driven by low abundance bacteria with high heterogeneity. Over time, we conjecture that the microbiota then stabilizes and reverts to an ecosystem more characteristic of healthy controls, with the reacquisition of some low abundance species and normalization of diversity. These results highlight the importance of stratifying by disease duration to identify features relevant to disease progression.
ME/CFS is distinguishable with microbiome and metabolomics features
Currently, there are no approved laboratory diagnostics available for ME/CFS 6, likely due to the heterogeneity of the disease. We hypothesized that the combination of environmental and clinical factors may assist in a more comprehensive disease classification. We constructed multiple state-of-the-art classifiers for deep profiling. For metagenomic data, we used both microbial species as well as gene relative abundance (identified by KEGG gene profiling, see Methods, Table S4) for our classifier, as we hypothesized that both species relative abundance as well as gene-level differences could differ between cohorts. Thus, we constructed four models based on 1) species and 2) KEGG gene relative abundances, 3) normalized abundance of plasma metabolites or 4) a combination of all three (multi-‘omics, see Methods), in addition to the clinical classifier described above. Irrespective of the model, results obtained using multi-‘omics data (gradient boosting model, AUC=0.90) outperformed any individual dataset, followed by the metabolome (AUC = 0.82), KEGG gene profile (AUC = 0.73), and species relative abundance (AUC = 0.73, gradient boosting model, Figure 4B; LASSO logistic, SVM, and Random Forest, Figure S4). This improved performance of multi-‘omics for differentiating ME/CFS patients from healthy controls underscores the complementarity of different ‘omics in describing the molecular processes that can occur with shifts in the host physiological state.
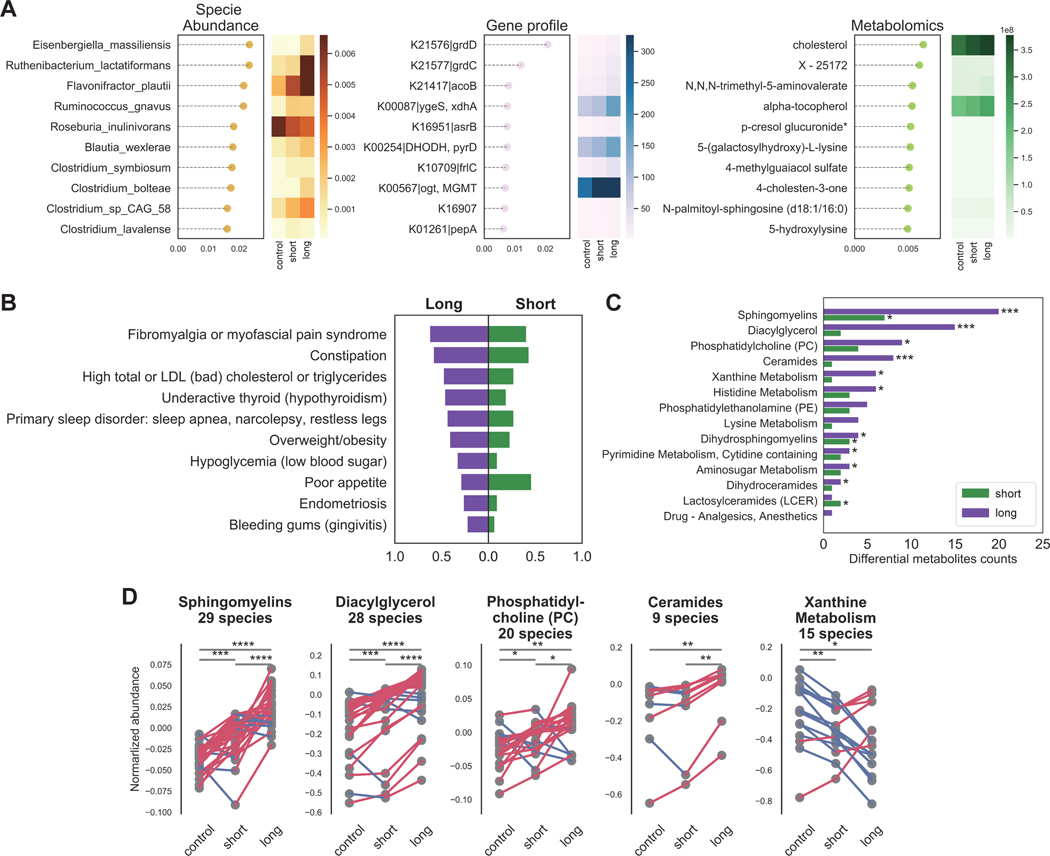
Figure 4. Out-performing multi-‘omics model identifies microbial, metagenomic, and metabolic biomarkers for ME/CFS compared to controls.
A) Biomarkers from three supervised Gradient Boosting (GDBT) models are shown. Models from top to bottom: species relative abundance, relative abundance of KEGG gene profile, normalized abundance of plasma metabolomics. The top ten most important features in each model are shown together with their general functional class, raw abundance, and variance. From left to right: 1. Functional annotations: species relative abundance model - the metabolic function (capacity of butyrate, tryptophan, and propionate pathway); KEGG gene profile model - the class identification of the enzyme; metabolomics models - the superfamily for the metabolite; 2. Feature importance: features were ranked by their contribution to the model on the y-axis; the x-axis indicates the feature importance value from each model; 3. Average feature abundance in control and patient groups (Figure S4); 4. Variation in mean relative abundance in control and patient groups with coefficient of variation. B) Performance of the classifiers using area under the curve (AUC) was evaluated using 10 randomized and 10-fold cross-validations for each model: species relative abundance (pink), KEGG gene profile (blue) or metabolites (orange) alone, or taken altogether (‘omics, green), which used the combination of the top 30 features from three models. See also Figure S4 and Table S3–5.
We then examined the most discriminatory features for each of the individual ‘omics models to identify potential biomarkers and to further biological interpretation (Figure 4A, S5). Low abundance microbes comprised the most discriminatory features, including microbes implicated in tryptophan, butyrate and propionic acid production that were largely depleted in ME/CFS (Wilcoxon rank-sum test with Bonferroni correction with over N=384 species, p < 0.01, Figure S4). Metabolites of both pathways are key immunomodulatory molecules that regulate metabolic and endocrine functions 22,43–46.
Microbial genes that discriminated ME/CFS and were decreased included genes involved in betaine production (grdD, grdE, grdI), an anti-inflammatory metabolite 47,48. Besides betaine, sphingomyelin, serotonin, and cholesterol were highly discriminatory features, and have also been previously reported to be altered in ME/CFS patients 6, 49, 50, 51.
Finally, because of ME/CFS’ inherent heterogeneity, it is possible that clinical or lifestyle factors could confound this analysis. We sought to determine whether biomarkers of interest were disease- or confounder- driven. We applied an unbiased general linear test (see Methods, Table S7) with an extensive array of metadata features. We found none of these biomarkers were significantly correlated with confounders. Additionally, from this general association study, we observed that broader microbiome features, such as alpha diversity, were not biased by metadata. However, a few metabolites (importantly, not those features selected in the ME/CFS classification model below) were influenced by gender, age, and IBS score (Figure S6). Despite this, we concluded our microbiome and metabolomic analyses are largely unconfounded by individual clinical and behavioral features.
Severe phenotypic and metabolic abnormalities in long-term ME/CFS
We then constructed individual and multi-‘omics models to differentiate disease durations and controls. We followed this by over representation analysis (ORA) on metabolic pathways and Bayesian classification on phenotypic abnormalities to further pinpoint distinctive patterns in different stages of ME/CFS (see Methods). Overall classification accuracy was lower than for overall disease but still relatively accurate leveraging multi-‘omics data (AUC=0.82, gradient boosting model, Figure S5). Low (<0.5%) relative abundance bacteria, including several putative butyrate producers (Clostridium sp.) 52, were identified as potential biomarkers. The metabolomics model also identified two cholesterol and several lipid metabolites as discriminatory (Figure 5A, Table S6), consistent with the phenotypic classifier which identified many metabolic-related abnormalities (Figure 5C) 53,54, 55, 56, 57, 58, 59, 60 to be more predictive in long-term ME/CFS. However, we note that some of these phenotypes also can increase with age, which is largely matched in our cohort but may contribute to these differences 61 ,62. In addition, we found that fibromyalgia 63 was a key feature distinguishing short-vs. long-term disease, as was a trend towards worsening sleep problems and more pronounced post-exertional malaise in the later stage of the disease (Figure 5B). Interestingly, we identified poor appetite 64 to be the most distinguishable phenotype in the short-term group, which is consistent with a trend of more GI disturbances in the early stage (Figure 5B, 3A).
Figure 5. Phenotypical and metabolic abnormality are most pronounced in long-term patients.
A) Multi gradient boosting models identified most important species, genes and metabolites differentiating controls, short-, or long-term ME/CFS. In each model, the top ten features are ranked by their contribution to the model on the y-axis, and the x-axis indicates the feature importance value. The heatmap shows the average feature abundance or relative abundance in each group. For full classification model performance (AUCs), see also Figure S5. B) Naïve Bayesian model based on medical history records classified the stage of disease and identified nine significant clinical phenotypes in the long-term cohort and one significant phenotype in the short-term cohort. For each feature, the probability of experiencing the symptom in the long-term patients was presented to the left on the x-axis and the probability in the short-term patients was presented to the right. C) Overrepresentation analysis (ORA) on the plasma metabolome identified the most differential metabolites and pathways in the long-term group. For each pathway, two comparisons were conducted, control vs. short-term and control vs. long-term. P-values were computed by linear global t-test and the counts of differential metabolites presented on the x-axis. D) The trend of gradually changing metabolic irregularities along with the progression of disease are indicated by the difference between control, short-term and long-term cohorts. Here, each point represents the average normalized abundance for a given metabolite in the top five most abundant superfamilies, connected with a line that is colored by if increasing (red) or decreasing (blue). p-values were computed by Wilcoxon signed-rank test. p-value annotation legend: *: 0.01 < p <= 0.05, **: 0.001 < p <= 0.01, ***: 1e-04 < p <= 0.001, ****: p <= 1e-4. See also Table S5–6.
ORA with the metabolomics profiles identified the most striking differences between short- and long-term patients and controls. Unlike trends observed in the microbiome data with the greatest dysbiosis observed in short-term patients, long-term patients had more metabolites differentiating them from healthy controls, especially in sphingolipids and diacylglycerol metabolites (Figure 5C), confirming previous metabolomic findings. Interestingly, most metabolic species either decreased across experimental groups (control>short-term>long-term, e.g., xanthine;), or increased (control<short-term<long-term; e.g. sphingomyelins, diacylglycerol, phosphatidylcholine, and ceramides, p < 0.01, pairwise Wilcoxon rank-sum test with Bonferroni correction with N=1278 metabolites, see Figure 5D), suggesting that metabolic irregularities associated with ME/CFS may gradually worsen over time (Figure 5D). Taken together, we postulate that long-term ME/CFS patients have developed a unique but stable pathophysiology characterized by more severe clinical symptoms and an array of altered host metabolic reactions.
Gut and plasma butyrate is reduced in early-stage disease and is associated with host abnormal physiology
We noted in our metagenomics classification model a particular refrain of a depletion of butyrate-synthesizing microbes, including Roseburia and F. prausnitzii in ME/CFS. Butyrate is a major energy source for colonic epithelial cells and one of the main intestinal anti-inflammatory metabolites 65, 43. We thus performed a focused metagenomic and metabolomic analysis of the butyrate pathway to better understand its potential role in the crosstalk between the gut microbiota and host physiology in ME/CFS.
Strikingly, we found a depletion in plasma isobutyrate in the short-term group (Figure 6A, p = 0.03, Wilcoxon rank-sum test with Bonferroni correction with over N=1278 metabolites) 66. Thus, we sought to link plasma metabolite abundance to gene-coding potential of the microbiome. We performed differential abundance analysis of KEGG gene matrix (p value computed by Wilcoxon rank-sum test with Bonferroni correction over N=9652 genes) and found that butanoate synthesis (KEGG map00650) differentiated between patients and controls (Figure 6C; fold change and p value of each gene in butyrate pathway is shown in Figure 6D, also in Table S4). Finally, we inferred gut butyrate abundance from metagenomic data using a Markov Chain Monte Carlo (MCMC) metabolite prediction algorithm (see Methods), in the absence of matched gut metabolomic data. Inferred concentrations of isobutyrate were significantly decreased in ME/CFS (Wilcoxon rank-sum test with Bonferroni correction, p = 0.05), especially in the short-term group (Figure 6B, pairwise Wilcoxon rank-sum test with Bonferroni correction, p < 0.01).
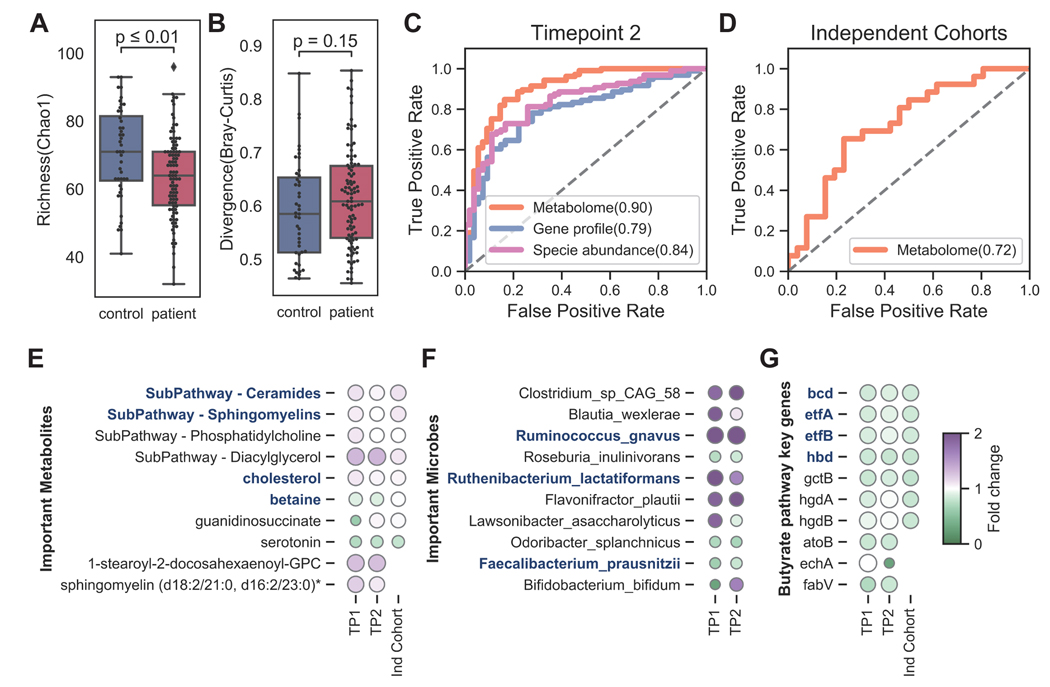
Figure 6. Limited microbial butyrate biosynthesis capacity associates with reduced plasma isobutyrate and multiple blood metabolites.
In the blood and gut environment, decreased butyrate abundance in the short-term patient was indicated by: A) significantly reduced plasma isobutyrate normalized abundance; B) decreased predicted gut isobutyrate in patients, especially in short-term patients. Boxes show median relative abundance and interquartile ranges (IQR); whiskers specify ±1.5*IQR from the box’s quartile. P-values were computed by Wilcoxon rank-sum test. C) The reduced abundance of most key enzymes in the butanoate mechanism (KEGG pathway map00650) indicated a more limited microbial butyrate biosynthesis capacity in ME/CFS. Differentiating enzymes were colored and annotated on the map (decreased in blue and increased in red). D) Correlation of plasma metabolite normalized abundance and relative abundance of microbial butyrate biosynthesis features, with fold changes. Heatmap shows significant correlations (Spearman, p < 0.05) with the top bar indicating the metabolite superfamily. The top half shows the key enzymes in the KEGG butanoate pathway. On the left, different fold changes between the two patient cohorts (short-term vs. control and long-term vs. control, respectively) indicated a significant decrease in butyrate biosynthetic capacity in the early stages of ME/CFS. P-values were calculated in each group with Wilcoxon rank-sum test. Finally, the bottom half shows the correlation between the relative abundance of predicted butyrate producers and plasma metabolites. Microbes were ordered by relative abundance. For each microbe, the size of the dot indicates the mean abundance in each group and the color indicated fold change over the control group. P-value was computed by Kruskal–Wallis H test. p-value annotation legend: *: 0.01 < p <= 0.05, **: 0.001 < p <= 0.01, ***: 1e-04 < p <= 0.001. See also Table S6.
Finally, we performed a Spearman correlation analysis with the relative abundance of 1) butyrate producing microbes or 2) KEGG enzymes with plasma metabolites to identify the degree to which this pathway may influence circulating metabolite levels (Figure 6D, Table S4, p value computed by Spearman correlation and correlated by Holm’s Method with N=18 species + N=113 butyrate genes ~ N=1278 metabolites, see Methods). From the thousands of plasma metabolites tested, we identified 24 positive correlations, including moieties from propionates, succinates, tryptophans, and hippurates, consistent with results of our differential abundance analysis, as well as 12 negative correlations, including sulfate and ursodeoxycholate moieties. The further suggests that changes in the ability of the gut microbiome to metabolize or synthesize short-chain-fatty acids (SFCA) is reflected in dysbiosis of these and related metabolites in plasma.
Microbiome and metabolomic biomarkers are shared across additional ME/CFS cohorts
To explore the generalizability of our findings, we compared our findings with those identified in 1) an additional timepoint for our current cohort: we collected metagenomics and metabolomics data again õne year after their initial recruitment (n=107 patients, n=59 controls); 2) two independent cohorts: the microbiome cohort from Guo et al., 202367 with 197 long-term participants (n=106 patients, n=91 controls) and the metabolome cohort from Germain et al., 202049 with 56 female individuals (n=26 patients, n=26 controls). While there are notable biological differences in study design (e.g., long-term patients only vs. our short- and long-term cohort, gender), we found that the main conclusions and the identified biomarkers in ME/CFS were largely shared across all cohorts, supporting the robustness of our models and the validity of our findings.
First, the loss of gut microbial diversity in ME/CFS was observed in both the external microbiome cohort and our second timepoint. Guo et al. also found decreased evenness in the disease (Figure 2B). Most microbiome features that we at timepoint 1 were similar one year later (Figure 7A–B). Similarly, external cohorts and our second timepoint supported the depletion of butyrate producers and key butyrate-producing genes, indicating that the microbial butyrate biosynthesis capability was reduced in ME/CFS. Guo’s cohort measured fecal SCFAs (acetate, propionate, butyrate) by GC-MS, and found significant decreases of acetate and butyrate in ME/CFS.(Figure 7G). We then validated our classifiers with plasma metabolomics data from our second timepoint (Figure 7C) and Germain’s cohort (Figure 7D). The performance of the models was strong (accuracy > 70%) and numerous shared biomarkers among cohorts. Out of ten metabolic biomarkers identified from timepoint 1, six were shared among cohorts (Figure 7E).
Figure 7. Microbial and metabolomic features of ME/CFS identified in additional cohorts.
A) Decreased richness and B) increased heterogeneity (Bray-Curtis dissimilarity) in the gut microbiota were consistent in our second timepoint (Wilcoxon rank-sum test). B) The performance of our classifiers was assessed using ROC curves in C) our second timepoint and D) an independent cohort (Germain et al., 2020). The models were colored by species relative abundance (pink), KEGG gene profile (blue), and metabolites (orange). E) The fold change of important metabolic subpathways and biomarkers are shown from three cohorts: timepoint 1, 2, and an independent cohort. The shared features with Germain’s study were highlighted in blue. F) The fold change of microbial biomarkers from the classifier in timepoints 1 and 2. The shared biomarkers with Guo’s study are highlighted in blue. G) The fold change of key genes in the butyrate pathway across three cohorts. The shared essential genes from the Acetyl-CoA branch are highlighted in blue. See also Figure S7.
Taken together, we found that our microbial and metabolic biomarkers were robust to cohort, supporting the reproducibility of our conclusions in ME/CFS.
Discussion
Here, we performed a large-scale multi-‘omics investigation integrating detailed clinical and lifestyle data, gut metagenomics, and plasma metabolomics in short- and long-term ME/CFS patients compared to healthy controls. Several studies have reported a disrupted gut microbiome in ME/CFS 11, 13 as well as changes in blood cytokine and metabolite levels. However, our cohort design differentiating short- and long-term patients was important to identify microbial and metabolic features that may contribute to disease progression.
Notably, the most significant microbial dysbiosis occurred in short-term ME/CFS. Compositional differences in short-term ME/CFS were consistent with previous studies using lower resolution sequencing 68 and an independent microbiome study from Guo et al. 67 co-published in this issue, identifying a broad reduction in microbial diversity 69, alterations in the ratio of Bacteroides:Firmicutes microbiota 10, and increased heterogeneity of low abundance organisms. This latter feature has recently been associated with the microbiome of frail older adults and supports its general association with reduced health outcomes 35. There are several potential explanations for this early-stage dysbiosis. First, short-term patients suffer more GI disturbances, and gut microbiome changes may reflect these environmental changes. Second, it is possible that patients may try a range of interventions that impact their gut microbiome, which is dynamic and influenced by numerous intrinsic and extrinsic factors, including age and diet 70. Diet can not only dramatically shift gut microbial community composition, but it can alter the metabolic potential of the microbes and production of immunomodulatory metabolites. We also analyzed the detailed dietary metadata, showing that most dietary habits are comparable among our cohorts, except infrequent sugar intake in our short-term patients, which might also contribute to the microbial differences observed in early stages of disease (Table S1, Figure S1).
The return of the gut microbiome of long-term patients to a configuration more similar to healthy controls (with notable differences nonetheless, in low abundance species and in heterogeneity) as well as the reduced occurrence of gastrointestinal illness in this cohort, suggests a return to a relative homeostasis. However, we conjecture that microbial dysbiosis seen in short-term patients may have cumulative and long-term effects, where damage may be caused by an initial trigger, resulting in cascading events. Long-term patients, despite their relatively more ‘control-like’ gut microbiome, have more severe clinical symptoms and metabolic dysbiosis. Thus, we hypothesize that ME/CFS progression may begin with loss of beneficial microbes, particularly SCFA producers, resulting in more pervasive gastrointestinal phenotypes that is later reflected in plasma metabolite levels. Individual-specific changes then lead to the irreversible metabolic and phenotypic changes and unrecoverable ME/CFS. Other possibilities for this ‘normalization’ observed in long-term ME/CFS include survival bias, as the cohort design for this study is cross-sectional, not truly longitudinal, and it is possible that the >10y cohort is comprised of individuals who have not seen significant improvement or decline in their symptoms, not that disease progression normalizes by default.
The abnormalities in the short-term cohort could result in potential increases in aberrant translocation of microbial metabolites that could affect host immune and metabolic processes. For example, one of the changes we noted in short-term patients was a reduction in potential immunomodulatory organisms (butyrate and tryptophan producers, e.g., F. prausnitzii), which we speculate could lead to long-term metabolic dysbiosis. The reduced prevalence of the butanoate synthesis pathway and the reduced relative abundance of butyrate-producing bacteria among all patients, but especially in the short-term group, suggested a loss of butyrate in the intestinal environment. This was consistent with the decrease of isobutyrate measured in the blood. We additionally note that some of these microbial signatures, such as these changes in SCFA producers, have been identified in other disease cohorts. For example, a particularly interesting study examining the gut microbiome and metabolome of cardiovascular disease 71 suggested that major alterations in both feature sets might begin significantly before clinical onset of disease, including a depletion of butyrate producers. One possibility is that the microbial and metabolic features identified here are less specific to ME/CFS but more generally biomarkers of future disease risk.
Butyrate, tryptophan, and other microbial metabolites have been linked to mucosal immune regulation, and our team previously showed a striking immune dysbiosis in different blood immune markers, including changes in the functional capacity of mucosal associated invariant T (MAIT) cells and Th17 cells, and a decrease in the frequency of CD8+ T cells and natural killer cells in long-term ME/CFS patients 72. This is an exciting association because each of these cell types have been linked to bacterial or fungal infections, respond to microbial metabolites, and have been linked to the pathogenesis of autoimmune or chronic inflammatory diseases. Thus, it is possible that the microbiome primes or sustains an aberrant immune response following disease onset. This is supported by an observed shift from a predominantly Th1 to Th2 immune response in ME/CFS 73.
There is currently no standard diagnostic test for ME/CFS because of many phenotypes of ME/CFS are shared with other disorders 5,6, such as fibromyalgia. Here, our integration of multiple ‘omics data significantly increased classification accuracy and identified microbial and metabolic features that could pinpoint potential hypotheses for further investigation and therapeutic strategies. Longitudinal sampling of short-term patients, particularly as they progress to long-term disease, would help to untangle directionality of microbial dysbiosis and potential effects on the blood metabolome. For example, recent studies performing large-scale associations with the gut microbiome and blood metabolome have identified that a significant fraction (upwards of 15%) of blood metabolites can be predicted by gut microbiome composition 74. However, understanding of the temporal nature of this association is limited. Here, our ‘omics workflows could be one of the guiding frameworks to intergrading microbiome, metabolome, and host phenotypes, and thus, bring a more understanding to host-microbiome interactions.
Finally, we believe that recent potential associations between the chronic immune dysfunctions in ME/CFS patients and ‘long COVID’ increase the relevance of the results reported here. ‘Long COVID’ refers to phenotypes suffered by numerous patients infected by SARS-CoV-2 (COVID-19) that have ‘recovered’, but did not return to full health. Notably, it manifests as numerous phenotypical abnormalities shared with ME/CFS, including lingering chronic fatigue and myalgias. In ME/CFS, key symptoms might also be triggered by acute infections including SARS coronavirus, MERS 75, the Epstein-Barr Virus (EBV) 76, or other agents, and such infections were reported to be more frequent in the medical histories of our patient cohort, even preceding the onset of ME/CFS symptoms 77, 78 Understanding the biological mechanisms underlying ME/CFS may now have further urgency and generalizability in the worldwide COVID-19 pandemic. Taken together, we have established a framework to study host-microbiome interactions leveraging ‘omics to identify host and microbial metabolites and functions implicit in ME/CFS, presenting a rich clinical and ‘omics dataset to further mechanistic hypotheses to better understand this debilitating disease.
LIMITATIONS OF THE STUDY
This study was designed to recruit age-, sex- and diet- matched healthy cohort and patient cohort. However, we note that our long-term cohort is slightly but significantly older than the short-term cohort (though age-matched in our healthy controls), which could contribute to some of the phenotypes, particularly clinical abnormalities, observed. Additionally, we do not have matched cohorts with other disorders with overlapping phenotypes, such as IBS, neuroinflammatory disorders, and others. This is particularly relevant because ME/CFS is often misdiagnosed. While our results support that we can very accurately discriminate healthy individuals from ME/CFS patients, future studies would include cohorts with matched individuals with differing diagnoses to pinpoint microbial/metabolomic changes that are specific to ME/CFS rather than general disease risk. In addition, a rigorous control of exposures and co-morbidities could more effectively narrow down on microbial and metabolomic features that are ME/CFS-specific vs. a generalizable risk or protective factors for inflammatory disease. In advance of these data, the use of these features as potential diagnostic biomarkers may be premature.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Julia. Oh (Julia.Oh@jax.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Metagenomics data have been deposited at PRJNA878603 and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. All original code has been deposited at https://github.com/ohlab/MECFS_2021 and is publicly available as of the date of publication.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Stool samples from ME/CFS patients and healthy donors | This study | N/A |
| Plasma samples from ME/CFS patients and healthy donors | This study | N/A |
| Critical commercial assays | ||
| QIAamp 96 DNA QIAcube HT Kit | QIAGEN | #51331 |
| BioCollector fecal collection kit | The BioCollective, Denver, CO | https://www.thebiocollective.com/products-services |
| OMNIgene•GUT tube | DNA Genotek | OMR-200 |
| Deposited data | ||
| Metagenomics sequencing raw data | This study | NCBI-SRA: Bioproject PRJNA878603 |
| Software and algorithms | ||
| R version (v4.0.4) | ||
| Python (v3.10.4) | ||
| scythe (v0.994) | Buffalo, 2014 | https://github.com/vsbuffalo/scythe |
| sickle (v1.33) | Joshi and Fass, 2011 | https://github.com/najoshi/sickle |
| MetaPhlAn3 | Beghini et al., 2021 | https://huttenhower.sph.harvard.edu/metaphlan/ |
| Bowtie2 (v2.3.1) | Langmead and Salzberg, 2012 | https://github.com/BenLangmead/bowtie2 |
| GRiD (v1.3) | Emiola et al., 2018 | https://github.com/ohlab/GRiD |
| USEARCH (v8.0.1517) | Edgar, 2010 | https://www.drive5.com/usearch/ |
| dplyr_1.0.5 | R package | https://dplyr.tidyverse.org/index.html |
| microbiome_1.12.0 | R package | https://microbiome.github.io/tutorials/ |
| phyloseq_1.34.0 | R package | https://joey711.github.io/phyloseq/ |
| maaslin2_1.8.0 | R package | https://huttenhower.sph.harvard.edu/maaslin/ |
| globaltest_5.44.0 | R package | https://www.bioconductor.org/packages/release/bioc/html/globaltest.html |
| bcdstats_0.0.0.9005 | R package | https://github.com/bcdudek/bcdstats/ |
| lme4_1.1–29 | R package | https://cran.rproject.org/web/packages/lme4/index.html |
| vegan_2.6–2 | R package | https://cran.r-project.org/web/packages/vegan/index.html |
| deseq2_v1.18.1 | R package | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| scipy_1.8.0 | python package | https://scipy.org/ |
| jupyter_4.9.2 | python package | https://jupyter.org/ |
| numpy_1.22.3 | python package | https://numpy.org/ |
| pandas_1.4.2 | python package | https://pandas.pydata.org/ |
| scikit-learn_1.0.2 | python package | https://scikit-learn.org/stable/index.html |
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cohort and study design
All subjects were recruited at Bateman Horne Center, Salt Lake City, UT, based on who met the 1994 CDC Fukuda (Fukuda et al., 1994) and/or Canadian consensus criteria for ME/CFS (Carruthers, 2007). Healthy controls were frequency-matched to cases on age, sex, race/ethnicity, geographic/clinical site, and season of sampling. Patients or controls taking antibiotics or who had any infections in the prior one month, or who were taking any immunomodulatory medications were excluded from the study. The study was approved by The Jackson Laboratory IRB (Study number 17-JGM-13) and written informed consent and verbal assent when appropriate were obtained from all participants in this study. We enrolled a total of 149 ME/CFS patients (of which 74 had been diagnosed with ME/CFS <4 years before recruitment and 75 had been diagnosed with ME/CFS >10 years before recruitment) and 79 healthy controls. Among them, 107 patients and 59 healthy controls were followed one year after the recruitment as timepoint 2. Subject characteristics are shown in Supplemental Table 1.
Participants
149 ME/CFS patients who had been seen at Bateman Horne Center (Salt Lake City, UT) for routine clinical care between February 2018 and September 2019 and 79 matched HCs were recruited for the study. The 149 MECFS subjects included 74 sick with ME/CFS for <4 years and 75 sick for greater than 10 years. The age range of ME/CFS participants and HCs was 18–65 years at the time of informed consent. HCs were matched with <4 ME/CFS participants by age (± 5 years), gender and ethnicity. Enrolled ME/CFS participants were required to fulfill the International Chronic Fatigue Syndrome Study Group research criteria 79, the Canadian Consensus Criteria 80, and the IOM clinical diagnostic criteria 81. HCs were recruited from the Salt Lake City metropolitan area using advertisements posted on social media, the clinic webpage or by phone contact with a volunteer pool from previous studies. HCs were considered generally healthy and between 18 to 65 years of age. HCs were excluded if they fulfilled ME/CFS diagnostic criteria or had a history of illness, had a BMI>40 or had been treated with long-term (longer than 2 weeks) antiviral medication or immune modulatory medications within the past 6 months or had been treated with short-term (less than 2 weeks) antiviral or antibiotic medication within the past 30 days.
METHOD DETAILS
Clinical metadata collection and preprocess
Clinical symptoms and baseline health status was assessed on the day of physical examination and biological sample collection from both case and control subjects. For each participant, we collected demographic information (including age, gender, diet, race, family, work, and education), medical histories, and three questionnaires regarding the general physical and mental health condition (RAND-36 form), sleep quality (PSQI form) and gastrointestinal health (GSRS form). The summary of analyzed clinical features and questionnaires are shown in supplemental Table 2. Age and diet were analyzed and discussed as potential confounders (Figure S1 and S4). Medical histories were simplified into binary features (0 - no records, 1, had/having the disease) and further constructed naïve Bayesian classification models. Every questionnaire was transformed into a 0–100 scale to facilitate combination and comparison wherein a score of 100 is equivalent to maximum disability or severity and a score of zero is equivalent to no disability or disturbance.
Plasma sample collection and preparation
Healthy and patient blood samples were obtained from Bateman Horne Center, Salt Lake City, UT and approved by JAX IRB. One 4 mL lavender top tube (K2EDTA) was collected, and tube slowly inverted 8–10 times immediately after collection. Blood was centrifuged within 30 minutes of collection at 1000 x g with low brake for 10 minutes. 250 uL of plasma was transferred into three 1 mL cryovial tubes, and tubes were frozen upright at −80°C. Frozen plasma samples were batch shipped overnight on dry ice to The Jackson Laboratory, Farmington, CT, and stored at −80°C.
Plasma untargeted metabolome by UPLC-MS/MS
Plasma samples were sent to Metabolon platform and processed by Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS) following the CFS cohort pipeline. In brief, samples were prepared using the automated MicroLab STAR® system from Hamilton Company. The extract was divided into five fractions: two for analysis by two separate reverse phases (RP)/UPLC-MS/MS methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by HILIC/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. QA/QC were analyzed with several types of controls were analyzed including a pooled matrix sample generated by taking a small volume of each experimental sample (or alternatively, use of a pool of well-characterized human plasma), extracted water samples, and a cocktail of QC standards that were carefully chosen not to interfere with the measurement of endogenous compounds were spiked into every analyzed sample, allowed instrument performance monitoring, and aided chromatographic alignment. Compounds were identified by comparison to Metabolon library entries of purified standards or recurrent unknown entities. The output raw data included the annotations and the value of peaks quantified using area-under-the-curve for metabolites.
Fecal sample collection and DNA extraction
Stool was self-collected at home by volunteers using a BioCollector fecal collection kit (The BioCollective, Denver, CO) according to manufacturer instructions for preservation for sequencing prior to sending the sample in a provided Styrofoam container with a cold pack. Upon receipt, stool and OMNIgene samples were immediately aliquoted and frozen at –80°C for storage. Prior to aliquoting, OMNIgene stool samples were homogenized by vortexing (using the metal bead inside the OMNIgene tube), then divided into 2 microfuge tubes, one with 100μL aliquot and one with 1mL. DNA was extracted using the Qiagen (Germantown, MD, USA) QIAamp 96 DNA QIAcube HT Kit with the following modifications: enzymatic digestion with 50μg of lysozyme (Sigma, St. Louis, MO, USA) and 5U each of lysostaphin and mutanolysin (Sigma) for 30 min at 37 °C followed by bead-beating with 50 μg 0.1 mm of zirconium beads for 6 min on the Tissuelyzer II (Qiagen) prior to loading onto the Qiacube HT. DNA concentration was measured using the Qubit high sensitivity dsDNA kit (Invitrogen, Carlsbad, CA, USA).
Metagenomic shotgun sequencing
Approximately 50μL of thawed OMNIgene preserved stool sample was added to a microfuge tube containing 350 μL Tissue and Cell lysis buffer and 100 μg 0.1 mm zirconia beads. Metagenomic DNA was extracted using the QiaAmp 96 DNA QiaCube HT kit (Qiagen, 5331) with the following modifications: each sample was digested with 5μL of Lysozyme (10 mg/mL, Sigma-Aldrich, L6876), 1μL Lysostaphin (5000U/mL, Sigma-Aldrich, L9043) and 1μL oh Mutanolysin (5000U/mL, Sigma-Aldrich, M9901) were added to each sample to digest at 37°C for 30 minutes prior to the bead-beating in the in the TissueLyser II (Qiagen) for 2 × 3 minutes at 30 Hz. Each sample was centrifuged for 1 minute at 15000 x g prior to loading 200μl into an S-block (Qiagen, 19585) Negative (environmental) controls and positive (in-house mock community of 26 unique species) controls were extracted and sequenced with each extraction and library preparation batch to ensure sample integrity.
Sequencing adapters and low-quality bases were removed from the metagenomic reads using scythe (v0.994) and sickle (v1.33), respectively, with default parameters. Host reads were removed by mapping all sequencing reads to the hg19 human reference genome using Bowtie2 (v2.3.1), under ‘very-sensitive’ mode. Unmapped reads (i.e., microbial reads) were used to estimate the relative abundance profiles of the microbial species in the samples using MetaPhlAn3.
QUANTIFICATION AND STATISTICAL ANALYSIS
Metabolome enrichment study
From the raw data (peaks area-under-the-curve), we first kept metabolic features present in >50% of the samples for further analysis, and missing values were imputed with the minimum value. To remove batch variability, for each metabolite, the values in the experimental samples are divided by the median of those samples in each instrument batch, giving each batch and thus the metabolite a median of one. For each metabolite, the minimum value across all batches in the median scaled data is imputed for the missing values. We first applied qualitative enrichment analysis (Over Representation Analysis ORA) in two patient cohorts (short-term vs. control, and long-term vs. control). We conducted Wilcoxon rank-sum test on all metabolites and counted the significantly differential metabolites in every sub pathway. Fisher test with Benjamini-Hochberg adjustment was followed to identify the distributions of the over-representated genes in the pathway. For every sub pathway, we also applied a global quantitative enrichment analysis with linear globaltest in R to compute the association between a group of metabolites from that pathway and the duration of disease (control, short-term, and long-term).
Taxonomic and KEGG gene profiling of metagenomics samples
Taxonomic compositions were profiled using Metaphlan3.0 82 and the species whose average relative abundance > 1e-5 were kept for further analysis, giving 384 species. The gene profiling was computed with USEARCH(v8.0.15) 83 (with parameters: evalue 1e-9, accel 0.5, top_hits_only) to KEGG Orthology (KO) database v54, giving a total of 9452 annotated KEGG genes. The reads count profile was normalized by DeSeq2 in R 84. Metagenomics read depth was considered as potential confounders and analyzed as below (See Method Confounder analysis).
Microbial community structure analysis
The overall community structures was examined by Correspondence Analysis (PCoA) and PERMANOVA were performed in R with the adonis function in the R package vegan to analyze the partitioning of variation giving potential confounders including age and gender. The heterogeneity index (Inter-individual divergence) and community indexes including chao1, evenness(evar), rarity(low_abundance) and inequality(dominance_gini) were computed by R package microbiome. The species replication rate was predicted using GRiD with default settings 42. Metagenomics read depth was considered as potential confounders and analyzed as below (See Method Confounder analysis).
Gut metabolic status prediction
Here, we adapted from the MAMBO 85 pipeline and predicted 224 metabolites status of the microbial community in our cohort. We first constrained the Genome-scale metabolic models (GSMMs) community of all 384 microbes identified in the species profile. We started with the same gut metabolic environment for all samples giving randomized 224 metabolites initialization and using a Markov chain Monte Carlo (MCMC) to sample metabolites. In each step, we sampled one metabolite and used the Flux balance analysis to model reaction flux with a slight change of the target metabolites and accepted the step only if the probed growth rate is correlated with the species relative abundance. Samples were first subjected to 100,000 search steps, and 100,000 steps were subsequently added until a high Pearson correlation (ρ > 0.6) with the target metagenomic abundance profile was achieved. Finally, the 10% time points with the highest Pearson correlation scores between the biomass profile and the metagenomic abundance profile were averaged, yielding a robustly predicted metabolome.
Multi-‘omics classification models
To identify phenotypic, metagenomic, and metabolomic markers of the onset(control/patient) of the disease, we constructed a naïve Bayesian classification model with medical history records and three individual classification models based on the species abundance, normalized KEGG gene abundance, and normalized metabolite profile and one combination multi-omics model with all top ten features collected from each model. We also tested four different classification methods, LASSO logistic regression, Support vector machine (SVM), Random Forest (RF), and Gradient Boosting (GDBT). The same Multi-‘omics classification model system was also applied to classify the duration(control/short-term/long-term) of the disease.
All analyses were carried out using the Python package ‘scikit-learn’. Normalized KEGG gene and normalized plasma metabolome were standardized (by centering to mean 0 and dividing by the standard deviation of each feature) before fitting into the models. The models were optimized by five-fold RandomizedSearchCV to probe the best parameters giving lists of candidates. Models were then validated by 10-fold stratified cross-validation testing (we resampled dataset partitions 10 times). In each test, the accuracy of the model was examined using ROC (area under the curve). The comparison among models showed that GDBT outcompeted the rest three and reached the best performance. In the Gradient boosting model, two steps were carried out. In the first step, the model was constructed using each of the three profiles (species abundance, KEGG gene abundance, and metabolite profile) individually to compute the feature importance as the feature contributions to the classification. In the second step, the collective model was constructed using a combination of top ten important features determined from the species relative abundance model + top ten important features determined from the KEGG gene model + top ten important features determined from the plasma metabolite model.
Confounder analysis
Confounder analysis was done by R package MaAsLin286. The demographic features (including age, gender, ethnicity, and race), diet records, antivirals, antifungals, antibiotics, and probiotics medications, and self-reported IBS scores are considered potential confounders. We ran general linear tests to determine the multivariable association between each meta feature and each microbial and metabolomic features (including microbiome diversity indexes, specie abundance, KEGG gene abundance, plasma metabolites). The three formulas for fixed effects: 1) Demographic model: expr ~ age + gender + ethnic + race + IBS; 2) Diet model: expr ~ diet_meat + diet_sugar + diet_veg + diet_grains + diet_fruit; 3) expr ~ antifungals + antibiotics + probiotics + antivirals. The expr in these formulas have combined microbiome diversity indexes and 3 ‘omics in the model training and covered all features in the differentiation analysis. Metagenomics reads depth was also considered as another confounder. The general linear tests were computed with all microbiome features by the formula: reads_count ~ microbiome diversity indexes + specie abundance + KEGG gene abundance. No microbiome features were significantly selected by the model. All significant features identified by the association models are shared in supplemental table 7. The codes and run log files are shared on our GitHub.
Interaction study and targeted pathway analysis
We computed the Spearman correlation for the butyrate pathway genes (N = 113) and butyrate producers (N = 18) with metabolites (N = 1278) along with the fold change of the elements. P-value was correlated by Holm’s method.
Statistical Analysis
The dimensionality reduction analysis was conducted by Principal Correspondence Analysis (PCoA) using sklearn.manifold.MDS function for both gut microbiome Bray-Curtis dissimilarity distance matrix and normalized plasma metabolome profile. The statistically significant differences among independent groups (healthy/patient/short-term/long-term) were determined by nonparametric test using pairwise Wilcoxon rank-sum test two-sided with Bonferroni correction. The average abundances of each species, genes and metabolites were determined to be significantly elevated or depleted in short-term or long-term groups by pairwise nonparametric comparison using Wilcoxon signed-rank test with Bonferroni correction over N=384 species, N=9652 genes and N=1278 metabolites, respectively. Chi-squared test was used to compare the infection frequencies between healthy and patient groups. The correlation analysis was done by Spearman’s rank correlation, and the p-values were correlated by Holm’s method. P value annotations: ns: p > 0.05, *: 0.01 < p <= 0.05, **: 0.001 < p <= 0.01, ***: 1e-04 < p <= 0.001, ****: p <= 1e-04.
Supplementary Material
Supplementary Table 1 Demographic information of cohorts and Clinical metadata summary. Related to STAR Methods.
Supplementary Table 2 Metadata. Related to STAR Methods.
Supplementary Table 3 Species relative abundances derived from MetaPhlAn3. Related to Figure 2, 3 and 4.
Supplementary Table 4 KEGG gene reads count from metagenomic data. Related to Figure 4.
Supplementary Table 5 Plasma metabolome raw data (peaks area-under-the-curve). Related to Figure 4 and 5.
Supplementary Table 6 Feature importance and annotation from multi-‘omics classification model and Butyrate pathway: the statistics of key microbes and key enzymes in KEGG butanoate metabolism, and their correlations with key plasma metabolites. Related to Figure 5 and 6.
Supplementary Table 7 Confounder driven features identified by MaAsLin2, Related to STAR Methods
Highlights.
Multi-omics identified phenotypic, gut microbial, and metabolic biomarkers for ME/CFS
Reduced gut microbial diversity and increased plasma sphingomyelins in ME/CFS
Short-term patients had more severe gut microbial dysbiosis with decreased butyrate
Long-term patients had more significant metabolic and clinical aberrations
ACKNOWLEDGEMENTS
We are thankful to the Oh, Unutmaz, and Li laboratories for inspiring discussions and acknowledge the contribution of the Genome Technologies Service at The Jackson Laboratory for expert assistance with sample sequencing for the work described in this publication. We also thank the clinical support team at the Bateman Horne Center and all the individuals who participated in this study. This work was funded by 1U54NS105539. JO is additionally supported by the NIH (1 R01 AR078634-01, DP2 GM126893-01, 1 U19 AI142733, 1 R21 AR075174).
Footnotes
DECLARATION OF INTERESTS
Dr. Suzanne D. Vernon is affiliated and has a financial interest with The BioCollective, a company that provided the BioCollector, the collection kit used for at home stool collection discussed in this manuscript. No other authors have competing interests.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Afari N, and Buchwald D. (2003). Chronic fatigue syndrome: a review. Am. J. Psychiatry 160, 221–236. 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Nacul L, O’Boyle S, Palla L, Nacul FE, Mudie K, Kingdon CC, Cliff JM, Clark TG, Dockrell HM, and Lacerda EM (2020). How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: The Natural History of ME/CFS. Front. Neurol. 11, 826. 10.3389/fneur.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holgate ST, Komaroff AL, Mangan D, and Wessely S. (2011). Chronic fatigue syndrome: understanding a complex illness. Nat. Rev. Neurosci 12, 539–544. 10.1038/nrn3087. [DOI] [PubMed] [Google Scholar]
- 4.Bested AC, and Marshall LM (2015). Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health 30, 223–249. 10.1515/reveh-2015-0026. [DOI] [PubMed] [Google Scholar]
- 5.Pheby DFH, Araja D, Berkis U, Brenna E, Cullinan J, de Korwin J-D, Gitto L, Hughes DA, Hunter RM, Trepel D, et al. (2021). A Literature Review of GP Knowledge and Understanding of ME/CFS: A Report from the Socioeconomic Working Group of the European Network on ME/CFS (EUROMENE). Medicina (Mex.) 57, 7. 10.3390/medicina57010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naviaux RK., Naviaux JC., Li K., Bright AT., Alaynick WA., Wang L., Baxter A., Nathan N., Anderson W., and Gordon E. (2016). Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci 113, E5472–E5480. 10.1073/pnas.1607571113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaneetharaja N, Griffiths V, Wileman T, and Carding SR (2016). A Role for the Intestinal Microbiota and Virome in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)? J. Clin. Med 5, E55. 10.3390/jcm5060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheedy JR, Wettenhall REH, Scanlon D, Gooley PR, Lewis DP, McGregor N, Stapleton DI, Butt HL, and DE Meirleir KL (2009). Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. Vivo Athens Greece 23, 621–628. [PubMed] [Google Scholar]
- 9.Frémont M, Coomans D, Massart S, and De Meirleir K. (2013). High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 22, 50–56. 10.1016/j.anaerobe.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, and Frank DN (2015). Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PloS One 10, e0145453. 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, and Hanson MR (2016). Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 4, 30. 10.1186/s40168016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giloteaux L, Hanson MR, and Keller BA (2016). A Pair of Identical Twins Discordant for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Differ in Physiological Parameters and Gut Microbiome Composition. Am. J. Case Rep 17, 720–729. 10.12659/ajcr.900314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy-Szakal D, Williams BL, Mishra N, Che X, Lee B, Bateman L, Klimas NG, Komaroff AL, Levine S, Montoya JG, et al. (2017). Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 5, 44. 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visconti A., Le Roy CI., Rosa F., Rossi N., Martin TC., Mohney RP., Li W., de Rinaldis E., Bell JT., Venter JC., et al. (2019). Interplay between the human gut microbiome and host metabolism. Nat. Commun 10, 4505. 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, and Siuzdak G. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci 106, 3698–3703. 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, and Gordon JI (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 17.Hooper LV, Littman DR, and Macpherson AJ (2012). Interactions Between the Microbiota and the Immune System. Science 336, 1268–1273. 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, and Pettersson S. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 19.Valdes AM, Walter J, Segal E, and Spector TD (2018). Role of the gut microbiota in nutrition and health. BMJ 361, k2179. 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, and Vinolo MAR (2016). Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol 5, e73. 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorucci S, Biagioli M, Zampella A, and Distrutti E. (2018). Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol 9, 1853. 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, and Deyn PPD (2019). Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol 10, 2565. 10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, and Julius D. (2017). Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 170, 185–198.e16. 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian X, Chi L, Gao B, Tu P, Ru H, and Lu K. (2017). The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLOS ONE 12, e0178426. 10.1371/journal.pone.0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin AM, Sun EW, Rogers GB, and Keating DJ (2019). The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol 10, 428. 10.3389/fphys.2019.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh KS., Kumar S., Mohanty AK., Grover S., and Kaushik JK. (2018). Mechanistic insights into the host-microbe interaction and pathogen exclusion mediated by the Mucusbinding protein of Lactobacillus plantarum. Sci. Rep 8, 14198. 10.1038/s41598-018-32417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Sun L, Zhang S, Wang S, and Lu M. (2019). High-Resolution Profiling of Gut Bacterial Communities in an Invasive Beetle using PacBio SMRT Sequencing System. Insects 10, E248. 10.3390/insects10080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noecker C, McNally CP, Eng A, and Borenstein E. (2017). High-Resolution Characterization of the Human Microbiome. Transl. Res. J. Lab. Clin. Med 179, 7–23. 10.1016/j.trsl.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, Graeber TG, Sonnenburg JL, Horvath S, Huttenhower C, et al. (2013). Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome 1, 17. 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim E-J, Ahn Y-C, Jang E-S, Lee S-W, Lee S-H, and Son C-G (2020). Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med 18, 100. 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikova E, Reshkova V, Kumanova А, Raleva S, Alexandrova D, Capo N, Murovska M, and European Network on ME/CFS (EUROMENE) (2020). Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J. Med. Virol 10.1002/jmv.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clmc van C., Fwa V, Pc R, and Fc V. (2020). Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract 5. 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Becker P, McGregor N, and De Meirleir K. (2001). A definition-based analysis of symptoms in a large cohort of patients with chronic fatigue syndrome. J. Intern. Med 250, 234–240. 10.1046/j.1365-2796.2001.00890.x. [DOI] [PubMed] [Google Scholar]
- 34.Roma M, Marden CL, Flaherty MAK, Jasion SE, Cranston EM, and Rowe PC (2019). Impaired Health-Related Quality of Life in Adolescent Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Impact of Core Symptoms. Front. Pediatr 7, 26. 10.3389/fped.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson PJ, Oh J, Robison J, Grady J, and Kuchel G. (2020). 1206. Association of Aging, Frailty and Place of Residence with Skin, Oral and Gut Microbiome Characteristics and Pathogenicity Reservoirs. Open Forum Infect. Dis 7, S625. 10.1093/ofid/ofaa439.1391. [DOI] [Google Scholar]
- 36.Larson PJ., Zhou W., Santiago A., Driscoll S., Fleming E., Voigt AY., Chun OK., Grady JJ., Kuchel GA., Robison JT., et al. (2022). Associations of the skin, oral and gut microbiome with aging, frailty and infection risk reservoirs in older adults. Nat. Aging 2, 941–955. 10.1038/s43587-022-00287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosca A, Leclerc M, and Hugot JP (2016). Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol 7, 455. 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haahtela T. (2019). A biodiversity hypothesis. Allergy 74, 1445–1456. 10.1111/all.13763. [DOI] [PubMed] [Google Scholar]
- 39.Behary J, Amorim N, Jiang X-T, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, et al. (2021). Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun 12, 187. 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin AM, Sun EW, Rogers GB, and Keating DJ (2019). The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol 10, 428. 10.3389/fphys.2019.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñiz Pedrogo DA, Chen J, Hillmann B, Jeraldo P, Al-Ghalith G, Taneja V, Davis JM, Knights D, Nelson H, Faubion WA, et al. (2019). An Increased Abundance of Clostridiaceae Characterizes Arthritis in Inflammatory Bowel Disease and Rheumatoid Arthritis: A Cross-sectional Study. Inflamm. Bowel Dis. 25, 902–913. 10.1093/ibd/izy318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emiola A, and Oh J. (2018). High throughput in situ metagenomic measurement of bacterial replication at ultra-low sequencing coverage. Nat. Commun 9, 4956. 10.1038/s41467-018-07240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, and Calignano A. (2011). Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. WJG 17, 1519–1528. 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrer-Picón E, Dotti I, Corraliza AM, Mayorgas A, Esteller M, Perales JC, Ricart E, Masamunt MC, Carrasco A, Tristán E, et al. (2020). Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 26, 43–55. 10.1093/ibd/izz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseini E, Grootaert C, Verstraete W, and Van de Wiele T. (2011). Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev 69, 245–258. 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 46.Taleb S. (2019). Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol 10, 2113. 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Go EK, Jung KJ, Kim JY, Yu BP, and Chung HY (2005). Betaine Suppresses Proinflammatory Signaling During Aging: The Involvement of Nuclear Factor-κB via Nuclear Factor-Inducing Kinase/IκB Kinase and Mitogen-Activated Protein Kinases. J. Gerontol. Ser. A 60, 1252–1264. 10.1093/gerona/60.10.1252. [DOI] [PubMed] [Google Scholar]
- 48.Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, and Peng Y. (2018). Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol 9, 1070. 10.3389/fimmu.2018.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Germain A, Barupal DK, Levine SM, and Hanson MR (2020). Comprehensive Circulatory Metabolomics in ME/CFS Reveals Disrupted Metabolism of Acyl Lipids and Steroids. Metabolites 10, 34. 10.3390/metabo10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nkiliza A., Parks M., Cseresznye A., Oberlin S., Evans JE., Darcey T., Aenlle K., Niedospial D., Mullan M., Crawford F., et al. (2021). Sex-specific plasma lipid profiles of ME/CFS patients and their association with pain, fatigue, and cognitive symptoms. J. Transl. Med 19, 370. 10.1186/s12967-021-03035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noda M, Ifuku M,Hossain Md.S., and Katafuchi T. (2018). Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front. Psychiatry 9, 589. 10.3389/fpsyt.2018.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo P, Zhang K, Ma X, and He P. (2020). Clostridium species as probiotics: potentials and challenges. J. Anim. Sci. Biotechnol 11, 24. 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brkic S, Tomic S, Maric D, Mikic AN, and Turkulov V. (2010). Lipid peroxidation is elevated in female patients with chronic fatigue syndrome. Med. Sci. Monit 16, CR628–CR632. [PubMed] [Google Scholar]
- 54.Tomic S, Brkic S, Maric D, and Mikic AN (2012). Lipid and protein oxidation in female patients with chronic fatigue syndrome. Arch. Med. Sci. AMS 8, 886–891. 10.5114/aoms.2012.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Núñez B, Tarasse R, Vogelaar EF, Janneke Dijck-Brouwer DA, and Muskiet FAJ (2018). Higher Prevalence of “Low T3 Syndrome” in Patients With Chronic Fatigue Syndrome: A Case–Control Study. Front. Endocrinol 9, 97. 10.3389/fendo.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores S, Brown A, Adeoye S, Jason L, and Evans M. (2013). Examining the Impact of Obesity on Individuals with CFS. Workplace Health Saf. 61, 299–307. 10.3928/2165079920130617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Germain A, Ruppert D, Levine SM, and Hanson MR (2018). Prospective Biomarkers from Plasma Metabolomics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Implicate Redox Imbalance in Disease Symptomatology. Metabolites 8, 90. 10.3390/metabo8040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakhan SE., and Kirchgessner A. (2010). Gut inflammation in chronic fatigue syndrome. Nutr. Metab 7, 79. 10.1186/1743-7075-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson ML, and Bruck D. (2012). Sleep Abnormalities in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Review. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 8, 719–728. 10.5664/jcsm.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boneva RS, Lin J-MS, Wieser F, Nater UM, Ditzen B, Taylor RN, and Unger ER (2019). Endometriosis as a Comorbid Condition in Chronic Fatigue Syndrome (CFS): Secondary Analysis of Data From a CFS Case-Control Study. Front. Pediatr 7, 195. 10.3389/fped.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pataky MW, Young WF, and Nair KS (2021). Hormonal and Metabolic Changes of Aging and the Influence of Lifestyle Modifications. Mayo Clin. Proc 96, 788–814. 10.1016/j.mayocp.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jura M, and Kozak Leslie.P. (2016). Obesity and related consequences to ageing. Age 38, 23. 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natelson BH (2019). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: Definitions, Similarities and Differences. Clin. Ther 41, 612–618. 10.1016/j.clinthera.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris S, Gilbert M, Beasant L, Linney C, Broughton J, and Crawley E. (2017). A qualitative investigation of eating difficulties in adolescents with chronic fatigue syndrome/myalgic encephalomyelitis. Clin. Child Psychol. Psychiatry 22, 128–139. 10.1177/1359104516646813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, and Bultman SJ (2011). The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 13, 517–526. 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, van Eijk H, Canfora EE, and Blaak EE (2019). Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep 9, 12515. 10.1038/s41598-019-48775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo C, Che X, Briese T, Allicock O, Yates RA, Cheng A, Ranjan A, March D, Hornig M, Komaroff AL, et al. (2021). Deficient butyrate-producing capacity in the gut microbiome of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome patients is associated with fatigue symptoms. Cell Host & Microbe in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Proal A, and Marshall T. (2018). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity. Front. Pediatr 6, 373. 10.3389/fped.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lupo GFD, Rocchetti G, Lucini L, Lorusso L, Manara E, Bertelli M, Puglisi E, and Capelli E. (2021). Potential role of microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME). Sci. Rep 11, 7043. 10.1038/s41598-021-86425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Morate E, Gimeno-Mallench L, Stromsnes K, Sanz-Ros J, Román-Domínguez A, Parejo-Pedrajas S, Inglés M, Olaso G, Gambini J, and Mas-Bargues C. (2020). Relationship between Diet, Microbiota, and Healthy Aging. Biomedicines 8, 287. 10.3390/biomedicines8080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fromentin S., Forslund SK., Chechi K., Aron-Wisnewsky J., Chakaroun R., Nielsen T., Tremaroli V., J B., Prifti E., Myridakis A., et al. (2022). Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med 28, 303–314. 10.1038/s41591-022-01688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karhan E, Gunter CL, Ravanmehr V, Horne M, Kozhaya L, Renzullo S, Placek L, George J, Robinson PN, Vernon SD, et al. (2019). Perturbation of effector and regulatory T cell subsets in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) 10.1101/2019.12.23.887505. [DOI] [Google Scholar]
- 73.Russell L, Broderick G, Taylor R, Fernandes H, Harvey J, Barnes Z, Smylie A, Collado F, Balbin EG, Katz BZ, et al. (2016). Illness progression in chronic fatigue syndrome: a shifting immune baseline. BMC Immunol. 17, 3. 10.1186/s12865-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bar N, Korem T, Weissbrod O, Zeevi D, Rothschild D, Leviatan S, Kosower N, Lotan-Pompan M, Weinberger A, Le Roy CI, et al. (2020). A reference map of potential determinants for the human serum metabolome. Nature 588, 135–140. 10.1038/s41586-020-2896-2. [DOI] [PubMed] [Google Scholar]
- 75.Yancey JR, and Thomas SM (2012). Chronic fatigue syndrome: diagnosis and treatment. Am. Fam. Physician 86, 741–746. [PubMed] [Google Scholar]
- 76.Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A, and Dubbo Infection Outcomes Study Group (2006). Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333, 575. 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pendergrast T, Brown A, Sunnquist M, Jantke R, Newton JL, Strand EB, and Jason LA (2016). Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic Illn. 12, 292–307. 10.1177/1742395316644770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Komaroff AL, and Bateman L. (2021). Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med 7, 1132. 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, and Komaroff A. (1994). The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med 121, 953–959. 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 80.Carruthers BM (2007). Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J. Clin. Pathol 60, 117–119. 10.1136/jcp.2006.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, and Institute of Medicine (2015). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness (National Academies Press (US)). [PubMed] [Google Scholar]
- 82.Beghini F, McIver LJ, Blanco-Míguez A, Dubois L, Asnicar F, Maharjan S, Mailyan A, Manghi P, Scholz M, Thomas AM, et al. (2021). Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 10, e65088. 10.7554/eLife.65088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 84.Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-0140550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garza DR, van Verk MC, Huynen MA, and Dutilh BE (2018). Towards predicting the environmental metabolome from metagenomics with a mechanistic model. Nat. Microbiol 3, 456–460. 10.1038/s41564-018-0124-8. [DOI] [PubMed] [Google Scholar]
- 86.Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, Tickle TL, Weingart G, Ren B, Schwager EH, et al. (2021). Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol 17, e1009442. 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Demographic information of cohorts and Clinical metadata summary. Related to STAR Methods.
Supplementary Table 2 Metadata. Related to STAR Methods.
Supplementary Table 3 Species relative abundances derived from MetaPhlAn3. Related to Figure 2, 3 and 4.
Supplementary Table 4 KEGG gene reads count from metagenomic data. Related to Figure 4.
Supplementary Table 5 Plasma metabolome raw data (peaks area-under-the-curve). Related to Figure 4 and 5.
Supplementary Table 6 Feature importance and annotation from multi-‘omics classification model and Butyrate pathway: the statistics of key microbes and key enzymes in KEGG butanoate metabolism, and their correlations with key plasma metabolites. Related to Figure 5 and 6.
Supplementary Table 7 Confounder driven features identified by MaAsLin2, Related to STAR Methods
Data Availability Statement
Metagenomics data have been deposited at PRJNA878603 and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. All original code has been deposited at https://github.com/ohlab/MECFS_2021 and is publicly available as of the date of publication.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Stool samples from ME/CFS patients and healthy donors | This study | N/A |
| Plasma samples from ME/CFS patients and healthy donors | This study | N/A |
| Critical commercial assays | ||
| QIAamp 96 DNA QIAcube HT Kit | QIAGEN | #51331 |
| BioCollector fecal collection kit | The BioCollective, Denver, CO | https://www.thebiocollective.com/products-services |
| OMNIgene•GUT tube | DNA Genotek | OMR-200 |
| Deposited data | ||
| Metagenomics sequencing raw data | This study | NCBI-SRA: Bioproject PRJNA878603 |
| Software and algorithms | ||
| R version (v4.0.4) | ||
| Python (v3.10.4) | ||
| scythe (v0.994) | Buffalo, 2014 | https://github.com/vsbuffalo/scythe |
| sickle (v1.33) | Joshi and Fass, 2011 | https://github.com/najoshi/sickle |
| MetaPhlAn3 | Beghini et al., 2021 | https://huttenhower.sph.harvard.edu/metaphlan/ |
| Bowtie2 (v2.3.1) | Langmead and Salzberg, 2012 | https://github.com/BenLangmead/bowtie2 |
| GRiD (v1.3) | Emiola et al., 2018 | https://github.com/ohlab/GRiD |
| USEARCH (v8.0.1517) | Edgar, 2010 | https://www.drive5.com/usearch/ |
| dplyr_1.0.5 | R package | https://dplyr.tidyverse.org/index.html |
| microbiome_1.12.0 | R package | https://microbiome.github.io/tutorials/ |
| phyloseq_1.34.0 | R package | https://joey711.github.io/phyloseq/ |
| maaslin2_1.8.0 | R package | https://huttenhower.sph.harvard.edu/maaslin/ |
| globaltest_5.44.0 | R package | https://www.bioconductor.org/packages/release/bioc/html/globaltest.html |
| bcdstats_0.0.0.9005 | R package | https://github.com/bcdudek/bcdstats/ |
| lme4_1.1–29 | R package | https://cran.rproject.org/web/packages/lme4/index.html |
| vegan_2.6–2 | R package | https://cran.r-project.org/web/packages/vegan/index.html |
| deseq2_v1.18.1 | R package | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| scipy_1.8.0 | python package | https://scipy.org/ |
| jupyter_4.9.2 | python package | https://jupyter.org/ |
| numpy_1.22.3 | python package | https://numpy.org/ |
| pandas_1.4.2 | python package | https://pandas.pydata.org/ |
| scikit-learn_1.0.2 | python package | https://scikit-learn.org/stable/index.html |
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.